Introduction
The synthesis of polycyclic aromatic rings by
various methodologies has been extensively documented (1). The carcinogenic properties of these
compounds have been explained by advancing different mechanisms
(2). The use of polycyclic aromatic
compounds and their derivatives as anticancer agents has been
explored (3). The antitumor
activity of these compounds has been proposed to depend on
intercalation with or covalent binding to DNA (4). Many other sites of interaction such as
the cell membrane have been identified. We determined that suitably
substituted chrysene derivatives act on the cancer cell through
interactions with the membrane (5).
Although using potentially mutagenic compounds to derive antitumor
agents may appear to be a questionable approach, a large body of
information supports this concept. Many reports have claimed that
alteration of the structure of polycyclic aromatic hydrocarbons can
mitigate their deleterious effects, emphasizing their interaction
with specific cell organelles to evoke specific cytotoxic
reactions. As a result, many of the antitumor agents that are in
current clinical use are derived from compounds such as carbazoles,
anthracenes and related structures (6).
Materials and methods
The materials and methods used included:
dibenzofluorene, methylmagnesiumiodide, hydroiodic acid,
tetrahydrofuran, nitric acid, sulfuric acid, hydrazine,
palladium-carbon, ethanol, piperidine, N-methylpiperazine,
isobutylchloroformate, triethylamine, diborane, the MTT assay and
several cancer cell lines.
All reactions described in this study were carried
out under a well-ventilated hood. Some of the compounds described
here are potential carcinogens.
Results
We examined the synthesis and the antitumor
activities of structurally complex, angular dibenzofluorene [a,g]
polycyclic systems with a very reactive methyl substituted group.
The structure-activity relationships of several new diamides and
diamines (8,9) are also reported.
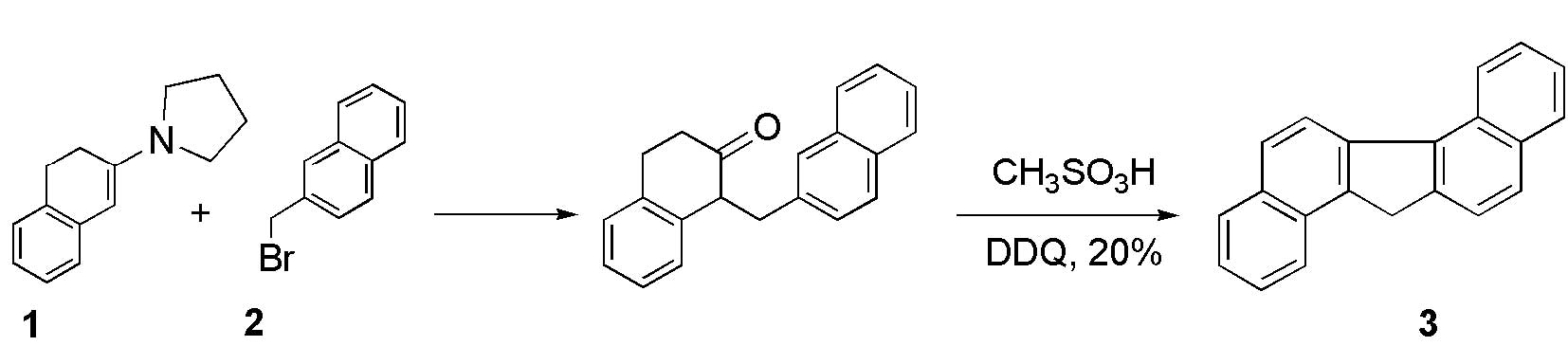
We prepared pentacyclic dibenzo[a,g]fluorene
(3) in 30% yield by following the
method reported by Harvey et al (7). Hydrocarbon 3 can be prepared via
alkylation of the enamine 1 with bromide 2 and
cyclodehydration-aromatization of the ketone (scheme 1).
Functionalization of aromatic derivatives by an
electrophilic reaction is routine organic chemistry. The
orientation of the electrophile in monocyclic or bicyclic
derivatives is predictable. However, a substitution reaction in a
polycyclic aromatic system is extremely difficult, and for a
polycyclic non-alternate hydrocarbon it is poorly predictable. The
electrophilic substitution reaction in the polycyclic aromatic
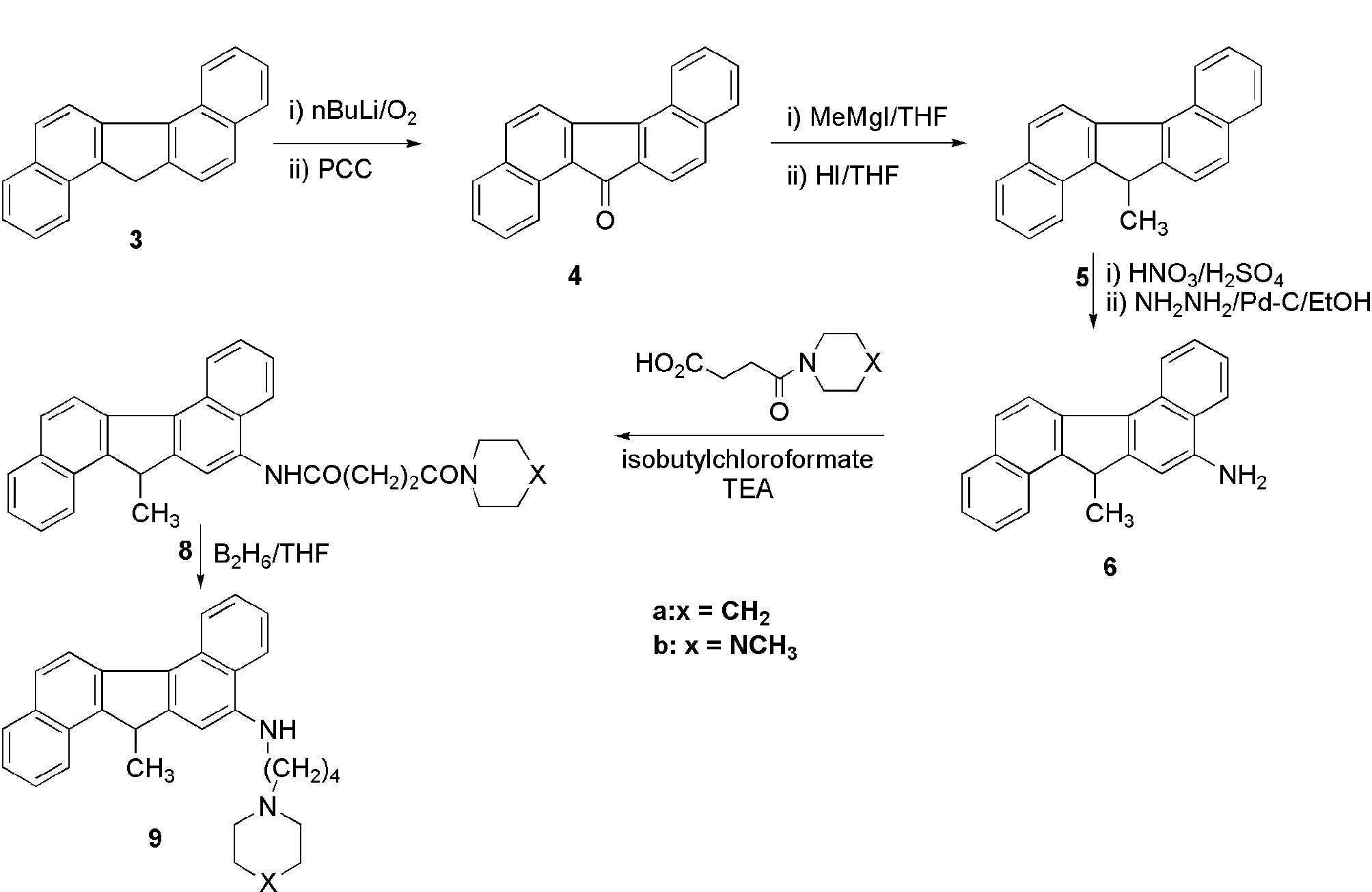
system has been poorly described (8). We planned to link a 4-carbon side
chain with a heterocyclic base at the end to the aromatic ring
through nitrogen. Therefore, we aimed to prepare amino
dibenzofluorene 6 for the subsequent derivatization. Towards this
goal, we reacted the ketone 4 with nitric acid in acetic acid under
different conditions but failed to produce the desired nitro
derivative. However, the hydrocarbon 3 produced a single nitro
compound with nitric acid-sulfuric acid at 0–5°C in an 80% yield.
Reduction of the nitro compound to the amino compound 6 was carried
out. Samarium can be used for this reduction (9). Our next task was to prepare the side
chains and to couple them to the amine 6. The amine 6 was then
condensed with the side chain acid by a mixed anhydride method.
Many other condensing agents, such as DCC and HOBt, failed to yield
the amides 8 (scheme 2). The
desired diamides 8a and 8b were isolated by column chromatography.
Reduction of the amide functionality to the amino group by lithium
aluminum hydride is a standard chemical transformation. Therefore,
in order to produce the diamino compounds, reduction of 8 was
carried out by this reagent under a variety of conditions. However,
neither of the desired diamino compounds 9a or 9b could be isolated
from the complex reaction mixtures by this method. After many
experiments, diborane was found to be the reagent of choice for
this reaction, and it resulted in a good yield of the amino
compound 9. These derivatives were tested against a number of tumor
cell lines. As an approach to an operational definition of in
vitro antitumor cytotoxicity (activity), we chose to compare
the effects of our compounds with those of cisplatin.
Discussion
The antitumor activity of these newly synthesized
dibenzofluorene derivatives 8 and 9 were tested, and a comparison
with respect to cisplatin is shown in Table I. The diamide with N-methyl
piperazine moiety was more potent than the diamide with piperidine.
However, all of the diamines were potent irrespective of the
terminal groups. A variation of 0.3–0.8 μm was observed.
 | Table IIC50 (μM) of compounds 8
and 9 using the MTT assay (a 72-h continuous exposure). |
Table I
IC50 (μM) of compounds 8
and 9 using the MTT assay (a 72-h continuous exposure).
| Cell Line | Cisplatin | 8a | 8b | 9a | 9b |
|---|
| B16 | 7.33 | >20 | 4.30 | 4.05 | 1.65 |
| BRO | 5.66 | >20 | 3.88 | 4.38 | 3.65 |
| HL-60 | 1.66 | >20 | 3.40 | 3.90 | 3.95 |
| MCF-7 | 15.99 | >20 | 4.35 | 4.85 | 4.36 |
| OVCAR 3 | 3.99 | >20 | 4.19 | 3.00 | 1.85 |
| P388/0 | - | >20 | 4.35 | 4.51 | 4.38 |
| PC 3 | 1.66 | >20 | 3.98 | 4.14 | 3.49 |
| HT-29 | 15.99 | >20 | 3.80 | 4.14 | 3.20 |
Acknowledgements
We gratefully acknowledge the financial support for
this research project from NIH/NCI P-2OCA 138022 (B.K.B.) and Cha
Family Fund (F.F.B.). We are also thankful to the Pharmacology and
Analytical Center Facility of the University of Texas M.D. Anderson
Cancer Center.
References
|
1
|
Harvey RG: Polycyclic Aromatic
Hydrocarbons. Wiley-VCH; 1997
|
|
2
|
Clar E: Polycyclic Hydrocarbons. Academic
Press; New York: 1964
|
|
3
|
Zhang FJ, Cortez C and Harvey RG: New
synthetic approaches to polycyclic aromatic hydrocarbons and their
carcinogenic oxidized metabolites: derivatives of benzo[s]picene,
benzo[rst]pentaphene and dibenzo[b,def]chrysene. J Org Chem.
65:3952–3960. 2000.PubMed/NCBI
|
|
4
|
Harvey RG: Chemistry and carcinogenicity.
Polycyclic Aromatic Hydrocarbons Chapter 4. Cambridge University
Press; 1991
|
|
5
|
Becker FF and Banik BK: Polycyclic
aromatic compounds as anticancer agents: synthesis and biological
evaluation of some chrysene derivatives. Bioorg Med Chem.
8:2877–2880. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palmer BD, Rewcastle GW, Atwell GJ,
Baguley BC and Denny WA: Potential antitumor agents. 54 Chromophore
requirements for in vivo antitumor activity among the general class
of linear tricyclic carboxamides. J Med Chem. 31:707–712. 1988.
View Article : Google Scholar
|
|
7
|
Harvey RG, Pataki J, Cortez C, DiRaddo P
and Yang C: A new general synthesis of polycyclic aromatic
compounds based on enamine chemistry. J Org Chem. 56:1210–1217.
1991. View Article : Google Scholar
|
|
8
|
Heaney H: Comprehensive Organic Synthesis.
Trost BM: Pergamon Press; Oxford: pp. 7531991, View Article : Google Scholar
|
|
9
|
Banik BK, Venkatraman MS, Mukhopadhyay C
and Becker FF: A facile reduction of aromatic nitro compounds to
aromatic amines by samarium and iodine. Tetrahedron Lett.
39:7243–7246. 1998. View Article : Google Scholar
|