Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor
that has a high incidence in East and Southeast Asia, with an
estimated ~72,000 people dying from NPC in 2018 (1,2).
According to the 2022 National Comprehensive Cancer Network
guidelines, the standard treatment approach for NPC is concurrent
chemoradiotherapy (3). Based on the
use of an effective and standardized treatment, the 5-year survival
rate of early-stage NPC is ~80%. However, 5–10% of patients
experience recurrence or metastasis after treatment, and even in
developed countries, the 5-year survival rate of these patients is
>40% (4,5). The ongoing phase III clinical study
(CONTINUUM), reported at the American Society of Clinical Oncology
in 2023, highlighted the promising efficacy of sintilimab combined
with concurrent chemoradiotherapy in the treatment of locally
advanced NPC (6). Therefore,
combination therapy is an inevitable trend, and understanding the
effect of conventional treatment on the immune system is of great
importance.
Radiotherapy (RT) is a local treatment strategy,
which is administered to regulate tumor growth via ionizing
radiation, thus promoting double-stranded DNA breaks in cells
(7). Similarly, ionizing radiation
can also directly damage immune cells. The immunosuppressive effect
of RT is associated with the reduction of lymphocyte count, the
activation of regulatory T cells and the recruitment of
myeloid-derived suppressor cells (8–11).
Previous studies have also reported that ionizing radiation could
increase the secretion of pro-inflammatory cytokines, including CXC
chemokine ligand (CXCL)16, transforming growth factor-β, CXCL10 and
CXCL4, and promote the expression of major histocompatibility
complex I, programmed cell death 1 ligand 1 (PD-L1) and programmed
cell death protein 1 (PD-1) (12–17).
In addition, RT exerts its antitumor effects via promoting T cell
infiltration and activation through the secretion of
pro-inflammatory factors and expression of particular molecules,
such as TNF-α, IL-1β and IL-6 (18). Different pathological types and RT
plans can affect the efficacy of RT in the immune system (19); however, there is still a lack of
qualified markers to dynamically evaluate the changes in the immune
microenvironment in vivo and elucidate the association
between RT and immunity.
The function of mitochondria is primarily, but not
exclusively, associated with energy supply (20). Based on their activation status,
immune cells can be divided into effector T (Te) cells
(CD62L−CD45RA+) and memory-effector T (Tem)
cells (CD62L−CD45RA−). Te cells are commonly
found in peripheral tissues and exert direct antitumor effects,
whereas Tem cells exist in peripheral and lymphoid tissues and show
antitumor effects after re-exposure to an antigen. CD4+
Te cells, which are involved in tumor immunity, can recruit
dendritic cells, activate CD8+ T cells and directly kill
tumor cells (21). A previous study
reported that the immune status of CD62L−CD4+
Te cells could predict short- and long-term responses to
immunotherapy in patients with non-small cell lung cancer (22).
Furthermore, immune cell activation is characterized
by enhanced mitochondrial mass (MM) and mitochondrial membrane
potential (MMP). Mitochondrial dysfunction-induced depleted immune
cells, and in particular, tumor-infiltrating T cells (TILs),
display reduced mitochondrial function (23–25).
Notably, a previous study reported that mitochondrial dysfunction
was not reversed by the PD-1 blockade of TILs with persistent
mitochondrial loss, suggesting that immune checkpoint blockade
alone could not yield desirable results in patients with more
complex tumors (23,25). The mitochondrial function grouping
of peripheral blood immune cells can provide a basis for evaluating
the immune status of patients with cancer (26). The maintenance of cellular function
requires sufficient MM to maintain oxidative phosphorylation
(27). Activated CD8+ T
cells and Te cells are characterized by enhanced MM, whilst
depleted T cells have low MM (28).
MMP is generated by the pumping of protons from the matrix into the
membrane space and is associated with adenosine triphosphate
production. When T cell receptors are activated, MMP and
mitochondrial metabolism are increased, thus promoting the
secretion of cytokines, such as interleukin (IL)-17A, IL-17F and
interferon-γ (IFN-γ), by several Te cells (29). However, whether MM and MMP can
predict the response to tumor therapy remains unknown.
The present study aimed to assess the dynamic
changes in immune status in patients with NPC during RT. Using a
novel immunofluorescence technique, the mitochondrial function
indicators of immune cells, namely MM and MMP, were evaluated.
Subsequently, the functional status of immune cells at different
time points during RT in patients with NPC and the association
between the functional status of immune cells and the efficacy of
RT were assessed, thus providing novel insights into the role of
MMP of immune cells in predicting the efficacy of RT.
Materials and methods
Study design and patient
enrollment
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Qingdao University
(Qingdao, China; approval no. MR-37-23-012058). The study was
designed in accordance with the ethical principles laid down in the
Declaration of Helsinki. A total of 28 patients with locally
advanced NPC were enrolled from the Affiliated Hospital of Qingdao
University, between September 2022 and November 2023. Due to the 1
patient contracting coronavirus disease-19 and being excluded from
the present study, a total of 27 patients completed the study. The
inclusion criteria were as follows: i) New diagnosis of
pathologically-confirmed NPC; ii) stage III–IVa NPC according to
the 8th edition of the American Joint Committee on Cancer staging
system (30); iii) refusal of
concurrent chemoradiotherapy due to physical reasons; iv) age of
18–70 years; and v) written informed consent provided. The
exclusion criteria were as follows: i) Diagnosis of autoimmune
diseases or primary immune dysfunction; ii) diagnosis of other
malignant tumors; iii) previous therapy with PD-1, PD-L1 or
cytotoxic T lymphocyte-associated antigen-4 inhibitors; iv)
presence of inflammatory reactions, such as cold and fever, or
infection, including hepatitis B and acquired immune deficiency
syndrome; v) diagnosis of metabolic syndrome (diabetes); vi)
hemodialysis or organ transplantation performed; and vi)
unwillingness to participate in the study. All patients provided
written informed consent prior enrollment in the study.
Delineation of the target volume and RT plan design
was performed according to International Guidelines for NPC
(31). The RT plan was implemented
in accordance with the International Commission of Radiation Units
reports 50 and 62 (32,33). All patients previously refused
concurrent chemotherapy and had received concurrent targeted
therapy during RT after induction chemotherapy. Targeted therapy
involved the administration of 100 mg nitocilizumab once a week for
8 weeks during RT. Following RT for 4 weeks, the clinical efficacy,
which was classified as complete response (CR), partial response
(PR), stable disease (SD) and progressive disease (PD), was
evaluated by two attending physicians according to the Response
Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines
(34). The clinical and
pathological data were obtained from the electronic medical records
of the Affiliated Hospital of Qingdao University.
Peripheral blood samples
At least 3 ml peripheral venous blood was collected
in ethylene diaminetetraacetic acid tubes at day 1 pre-RT, at the
10th fraction of RT and within 2 days after RT. The antibodies were
as follows: CD3 PE, CD4 FITC, CD45RA PerCP-Cy5.5, CD62L
PE-Cyamine7, CD8 APC-Cyanine7 contained in a complete antibody kit
(cat. no. KUB32479; Hunan Xiangyi Biotechnology Co. Ltd.) and the
mitochondrial staining fluorescent probes, MitoDye (structural
formula C34H36Cl2N2;
cat. no. UB32479-8; Hunan Xiangyi Biotechnology Co., Ltd.) and
phosphate buffer (DPBS; pH 7.4, 0.12%
NaH2PO4, 0.88% NaCl, 0.2% gelatin and 0.09%
sodium azide), purchased from UB Biotechnology Co. Ltd. A 20 µl
volume of antibody detection reagent was placed in a labelled flow
tube at room temperature. A 100 µl anticoagulated human whole blood
sample turnd upside down at least 7 times, was placed in the flow
tube for 3–5 secand incubated in the dark at room temperature for
15 min. A 2 ml hemolysin working solution (BD Biosciences) was
added to the flow tube and vortexed at a low speed for 3–5 sec,
then incubated in the dark for 15 min at room temperature. Samples
were centrifuged at 300 × g for 5 min at room temperature, and the
supernatant was discarded. The precipitate was resuspended in 200
µl of DPBS, transferred to an 8-linked tube of mitochondrial
staining reagent and incubated at 37°C in the dark for 30 min. The
lymphocyte subtypes in peripheral blood, including CD3+,
CD4+ and CD8+ T cells, were assessed using
flow cytometry. The CD62L−CD45RA+ effector
and CD62L−CD45RA− memory-effector subsets
were also detected (35). The
mitochondrial indices determined were MM and low MMP
(MMPlow). Mitochondria were stained with MitoDye-APC as
aforementioned and detected using a flow cytometer (DiagCyto 6C2L;
Changchun YouBi Biotechnology Co., Ltd.), followed by passing
through an antigen-assisted loop gate (36). Using the antibody-assisted ring
gate, each target cell gate was read and a binary gate was then
drawn to distinguish the low from the high mitochondrial expression
groups. The low percentage group represented MMPlow,
whilst the total fluorescence intensity group indicated MM.
Analysis was performed using NovoExpress (version 1.4.1; Agilent
Technologies, Inc.). The screening strategy is illustrated in
Fig. 1A-D.
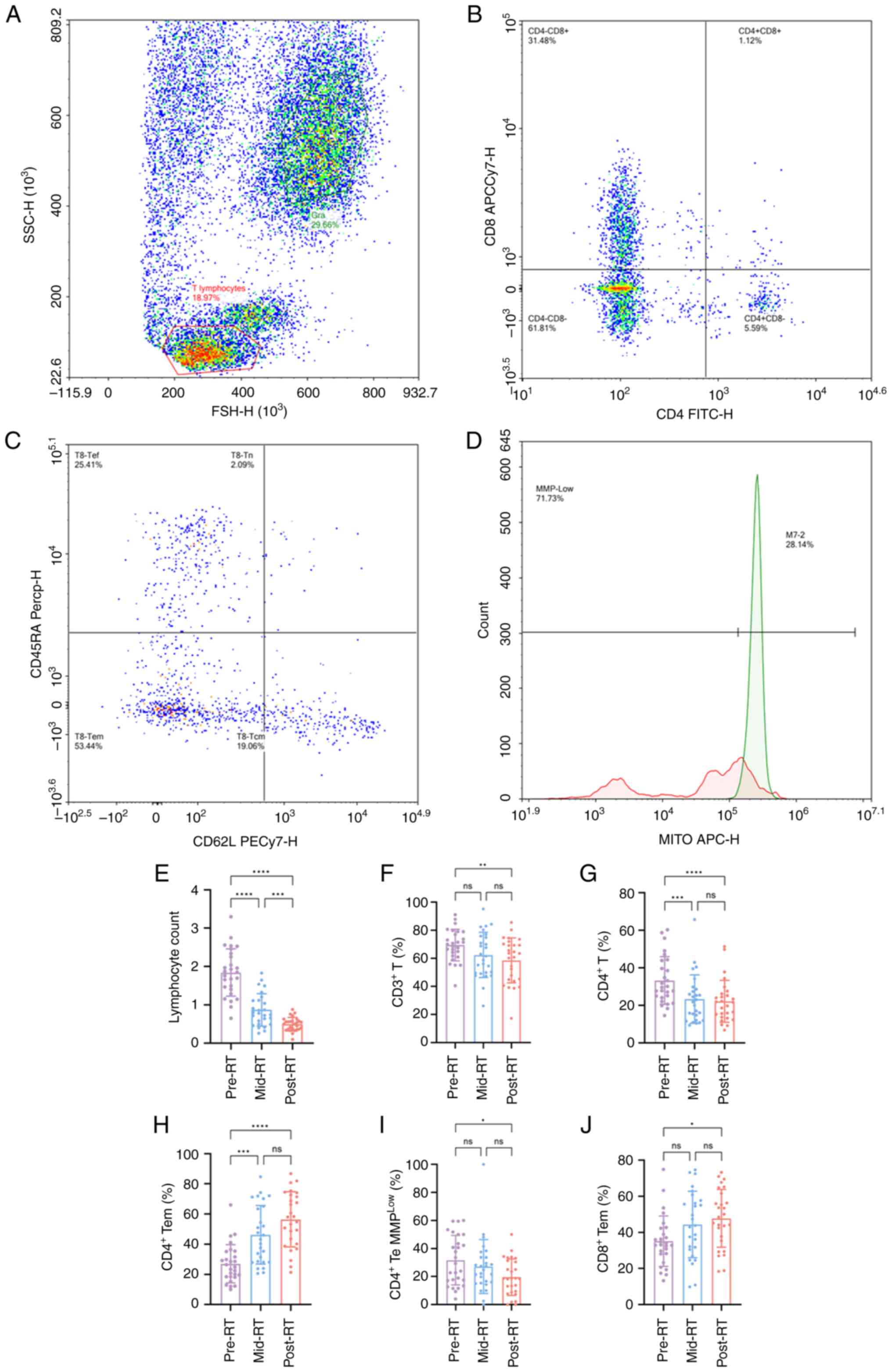 | Figure 1.Flow cytometry and changes in immune
cells during RT. Gating strategy in flow cytometry. (A) FSC/SSC
plot of the lymphocyte populations. (B) CD4/CD8 scatter of the
CD4+CD8− and CD4−CD8+
cell populations. (C) CD62L/CD45RA scatter plots of the Tn, Tcm,
Tem and Tef cell populations. (D) Count/MITO APC-H of the Tem, Tn,
Tcm and Tef cell populations, using a dichotomous gate to calculate
the percentage of lymphocytes and fluorescence intensity in each
population. Changes in the (E) number of lymphocytes, proportion of
(F) CD3+ T cells, (G) CD4+ T cells and (H)
CD4+Tem cells, (I) MMPlow in CD4+
Te cells and (J) proportion of CD8+ Tem cells during RT.
n=27 per group. *P<0.05; **P<0.01;
***P<0.001; ****P<0.0001. RSC, forward scatter;
SSC, side scatter; ns, not significant; RT, radiotherapy; Tem,
memory-effector T cells; Te, effector cells T; Tef, effector T
cells; Tn, Naïve T cells; Tcm, central memory T cells; MMP,
mitochondrial membrane potential. |
Statistical analysis
All statistical analyses were performed using SPSS
version 26.0 (IBM Corp.), whilst GraphPad Prism 10.0 software
(Dotmatics) was used to draw graphs. Continuous variables are
expressed as the mean ± standard deviation and categorical
variables as n (%). When the changes in the immune cells during RT
were normally distributed, the repeated measures ANOVA test was
performed. For non-normally distributed changes, the Friedman's
test was performed. For multiple comparisons between two groups,
Bonferroni test or Nemenyi test was performed for statistically
significant differences. To analyze the potential prognostic
factors for clinical response (CR/PR), univariate logistic
regression analysis was performed. Prognostic factors with P<0.2
were subjected to multivariate logistic regression analysis.
Receiver operating characteristic (ROC) curves were utilized to
describe the predictive function of mitochondria in immune cells
and lymphocytes. To analyze the changes in PD-1 expression in T
cell subsets before and after RT, the paired t test was used if the
distribution was normal, and the Wilcoxon Rank-Sign test was used
if the distribution was not normal. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
A total of 27 patients, including 16 men and 11
women, with locally advanced NPC were enrolled from the Affiliated
Hospital of Qingdao University (Table
I). The median age of patients was 49 years (range, 18–70
years). The majority of the included patients had stage III NPC
(63.0%), whilst they all had pathologically confirmed EGFR
mutations. Among the 27 patients, 20 were treated with helical
tomotherapy, whilst the remaining seven patients were subjected to
intensity-modulated RT.
 | Table I.Clinical characteristics of patients
with nasopharyngeal carcinoma in the present study. |
Table I.
Clinical characteristics of patients
with nasopharyngeal carcinoma in the present study.
| Characteristic | Patients
(n=27) |
|---|
| Sex |
|
|
Male | 16 (59.3) |
|
Female | 11 (40.7) |
| Age, years | 49.0±15.2 |
| T stage |
|
| T1 | 3 (11.1) |
| T2 | 16 (59.3) |
| T3 | 5 (18.5) |
| T4 | 3 (11.1) |
| N stage |
|
| N0 | 2 (7.4) |
| N1 | 2 (7.4) |
| N2 | 16 (59.3) |
| N3 | 7 (25.9) |
| Overall stage |
|
|
III | 17 (63.0) |
|
IVa | 10 (37.0) |
| World Health
Organization pathological type |
|
|
Keratinizing carcinoma | 2 (7.4) |
|
Differentiated
non-keratinizing carcinoma | 21 (77.8) |
|
Undifferentiated
non-keratinizing carcinoma | 4 (14.8) |
Immune status of peripheral blood
cells during RT
All 27 patients underwent pre-RT, mid-RT and
post-RT. After RT, the number of peripheral blood lymphocytes
(F=104.554; P<0.001; Fig. 1E)
and the proportion of CD4+ T cells were significantly
decreased (F=19.185; P<0.001; Fig.
1G), whilst no significant change was demonstrated for the
percentage of CD8+ T cells. In addition, the proportion
of CD4+ (F=30.519; P<0.001; Fig. 1H) and CD8+ (F=5.827;
P=0.005; Fig. 1J) Tem cells was
significantly increased, whilst no changes were demonstrated for
CD4+ and CD8+ Te cells (Table II). Previous studies have reported
that the mitochondrial function of immune cells could be associated
with response to cancer therapy (37,38).
Therefore, the functional status of mitochondria was used to
reflect the changes in immune cell status. A novel
immunofluorescence technique was used to detect mitochondrial
function indicators, namely MMP and MM. No significant change was
revealed for the mitochondrial function of CD4+ T cells
after RT (Table II). Additionally,
no significant changes in MM were demonstrated in CD4+
Te cells. However, MMPlow was significantly reduced in
these cells (F=3.294; P=0.047; Fig.
1I). The aforementioned results indicate that the effector
subsets of CD4+ T cells were activated during RT, whilst
their mitochondrial function was significantly increased. Notably,
the mitochondrial function of CD8+ T cells and their
subsets did not change significantly.
 | Table II.Analysis of changes in the number of
immune cells and mitochondrial function during radiotherapy. |
Table II.
Analysis of changes in the number of
immune cells and mitochondrial function during radiotherapy.
| Parameter | Pre-RT (n=27) | Mid-RT (n=27) | Post-RT (n=27) | F | P-value |
|---|
| White blood cell
count, 109/l | 5.86±2.46 | 5.57±1.68 | 5.32±2.06 | 2.889 | 0.236 |
| Lymphocyte count,
109/l | 1.85±0.62 | 0.87±0.42 | 0.50±0.18 | 104.554 | <0.001 |
| CD3+ T,
% | 69.47±11.26 | 62.41±16.15 | 58.54±15.99 | 6.016 | 0.009 |
| CD4+ T,
% | 33.31±12.7 | 23.41±12.89 | 22.20±11.14 | 19.185 | <0.001 |
| CD4+ T
MMPlow, % | 31.57±10.68 | 35.95±12.82 | 38.12±13.77 | 1.855 | 0.167 |
| CD4+ T
MM | 3.13±3.13 | 2.78±3.65 | 1.70±0.77 | 2.296 | 0.317 |
| CD4+ Te,
% | 18.34±13.99 | 19.98±18.25 | 17.28±17.31 | 0.519 | 0.772 |
| CD4+ Te
MMPlow, % | 31.71±17.67 | 27.18±19.18 | 19.62±13.04 | 3.294 | 0.047 |
| CD4+ Te
MM | 2.37±1.54 | 2.26±0.83 | 2.48±1.48 | 0.222 | 0.895 |
| CD4+
Tem, % | 27.04±12.67 | 46.24±19.39 | 56.47±18.30 | 30.519 | <0.001 |
| CD4+ Tem
MMPlow, % | 40.41±14.54 | 43.30±15.75 | 44.99±17.81 | 0.621 | 0.541 |
| CD4+ Tem
MM | 1.86±1.27 | 1.46±0.64 | 1.35±0.71 | 5.505 | 0.064 |
| CD8+ T,
% | 33.16±12.44 | 33.29±12.52 | 33.45±14.94 | 0.963 | 0.618 |
| CD8+ T
MMPlow, % | 46.66±17.30 | 38.89±16.00 | 43.03±15.47 | 2.005 | 0.145 |
| CD8+ T
MM | 1.96±1.73 | 2.06±2.16 | 1.39±0.82 | 3.852 | 0.146 |
| CD8+ Te,
% | 27.52±15.29 | 29.63±20.26 | 25.79±12.33 | 0.074 | 0.964 |
| CD8+ Te
MMPlow, % | 40.75±30.13 | 40.48±26.69 | 39.08±16.63 | 0.222 | 0.895 |
| CD8+ Te
MM | 2.14±1.88 | 1.65±0.93 | 1.68±1.09 | 0.296 | 0.862 |
| CD8+
Tem, % | 35.12±13.98 | 44.38±18.40 | 47.75±16.01 | 5.827 | 0.005 |
| CD8+ Tem
MMPlow, % | 53.08±26.02 | 53.50±24.62 | 47.04±18.47 | 0.802 | 0.454 |
| CD8+ Tem
MM | 1.62±1.91 | 1.17±0.72 | 1.34±0.86 | 1.364 | 0.505 |
| CD4/CD8 | 1.25±1.13 | 0.80±0.48 | 0.85±0.68 | 15.407 | <0.001 |
Mitochondrial function as a prognostic
indicator
At 4 weeks after RT, clinical efficacy was assessed
using the RECIST 1.1. guidelines. A total of 22 patients achieved
CR or PR, whilst the remaining patients were classified as PD (n=3)
or SD (n=2). It was therefore considered that patients with CR and
PR had a good response, whilst the remaining patients displayed a
poor response to RT. The clinical characteristics of patients, such
as age, tumor stage, lymph node stage and pathological features, as
well as immunological parameters that changed during RT, were
included in the regression analysis. Indicators with P<0.2 in
the univariate logistic regression analysis were included in the
multivariate logistic regression analysis. Therefore, the
multivariate logistic regression analysis demonstrated that
lymphocyte count and MMPlow in CD4+ Te cells were
independent factors affecting clinical efficacy. The higher the
lymphocyte count, the greater the clinical efficacy [odds ratio
(OR), 47.317; 95% confidence interval (CI), 1.240–1806.065]. In
addition, the higher the MMPlow in CD4+ Te
cells, the worse the clinical efficacy (OR, 0.889; 95% CI,
0.792–0.997) (Table III). These
results suggest that increased MMP in CD4+ Te cells is
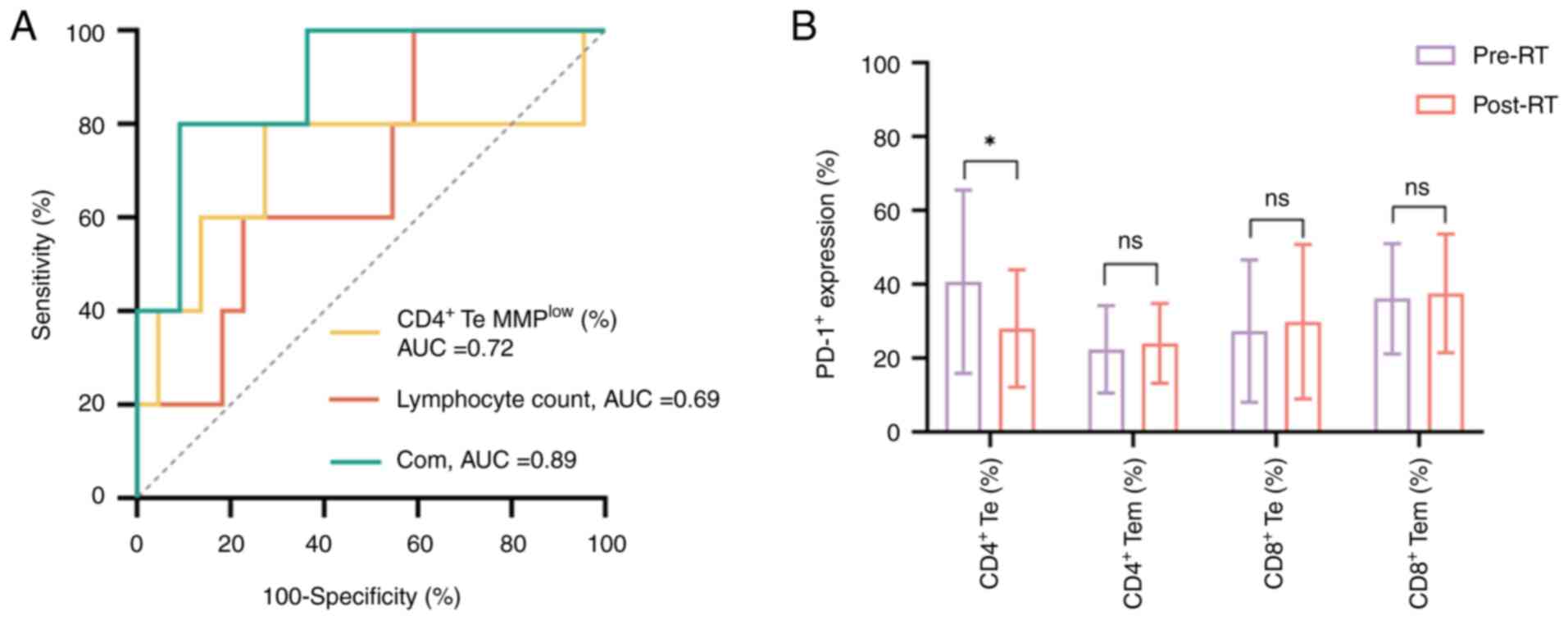
associated with improved clinical efficacy. Furthermore, the ROC
curve analysis revealed that the combination of MMPlow
in CD4+ Te cells with lymphocyte count exerted an
improved area under the curve (AUC) value for predicting clinical
efficacy, compared with MMPlow in CD4+ Te
cells and lymphocyte counts. The AUC values for MMPlow
in CD4+ Te cells, lymphocyte count and their combination
were 0.72 (P=0.13), 0.69 (P=0.19) and 0.89 (P=0.0073), respectively
(Fig. 2A). These findings suggest
that mitochondrial function in lymphocytes could be considered as
an indicator to predict the efficacy of RT in patients with locally
advanced NPC.
 | Table III.Univariate and multivariate analysis
of radiotherapy efficacy. |
Table III.
Univariate and multivariate analysis
of radiotherapy efficacy.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | P-value | OR | 95% CI | P-value | OR | 95% CI |
|---|
| Age | 0.63 | 1.02 | 0.95–1.10 |
|
|
|
| T stage | 0.76 | - | - |
|
|
|
| N stage | 0.77 | - | - |
|
|
|
| Stage | 0.88 | 0.86 | 0.12–6.26 |
|
|
|
| Pathological
type | 0.49 | - | - |
|
|
|
| Lymphocyte
count | 0.10 | 4.73 | 0.73–30.66 | 0.038 | 47.317 | 1.240–1806.065 |
| CD3+
T | 0.24 | 1.06 | 0.96–1.17 |
|
|
|
| CD4+
T | 0.40 | 1.03 | 0.96–1.11 |
|
|
|
| CD4+ Te
MMPlow | 0.17 | 0.95 | 0.89–1.02 | 0.045 | 0.889 | 0.792–0.997 |
| CD4+
Tem | 0.56 | 0.97 | 0.89–1.07 |
|
|
|
| CD8+
Tem | 0.86 | 0.99 | 0.92–1.07 |
|
|
|
| CD4/CD8 | 0.22 | 2.76 | 0.55–13.80 |
|
|
|
PD-1 expression in peripheral blood T
cell subsets
It has been reported that the enhanced expression of
PD-1 in peripheral blood immune cells is associated with cell
failure and worse clinical efficacy (39,40).
Therefore, the present study assessed the expression levels of PD-1
in CD4+ and CD8+ T cell subsets before and
after RT. The results revealed that the expression levels of PD-1
in CD4+ Te cells displayed a downward trend and the
difference was statistically significant (Z=−2.162; P=0.031;
Fig. 2B). By contrast, the
expression levels of PD-1 in the other cell subsets were notably
increased; however, statistical significance was not reached.
Discussion
The application of immunotherapy in patients with
advanced NPC has reached a consensus, whilst the combination of RT
with immunotherapy in patients with locally advanced NPC remains in
the exploratory stage (41).
Currently, the RT-mediated changes in immune status in patients
with locally advanced NPC are unclear. Therefore, the timing of
combination therapy remains an urgent problem to be solved.
Consequently, the present study assessed the mitochondrial changes
in immune cells in the peripheral blood of patients with locally
advanced NPC before and after RT. The results demonstrated that RT
could activate CD4+ Te cells and increase MMP, whilst
MMPhigh was associated with the efficacy of RT.
Therefore, we hypothesize that MMP in peripheral blood
CD4+ Te cells could be used as an index for evaluating
the immune status of patients with locally advanced NPC.
RT can induce the immunogenic death of tumor cells
and promote inflammatory and antitumor responses; however, it has
been reported that RT can increase the secretion of
immunosuppressive antibodies, which in turn deplete immune cells
(42). Nevertheless, how to
regulate the immunomodulatory effect of RT remains unresolved. RT
is the main treatment strategy for NPC; however, ~30% of patients
will relapse after systemic treatment (5). In our previous study, it was
demonstrated that the compliance of patients with NPC treated with
concurrent chemoradiotherapy was poor and patients could not
tolerate concurrent chemoradiotherapy (38). In addition, You et al
(43) and Cao et al
(44) reported that the clinical
effect of synchronous targeted therapy during RT was not markedly
different from that of synchronous chemoradiotherapy, and there
were fewer adverse effects, which was easier for patients to
accept. Moreover, Teng et al (45) reported that RT combined with
targeted therapy had a small inhibitory effect on the immune
system, which enhanced the activation of autoimmune mechanisms in
patients with locally advanced NPC and established a foundational
framework for combination immunotherapy strategies. Due to patient
refusal of concurrent chemotherapy, the inherent limitations of the
single-center study design and predefined research objectives, all
participants underwent radiotherapy with concurrent targeted
therapy instead of the standard chemoradiotherapy regimen.
Single-cell transcriptome analysis of the tumor
microenvironment in patients with locally advanced NPC revealed
that PD-1 was highly expressed in TILs, accompanied by an increased
number of immunosuppressive cells, which may have originated from
their migration into peripheral blood (46). Patients with locally advanced NPC
have a unique tumor immune microenvironment, which provides the
basis for the application of RT combined with immunotherapy.
However, the changes in the immune status of patients with locally
advanced NPC during RT have not been elucidated (47). Consistent with the study by Xie
et al (48), in the present
study, the number of lymphocytes decreased during RT. Radiation can
directly damage the DNA of circulating lymphocytes, and circulating
lymphocytes are sensitive to radiation; therefore, DNA
fragmentation can be caused by a radiation dose of 2 Gy (49). Previous studies have reported that
CD4+ T cells could be more susceptible to radiation
compared with CD8+ T cells, thus highlighting the
different radiosensitivity of different cells, which could lead to
differential lymphocyte subtype distribution (50). Tem cells, as memory effector cells,
are located in peripheral lymphoid tissues and respond rapidly when
T cell receptors are activated (51). However, Te cells are terminally
differentiated effector cells and their proliferation ability is
weak (51). Therefore, this
explains why the proportion of Tem cells increased during RT in the
present study. By contrast, the proportion of Te cells did not
change significantly.
The current study demonstrated that RT could
activate circulating CD4+ Te cells in patients with
locally advanced NPC. Additionally, higher MMP was associated with
the efficacy of RT. Previous studies have reported that elevated
MMP induced glycolysis and the secretion of different cytokines,
such as IL-17A, IL-17F and IFN-γ (29,52).
Ma et al (53) reported that
the MMP and MM of lymphocyte could predict early liver
inflammation, indicating that MMP could reflect the inflammatory
state of the body. However, the present study is the first to
predict MMP and MM during RT in patients with tumor, to the best of
our knowledge. Unlike the study by Ma et al, the
inflammatory state induced by RT in patients with NPC stimulated an
antitumor immune response, with the increased metabolism of immune
cells promoting their antitumor function. Moreover, it has been
reported that T-cell exhaustion is characterized by increased PD-1
expression (54). However,
consistent with membrane potential, PD-1 expression decreased in
CD4+ Te cells after RT in the present study. These
results indicate that RT could activate CD4+ Te
cell-mediated immunity. Ogando et al (55) reported that PD-1 expression in
CD8+ T cells limited the mitochondrial contact site and
downregulated cristae organizing system complex proteins, namely
coiled-coil-helix-coiled-coil-helix domain containing (CHCHD)3 and
CHCHD10, thus promoting the loss of mitochondrial cristae
morphology and MMP, eventually resulting in T cell dysfunction.
However, the mechanism underlying the effect of PD-1 signaling on
MMP in CD4+ Te cells has not been elucidated. Yang et
al (56) reported that the
activation of CD4+ Te cells during chemoimmunotherapy
was associated with an improved prognosis in patients with
non-small cell lung cancer. Furthermore, it has been reported that
CD4+ T cells are associated with the efficacy of RT
against malignant tumors, such as ovarian cancer, head and neck
squamous cell carcinoma, malignant melanoma, rectal cancer and
breast cancer (57,58). This could be due to the RT-mediated
activation CD4+ T cells and the release of cytokines,
such as IFN-γ and TNF-α, thus enhancing the sensitivity of tumor
cells to RT (59). Another study
also reported that the production of IFN-γ and that of other
cytokines was closely associated with mitochondria-mediated
reactive oxygen species generation. The aforementioned process was
regulated by the Fas/FasL pathway (60). Herrera et al (61) reported that RT could promote the
expression of natural killer group 2 member D (NKG2D) by
CD4+ Te cells and activate dendritic cells. In turn, the
expression of NKG2D was notably associated with glucose metabolism
regulated by mitochondria. However, when T cells with stem cell
properties are needed, T cells with lower MMP should be screened
for CAR-T therapy (62). By
contrast, Te cells with high MMP are required to achieve antitumor
effects (29). Therefore, the
immune system of patients with high MMP in CD4+ Te cells
could be activated during RT and therefore, patients who could be
more likely to benefit from immunotherapy should be selected. At
the same time, as a non-invasive index, MMP could be easier
measured.
The current study has certain limitations. Firstly,
due to the limitation of time and region, the sample size was
relatively small. A strict inclusion and exclusion criteria was
adopted to reduce the heterogeneity of patients and improve the
universality of the study; however, due to the small number of
patients included, heterogeneity was still present. As a result,
certain results were not significant, the research data were skewed
and the universality of the research requires improvement.
Additionally, as long-term follow up was not performed, several
P-values could be underestimated. Therefore, further studies with
an increased follow-up duration should be performed. Secondly, only
the effect of RT on mitochondrial function in peripheral blood
immune cells from patients with locally advanced NPC was assessed.
However, the underlying mechanism at the cellular level remains
unclear. Thus, further in vivo and in vitro
experiments are needed to clarify the underlying mechanism.
Furthermore, due to the limitation of clinical practical
application, only patients treated with RT concurrent with targeted
therapy were included, whilst those treated with RT combined with
chemotherapy were excluded. Therefore, the generalizability of the
study is lacking and more studies, including more clinical
treatment centers, are needed to enhance the clinical data and
ensure more comprehensive and reliable experimental results.
In conclusion, the present study was the first to
assess the changes in mitochondrial function in peripheral blood
immune cells during RT, to the best of our knowledge. The results
revealed that RT could increase the MMP of circulating
CD4+ Te cells in patients with locally advanced NPC and
that MMPhigh was associated with the efficacy of RT.
Therefore, MMPlow in CD4+ Te cells could be
used as a marker for evaluating the immune status of patients with
locally advanced NPC, thus providing a potential treatment approach
for NPC.
Acknowledgements
Not applicable.
Funding
The present study received funding from the National Key
Research and Development Program of China (grant no.
2022YFC2401500).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conception and design of the research and drafting
of the manuscript was conducted by QuW. Acquisition of data and
revision of the manuscript for intellectual content was conducted
by XY and LZ. Analysis and interpretation of data was conducted by
HoL, YW, QiW and XY. Statistical analysis was conducted by HaL. QuW
and XY confirm the authenticity of all the raw data. All authors
contributed to the manuscript. All author read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Qingdao University
(approval no. MR-37-23-012058). All patients provided written
informed consent prior to enrollment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
MM
|
mitochondrial mass
|
|
MMPlow
|
low mitochondrial membrane
potential
|
|
RT
|
radiotherapy
|
|
CXCL
|
CXC chemokine ligand
|
|
PD-L1
|
programmed cell death 1 ligand 1
|
|
TILs
|
tumor-infiltrating T cells
|
|
IL
|
interleukin
|
|
IFN-γ
|
interferon-γ
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
ROC
|
receiver operating characteristic
|
References
|
1
|
Chang ET, Ye W, Zeng YX and Adami HO: The
evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 30:1035–1047. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caudell JJ, Gillison ML, Maghami E,
Spencer S, Pfister DG, Adkins D, Birkeland AC, Brizel DM, Busse PM,
Cmelak AJ, et al: NCCN Guidelines® insights: Head and
neck cancers, version 1.2022. J Natl Compr Canc Netw. 20:224–234.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lechner M, Schartinger VH, Steele CD, Nei
WL, Ooft ML, Schreiber LM, Pipinikas CP, Chung GT, Chan YY, Wu F,
et al: Somatostatin receptor 2 expression in nasopharyngeal cancer
is induced by Epstein Barr virus infection: Impact on prognosis,
imaging and therapy. Nat Commun. 12:1172021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Rumgay H, Li M, Cao S and Chen W:
Nasopharyngeal cancer incidence and mortality in 185 countries in
2020 and the projected burden in 2040: Population-based global
epidemiological profiling. JMIR Public Health Surveill.
9:e499682023. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma J, Sun Y, Liu X, Yang KY, Zhang N, Jin
F, Zou G and Chen YP: PD-1 blockade with sintilimab plus induction
chemotherapy and concurrent chemoradiotherapy (IC-CCRT) versus
IC-CCRT in locoregionally-advanced nasopharyngeal carcinoma
(LANPC): A multicenter, phase 3, randomized controlled trial
(CONTINUUM). Head And Neck. 41 (Suppl 17):LBA60022023.
|
|
7
|
Schwartz JL, Mustafi R, Beckett MA and
Weichselbaum RR: DNA double-strand break rejoining rates, inherent
radiation sensitivity and human tumour response to radiotherapy. Br
J Cancer. 74:37–42. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heylmann D, Rödel F, Kindler T and Kaina
B: Radiation sensitivity of human and murine peripheral blood
lymphocytes, stem and progenitor cells. Biochim Biophys Acta.
1846:121–129. 2014.PubMed/NCBI
|
|
9
|
Trowell OA: The sensitivity of lymphocytes
to ionising radiation. J Pathol Bacteriol. 64:687–704. 1952.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marciscano AE, Ghasemzadeh A, Nirschl TR,
Theodros D, Kochel CM, Francica BJ, Muroyama Y, Anders RA, Sharabi
AB, Velarde E, et al: Elective nodal irradiation attenuates the
combinatorial efficacy of stereotactic radiation therapy and
immunotherapy. Clin Cancer Res. 24:5058–5071. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karapetyan L, Iheagwara UK, Olson AC,
Chmura SJ, Skinner HK and Luke JJ: Radiation dose, schedule, and
novel systemic targets for radio-immunotherapy combinations. J Natl
Cancer Inst. 115:1278–1293. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng L, Liang H, Xu M, Yang X, Burnette B,
Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al:
STING-Dependent cytosolic DNA sensing promotes radiation-induced
type i interferon-dependent antitumor immunity in immunogenic
tumors. Immunity. 41:843–852. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sen T, Rodriguez BL, Chen L, Corte CMD,
Morikawa N, Fujimoto J, Cristea S, Nguyen T, Diao L, Li L, et al:
Targeting DNA damage response promotes antitumor immunity through
STING-mediated T-cell activation in small cell lung cancer. Cancer
Discov. 9:646–661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du SS, Chen GW, Yang P, Chen YX, Hu Y,
Zhao QQ, Zhang Y, Liu R, Zheng DX, Zhou J, et al: Radiation therapy
promotes hepatocellular carcinoma immune cloaking via PD-L1
upregulation induced by cGAS-STING activation. Int J Radiat Oncol
Biol Phys. 112:1243–1255. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reits EA, Hodge JW, Herberts CA, Groothuis
TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH,
Neijssen J, et al: Radiation modulates the peptide repertoire,
enhances MHC class I expression, and induces successful antitumor
immunotherapy. J Exp Med. 203:1259–1271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parikh F, Duluc D, Imai N, Clark A,
Misiukiewicz K, Bonomi M, Gupta V, Patsias A, Parides M, Demicco
EG, et al: Chemoradiotherapy-induced upregulation of PD-1
antagonizes immunity to HPV-related oropharyngeal cancer. Cancer
Res. 74:7205–7216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vanpouille-Box C, Diamond JM, Pilones KA,
Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH and Demaria S:
TGFβ is a master regulator of radiation therapy-induced antitumor
immunity. Cancer Res. 75:2232–2242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Filatenkov A, Baker J, Mueller AM, Kenkel
J, Ahn GO, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S,
et al: Ablative tumor radiation can change the tumor immune cell
microenvironment to induce durable complete remissions. Clin Cancer
Res. 21:3727–3739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galluzzi L, Humeau J, Buqué A, Zitvogel L
and Kroemer G: Immunostimulation with chemotherapy in the era of
immune checkpoint inhibitors. Nat Rev Clin Oncol. 17:725–741. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Zhang W, Li Z, Lin S, Zheng T,
Hao B, Hou Y, Zhang Y, Wang K, Qin C, et al: Mitochondria
dysfunction in CD8+ T cells as an important contributing factor for
cancer development and a potential target for cancer treatment: A
review. J Exp Clin Cancer Res. 41:2272022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krishna S, Lowery FJ, Copeland AR,
Bahadiroglu E, Mukherjee R, Jia L, Anibal JT, Sachs A, Adebola SO,
Gurusamy D, et al: Stem-like CD8 T cells mediate response of
adoptive cell immunotherapy against human cancer. Science.
370:1328–1334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kagamu H, Kitano S, Yamaguchi O, Yoshimura
K, Horimoto K, Kitazawa M, Fukui K, Shiono A, Mouri A, Nishihara F,
et al: CD4(+) T-cell immunity in the peripheral blood correlates
with response to anti-pd-1 therapy. Cancer Immunol Res. 8:334–344.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scharping NE, Menk AV, Moreci RS,
Whetstone RD, Dadey RE, Watkins SC, Ferris RL and Delgoffe GM: The
tumor microenvironment represses T cell mitochondrial biogenesis to
drive intratumoral T cell metabolic insufficiency and dysfunction.
Immunity. 45:374–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H, Zhao X, Hochrein SM, Eckstein M,
Gubert GF, Knöpper K, Mansilla AM, Öner A, Doucet-Ladevèze R,
Schmitz W, et al: Mitochondrial dysfunction promotes the transition
of precursor to terminally exhausted T cells through
HIF-1α-mediated glycolytic reprogramming. Nat Commun. 14:68582023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong X, Wu H, Ouyang C, Zhang W, Shi Y,
Wang YC, Ann DK, Gwack Y, Shang W and Sun Z: Ncoa2 Promotes CD8+ T
cell-mediated antitumor immunity by stimulating T-cell activation
via upregulation of PGC-1α critical for mitochondrial function.
Cancer Immunol Res. 11:1414–1431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dyikanov D, Zaitsev A, Vasileva T, Wang I,
Sokolov AA, Bolshakov ES, Frank A, Turova P, Golubeva O, Gantseva
A, et al: Comprehensive peripheral blood immunoprofiling reveals
five immunotypes with immunotherapy response characteristics in
patients with cancer. Cancer Cell. 42:759–779.e12. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Menk AV, Scharping NE, Rivadeneira DB,
Calderon MJ, Watson MJ, Dunstane D, Watkins SC and Delgoffe GM:
4-1BB costimulation induces T cell mitochondrial function and
biogenesis enabling cancer immunotherapeutic responses. J Exp Med.
215:1091–1100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fischer M, Bantug GR, Dimeloe S, Gubser
PM, Burgener AV, Grählert J, Balmer ML, Develioglu L, Steiner R,
Unterstab G, et al: Early effector maturation of naïve human CD8(+)
T cells requires mitochondrial biogenesis. Eur J Immunol.
48:1632–1643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sukumar M, Liu J, Mehta GU, Patel SJ,
Roychoudhuri R, Crompton JG, Klebanoff CA, Ji Y, Li P, Yu Z, et al:
Mitochondrial membrane potential identifies cells with enhanced
stemness for cellular therapy. Cell Metab. 23:63–76. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC,
AlHussain H, Corry J, Grau C, Grégoire V, Harrington KJ, et al:
International guideline for the delineation of the clinical target
volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol.
126:25–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
International Commission on Radiation
Units and Measurements, . Prescribing, recording, and reporting
photon beam therapy. ICRU Report 50. ICRU; Bethesda, MD: 1993
|
|
33
|
International Commission on Radiation
Units and Measurements, . Prescribing, recording, and reporting
photon beam therapy (Supplement to ICRU Report 50). ICRU Report 62.
ICRU; Bethesda, MD: 1999
|
|
34
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maecker HT, McCoy JP and Nussenblatt R:
Standardizing immunophenotyping for the human immunology project.
Nat Rev Immunol. 12:191–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang B, Chen Z, Huang Y, Ding J, Lin Y,
Wang M and Li X: Mitochondrial mass of circulating NK cells as a
novel biomarker in severe SARS-CoV-2 infection. Int
Immunopharmacol. 124:1108392023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liao R, Wu Y, Qin L, Jiang Z, Gou S, Zhou
L, Hong Q, Li Y, Shi J, Yao Y, et al: BCL11B and the NuRD complex
cooperatively guard T-cell fate and inhibit OPA1-mediated
mitochondrial fusion in T cells. EMBO J. 42:e1134482023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scharping NE, Rivadeneira DB, Menk AV,
Vignali PDA, Ford BR, Rittenhouse NL, Peralta R, Wang Y, Wang Y,
DePeaux K, et al: Mitochondrial stress induced by continuous
stimulation under hypoxia rapidly drives T cell exhaustion. Nat
Immunol. 22:205–215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong Y, Li X, Zhang L, Zhu Q, Chen C, Bao
J and Chen Y: CD4(+) T cell exhaustion revealed by high PD-1 and
LAG-3 expression and the loss of helper T cell function in chronic
hepatitis B. BMC Immunol. 20:272019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pandit M, Kil YS, Ahn JH, Pokhrel RH, Gu
Y, Mishra S, Han Y, Ouh YT, Kang B, Jeong MS, et al: Methionine
consumption by cancer cells drives a progressive upregulation of
PD-1 expression in CD4 T cells. Nat Commun. 14:25932023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu X, Zhang Y, Yang KY, Zhang N, Jin F,
Zou GR, Zhu XD, Xie FY, Liang XY, Li WF, et al:
Induction-concurrent chemoradiotherapy with or without sintilimab
in patients with locoregionally advanced nasopharyngeal carcinoma
in China (CONTINUUM): A multicentre, open-label, parallel-group,
randomised, controlled, phase 3 trial. Lancet. 403:2720–2731. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Zhao Q, Tan L, Wu X, Huang R, Zuo
Y, Chen L, Yang J, Zhang ZX, Ruan W, et al: Neutralizing IL-8
potentiates immune checkpoint blockade efficacy for glioma. Cancer
Cell. 41:693–710.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
You R, Hua YJ, Liu YP, Yang Q, Zhang YN,
Li JB, Li CF, Zou X, Yu T, Cao JY, et al: Concurrent
chemoradiotherapy with or without anti-EGFR-targeted treatment for
stage II–IVb nasopharyngeal carcinoma: Retrospective analysis with
a large cohort and long follow-up. Theranostics. 7:2314–2324. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao C, Fang Y, Jiang F, Jin Q, Jin T,
Huang S, Hu Q, Chen Y, Piao Y, Hua Y, et al: Concurrent nimotuzumab
and intensity-modulated radiotherapy for elderly patients with
locally advanced nasopharyngeal carcinoma. Cancer Sci.
115:2729–2737. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Teng F, Cui G, Qian L and Zhao L: Changes
of T Lymphocyte subsets in peripheral blood of patients with
intermediate and advanced cervical cancer before and after
nimotuzumab combined with chemoradiotherapy. Int Arch Allergy
Immunol. 184:85–97. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Y, He S, Wang XL, Peng W, Chen QY, Chi
DM, Chen JR, Han BW, Lin GW, Li YQ, et al: Tumour heterogeneity and
intercellular networks of nasopharyngeal carcinoma at single cell
resolution. Nat Commun. 12:7412021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lv J, Wei Y, Yin JH, Chen YP, Zhou GQ, Wei
C, Liang XY, Zhang Y, Zhang CJ, He SW, et al: The tumor immune
microenvironment of nasopharyngeal carcinoma after gemcitabine plus
cisplatin treatment. Nat Med. 29:1424–1436. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie X, Gong S, Jin H, Yang P, Xu T, Cai Y,
Guo C, Zhang R, Lou F, Yang W, et al: Radiation-induced lymphopenia
correlates with survival in nasopharyngeal carcinoma: Impact of
treatment modality and the baseline lymphocyte count. Radiat Oncol.
15:652020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Stratton JA, Byfield PE, Byfield JE, Small
RC, Benfield J and Pilch Y: A comparison of the acute effects of
radiation therapy, including or excluding the thymus, on the
lymphocyte subpopulations of cancer patients. J Clin Invest.
56:88–97. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Q, Li S, Qiao S, Zheng Z, Duan X and
Zhu X: Changes in T lymphocyte subsets in different tumors before
and after radiotherapy: A meta-analysis. Front Immunol.
12:6486522021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mahnke YD, Brodie TM, Sallusto F, Roederer
M and Lugli E: The who's who of T-cell differentiation: Human
memory T-cell subsets. Eur J Immunol. 43:2797–2809. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gicobi JK, Mao Z, DeFranco G, Hirdler JB,
Li Y, Vianzon VV, Dellacecca ER, Hsu MA, Barham W, Yan Y, et al:
Salvage therapy expands highly cytotoxic and metabolically fit
resilient CD8(+) T cells via ME1 up-regulation. Sci Adv.
9:eadi24142023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma L, Han Q, Cheng L, Song H, Qiang R, Xu
P, Gao F, Zhu L and Xu J: Altered mitochondrial mass and low
mitochondrial membrane potential of immune cells in patients with
HBV infection and correlation with liver inflammation. Front
Immunol. 15:14776462024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Andrews LP, Butler SC, Cui J, Cillo AR,
Cardello C, Liu C, Brunazzi EA, Baessler A, Xie B, Kunning SR, et
al: LAG-3 and PD-1 synergize on CD8(+) T cells to drive T cell
exhaustion and hinder autocrine IFN-γ-dependent anti-tumor
immunity. Cell. 187:4355–4372.e22. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ogando J, Sáez ME, Santos J,
Nuevo-Tapioles C, Gut M, Esteve-Codina A, Heath S, González-Pérez
A, Cuezva JM, Lacalle RA and Mañes S: PD-1 signaling affects
cristae morphology and leads to mitochondrial dysfunction in human
CD8(+) T lymphocytes. J Immunother Cancer. 7:1512019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang X, Li Q and Zeng T: Peripheral CD4(+)
T cells correlate with response and survival in patients with
advanced non-small cell lung cancer receiving chemo-immunotherapy.
Front Immunol. 15:13645072024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu M and Li X, Cheng X, Yi X, Ye F and Li
X, Hu Z, Zhang L, Nie J and Li X: Association of the tissue
infiltrated and peripheral blood immune cell subsets with response
to radiotherapy for rectal cancer. BMC Med Genomics. 15 (Suppl
2):S1072022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kresovich JK, O'Brien KM, Xu Z, Weinberg
CR, Sandler DP and Taylor JA: Circulating leukocyte subsets before
and after a breast cancer diagnosis and therapy. JAMA Netw Open.
7:e23561132024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Y, Radfar S and Khong HT: Activated
CD4+ T cells enhance radiation effect through the cooperation of
interferon-gamma and TNF-alpha. BMC Cancer. 10:602010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rackov G, Zaniani PT, Del Pino S, Shokri
R, Monserrat J, Alvarez-Mon M, Martinez-A C and Balomenos D:
Mitochondrial reactive oxygen is critical for IL-12/IL-18-induced
IFN-γ production by CD4(+) T cells and is regulated by Fas/FasL
signaling. Cell Death Dis. 13:5312022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Herrera FG, Ronet C, de Olza MO, Barras D,
Crespo I, Andreatta M, Corria-Osorio J, Spill A, Benedetti F,
Genolet R, et al: Low-Dose radiotherapy reverses tumor immune
desertification and resistance to immunotherapy. Cancer Discov.
12:108–133. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fraietta JA, Lacey SF, Orlando EJ,
Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y,
O'Connor RS, Hwang WT, et al: Determinants of response and
resistance to CD19 chimeric antigen receptor (CAR) T cell therapy
of chronic lymphocytic leukemia. Nat Med. 24:563–571. 2018.
View Article : Google Scholar : PubMed/NCBI
|