Introduction
The role of chemotherapy in advanced lung cancer was
not proven until the mid-1990s. In 1995, a meta-analysis by the
Non-small Cell Lung Cancer Collaborative Group showed that
cisplatin-based chemotherapy was effective in patients with
advanced non-small cell lung cancer (NSCLC) (1). More recently, in 2000, second-line
chemotherapy with docetaxel was shown to prolong survival in NSCLC
patients previously treated with platinum-based chemotherapy as a
first-line chemotherapy treatment (2). As more novel agents have been been
shown to be effective against lung cancer, such as EGFR inhibitors,
third-line chemotherapy can be accepted as a reasonable therapeutic
option (3,4). These types of chemotherapy were also
shown to improve quality of life (QOL) (5).
The role of additional palliative chemotherapy for
patients with progressive disease after third-line chemotherapy
remains unclear. If additional chemotherapy improves survival or
QOL of patients even after third-line chemotherapy, further
treatment may be a reasonable option in patients with a favorable
performance status. However, little data are available on the
results of additional treatments including more than third-line
chemotherapy. Therefore, we report on treatment outcomes including
more than third-line chemotherapy for advanced NSCLC patients.
Patients and methods
Eighty-two patients with inoperable advanced NSCLC
were admitted for platinum-based chemotherapy as a first-line
treatment at the Korea University Hospitals between March 2003 and
February 2007. All 82 patients were initially treated with
cisplatin-based first-line chemotherapy in combination with
gemcitabine or taxanes. When disease progression was confirmed, the
patients moved on to additional lines of chemotherapy with drugs
such as docetaxel, gefitinib or erlotinib, vinorelbine, irinotecan
and pemetrexed. Vinorelbine and irinotecan were known to be
effective in combination with the platinum agent in first-line
chemotherapy (6). These drugs were
provided as monotherapy based on the attending physician’s decision
based on patient performance status and drug toxicity. Selection of
drugs with same toxicity profiles as the previous treatment was
avoided to minimize cumulative toxicity. After every 2 cycles of
chemotherapy, the patients were evaluated for a response. Toxicity
was evaluated after every cycle of chemotherapy. The total cycles
of chemotherapy and withdrawal of treatment were based on the
physician’s decision, as well as the benefit of treatment and risk
of toxicity. Chemotherapy was changed or withdrawn if disease
progression or unacceptable toxicity was observed. In cases of
grade 4 hematological toxicities and more than a grade 3
non-hematological toxicity, the chemotherapy dose was reduced by
25%.
The electronic medical records of the enrolled
patients were retrospectively reviewed using the hospital’s
computer network. Information on the patients including performance
status, based on the Eastern Cooperative Oncology Group (ECOG);
performance scale; histological type of malignancy, chemotherapy
regimens; overall survival; initial response; duration of response;
total chemotherapy treatment lines and number of cycles of
treatment were recorded. Collected information was then analyzed
and evaluated for overall survival, duration of response and the
total number of chemotherapy cycles provided. Patients with an
initial response of partial response or better were further
classified as the response group. The subgroup analysis for overall
survival, total number of chemotherapy cycles and duration of
initial response was determined and compared among the different
response groups. In addition, a subgroup analysis for overall
survival between smokers and non-smokers was performed.
The response to chemotherapy was classified
according to the Response Evaluation Criteria in Solid Tumors
criteria (7). Definitions of
complete response (CR), partial response (PR), stable disease (SD)
and progressive disease (PD) were as follows: CR, disappearance of
all target lesions; PR, at least a 30% decrease in the sum of the
longest diameters of target lesions compared to the baseline; PD,
at least a 20% increase in the sum of the longest diameters of the
target lesions, using as a reference the smallest sum of the
longest diameters recorded since onset of the treatment or the
appearance of one or more new lesions and SD, neither sufficient
shrinkage to qualify for PR nor sufficient increase to qualify for
PD.
The survival curves were plotted according to the
method of Kaplan-Meyer. Log-rank and Chi-square analyses were
performed to determine statistical significance.
Results
The median age of the enrolled patients was 65 and
there were more males. At the time of diagnosis most patients had
stage IV lung cancer except for three patients with stage IIIb
disease. Most patients had a favorable performance status.
Adenocarcinoma (47.5%) was the most common histological type of
cancer, followed by squamous cell carcinoma (26.8%; Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| No. of patients | 82 |
| Median age | 65 (23–86) |
| Gender |
| Male | 24 (29.3%) |
| Female | 58 (70.1%) |
| Stage |
| IIIb | 3 (3.7%) |
| IV | 79 (96.3%) |
| Performance status
(ECOG) |
| 0 | 14 (17.1%) |
| 1 | 40 (48.8%) |
| 2 | 21 (25.6%) |
| 3 | 7 (8.5%) |
| Histological type of
NSCLC |
| Adenocarcinoma | 39 (47.5%) |
| Squamous cell
carcinoma | 22 (26.8%) |
| Poorly
differentiated | 19 (23.2%) |
| Large cell
carcinoma | 2 (2.4%) |
| First-line
chemotherapy regimen |
| Gemcitabine +
cisplatin | 46 (56.1%) |
| Taxene +
cisplatin | 36 (43.9%) |
| Initial response of
first-line chemotherapy |
| CR | 2 (2.4%) |
| PR | 31 (37.8%) |
| SD | 26 (31.7%) |
| PD | 23 (28.0%) |
| Median no. of
chemotherapy lines | 2 (1–8) |
| Median no. of
chemotherapy cycles | 7 (1–33) |
| Median overall
survival | 15 months |
| No. of patients
treated with third-line chemotherapy or more | 33 (40.2%) |
All patients started first-line chemotherapy with a
regimen of cisplatin combined with either gemcitabine (56.1%) or
taxanes (43.9%). Thirty-three out of 82 patients (40.2%) had a PR
or better on their initial response evaluation, including two
patients who were observed with a CR. The median number of
chemotherapy lines and cycles received were 2 and 7, respectively.
A total of 33 patients were candidate third-line chemotherapy or
more. The highest number of lines and cycles of chemotherapy was
eighth-line and total 33 cycles (Table
I). The median overall survival of the studied patients was 15
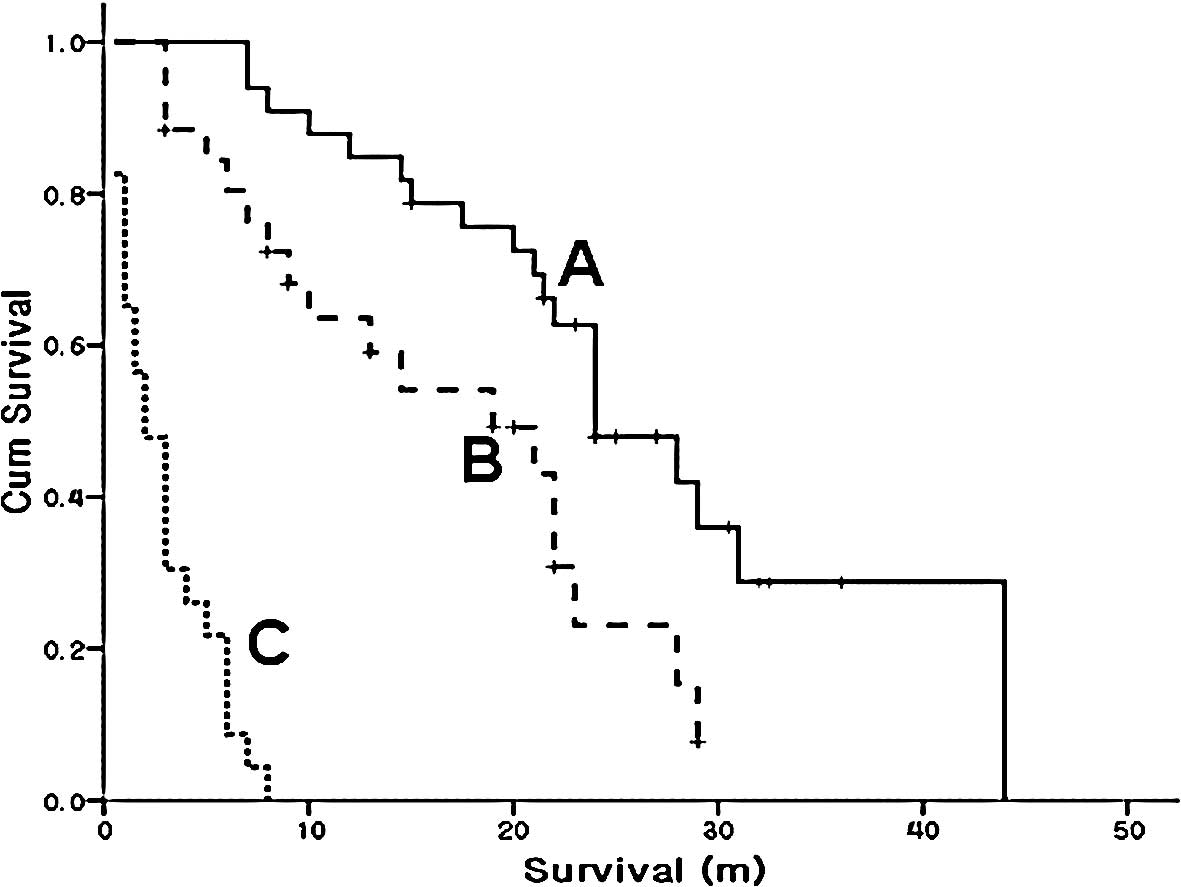
months (Fig. 1A).
Overall survival according to response in
the first line chemotherapy
In the subgroup analysis, a total of 33 patients
were classified as the response group for first-line chemotherapy.
The median overall survival was increased to 24 months (95% CI,
18.8–29.2) in the response group compared to 15 months (95% CI,
4.7–25.3) for the entire study group. Fig. 1 shows that survival increased as the
response to initial chemotherapy improved. The patients of the
response groups in first-line chemotherapy received on average 1
more additional line of chemotherapy compared to the entire group
of patients. This group received a median of 3 lines of
chemotherapy. The median number of chemotherapy cycles received was
15 in the response group. The median number of chemotherapy cycles
was 7 for the 82 patients (Table
II).
 | Table IIMedian survival, duration of initial
response, median number of chemotherapy lines and cycles in all
patients and the response group. |
Table II
Median survival, duration of initial
response, median number of chemotherapy lines and cycles in all
patients and the response group.
| All patients | Response |
|---|
| No. of patients | 82 | 33 |
| Median overall
survival (months) | 15 | 24 |
| Median no. of
chemotherapy lines | 2 | 3 |
| Median no. of
chemotherapy cycles | 7 | 15 |
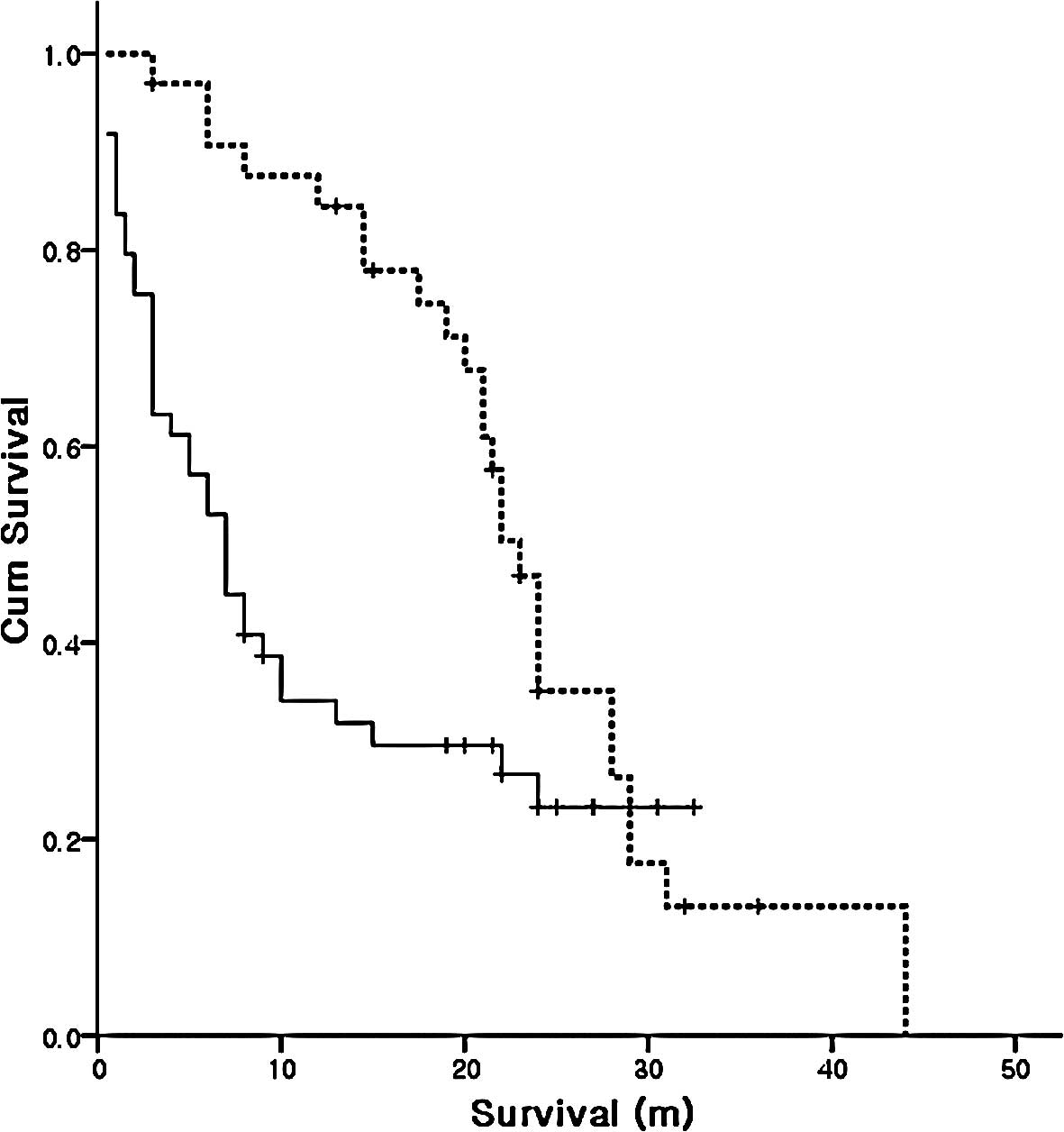
Number of chemotherapy lines
The subgroup analysis showed that 49 patients were
treated with less than second-line chemotherapy and 33 patients
(40.2%) were treated with more than third-line chemotherapy. The
median survival was 23 months (95% CI, 20.9–25.1) in patients
treated with more than third-line chemotherapy, compared to 7
months (95% CI, 4.7–9.3) in patients treated with less than
second-line chemotherapy (Fig.
2).
Smokers and non-smokers
Fifty-three smokers and 29 non-smokers participated
in this study. A significant difference of 10 and 28 months,
respectively, was noted in the median overall survival of smokers
compared to non-smokers (Fig.
3).
Toxicities
Concerns were raised regarding the increase of
cumulative toxicities in patients treated with multiple lines and
cycles of chemotherapy. Although nephrotoxicity, neurotoxicity and
bone marrow suppression occurred during additional cycles of
chemotherapy, no unmanageable cumulative toxicities among patients
treated with additional chemotherapy were noted. Selection of drugs
with same toxicity profiles as the previous treatment was avoided
to minimize cumulative toxicity. Monotherapy may also contribute to
decreasing the toxicity of additional chemotherapy (data not
shown).
Discussion
In the treatment of patients with inoperable,
advanced lung cancer, first- and second-line chemotherapy have
proven to be effective (1,2). Agents such as docetaxel, pemetrexed
and EGFR inhibitors are widely used for second- and third-line
chemotherapy (3,4,8).
However, even after completion of third-line chemotherapy, some
patients still exhibit a favorable performance status despite
disease progression. The question is whether present with a
favorable performance status after third-line chemotherapy.
Chemotherapy beyond third-line treatment is supported by medical
insurance in Korea. Thus, in practice, when these treatments are
shown to improve QOL even after third-line chemotherapy, a
physician may approve additional cyles of chemotherapy after
disease progression.
In this study, the subgroup analysis showed that 49
patients were treated with less than second-line chemotherapy and
33 patients (40.2%) were treated with more than third-line
chemotherapy. This result is similar to that of Murillo and
Koeller, who also reported that 41% patients received more than
third-line chemotherapy (9).
Vigorous efforts are ongoing to prolong survival by
increasing drug exposure or duration. Sequential, alternating and
maintenance therapy have been proposed in an attempt to improve
survival. These treatments are appealing as they may increase the
number of non-cross resistant agents and/or duration of
chemotherapy. As maintenance therapy, paclitaxel has shown success
(10–12), whereas agents such as docetaxel and
gemcitabine have failed to show significant benefits (10,13,14).
Based on the studies published thus far, however, it is unlikely
that sequential and alternating treatment will prove to be superior
to the current standard therapy for all advanced NSCLC patients
(15). However, selective groups,
especially chemo-sensitive patients, may benefit from such
treatments.
In this retrospective study, the overall treatment
results for patients with inoperable advanced NSCLC had a median
overall survival of 15 months. This finding was not significantly
different from the results of previous reports. However, in the
subgroup analysis of patients with a partial response or better
(response group) to first-line chemotherapy, the median overall
survival increased to 24 months. Compared to the entire study
group, patients of the response group in first-line chemotherapy
received a median of 1 more line and 8 more cycles of chemotherapy.
They were exposed to more chemotherapeutic agents than the
non-responsive group. The patient, for example, who was treated
with the highest number of chemotherapy lines and cycles in this
study received as many as 8 lines and 33 cycles of chemotherapy.
The median survival was 23 months in patients treated with more
than third-line chemotherapy, compared to 7 months in patients
treated with less than second-line chemotherapy.
Notably, since the patients lived longer, they could
be potential candidates for additional chemotherapy. However, the
increase of the median overall survival of 24 months in the
response group is difficult to explain solely by additional
chemotherapy. In a study that identified an objective response to
chemotherapy as a marker for further survival, a median survival
time of 41 and 19 weeks was reported for the responding and
non-responding patients, respectively (16).
For patients with colorectal cancer, exposure to 3
active cytotoxic drugs prolonged survival regardless of whether the
drugs were administered as monotherapy or in combination (17–20,
Pitot HC, et al, J Clin Oncol 23: abs. 3506, 2005). It is
possible that a similar mechanism applies to patients with NSCLC.
Multi-drug exposure shown to be non-cross resistant may increase
survival in the response group. If this is also true for NSCLC
patients, additional chemotherapy after second- or third-line
chemotherapy may be an effective and feasible strategy for treating
patients with inoperable NSCLC. Careful patient selection is
important, since many of the patients that were resistant to the
initial chemotherapeutic agents appeared to be resistant to second-
and third-line drugs as well.
The subgroup analysis comparing the survival of
NSCLC patients who were smokers to non-smokers showed that the
smokers had a significantly worse prognosis. This finding is
comparable to the results of previously published studies (21). Certain investigators have suggested
that NSCLC among non-smokers exhibits different clinical and
pathological features compared to that of smokers (22–24).
Treatment-related toxicities are always a concern
when further chemotherapy is required. Although not studied
intensively, we did not encounter unmanageable toxicities
associated with increased chemotherapy exposure and related
cumulative toxicities. The fact that most of the additional lines
were single agent lines of chemotherapy may have played a role in
reducing the toxicities. In addition, chemotherapeutic agents were
carefully selected in order that the major toxicities of these
drugs did not overlap the major toxicities of previously used
drugs.
Significant limitations of this study were that it
was not a prospective randomized study, and that the sample size is
small. However, the results suggested that NSCLC patients with a
favorable response to initial chemotherapy benefit from further
chemotherapy beyond third-line treatment by multi-drug exposure.
Chemotherapy may, therefore, benefit NSCLC patients who remain fit
for treatment following the completion of third-line cycles of
chemotherapy. Prospective large scale studies are needed to confirm
these results.
References
|
1
|
Non-small Cell Lung Cancer Collaborative
Group. Chemotherapy in non-small cell lung cancer: a meta-analysis
using updated data on individual patients from 52 randomised
clinical trials. BMJ. 311:899–909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shepherd FA, Dancey J, Ramlau R, et al:
Prospective randomized trial of docetaxel versus best supportive
care in patients with non-small-cell lung cancer previously treated
with platinum-based chemotherapy. J Clin Oncol. 18:2095–2103.
2000.
|
|
3
|
Cappuzzo F, Gregorc V, Rossi E, et al:
Gefitinib in pretreated non-small cell lung cancer (NSCLC):
analysis of efficacy and correlation with HER2 and epidermal growth
factor receptor expression in locally advanced or metastatic NSCLC.
J Clin Oncol. 21:2658–2663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, et al: Erlotinib in previously treated non-small cell lung
cancer. N Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bunn PA Jr and Kelly K: New
chemotherapeutic agents prolong survival and improve quality of
life in non-small cell lung cancer: a review of the literature and
future directions. Clin Cancer Res. 4:1087–1100. 1998.PubMed/NCBI
|
|
6
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
8
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murillo JR Jr and Koeller J: Chemotherapy
given near the end of life by community oncologists for advanced
non-small cell lung cancer. Oncologist. 11:1095–1099. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belani CP, Barstis J, La Rocca RV, et al:
Meta-analysis of weekly paclitaxel as maintenance therapy for
advanced non-small cell lung cancer patients following initial
chemotherapy. Lung Cancer. 49:332005. View Article : Google Scholar
|
|
11
|
Belani CP, Barstis J, Perry MC, et al:
Multicenter, randomized trial for stage IIIB or IV non-small cell
lung cancer using weekly paclitaxel and carboplatin followed by
maintenance weekly paclitaxel or observation. J Clin Oncol.
21:2933–2939. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramalingam S and Belani C: Systemic
chemotherapy for advanced non-small cell lung cancer: recent
advances and future directions. Oncologist. 13:5–13. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brodowicz T, Krzakowski M, Zwitter M, et
al: Cisplatin and gemcitabine first-line chemotherapy followed by
maintenance gemcitabine or best supportive care in advanced
non-small cell lung cancer: a phase III trial. Lung Cancer.
52:155–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fidias P, Dahkil SR, Lyss AP, et al:
Updated report of a phase III study of induction therapy with
gemcitabine + carboplatin (GC) followed by either delayed vs.
immediate second-line therapy with docetaxel (D) in advanced
non-small cell lung cancer. J Clin Oncol. 24:70322006.
|
|
15
|
Grossi F, Aita M, Follador A, et al:
Sequential, alternating and maintenance/consolidation chemotherapy
in advanced non-small cell lung cancer: a review of the literature.
Oncologist. 12:451–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paesmans M, Sculier JP, Libert P, et al:
Response to chemotherapy has predictive value for further survival
of patients with advanced non-small cell lung cancer: 10 years
experience of the European Lung Cancer Working Party. Eur J Cancer.
33:2326–2332. 1997.PubMed/NCBI
|
|
17
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan and oxaliplatin combinations in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 22:23–30.
2004. View Article : Google Scholar
|
|
18
|
Grothey A and Sargent D: Overall survival
of patients with advanced colorectal cancer correlates with
availability of fluorouracil, irinotecan and oxaliplatin regardless
of whether doublet or single-agent therapy is used first line. J
Clin Oncol. 23:9441–9442. 2005. View Article : Google Scholar
|
|
19
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tournigand C, Andre T, Achille E, et al:
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: a randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scagliotti GV, Parikh P, Von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Einhorn LH: First-line chemotherapy for
non-small cell lung cancer: is there a superior regimen based on
histology? J Clin Oncol. 26:3485–3486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yano T, Miura N, Takenaka T, et al:
Never-smoking non-small cell lung cancer as a separate entity:
clinicopathologic features and survival. Cancer. 113:1012–1018.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou W, Heist RS, Liu G, et al: Smoking
cessation before diagnosis and survival in early stage non-small
cell lung cancer patients. Lung Cancer. 53:375–380. 2006.
View Article : Google Scholar : PubMed/NCBI
|