Introduction
Myoepithelial carcinoma (MEC) of the salivary gland
is a rare malignant tumour that primarily affects the parotid
gland, accounting for ~1% of all salivary gland tumours (1). Its intricate and varied morphology,
derived from the epithelial cells of the excretory ducts, often
leads to confusion with similar tumours (2). Factors such as cell type, growth
pattern and treatment approach may contribute to a lower
disease-free survival rate (3).
Clinically, MEC typically presents as a slowly growing,
asymptomatic mass. Early symptoms are subtle and the clinical
manifestations are non-specific, making it easy for both patients
and clinicians to overlook the condition. MEC is characterised by
the cellular, uniform growth of myoepithelial cells and a
multinodular, expansile, invasive pattern with zonal cellular
distribution (4). Myoepithelial
tumours, including myoepithelioma and MED, demonstrate
myoepithelial differentiation and lack a ductal component (5). These neoplasms can be particularly
challenging to diagnose, as they are often mistaken for other
cutaneous or soft tissue neoplasms (6). The primary criteria for diagnosis are
histopathology and immunophenotype. MEC, a highly malignant
salivary gland tumour, has a higher propensity to involve cervical
or distal lymph nodes than epithelial-MEC, carcinoma ex pleomorphic
adenoma or mucinous adenocarcinoma. In addition, MEC has a high
risk of recurrence or multiple recurrences following surgery and
the prognosis is poor (7). A
retrospective analysis was conducted on a single case of MEC of the
submandibular gland who was admitted to our hospital. The following
aspects were examined: Clinical features, pathological
characteristics, differential diagnosis and patient prognosis.
Case report
An 82-year-old man was admitted to the Affiliated
Hospital of Jining Medical University (Jining, China) in May 2024
with a 60-year history of a mass on the upper left side of the
neck. Approximately 60 years previously, the patient had noticed a
lesion on the left side of the neck, initially about the size of a
pea. The swelling was asymptomatic, with no itching, numbness or
discomfort, and no treatment was sought. However, the swelling
gradually increased in size over time. At one month prior, the
patient had undergone an ultrasound examination at Juancheng
Friendship Hospital (Heze, China), which revealed a homogeneous
paramedian gland mass on the left side of the neck. The nature of
the irregular lesion adjacent to the left submandibular gland was
unclear. The patient was advised to monitor the condition and no
treatment was administered at that time. Five days later, a
follow-up ultrasound at the same hospital showed an inhomogeneous
mass adjacent to the left submandibular gland, distinct from the
mass within the gland itself. Referral to a higher-level hospital
for further evaluation and treatment was recommended. Subsequently,
the patient was admitted to the stomatology department of the
Affiliated Hospital of Jining Medical University (Jining, China)
for further management. Surgical treatment was advised and the
patient was admitted to the outpatient clinic with the diagnosis of
a ‘left cervical mass’. Throughout this period, the patient's level
of consciousness remained clear and the patient's general condition
was stable, with a consistent diet, normal sleep patterns, regular
bowel movements and no significant changes in body weight or
physical strength since the onset of the swelling. The patient had
a five-year history of ‘cerebral infarction’ but had not been on
any medication for this condition, which had remained stable. The
patient's past medical history was otherwise unremarkable. Although
the patient did not smoke, there was a history of occasional
alcohol consumption, drinking ~250 ml 40-degree alcohol per
occasion for the past 50 years. There was no family history of
hereditary or infectious diseases and no similar ailments had been
previously reported.
Stomatology specialist examination indicated the
following: The face of the patient showed no sores or carbuncles
and maxillofacial symmetry was intact. There was a noticeable
swelling in the left upper part of the neck, measuring ~6×5 cm. The
skin over the lesion had a medium texture, with a distinct boundary
and poor mobility. The local skin temperature, colour and tension
were normal and no numbness or paralysis were observed on the left
side of the neck. The patient's bite was satisfactory and the mouth
opening and shape were acceptable. The secretion from the salivary
glands was clear and the entrance to the parotid ducts showed no
redness or swelling on either side. The patient was admitted to the
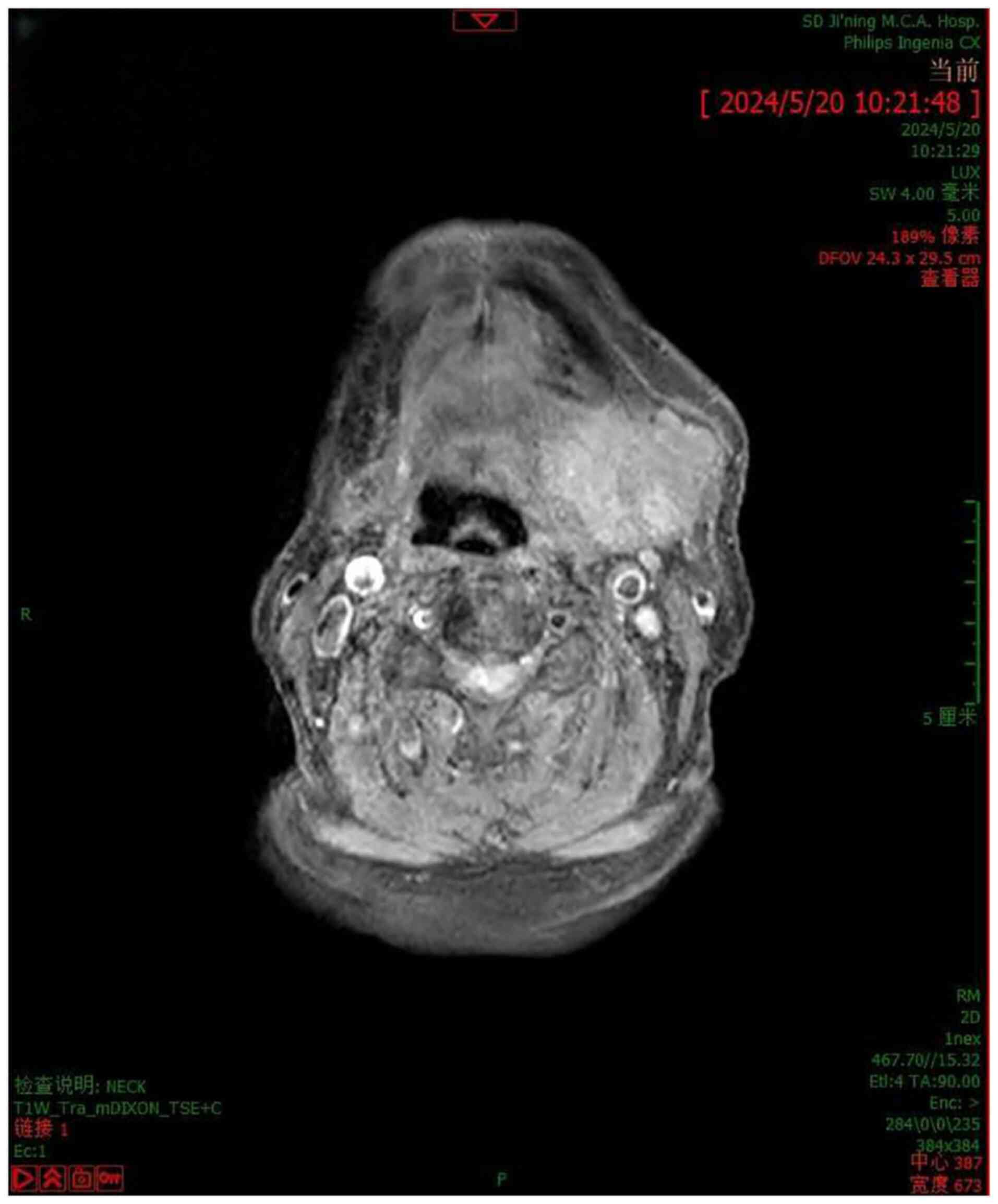
hospital for further necessary examinations. On admission in May
2024, neck MRI (Fig. 1) indicated
the following: i) An occupying lesion in the left submandibular
gland area, not clearly confined to the gland itself; enhancement
scanning was recommended, but enhancement was not performed; ii)
numerous lymph node shadows of varying sizes were present in the
bilateral submandibular region. The patient was then subjected to
surgery. Pathological examination (macroscopy) indicated a single
grey-red mass measuring 5×5×4 cm with a rough texture and a cut
surface showing grey-white and grey-yellow areas. The report of
intraoperative frozen pathology described the presence of a
malignant tumour in the left upper neck, confirmed by paraffin
section-based analysis. The tissue was paraffin-embedded or was
rapidly frozen. For the paraffin-embedding samples, 10% formalin
was used to fix the tissue for ~18 h at 36°C. The sections were ~4
µm-thick, and were placed in melted paraffin, and soaked at ~58°C.
The wax dipping time was determined according to the size and type
of the tissue, and was typically 2–4 h. The wax-impregnated tissue
sample was placed in an embedding mould. For the intraoperative
frozen samples, the fresh tissue was quickly placed in a frozen
microtome at −20°C for rapid freezing for 7–15 min. The thickness
of ordinary tissue sections was 7 µm. The section thickness of
lymph nodes or small cell tumours was 4–5 µm. The adipose tissue
sections were 10 µm-thick, and the pure adipose tissue sections
were 12–15 µm-thick. A paraffin slicer was used to cut the paraffin
embedding block into thin slices. The samples were fixed with 95%
ethanol and ice acetone for 20 min. After which, the sample was
washed with PBS twice for 1 min each time. The sample was incubated
for 2–3 min with haematoxylin dye solution to nucleate the sample
at 26°C and then rinsed with tap water. After which, the sample was
immersed in eosin dye solution for 1 min for cytoplasmic staining
at 26°C and then rinsed with tap water. The diagnosis was made by
observing the sections with an optical microscope and combining the
immunohistochemical result(s).
Malignancy was defined by the presence of severe
nuclear atypia with easily discerned nucleoli with or without high
mitotic count and necrosis (8).
Microscopic observation indicated that the cells of the tissue were
closely arranged, the nuclei were large and deeply stained and the
tissue had atypia; accordingly, it was not a benign tumour.
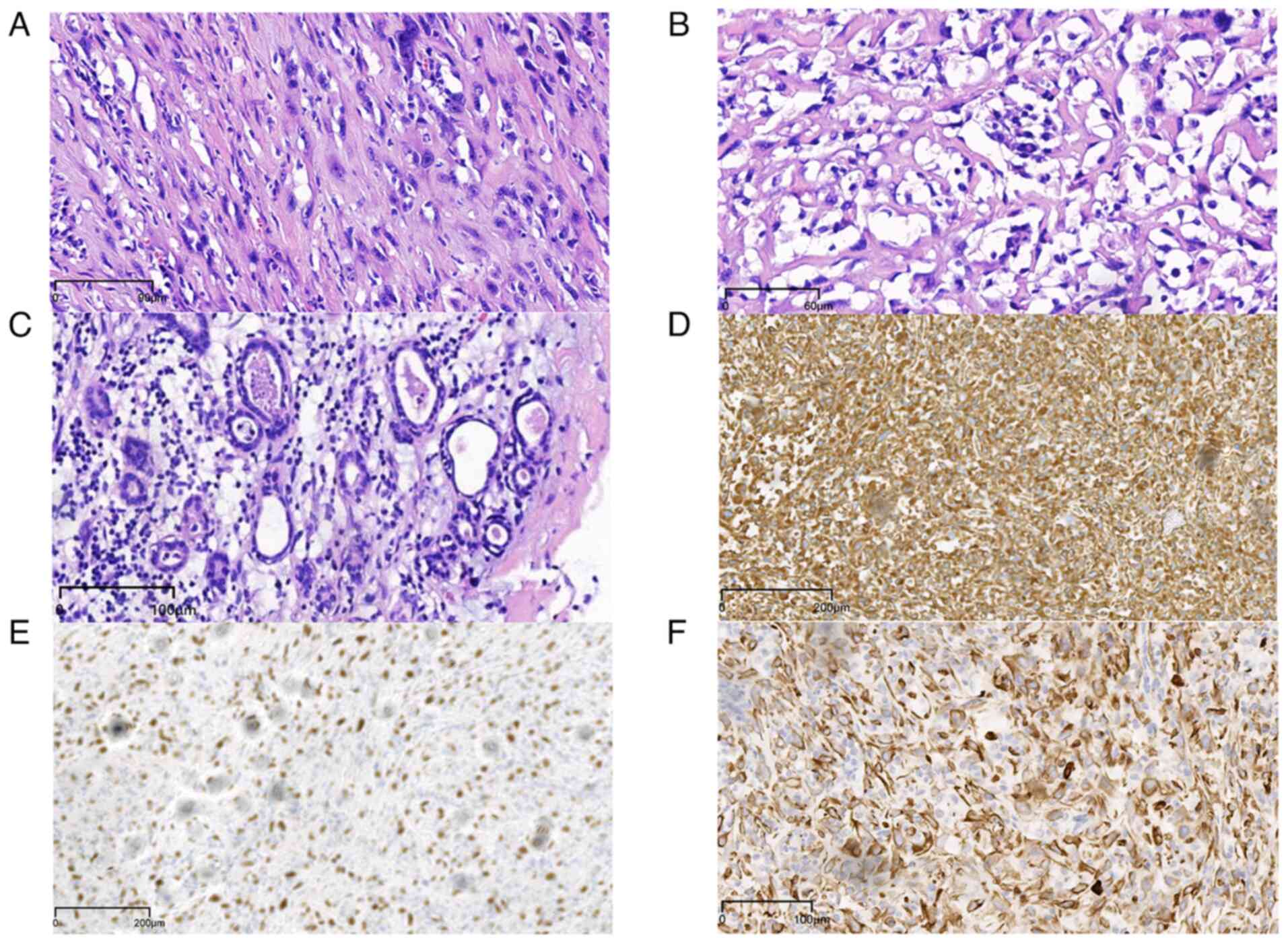
Pathological microscopic observations showed that tumour cells
varied in size, with deeply stained nuclei and distinct nucleolus
(Fig. 2). The immunohistochemistry
data showed that the myoepithelium expressed smooth muscle actin,
calponin, vimentin and P40 (9). In
the routine pathology report from the end of May 2024, the paraffin
section was confirmed as a malignant tumour in the left upper neck,
with features consistent with pleomorphic adenoma and MEC, based on
immunohistochemistry. First, paraffin embedding of the tissue
samples, dewaxing in xylene and rehydration in an ethanol series
were performed. The samples were then soaked in PBS twice for 5 min
each at room temperature. For antigen repair, samples were heated
under 100 kpa in 0.01 mol/l citric acid buffer, at 99°C for 10 min.
After soaking in PBS twice for 5 min each at room temperature,
samples were blocked with 100 ml non-immune normal goat serum
(Beyotime Institute of Biotechnology) at room temperature for 60
min. Following the aspiration of the sealing liquid, antibody
incubation was performed. A drop of the monoclonal antibody was
added at a dilution ratio of 1:100 and incubated at 4°C for 14 h.
Slides were fully soaked in PBS with Tween-20 (PBST) 3 times for 5
min each. Subsequently, an immunohistochemical secondary antibody
was applied with a dilution ratio of 1:200 and incubation at room
temperature for 30 min. Samples were fully soaked in PBST 5 times
for 5 min each. Subsequently, visualisation was performed with
diaminobenzidine. Following counterstaining with hematoxylin,
samples were dehydrated with ethanol, sealed with neutral gum and
and observed under a microscope.
The following primary antibodies were used (all from
Beyotime Institute of Biotechnology): CK pan-antibody (PanCK)
rabbit antibody, cat. no. AG8362; P40 rabbit antibody, cat. no.
AG8590; anaplastic lymphoma kinase (ALK) rabbit antibody, cat. no.
AG8114; CD34 rabbit antibody, cat. no. AG8229; Desmin rabbit
antibody, cat. no. AG8366. epithelial membrane antigen (EMA) rabbit
antibody, cat. no. AF2191; CD31 rabbit antibody, cat. no. AG8226;
vimentin rabbit antibody, cat. no. AG8772; Ki-67 rabbit antibody,
cat. no. AG8471. Secondary antibodies were as follows:
Biotin-labeled goat anti-rabbit IgG(H+L), cat. no. A0277;
biotin-labeled goat anti-mouse IgG(H+L), cat. no. A0288 (both from
Beyotime Institute of Biotechnology).
Due to the high variability of salivary gland
myoepithelial cells, a combination of immunohistochemical tests
with a number of markers of myoepithelial origin, including SMA,
vimentin, p40 and p63, are required to confirm the diagnosis
(9). The tumour cells in the
present study were positive for myoepithelial cells. S-100, p63 and
CK5/6 can play a certain auxiliary role in the diagnosis of MEC,
and in combination with other myoepithelial-specific antigenic
markers, they have significance in the qualitative diagnosis of MEC
(10). It is hypothesized that the
use of Ki-67 antibody immunohistochemical staining to identify the
proliferative activity of cells may be helpful in the differential
diagnosis of benign and malignant myoepithelioma (11). The immunohistochemistry results were
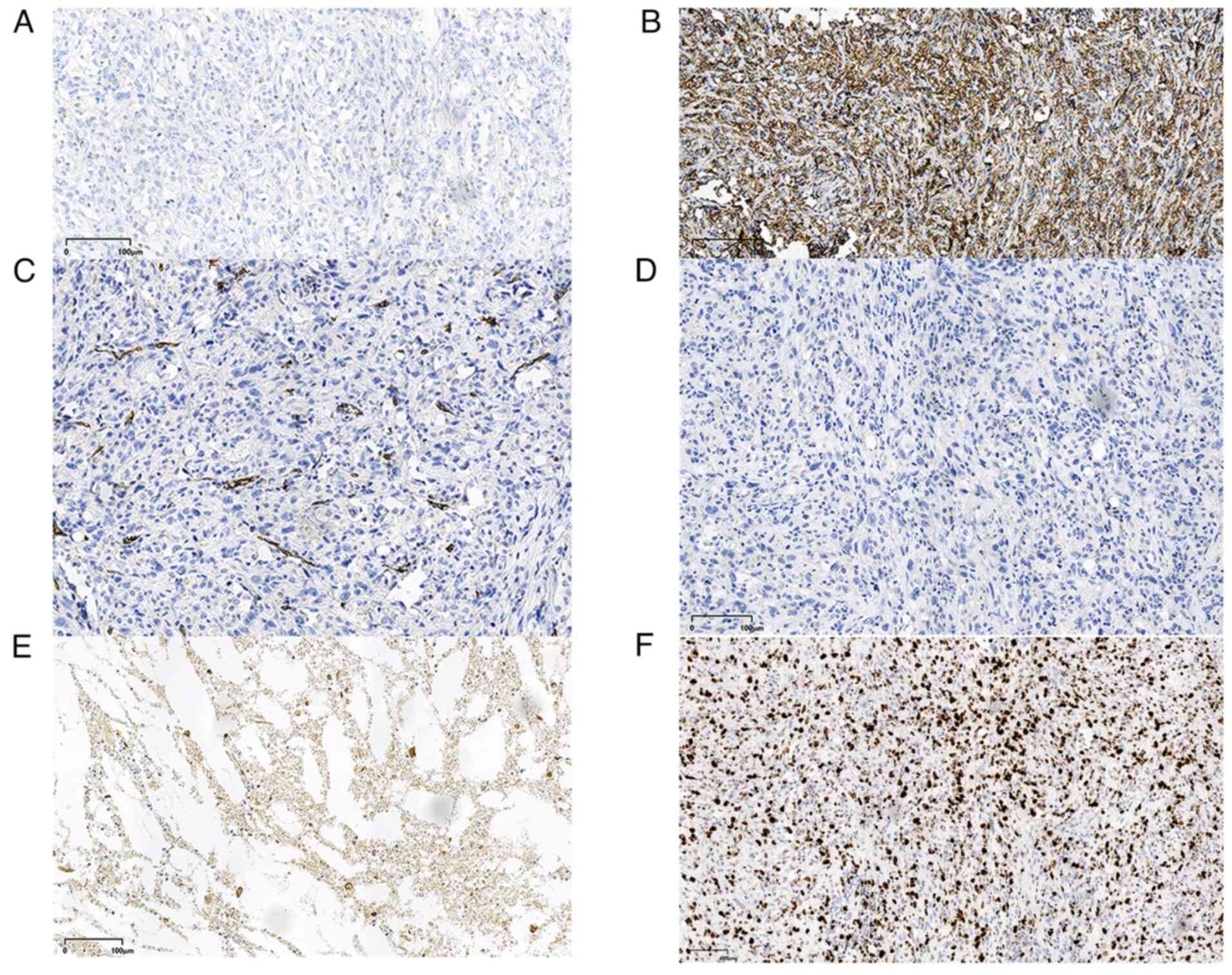
as follows: CD31 (−) (Fig. 3B),
CD34 (−) (Fig. 3C), cytokeratin
(CK)7 (+), vimentin (+) (Fig. 2D),
Ki-67 (+, 70%), erythroid growth factor receptor (−), desmin (−)
(Fig. 3D), ALK (−) (Fig. 3A), P40 (+) (Fig. 2E), PanCK (+) (Fig. 2F) and EMA (−) (Fig. 3E). The patient underwent neck mass
resection under sedation with complex anaesthesia 5 days after
admission. No surgical contraindications were identified. The
postoperative course was uneventful and no radiotherapy or
chemotherapy was administered, as myoepithelial carcinoma is not
sensitive to radiotherapy or chemotherapy (12). At 3 months after the surgery,
patient visited the Affiliated Hospital to see the stomatology
specialist and no evidence of recurrence or metastasis was found.
The patient is being followed up by telephone calls at fortnightly
intervals to enquire if the patient has any abnormalities, and the
patient is coming for monthly visits to the hospital's Dental
Department.
Histological analysis performed according to
standard procedures indicated that the tumour exhibited
infiltrative growth with uneven cell density. Observation under the
microscope revealed that the tumour had grown into the surrounding
adipose tissue, showing invasive growth, which is consistent with
the growth pattern of malignant tumours, and the density of
different areas of the tumour was different. The tumour cells were
primarily spindle-shaped, consistent with myoepithelial cell
morphology. Furthermore, certain cells were polygonal or oval, with
abundant translucent cytoplasm, centrally located rounded nuclei
and clear nuclear pleomorphism, including nuclear schizophrenia,
and these were all showing a high degree of antypia. Certain areas
of the tumour displayed features of pleomorphic adenoma,
characterised by a two-layered structure of inner adenoepithelium
and outer myoepithelium, with a mucus-like mesenchymal stroma and a
peritoneal membrane on the surface.
Discussion
MEC, also known as malignant myoepithelioma, is a
morphologically diverse tumour type that can arise de novo
or result from the malignant transformation of its benign
counterpart (13). MEC was first
recognised as a distinct entity in 1991, when it was included in
the second edition of the World Health Organization's histological
classification of salivary gland tumours (14). It is characterised by the absence of
duct formation and typically exhibits only myoepithelial
differentiation (15). The most
common site for MEC is the parotid gland, followed by the minor
salivary glands (16). Beyond the
salivary glands, MEC can also occur in other sites, such as the
epidermis, soft tissues, breast, nasal sinuses, nasopharynx,
tongue, lacrimal gland, as well as in the lungs, heart, liver and
stomach (16). Clinically, MEC
frequently presents as a mass in the parotid gland, oral cavity or
neck. These masses are typically slow-growing, painless and
movable, with no apparent signs of malignancy during initial
imaging (17). As a result, they
are frequently misdiagnosed as benign tumours. In advanced stages,
the tumour often invades surrounding tissues, leading to
infiltrative growth and dysfunction of adjacent organs. As the
tumour enlarges, patients may experience a variety of symptoms,
including pain or numbness of the tongue if the lingual nerve is
affected (18). MEC tumour cells
exhibit a range of histological patterns, including spindle- and
plasma-like, epithelial and hyaline cells. Different cell patterns
may coexist within the same tumour (19). Current diagnostic criteria for MEC
include both morphology and immunohistochemistry, which demonstrate
significant myoepithelial differentiation and clear infiltration
into adjacent salivary glands or other tissues (20). Malignancy is determined by assessing
cellular heterogeneity, regional necrosis and infiltration
(21). The presence or absence of
tumour necrosis is critical in determining prognosis. Tumours
without necrosis tend to have a lower incidence of distant
metastases, while necrosis is associated with a lower disease-free
survival rate (22). The Ki-67
proliferation index is a useful tool in distinguishing between
benign and malignant myoepitheliomas (10). A Ki-67 proliferation index >10%
supports a diagnosis of MEC (11),
while an index >50% is linked to a poor prognosis (10). In the present case, the mass
infiltrated the surrounding fatty tissue and the Ki-67
proliferation index exceeded 10%. The densely packed spindle cell
area displayed significant heterogeneity. Immunohistochemistry
results were consistent with the diagnosis of MEC.
MEC must be distinguished from the following
tumours: i) Epithelial-MEC: This tumour is most commonly found in
the parotid gland (57.7%) and the submandibular gland (20). It is more prevalent in females, with
an average age of diagnosis at 60 years (23). Epithelial-MEC is characterised by a
dual population of epithelial and myoepithelial cells, showing
epithelial differentiation (24).
Immunohistochemistry reveals that the inner layer of the glandular
epithelium expresses CK, EMA and other specific markers, while the
outer myoepithelial cells express vimentin, S-100 and P63 to
varying degrees. P63 has the highest diagnostic accuracy (10). Both adenoepithelial and
myoepithelial cells express PanCK (+). ii) yoepithelioma: This is a
benign tumour with well-defined boundaries a peripheral membrane,
low cellular heterogeneity, rare nuclear pleomorphism, a low cell
proliferation index and a low cellular proliferation rate. Unlike
MEC, myoepithelioma does not exhibit any infiltrative growth
pattern, and MEC shows significant heterogeneity (25).
In summary, MEC is relatively rare but exhibits a
high rate of metastasis and local recurrence (13). Therefore, early diagnosis is
critical (26). Complete surgical
excision remains the treatment of choice (27). Factors such as age at diagnosis,
American Joint Committee on Cancer stage, Tumour-Nodes-Metastasis
stage and treatment type significantly impact survival (28). To reduce the risk of postoperative
recurrence, the initial surgery should be comprehensive, with
adequate safety margins. Early diagnosis and treatment can
significantly reduce the local recurrence rate and improve both the
survival rate and prognosis for patients.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Jining Medical
University High-level Research Project Cultivation Programme (grant
no. JYGC2022FKJ014).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL performed case data collection and manuscript
drafting. QW modified the manuscript, analyzed the results of the
HE sections and immunohistochemistry and made corresponding
diagnosis, which provided guidance for the definition, diagnostic
points and differential diagnosis and differentiation knowledge of
myoepithelial carcinoma. YL and QW confirmed the authenticity of
the raw data. Both authors agreed on the journal to which the
article was submitted and agreed to be accountable for all aspects
of the work. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim SH, Park SE, Bae HG, Song DH, Oh HH,
Cho KR, Kim HJ and Sohn BS: Epithelial-myoepithelial carcinoma of
the nasopharynx: A case report and review of the literature. Oncol
Lett Aug. 10:927–930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah AA, Mulla AF and Mayank M:
Pathophysiology of myoepithelial cells in salivary glands. J Oral
Maxillofac Pathol. 20:480–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lavareze L, Scarini JF, de Lima-Souza RA,
Kimura TC, Gondak RO, Egal ESA, Altemani A and Mariano FV:
Clinicopathological and survival profile of patients with salivary
gland myoepithelial carcinoma: A systematic review. J Oral Pathol
Med. 52:101–108. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu B and Katabi N: Myoepithelial
carcinoma. Surg Pathol Clin Mar. 14:67–73. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosen LE, Singh RI, Vercillo M and Gattuso
P: Myoepithelial carcinoma of the lung: A review. Appl
Immunohistochem Mol Morphol. 23:397–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henning A, Pennington G, Deeken A and
Srivastava S: Myoepithelial carcinoma of the digit. J Cutan Pathol.
49:111–115. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanesv SV and Bagwanin IN:
Myoepithelialcarcinoma of the salivary glands: A clinicopathologic
study of 51 cases in atertiary cancer center. Arch Otolaryngol Head
Neck Surg. 136:702–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jo VY, Antonescu CR, Hornick JL and Patel
RM: Myoepithelioma, myoepithelial carcinoma, and mixed tumor. In:
WHO Classification of Tumours Editorial Boardeditor. WHO
classification of tumours, soft tissue and bone tumours. 5th
edition. Geneva, Switzerland: World Health Organization; pp.
277–279. 2020
|
|
9
|
Seethala RR, Barnes EL and Hunt JL:
Epithelial-myoepithelial carcinoma: A review of the
clinicopathologic spectrum and immunophenotypic characteristics in
61 tumors of the salivary glands and upper aerodigestive tract. Am
J Surg Pathol. 31:44–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peilong CA, Shaoqiang Z and Jiyuan ZH:
Clinicopathological analysis of 12 cases of myoepithelial carcinoma
of salivary gland. J Clin Exp Pathol. 33:174–177. 2017.
|
|
11
|
Nagao T, Sugano I and Ishida Y: Salivary
gland malig-nant myoepithelioma: A clinicopathologic and
immunohisto-chemical study of ten cases. Cancer. 83:1292–1299.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo-Liang PI, Peng XU and Guang H:
Research progress of myoepithelial carcinoma. China Cancer Clinics.
44:253–257. 2017.(In Chinese).
|
|
13
|
Aiba H, Errani C, Ciani G, Gambarotti M,
Righi A, Maioli M, Spinnato P, Frega G, Ibrahim T and Longhi A:
Myoepithelial carcinoma of soft tissues and bone. Eur J Cancer.
194:1133532023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dinker D, Rajan K, Sharma S and Kumar NA:
Myoepithelial carcinoma of sinonasal cavity: Peculiar diagnosis,
conventional treatment. Indian J Otolaryngol Head Neck Surg.
75:3929–3935. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vilar-González S, Bradley K, Rico-Pérez J,
Vogiatzis P, Golka D, Nigam A, Sivaramalingam M and Kazmi S:
Epithelial-myoepithelial Carcinoma-review of clinicopathological
and immunohistochemical features. Clin Transl Oncol. 17:847–855.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masuya D, Haba R, Huang CL and Yokomise H:
Myoepithelial carcinoma of the lung. Eur J Cardiothorac Surg.
28:775–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nicholas RG, Hanson JA and Meiklejohn DA:
Myoepithelial cell carcinoma of the oral tongue: Case report and
review of the literature. Clin Med Insights Oncol.
13:11795549198382542019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu H, Zhang F, Peng J, Wu Z, Zhang X and
Wu X: Epithelial-myoepithelial carcinoma of the esophagus: A Case
Report. Front Surg. 9:9420192022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shaoyan L, Song NI, Yiming Z and Jian W:
Analysis of diagnosis and treatment of myoepithelial carcinoma of
the submandibular gland. Chin Med J. 50:28–29. 2015.
|
|
20
|
Waitzman J, Waitzman A, Powers J and
Deraniyagala R: Epithelial-myoepithelial carcinoma of the nasal
cavity: A case report. Ear Nose Throat J. 31:14556132311899622023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao F'Zhu H and Huang Y: Myoepithelial
carcinoma inside of maxilla bone: A case report. Mol Clin Oncol.
1:315–317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Passador-Santos F, Grönroos M, Irish J,
Gilbert R, Gullane P, Perez-Ordonez B, Mäkitie A and Leivo I:
Clinicopathological characteristics and cell cycle proteins as
potential prognostic factors in myoepithelial carcinoma of salivary
glands. Virchows Arch. 468:305–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaur R, Melgandi W, Rathi AK, Singh K,
Rahman F and Mandal S: Epithelial myoepithelial carcinoma: A case
report. J Cancer Res Ther. Jan 22–2024.(Epub ahead of print).
|
|
24
|
Ru K, Srivastava A and Tischler AS:
Primary myoepithelial carcinoma of palate. Arch Pathol Lab Med.
128:92–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thompson LDR and Xu B: Top ten
differentials to mull over for head and neck myoepithelial
neoplasms. Head Neck Pathol. 17:1–15. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yokose C, Asai J, Kan S, Nomiyama T,
Takenaka H, Konishi E, Goto K, Ansai SI and Katoh N: Myoepithelial
carcinoma on the right shoulder: A case report with published work
review. J Dermatol. 43:1083–1087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren J, Liu Z, Liu X, Li Y, Zhang X, Li Z,
Yang Y, Yang Y, Chen Y and Jiang S: Primary myoepithelial carcinoma
of palate. World J Surg Oncol. 9:1042011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gore MR: Epithelial-myoepithelial
carcinoma: A population-based survival analysis. BMC Ear Nose
Throat Disord. 18:152017. View Article : Google Scholar : PubMed/NCBI
|