Introduction
Globally, ovarian cancer is a primary cause of
cancer-related death among women with malignant tumors of the
reproductive system (1). Krukenberg
tumors, a specialized form of metastatic ovarian cancer, are
histologically defined by the presence of mucin-filled signet ring
cells (2). Predominantly, these
tumors originate from the stomach (76%), with the colorectum being
the next most common primary site (11%), classifying them as rare
and highly malignant metastatic neoplasms with a poor patient
prognosis (2–4). The rarity of Krukenberg tumors,
coupled with the typically short survival time of those affected,
results in a lack of consensus regarding their diagnosis and
treatment (3,5). Furthermore, the absence of
comprehensive genomic data impedes research into the underlying
molecular mechanisms of this disease (6). These factors constrain the
comprehension of this rare disease and impede the advancement of
targeted treatment strategies.
The present study focused on two critical oncogenes:
AT-rich interaction domain 1A (ARID1A) and KRAS. The
KRAS gene is commonly mutated in multiple types of cancer,
including pancreatic cancer, colorectal cancer (CRC) and lung
adenocarcinoma (7–9). KRAS encodes a protein that is a
key component of the MAPK/ERK signaling pathway (10), which serves a notable role in cell
proliferation, differentiation and survival (11). Mutations in KRAS typically
lead to the constitutive activation of this pathway, promoting the
continuous proliferation of tumor cells, and are associated with
tumor aggressiveness and therapeutic resistance (12). The ARID1A gene is an
N-terminal acetyltransferase (13),
and its function is closely related to the occurrence, progression
and metastasis of various types of cancer, including ovarian
cancer, endometrial cancer and gastric cancer (GC) (14–17).
ARID1A regulates the activity of cyclin proteins by
inactivating β-catenin, thereby affecting the progression of the
cell cycle (18). Moreover,
mutations in ARID1A are also associated with alterations in
the tumor immune microenvironment, potentially enhancing the
sensitivity of tumors to immunotherapy by affecting the stimulator
of interferon (IFN) genes (STING)/IFN signaling pathway and
promoting a robust antitumor T-cell response (14,19,20).
The present report describes the case a patient who
underwent adjuvant chemotherapy with oxaliplatin and tegafur
following curative resection for GC. The present study aims to
provide further genomics research into Krukenberg tumors, but also
to provide novel insights into understanding the pathogenesis of
Krukenberg tumors and exploring personalized treatment
strategies.
Case report
In September 2023, a 46-year-old female patient was
admitted to the Department of Obstetrics and Gynecology, Dongguan
Songshan Lake Tungwah Hospital (Dongguan, China), presenting with
abdominal distension and difficulty urinating. The patient had
experienced progressively worsening abdominal distension within 2
months and had lost 3 kg in weight within the 2 weeks prior to
admission. The patient had undergone curative surgery for GC in
July 2021, with the pathological results indicating poorly
differentiated adenocarcinoma of the stomach, with some areas
presenting characteristic signet ring cells. Postoperatively, the
patient received chemotherapy with oxaliplatin (150 mg,
intravenous) and tegafur (160 mg, oral) for eight cycles. The
patient denied experiencing any abnormal vaginal bleeding. On
physical examination in September 2023, a 10-cm diameter ovarian
mass was palpated behind and to the right of the uterus, which was
hard in texture, poorly mobile and non-tender. The laboratory tests
revealed CA199 at 96.0 U/ml (normal range 0–30 U/ml), CEA at 1.66
ng/ml (normal range 0–5 ng/ml), CA125 at 33.4 U/ml (normal range
0–47 U/ml), human epididymis protein 4 at 420.6 pmol/l (normal
range 0–76.2 pmol/l) and AFP at 2.6 IU/ml (normal range 0–7 IU/ml).
Both CA199 and human epididymis protein 4 were above the normal
levels; hemoglobin was 54 g/l (normal range 115–155 g/l) and
creatinine was 135.2 µmol/l (normal range 44–97 µmol/l).
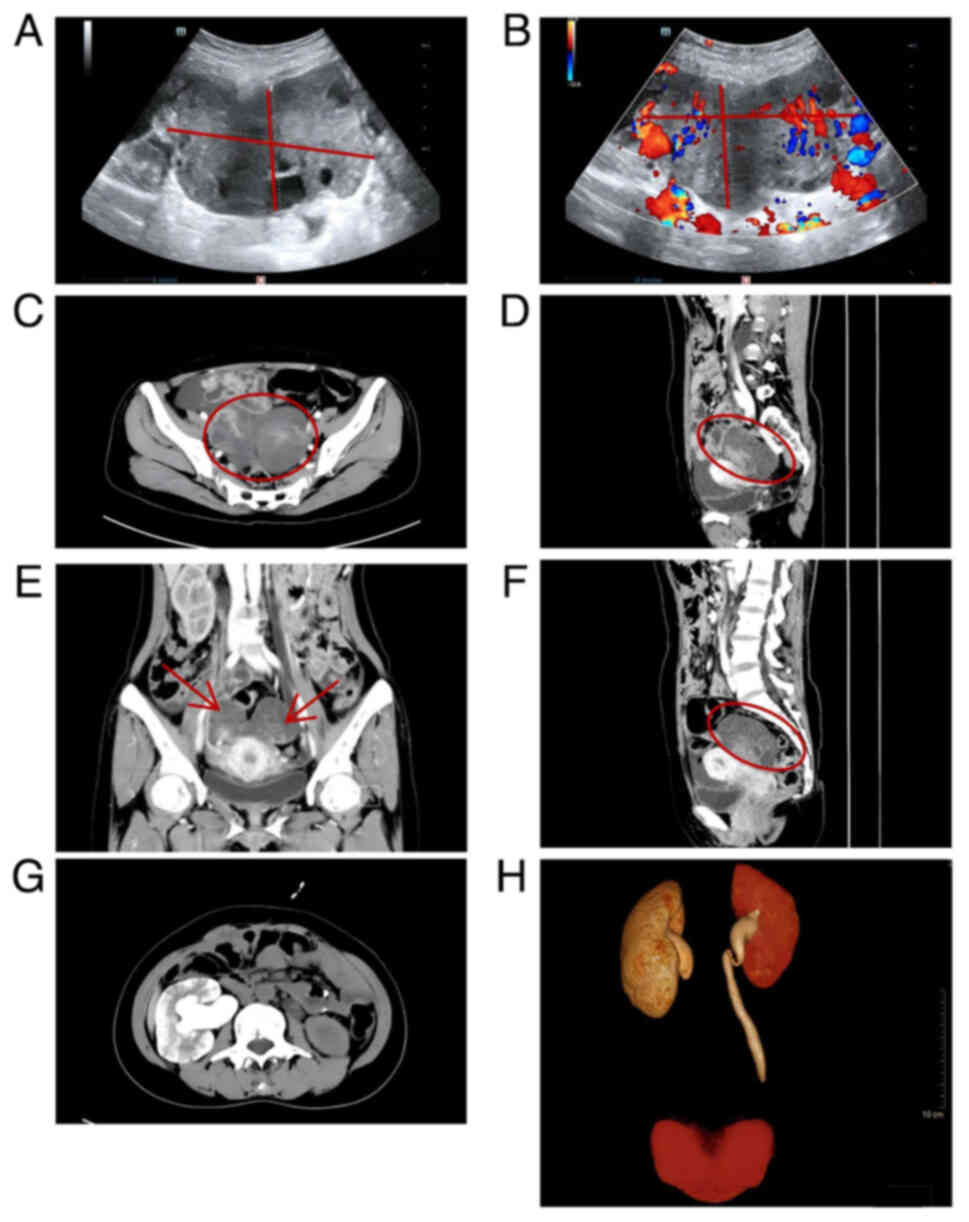
An abdominal color Doppler ultrasound indicated a
hypoechoic mass behind the uterus, measuring ~110×67×47 mm, with
imaging revealing abundant blood flow signals within the mass
(Fig. 1A and B). Enhanced abdominal
computed tomography (CT) clearly depicted the tumor vasculature and
necrotic lesions. An irregular cystic-solid mass was observed in
the pelvic cavity, which began to enhance earlier than the uterine
wall after the injection of contrast medium Ioversol, showing
heterogeneous hyperenhancement. A large vessel could be observed
entering the mass from one side, and heterogeneous enhancement
areas within the mass were noted, which are indicative of tumor
necrosis or liquefaction, signs suggestive of a malignant tumor
(Fig. 1C-F). Due to the
accompanying symptoms of difficulty urinating, further examination
with CT urography and three-dimensional reconstruction revealed an
irregular mass in the pelvic cavity, with compression and narrowing
of the lower segments of both ureters, leading to dilatation of the
upper ureteral segments and bilateral hydronephrosis. There was a
notable delay in the excretion of the right urinary system, with
local suspected obstruction, surrounding exudate and possible tumor
invasion requiring further investigation (Fig. 1G and H).
The patient was informed that the treatment options
included chemotherapy or cytoreductive surgery. The following
treatment plan was determined based on the preference of the
patient: Total abdominal hysterectomy, bilateral
salpingo-oophorectomy, pelvic adhesiolysis, transurethral bilateral
ureteroscopy and bilateral ureteral stent placement. The patient
initially underwent a challenging surgical procedure with thorough
exploration of the pelvic and abdominal cavities, the observations
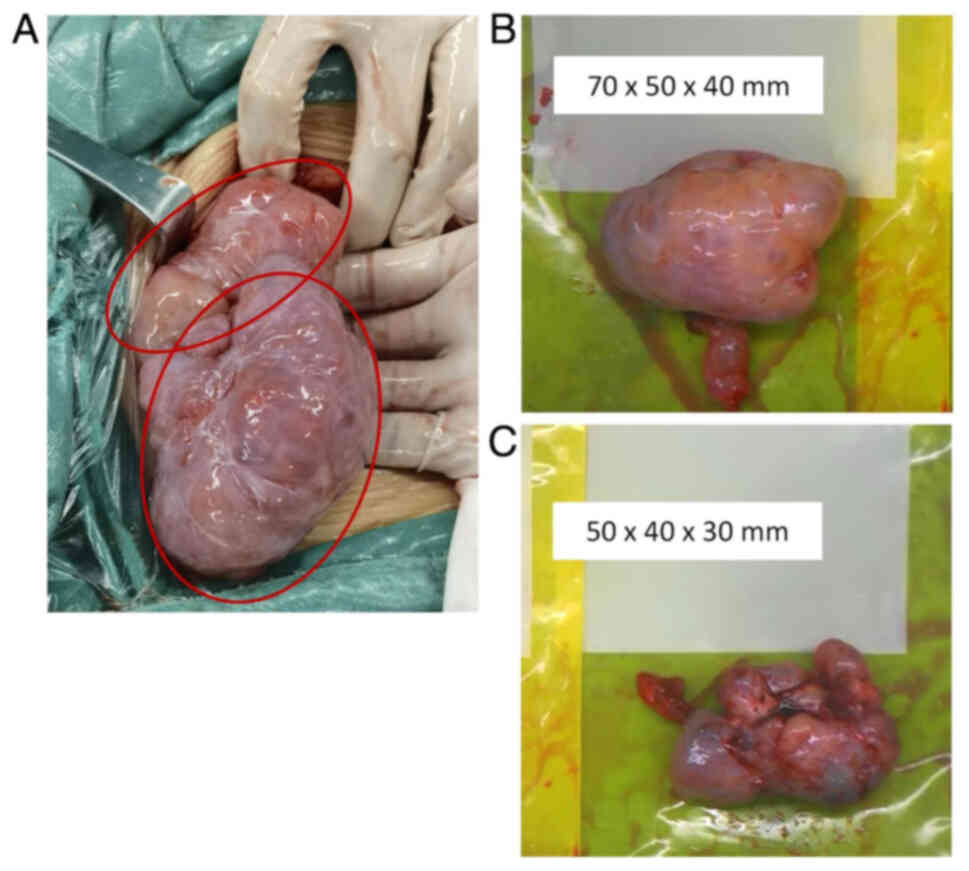
included: ~100 ml mucinous ascites in the pelvic and abdominal
cavities, as well as two solid masses behind the uterus,
originating from the ovaries, measuring ~70×50×40 mm on the left
and 50×40×30 mm on the right. The surfaces of the masses were
smooth with intact capsules, exhibiting a reniform shape. The rest
of the abdomen and organ surfaces were smooth with no obvious
lesions. The bilateral adnexa and masses were resected (Fig. 2A-C). Frozen section diagnosis
revealed poorly differentiated carcinoma of the ovaries on both
sides, with some cancer cells exhibiting signet ring cell carcinoma
(SRCC), consistent with metastatic GC. Consequently, an additional
total hysterectomy was performed and transurethral ureteroscopy
plus bilateral ureteral stent placement was conducted
intraoperatively. The surgical exploration revealed pale mucosa of
the bilateral ureters, with the middle and upper segments becoming
narrow and rigid due to compression, and no obvious tumor invasion
was seen on the ureteral wall. At the end of the surgery, a single
intraperitoneal chemotherapy dose of cisplatin was administered (70
mg in 1,000 ml distilled water).
Postoperatively, further pathological histological
examination was conducted. Tissue samples were fixed in 10%
neutral-buffered formalin at room temperature for 24 h and embedded
in paraffin. Sections 4-µm thick were prepared and stained with
hematoxylin for 5 min and eosin for 2 min at room temperature
(Fig. 3A-C). The stained sections
were examined under a light microscope to identify infiltrating
tumor cells in the stroma. Mucin-laden signet ring cells with
eccentric hyperchromatic nuclei were observed. These cells were
morphologically compatible with the cells of the previously
resected gastric adenocarcinoma tissue.
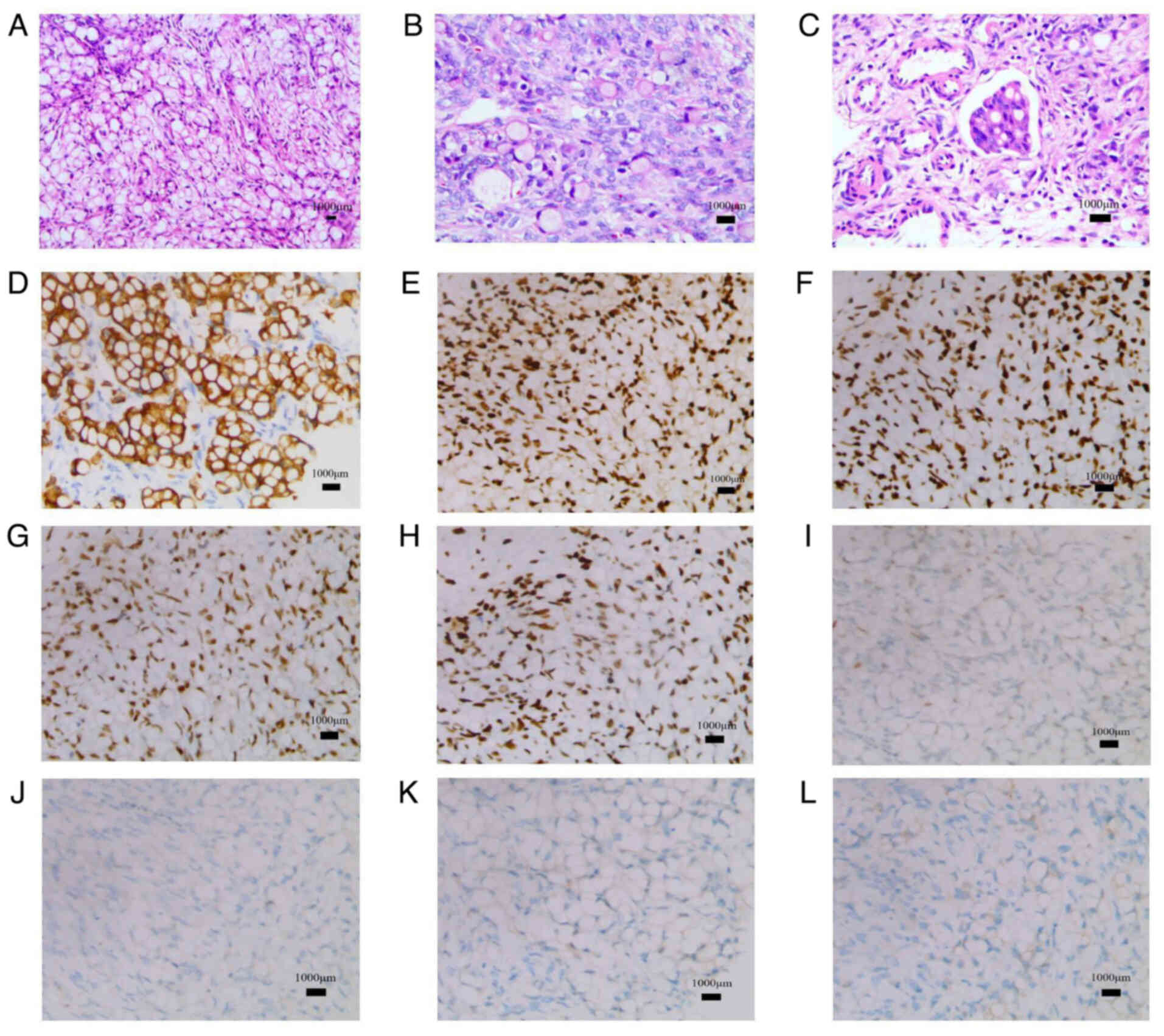 | Figure 3.Histological and immunohistochemical
findings. (A) Hematoxylin and eosin staining demonstrated the
overall morphology of the tissue (magnification, ×10). (B and C)
Microscopic hematoxylin and eosin findings showed a signet-ring
cell carcinoma composed of poorly cohesive tumor cells with
abundant intracytoplasmic mucin and eccentric nuclei
(magnification, ×20). (D) Immunohistochemical staining for
cytokeratin 7 highlighted epithelial cells (magnification, ×20).
(E) Immunohistochemical staining for MLH1, a marker to determine
the mismatch repair status (magnification, ×20).
Immunohistochemical staining for (F) MLH2 (magnification, ×20), (G)
PMS1 homolog 2 (magnification, ×20), (H) mutS homolog 6
(magnification, 20), (I) p53 (magnification, ×20), (J) paired box 8
(magnification, ×20), (K) SATB homeobox 2 (magnification, ×20) and
(L) HER-2 (magnification, ×20). MLH, mutL homolog. |
For immunohistochemical analysis, formalin-fixed,
paraffin-embedded tissue sections (4 µm) were used. Antigen
retrieval was performed by heating at 95°C in citrate buffer (pH
6.0) for 20 min, followed by washing with xylene and rehydration
through a graded ethanol series. Endogenous peroxidase activity was
blocked using 3% hydrogen peroxide for 10 min at room temperature.
Sections were then incubated with primary antibodies at 4°C
overnight, followed by incubation with HRP-conjugated secondary
antibodies for 30 min at room temperature. Immunoreactivity was
detected using DAB chromogen, and counterstaining was performed
with hematoxylin. The slides were examined under a light
microscope. In the immunohistochemical diagnosis, the following
markers were positive: Cytokeratin (CK)7, mutL homolog (MLH)1,
MLH2, PMS1 homolog 2 and mutS homolog 6, whereas p53 was wild-type
and there was no amplification of paired box 8 (PAX8), SATB
homeobox 2 (SATB2) or HER-2 (Fig.
3D-L). Based on the pathological results, it was confirmed that
the ovarian masses were metastatic carcinoma from the previously
treated GC, and the mucin-laden signet ring cells confirmed the
diagnosis of Krukenberg tumors.
In September 2023, to identify personalized
treatment strategies, whole exome sequencing was performed on
ovarian tumor tissue by Letu Biotechnology Co., Ltd. DNA was
extracted using the QIAamp DNA Mini Kit (Qiagen, Inc.). Library
preparation was conducted using the Agilent SureSelect XT Human All
Exon V8 Kit (Agilent Technologies, Inc.) following the
manufacturer's instructions. Sequencing was performed on an
Illumina NovaSeq 6000 platform using a paired-end 150 bp strategy.
The final library was loaded at a concentration of 10 nM, measured
using a Qubit 4 Fluorometer (Thermo Fisher Scientific, Inc.).
Genomic DNA was extracted using the FFPE Tissue Genomic DNA
One-Step Extraction Kit (Hangzhou Simgen Biotechnology Co., Ltd.)
and the QIAamp Circulating Nucleic Acid Kit (Qiagen, Inc.). The
xGen Lockdown Probe (Integrated DNA Technologies, Inc.), customized
with the KAPA Hyper Prep Kit (Kapa Biosystems; Roche Diagnostics),
was used for capture reactions, which were conducted with Dynabeads
M-270 (Thermo Fisher Scientific, Inc.) and the xGen Lockdown
Hybridization and Wash Kit (Integrated DNA Technologies, Inc.). The
libraries were amplified with Illumina p5 and p7 primers using the
KAPA HiFi HotStart ReadyMix (Kapa Biosystems; Roche Diagnostics),
and the library fragment sizes were determined using the KAPA
Library Quantification Kit (Kapa Biosystems; Roche Diagnostics) and
a Bioanalyzer 2100 (Agilent Technologies, Inc.). Target-enriched
libraries were sequenced on the HiSeq4000 NGS platform (Illumina,
Inc.). Data analysis included quality control with Trimmomatic
(version 0.39; http://github.com/usadellab/Trimmomatic), alignment
with BWA (version 0.7.17; http://github.com/lh3/bwa), PCR duplicate removal with
Picard (version 2.23.8; http://github.com/broadinstitute/picard) and variant
calling with Mutect2 [version 1.1.7 (legacy) or part of the GATK
4.x series (current); https://gatk.broadinstitute.org/hc/en-us/articles/360037593851-Mutect2]
and Scalpel (version 0.5.3; http://github.com/sleuthkit/scalpel). Annotations were
performed with vcf2maf (version 1.6.19; http://github.com/mskcc/vcf2maf). The sequencing depth
for the tumor tissue control sample was 1,500×.
The DNA sequencing results revealed two genetic
mutations (Table I). The
ARID1A gene harbored a frameshift mutation (c.4720delC),
where a cytosine was deleted at the 4,720th position of the DNA
sequence, resulting in the change of proline to histidine at the
1,575th amino acid position of the corresponding protein sequence.
Additionally, a missense mutation (c.35G>C) was detected in the
KRAS gene, with a guanine being replaced by a cytosine at
the 35th position of the DNA sequence, leading to the change of the
12th amino acid from glycine to alanine in the protein sequence. No
abnormalities in terms of gene fusions or copy number variations
were observed, and no mutations were found in genes related to the
homologous recombinational repair pathway. Microsatellite
instability (MSI) indicated a stable microsatellite phenotype.
 | Table I.Genome location of single nucleotide
variants, and insertion-deletions in tumor tissue. |
Table I.
Genome location of single nucleotide
variants, and insertion-deletions in tumor tissue.
| Gene | Transcript
number | Exon | Base change | Amino acid
change |
|---|
| ARID1A | NM_006015 | Exon 18 | c.4720delC | p.P1575Hfs*37 |
| KRAS | NM_004985 | Exon 2 | c.35G>C | p.G12A |
Postoperatively, the creatinine level of the patient
decreased from 135.2 µmol/l (normal range 44–97 µmol/l)
preoperatively to 66.0 µmol/l on day 4 after surgery, indicating
the restoration of normal kidney function. Given the stage IV
(International Federation of Gynecology and Obstetrics, 2021)
(21) poorly differentiated
adenocarcinoma, adjuvant systemic chemotherapy was recommended.
However, the patient, considering their predicted survival rate and
financial capacity, chose to decline chemotherapy. Finally, the
patient succumbed to the disease in January 2024. The overall
survival (OS) time, from the initial diagnosis of GC in July 2021
to the time of death, totaled 30 months. Specifically, the survival
period following recurrence with distant metastatic Krukenberg
tumors, identified in September 2023, was 4 months.
Discussion
Ovarian metastatic tumors result from the spread of
a primary cancer from another site to the ovaries (22), accounting for 10–25% of all ovarian
malignancies (23). Krukenberg
tumors, as a rare and distinct type of malignant tumor, represent
only 1–2% of these metastatic tumors (23,24).
Krukenberg tumors are diagnosed at a markedly younger age compared
with epithelial ovarian cancer, predominantly affecting
premenopausal women with an average age of diagnosis of 45 years
(3). A previous retrospective
analysis of Krukenberg tumors revealed that the median age at
diagnosis for these patients was 48 years, with ages ranging
between 22 and 71 years (25). The
patient reported in the present study was diagnosed at 46 years
old, which is in accordance with the age distribution cited in the
literature. Unlike tumors with overt mucin-laden signet ring cells
in the pathological histology, the clinical presentation of this
type of lesion is often more subtle, manifesting as an abdominal
mass with abdominal distension and insidious abdominal pain. Due to
its covert onset and lack of distinctive clinical features, when
ascites is present, the disease is typically at an advanced stage
(23,26). In the present case, the clinical
symptoms were limited to a 2-month history of abdominal distension,
and physical examination revealed only pelvic masses and dullness
to percussion, indicative of ascites.
Preoperative imaging reports also provide a certain
reference for the diagnosis of ovarian metastatic tumors. In the
present case report, imaging examinations confirmed no notable
lesions in other areas besides the adnexal region. Ultrasound
indicated a mass behind the uterus with abundant blood flow
signals, and enhanced CT suggested that the mass was a complex
cystic-solid lesion with irregular shape and heterogeneous
enhancement. Metastatic ovarian tumors typically manifest as solid
or mixed cystic-solid masses, often bilateral and multiple
(26), whereas primary ovarian
tumors typically present as cystic masses or masses with areas of
liquefied necrosis, often unilateral (27,28).
In the present case, the imaging results revealed a solitary
complex cystic-solid mass with significant enhancement areas,
considered to be areas of tumor necrosis or liquefaction, which is
different from the presentation of primary ovarian cancer,
increasing the difficulty of diagnosis.
The examination of tumor markers also provides a
basis for diagnosis. Elevated CA199 levels are often seen in
mucinous ovarian cancer, borderline tumors and gastrointestinal
metastatic ovarian cancer (29–32),
whereas increased CEA levels are common in gastrointestinal
metastatic ovarian cancer (33–36).
In the present case, the elevation of CA199 supported the diagnosis
of metastatic ovarian cancer, while CEA did not show a significant
increase. However, these tumor marker tests aid in diagnosis but
lack specificity. A definitive diagnosis of ovarian metastatic
tumors is more reliant on pathological and immunohistochemical
examinations. Negative expression of PAX8 can clearly rule out a
primary tumor and support a metastatic origin (37), CK7 and CK20 are important markers
for distinguishing ovarian tumors (2), and SATB2 is typically highly expressed
in the lower epithelial tissues of the gastrointestinal tract
(38). In the present case, SATB2
exhibited negative expression.
At present, there is a lack of standards or
consensus on the treatment of metastatic ovarian cancer due to an
insufficient number of case studies (3,5). While
appropriate surgical intervention can prolong the survival time of
patients with primary tumors, it is less effective for those with
metastatic tumors requiring combined treatment with radiotherapy or
chemotherapy, with recurrence often occurring within 2–5 years
(39). In the present case, NGS was
utilized for genomic analysis of the tumor tissue, revealing
mutations at c.4720delC in the ARID1A gene and c.35G>C in
the KRAS gene, with the aim of identifying potential
targeted therapies.
ARID1A, also known as NAA10 (40), is recognized as a tumor suppressor
gene that is integral to the SWI/SNF chromatin remodeling complex
(41). ARD1A has been
identified as one of the frequently mutated chromatin remodeling
genes in GC (42). Mutations in
ARD1A across the entire coding region are prevalent in GC,
typically resulting in inactivating mutations, including truncating
mutations and insertions/deletions that lead to frameshifts
(43,44), which have a pivotal role in the
initiation, progression and metastasis of cancer, as well as cell
cycle arrest (14,18). In terms of pathogenesis, mutations
in ARID1A can inactivate β-catenin, subsequently reducing
the transcription of cyclin D1, leading to cell cycle arrest at the
G0/G1 phase (18). Although cell cycle arrest is
generally considered an anticancer mechanism, certain studies have
proposed the concept of the DNA damage model, where cell cycle
arrest can, in some cases, drive cells into senescence, increasing
the risk of genetic mutations and thereby promoting cancer
development (45–47). This suggests that cell cycle arrest
induced by ARID1A mutations can exert stress on cells,
leading to the accumulation of DNA damage and further genetic
mutations, thus promoting cancer development.
Previous studies have underscored the importance of
ARID1A mutations in a spectrum of cancer types, including GC
and CRC (14–17); however, their role in metastatic
ovarian cancer, such as in Krukenberg tumors, remains inadequately
explored. In murine models, ARD1A has been demonstrated to
stabilize nuclear factor erythroid 2-related factor 2 through
direct interaction, thereby promoting the progression of CRC
(48). Furthermore, research has
established an association between ARID1A mutations and OS
in patients with GC. In a cohort of 518 patients with GC,
immunohistochemical assessments revealed that, compared with those
without ARID1A mutations, patients with
ARID1A-mutated GC were older, exhibited higher tumor MSI and
had a greater prevalence of PI3K/AKT pathway
mutations. Multivariate analysis indicated that ARID1A
mutations were an independent prognostic factor for diffuse-type GC
(49). These findings align with
the present case, suggesting that ARID1A mutations are not
isolated events and may have a role in the pathogenesis of
Krukenberg tumors. However, large-scale studies specifically
targeting Krukenberg tumors are lacking, underscoring the need for
further research to substantiate these observations.
In terms of treatment, certain clinical trials of
immune checkpoint inhibitors (ICIs) have revealed that
ARID1A mutations are significantly enriched in responders to
immunotherapy across various solid tumor types, independent of MSI
(50–54). Patients with ARID1A-mutated
gastrointestinal tumors have been shown to exhibit more favorable
treatment responses to programmed cell death protein 1
(PD-1)/programmed death-ligand 1 (PD-L1) therapies and have
improved survival outcomes compared with those with ARID1A
wild-type tumors (55). Preclinical
studies using mouse models have confirmed that the loss of
ARID1A in tumor cells induces R-loops, and the cellular
membrane DNA species produced by R-loops activate the STING/type I
IFN signaling pathway, inducing an ARID1A-IFN gene
expression signature that promotes antitumor immunity (14,19,20),
explaining the enhanced responsiveness of ARID1A-mutated
human tumors to ICIs. ICI therapy has also demonstrated favorable
response rates in a multitude of clinical trials involving GC.
Interim results from the Phase III MATTERHORN trial have indicated
that the application of a PD-L1 monoclonal antibody (durvalumab) in
combination with the FLOT regimen (docetaxel, 5-FU, leucovorin and
oxaliplatin) in patients with resectable GC and gastroesophageal
junction adenocarcinoma (GEJAC) yields a higher rate of
pathological complete response compared with the placebo group (19
vs. 7%) (56). Similarly,
preliminary results from the DANTE trial have suggested that the
combination of a PD-1 inhibitor (atezolizumab) with chemotherapy is
associated with improved safety and efficacy compared with the FLOT
regimen alone in patients with resectable GEJAC (57). These clinical data on immunotherapy
for GC indicate that ICIs, particularly PD-1 inhibitors, may
exhibit promising efficacy and safety profiles for patients with
Krukenberg tumors harboring ARID1A mutations.
KRAS gene mutations are closely associated
with tumor development, and the encoded protein is a key component
of the MAPK/ERK signaling pathway (10,11),
which can directly promote cell metabolism and proliferation,
participate in tumor immune evasion and modulate the immune system
response to tumor cells, thereby affecting the tumor
microenvironment (12). In the
present case, DNA sequencing revealed a KRAS mutation
corresponding to the amino acid change, p.G12A (glycine at the 12th
position), located in a key structural domain of the protein and a
known mutation hotspot (58,59).
Mutations at G12 affect the binding of KRAS protein to the GTP/GDP
cycle, leading to continuous activation of the KRAS protein,
abnormal activation of the MAPK/ERK signaling pathway, uncontrolled
cell proliferation and ultimately contributes to tumor invasion and
metastasis (60–62). KRAS mutations are among the
most extensively studied and are distinctively characterized
oncogenic alterations, occurring in 17–25% of all cancers, with a
prevalence of ~9% in GC and 30–40% in CRC (63,64).
In a cohort analysis of 595 patients with GC, those with
KRAS mutations and an MSI status exhibited a longer survival
time compared with patients without KRAS mutations and a
microsatellite stable status (65).
Furthermore, KRAS harboring the G12 mutation is associated
with poorer patient survival in GC (66,67).
Warneke et al (67)
evaluated the prognostic significance of different phenotypic and
genotypic markers, including KRAS, PIK3CA and MSI, in GC and
predicted the feasibility of applying these markers in personalized
therapy for GC. The results indicated that patients with proximal
GC harboring KRAS mutations had shorter survival times than
those without mutations (3.5 vs. 12.7 months). Additionally, in a
study of SRCC of the stomach, immunohistochemistry revealed
positive KRAS expression in the majority of SRCC samples, which was
higher than in the intestinal-type cohort (28 vs. 12.6%).
Concurrently, patients with KRAS mutations had a median OS
time of 12.5 months, compared with 19.5 months for those without
KRAS mutations, demonstrating a notable reduction in OS
(66).
In the therapeutic domain, studies have confirmed
that KRASG12C inhibitors, such as sotorasib and
adagrasib, have shown clinical activity in clinical trials for
KRASG12C-mutated non-small cell lung cancer (NSCLC) and
CRC (60,61,68,69).
Specifically, in patients with NSCLC, a confirmed response rate of
53.4% was observed, with a median progression-free survival (PFS)
time of 13.1 months. In CRC, 29.1% of patients exhibited a
response, with a PFS time of 5.6 months. Although treatment-related
adverse events occurred in 93% of patients, the most common being
nausea (74%), diarrhea (61%) and vomiting (58%), the majority were
grade 1–2 (94%) and resolved following symptomatic management and
drug discontinuation (68).
Overall, based on the current clinical data, KRASG12C
inhibitors demonstrate a favorable safety profile in other
malignancies, providing preliminary support for their potential
application in Krukenberg tumors.
While the present study underscores potential
therapeutic strategies based on ARID1A mutations and
KRASG12C inhibitors, their clinical application in
Krukenberg tumors remains speculative. The rarity of this disease
contributes to a scarcity of therapeutic references and research.
Considering the unique tumor microenvironment and immune
characteristics of Krukenberg tumors, larger cohort studies are
necessary to further evaluate the efficacy and safety of
immunotherapy in this tumor type.
In conclusion, the present study describes the case
of a patient with Krukenberg tumors harboring two mutated genes,
and discusses the molecular mechanisms and clinical significance of
tumor invasion caused by ARID1A and KRAS mutations.
The mutation in the ARID1A gene may increase sensitivity to
ICI therapy, whereas the successful application of
KRASG12C inhibitors in other cancer types provides novel
insights for targeted therapy of Krukenberg tumors. The patient in
the present case achieved an OS time of 30 months from the initial
diagnosis of GC, but only 4 months following the recurrence of
Krukenberg tumors. This highlights the poor prognosis of this
disease and the urgent need for the development of effective
therapeutic strategies. Nevertheless, as the first study, to the
best of our knowledge, to explore the genomic alterations of
Krukenberg tumors, it offers novel insights into the molecular
mechanisms of this rare disease. These preliminary findings may
stimulate further research to validate the efficacy of these
molecular targets, ultimately improving patient treatment
outcomes.
Acknowledgements
The authors would like to thank Dr Feng Na
(Department of Pathology, Dongguan Songshan Lake Hospital,
Dongguan, China) for the assistance and expertise contributed to
the present study.
Funding
This study was supported by a grant from the Dongguan Social
Development and Technology Program (grant no. 20211800901802).
Availability of data and materials
The datasets generated in the present study may be
found in the NCBI database under accession number PRJNA1240087 or
at the following URL: https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1240087.
Authors' contributions
JW was responsible for the conception and design of
the study, and drafted the manuscript. Data collection and analysis
were carried out by JW, SJ and QS. JW and HG confirm the
authenticity of all the raw data. HG contributed to the study
design and participated in the interpretation of the results. The
entire study was supervised by HG. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was carried out in accordance with
institutional guidelines and was approved by the Ethics Committee
of Dongguan Songshan Lake Tungwah Hospital (Dongguan, China;
approval no. SDHKY-2024-008-01), as it involved detailed clinical
features and sequencing data analysis beyond standard case
reporting. The study was conducted in accordance with institutional
guidelines and The Declaration of Helsinki. Written informed
consent was obtained from the patient for participation.
Patient consent for publication
The patient provided written informed consent for
the publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kossai M, Leary A, Scoazec JY and Genestie
C: Ovarian cancer: A heterogeneous disease. Pathobiology. 85:41–49.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Agha OM and Nicastri AD: An in-depth
look at Krukenberg tumor an overview. Arch Pathol Lab Med.
130:1725–1730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiyokawa T, Young RH and Scully RE:
Krukenberg tumors of the ovary a clinicopathologic analysis of 120
cases with emphasis on their variable pathologic manifestations. Am
J Surg Pathol. 30:277–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yada-Hashimoto N, Yamamoto T, Kamiura S,
Seino H, Ohira H, Sawai K, Kimura T and Saji F: Metastatic ovarian
tumors: A review of 64 cases. Gynecol Oncol. 89:314–317. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kodama M, Moeini A, Machida H, Blake EA,
Grubbs BH and Matsuo K: Feto-maternal outcomes of pregnancy
complicated by Krukenberg tumor: A systematic review of literature.
Arch Gynecol Obstet. 294:589–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang B, Tang Q, Xu L, Teng X, Ding W, Ren
G and Wang X: A comparative study of RTK gene status between
primary tumors, lymph-node metastases, and Krukenberg tumors. Mod
Pathol. 34:42–50. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Timar J and Kashofer K: Molecular
epidemiology and diagnostics of KRAS mutations in human cancer.
Cancer Metastasis Rev. 39:1029–1038. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prior IA, Hood FE and Hartley JL: The
frequency of ras mutations in cancer. Cancer Res. 80:2969–2974.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cook JH, Melloni GEM, Gulhan DC, Park PJ
and Haigis KM: The origins and genetic interactions of KRAS
mutations are allele- and tissue-specific. Nat Commun. 12:18082021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cox AD, Fesik SW, Kimmelman AC, Luo J and
Der CJ: Drugging the undruggable RAS: Mission possible? Nat Rev
Drug Discov. 13:828–851. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu WH, LaBella KA, Lin Y, Xu P, Lee R,
Hsieh CE, Yang L, Zhou A, Blecher JM, Wu CJ, et al: Oncogenic KRAS
drives lipofibrogenesis to promote angiogenesis and colon cancer
progression. Cancer Discov. 13:2652–2673. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee D, Jang MK, Seo JH, Ryu SH, Kim JA and
Chung YH: ARD1/NAA10 in hepatocellular carcinoma: Pathways and
clinical implications. Exp Mol Med. 50:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vokshi BH and Toska E: Mutant ARID1A:
Igniting cancer immunotherapy. Trends Immunol. 45:565–567. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mullen J, Kato S, Sicklick JK and Kurzrock
R: Targeting ARID1A mutations in cancer. Cancer Treat Rev.
100:1022872021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeda T, Banno K, Okawa R, Yanokura M,
Iijima M, Irie-Kunitomi H, Nakamura K, Iida M, Adachi M, Umene K,
et al: ARID1A gene mutation in ovarian and endometrial cancers
(Review). Oncol Rep. 35:607–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fontana B, Gallerani G, Salamon I, Pace I,
Roncarati R and Ferracin M: ARID1A in cancer: Friend or foe? Front
Oncol. 13:11362482023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim JH, Park JW and Chun YS: Human arrest
defective 1 acetylates and activates beta-catenin, promoting lung
cancer cell proliferation. Cancer Res. 66:10677–10682. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maxwell MB, Hom-Tedla MS, Yi J, Li S,
Rivera SA, Yu J, Burns MJ, McRae HM, Stevenson BT, Coakley KE, et
al: ARID1A suppresses R-loop-mediated STING-type I interferon
pathway activation of anti-tumor immunity. Cell.
187:3390–3408.e3319. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goswami S and Sharma P: Loss of ARID1A
‘loops’ in STING. Trends Immunol. 45:568–570. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berek JS, Renz M, Kehoe S, Kumar L and
Friedlander M: Cancer of the ovary, fallopian tube, and peritoneum:
2021 update. Int J Gynaecol Obstet. 155 (Suppl 1):S61–S85. 2021.
View Article : Google Scholar
|
|
22
|
Classic pages in obstetrics and
gynecology, . Friedrich Ernst Krukenberg: Fibrosarcoma ovarii
mucocellulare (carcinomatodes). Archiv für Gynäkologie, vol 50, pp.
287–321, 1896. Am J Obstet Gynecol. 117:5751973.PubMed/NCBI
|
|
23
|
Kubeček O, Laco J, Špaček J, Petera J,
Kopecký J, Kubečková A and Filip S: The pathogenesis, diagnosis,
and management of metastatic tumors to the ovary: A comprehensive
review. Clin Exp Metastasis. 34:295–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Z, Li C, Wang Z, Haybaeck J and Zhang
C: Cd44v6 acts as a directional responding factor in the process of
transcoelomic metastasis from gastric carcinoma to Krukenberg
tumor. Expert Rev Mol Diagn. 23:583–588. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thakur P, Sharma M, Chauhan A, Pal KM,
Thakur S, Gupta M and Kaushal S: Colorectal origin: A marker of
favorable outcome in Krukenberg Tumor? Results from Clinical and
Prognostic Analysis. South Asian J Cancer. 13:99–105. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu F and Zhao X, Mi B, Feng LU, Yuan NA,
Lei F, Li M and Zhao X: Clinical characteristics and prognostic
analysis of Krukenberg tumor. Mol Clin Oncol. 3:1323–1328. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scully RE and Sobin LH: Histologic typing
of ovarian tumors. Arch Pathol Lab Med. 111:794–795.
1987.PubMed/NCBI
|
|
28
|
Karaosmanoglu AD, Onur MR, Salman MC,
Usubutun A, Karcaaltincaba M, Ozmen MN and Akata D: Imaging in
secondary tumors of the ovary. Abdom Radiol (NY). 44:1493–1505.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang M, Shi M, Yu Y, Sang J, Wang H, Shi
J, Duan P and Ge R: The immune subtypes and landscape of
advanced-stage ovarian cancer. Vaccines (Basel). 10:14512022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo J, Yu J, Song X and Mi H: Serum CA125,
CA199 and CEA combined detection for epithelial ovarian cancer
diagnosis: A meta-analysis. Open Med (Wars). 12:131–137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Liu L and Yu Y: Mucins and
mucinous ovarian carcinoma: Development, differential diagnosis,
and treatment. Heliyon. 9:e192212023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsas A, Stefanoudakis D, Troupis T,
Kontzoglou K, Eleftheriades M, Christopoulos P, Panoskaltsis T,
Stamoula E and Iliopoulos DC: Tumor markers and their diagnostic
significance in ovarian cancer. Life (Basel).
13:16892023.PubMed/NCBI
|
|
33
|
Hu J, Khalifa RD, Roma AA and Fadare O:
The pathologic distinction of primary and metastatic mucinous
tumors involving the ovary: A re-evaluation of algorithms based on
gross features. Ann Diagn Pathol. 37:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hall C, Clarke L, Pal A, Buchwald P,
Eglinton T, Wakeman C and Frizelle F: A review of the role of
carcinoembryonic antigen in clinical practice. Ann Coloproctol.
35:294–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Locker GY, Hamilton S, Harris J, Jessup
JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF and Bast RC Jr;
ASCO, : ASCO 2006 update of recommendations for the use of tumor
markers in gastrointestinal cancer. J Clin Oncol. 24:5313–5327.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sturgeon CM, Duffy MJ, Stenman UH, Lilja
H, Brünner N, Chan DW, Babaian R, Bast RC Jr, Dowell B, Esteva FJ,
et al: National Academy of clinical biochemistry laboratory
medicine practice guidelines for use of tumor markers in
testicular, prostate, colorectal, breast, and ovarian cancers. Clin
Chem. 54:e11–e79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ozcan A, Shen SS, Hamilton C, Anjana K,
Coffey D, Krishnan B and Truong LD: PAX 8 expression in
non-neoplastic tissues, primary tumors, and metastatic tumors: A
comprehensive immunohistochemical study. Mod Pathol. 24:751–764.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berg KB and Schaeffer DF: SATB2 as an
immunohistochemical marker for colorectal adenocarcinoma: A concise
review of benefits and pitfalls. Arch Pathol Lab Med.
141:1428–1433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuroki L and Guntupalli SR: Treatment of
epithelial ovarian cancer. BMJ. 371:m37732020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuhns KJ, Zhang G, Wang Z and Liu W:
ARD1/NAA10 acetylation in prostate cancer. Exp Mol Med. 50:1–8.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu RC, Wang TL and Shih IM: The emerging
roles of ARID1A in tumor suppression. Cancer Biol Ther. 15:655–664.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chia NY and Tan P: Molecular
classification of gastric cancer. Ann Oncol. 27:763–769. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zang ZJ, Cutcutache I, Poon SL, Zhang SL,
McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A, et al:
Exome sequencing of gastric adenocarcinoma identifies recurrent
somatic mutations in cell adhesion and chromatin remodeling genes.
Nat Genet. 44:570–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang K, Kan J, Yuen ST, Shi ST, Chu KM,
Law S, Chan TL, Kan Z, Chan AS, Tsui WY, et al: Exome sequencing
identifies frequent mutation of ARID1A in molecular subtypes of
gastric cancer. Nat Genet. 43:1219–1223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Halazonetis TD, Gorgoulis VG and Bartek J:
An oncogene-induced DNA damage model for cancer development.
Science. 319:1352–1355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xue W, Zender L, Miething C, Dickins RA,
Hernando E, Krizhanovsky V, Cordon-Cardo C and Lowe SW: Senescence
and tumour clearance is triggered by p53 restoration in murine
liver carcinomas. Nature. 445:656–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fang X, Lee YH, Jang JH, Kim SJ, Kim SH,
Kim DH, Na HK, Kim KO, Baek JH and Surh YJ: ARD1 stabilizes NRF2
through direct interaction and promotes colon cancer progression.
Life Sci. 313:1212172023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu CH, Tseng CH, Huang KH, Fang WL, Chen
MH, Li AFY and Wu CW: The clinical signiicance of ARID1A mutations
in gastric cancer patients. Formosan J Surg. 53:93–100. 2020.
View Article : Google Scholar
|
|
50
|
Okamura R, Kato S, Lee S, Jimenez RE,
Sicklick JK and Kurzrock R: ARID1A alterations function as a
biomarker for longer progression-free survival after
anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer. 8:e0004382020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Botta GP, Kato S, Patel H, Fanta P, Lee S,
Okamura R and Kurzrock R: SWI/SNF complex alterations as a
biomarker of immunotherapy efficacy in pancreatic cancer. JCI
Insight. 6:e1504532021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Goswami S, Chen Y, Anandhan S, Szabo PM,
Basu S, Blando JM, Liu W, Zhang J, Natarajan SM, Xiong L, et al:
ARID1A mutation plus CXCL13 expression act as combinatorial
biomarkers to predict responses to immune checkpoint therapy in
mUCC. Sci Transl Med. 12:eabc42202020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li L, Li M, Jiang Z and Wang X: ARID1A
mutations are associated with increased immune activity in
gastrointestinal cancer. Cells. 8:6782019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu
XC, Liao W, Jiang Y, Lin XY, Zhang QY, et al: Efficacy, safety, and
correlative biomarkers of toripalimab in previously treated
recurrent or metastatic nasopharyngeal carcinoma: A phase II
clinical trial (POLARIS-02). J Clin Oncol. 39:704–712. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gu Y, Zhang P, Wang J, Lin C, Liu H, Li H,
He H, Li R, Zhang H and Zhang W: Somatic ARID1A mutation stratifies
patients with gastric cancer to PD-1 blockade and adjuvant
chemotherapy. Cancer Immunol Immunother. 72:1199–1208. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Janjigian YY, Al-Batran SE, Wainberg ZA,
Cutsem EV, Molena D, Muro K, Hyung WJ, Wyrwicz LS, Oh DY, Omori T,
et al: Pathological complete response (pCR) to 5-fluorouracil,
leucovorin, oxaliplatin and docetaxel (FLOT) with or without
durvalumab (D) in resectable gastric and gastroesophageal junction
cancer (GC/GEJC): Subgroup analysis by region from the phase 3,
randomized, double-blind MATTERHORN study. J Clin Oncol. 42:LBA246.
2024. View Article : Google Scholar
|
|
57
|
Lorenzen S, Götze TO, Thuss-Patience P,
Biebl M, Homann N, Schenk M, Lindig U, Heuer V, Kretzschmar A,
Goekkurt E, et al: Perioperative atezolizumab plus fluorouracil,
leucovorin, oxaliplatin, and docetaxel for resectable
esophagogastric cancer: Interim results from the randomized,
multicenter, phase II/III DANTE/IKF-s633 trial. J Clin Oncol.
42:410–420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Prior IA, Lewis PD and Mattos C: A
comprehensive survey of Ras mutations in cancer. Cancer Res.
72:2457–2467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Forbes SA, Bindal N, Bamford S, Cole C,
Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al:
COSMIC: Mining complete cancer genomes in the catalogue of somatic
mutations in cancer. Nucleic Acids Res. 39:D945–D950. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Reck M, Carbone DP, Garassino M and
Barlesi F: Targeting KRAS in non-small-cell lung cancer: Recent
progress and new approaches. Ann Oncol. 32:1101–1110. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Melosky B, Wheatley-Price P, Juergens RA,
Sacher A, Leighl NB, Tsao MS, Cheema P, Snow S, Liu G, Card PB and
Chu Q: The rapidly evolving landscape of novel targeted therapies
in advanced non-small cell lung cancer. Lung Cancer. 160:136–151.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Scheffzek K, Ahmadian MR, Kabsch W,
Wiesmüller L, Lautwein A, Schmitz F and Wittinghofer A: The
Ras-RasGAP complex: Structural basis for GTPase activation and its
loss in oncogenic Ras mutants. Science. 277:333–338. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Arrington AK, Heinrich EL, Lee W, Duldulao
M, Patel S, Sanchez J, Garcia-Aguilar J and Kim J: Prognostic and
predictive roles of KRAS mutation in colorectal cancer. Int J Mol
Sci. 13:12153–12168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Polom K, Das K, Marrelli D, Roviello G,
Pascale V, Voglino C, Rho H, Tan P and Roviello F: KRAS Mutation in
gastric cancer and prognostication associated with microsatellite
instability status. Pathol Oncol Res. 25:333–340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu N, Huang Y, Liu F, Xu X, Liu B and Wei
J: KRAS gene status in gastric signet-ring cell carcinoma patients
and acts as biomarker of MEK inhibitor. J Gastrointest Oncol.
12:1020–1030. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Warneke VS, Behrens HM, Haag J, Balschun
K, Böger C, Becker T, Ebert MP, Lordick F and Röcken C: Prognostic
and putative predictive biomarkers of gastric cancer for
personalized medicine. Diagn Mol Pathol. 22:127–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sacher A, LoRusso P, Patel MR, Miller WH
Jr, Garralda E, Forster MD, Santoro A, Falcon A, Kim TW, Paz-Ares
L, et al: Single-Agent Divarasib (GDC-6036) in solid tumors with a
KRAS G12C mutation. N Engl J Med. 389:710–721. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Canon J, Rex K, Saiki AY, Mohr C, Cooke K,
Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, et al: The
clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity.
Nature. 575:217–223. 2019. View Article : Google Scholar : PubMed/NCBI
|