Introduction
Acute myeloid leukemia (AML) is a hematologic
malignancy characterized by a block of terminal differentiation of
the hematopoietic progenitors at early stages in myelopoiesis. AML
accounts for ~25% of all leukemias diagnosed in adults and its
incidence is stably increasing (1).
In the past two decades, there has been little improvement in
chemotherapeutic regimens and hence the overall survival for
patients with AML remains poor, with a 5-year survival rate of ~20%
(2,3) and median survival times of only a few
months for elderly patients (4).
This is partly due to a higher prevalence of unfavorable
cytogenetics and myelodysplasia, a higher incidence of MDR, and
more frequent comorbidities that often render them unsuitable for
intensive treatment (4,5). Therefore, it is urgent to seek new
novel therapeutic agents and strategies with less systemic toxicity
for improving the treatment outcomes of elderly patients.
In the past decades, natural products have played a
critical role in drug discovery and development. The potential of
natural products from plants, notably from traditional Chinese
medicine (TCM), has been recognized by the scientific community in
the Western world for its low toxicity, and considerable efforts
have been made to systematically investigate the active component
of TCM for cancer therapy. A series of natural products, such as
Homoharringtonine (6), arsenic
trioxide (ATO) (7) and paclitaxel
(8), have been found and clinically
proved for their potential use in leukemia therapy. They
demonstrate the translation of basic knowledge of purified agents
of complex mixtures from TCM into the clinic. In addition, the
successful application of combination of all-trans retinoic
acid (ATRA) and ATO therapy in APL demonstrates that the
combination of TCM with synthesized compound provides an attractive
strategy for the development of novel and improved cancer
therapeutics (9,10).
We have focused on studies of TCM treatment in
leukemia for decades and have tested more than 10 chemically
characterized compounds from TCM treatment in leukemia cell lines
and found several active compounds such as icariin (11) and baicalin (12). Baicalin is a flavonoid compound
isolated from Scutellaria baicalensis, a Chinese traditional
medicinal herb widely used as an anti-inflammatory (13), antibacterial, anxiolytic and
hepatoprotective drug (14).
Accumulating evidence demonstrates that baicalin exhibits potent
antitumor properties by suppressing cell growth, modulating cell
cycle, inducing differentiation or apoptosis in leukemia cell lines
(15,16), without affecting primary or normal
cells (17,18), raising the possibility of treating
even older patients with AML.
Hexamethylene bisacetamide (HMBA), a hybrid polar
compound, was used in phase I and II clinical trials for the
treatment of myelodysplastic syndrome (MDS) and AML (19). Previous studies demonstrated that
HMBA exerted dose-dependent dual effects on AML cells. At low
concentrations, HMBA induces terminal differentiation in a variety
of leukemic cell lines (20,21),
while it inhibits cell growth significantly at high concentrations
through apoptosis and cell cycle delay rather than through
differentiation. Evidence indicates that HMBA induced apoptosis
associated with downregulation of Bcl-2 gene expression,
upregulation of expression of p21, p53 (22), or HMBA induced the activation of a
caspase-independent cell death pathway (23). Additionally, other studies
documented that HMBA can be efficacious as an adjunct agent for
enhancing the antitumor effects of antineoplastic drugs (24,25).
However, some serious side-effects were induced when administered
at higher concentrations, such as thrombocytopenia, thereby
limiting the utilization of this agent in cancer chemoprevention
(21).
It was previously reported that suberoylanilide
hydroxamic acid (SAHA) and flavopiridol (FP) interact
synergistically to induce mitochondrial damage and apoptosis in
human leukemia cells (26,27). In view of the similar molecular
structure between SAHA and HMBA, and that between FP and baicalin,
together with their potential antileukemic efficacy, the
enhancement of baicalin by HMBA in growth arrest and apoptosis
induction of AML cells in the present study were detected and the
underlying mechanism of their effect was also elucidated.
Materials and methods
Reagents
Baicalin with a purity of up to 99.5% was kindly
provided by Professor Xiao Wang (Shandong Analysis and Test Center,
Shandong Academy of Sciences) and 10 mg/ml solution dissolved with
DMSO was stored at -20°C. HMBA was obtained from Sigma (St. Louis,
MO, USA) and 0.5 M stock solution was made by dissolving it in
RPMI-1640 medium (Gibco, Grand Island, NY, USA). Propidium iodide
(PI), rhodamine 123 (Rh123) were also purchased from Sigma. Hoechst
33342 was obtained from Beyotime Biotechnology, Inc. (Nantong,
China). Annexin V fluorescein isothiocyanate (FITC) kit was
obtained from BD Biosciences (San Diego, CA, USA). Antibodies for
detecting Bax, Bcl-2, cleaved caspase-3, -8, -9, Fas were purchased
from Cell Signaling Technology (Beverly, MA, USA). β-actin antibody
was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Horseradish peroxidase-labeled IgG anti-mouse and anti-rabbit
antibodies were supplied by Zhongshan Golden Bridge Biotechnology
Co. (Beijing, China). Cell Counting Kit-8 (CCK-8) was obtained from
Dojindo Laboratories (Kumamoto, Japan).
Cell culture
AML cell lines NB4 and THP-1 were purchased from
American Type Culture Collection (ATCC, Bethesda, MD, USA); HL-60
cells and K562 cells were conserved by our laboratory. Cells were
cultured in RPMI-1640 medium supplemented with 10% heat-inactivated
newborn calf serum (NCS), 100 IU/ml penicillin and 100 IU/ml
streptomycin at 37°C in an atmosphere with 5% CO2.
Logarithmically growing cells were exposed to drugs for the
indicated time periods.
Human peripheral blood mononuclear cells (PBMCs)
were isolated from heparinized venous blood of healthy volunteers
by Ficoll-Paque density gradient centrifugation. After washing with
PBS twice, PBMCs were cultured in RPMI-1640 with 10% FCS at 37°C in
a humidified atmosphere containing 5% CO2. After
incubating on a plastic plate for 6 h, non-adherent cells were
collected and used for cytotoxicity assay (18).
Cell viability assay
Cellular viability was detected using CCK-8
according to the manufacturer’s instructions. In brief,
logarithmically growing cells were seeded on 96-well plates at a
density of 1×104 cells/well in 100 μl of medium in
triplicate and treated with baicalin (5, 10, 20, 40, 80 μg/ml) or
HMBA (0.5, 1, 2, 4 mM) or in combination. Cells treated with 0.1%
(v/v) DMSO or RPMI-1640 medium were used as control. Following
incubation for 24 h, 10 μl of CCK-8 solution was added to each well
in the assay plate and incubated for an additional 2 h at 37°C.
Absorbance was measured at 450 nm using a microplate reader (Model
550; Bio-Rad, USA). Each group had triplicate samples. The
inhibition rate was calculated by the following formula: cell
inhibition rate (%) = 1 − average absorbance of treated
group/average absorbance of control group ×100%. Data were
calculated as the means ± SD of triplicate samples and are
representative of at least three independent assays.
The cytotoxicity of baicalin and HMBA on PBMCs was
assayed by the trypan blue exclusion test. Briefly, PBMCs from two
healthy volunteers were cultured with 20 μg/ml baicalin and/or 2 mM
HMBA for 3 days, then 1 μl of trypan blue dye was added to cell
suspension, mixed and incubated for 2 min. Dye-cell suspension was
loaded to a counting chamber and counted under a microscope to
determine whether cells take up or exclude dye. Percentage of
viable cells was calculated by the following formula: % of viable
cells = number of viable cells counted/total number cells
counted.
Cell cycle analysis
Following baicalin (20 μg/ml) and/or HMBA (2 mM)
treatment for 24 h, HL-60 cells (3×105) were washed
twice with ice-cold PBS, and fixed in cold 75% ethanol at 4°C for
at least 24 h. Then the cells were rinsed with PBS, resuspended in
1 ml of cell cycle buffer (0.38 mm Na-Citrate, 0.5 mg/ml RNase A
and 20 μg/ml PI) at room temperature for 30 min and analyzed using
an EPICS XL flow cytometer with EXPO32™ ADC software (Beckman
Coulter, Miami, FL, USA).
Morphological assessment of
apoptosis
HL-60 cells were plated (3×105
cells/well) after baicalin (20 μg/ml) and/or HMBA (2 mM) treatment
for 24 h; cell morphology was observed with light microscopy. For
nuclear morphology, cells were washed twice with PBS, fixed with 4%
paraformaldehyde for 15 min and stained with Hoechst 33342 (10
μg/ml) for 5 min at room temperature in the dark. After washing
three times, cells were resuspended by PBS. Stained nuclei were
observed by a Nikon ECLIPSE Ti Fluorescence Microscope (Nikon,
Japan) and photographed.
Apoptosis assessment by Annexin V/PI
staining
Following drug exposure for 24 h, 3×105
cells were harvested, washed twice with cold PBS and resuspended in
100 μl of 1X binding buffer containing 5 μl Annexin V and 10 μl PI
for 15 min at room temperature in the dark. Flow cytometry
measurements were made on a Beckman Coulter EPICS XL cytometer.
Assays for analysis of mitochondrial
membrane potential (ΔΨm)
ΔΨm was assessed using fluorescent dye Rh123 and
flow cytometric analysis. Briefly, following drug treatments for 6
h, the cells were washed twice with PBS; 1×106 cells in
different groups were incubated with 10 mg/ml Rh123 for 30 min at
37°C. Following incubation, cells were washed twice and resuspended
in PBS followed by flow cytometric analysis. The change in the mean
fluorescence intensity reflects the modification of ΔΨm, which
drives the uptake and accumulation of Rh123 in the
mitochondria.
Reverse transcription (RT)-PCR
analysis
Following baicalin (20 μg/ml) and/or HMBA (2 mM)
treatment for 24 h, HL-60 cells were collected and total RNA was
extracted from each sample of 1×106 cells by TRIzol
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
protocol. The RNA samples were resuspended in RNase-free water and
frozen at −80°C until use. RT-PCR was performed as previously
described (28). The PCR products
were electrophoresed in 1.5% agarose gels. The primers used were
all synthesized by Sangon Co., Ltd. (Shanghai, China). The
sequences are listed in Table
I.
 | Table IThe sequence of primers, size of
products and annealing temperatures for reverse
transcription-PCR. |
Table I
The sequence of primers, size of
products and annealing temperatures for reverse
transcription-PCR.
| Primers | Sequence of
primers | Size of products
(bp) | Annealing
temperatures (°C) |
|---|
| β-actin | Sense:
5′-GTGGGGCGCCCCAGGCAGGCACCA-3′
Antisense: 5′-CTCCTTAATGTCACGCACGATTTC-3′ | 540 | 55 |
| β-actin | Sense:
5′-ACTATGTTTGAGACCTTCAACA-3′
Antisense: 5′-CATCTCTTGCTCGAAGTCCA-3′ | 318 | 55 |
| Bcl-2 | Sense:
5′-AGGCACCCAGGGTGATGCAA-3′
Antisense: 5′-GTGGAGGAGCTCTTCAAGGA-3′ | 304 | 56 |
| Bax | Sense:
5′-ATGTCAAACGTGCGAGTGTC-3′
Antisense: 5′-TCTGTAGTAGAACTCGGGCAA-3′ | 289 | 55 |
|
Bcl-XL | Sense:
5′-GGAGCTGGTGGTTGACTTTCT-3′
Antisense: 5′-GTACCGCAGTTCAAACTCGTC-3′ | 286 | 55 |
| Caspase-9 | Sense:
5′-CTAGTTTGCCCACACCCAGT-3′
Antisense: 5′-GCATTAGCGACCCTAAGCAG-3′ | 172 | 55 |
| Caspase-8 | Sense:
5′-GGACAGGAATGGAACACACTT-3′
Antisense: 5′-TCAGGATGGTGAGAATATCATC-3′ | 557 | 50 |
Western blot analysis
After exposing to baicalin (20 μg/ml) and/or HMBA (2
mM) for 24 h, HL-60 cells were washed twice with cold PBS and lysed
in extraction buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM
PMSF, 1 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 1 μg/ml
leupeptin, 2 μg/ml aprotinin, 1 mM Na3VO4 and
0.1% SDS) for 30 min on ice. The lysates were centrifuged at 12,000
× g for 15 min and quantified using Bradford protein assay.
Proteins were separated by SDS-PAGE and electroblotted onto PVDF
membranes. The membranes were blocked with 5% milk for 1 h and
incubated with primary monoclonal antibodies against caspase-8
(1:1,000), caspase-9 (1:1,000), cleaved caspase-3 (1:1,000), Fas
(1:1,000), Bcl-2 (1:1,000) and Bax (1:1,000) (Cell Signaling
Technology) overnight at 4°C followed by incubation with
HRP-conjugated secondary antibodies (Zhongshan Golden Bridge
Biotechnology Co.) for 1 h. The protein bands were visualized using
Immobilon Western Chemiluminescent HRP Substrate (Millipore,
Billerica, MA, USA) and pictured by LAS-4000 Mini luminescent image
analyzer (Fujifilm, Tokyo, Japan).
Statistical analysis
Data presented are the means ± SD from at least
three independent experiments and the significance of difference
between two groups was compared by one-way analysis of variance
(ANOVA) followed by Tukey’s test using SPSS 13.0 (SPSS, Chicago,
IL, USA). P<0.05 was considered to indicate statistically
significant differences.
Results
Combined treatment with baicalin and HMBA
synergistically inhibits the proliferation of AML cell lines
To determine the optimal combined concentration,
HL-60 cells were initially treated with various concentrations of
baicalin or HMBA alone. As shown in Fig. 1A, at the indicated concentration
scope, baicalin inhibited cell proliferation in a dose-dependent
manner. The IC50 value of baicalin was 21.8 μg/ml
calculated according to the dose-response curve. At this
concentration baicalin showed almost no cytotoxicity in PBMCs even
after 5.5 days of culture (18).
Therefore, 20 μg/ml was selected for the subsequent analysis. HMBA
has been shown to induce differentiation in multiple leukemia types
at a concentration of 2–5 mM (29).
Thus, the 0–4 mM of HMBA concentration range was selected for cell
viability assay. CCK-8 assay indicated that HMBA induced cell
growth arrest in a dose-dependent manner, but more mildly than
baicalin (Fig. 1B). Based on this
growth inhibition of HMBA and the low efficient concentration used
in the clinic (30), 2 mM was
determined for the combined concentration of HMBA.
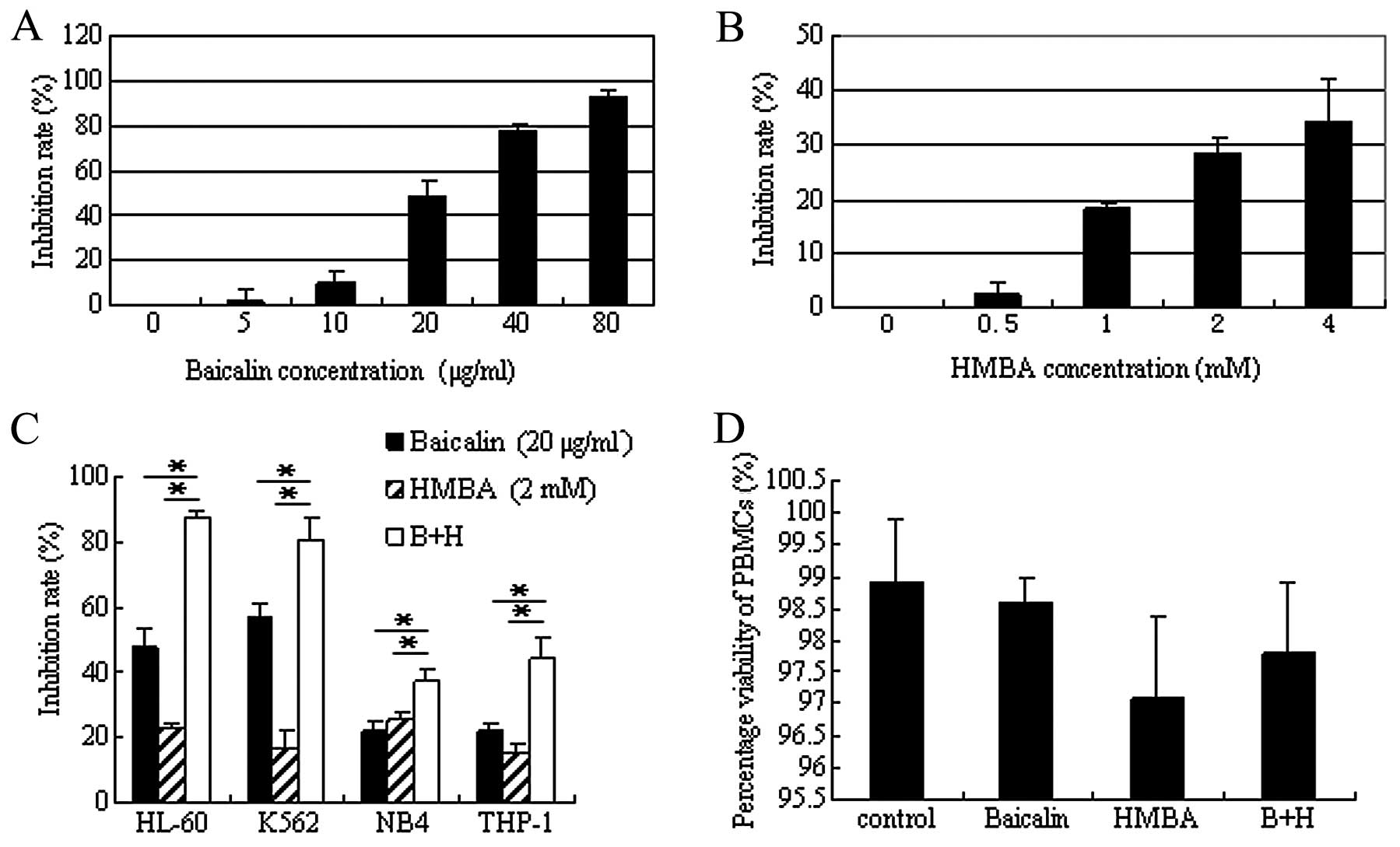 | Figure 1Inhibitory effect of baicalin and
hexamethylene bisacetamide (HMBA) alone or in combination on acute
myeloid leukemia cell lines. (A and B) HL-60 cells were treated
with various concentrations of baicalin (0, 5, 10, 20, 40, 80
μg/ml) or HMBA (0, 0.5, 1, 2, 4 mM) for 24 h. Cell viability was
measured by CCK-8 assay and cell growth inhibition rate were
calculated as described in Materials and methods. Values represent
the means ± SD in triplicates in three separate experiments. (C)
HL-60, K562, NB4, THP-1 cells were simultaneously exposed to 20
μg/ml baicalin and 2 mM HMBA for 24 h, then cell viability was
detected and analyzed as described in (A). (D) Peripheral blood
mononuclear cells (PBMCs) from three healthy volunteers were
cultured with 20 μg/ml baicalin and/or 2 mM HMBA for 3 days. The
cell viability was analyzed by trypan blue exclusion test. Values
represent the means ± SD of three separate experiments.
*P<0.01 compared with combination treatment. |
Next, studies were performed to detect the
inhibitory effect of the combination treatment. Following
coadministration of 20 μg/ml baicalin with 2 mM HMBA for 24 h, the
inhibition rate strikingly increased from 47.5±6.3 or 22.6±1.5 to
87.4±2.6% (P<0.01), which indicated that combination treatment
exerted a synergistic inhibitory effect on the proliferation of
HL-60 cells. To determine whether the synergistic effect was
restricted to HL-60 cells, parallel studies were performed in other
human leukemia cell lines K562, THP-1 and NB4 and the same results
were obtained (Fig. 1C). Of these
cell lines, the combination treatment showed the most significant
inhibitory effect on HL-60 cells. Therefore the following
experiments were performed with HL-60 cells.
In order to confirm that the combination of baicalin
and HMBA has selectivity for malignant cells, rather than also
indiscriminately killing normal cells, PBMCs were separated from
healthy volunteers and cultured with baicalin and/or HMBA. The
trypan blue staining results demonstrated that single agent or
combination treatment had only slight cytotoxicity on PBMCs even
after 3 days of culture (Fig.
1D).
Combined treatment with baicalin and HMBA
induces a slight G0/G1 arrest in HL-60
cells
To investigate the mechanisms underlying the
enhanced growth inhibitory effect of the combination of baicalin
and HMBA in HL-60 cells, we performed the cell cycle analysis by
flow cytometry. As shown in Fig. 2,
no significant changes in cell distribution were observed following
exposure of HL-60 cells to 20 μg/ml baicalin or 2 mM HMBA for 24 h,
although both cause G0/G1 phase arrest for 48
h or longer (data not shown). However, combined treatment with
baicalin and HMBA slightly increased the proportion of cells in the
G0/G1 phase from 29.2% in the vehicle-treated
cells to 37.6% (P<0.05). The data indicated that a block in the
cell cycle may be partly associated with the synergistic inhibitory
effect on the proliferation of HL-60 cells after 24 h combined
treatment.
Combination of baicalin and HMBA enhances
induction of apoptosis in HL-60 cells
To determine whether the synergistic inhibitory
effect on combined treatment of HL-60 cells with baicalin and HMBA
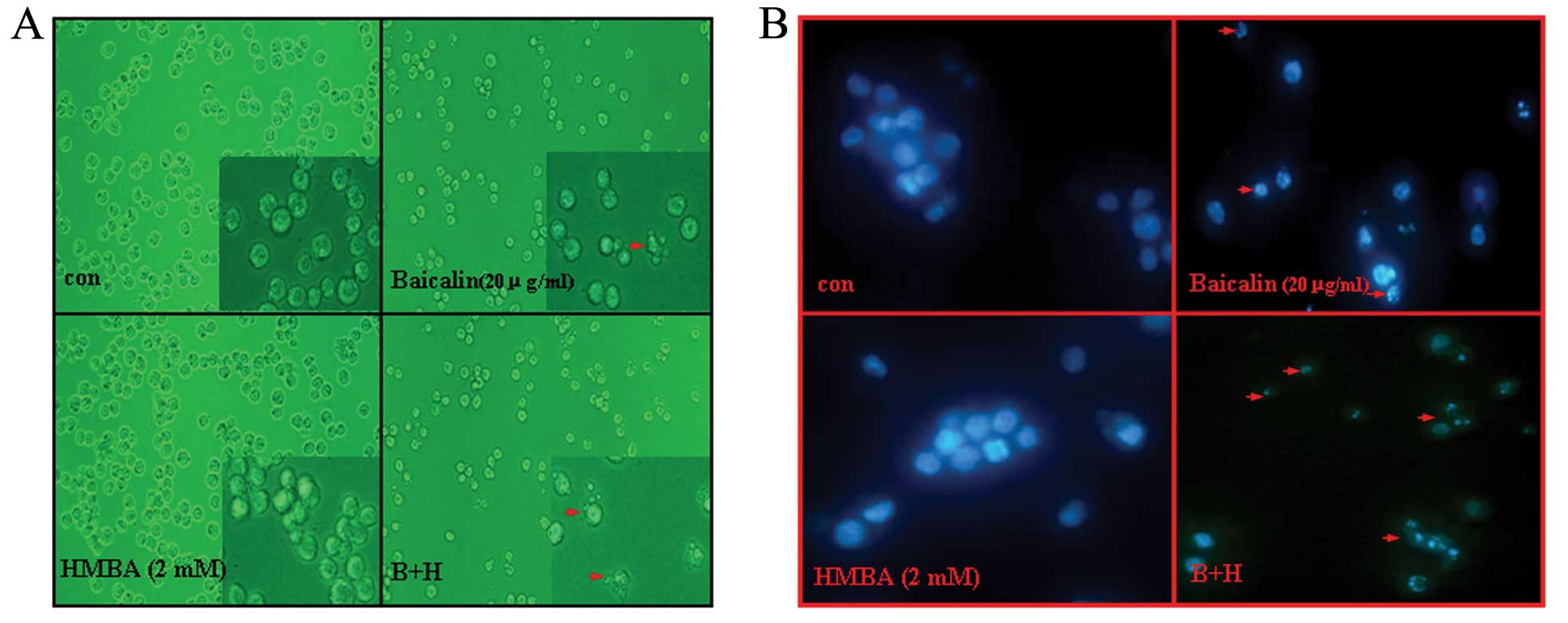
was due to the induction of apoptosis, cell morphological changes
were observed under a light micrograph and under an inverted
fluorescence microscope after Hoechst 33342 staining. It was noted
that HMBA-treated cells presented no significant morphological
changes but only with the numbers decreased compared to control
cells. However, after exposure to 20 μg/ml baicalin for 24 h, the
number of HL-60 cells significantly decreased and, also, part of
the cells exhibited typical morphological characteristics of
apoptosis (such as cell shrinkage, membrane blebbing and formation
of apoptotic bodies) (Fig. 3A).
Combined with HMBA, the phenomenon became stronger. Accordingly,
Hoechst 33342 staining results showed that the nuclei of untreated
cells and HMBA-treated cells were round and large in size,
exhibiting homogeneous blue fluorescence. By contrast, parts of
cells treated with baicalin for 24 h were observed with condensed
or fragmented nuclei which is characteristic of cell apoptosis.
Moreover, the apoptosis events in the combination group were more
distinguished than in the baicalin treatment group (Fig. 3B).
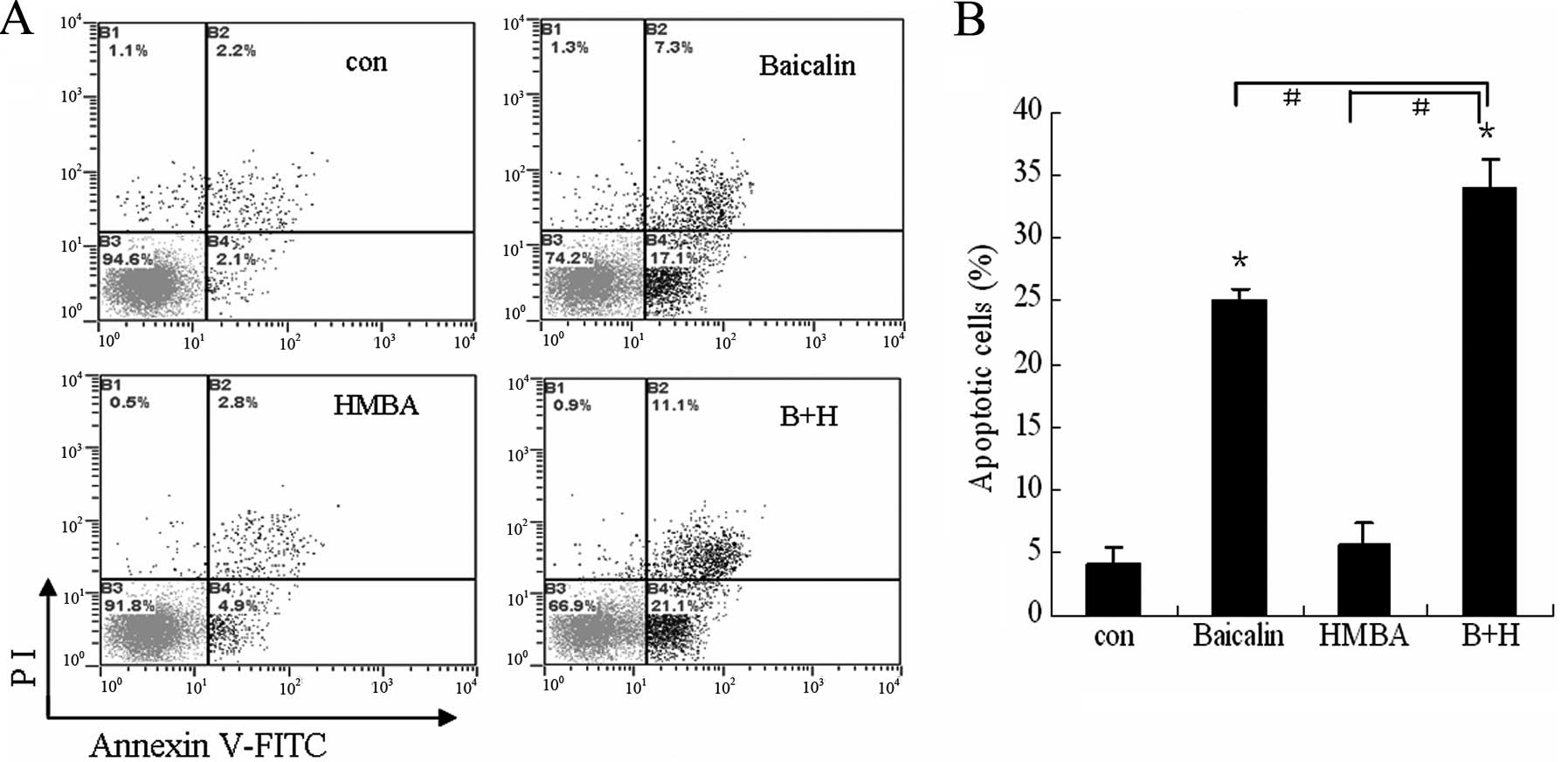
To further validate the enhanced effect of combined
treatment on cell apoptosis, the extent of apoptosis was evaluated
by Annexin V/PI assay. As indicated in Fig. 4, cotreatment with 20 μg/ml baicalin
and 2 mM HMBA showed a synergistic effect (34% total apoptotic
cells) on the induction of apoptosis in HL-60 cells compared to
24.4 and 7.7% for 20 μg/ml baicalin and 2 mM HMBA treatment alone,
respectively. These findings suggest that HMBA enhances apoptosis
induced by baicalin on HL-60 cells.
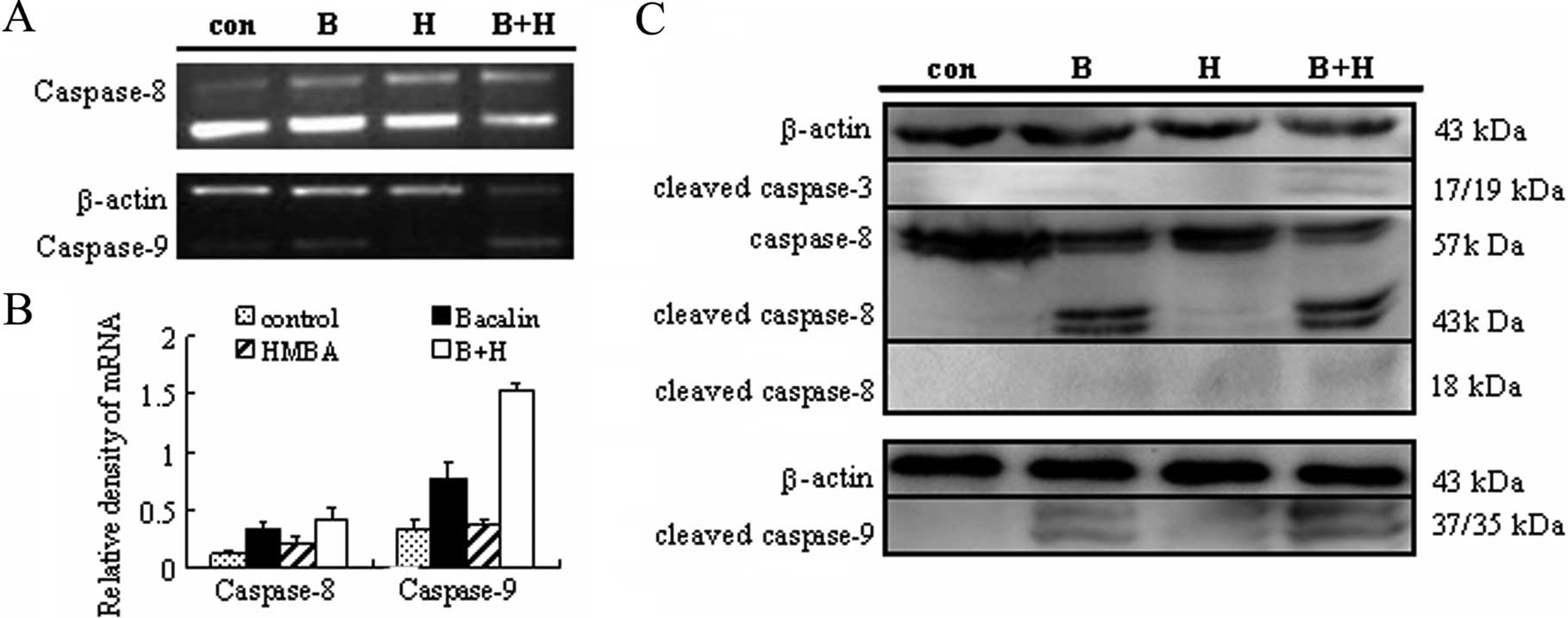
Combined treatment with baicalin and HMBA
induces the activation of caspase-3, -8 and -9
The caspase family of cysteinyl-proteases plays the
key role in the initiation and execution of programmed cell death
(31). Thus, the mRNA expression of
caspase-8, caspase-9 was first detected by semiquantitative RT-PCR.
As shown in Fig. 5A, baicalin
treatment alone caused a 2.82±0.13-fold and 2.29±0.11-fold increase
at the transcriptional level of caspase-8 and caspase-9,
respectively, as compared with the control group. When combined
with HMBA, the mRNA expression of caspase-8, caspase-9 rose to
3.43±0.10-fold and 4.48±0.26-fold, respectively. These data suggest
that caspase-8 and caspase-9 may be involved in the apoptosis
induced by baicalin/HMBA. To further confirm the involvement of
caspases, activation of caspase-8, -9 and -3 was monitored by
western blotting. As shown in Fig.
5B, 24 h exposure to 2 mM HMBA failed to increase cleavage and
activation of caspase-3 while 20 μg/ml baicalin did. Moreover,
combined treatment led to a clear increase in caspase-3 activation
which was reflected by the appearance of a 17 kDa caspase-3
cleavage fragment. Similarly, the cleavages of caspase-8 and -9
were significantly enhanced by combined treatment of baicalin and
HMBA. These results from RT-PCR and western blot analyses taken
together thus indicate that baicalin/HMBA-induced apoptosis is
mediated through the activation of caspase-3, -8, and -9.
Combined treatment-induced apoptosis is
mediated through both the mitochondrial- and Fas-mediated
pathways
Since combination treatment induced the activation
of caspase-8 and -9, it suggests that both extrinsic and intrinsic
pathways are involved in the apoptosis signaling. To address the
intrinsic pathway, ΔΨm was monitored by flow cytometry using Rh123.
The reduction of Rh123 fluorescence intensity presented dissipation
of ΔΨm. As shown in Fig. 6A, HMBA
administered alone for 6 h had little effect on ΔΨm compared with
controls, whereas baicalin alone led to a slight reduction of ΔΨm.
However, combined treatment of HL-60 cells to baicalin/HMBA
resulted in a marked increase in loss of ΔΨm, as compared with
either agent alone. These findings were consistent with the
activation of caspase-9, which is often the result of disruption of
ΔΨm.
To characterize the role of the extrinsic pathway in
baicalin/HMBA-induced apoptosis, we detected Fas protein expression
by western blot analysis. The results showed that exposure to
baicalin or HMBA alone triggered the Fas expression. Moreover,
combination treatment significantly increased its expression
(Fig. 6B). The data presented
herein suggest that activation of the extrinsic Fas-related pathway
plays a major role in the enhanced apoptosis observed in
baicalin/HMBA-treated cells.
Effect of baicalin and HMBA combined
treatment on the mRNA and protein expression of the Bcl-2
family
Proapoptotic and antiapoptotic members of the Bcl-2
family regulate the mitochondrial pathway (32). To further determine whether
baicalin/HMBA-induced apoptosis in HL-60 cells is associated with
the mitochondrial pathway, the expression of proapoptotic factor
Bax, as well as antiapoptotic factor Bcl-2 and Bcl-XL,
were tested at the transcriptional and post-transcriptional level.
As shown in Fig. 7A, 24 h exposure
to either 20 μg/ml baicalin or 2 mM HMBA upregulated the expression
of Bax mRNA, while no evident augmentation was observed in the
combination group as compared with single agent. In contrast to the
increase in the Bax mRNA levels, the mRNA expression of Bcl-2 and
Bcl-XL decreased more clearly in combination-treated
cells than in single agent-treated cells. Consequently, the ratio
of Bcl-2/Bax and the ratio of Bcl-XL/Bax markedly
declined. In parallel studies, western blot analysis revealed that
the protein expression of Bax and Bcl-2 changed in line with the
mRNA expression (Fig. 7B).
Discussion
Similar to previous findings observed in leukemic
cells treated with FP in combination with SAHA (26,27),
the present study highlighted the synergistic antileukemic effect
of baicalin in combination with HMBA in AML cell lines. It was
demonstrated for the first time that the administration of a
subtoxic concentration of baicalin and a pharmacologically relevant
concentration of HMBA results in a synergistic effect in growth
inhibition with only slight toxic effect on normal human cells.
To determine whether the synergistic inhibitory
effect of the combined treatment of HL-60 cells with baicalin and
HMBA is associated with the cell cycle arrest, we performed the
cell cycle analysis by flow cytometry. Cell cycle analysis was
studied in the way of time course as in our previous study
(20), the data confirmed that HMBA
alone clearly induced G0/G1 cell cycle arrest
after treatment for 48 h or later. Ikezoe et al(33) reported that baicalin arrested
G2/M phase in HL-60 cells; however, our results showed
that baicalin induced G0/G1 arrest 48 h
later. By contrast, a 24-h exposure to 2 mM HMBA or 20 μg/ml
baicalin alone had almost no effect on cell cycle distribution,
while they could slightly induce G0/G1
arrest, which may at least partly contribute to the synergistical
inhibition of cell proliferation (Fig.
2). In general, Cdk inhibitors, including p21waf1
and p27kip1, are involved in G1 to S
transition (34). Previous studies
demonstrated that baicalin induced expression of p27kip1
as well as slightly upregulated the expression of
p21waf1 in HL-60 cells and concomitantly induced
differentiation, cell cycle arrest and apoptosis in these cells
(33). Buonamici and Aifantis
(35) found p21 is also involved in
HMBA-induced apoptosis and a short delay in cell cycle. Consistent
with the above mentioned studies, herein we also found the
upregulation of p21 and p27 in HMBA- or baicalin-treated cells,
even more evidently with combination treatment, which may account
for the G0/G1 cell cycle arrest (data not
shown). However, the result of cell cycle distribution is not fully
consistent with the synergistic growth inhibition detected by CCK-8
assay, suggesting that other mechanisms must exist which associate
with the antiproliferative effect of drugs in combination.
To confirm whether the synergistic effect on cell
growth arrest is associated with apoptosis, cell morphology and
Annexin V/PI staining assay were performed. Data showed that
treatment of HMBA alone at a concentration of 2 mM had almost no
effect on the apoptosis of HL-60 cells. While treatment with
baicalin alone induced significant apoptosis. However, the
combination treatment of baicalin and HMBA induced more apoptotic
cells, which suggested that HMBA may enhance the apoptosis induced
by baicalin. This phenomenon is consistent with the pronounced
inhibition of cell proliferation (Figs.
3 and 4).
To further delineate the convergence in apoptosis
signaling, the caspases, proapoptotic and antiapoptotic proteins
were detected in cells with combined treatment of baicalin and
HMBA. It is well known that two major apoptotic pathways exist in
mammalian cells: the extrinsic death receptor pathway and the
intrinsic mitochondrial pathway (36). In the death receptor (extrinsic)
pathway, binding of death ligands such as Fas ligand or TNF-α to
their cognate death receptors triggers receptor trimerization,
recruitment of the adaptor molecule FADD and caspase-8 to the
death-inducing signaling complex (DISC). This, in turn, leads to
activation of caspase-8, which then either directly cleaves and
activates the effector caspase-3 and -7 or cleaves Bid, a Bcl-2
family protein with a BH3 domain only that translocates to
mitochondria upon cleavage to initiate a mitochondrial pathway
(37). In the mitochondrial
(intrinsic) pathway, a variety of extra- and intracellular
stresses, including oxidative stress, DNA damage, heat shock and
treatment with cytotoxic drugs, converge to induce the release of
cytochrome c from the mitochondrial intermembrane space to
the cytosol. Cytochrome c cooperates with dATP and Apaf-1 to
induce the activation of caspase-9 that can cleave and activate
caspase-3 (31), culminating in
cell death. Previous studies demonstrated that baicalin acted as a
pro-oxidant and induced caspase-3 activation and apoptosis in
Jurkat cells (18)or HL-60 cells
(15) via the mitochondrial
pathway. Recently, the intrinsic (mitochondrial) pathway was
confirmed to play a pivotal role in apoptosis induced by baicalin
in CA46 Burkitt lymphoma cells (38). Consistent with previous reports, we
also observed increase of caspase-3 in cleaved form and caspase-9
mRNA expression in HL-60 cells after exposure to baicalin for 24 h,
concomitant with the loss of ΔΨm. Furthermore, 2 mM HMBA failed to
induce activation of caspase-9 and -3, although HMBA can do so in
P-glycoprotein cells at concentrations as high as 10 mmol/l
(23). However, coadministration of
baicalin with HMBA resulted in enhanced dissipation of ΔΨm and
increased activation of caspase-9, caspase-3 (Figs. 5 and 6A). The data suggested that HMBA lowers
the threshold for baicalin-mediated mitochondrial injury and
subsequent activation of the caspase cascade, increasing the
apoptotic effects induced by baicalin.
The Bcl-2 family proteins are well known for
regulating the intrinsic pathway of apoptosis through tightly
regulating mitochondrial outer membrane permeabilization (MOMP),
which leads to the loss of ΔΨm and therefore the release of
proapoptotic molecules, including cytochrome c from
mitochondria to the cytosol (39).
The family consists of antiapoptotic proteins, such as Bcl-2 and
Bcl-XL, as well as proapoptotic members, such as Bax,
Bid, Bax and Bak. Accumulating evidence suggests that it is the
relative ratio of antiapoptotic and proapoptotic Bcl-2 family
proteins rather than the levels of individual proteins that play a
major role in determining the survival or death of cells (40). Consistent with previous reports, our
data indicated that either baicalin or HMBA induced negative
modulation expression of Bcl-2/Bax and Bcl-XL/Bax
ratios. Moreover, the ratios decreased more significantly with
combination treatment (Fig. 7). Our
data suggested that HMBA at a concentration not sufficient to
induce cell death per se, reduced the ratio between
antiapoptotic and proapoptotic Bcl-2 family members, thereby
lowering the threshold for cell death commitment and sensitizing
HL-60 cells to the apoptosis induced death by baicalin. This
finding is supported by Palumbo et al(24). Collectively, the modification of
Bcl-2 family proteins further indicated that the intrinsic
apoptotic pathway is involved in baicalin/HMBA-induced
apoptosis.
Previous studies had not referred to the effect of
baicalin on change of caspase-8 protein. In the present study,
RT-PCR and western blot analyses results showed for the first time
that the cleavage/activation of caspase-8 increased after exposure
to baicalin for 24 h, more significantly in the combination
treatment group for 24 h (Fig. 5),
which may partly contribute to the increase of caspase-3
activation. Consistent with the above findings, our data also
showed that baicalin upregulated the expression of Fas protein
(Fig. 6B). However, this is
contrary to another report indicating that baicalin had no effect
on the expression of Fas protein in TALL cell lines CCRF-CEM
(16). The difference is likely due
to the specific cell type. Moreover, baicalin markedly elevated the
expression of Fas in combination with HMBA. Taking into account the
pronounced upregulation of Fas expression and increased activation
of caspase-8, we postulate that the extrinsic pathway is likely to
be involved in baicalin/HMBA-induced apoptosis in HL-60 cells.
In summary, we showed that the combination of
baicalin and HMBA could synergistically inhibit the proliferation
of AML cell lines with little toxic effect on normal human cells.
In addition, a slight G0/G1 phase arrest and
significant apoptosis were observed. The combination treatment
triggers apoptosis through the intrinsic pathway, which involves
loss of MMP, decreased Bcl-2/Bax ratio and Bcl-XL/Bax
ratio, caspase-9 activation, as well as through the extrinsic
pathway mediated by Fas and caspase-8 activation. Our results raise
the possibility that the novel combination of baicalin and HMBA may
be a promising regimen for the treatment of AML.
Acknowledgements
The authors thank Dr Guihai Li (Shandong Academy of
Chinese Medicine) for providing purified baicalin and Dr Dongdong
Yu and Lingzhi Huang for revising the manuscript. This study was
supported by grants from the Natural Science Foundation of Shandong
Province of China (ZR2011HL045, Y2008C165), the Youth Fund Project
of the Health Department of Shandong Province (2007QZ023), and the
Science and Technology Development Grant of the State
Administration of Traditional Chinese Medicine of Shandong Province
(2011-234), the Project of Scientific Research of Shandong Province
(2007GG2002023).
References
|
1
|
Smith M, Barnett M, Bassan R, Gatta G,
Tondini C and Kern W: Adult acute myeloid leukaemia. Crit Rev Oncol
Hematol. 50:197–222. 2004. View Article : Google Scholar
|
|
2
|
Kumar CC: Genetic abnormalities and
challenges in the treatment of acute myeloid leukemia. Genes
Cancer. 2:95–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tallman MS, Gilliland DG and Rowe JM: Drug
therapy for acute myeloid leukemia. Blood. 106:1154–1163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar
|
|
5
|
Kantarjian H, O’brien S, Cortes J, et al:
Results of intensive chemotherapy in 998 patients age 65 years or
older with acute myeloid leukemia or high-risk myelodysplastic
syndrome: predictive prognostic models for outcome. Cancer.
106:1090–1098. 2006.
|
|
6
|
Luo CY, Tang JY and Wang YP:
Homoharringtonine: a new treatment option for myeloid leukemia.
Hematology. 9:259–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen ZX, Chen GQ, Ni JH, et al: Use of
arsenic trioxide (As2O3) in the treatment of acute promyelocytic
leukemia (APL): II. Clinical efficacy and pharmacokinetics in
relapsed patients. Blood. 89:3354–3360. 1997.PubMed/NCBI
|
|
8
|
Lichtman SM, Hollis D, Miller AA, et al:
Prospective evaluation of the relationship of patient age and
paclitaxel clinical pharmacology: Cancer and Leukemia Group B
(CALGB 9762). J Clin Oncol. 24:1846–1851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou GB, Zhang J, Wang ZY, Chen SJ and
Chen Z: Treatment of acute promyelocytic leukaemia with all-trans
retinoic acid and arsenic trioxide: a paradigm of synergistic
molecular targeting therapy. Philos Trans R Soc Lond B Biol Sci.
362:959–971. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen ZX, Shi ZZ, Fang J, et al: All-trans
retinoic acid As2O3 combination yields a high quality remission and
survival in newly diagnosed acute promyelocytic leukemia. Proc Natl
Acad Sci USA. 101:5328–5335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Cui Z and Zhang L: Effects of
icariin on the differentiation of HL-60 cells. Zhonghua Zhong Liu
Za Zhi. 19:53–55. 1997.(In Chinese).
|
|
12
|
Ren X, Li CL, Wang HX, Wen PE, Yuan CJ, Li
YM and Jiang GS: Molecular mechanism of HL-60 cells apoptosis
induced by baicalin. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
20:847–851. 2012.(In Chinese).
|
|
13
|
Huang WH, Lee AR and Yang CH:
Antioxidative and anti-inflammatory activities of polyhydroxy
flavonoids of Scutellaria baicalensis GEORGI. Biosci
Biotechnol Biochem. 70:2371–2380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shang X, He X, Li M, Zhang R, Fan P, Zhang
Q and Jia Z: The genus Scutellaria an ethnopharmacological
and phytochemical review. J Ethnopharmacol. 128:279–313.
2010.PubMed/NCBI
|
|
15
|
Lu HF, Hsueh SC, Ho YT, et al: ROS
mediates baicalin-induced apoptosis in human promyelocytic leukemia
HL-60 cells through the expression of the Gadd153 and
mitochondrial-depedent pathway. Anticancer Res. 27:117–125.
2007.PubMed/NCBI
|
|
16
|
Shieh DE, Cheng HY, Yen MH, Chiang LC and
Lin CC: Baicalin-induced apoptosis is mediated by Bcl-2-dependent,
but not p53-dependent, pathway in human leukemia cell lines. Am J
Chin Med. 34:245–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parajuli P, Joshee N, Rimando AM, Mittal S
and Yadav AK: In vitro antitumor mechanisms of various
Scutellaria extracts and constituent flavonoids. Planta Med.
75:41–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueda S, Nakamura H, Masutani H, Sasada T,
Takabayashi A, Yamaoka Y and Yodoi J: Baicalin induces apoptosis
via mitochondrial pathway as prooxidant. Mol Immunol. 38:781–791.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andreeff M, Stone R, Michaeli J, et al:
Hexamethylene bisacetamide in myelodysplastic syndrome and acute
myelo-genous leukemia: a phase II clinical trial with a
differentiation-inducing agent. Blood. 80:2604–2609. 1992.
|
|
20
|
Ren X, Wen PE, Yang WH, et al: Molecular
mechanism underlying differentiation of HL-60 cells induced by
hexamethylene bisacetamide. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
16:1030–1034. 2008.(In Chinese).
|
|
21
|
Marks PA, Richon VM, Kiyokawa H and
Rifkind RA: Inducing differentiation of transformed cells with
hybrid polar compounds: a cell cycle-dependent process. Proc Natl
Acad Sci USA. 91:10251–10254. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cecchinato V, Erba E, Basile A, et al:
Hexamethylene bisacetamide inhibits malignant phenotype in T-ALL
cell lines. Leuk Res. 32:791–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruefli AA, Smyth MJ and Johnstone RW: HMBA
induces activation of a caspase-independent cell death pathway to
overcome P-glycoprotein-mediated multidrug resistance. Blood.
95:2378–2385. 2000.PubMed/NCBI
|
|
24
|
Palumbo C, Albonici L, Bei R, Bocci C,
Scarpa S, Di Nardo P and Modesti A: HMBA induces cell death and
potentiates doxorubicin toxicity in malignant mesothelioma cells.
Cancer Chemother Pharmacol. 54:398–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waxman S, Scher BM, Hellinger N and Scher
W: Combination cytotoxic-differentiation therapy of mouse
erythroleukemia cells with 5-fluorouracil and hexamethylene
bisacetamide. Cancer Res. 50:3878–3887. 1990.
|
|
26
|
Almenara J, Rosato R and Grant S:
Synergistic induction of mitochondrial damage and apoptosis in
human leukemia cells by flavopiridol and the histone deacetylase
inhibitor suberoylanilide hydroxamic acid (SAHA). Leukemia.
16:1331–1343. 2002. View Article : Google Scholar
|
|
27
|
Dasmahapatra G, Almenara J and Grant S:
Flavopiridol and histone deacetylase inhibitors promote
mitochondrial injury and cell death in human leukemia cells that
overexpress Bcl-2. Mol Pharmacol. 69:288–298. 2006.PubMed/NCBI
|
|
28
|
Liu J, Bi G, Wen P, et al: Down-regulation
of CD44 contributes to the differentiation of HL-60 cells induced
by ATRA or HMBA. Cell Mol Immunol. 4:59–63. 2007.PubMed/NCBI
|
|
29
|
Smith KM, Ketchart W, Zhou X, Montano MM
and Xu Y: Determination of hexamethylene bisacetamide, an
antineoplastic compound in mouse and human plasma by LC-MS/MS. J
Chromatogr B Analyt Technol Biomed Life Sci. 879:2206–2212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Conley BA, Egorin MJ, Sinibaldi V, et al:
Approaches to optimal dosing of hexamethylene bisacetamide. Cancer
Chemother Pharmacol. 31:37–45. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghavami S, Hashemi M, Ande SR, et al:
Apoptosis and cancer: mutations within caspase genes. J Med Genet.
46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zimmermann KC, Bonzon C and Green DR: The
machinery of programmed cell death. Pharmacol Ther. 92:57–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikezoe T, Chen SS, Heber D, Taguchi H and
Koeffler HP: Baicalin is a major component of PC-SPES which
inhibits the proliferation of human cancer cells via apoptosis and
cell cycle arrest. Prostate. 49:285–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Steinman RA: Cell cycle regulators and
hematopoiesis. Oncogene. 21:3403–3413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Buonamici S and Aifantis I: Hexamethylene
bisacetamide as a treatment for T-cell leukemia (T-ALL). Leuk Res.
32:689–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fulda S: Caspase-8 in cancer biology and
therapy. Cancer Lett. 281:128–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang Y, Hu J, Zheng J, Li J, Wei T, Zheng
Z and Chen Y: Down-regulation of the PI3K/Akt signaling pathway and
induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin.
J Exp Clin Cancer Res. 31:482012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Autret A and Martin SJ: Bcl-2 family
proteins and mitochondrial fission/fusion dynamics. Cell Mol Life
Sci. 67:1599–1606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reed JC, Jurgensmeier JM and Matsuyama S:
Bcl-2 family proteins and mitochondria. Biochim Biophys Acta.
1366:127–137. 1998. View Article : Google Scholar : PubMed/NCBI
|