Introduction
The incidence of malignant tumors in the heart is
low (1) and secondary tumors are
more common (2). Cardiac lymphoma,
which includes both primary and secondary tumors, refers to a
lymphoma that invades the heart and pericardium. Secondary cardiac
lymphoma is more prevalent than its primary counterpart, accounting
for 20% of all secondary cardiac tumors, with mortality rates of
~8.5–25% (2). It is most common in
men, particularly for patients with immunosuppression, with a
median age of 60 years (3,4). It is more frequently associated with
non-Hodgkin's lymphoma of B-cell origin, particularly diffuse large
B-cell lymphoma (DLBCL) (5), and
typically presents as a right-sided heart mass (6).
The diagnosis of secondary cardiac lymphoma is
challenging due to the lack of symptoms and numerous secondary
cardiac lymphomas are identified at autopsy. In the few symptomatic
patients, clinical manifestations are frequently nonspecific. These
may include characteristic features such as superior vena cava
syndrome, respiratory distress (dyspnea), constitutional symptoms
and thoracic pain (7–9). In addition, patients who undergo
current combination chemotherapy regimens have good prognoses.
However, secondary cardiac lymphoma outcomes remain poor due to
delayed diagnoses caused by the lack of symptoms (6). Thus, an understanding of the
manifestations of secondary cardiac lymphoma may lead to early
diagnosis and improved survival rates for these patients. The
present case report describes the clinical presentation and
treatment of a 53-year-old male patient diagnosed with secondary
DLBCL of the heart. The primary imaging feature before treatment
was diffuse lymphoma infiltration in the myocardium, resulting in
cardiac enlargement. Following chemotherapy, the cardiac tumor
resolved and myocardial necrosis occurred, leading to the formation
of a left ventricular aneurysm. Throughout the diagnosis and
treatment process, multimodal imaging was used to diagnose and
monitor the patient's cardiac condition.
Case report
A 53-year-old male presented at Mianyang Central
Hospital (Mianyang, China) in May 2023 with pain and swelling
behind their sternum for ~20 days. The patient denied having had
any fever or weight loss but reported fatigue and dyspnea. The
patient's initial blood pressure was normal at 104/79 mmHg and the
heart rate was 107 beats per minute (normal range, 60–100 beats per
minute). The patient had no history of lymphoma. The clinical
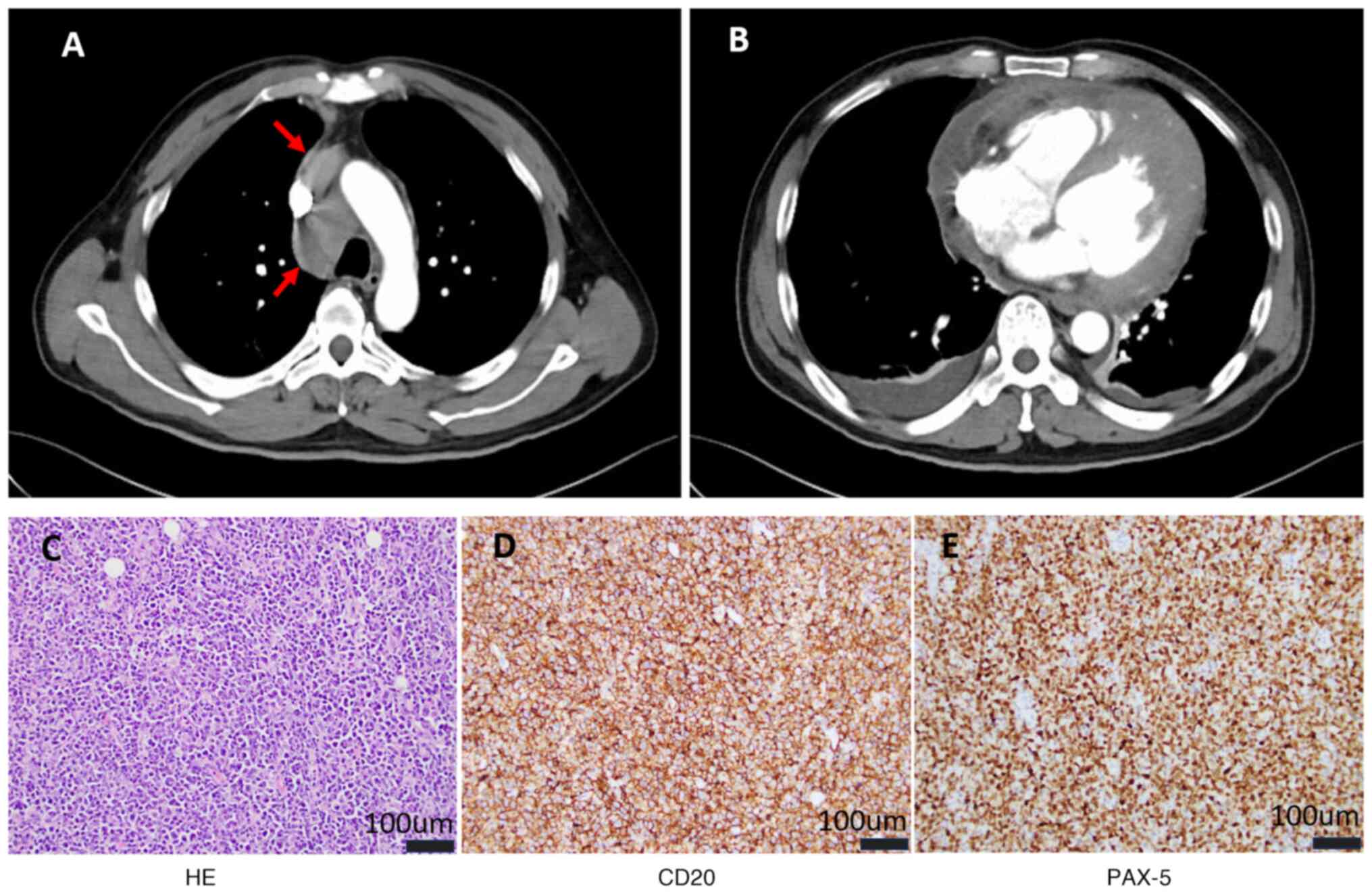
examination results were unremarkable. Enhanced chest computed
tomography (CT) revealed a mediastinal mass, enlargement of the
heart with predominant enlargement of the left ventricle,
thickening of the pericardial wall and a small amount of fluid
accumulation in the pericardial cavity (Fig. 1A and B). Minor bilateral pleural
effusion was also observed. Electrocardiography indicated T-wave
inversion in leads I, II, aVL and V2-V6, and the persistent T-wave
inversions may reflect chronic structural remodeling of the left
ventricle.
Due to the risk associated with cardiac puncture,
the patient refused to undergo cardiac biopsy. Subsequently, the
patient underwent pathological biopsy of the mediastinal mass with
a punch needle. Subsequently, hematoxylin and eosin staining and
immunohistochemical staining were performed according to standard
protocols (10). Hematoxylin and
eosin staining showed proliferative lesions in lymphoid tissue,
disappearance of follicular structures and significant cellular
pleomorphism (Fig. 1C). The
immunohistochemical staining was positive for CD20 (cat. no.
kit-0001), CD79α (cat. no. RMA-0552), Bcl-2 (~90%; cat. no.
RMA-0660), multiple myeloma oncogene 1 (cat. no. RMA-0310), paired
box gene 5 (cat. no. MAB-0706) and Kiel-67 (~80%; cat. no.
RMA-0731); scattered positive for CD3 (cat. no. MAB-0740) and Bcl-6
(cat. no. MAB-0746); negative for CD5 (cat. no. MAB-0827), CD23
(cat. no. MAB-0504), cellular myelocytomatosis oncogene (cat. no.
RMA-0552), CD10 (cat. no. MAB-0668), pan-cytokeratin (cat. no.
kit-0009) and terminal deoxynucleotidyl transferase (cat. no.
RMA-0651) (Fig. 1D and E). The
ready-to-use antibodies used for immunohistochemical staining were
obtained from Maxim Biotechnology Co., Ltd. The mediastinal mass
was diagnosed as a DLBCL of thymic origin according to the National
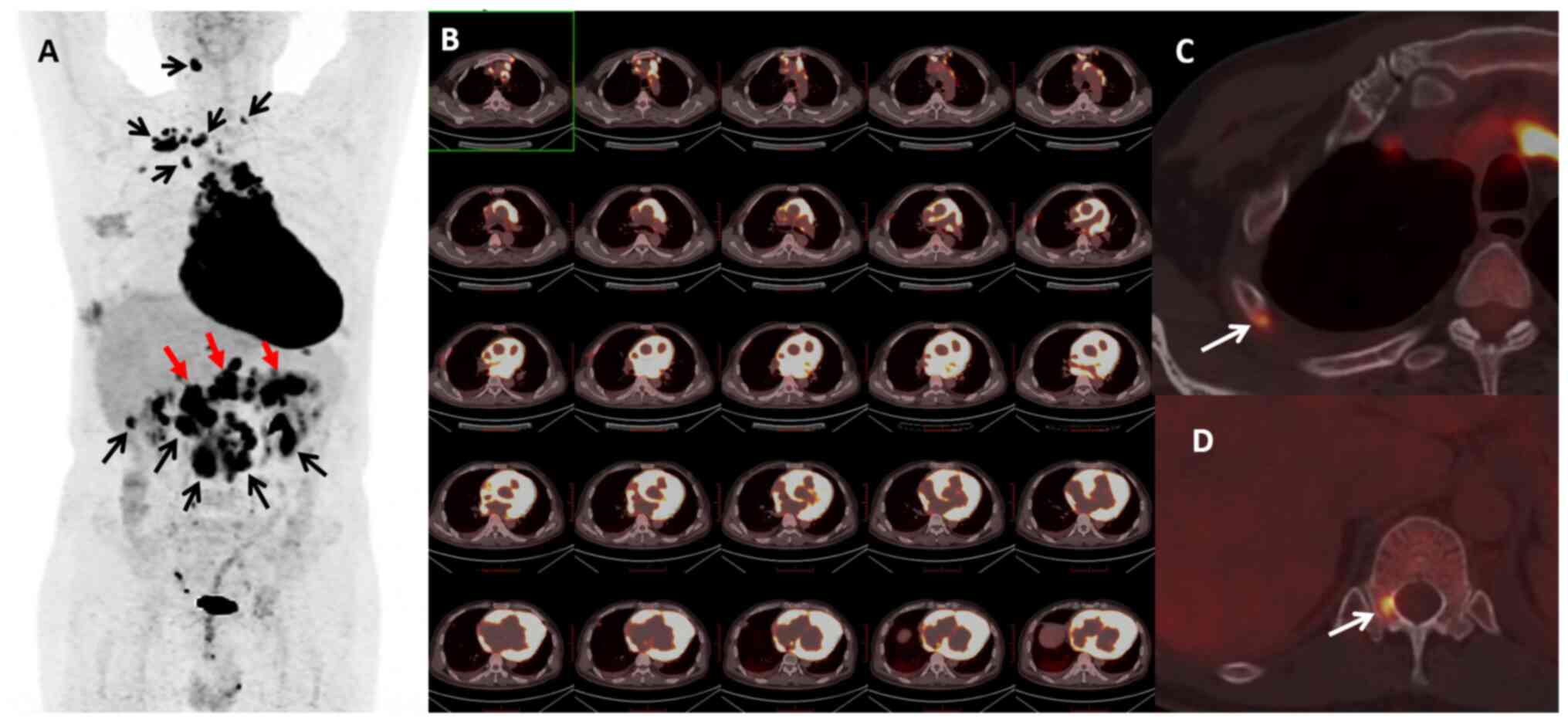
Comprehensive Cancer Network guideline (11). 18F-fluorodeoxyglucose
positron emission tomography/CT (18F-FDG PET/CT)
revealed global cardiac enlargement and a diffuse increase in
cardiac glucose metabolism (Fig. 2A and
B). In addition, it demonstrated mediastinal invasion and
involvement of the upper and lower mediastinal lymph nodes,
pancreas and bone (Fig. 2A, C and
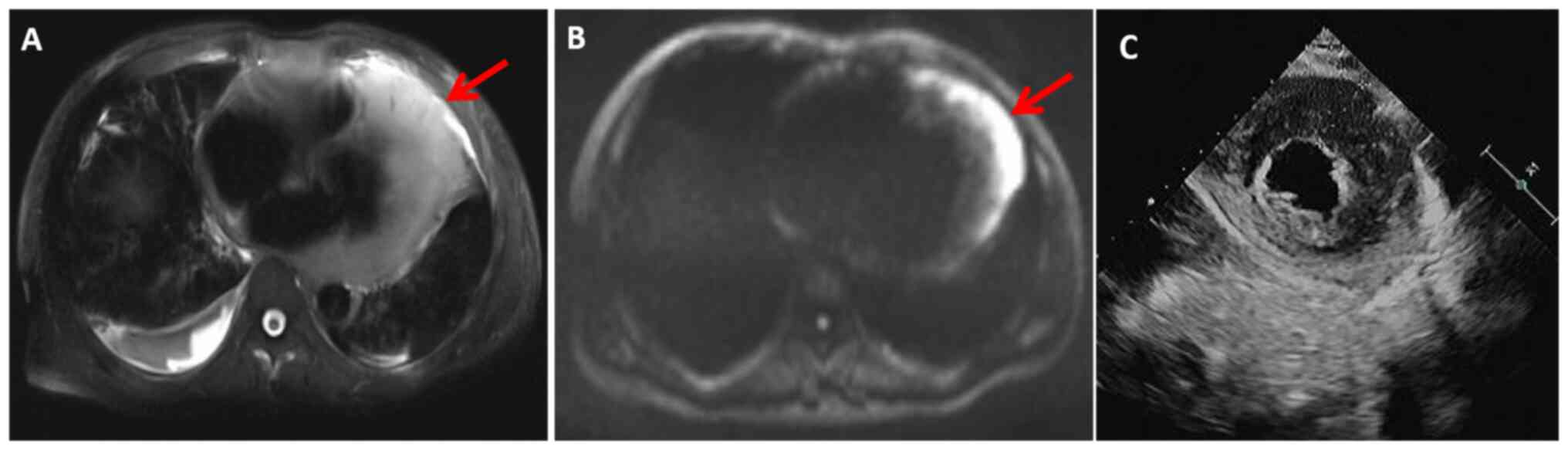
D). Magnetic resonance imaging (MRI) demonstrated an increased
T2 and diffusion-weighted imaging (DWI) signal within the heart
(Fig. 3A and B). Cardiac
ultrasonography revealed widespread thickening of the myocardial
wall with an ejection fraction (EF) of 64% and a stroke volume (SV)
of 92 ml (Fig. 3C). Multimodal
imaging suggested cardiac invasion. After ruling out
contraindications, chemotherapy was initiated with the first-line
regimen R-CHOP (Rituximab 600 mg on day 0, cyclophosphamide 1 g on
day 1, doxorubicin 60 mg on day 1, vincristine 2 mg on day 1 and
prednisone 100 mg on days 1–5). At 5 days after the initial
chemotherapy cycle, the patient exhibited a significant improvement
in fatigue and dyspnea.
During chemotherapy, ultrasonography indicated a
gradual improvement in heart size, with no adverse cardiac events.
After completing six cycles of chemotherapy at 5 months, the
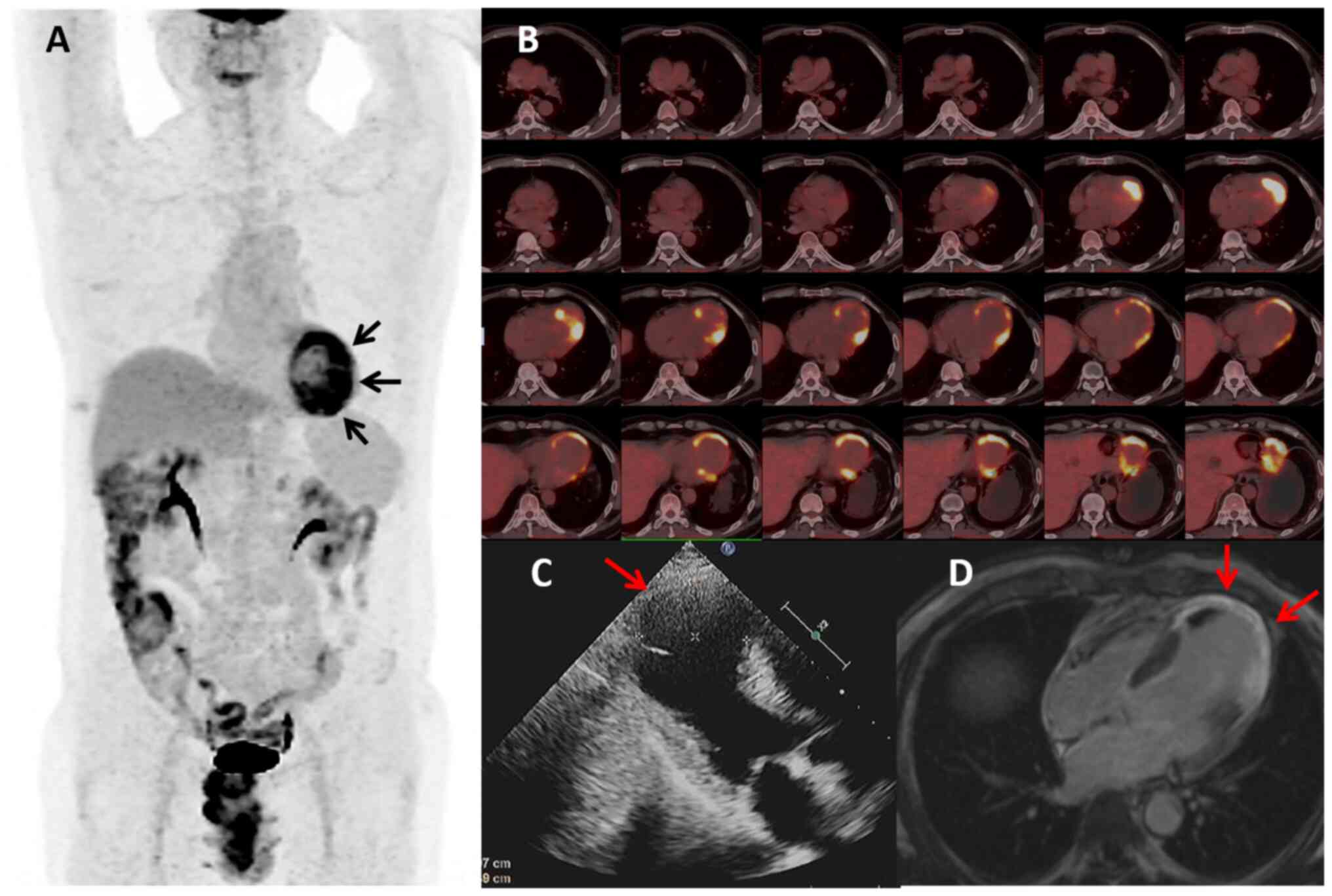
patient showed no discomfort. 18F-FDG PET/CT revealed a
reduction in heart volume, ventricular wall thinning, increased
glucose metabolism in certain areas of the left ventricular wall
and complete remission of the remaining lymphoma lesions (Fig. 4A and B). Cardiac ultrasonography
showed an aneurysm in the left ventricular apex with associated
wall thrombus formation, an EF of 30% and an SV of 42 ml (Fig. 4C). Cardiac MRI (CMR) demonstrated
extensive enhancement of the ventricular wall, indicating possible
residual myocardial fibrosis or lymphoma (Fig. 4D). Except for the myocardial
abnormalities, all other lesions in the patient had resolved
completely. Further antitumor treatments were not pursued due to
the uncertainty of whether myocardial abnormalities represented
residual lymphoma and the potential for chemotherapy or local
radiotherapy to exacerbate myocardial necrosis and cause heart
rupture (4). Enhanced CT was
performed every 3 months after the completion of chemotherapy. At
the time of writing this study (12 months post-chemotherapy), the
patient's general condition was stable, with no evidence of tumor
progression. The timeline of clinical treatment and the state of
the disease are shown in Table
I.
 | Table I.Timeline of clinical treatment and the
state of the disease. |
Table I.
Timeline of clinical treatment and the
state of the disease.
| Time-point |
Presentation/event |
|---|
| 20 days prior to
admission | Pain and swelling
behind the sternum. |
| Day 1; May 2023 | Enhanced chest CT
revealed a mediastinal mass, enlargement of the heart, thickening
of the pericardial wall and a small amount of fluid accumulation in
the pericardial cavity. |
| Days 5 to 15 | The patient underwent
pathological biopsy of the mediastinal mass and it was diagnosed as
a diffuse large B-cell lymphoma of thymic origin. |
| Day 20 | Multimodal imaging
suggested cardiac invasion. |
| Day 23 | After ruling out
contraindications, chemotherapy was initiated, with the first-line
regimen chosen as R-CHOP. |
| 5 months | After completing six
cycles of chemotherapy, multimodal imaging suggested that, except
for the myocardial abnormalities, all other lesions achieved
complete remission. The cardiac ultrasound showed an aneurysm of
the left ventricular apex with associated wall thrombus
formation. |
| Till 17 months | CT reexamination
indicated no evidence of tumor progression. |
Discussion
Secondary cardiac lymphoma is more prevalent in the
right side of the heart and is characterized by invasive,
intramural and pericardial growth patterns. It may present as
solitary or multiple masses. The patient with secondary cardiac
lymphoma described in this case report exhibited extensive lymphoma
infiltration in the ventricular wall, manifesting as infiltrative
hypertrophic cardiomyopathy that extended to the mediastinal
spaces. This condition is relatively rare, with only a few case
studies reporting similar manifestations (3,12–14),
leading to diagnostic challenges. Cardiac ultrasonography is the
preferred imaging method for cardiac lymphoma invasion screening
and can detect cardiac masses and abnormal myocardial echoes
(15). CT can delineate the
morphology, location and extent of the cardiac or mediastinal
masses (16). In the present case,
ultrasonography and CT scans only identified abnormal cardiac
morphology without a definitive diagnosis of secondary cardiac
lymphoma. CMR offers superior temporal and spatial resolutions.
However, compared with myocarditis, cardiac lymphoma lacks specific
MRI signals and enhancement characteristics (17). DWI and apparent diffusion
coefficient (ADC) sequences are important for determining tumor
malignancy. However, their application to the heart is limited
owing to poor display effects, and CMR typically does not include
DWI and ADC scans. In the present case, partial cardiac images were
obtained during upper abdominal MRI examination. Owing to
significant myocardial thickening, the DWI sequence revealed
diffusion limitations in the myocardial tissue, enhancing the
accuracy of the myocardial property diagnosis. For patients with
substantial myocardial thickening, DWI and ADC sequences may be
used to ascertain the nature of myocardial abnormalities.
18F-FDG PET/CT is the preferred examination method for
most lymphoma types and is indispensable for pretreatment staging,
posttreatment restaging and efficacy evaluation. It can also detect
asymptomatic lymphomas with cardiac invasion (3,17). In
the present case, no cardiac or mediastinal space invasion by the
lymphoma was identified on CT or cardiac ultrasonography prior to
treatment. 18F-FDG PET/CT clearly delineated the extent
of lymphoma invasion in the mediastinum and heart and was used to
compare changes in cardiac morphology and glucose metabolism
post-chemotherapy, providing an accurate assessment of the efficacy
of chemotherapy.
Currently, there are no systematic guidelines for
the diagnosis and treatment of secondary cardiac lymphomas. The two
fundamental treatment principles are early chemotherapy and the
prevention of complications (18).
As the sole effective treatment, chemotherapy often aims for
remission and, in rare cases, may be associated with fatal events
during its initiation (16). In
accordance with the National Comprehensive Cancer Network
guidelines (11), the R-CHOP
regimen represents the standard first-line therapeutic approach for
diffuse large B-cell lymphoma. Based on these evidence-based
recommendations, R-CHOP was initiated as the primary treatment
modality for the patient of the present study. The R-CHOP regimen
administered in this case led to significant improvements in
cardiac-related symptoms within five days after the first
chemotherapy cycle, suggesting that the chemotherapeutic agents
were effective against cardiac lesions and further confirming that
the cardiac abnormalities were due to lymphoma invasion. After
completing six cycles of chemotherapy, multimodal imaging
techniques showed that, apart from a few suspected lymphoma lesions
remaining in the left ventricular wall, the remaining lymphoma
lesions had resolved completely. The real-time dynamic imaging
capabilities of cardiac ultrasonography revealed the presence of a
ventricular aneurysm. Although the CMR cardiac movie sequence can
also be used to visualize the heartbeat, the hypertrophy of the
patient's heart and the presence of an apical ventricular aneurysm
limited full visualization during the scan. Although the EF and SV
measured using ultrasound significantly decreased after treatment,
the patient did not exhibit any symptoms. To date, no tumor
recurrence has been observed during follow-up after treatment. The
patient's progression-free survival and overall survival so far
were longer than 17 months.
To the best of our knowledge, only three published
studies have systematically investigated cardiac involvement in
DLBCL, each demonstrating distinct clinical trajectories. Soens
et al (19) reported on a
59-year-old male presenting with acute cardiac tamponade
manifesting as severe dyspnea. Despite undergoing pericardial
fenestration, the patient developed fatal biventricular failure due
to extensive lymphomatous myocardial infiltration within one month
of intervention. Notably, the diagnosis of secondary cardiac DLBCL
was only confirmed at autopsy, underscoring the diagnostic
challenges associated with this condition. Li et al
(20) conducted a retrospective
analysis of 10 histologically confirmed cardiac lymphoma cases,
revealing that 6 patients (60%) exhibited secondary cardiac
involvement of DLBCL. Their therapeutic protocol primarily
incorporated CHOP/R-CHOP chemotherapy regimens, with 3 patients
(30%) receiving supplemental thoracic radiotherapy. The observed
survival outcomes, with progression-free survival ranging from 3 to
12 months and overall survival extending from 6 to >28 months,
emphasize the heterogeneous, yet generally poor prognosis
associated with cardiac DLBCL. In a more recent and comprehensive
report, Yang et al (21)
detailed the clinical course of a 59-year-old male patient with
DLBCL confirmed through combined histopathological examination of
mediastinal and peripancreatic masses, further validated by
fluorescence in situ hybridization analysis. This case was
particularly notable for its extensive treatment protocol, which
sequentially incorporated R-CHOP chemotherapy, anti-CD19 chimeric
antigen receptor T-cell immunotherapy, chimeric antigen receptor
natural killer cell immunotherapy and ultimately allogeneic
hematopoietic stem cell transplantation. Despite this aggressive
multimodal approach, disease progression ensued, culminating in an
OS of 18 months. The therapeutic paradigms outlined in these
studies exhibit substantial concordance with the treatment strategy
applied in the present study. Collectively, these clinical
observations, coupled with the existing literature, strongly
suggest that early detection and timely intervention are pivotal
factors influencing therapeutic efficacy in cardiac DLBCL. This
conclusion is particularly salient given the typically aggressive
disease course and diagnostic complexities associated with cardiac
involvement in DLBCL (Table II)
(19–21).
 | Table II.Treatment outcomes of other similar
cases. |
Table II.
Treatment outcomes of other similar
cases.
| Author/s, year | Article type | Number of cases | Pathological
type | Chemotherapy
regimen | PFS, months | OS, months | (Refs.) |
|---|
| Soens et al,
2012 | Case report | 1 | DLBCL | - | 1 | 1 | (19) |
| Li et al,
2017 | Case series | 6 | DLBCL | CHOP/R-CHOP | 3–12 | 6–28+ | (20) |
| Yang et al,
2023 | Case report | 1 | DLBCL | CHOP, CAR-T/NK | 7 | 18 | (21) |
This case report has several limitations that
warrant consideration. Primarily, the absence of a post-treatment
18F-FDG PET/CT scan precluded the assessment of
metabolic activity changes in the lesions. In addition, the current
follow-up duration remains insufficient to comprehensively evaluate
the long-term therapeutic outcomes and potential disease
progression.
The diagnosis of secondary cardiac lymphoma with
diffuse myocardial infiltration is challenging. The use of
multimodal imaging examinations is essential to enhance the
diagnostic accuracy. Physicians should promptly initiate
chemotherapy and monitor patients for potential cardiac
complications throughout the treatment. Posttreatment multimodal
imaging techniques remain crucial for assessing myocardial
morphology, metabolic alterations, necrosis and fibrosis to
effectively manage the adverse outcomes associated with
chemotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BL treated the patient. DH and LX acquired data. DH,
CW and BL performed the literature search and data analysis. DH
drafted the manuscript. CH and XD designed the study and revised
the manuscript. All authors contributed to the manuscript and have
read and approved the submitted version. DH and BL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Biomedical
Ethics Committee of Mianyang Central Hospital (Mianyang, China;
approval no. S20230350-01) and conducted according to the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards.
Patient consent for publication
The patient provided written informed consent for
the publication of his data and the medical images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basso C, Valente M, Poletti A, Casarotto D
and Thiene G: Surgical pathology of primary cardiac and pericardial
tumors. Eur J Cardiothorac Surg. 12:730–738. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeudy J, Burke AP and Frazier AA: Cardiac
lymphoma. Radiol Clin North Am. 54:689–710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonelli A, Paris S, Bisegna S, Milesi G,
Gavazzi E, Giubbini R, Cattaneo C, Facchetti F and Faggiano P:
Cardiac lymphoma with early response to chemotherapy: A case report
and review of the literature. J Nucl Cardiol. 29:3044–3056. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voigt P, Wienbeck S, Weber MA,
Oyama-Manabe N, Beimler M, Schob S, Kahn T, Meyer HJ, Randaxhe JF
and Surov A: Cardiac hematological malignancies: Typical growth
patterns, imaging features, and clinical outcome. Angiology.
69:170–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakagawa Y, Ikeda U, Hirose M, Ubukata S,
Katsuki TA, Kaminishi Y, Saito T, Hironaka M, Izumi T and Shimada
K: Successful treatment of primary cardiac lymphoma with monoclonal
CD20 antibody (rituximab). Circ J. 68:172–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Huang S, Ma C, Zhu H and Bo J:
Clinical features of cardiac lymphoma: An analysis of 37 cases. J
Int Med Res. 49:3000605219995582021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kondo S, Osanai H, Sakamoto Y, Uno H,
Tagahara K, Hosono H, Miyamoto S, Hiramatsu S, Matsumoto H,
Sakaguchi T, et al: Secondary cardiac lymphoma presenting as sick
sinus syndrome and atrial fibrillation which required leadless
pacemaker implantation. Intern Med. 60:431–434. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bussani R, Castrichini M, Restivo L,
Fabris E, Porcari A, Ferro F, Pivetta A, Korcova R, Cappelletto C,
Manca P, et al: Cardiac tumors: Diagnosis, prognosis, and
treatment. Curr Cardiol Rep. 22:1692020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ido T, Minamiguchi H, Ichibori Y, Hayashi
T, Makino N, Hirayama A and Higuchi Y: Cardiac involvement of
diffuse large B-cell lymphoma presenting as various arrythmias.
Clin Case Rep. 10:e65042022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi X, Yang Y, Zhang W, Wang J, Xiao D,
Ren H, Wang T, Gao F, Liu Z, Zhou K, et al: FLASH X-ray spares
intestinal crypts from pyroptosis initiated by cGAS-STING
activation upon radioimmunotherapy. Proc Natl Acad Sci USA.
119:e22085061192022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Comprehensive Cancer Network
(NCCN), . Clinical Practice Guidelines in Oncology (NCCN
Guidelines®): B-Cell Lymphomas, Version 2. NCCN;
Plymouth Meeting, PA: 2025
|
|
12
|
Fujisaki J, Tanaka T, Kato J, Saito T,
Yano K, Shimizu Y, Sada T, Kitazume K, Fujita A and Kira Y: Primary
cardiac lymphoma presenting clinically as restrictive
cardiomyopathy. Circ J. 69:249–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuchynka P, Palecek T, Lambert L, Masek M
and Knotkova V: Cardiac involvement in lymphoma mimicking
hypertrophic cardiomyopathy. Kardiol Pol. 76:12782018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lau L, Mozolevska V, Kirkpatrick IDC,
Jassal DS and Kansara R: Diffuse large B-cell lymphoma mimicking
cardiac amyloidosis. Clin Case Rep. 5:1034–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poterucha TJ, Kochav J, O'Connor DS and
Rosner GF: Cardiac tumors: Clinical presentation, diagnosis, and
management. Curr Treat Options Oncol. 20:662019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Mehisen R, Al-Mohaissen M and Yousef H:
Cardiac involvement in disseminated diffuse large B-cell lymphoma,
successful management with chemotherapy dose reduction guided by
cardiac imaging: A case report and review of literature. World J
Clin Cases. 7:191–202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maleszewski JJ, Anavekar NS and Moynihan
TJ: KW: Pathology, imaging, and treatment of cardiac tumours. Nat
Rev Cardiol. 14:536–549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vinicki JP, Cianciulli TF, Farace GA,
Saccheri MC, Lax JA, Kazelian LR and Wachs A: Complete regression
of myocardial involvement associated with lymphoma following
chemotherapy. World J Cardiol. 5:364–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soens L, Schoors D and Van Camp G: Acute
heart failure due to fulminant myocardial infiltration by a diffuse
large B-cell lymphoma. Acta Cardiol. 67:101–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YH, Shi CY, Duan FQ, Pang Y, Li HB,
Zhang LQ, Liu ZH, Ouyang L, Yue CY, Xie MC, et al: A clinical
analysis of 10 cases with cardiac lymphoma. Zhonghua Xue Ye Xue Za
Zhi. 38:102–106. 2017.(In Chinese). PubMed/NCBI
|
|
21
|
Yang Y, Li Z, Li Y, Zhao Y and Shi M:
Relapsed/refractory diffuse large B cell lymphoma with cardiac
involvement: A case report and literature review. Front Oncol.
13:10910742023. View Article : Google Scholar : PubMed/NCBI
|