Introduction
In order to determine whether microscopic
involvement of pseudoangiomatous stromal hyperplasia (PASH) by
breast cancer is associated with lymph node metastases, 80 cases of
breast carcinoma in different categories were examined for
microscopic foci of PASH. Of the 80 cases, 4 contained microscopic
foci of PASH permeated by carcinoma. The results support the
premise that PASH is an underestimated pathway of tumour
spread.
Materials and methods
A total of 80 cases of infiltrating breast carcinoma
in four different categories were examined. The materials used in
this study were archived consecutive cases (mastectomy or wide
local excisions) obtained from the files of the Department of
Pathology at the University Hospital of South Manchester and were
selected based on histological type, with and without nodal
involvement. Carcinomas with vascular invasion were excluded. The
categories included 10 grade 2 infiltrating ductal carcinomas with
lymph node metastases, 10 grade 2 without lymph node metastases, 10
grade 3 infiltrating ductal carcinomas with lymph node metastases
and 10 grade 3 carcinomas without lymph node metastases,
respectively. In addition, four groups of infiltrating lobular
carcinoma grades 2 and 3, with and without lymph node metastases
were examined. All of the slides from the cases were reviewed. Any
cases showing evidence of vascular invasion were excluded and a
block from each case was randomly selected for
immunohistochemistry. The sections were stained with CD34
(Novocastra) and smooth muscle actin (Sigma) to confirm or negate
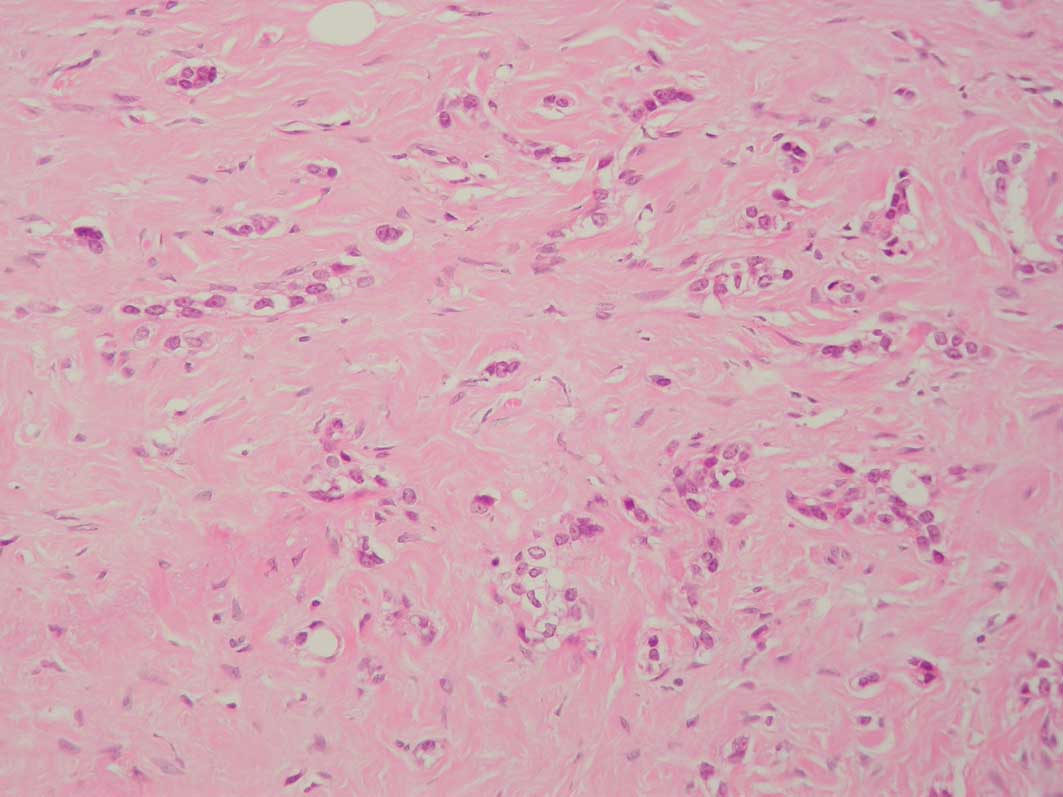
the presence of PASH with involvement by carcinoma (Figs. 1 and 2). A total of 4 cases with foci showing
such involvement were additionally stained with antibodies to
podoplanin (AngioBio), D2-40 (Zymed) and CD31.
Results
Of the 80 cases, 4 (2 grade 2 and 2 grade 3
infiltrating ductal carcinomas) contained microscopic foci of PASH
measuring up to one high-power field, confirmed
immunohistochemically, which was permeated by carcinoma. None of
the cases displayed foci of vascular invasion. The 4 cases were
also stained with antibodies to podoplanin (AngioBio), D2-40
(Zymed) and CD31 (Dako), and the CD34+ve spaces were unstained with
these lymphatic and vascular markers. Lymph node involvement was
noted in 3 of the 4 cases (Table
I).
 | Table IPseudoangiomatous stromal hyperplasia;
an observation on its presence in breast carcinoma and lymph node
metastases. |
Table I
Pseudoangiomatous stromal hyperplasia;
an observation on its presence in breast carcinoma and lymph node
metastases.
| Case no. | Age (years) | Carcinoma | Size (mm) | Vascular
invasion | Nodes |
|---|
| 1 | 64 | Infiltrating ductal
grade 2 | 18 | Not found | 1 of 13 |
| 2 | 56 | Infiltrating ductal
grade 3 | 15 | Not found | 4 of 15 |
| 3 | 72 | Infiltrating ductal
grade 3 | 25 | Not found | 1 of 24 |
| 4 | 59 | Infiltrating ductal
grade 2 | 10 | Not found | 0 of 17 |
Discussion
Pseudoangiomatous stromal hyperplasia consists of
slit-like anastomosing spaces lined by flattened elongated
myofibroblasts with small nuclei and scanty cytoplasm. The spaces
are separated by hyalinised connective tissue, and the cells are
negative for vascular endothelial markers, including factor VIII
and CD31, and for the lymphatic endothelial marker D2-40, but are
positive for CD34 and smooth muscle actin (1,2).
Vuitch et al described PASH as a form of stromal hyperplasia
considered to be the result of artefactual disruption and
separation of collagen fibres with resulting open
inter-anastomosing spaces (3).
Findings by Hartveit showed that the ultrastructure of attenuated
lymphatic endothelial cells form sheets rather than vessels within
the breast stroma and that these potential spaces form the missing
lymphatic system of the breast, the lymphatic labyrinth (4,5).
Fisher et al concluded that PASH and this lymphatic
labyrinth (spaces ultrastructurally lined by slender cells with
tapering cytoplasmic processes that are either fibroblasts or
lymphatic endothelial cells) are related structures (6). Recently, Asioli et al, in a
three-dimensional study of two cases of normal breast tissue and
one case of PASH, demonstrated direct anastomoses between
pre-lymphatic channels and true lymphatics of the breast (2).
The missing lymphatic labyrinth described by
Hartveit is now considered to be the normal counterpoint of the
spaces that constitute pseudoangiomatous hyperplasia (7). Due to the fact that these channels
communicate between breast epithelial/stromal structures and the
main lymphatic system, it is also suggested that these
pre-lymphatics should be considered in the intramammary spread of
tumours, a suggestion previously posited by Damiani et al
(1). These authors’ observations,
although not statistically valid, are supportive of this
premise.
Axillary lymph node involvement is a powerful
prognostic indicator (7).
Undetected or unsampled lymphatic involvement in these 4 cases
cannot be excluded, while the correlation of two findings does not
necessarily establish a cause and effect relationship. However, the
involvement of PASH may be a marker of such involvement, given the
results of this study and the findings of Damiani et al
(1) and Asioli et al
(2). Furthermore, we cannot exclude
such undetected or unsampled lymphatic involvement in other cases
with lymph node metastases, but without vascular involvement and
the absence of PASH foci. A recent study using antibodies to D2-40,
podoplanin and Prox-1 concluded that lymphangiogenesis does not
occur in breast cancer (8). Three
additional studies documented the correlation between prominent
separation/retraction artefact in breast cancer and lymph node
metastases (10–12). One of these studies suggested that
separation artefact may be early ‘lymphovasculogenesis’ prior to
the mesenchymal cell being converted to the endothelial cell
(10). An additional study
postulates that retraction spaces are likely related to altered
tumour-stromal interactions and are possibly an early stage of
lymphatic tumour spread (12).
References
|
1
|
Damiani S, Peterse JL and Eusebi V:
Malignant neoplasms infiltrating ‘pseudoangiomatous’ stromal
hyperplasia of the breast: an unrecognised pathway of tumour
spread. Histopathology. 41:208–215. 2002.
|
|
2
|
Asioli S, Eusebi V, Gaetano L, Losi L and
Bussolati G: The pre-lymphatic pathway, the roots of the lymphatic
system in breast tissue: a 3D study. Virchows Arch. 453:401–406.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vuitch MF, Rosen PP and Erlandson RA:
Pseudoangiomatous hyperplasia of mammary stroma. Hum Pathol.
17:185–191. 1986. View Article : Google Scholar
|
|
4
|
Hartveit F: Attenuated cells in breast
stroma; the missing lymphatic system of the breast. Histopathology.
16:533–543. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartveit F: Pericytes in human breast
stroma and the cells to which they relate. Eur J Morphol.
30:289–296. 1992.PubMed/NCBI
|
|
6
|
Fisher CJ, Hanby AM, Robinson L and Mills
RR: Mammary hamartoma – a review of thirty-five cases.
Histopathology. 20:99–106. 1992.
|
|
7
|
Tavassoli FA and Eusebi V: Tumors of the
Mammary Gland. AFIP Atlas of Tumor Pathology, 4th Series, Fasicle
10. ARP Press; Maryland: pp. 269–274. 2009
|
|
8
|
Cserni G: Evaluation of sentinel lymph
nodes in breast cancer. Histopathology. 46:697–702. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agarmal B, Saxena R, Morimiya A, Mehrotra
S and Badve S: Lymphoangiogenesis does not occur in breast cancer.
Am J Surg Pathol. 29:1449–1455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barsky SH, Ye Y and Karlin NJ: ‘Separation
Artefact’ V lymphovascular invasion: are mimics only mimics? Mod
Pathol. 86(Suppl 1): 289A2006.
|
|
11
|
Acs G, Dumoff KL, Solin LJ, Pasha T, Xu
Xiaowei and Zhang PJ: Extensive retraction artifact correlates with
lymphatic and nodal metastasis and predicts poor outcome in early
stage breast carcinoma. Am J Surg Pathol. 31:129–140. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Acs G, Paragh G, Shang-Tian C, Laronga C
and Zhang PJ: The presence of micropapillary features and
retraction artifact in core needle biopsy material predict lymph
node metastasis in breast carcinoma. Am J Surg Pathol. 33:202–210.
2009. View Article : Google Scholar : PubMed/NCBI
|