Introduction
The extracellular matrix (ECM), which provides
structural support to cells, is the extracellular material in
tissue that generally has a role in the regulation of cell
proliferation, differentiation and wound healing. Components of the
ECM are now being recognized as key signaling molecules that affect
the invasion and metastasis of cancer. It has become evident that
the ECM modulates cellular proliferation and differentiation by
affecting not only growth factors, but also various receptors
involved in controlling morphogenesis and cell growth (1). Decorin is a component of the ECM that
is synthesized primarily by fibroblasts and myofibroblasts
(2) and that regulates collagen
fibrillogenesis (3,4). Based on in vitro assays,
decorin is a potent inhibitor of tumor cell proliferation as it
interacts with transforming growth factor-β (TGF-β) (5) and affects several receptors, epidermal
growth factor receptor (EGFR), insulin-like growth factor receptor
(IGF-IR) and low-density lipoprotein receptor-related protein (LRP)
(2). Evidence from in vitro
experiments indicates that decorin is a potent inhibitor of tumor
cell proliferation, therefore, the antitumor effects of decorin
have been tested in vivo. A cytomegaloviral vector
containing the decorin transgene has been shown to inhibit
tumorigenesis and metastatic spreading of breast carcinoma
(6). A recombinant protein that
comprises only the core of the human decorin protein inhibits
metastatic spreading to the lung in xenograft models of breast
carcinoma (7).
The natural history of breast cancer involves a
progression that begins with abnormal proliferation of epithelial
cells, which can progress to ductal carcinoma in situ
(DCIS), which in turn can become invasive breast carcinoma (IBC).
This progression can culminate in metastatic disease (8). Cancer invasion is the process in which
malignant cells break away from a primary tumor and spread through
surrounding tissue by interaction with components of the stroma.
The development of invasiveness is a critical event for patients
since malignant cells acquire the capacity to metastasize during
this process. The mechanisms by which primary breast cancer cells
acquire an invasive phenotype and break the basal membrane are not
fully understood.
During invasion, the stroma is altered by
interactions with breast cancer cells, and the stromal environment
becomes susceptible to invasion. Changes in the stroma may occur at
the early stage of the transition from DCIS to IBC. Several of the
critical changes in the tumor stroma that accompany cancer
progression have yet to be identified. To identify critical changes
in the stroma that promote invasion, we focused on changes in the
expression of decorin which has anticancer effects and is a
component of ECM. In particular, we hypothesized that expression of
decorin in the stroma may decrease during the development and
progression of breast cancer. To test this hypothesis, we examined
decorin expression in the stroma of tissue samples in different
histological categories [normal glands, flat epithelial atypia
(FEA), atypical ductal hyperplasia (ADH), in situ component,
invasive component] in individual IBC patients. We also compared
pure DCIS samples to IBC samples.
This is the first study to demonstrate that stromal
decorin expression decreases during the progression of breast
cancer. Reduced decorin expression may facilitate tumorigenesis,
tumor invasion and/or tumor growth.
Materials and methods
Patients and tissue samples
We obtained 120 tumor samples: 98 were from patients
with IBC and 22 were from patients with DCIS. All patients
underwent surgical resection in the Department of Breast Surgery at
Tokyo Medical and Dental University, Japan, between January 1999
and November 2003. Mean patient age was 54.6 years (range, 30–91
years). None of the patients had distant metastasis. All specimens
were formalin-fixed and paraffin-embedded. The Institutional Review
Board approved the study, and written informed consent was obtained
from each patient before surgery.
Examination of clinicopathological and
biological features
After tissue samples were stained with
hematoxylin-eosin (H&E), histopathological examination was
performed using the International Union Against Cancer
Tumor-Node-Metastasis classification criteria (9). Expression of estrogen receptor (ER)
and progesterone receptor (PgR) were evaluated using
immunohistochemistry. The status of each tumor with regard to ER
and PgR expression was determined by calculating the percentage of
all cancer cells within a given tumor with positive nuclear
staining; the cut-off value was set at 10%.
Decorin immunohistochemical staining
For immunohistochemical analyses, tissue sections
(4-μm) were deparaffinized over the course of five 10-min
incubations in xylene. Tissue sections were rehydrated, and antigen
retrieval was then performed by incubating the sections in 10
mmol/l sodium citrate buffer (pH 6.0) in a temperature-controllable
microwave (MW) processor (MI-77; Azumaya Co., Tokyo, Japan) at 98°C
for 25 min. Endogenous peroxidase activity was blocked using a
solution of 3% hydrogen peroxide in absolute methanol for 15 min.
Sections were incubated with anti-decorin mouse monoclonal
antibodies (ab54728, Abcam, Cambridge, UK) (1:1,000 dilution); the
sections were then beam irradiated with the MW processor at 27°C
for 15 min. The Histofine SAB-PO kit (Nichirei Corp., Tokyo, Japan)
was used according to the manufacturer's instructions to block
non-specific binding and to detect bound primary antibody. Color
development was carried out with DAB (0.02% 3-3′-diaminobenzidine
tetrahydrochloride; Nichirei) for 10 min at room temperature. The
sections were then counterstained with Mayer's hematoxylin.
Immunohistochemical evaluation
The immunostaining of decorin was analyzed under a
light microscope. The evaluation of stromal decorin expression was
performed around normal gland tissue, pre-malignant components and
in situ components. In the cases of IBC, the stroma adjacent
to malignant cells was evaluated. First, areas of tissue that
represented each of the different histological categories (normal
gland, FEA, ADH, in situ component, invasive component)
within samples from each individual IBC patient were evaluated
separately. Additionally, samples from patients with pure DCIS were
compared to those from patients with IBC. We performed
semiquantitative digital image analysis. The intensity of decorin
signal was evaluated using the ImageJ software (version 1.43u,
National Institutes of Health, Bethesda, MD, USA), according to the
method described by Augoff et al (10). Briefly, stained specimens were
viewed using a light microscope, and random areas at the periphery
of lesions were captured as digital images (680×512 pixels) with a
digital camera. For each digital image, the signal from 10
representative areas was digitized into a grayscale ranging from 0
(white) to 255 (black), and these data were used to generate a
histogram. Cellular nuclei were omitted from this analysis since
they were counterstained with hematoxylin, which would have
artificially increased the gray-level values. The stroma in the
negative control samples (samples without primary antibody) was
used as an internal control. The intensity of decorin signal was
standardized by subtracting the mean gray value of the internal
control.
Statistical analysis
The Wilcoxon signed-rank test was used for
comparisons of different histological categories within individual
IBC patients. Quantitative decorin expression for comparison of IBC
and pure DCIS were analyzed using the Mann-Whitney U test. The
Mann-Whitney U test was also used to examine the association
between decorin expression and clinicopathological/biological
features. P-values <5% were considered to indicate statistically
significant differences.
Results
Decorin expression
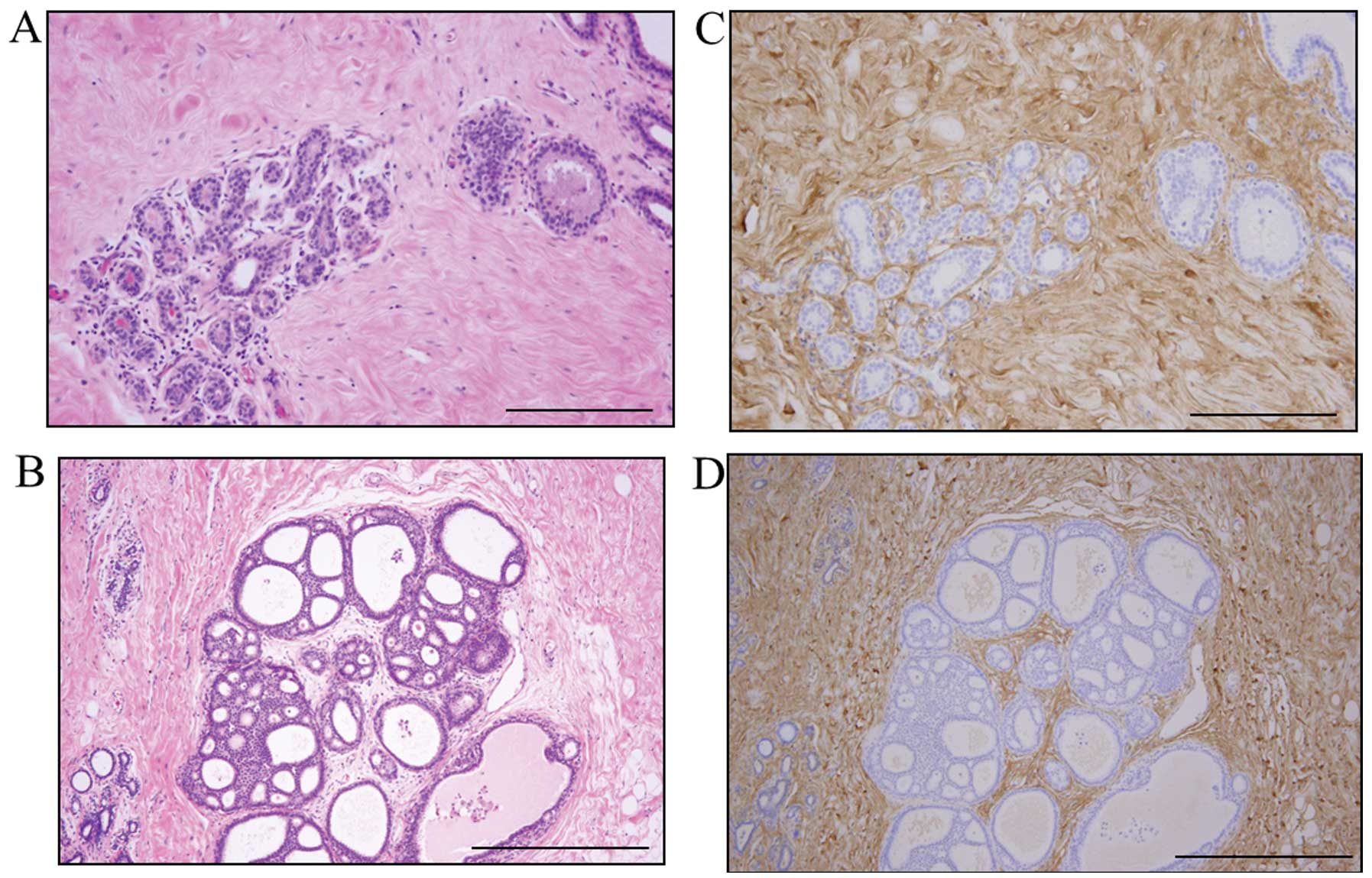
Decorin was present mainly in the ECM and was
clearly expressed by stromal cells, such as fibroblasts and some
inflammatory cells. Decorin expression was also evident around
normal gland tissue and the in situ component (Fig. 1). However, in some cases, the
decorin expression in the stroma around the in situ
component was weak. Decorin expression in stroma tended to be
weaker in the invasive components than in the in situ
components (Fig. 2). Decorin
signals were almost completely absent from epithelial cells.
Initially, we compared different histological
categories (normal glands, FEA, ADH, in situ component,
invasive component) in individual IBC patients. When a single IBC
had regions with different histological features, the stromal
decorin expression around each feature was evaluated separately;
these results are shown in Fig. 3.
Stromal decorin expression was significantly lower in invasive
components than in the other components. Stromal decorin expression
was also lower in the in situ components than in normal
glands, FEA, or ADH.
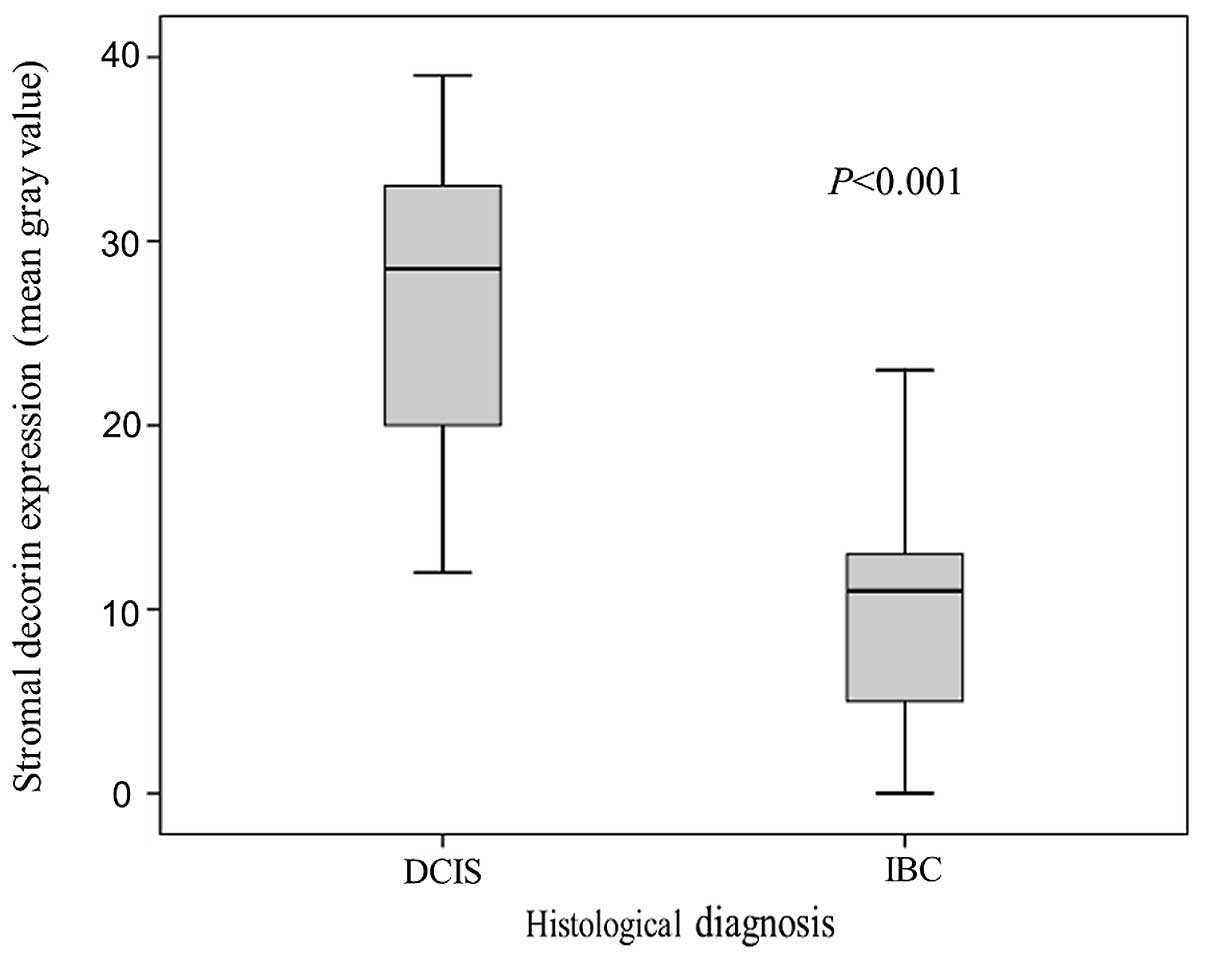
Subsequently, we compared pure DCIS samples to IBC
samples. The mean value of stromal decorin expression around DCIS
(in situ component) was 27±8.1. In 6 cases of DCIS, very
weak decorin staining in the stroma was detected (Fig. 4). In IBC patients, the mean value of
stromal decorin expression around invasive components was 10±6.1.
The values of decorin expression were significantly different in
DCIS and IBC samples (P<0.001, Fig.
5).
Decorin expression and
clinicopathological/biological features
Correlations between stromal decorin expression
adjacent to malignant cells and clinicopathological/biological
features are summarized in Table I.
Stromal decorin expression was significantly lower in IBC samples
than in DCIS samples. There was no statistically significant
correlation between stromal decorin expression and age, breast
density, menopausal status, tumor location or hormone receptor
expression.
 | Table IStromal decorin expression and
clinicopathological/biological features. |
Table I
Stromal decorin expression and
clinicopathological/biological features.
| Characteristics | No. of patients
(n=120) | Stromal decorin
expression (median gray value) | P-value |
|---|
| Age |
| ≤50 | 50 | 12 | |
| >50 | 70 | 11 | NS |
| Breast
densitya |
| Low | 70 | 11 | |
| High | 50 | 12 | NS |
| Menopausal
status |
| Pre-menopausal | 65 | 12 | |
| Post-menopausal | 55 | 11 | NS |
| Tumor site |
| Inner | 44 | 13 | |
| Outer | 76 | 11 | NS |
| ER |
| Positive
(≥10%) | 77 | 12 | |
| Negative | 43 | 11 | NS |
| PgR |
| Positive
(≥10%) | 70 | 12 | |
| Negative | 50 | 11 | NS |
| Histological
diagnosis |
| DCIS | 22 | 28.5 | |
| IBC | 98 | 11 | <0.001 |
Discussion
This is the first immunohistochemistry study to
thoroughly assess decorin expression around normal glands, ductal
carcinoma in situ (DCIS) and invasive breast carcinoma
(IBC). We showed direct evidence that stromal decorin expression
was highest in normal tissue, lower in in situ components
and the lowest in invasive components. We also demonstrated that
stromal decorin expression was significantly lower in cancer
tissues from patients with IBC than in cancer tissues from patients
with DCIS.
Our results indicate the possibility that
downregulation of stromal decorin expression may be involved in the
progression of breast cancer. Similar results have been reported in
other types of cancer. In colorectal tumors, the stromal decorin of
adenocarcinoma tends to have significantly lower expression than
normal tissue and adenoma (11).
These results support the hypothesis that the downregulation of
decorin expression is associated with cancer progression and
carcinogenesis. However, to date, there have been no detailed
immunohistochemical studies on decorin expression in breast
cancer.
The biological implications of decreased decorin
expression with respect to disease aggressiveness have yet to be
determined. One explanation of the reduced decorin expression is
that a reduction in decorin expression might weaken the functional
and physical barriers to tumor invasion since decorin plays an
important role in maintaining normal collagen structure (12). A second possible explanation is that
decreased decorin expression might allow for the accumulation of
active TGF-β. Cell growth inhibition is reported to be due to the
ability of decorin to bind TGF-β (5,13).
Although there are multiple opinions on this issue, TGF-β may
actually be stimulatory at early stages of epithelial tumorigenesis
(14,15). A third possibility is that low
levels of stromal decorin might promote tumor invasion by affecting
the signaling pathways associated with EGFR and other Erb-B family
receptors. Decorin protein is a biological ligand of EGFR, but its
interaction with EGFR is different from that of other typical
ligands (16). Decorin binding to
EGFR leads to transient activation of the receptor tyrosine kinase,
and this activation is followed by the phosphorylation of MAP
kinase, the induction of p21 and growth suppression (16,17).
The mechanisms that cause downregulation of stromal
decorin are not known. Production of decorin from myofibroblasts
and fibroblasts around the periphery of the cancerous tissue might
decrease. Decorin originates from stromal fibroblasts and
myofibroblasts (2), but there is no
evidence that cancer-associated-fibroblasts produce decorin.
Alternatively or additionally, matrix metalloproteinases (MMPs),
which are secreted by breast carcinoma or stromal cells (18), may cleave the decorin around the
duct and thereby accelerate cancer invasion. Decorin is reported to
be degraded by MMP-2, −3, and −7 (19) and MMP-2 and MMP-7 are expressed in
breast carcinoma (20–23). As there is no direct evidence that
MMP-2 or MMP-7 cleaves decorin in breast cancer, this question
should be addressed directly in future studies.
We also demonstrated that stromal decorin expression
was lower in the in situ component compared to normal
glands, flat epithelial atypia (FEA) and atypical ductal
hyperplasia (ADH). One possible explanation for this difference is
that the stroma was altered and became favorable to cancer. Another
possible explanation is that transformation of stroma before
invasion might induce carcinogenesis. Quante et al suggested
that, based on studies of mouse models, bone marrow-derived
myofibroblasts contribute to the mesenchymal stem cell niche and
promote tumor growth (24). In any
case, decreased expression of decorin around DCIS may represent a
local, negative host response that contributes to invasion of
cancer.
Based on PCR and western blot studies, decorin
expression and prognostic factors of malignant tumors are related.
For example, stromal decorin expression is reduced in soft tissue
sarcoma and reduction of decorin expression is associated with poor
outcomes (25). In breast cancer,
reduced decorin expression in the peritumoral stroma of breast
cancer worsens the prognosis in node-negative patients (26). However, to date, there have been no
detailed immunohistochemical studies on decorin expression in
breast cancer. Further immunohistochemical studies are required to
fully assess the relationship between prognosis and stromal decorin
expression.
Systemic injection of a recombinant protein
comprising the decorin core protein can reduce breast tumor growth
and halt metastatic spread to the lungs (7). Decorin may play an important role for
the treatment of breast cancer.
In conclusion, we have shown that the stromal
decorin expression around DCIS or IBC tumors is markedly different
from that in normal gland tissue. Reduced decorin expression may
facilitate tumorigenesis, tumor invasion and/or tumor growth. A
future study to confirm and further assess the prognostic
significance of stromal decorin expression in breast carcinoma is
warranted.
Acknowledgements
The authors thank Y. Takagi for excellent technical
assistance.
Abbreviations:
|
DCIS
|
ductal carcinoma in situ
|
|
IBC
|
invasive breast carcinoma
|
|
ECM
|
extracellular matrix
|
|
TGF-β
|
transforming growth factor-β
|
|
EGFR
|
epidermal growth factor receptor
|
|
IGF-IR
|
insulin-like growth factor
receptor
|
|
LRP
|
low-density lipoprotein
receptor-related protein
|
|
MW
|
microwave
|
|
FEA
|
flat epithelial atypia
|
|
ADH
|
atypical ductal hyperplasia
|
|
ER
|
expression of estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
MMPs
|
matrix metalloproteinases
|
References
|
1
|
Kim SH, Turnbull J and Guimond S:
Extracellular matrix and cell signalling: the dynamic cooperation
of integrin, proteoglycan and growth factor receptor. J Endocrinol.
209:139–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldoni S and Iozzo R: Tumor
microenvironment: Modulation by decorin and related molecules
harboring leucine-rich tandem motifs. Int J Cancer. 123:2473–2479.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iozzo R: Matrix proteoglycans: from
molecular design to cellular function. Annu Rev Biochem.
67:609–652. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reed CC and Iozzo RV: The role of decorin
in collagen fibrillogenesis and skin homeostasis. Glycoconj J.
19:249–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi Y, Mann D and Ruoslahti E:
Negative regulation of transforming growth factor-beta by the
proteoglycan decorin. Nature. 346:281–284. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Araki K, Wakabayashi H, Shintani K, et al:
Decorin suppresses bone metastasis in a breast cancer cell line.
Oncology. 77:92–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldoni S, Seidler D, Heath J, et al: An
antimetastatic role for decorin in breast cancer. Am J Pathol.
173:844–855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burstein HJ, Polyak K, Wong JS, Lester SC
and Kaelin CM: Ductal carcinoma in situ of the breast. N Engl J
Med. 350:1430–1441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumors. 6th edition. John Wiley &
Sons; New York: 2002
|
|
10
|
Augoff K, Grabowski K, Rabczynski J,
Kolondra A, Tabola R and Sikorski A: Expression of decorin in
esophageal cancer in relation to the expression of three isoforms
of transforming growth factor-beta (TGF-beta1, -beta2, and -beta3)
and matrix metalloproteinase-2 activity. Cancer Invest. 27:443–452.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Augoff K, Rabczynski J, Tabola R, Czapla
L, Ratajczak K and Grabowski K: Immunohistochemical study of
decorin expression in polyps and carcinomas of the colon. Med Sci
Monit. 14:CR530–CR535. 2008.PubMed/NCBI
|
|
12
|
Ferdous Z, Wei V, Iozzo R, Höök M and
Grande-Allen K: Decorin-transforming growth factor-interaction
regulates matrix organization and mechanical characteristics of
three-dimensional collagen matrices. J Biol Chem. 282:35887–35898.
2007. View Article : Google Scholar
|
|
13
|
Ständer M, Naumann U, Dumitrescu L, et al:
Decorin gene transfer-mediated suppression of TGF-beta synthesis
abrogates experimental malignant glioma growth in vivo. Gene Ther.
5:1187–1194. 1998.PubMed/NCBI
|
|
14
|
Reiss M and Barcellos-Hoff MH:
Transforming growth factor-beta in breast cancer: a working
hypothesis. Breast Cancer Res Treat. 45:81–95. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akhurst RJ and Balmain A: Genetic events
and the role of TGF beta in epithelial tumour progression. J
Pathol. 187:82–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iozzo R, Moscatello D, McQuillan D and
Eichstetter I: Decorin is a biological ligand for the epidermal
growth factor receptor. J Biol Chem. 274:4489–4492. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Csordás G, Santra M, Reed C, et al:
Sustained down-regulation of the epidermal growth factor receptor
by decorin. A mechanism for controlling tumor growth in vivo. J
Biol Chem. 275:32879–32887. 2000.PubMed/NCBI
|
|
18
|
Polette M, Gilbert N, Stas I, et al:
Gelatinase A expression and localization in human breast cancers.
An in situ hybridization study and immunohistochemical detection
using confocal microscopy. Virchows Arch. 424:641–645. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Imai K, Hiramatsu A, Fukushima D,
Pierschbacher M and Okada Y: Degradation of decorin by matrix
metalloproteinases: identification of the cleavage sites, kinetic
analyses and transforming growth factor-beta1 release. Biochem J.
322:809–814. 1997.PubMed/NCBI
|
|
20
|
Iwata H, Kobayashi S, Iwase H, Masaoka A,
Fujimoto N and Okada Y: Production of matrix metalloproteinases and
tissue inhibitors of metalloproteinases in human breast carcinomas.
Jpn J Cancer Res. 87:602–611. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones JL, Glynn P and Walker RA:
Expression of MMP-2 and MMP-9, their inhibitors, and the activator
MT1-MMP in primary breast carcinomas. J Pathol. 189:161–168. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garbett EA, Reed MW, Stephenson TJ and
Brown NJ: Proteolysis in human breast cancer. Mol Pathol.
53:99–106. 2000. View Article : Google Scholar
|
|
23
|
Jiang WG, Davies G, Martin TA, et al:
Targeting matrilysin and its impact on tumor growth in vivo: the
potential implications in breast cancer therapy. Clin Cancer Res.
11:6012–6019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quante M, Tu SP, Tomita H, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsumine A, Shintani K, Kusuzaki K, et
al: Expression of decorin, a small leucine-rich proteoglycan, as a
prognostic factor in soft tissue tumors. J Surg Oncol. 96:411–418.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Troup S, Njue C, Kliewer E, et al: Reduced
expression of the small leucine-rich proteoglycans, lumican, and
decorin is associated with poor outcome in node-negative invasive
breast cancer. Clin Cancer Res. 9:207–214. 2003.PubMed/NCBI
|