Introduction
Complete resection of the tumor is necessary for
curing gastric cancer. Macroscopic residue of the tumor is
indicative of a poor patient prognosis since chemotherapy is unable
to eliminate large gastric cancer cell masses. Unfortunately, a
considerable number of patients with gastric cancer present locally
advanced disease at diagnosis. Consequently, preoperative adjuvant
chemotherapy (neoadjuvant chemotherapy, NAC), which can decrease
the extent of the invasion to adjacent organs and/or decrease the
number of lymph nodes involved, is considered to be a promising
approach in the treatment of advanced gastric cancer.
Combined chemotherapy with TS-1 plus cisplatin
(CDDP) is one of the most powerful regimens for advanced or
metastatic gastric cancer. In a Japanese phase III study (SPIRITS
trial) (1), TS-1/CDDP showed a
median overall survival of 13 months for metastatic gastric cancer
patients, which was significantly longer than the survival rate of
11 months with TS-1 alone. TS-1 is a mixed compound of tegafur,
gimeracil and oteracil potassium. Tegafur is a precursor of
5-fluorouracil (5-FU), while gimeracil and oteracil have no
anti-cancer effect. Consequently, TS-1/CDDP is a modified 5-FU/CDDP
therapy.
Safety, as well as efficacy, are extremely important
in NAC. NAC is associated with complications such as pneumonia,
enterocutaneous fistula and delay of wound healing (2–5).
However, the timing of 5-FU therapy for gastric cancer in relation
to the surgical wound healing process has yet to be
investigated.
Measurement of the urinary excretion of
phenolsulfonphthalein (PSP) following oral administration is a
standard method used to estimate intestinal permeability, which is
correlated with mucosal integrity. The migration of fibroblasts and
hydroxyproline (HP) production is essential for wound healing.
Utilizing the above techniques we investigated the effect of the
interval between NAC and surgery on enteric anastomotic and skin
wound healing, and intestinal permeability.
Materials and methods
Twenty-four male Sprague Dawley rats weighing
between 315 and 336 g were included in this study. The rats were
housed under barrier-sustained conditions in temperature-controlled
rooms under a light-dark cycle and fed standard rat food.
Experimental protocols were approved by the National Research
Council’s Guide for the Care and Use of Laboratory Animals. The
rats were divided into four groups, each containing 6 subjects
(Fig. 1).
Group 1 (control)
Animals were injected daily with 0.2 ml/kg of saline
in the peritoneal cavity from day 1 to 5 and from day 8 to 12, and
with 0.2 ml/kg of saline in the tail vein on days 2 and 9.
Groups 2, 3 and 4 (NAC groups)
Animals were injected daily with 20 mg/kg of 5-FU
(Kyowa Hakkoh, Tokyo, Japan) in the peritoneal cavity from day 1 to
5 and from day 8 to 12, and with 2 mg/kg of cisplatin (CDDP; Nihon
Chemical, Tokyo, Japan) in the tail vein on days 2 and 9.
Surgical procedure
The rats in Groups 1 and 2 underwent surgery on the
day after drug administration was completed (day 13). Group 3
underwent surgery on day 20, and Group 4 on day 27.
The rats were fasted 24 h before surgery, and
anesthetized with 40 mg/kg of pentobarbital sodium,
intraperitoneally. Abdominal hair was shaved with electric
clippers, and a laparotomic incision of 3 cm was made in the
midline. A 1.0-cm incision was made on the gastric corpus, and an
interrupted suture was performed using 5-0 Biocyn with 0.3-cm
intervals in one layer. After closure of the gastrotomy, the small
intestine was displaced from the abdominal cavity for 30 min and
subsequently returned to the abdomen. The laparotomy was closed
with an interrupted suture at a 0.6-cm interval in one layer using
4-0 Vicryl. The rats were placed in their cages and allowed food
and drinking water from the day following the initial surgery.
Utilizing the same anesthesia technique as that
mentioned above, the animals underwent a second surgical procedure
on day 7, where the abdominal wall was removed (3 × 3 cm). Blood
was drawn from the inferior vena cava for a serum assay, and the
whole stomach was resected from the thorax-esophagus to the
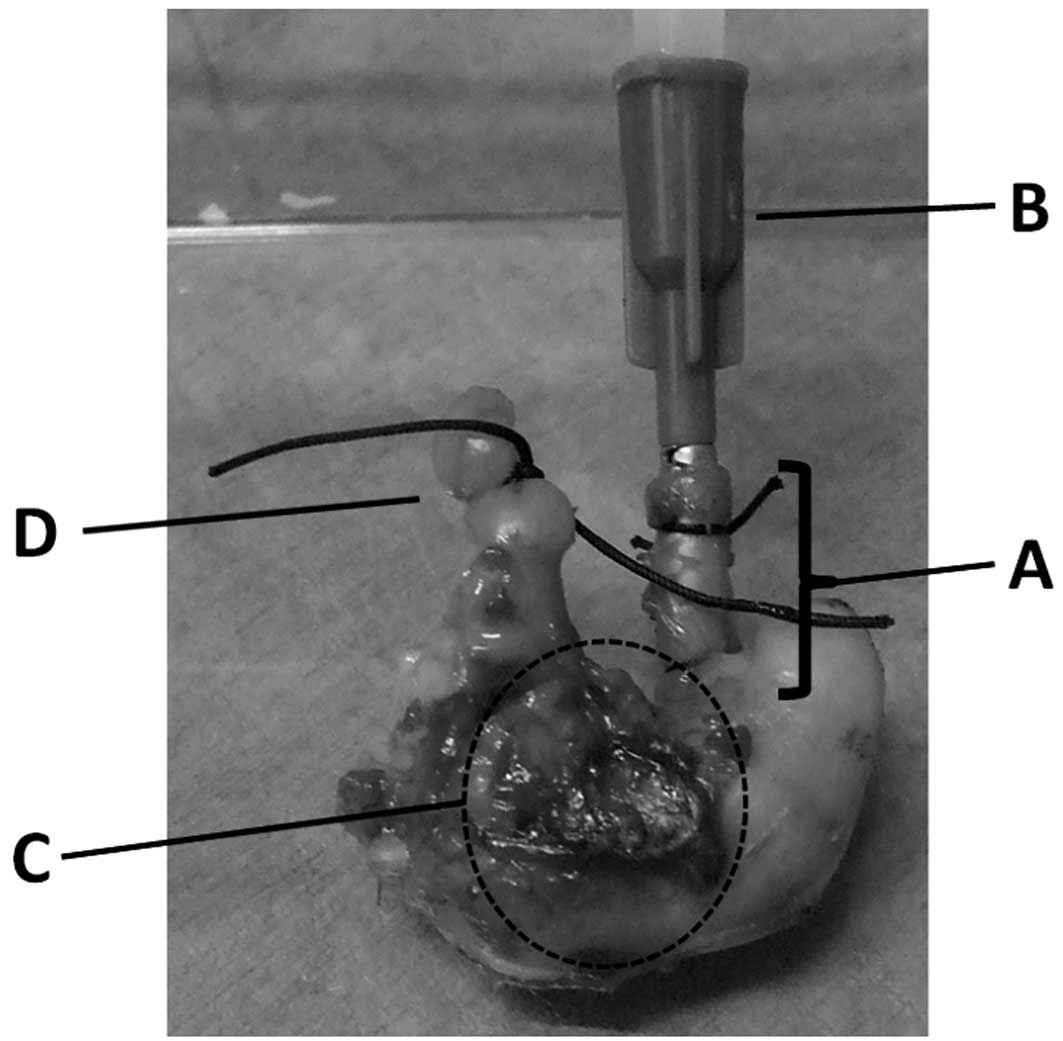
duodenum (Fig. 2).
Bursting pressure of the stomach
The bursting pressure (BP) of the resected stomach
was measured using a blood sphygmomanometer. The end of the
duodenum was tied with 2-0 silk, and a catheter was fixed to the
esophagus, which was 1 cm proximal from the cardia portion. The
catheter was connected to the manometer, and the segment was
inflated with saline. BP was defined as the pressure level at which
saline was able to spout from the resected stomach.
Tensile strength of the abdominal
wound
The removed portion of the abdominal wall was fixed
to one end so that the suture line was situated in the middle. A
tensiometer was fixed to the other end, and was pulled. The number
recorded when the tissue in the suture line began to separate was
considered to be the tensile strength.
Measurement of tissue hydroxyproline
levels
For the measurement of the tissue HP level, a sample
(3 × 0.7 cm) was taken from the abdominal wall incision line and
was preserved in deep freeze at −83°C. Tissue HP levels were
measured using the method of direct dissolution with HCl (SRL Inc.,
Tokyo). The samples were weighed, hydrolyzed with 6 N HCl, and ion
exchange water was added. The samples were then hydrolyzed at 100°C
for 20 h. A 0.1-ml portion of the hydrolyzed sample was removed,
added and mixed with 1.5 ml of 0.3 N LiOH. The sample was then
analyzed by high performance liquid chromatography. The HP
concentration in the tissue was calculated using the formula: HP
(μmol/g) = [measured HP (μmo/l) × volume of HCl (ml)]/[weight of
wet tissue (g) × 1000]
Bowel mucosal permeability test
The rats were fasted for 24 h prior to the initial
surgery, after which 10 mg of phenolsulfonphthalein (PSP; Daiichi
Sankyo, Tokyo, Japan) was orally administered to the animals. The
24-h urinary excretion of PSP was then quantified. The collected
urine was alkalinized in 0.2 ml of 1 N NaOH and brought to a volume
of 200 ml with distilled water. The PSP concentration was then
determined using a spectrophotometer at 562 nm.
Fibroblast cell counts
Four different sections were removed from the
gastric suture line for each animal, and fibroblast cells were
counted in a ×40 magnification area. The total of these counts was
recorded. Paraffin blocks of the tissue samples removed from the
gastric suture line were prepared by staining with α-smooth muscle
actin (α-SMA). Adobe Photoshop software was used to select
SMA-positive cells. The quantitative parameters were assessed using
WinROOF image-processing software (Mitani Corp., Tokyo, Japan).
Measurement of blood cell and serum
biochemical parameters
Total leukocyte count (TCL), blood hemoglobin (Hb),
blood platelet (Plt), serum total protein (TP), serum albumin
(Alb), serum basic-FGF and serum TGF-β were assayed for each rat.
Basic-FGF and TGF-β were measured at SRL Inc., while any remaining
measurements were carried out in the laboratory division of
Kanazawa University.
Statistical analysis
Results were expressed as the mean ± SD. BP, HP and
PSP were analyzed using non-repeated measures analysis of variance.
P<0.05 was accepted as statistically significant.
Results
Bursting pressure of the gastric suture
line
BP of the gastric suture line in Group 2 was
140.8±26 mmHg, which was significantly lower than that in the other
groups (Table I; P<0.01). In
Group 2, the stomach exploded at the suture line, while in the
other groups, the stomach exploded at the gastric fundus upon
compression.
 | Table ITissue hydroxyproline levels, bursting
pressure and Gut barrier function test. |
Table I
Tissue hydroxyproline levels, bursting
pressure and Gut barrier function test.
| Group 1 | Group 2 | Group 3 | Group 4 |
|---|
| Bursting pressure
(mmHg) | 204.5±19 | 140.8±26a | 230.7±45 | 237.0±43 |
| Tissue hydroxyproline
levels (μmol/g) | 43.7±5.2 | 41.0±6.5 | 42.4±4.8 | 41.1±4.9 |
| Bowel mucosal
permeability test (%) | 15.7±2.8 | 24.5±6.6a | 13.0±4.6 | 14.5±3.8 |
Tensile strength of the abdominal
wound
The tensile strength of the abdominal wound was
above the upper limit of measurement in all of the groups.
Measurement of tissue hydroxyproline
levels
No significant differences were noted in the HP of
the abdominal wall in Groups 1-4 (Table
I; P=0.34).
Bowel mucosal permeability test
The urinary excretion ratio of PSP in Group 2 was
24.5±6.6%, which was significantly higher than that in the other
groups (Table I; P<0.01).
Fibroblast cell counts
No significant differences were noted in the
fibroblast cell counts of the stomach wall in Groups 1-4 (Table II; P=1.75).
 | Table IIFibroblast cell counts. |
Table II
Fibroblast cell counts.
| Group 1 | Group 2 | Group 3 | Group 4 |
|---|
| Fibroblast cell
number (×103) | 3.17±0.3 | 3.65±0.7 | 2.07±1.2 | 2.62±0.7 |
Measurement of blood cell and serum
biochemical parameters
The serum levels of basic FGF were below the lower
limit of measurement in all of the groups. No significant
differences were found in TLC, Hb, TP, Alb and TGF-β in Groups 1-4.
The Plt of Group 2 was 13.2±1.6×104/mm3 and
that of Group 3 was 13.9±3.1×104/mm3, which
was higher than Group 1 (Table
III; P<0.01).
 | Table IIIBiochemical parameters of blood cells
and serum. |
Table III
Biochemical parameters of blood cells
and serum.
| Group 1 | Group 2 | Group 3 | Group 4 |
|---|
| WBC
(×103/mm3) | 7.21±2.1 | 6.26±2.8 | 6.06±0.9 | 6.95±1.7 |
| RBC
(×102/mm3) | 7.82±0.4 | 7.05±0.8 | 7.06±1.0 | 7.21±0.6 |
| Hb (g/dl) | 13.7±0.4 | 12.7±1.2 | 13.3±1.3 | 13.3±0.6 |
| HCT (%) | 43.1±1.2 | 41.2±2.6 | 42.9±2.6 | 42.7±2.0 |
| Plt
(×104/mm3) | 9.56±2.1 | 13.2±1.6a | 13.9±3.1a | 10.7±3.3 |
| TP (g/dl) | 6.03±0.2 | 5.91±0.4 | 6.06±0.3 | 5.9±0.1 |
| Alb (g/dl) | 0.75±0.1 | 0.73±0.1 | 0.77±0.1 | 0.68±0.1 |
| TGF-β (ng/ml) | 36.8±21 | 40.1±17 | 58.7±59 | 58.0±21 |
| Basic FGF
(pg/ml) | <10 | <10 | <10 | <10 |
Discussion
NAC is a promising approach in the treatment of
gastric cancer when R0 resection is difficult. NAC has various
advantages compared to postoperative adjuvant chemotherapy. Drug
delivery to the lesions is preferable since blood vessels are still
preserved. When the tumor stage is reduced, the possibility of R0
resection increases. Furthermore, the sensitivity of the tumor to
the regimen can be determined histopathologically using the
resected specimens. However, besides the aforementioned benefits,
disadvantages exist as well.
Gastrointestinal (GI) toxicities are common adverse
effects of cancer chemotherapy. 5-FU, one of the most frequently
used drugs in chemotherapy for gastric and colorectal cancer,
sometimes shows severe GI toxicities (6). It is known that 5-FU causes a decrease
in the height of intestinal villi on the one hand, but an increase
in intestinal secretion and diarrhea on the other. The decrease in
the height of villi indicates the derangement of numerous mucosal
functions, including barrier function and regeneration.
In this study, BP of the stomach showed a
significant decrease in Group 2 compared with the other three
groups. Furthermore, in this study, no rupture occurred in the
gastric suture lines of Groups 1, 3 and 4, while an interval of one
week appears to be adequate for recovery of the wound healing
ability of the gastric suture line.
Hananel et al (7) reported that the preoperative
administration of 5-FU beginning 4 weeks prior to surgery had no
effect on the healing of colonic anastomoses. Sahin et al
(8) reported that in colorectal
diseases wound healing was impaired in rats undergoing
chemotherapy, but following the second week after chemotherapy,
disrupted parameters returned to their normal levels.
In spite of the fragility of the gastric suture
line, the fibroblast counts in this suture line did not differ in
the four groups. Furthermore, the HP concentration in the abdominal
wound in Group 2 was comparable to that of the other three groups.
Since the HP concentration is a reliable indicator of collagen
synthesis (6,9,10), the
fragility of the gastric suture line in Group 2 did not result from
impairment of fibroblast migration or collagen production. These
findings suggest that the delay of wound healing following
chemotherapy was due to the impairment of collagen remodeling.
Several animal studies have shown TGF-β to play a
role in adhesion formation. TGF-β expression has been correlated
with the pathogenesis of several fibrotic disorders such as skin
scarring. It is secreted early in the immune cascade and stimulates
the release of other pro-inflammatory mediators. However, the
immunosuppressor often used after organ transplantation and
reflected by a decrease in TGF-β levels, resulted in a decrease in
postsurgical adhesion formation (11). Basic FGF is recognized as one of the
most common angiogenic growth factors. Additionally, Kuhn et
al (12) reported that the
release of basic FGF was indicative of chemosensitivity in lung
cancer patients. In this study, no significant difference was noted
for serum TGF-β or basic FGF in the four groups. From these data,
it appears that both TGF-β and basic FGF are not involved in the
delay of wound healing following chemotherapy.
The urinary PSP excretion rate following oral
administration is one of the indicators of intestinal mucosal
permeability (13,14). We reported an increase in the
urinary PSP excretion rate in rats that underwent surgery after
total parenteral nutrition was administered. Such an increase in
the urinary PSP excretion rate may be an indicator of decreased
mucosal integrity. Moreover, an increase in the urinary PSP
excretion rate was observed along with a simultaneous decrease in
IgA-positive mucosal cell number and serum diamine oxidase activity
(15). Nakamura et al
(16) reported that the recovery
rate of PSP correlated well with the extent of mucosal damage in
the small intestine of the rat. In this study, the urinary PSP
excretion rate showed a significant increase in Group 2 compared
with the other three groups. These findings suggest that the
permeability of intestinal mucosa is an indicator of wound healing
ability following chemotherapy.
The clinical effects of neoadjuvant chemotherapy for
locally advanced gastric cancer are currently under investigation.
As various powerful regimens against gastric cancer have been
developed, NAC should improve the clinical outcome of patients with
locally advanced gastric cancer. However, it is crucial to perform
NAC without increased morbidity or mortality. Thus, our findings
show that mucosal permeability may be a good indicator of wound
healing ability following NAC. However, further studies are
required to clarify the optimal interval between NAC and surgery
for human patients.
Acknowledgements
The authors wish to thank Dr You Zen and the staff
at the Pathology Department for the staining of the specimens.
References
|
1
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H and
Takeuchi M: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): a phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biert J, Seifert W, de Man B, Wobbes T,
Hoogenhout J and Hendriks T: Combined preoperative irradiation and
local hyperthermia delays early healing of experimental colonic
anastomoses. Arch Surg. 131:1037–1042. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Graf W, Weiber S, Glimelius B, Jiborn H,
Pahlman L and Zederfeldt B: Influence of 5-fluorouracil and folinic
acid on colonic healing: an experimental study in the rat. Br J
Surg. 79:825–828. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morris T: Retardation of healing of
large-bowel anastomoses by 5-fluorouracil. Aust NZ J Surg.
49:743–745. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldman LI, Lowe S and al-Saleem T: Effect
of fluorouracil on intestinal anastomoses in the rat. Arch Surg.
98:303–304. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiber S, Graf W, Glimelius B, Jiborn H,
Pahlman L and Zederfeldt B: Experimental colonic healing in
relation to timing of 5-fluorouracil therapy. Br J Surg.
81:1677–1680. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hananel N and Gordon PH: Effect of
5-fluorouracil and leucovorin on the integrity of colonic
anastomoses in the rat. Dis Colon Rectum. 38:886–890. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sahin M, Erikoglu M, Ozer S, Tekin A, Boz
S, Golcuk M, Avunduk MC and Akoz M: Determination of operation time
in colorectal diseases: preoperative chemotherapy application. J
Surg Res. 124:209–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiborn H, Ahonen J and Zederfeldt B:
Healing of experimental colonic anastomosis. III Collagen
metabolism in the colon after left colon resection. Am J Surg.
139:398–405. 1980.PubMed/NCBI
|
|
10
|
Hendriks T and Mastboom WJB: Healing of
experimental intestinal anastomoses. Parameters for repair. Dis
Colon Rectum. 33:891–901. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wasserberg N, Nunoo-Mensah JW, Ruiz P and
Tzakis AG: The effect of immunosuppression on peritoneal adhesion
formation after small bowel transplantation in rats. J Surg Res.
141:294–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuhn H, Weser L, Gessner C, Hammerschmidt
S and Wirtz H: Release of bFGF following apoptosis and necrosis in
NSCLC cells: Effects on chemosensitivity to cisplatin. Oncol Rep.
14:759–762. 2005.PubMed/NCBI
|
|
13
|
Inutsuka S, Takesue F, Yasuda M, Honda M,
Nagahama S, Kusumoto H, Nozoe T and Korenaga D: Assessment of the
intestinal permeability following postoperative chemotherapy for
human malignant disease. Eur Surg Res. 35:22–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toh Y, Korenaga D, Maekawa S, Matsumata T,
Muto Y, Ikeda T and Sugimachi K: Assessing the permeability of the
gastrointestinal mucosa after oral administration of
phenolsulfonphthalein. Hepatogastroenterology. 44:1147–1151.
1997.PubMed/NCBI
|
|
15
|
Ohta K, Omura K, Hirano K, Kanehira E,
Ishikawa N, Kato Y, Kawakami K and Watanabe G: The effects of an
additive small amount of a low residual diet against total
parenteral nutrition-induced gut mucosal barrier. Am J Surg.
185:79–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura J, Yoshizaki Y, Yasuhara M,
Kimura T and Muranishi S: Mechanisms of the absorption of
water-soluble dyes from the rat small intestine. Chem Pharm Bull.
24:683–690. 1976. View Article : Google Scholar
|