Introduction
Advancements in medical technology and increased
health awareness have improved prognoses for patients with cancer.
However, the overall prognosis of cancer remains poor,
necessitating the exploration of innovative approaches to enhance
patient outcomes. Long non-coding RNAs (lncRNAs) have been
identified as key regulators of cancer cell proliferation,
apoptosis and metastasis, as well as valuable diagnostic and
prognostic biomarkers (1–5). For example, Chen et al
(1) reported marked upregulation of
zinc finger homeobox 4-antisense RNA 1 (ZFHX4-AS1) in
adrenocortical carcinoma, with elevated expression being associated
with poor prognosis and serving as an independent prognostic risk
factor. Suppression of ZFHX4-AS1 expression has been reported to
inhibit cancer cell proliferation and migration in adrenocortical
carcinoma (1). Similarly, Wu et
al (2), observed a marked
downregulation of long intergenic non-protein coding RNA 1550
(LINC01550) in colorectal cancer (CRC) tissue, which was associated
with advanced cancer stages, increased metastasis and reduced
overall survival (OS) rate. Overexpression of LINC01550 suppressed
cell proliferation, migration, invasion and epithelial-mesenchymal
transition (EMT) and promoted apoptosis in CRC cells. These
findings highlight the potential of lncRNAs as biomarkers and
therapeutic targets to improve cancer prognosis.
Family with sequence similarity 83 member
H-antisense RNA 1 (FAM83H-AS1) is an oncogenic lncRNA overexpressed
in multiple cancers, including gastric, bladder and liver cancer.
Its overexpression has been associated with unfavourable diagnostic
and prognostic indicators, promoting cancer cell proliferation,
apoptosis and metastasis (6–35). Liu
et al (6) reported that
FAM83H-AS1 expression is markedly elevated in gastric cancer and
associated with poor prognosis. Increased FAM83H-AS1 expression
enhances proliferation, migration and invasion of gastric cancer
cells. To the best of our knowledge, however, comprehensive reviews
of the functional mechanisms of FAM83H-AS1 in cancers remain
limited. The present review aims to summarise its expression
patterns and elucidate its roles in cancer progression, diagnosis
and prognosis. Consequently, the present review may provide a
theoretical foundation for the development of cancer therapies.
FAM83H-AS1 overexpression in tumours
FAM83H-AS1 is overexpressed across several cancers
(Table I). Compared with normal
tissue, it exhibits marked upregulation in gastric, bladder, liver,
endometrial, CRC, pancreatic, breast, non-small cell lung (NSCLC),
ovarian, prostate, glioma and oesophageal cancer tissue. Similarly,
elevated expression of FAM83H-AS1 is observed in multiple cancer
cell lines (6–34).
 | Table I.Expression of family with sequence
similarity 83 member H antisense RNA 1 in cancer. |
Table I.
Expression of family with sequence
similarity 83 member H antisense RNA 1 in cancer.
| Cancer | Expression in
tissue or serum | N | Expression in
cancer cells | Cancer cell
lines | Relative normal
cell lines | (Refs.) |
|---|
| Gastric | High | 315 | High | SNU-1, NCI-N87,
AGS, SGC7901, MKN45, MKN74, SNU216, BGC823 | GES-1 | (6–8) |
| Bladder | High | 122 | High | T24, SW780,
HT-1197, BK10, HTB-9, RT4, J82 | SV-HUC-1 | (9,10) |
| Liver | High | 66 | High | HepG2, Huh-7,
SMCC-7721, MHCC97H | THLE-3 | (11) |
| Endometrial | High | 35 | - | - | - | (12) |
| Cervical | - | - | High | CASKI, W12/20863,
W12/201402, C-33A | HCK | (13) |
| Colorectal | High | 336 | High | SW480, LοVο,
HCT116, HT29 | HCoEpiC | (14–17) |
| Pancreatic | High | 89 | High | MIA PaCa-2, PANC-1,
SW 1990, AsPC-1, BxPC-3, Capan-2 | HPDE | (18) |
| Breast | High | 187 | High | MCF-7, MDA-MB-361,
MDA-MB-468, Hs-578, ZR75, BT20, MDA-MB-436, MDA-MB-231, T47D,
ZR-75-1 | MCF10A | (19–24) |
| Non-small cell
lung | High | 201 | High | H1299, H1650,
HCC827, A549, SPC-A1, H1975, H358, PC9 | BEAS-2B, HBE | (25–27) |
| Ovarian | High | 186 | High | ES-2, SKOV-3,
A2780, SW626 | IOSE386 | (28–30) |
| Prostate | High | 20 | High | LNCaP, 22Rv1,
C4-2B, PC-3, DU 145 | WPMY-1 | (31) |
| Glioma | High | 10 | High | U251, U87 | 1800 | (32) |
| Oesophageal | High | 201 | High | KYSE410, KYSE510,
KYSE520, KYSE30, KYSE170, TE1, KYSE150, Eca109 | NE1, Pools | (33,34) |
Roles of FAM83H-AS1 in cancer
FAM83H-AS1 serves a key role in regulating malignant
traits in cancer cells, including uncontrolled proliferation,
invasion, metastasis and drug resistance, as well as cell cycle
progression and apoptosis (6–14,16,18,19,23,25–28,31–34).
FAM83H-AS1 serves as a carcinogenic factor in
several cancers by promoting cell proliferation, facilitating cell
cycle progression and suppressing apoptosis (Table II). High FAM83H-AS1 expression is
associated with enhanced proliferation in gastric cancer (AGS,
NCI-N87, MKN74, MKN45), bladder cancer (T24, BK10, J82), liver
cancer (SMCC-7721, MHCC97H), endometrial cancer (HEC-1A, Ishikawa),
cervical cancer (CaSki), CRC (DLD1, RKO, SW480, HT29), pancreatic
cancer (PANC-1, SW1990), breast cancer (MCF7, MDA-MB-231,
MDA-MB-468), NSCLC (HCC827, H1650, A549, SPC-A1, PC9), ovarian
cancer (CAR-3), prostate cancer (LNCaP, DU145), glioma (U251, U87,
1800) and oesophageal cancer (KYSE30, KYSE510, TE1) cells (6,7,9–14,16,18,19,23,25–28,31–34).
FAM83H-AS1 overexpression facilitates cell cycle progression in
bladder (T24, BK10, J82) and endometrial cancer (HEC-1A, Ishikawa),
lung adenocarcinoma (PC9, H1650), prostate cancer (LNCaP, DU145)
and glioma (U251, U87) cells (9,10,12,27,31,32).
Furthermore, its overexpression inhibits apoptosis in bladder (T24,
BK10) and cervical cancer (CaSki), CRC (SW480, HT29), breast cancer
(MCF7), lung adenocarcinoma (A549, SPC-A1) and glioma (U251, U87)
cells (9,13,16,19,26,32).
In vivo studies corroborate these findings, demonstrating
that FAM83H-AS1 enhances tumour formation in bladder and
endometrial cancer, CRC, pancreatic and breast cancer, NSCLC and
lung adenocarcinoma models in nude mice (9,12,14,18,23,25,26).
 | Table II.In vitro functional
characterisation of family with sequence similarity 83 member H
antisense RNA 1 in cancer. |
Table II.
In vitro functional
characterisation of family with sequence similarity 83 member H
antisense RNA 1 in cancer.
| Cancer | Effect on
proliferation | Effect on cell
cycle | Effect on
apoptosis | Effect on
metastasis | Effect on
chemotherapy or radiotherapy resistance | (Refs.) |
|---|
| Gastric | Promotion | - | - | Promotion | Promotion | (6–8) |
| Bladder | Promotion | Promotion | Inhibition | Promotion | - | (9,10) |
| Liver | Promotion | - | - | Promotion | - | (11) |
| Endometrial | Promotion | Promotion | - | - | - | (12) |
| Cervical | Promotion | - | Inhibition | Promotion | - | (13) |
| Colorectal | Promotion | - | Inhibition | Promotion | Promotion | (14,16) |
| Pancreatic | Promotion | - | - | Promotion | - | (18) |
| Breast | Promotion | - | - | Promotion | - | (19,23) |
| Non-small cell
lung | Promotion | Promotion | - | Promotion | - | (25–27) |
| Ovarian | Promotion | - | - | Promotion | Promotion | (28,30) |
| Prostate | Promotion | Promotion | - | Promotion | - | (31) |
| Glioma | Promotion | Promotion | - | - | - | (32 |
| Oesophageal | Promotion | - | - | Promotion | - | (33,34) |
Roles of FAM83H-AS1 in tumour
metastasis
FAM83H-AS1 contributes to cancer metastasis,
influencing recurrence and progression (Table III). Overexpression of FAM83H-AS1
promotes cancer cell invasion and metastasis in gastric (AGS,
NCI-N87), bladder (T24, BK10, J82), liver (SMCC-7721, MHCC97H), CRC
(SW480, HT29), pancreatic (PANC-1, SW1990) and breast cancer
(MDA-MB-231, MDA-MB-468), lung adenocarcinoma (A549, SPC-A1, PC9,
H1650) and ovarian (ES-2, SKOV-3) and oesophageal cancer (KYSE150,
TE1) cells (6,9–11,16,18,23,26,27,29,34).
Additionally, elevated FAM83H-AS1 levels promote cell migration in
cervical (CaSki), breast (MCF7), ovarian (CAR-3), prostate (PC3,
DU145) and oesophageal cancer (KYSE30) (13,19,28,31,33).
It also facilitates cell invasion in NSCLC HCC827 cells (25).
 | Table III.Family with sequence similarity 83
member H antisense one promotes tumor growth in BALM/c nude
mice. |
Table III.
Family with sequence similarity 83
member H antisense one promotes tumor growth in BALM/c nude
mice.
| Cancer | Cell line | (Refs.) |
|---|
| Bladder | BK10, T24 | (9) |
| Endometrial | Ishikawa | (12) |
| Colorectal | RKO, DLD1 | (14) |
| Pancreatic | PANC-1 | (18) |
| Breast | MDA-MB-231,
MDA-MB-468 | (23) |
| Non-small cell
lung | HCC827 | (25) |
| Lung
adenocarcinoma | A549 | (26) |
Role of FAM83H-AS1 in chemotherapy and
radiotherapy resistance
FAM83H-AS1 contributes to cancer progression by
enhancing resistance to chemotherapy and radiotherapy (Table II). Elevated FAM83H-AS1 expression
has been observed in chemotherapy-resistant gastric cancer
(SGC7901/R) compared with parental SGC7901 cells. Silencing
FAM83H-AS1 sensitises drug-resistant SGC7901/R cells to cisplatin
and 5-fluorouracil, indicating its role in mediating
chemoresistance (8). Combining
FAM83H-AS1 with oxaliplatin/cisplatin markedly suppresses tumour
growth in CRC (14). Moreover, the
overexpression of FAM83H-AS1 can trigger metastasis and confer
radiation resistance in ovarian cancer cells (30).
Signalling mechanism of FAM83H-AS1 in
tumours
lncRNAs serve critical roles in cellular signalling
by regulating gene expression, the cell cycle and cell
differentiation (1–4). They also function as signalling
molecules, regulators or mediators in signal transduction. And
these interactions influence several physiological and pathological
processes, including cancer progression (23,25,31,34).
Studies have revealed that FAM83H-AS1 contributes to cancer cell
proliferation, apoptosis, migration and chemoresistance by
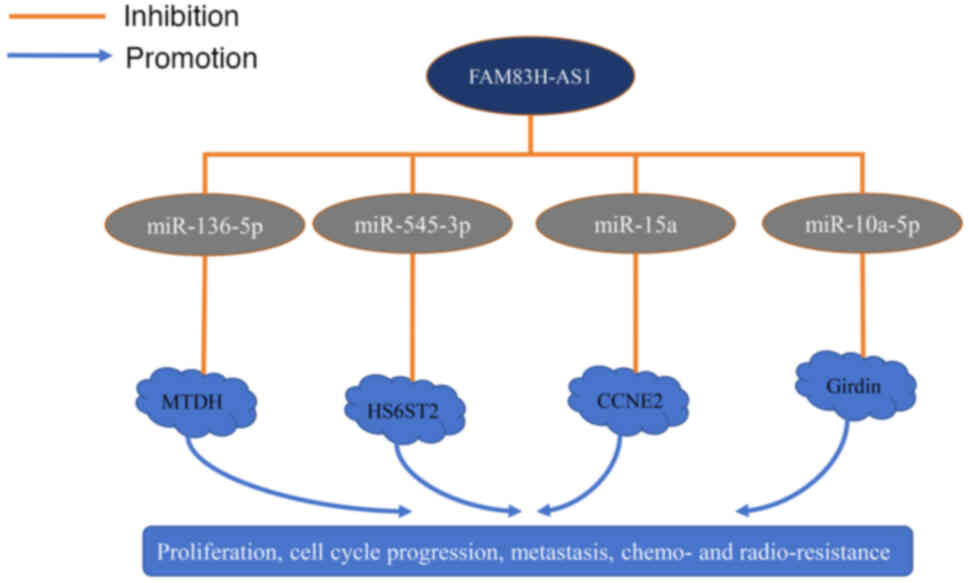
modulating signalling pathways such as microRNA (miR)-136-5p,
miR-545-3p, miR-15a, miR-10a-5p, Wnt/β-catenin and Notch receptor
(Table IV). Understanding these
signalling mechanisms offers insight into cellular signal
transduction regulation and presents potential therapeutic targets
for cancer treatment.
 | Table IV.FAM83H-AS1-miR-mRNA signalling
pathways in cancer. |
Table IV.
FAM83H-AS1-miR-mRNA signalling
pathways in cancer.
| FAM83H-AS1 target
miRs | Methods | Target genes | Cancer | (Refs.) |
|---|
| miR-136-5p | Luciferase reporter
assay, RT-PCR | MTDH | Breast | (23) |
| miR-545-3p | Luciferase reporter
assay, RIP, RT-PCR | HS6ST2 | NSCLC | (25) |
| miR-15a | Luciferase reporter
assay, RT-PCR | CCNE2 | Prostate | (31) |
| miR-10a-5p | Luciferase reporter
assay, RIP, RT-PCR | Girdin | ESCA | (34) |
FAM83H-AS1 serves as a competing
endogenous RNA
FAM83H-AS1 promotes cancer progression by
competitively binding miR-136-5p, miR-545-3p, miR-15a and
miR-10a-5p (23,25,31,34).
Han et al (23) reported
decreased expression of miR-136-5p in breast cancer tissue.
Overexpression of miR-136-5p inhibits the proliferation, migration
and invasion of breast cancer cells. Silencing FAM83H-AS1 reverses
these effects via the miR-136-5p/metadherin axis, thereby promoting
tumour growth and metastasis. Zhang et al (25) observed that miR-545-3p expression is
markedly decreased in lung cancer tissues and inhibition of
miR-545-3p increases heparan sulphate 6-O-sulfotransferase 2
(HS6ST2) protein levels, enhancing lung cancer cell invasion. By
targeting the miR-545-3p/HS6ST2 axis, FAM83H-AS1 facilitates NSCLC
progression. Liu et al (31)
reported the overexpression of FAM83H-AS1 in prostate cancer cells.
FAM83H-AS1 promotes cyclin E2 expression by sequestering miR-15a,
thus regulating cell proliferation, cell cycle progression and
migration. Additionally, FAM83H-AS1 is markedly upregulated in
oesophageal cancer tissue and sequesters miR-10a-5p, promoting
Girdin expression, thereby enhancing proliferation, migration and
invasion of oesophageal cancer cells (34).
Wnt/β-catenin signalling pathway
The Wnt/β-catenin signalling pathway, also known as
the canonical Wnt signalling pathway, is a highly conserved and
complex cascade that serves a key role in regulating cell
proliferation, differentiation, embryonic development and tissue
homeostasis (36). Understanding
the mechanisms of this pathway is essential for developing
therapeutic interventions targeting this pathway in various
diseases. Wang et al (8)
demonstrated that suppressing FAM83H-AS1 expression inhibits
gastric cancer progression via the Wnt/β-catenin pathway. Notably,
activation of this pathway counteracts the enhanced
chemosensitivity observed in gastric cancer cells following
FAM83H-AS1 knockdown. Similarly, Ma et al (11) demonstrated that silencing FAM83H-AS1
decreases the expression of β-catenin and WNT1, thereby suppressing
liver cancer cell proliferation and migration. In pancreatic ductal
adenocarcinoma, Zhou et al (18) revealed that FAM83H-AS1 promotes
proliferation, invasion and metastasis by stabilising FAM83H mRNA.
This stabilisation decreases β-catenin ubiquitination, thereby
enhancing pathway activation and tumour progression. Furthermore,
dysregulated Wnt/β-catenin signalling influences downstream target
genes such as transcriptional regulator c-Myc, cyclin D1 and axin
2. In bladder cancer, FAM83H-AS1 activates unc-51 like kinase 3
(ULK3) expression by binding transcription factor c-Myc,
contributing to tumour progression (9).
EMT signalling pathway
The EMT signalling pathway serves a key role in
cellular transformation into mesenchymal cell types. For example,
Liu et al (9) reported that
FAM83H-AS1 promotes bladder cancer cell proliferation by
upregulating N-cadherin, Snai1 and Slug proteins while
downregulating E-cadherin expression, thereby facilitating
EMT-mediated tumour progression. Similarly, Feng et al
(34) demonstrated that FAM83H-AS1
enhances oesophageal cancer cell proliferation, migration and
invasion by increasing N-cadherin levels and decreasing E-cadherin
expression, underscoring its role in EMT activation.
N6 methyladenine (m6A)
modification
m6A modification is a prevalent RNA modification
that regulates gene expression, stem cell fate determination and
the development of numerous diseases, and m6A-modified genes are
involved in cancer growth and metastasis (37,38).
Liu et al (6) reported that
the m6A-modified gene WT1 associated protein (WTAP) mediates
FAM83H-AS1 expression in an m6A-dependent manner. WTAP inhibition
reverses the effects of FAM83H-AS1 overexpression, thereby
decreasing gastric cancer cell proliferation, migration and
invasion. Additionally, Luo et al (14) observed that m6A modification, via
polypyrimidine tract binding protein 1 phosphorylation, decreases
FAM83H-AS1 expression, thereby inhibiting gastric cancer growth and
metastasis. This suggests that m6A can regulate the expression
levels of FAM83H-AS1 to affect cancer progression.
Other signalling pathways
In bladder cancer, FAM83H-AS1 suppresses the
Hedgehog signalling pathway, whereas ULK3 overexpression activates
this pathway. Targeting the Hedgehog signalling pathway effectively
counteracts the oncogenic effects of FAM83H-AS1 in bladder cancer
cells (9). In CRC, FAM83H-AS1
downregulates SMAD1 gene expression within the TGF-β signalling
pathway and promotes CRC cell proliferation via Notch1 in the Notch
signalling pathway (15,16). In lung adenocarcinoma cells,
FAM83H-AS1 enhances cell proliferation, migration and invasion by
modulating the MET proto-oncogene, receptor tyrosine kinase/EGFR
signalling pathway (27).
Additionally, it interacts with heterogeneous nuclear
ribonucleoprotein K to upregulate the anti-apoptotic oncogenes
RAB8B and RAB14, thereby inhibiting apoptosis in lung
adenocarcinoma cells (26). In
endometrial cancer, FAM83H-AS1 promotes tumour progression by
increasing the methylation of the CDO1 promoter via DNMT1
recruitment, resulting in decreased CDO1 expression. Decreased CDO1
levels inhibit iron-induced cell death and support cancer growth
(12). FAM83H-AS1 stabilises the
human antigen R (HuR) protein through cycloheximide, as
demonstrated by RNA immunoprecipitation and western blot assays
(30). Enhanced HuR expression
reverses the effects of FAM83H-AS1 silencing, restoring
radiotherapy resistance and metastasis in ovarian cancer cells
(30). Finally, in glioma,
FAM83H-AS1 regulates the cell cycle progression and proliferation
by recruiting enhancer of zeste homolog 2 to the CDK inhibitor 1
(CDKN1A) promoter, leading to increased CDKN1A expression (32).
FAM83H-AS1 serves as a potential prognostic
biomarker in patients with cancer
FAM83H-AS1 is overexpressed in numerous types of
cancer tissue and its elevated expression is associated with poor
prognosis in patients with cancer (Table V). Specifically, high levels of
FAM83H-AS1 are inversely associated with OS in patients with
gastric, bladder and liver cancer, CRC, pancreatic cancer, lung
adenocarcinoma, ovarian cancer, glioma and oesophageal squamous
cell carcinoma (7–11,15,16,18,20,26–28,30,32–34).
Additionally, in gastric cancer and oesophageal squamous cell
carcinoma, FAM83H-AS1 overexpression is associated with worse
disease-specific survival (7,8,32,34).
Elevated FAM83H-AS1 expression is associated with differentiation,
depth of invasion and chemotherapy response in gastric cancer. In
bladder cancer, its expression is associated with Ki-67 levels,
lymph node metastasis, pathological stage, differentiation,
invasion pattern and muscular invasion. Similarly, in liver cancer,
FAM83H-AS1 expression is associated with tumour size and vascular
invasion (7–11,15,16,20,28,30,32–34).
These findings highlight FAM83H-AS1 as a promising biomarker for
cancer diagnosis and prognosis. Furthermore, targeting FAM83H-AS1
to suppress its expression may slow cancer progression and improve
patient prognosis.
 | Table V.Family with sequence similarity 83
member H-antisense RNA 1 overexpression is associated with
prognosis in patients with cancer. |
Table V.
Family with sequence similarity 83
member H-antisense RNA 1 overexpression is associated with
prognosis in patients with cancer.
| Cancer | Prognostic
indicator | Associated clinical
features | (Refs.) |
|---|
| Gastric | OS, DFS | Diagnostic value,
differentiation, invasion depth, chemotherapy | (7,8) |
| Bladder | OS | Ki-67, lymph node
metastasis, pathological stage, differentiation, invasion depth,
muscularis invasion | (9,10) |
| Liver | OS | Tumor size,
vascular invasion | (11) |
| Colorectal | OS | TNM stage, tumor
size | (15,16) |
| Pancreatic | OS | - | (18) |
| Breast | - | Diagnostic value, T
stage, clinical stage, lymph node metastasis, distant metastasis,
ER status | (20) |
| Lung
adenocarcinoma | OS | - | (26,27) |
| Ovarian | OS | Tumor grade,
distant metastasis, TNM stage, tumor size, FIGO stage, lymph node
metastasis | (28,30) |
| Glioma | OS | Tumor grade | (32) |
| Oesophageal | OS, DFS | TNM stage, lymph
node metastasis, tumor grade | (33,34) |
Conclusions
Studies on FAM83H-AS1 in the context of cancer have
consistently demonstrated its notable role as an oncogene (6–34).
FAM83H-AS1 does not exhibit dual functionality as both an oncogene
and a tumour suppressor gene across different cancers. FAM83H-AS1
serves a key role in pathological processes, substantially
contributing to cancer progression (6–34).
This unique characteristic warrants further investigation.
Mechanistically, FAM83H-AS1 functions as a molecular sponge,
binding miRNAs to regulate downstream target genes, thereby
exerting oncogenic effects (Fig.
1). Its overexpression is associated with enhanced cell
proliferation, migration, invasion and drug resistance,
underscoring its potential as both a prognostic marker and
therapeutic target in cancer treatment. Moreover, FAM83H-AS1 is
associated with adverse prognostic factors such as advanced
pathological staging, increased lymph node metastasis and decreased
OS, establishing it as a novel biomarker with notable clinical
value for targeted therapy and prognosis assessment.
Despite these findings, several challenges remain.
Firstly, the function of FAM83H-AS1 in normal cells is unknown, and
the molecular mechanisms (such as Notch, Hedgehog and TGFβ-MET/EGFR
signalling) of FAM83H-AS1 vary across different cell types in
patients with cancer, complicating the development of universal
therapeutic strategies targeting FAM83H-AS1. In the future, the
association between FAM83H-AS1 and these mechanisms needs to be
further explored. Secondly, most data on FAM83H-AS1 stem from basic
research, highlighting the necessity of integrating preclinical
findings with clinical studies (6–34).
Furthermore, effective treatment options targeting FAM83H-AS1 are
limited, emphasising the need for further research into its
regulatory mechanisms and drug resistances.
In conclusion, advancing the understanding of the
biological roles and regulatory networks of FAM83H-AS1 in cancer is
key for its application in diagnostics and therapeutics.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZQC conceived the study. JLS reviewed the literature
and drafted the manuscript. JLS, CSL and MHG reviewed the
manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen G, Li S, Lu J, Liang A, Gao P, Ou F,
Wang Y, Li Y and Pan B: LncRNA ZFHX4-AS1 as a novel biomarker in
adrenocortical carcinoma. Transl Androl Urol. 13:1188–1205. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu W, Li A, He H, Ye S, Zhou Z, Quan JH
and Tan W: Long noncoding RNA LINC01550 inhibits colorectal cancer
malignancy by suppressing the Wnt/β-catenin signaling pathway. J
Biochem Mol Toxicol. 38:e237742024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma H, Weng F, Tong X, Li H, Yao Y and Yuan
J: LncRNA TRPM2-AS promotes endometrial carcinoma progression and
angiogenesis via targeting miR-497-5p/SPP1 axis. Cell Mol Biol
Lett. 29:932024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Shi J, Xiang Y, Wang ZW, Qi FF, Li
ZY, Zhao LL, Zhu GH, Duan YY, Yang ZY, et al: LINC00525 enhances
ZNF460-regulated CD24 expression through the sponge miR-125a-5p to
promote malignant progression of breast cancer. J Cancer Res Clin
Oncol. 150:3172024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D, Liu X, Jiang N, Ke D, Guo Q, Zhai K,
Han H, Xiao X and Fan T: Interfering with ITGB1-DT expression
delays cancer progression and promotes cell sensitivity of NSCLC to
cisplatin by inhibiting the MAPK/ERK pathway. Am J Cancer Res.
12:2966–2988. 2022.PubMed/NCBI
|
|
6
|
Liu N, Zhang C and Zhang L: WTAP-involved
the m6A modification of lncRNA FAM83H-AS1 accelerates the
development of gastric cancer. Mol Biotechnol. 66:1883–1893. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Da J, Liu P, Wang R and Bu L: Upregulation
of the long non-coding RNA FAM83H-AS1 in gastric cancer and its
clinical significance. Pathol Res Pract. 215:1526162019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, Guan G and Zhao D: Silence of
FAM83H-AS1 promotes chemosensitivity of gastric cancer through
Wnt/β-catenin signaling pathway. Biomed Pharmacother.
125:1099612020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu B, Gao W, Sun W, Li L, Wang C, Yang X,
Liu J and Guo Y: Promoting roles of long non-coding RNA FAM83H-AS1
in bladder cancer growth, metastasis, and angiogenesis through the
c-Myc-mediated ULK3 upregulation. Cell Cycle. 19:3546–3562. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shan H, Yang Y, Zhu X, Han X, Zhang P and
Zhang X: FAM83H-AS1 is associated with clinical progression and
modulates cell proliferation, migration, and invasion in bladder
cancer. J Cell Biochem. 120:4687–4693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma YK, Shen TH and Yang XY: Upregulation
of LncRNA FAM83H-AS1 in hepatocellular carcinoma promotes cell
proliferation, migration and invasion by Wnt/β-catenin pathway. Eur
Rev Med Pharmacol Sci. 23:7855–7862. 2019.PubMed/NCBI
|
|
12
|
Wang R, Yu X, Ye H, Ao M, Xi M and Hou M:
LncRNA FAM83H-AS1 inhibits ferroptosis of endometrial cancer by
promoting DNMT1-mediated CDO1 promoter hypermethylation. J Biol
Chem. 300:1076802024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barr JA, Hayes KE, Brownmiller T, Harold
AD, Jagannathan R, Lockman PR, Khan S and Martinez I: Long
non-coding RNA FAM83H-AS1 is regulated by human papillomavirus 16
E6 independently of p53 in cervical cancer cells. Sci Rep.
9:36622019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo XJ, Lu YX, Wang Y, Huang R, Liu J, Jin
Y, Liu ZK, Liu ZX, Huang QT, Pu HY, et al: M6A-modified lncRNA
FAM83H-AS1 promotes colorectal cancer progression through PTBP1.
Cancer Lett. 598:2170852024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Cui J, Wang Y and Tan J:
FAM83H-AS1 is upregulated and predicts poor prognosis in colon
cancer. Biomed Pharmacother. 118:1093422019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu S, Dong W, Zhao P and Liu Z: lncRNA
FAM83H-AS1 is associated with the prognosis of colorectal carcinoma
and promotes cell proliferation by targeting the Notch signaling
pathway. Oncol Lett. 15:1861–1868. 2018.PubMed/NCBI
|
|
17
|
Yang L, Xu L, Wang Q, Wang M and An G:
Dysregulation of long non-coding RNA profiles in human colorectal
cancer and its association with overall survival. Oncol Lett.
12:4068–4074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou M, Pan S, Qin T, Zhao C, Yin T, Gao
Y, Liu Y, Zhang Z, Shi Y, Bai Y, et al: LncRNA FAM83H-AS1 promotes
the malignant progression of pancreatic ductal adenocarcinoma by
stabilizing FAM83H mRNA to protect β-catenin from degradation. J
Exp Clin Cancer Res. 41:2882022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ríos-Romero M, Cedro-Tanda A, Peña-Luna M,
Mancera-Rodríguez MA, Hidalgo-Pérez L, Cisneros-Villanueva M,
Beltrán-Anaya FO, Arellano-Llamas R, Jiménez-Morales S, Alfaro-Ruíz
LA, et al: FAM83H-AS1 is a potential modulator of cancer driver
genes across different tumors and a prognostic marker for ER/PR +
BRCA patients. Sci Rep. 10:141452020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Ashmawy NE, Hussien FZ, El-Feky OA,
Hamouda SM and Al-Ashmawy GM: Serum LncRNA-ATB and FAM83H-AS1 as
diagnostic/prognostic non-invasive biomarkers for breast cancer.
Life Sci. 259:1181932020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian D, Qian C, Ye B, Xu M, Wu D, Li J, Li
D, Yu B and Tao Y: Development and validation of a novel
stemness-index-related long noncoding RNA signature for breast
cancer based on weighted gene co-expression network analysis. Front
Genet. 13:7605142022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deva Magendhra Rao AK, Patel K, Korivi
Jyothiraj S, Meenakumari B, Sundersingh S, Sridevi V, Rajkumar T,
Pandey A, Chatterjee A, Gowda H and Mani S: Identification of
lncRNAs associated with early-stage breast cancer and their
prognostic implications. Mol Oncol. 13:1342–1355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han C, Fu Y, Zeng N, Yin J and Li Q:
LncRNA FAM83H-AS1 promotes triple-negative breast cancer
progression by regulating the miR-136-5p/metadherin axis. Aging
(Albany NY). 12:3594–3616. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang F, Lv SX, Lv L, Liu YH, Dong SY, Yao
ZH, Dai XX, Zhang XH and Wang OC: Identification of lncRNA
FAM83H-AS1 as a novel prognostic marker in luminal subtype breast
cancer. Onco Targets Ther. 9:7039–7045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Yu Y, Cao X and Chen P: Role of
lncRNA FAM83H antisense RNA1 (FAM83H-AS1) in the progression of
non-small cell lung cancer by regulating the miR-545-3p/heparan
sulfate 6-O-sulfotransferase (HS6ST2) axis. Bioengineered.
13:6476–6489. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang S, Han C, Liu T, Ma Z, Qiu M, Wang J,
You Q, Zheng X, Xu W, Xia W, et al: FAM83H-AS1 is a noncoding
oncogenic driver and therapeutic target of lung adenocarcinoma.
Clin Transl Med. 11:e3162021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Feng S, Su W, Bai S, Xiao L, Wang
L, Thomas DG, Lin J, Reddy RM, Carrott PW, et al: Overexpression of
FAM83H-AS1 indicates poor patient survival and knockdown impairs
cell proliferation and invasion via MET/EGFR signaling in lung
cancer. Sci Rep. 7:428192017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gong YB and Zou YF: Clinical significance
of lncRNA FAM83H-AS1 in ovarian cancer. Eur Rev Med Pharmacol Sci.
23:4656–4662. 2019.PubMed/NCBI
|
|
29
|
Yuan X, Huang Y, Guo M, Hu X and Li P:
Long non-coding RNA FAM83H-AS1 acts as a potential oncogenic driver
in human ovarian cancer. J Ovarian Res. 14:62021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dou Q, Xu Y, Zhu Y, Hu Y, Yan Y and Yan H:
LncRNA FAM83H-AS1 contributes to the radioresistance,
proliferation, and metastasis in ovarian cancer through stabilizing
HuR protein. Eur J Pharmacol. 852:134–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu B, Qian D, Zhou W, Jiang H, Xiang Z
and Wu D: A novel androgen-induced lncRNA FAM83H-AS1 promotes
prostate cancer progression via the miR-15a/CCNE2 axis. Front
Oncol. 10:6203062021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bi YY, Shen G, Quan Y, Jiang W and Xu F:
Long noncoding RNA FAM83H-AS1 exerts an oncogenic role in glioma
through epigenetically silencing CDKN1A (p21). J Cell Physiol.
233:8896–8907. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bu L, Wang R, Liu P and Da J: Aberrantly
upregulated FAM83H-AS1 facilitates malignant progression of
esophageal squamous cell carcinoma. Oncol Lett. 20:3682020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng B, Wang G, Liang X, Wu Z, Wang X,
Dong Z, Guo Y, Shen S, Liang J and Guo W: LncRNA FAM83H-AS1
promotes oesophageal squamous cell carcinoma progression via
miR-10a-5p/Girdin axis. J Cell Mol Med. 24:8962–8976. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Ashmawy NE, Al-Ashmawy GM and Hamouda
SM: Long non-coding RNA FAM83H-AS1 as an emerging marker for
diagnosis, prognosis and therapeutic targeting of cancer. Cell
Biochem Funct. 39:350–356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li D, Li K, Zhang W, Yang KW, Mu DA, Jiang
GJ, Shi RS and Ke D: The m6A/m5C/m1A regulated gene signature
predicts the prognosis and correlates with the immune status of
hepatocellular carcinoma. Front Immunol. 13:9181402022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang X, Liu B, Nie Z, Duan L, Xiong Q,
Jin Z, Yang C and Chen Y: The role of m6A modification in the
biological functions and diseases. Signal Transduct Target Ther.
6:742021. View Article : Google Scholar : PubMed/NCBI
|