Introduction
Gliomas are the most common and aggressive type of
primary brain tumors. Despite recent advances in treatment by a
combination of surgery and chemotherapy and/or radiotherapy, the
prognosis of malignant glioma is still extremely poor. Gliomas can
be divided into low grade glioma (LGG, including astrocytomas,
oligodendrogliomas and oligoastrocytomas) and high grade glioma
(HGG, including anaplastic astrocytomas, anaplastic
oligodendrogliomas, anaplastic oligoastrocytomas and glioblastomas)
depending on the malignancy. Glioblastoma (GBM) is WHO grade IV
glioma, the median survival time of which is approximately only one
year (2,3). The increased invasive capacity of GBM
cells may be part of the reason for the poor prognosis. Thus,
research on invasion of glioma is important.
RAB, ras-related gtp-binding protein, is a member of
the small GTPase super family, it is located in specific
subcellular organelles and plays an important role in cell
secretion, endocytosis, signal transduction and development
(4). In recent years, the role in
the control of different aspects of tumor progression has emerged.
In tumors, main roles of RAB GTPases were cell migration, invasion,
proliferation, communication with stromal cells and the development
of drug resistance. Several RABs such as RAB1b, RAB24, RAB25,
RAB27a and RAB27b are disregulated in many cancers (5–8). The
function of RAB27a in glioma cell lines was also reported (9,10).
RAB38 is the gene responsible for oculocutaneous albinism (1), but the effect of RAB38 in tumors is
still unclear.
In the present study, we investigated mRNA
expression microarray data on 220 glioma samples from the Chinese
Glioma Genome Atlas (CGGA) database as discovery set and 3
additional published datasets as validation sets. Through
evaluating the RAB38 expression status, we obtained the prognostic
value. The protein expression level was validated in another 82
glioma samples from the Chinese Glioma Tissue Database (CGTD) by
immunohistochemistry. In all 4 datasets, we performed function
annotation of RAB38 by GO analysis and GSVA, as a result, its
correlation with cell migration mainly was found.
Materials and methods
Datasets
Whole genome mRNA expression microarray data and
clinical information on 220 glioma samples of all grades from the
Chinese Glioma Genome Atlas (CGGA) database (2) (http://www.cgga.org.cn) were obtained as discovery set
and 369 glioma expression files from the Cancer Genome Atlas (TCGA)
database (11) (http://cancergenome.nih.gov), the Repository for
Molecular Brain Neoplasia Data (REMBRANDT, http://caintegratorinfo.nci.nih.gov/rembrandt)
and GSE16011 data (12) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE16011)
were obtained as validation sets.
Immunohistochemistry (IHC)
Paraffin-embedded specimens were cut into 4 μm
sections and heated at 65°C for 30 min. The sections were
deparaffinized with xylenes and rehydrated. Sections were submerged
into EDTA (pH 8.0) and autoclaved for antigen retrieval, then
treated with 3% hydrogen peroxide, followed by incubation with 1%
FBS. Anti-RAB38 antibody (Antibodies; ABIN1390129) was added and
incubated overnight at 4°C. Horseradish peroxidase (HRP) labeled
secondary antibody in the MaxVision™ HRP-Polymer anti-rabbit IHC
kit (KIT-5930 Maixin Biology, Fuzhou, China) was applied and
incubated for 30 min at room temperature, followed by 5-min
incubation at room temperature with DAB provided in the kit for
color development. The sections were finally counterstained with
haematoxylin and mounted with Permount (BIOS, Beijing, China).
Results were visualized and photographed under a light microscope
(Olympus BX-51; Olympus Optical). The proportion of positively
stained tumor cells was graded as follows: 0, no positive tumor
cells; 1, <5% positive tumor cells; 2, 5–20% positive tumor
cells; and 3, >20% positive tumor cells. The intensity of
staining was recorded on a scale of 0 (no staining), 1 (weak
staining, light yellow), 2 (moderate staining, yellowish brown) and
3 (strong staining, brown). The staining index was calculated as
follows: staining index = staining intensity x tumor cell staining
grade. High RAB38 expression was defined as a staining index score
≥4, while low expression was defined as a staining index <4.
Gene Ontology (GO) analysis
After Pearson correlation analysis, Gene Ontology
analysis of the positively correlated genes (r>0.4; P<0.01)
were analyzed by DAVID (http://david.abcc.ncifcrf.gov/home.jsp).
Gene set variation analysis (GSVA)
Gene set variation analysis with RAB38 expression
was analyzed by GSVA package (13)
of R (14). Gene list was obtained
from the web (http://amigo.geneontology.org/) (GO:0030335, positive
regulation of cell migration and GO:0030336, negative regulation of
cell migration).
Statistical analysis
For molecular subtype annotation of the 4 datasets,
we applied prediction analysis of microarrays (PAM) as previously
reported (2). Quantitative results
are shown as mean ± standard deviation. The difference of RAB38
expression was tested by the Student’s t-test in microarray data
and by the Chi-square test in IHC results. Overall survival time
(OS) was calculated from the date of diagnosis until death or the
last follow-up. The survival curve of patients with high or low
expressed RAB38 was calculated with the Kaplan-Meier method and the
difference was analyzed using the two-sided log-rank test. A
P-value <0.05 was considered to indicate a statistically
significant result. All the data analysis was performed in MATLAB,
GraphPad Prism and R. Written informed consent was obtained from
the patient for publication of the present report and any
accompanying images. The study was performed with the approval of
the Ethics Committee of the Capital Medical University and it was
in compliance with the Helsinki Declaration.
Results
Characteristics of glioma patients
In 220 glioma patients, there were 97 WHO grade II
gliomas (astrocytomas, oligodendrogliomas and oligoastrocytomas),
34 WHO grade III gliomas (anaplastic astrocytomas, anaplastic
oligodendrogliomas and anaplastic oligoastrocytomas) and 89 WHO
grade IV gliomas (GBMs). Clinical information [age, gender,
preoperational Karnofsky performance scale (KPS) score and
treatment] were obtained from medical records of the CGGA database
(Table I).
 | Table IClinical characteristics of the 220
glioma patients. |
Table I
Clinical characteristics of the 220
glioma patients.
| Grade II | Grade III | Grade IV |
|---|
| Case number | 97 | 34 | 89 |
| Median age
(years) | 38 | 39 | 46 |
| Male % | 55.7 | 52.9 | 58.4 |
| Median KPS | 90 | 80 | 80 |
| Median OS (days) | ND | 633 | 420 |
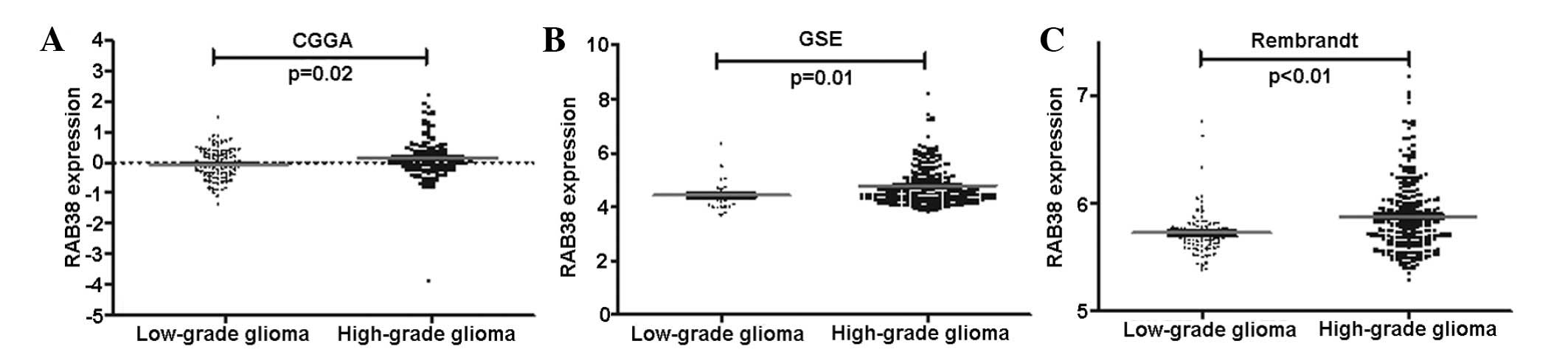
The expression of RAB38 in high grade
gliomas was higher than those of low grade
After analyzing of differently expressed genes in
the discovery dataset, we found that RAB38 was in the list of genes
significantly differently expressed between LGGs and HGGs. RAB38
expression increased along with grade progression of glioma both in
CGGA and the other 2 validation datasets (Fig. 1).
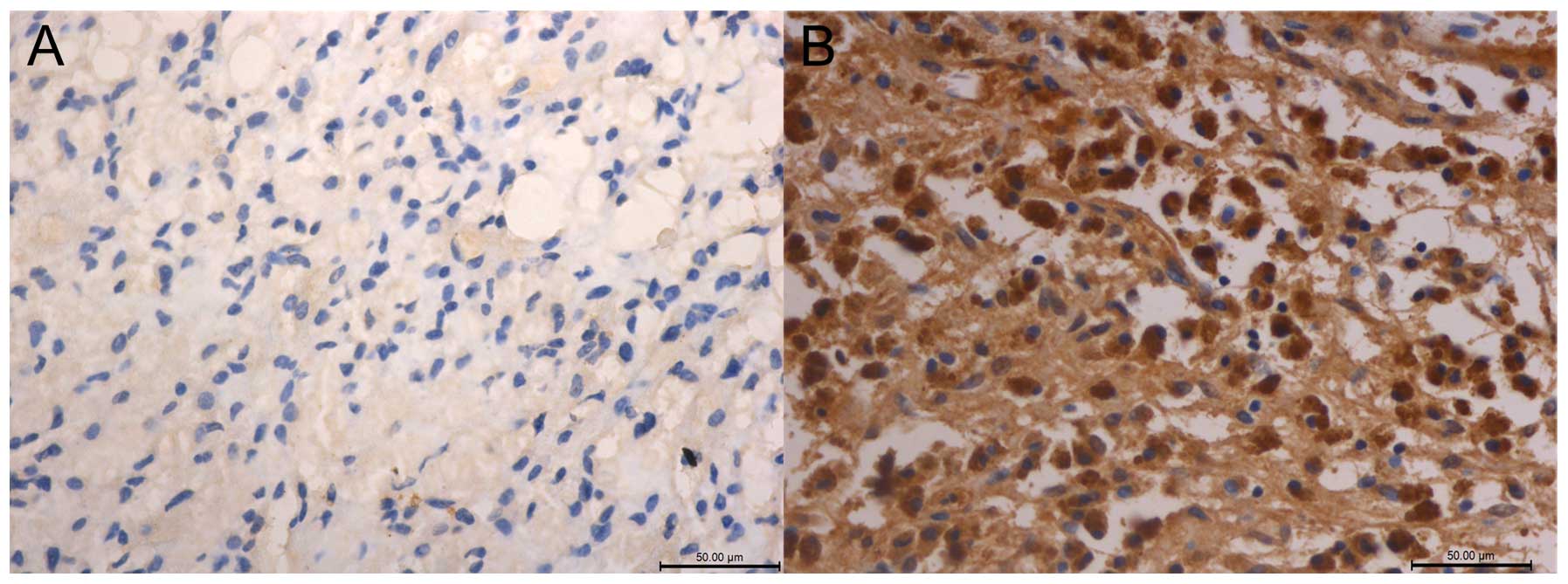
The protein expression level of RAB38 was
detected by IHC
Eighty-two glioma samples were used to detect the
expression status of RAB38 by IHC (Fig.
2). These samples included 38 LGGs and 44 HGGs. RAB38 showed a
higher expression level in HGGs than those in LGGs (P<0.01).
Thus, RAB38 expression showed high grade glioma preference both in
mRNA and in protein level.
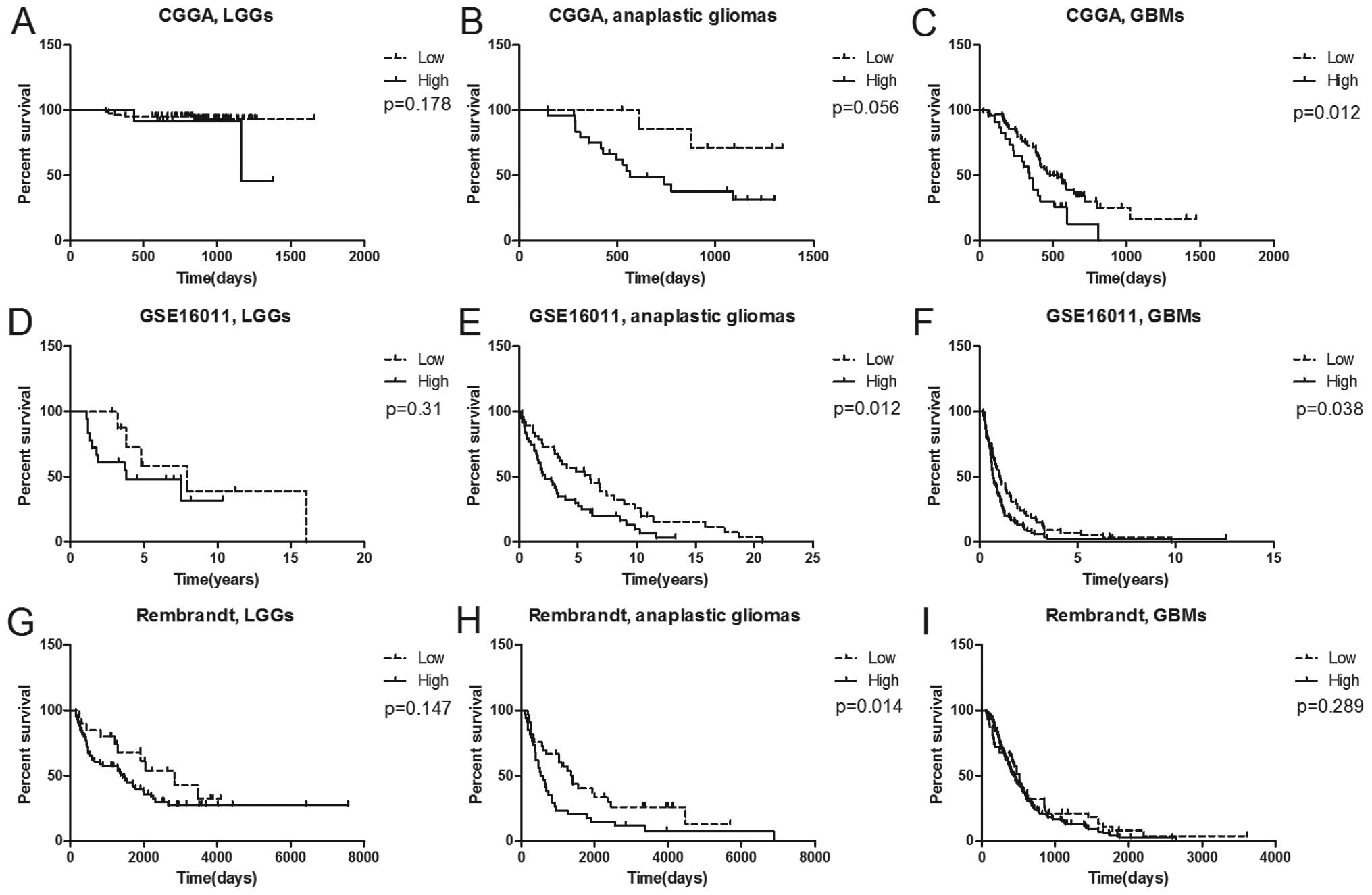
RAB38 may be a prognostic marker in
anaplastic gliomas and glioblastomas
In the 220 glioma patients of CGGA, 216 patients
with integrated prognostic information were further analyzed by
Kaplan-Meier. Although several P-values in survival analysis was no
statistical significance, the expression of RAB38 can be divided
into high and low expression with different percent survival of
each grade in CGGA and the other two datasets (Fig. 3). There was a marginal significance
in anaplastic gliomas in CGGA (Fig.
3B; P=0.056), but there was a significant different prognosis
in anaplastic gliomas of GSE16011 and Rembrandt cohort (Fig. 3E and H). In GBMs, there was a
significantly shorter survival time in high RAB38 expression than
those of low in CGGA (Fig. 3C) and
GSE16011 (Fig. 3F) except for
Rembrandt (Fig. 3I). Therefore,
RAB38 was a prognostic marker in anaplastic gliomas and
glioblastomas.
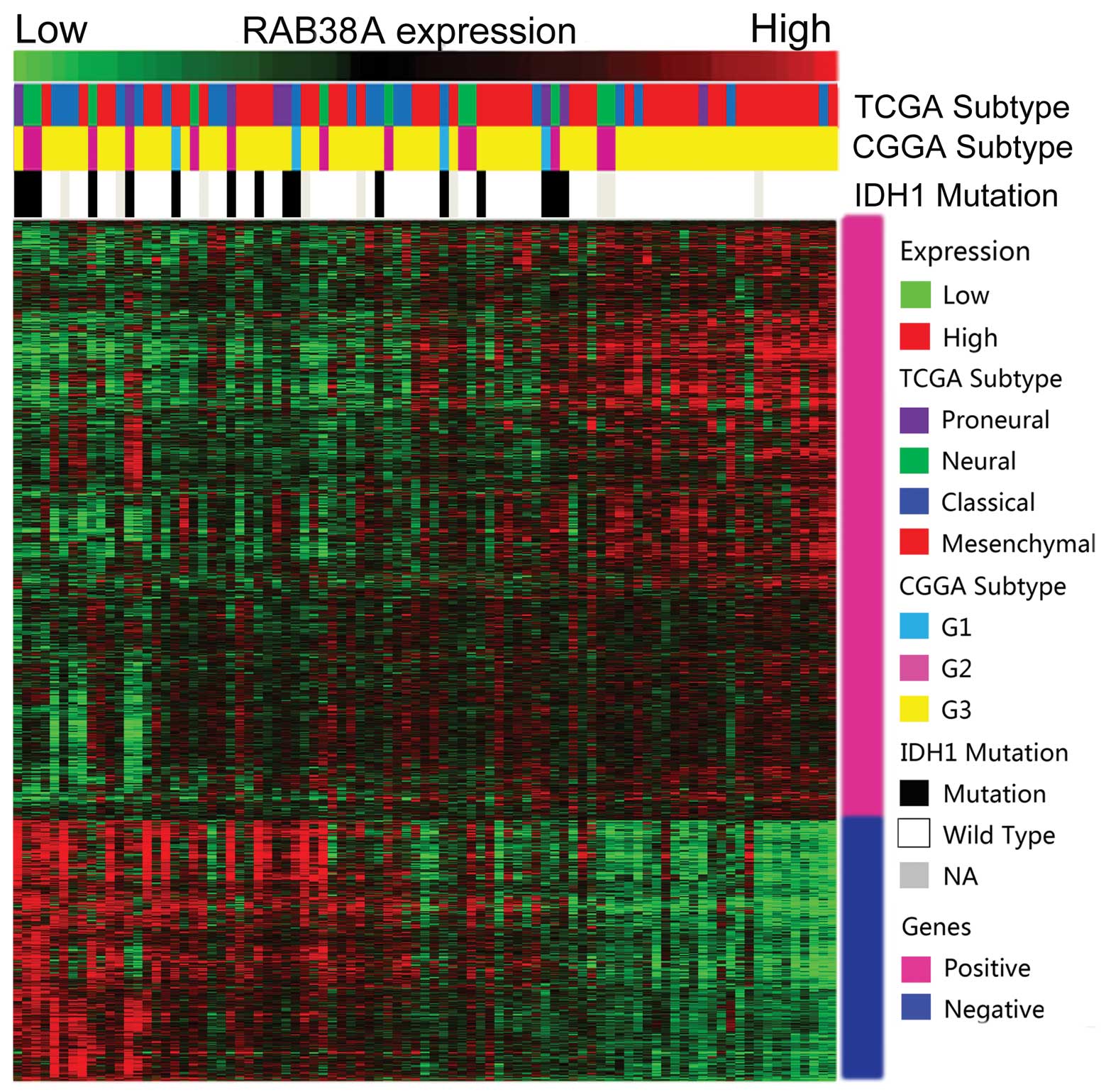
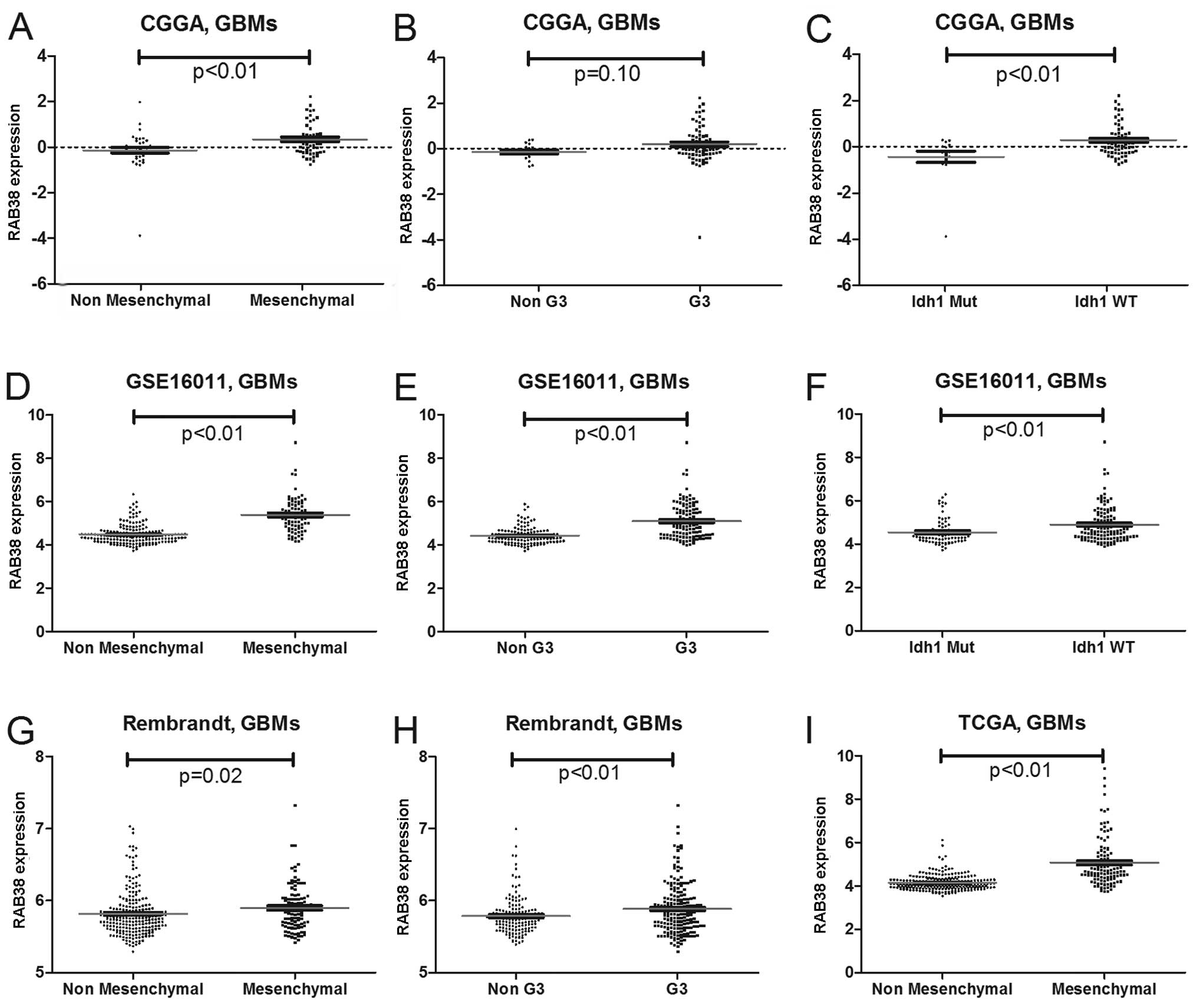
RAB38 expression showed a subtype
preference
As high RAB38 expression was mainly enriched in
HGGs, we screened its expression in different molecular subtypes of
GBMs. As previously reported, we annotated the 4 datasets by TCGA
and CGGA classification systems by PAM (2,11).
RAB38 with mesenchymal subtype and G3 subtype have high expression
level. Patients with IDH1 gene wild-type also showed higher
expression of RAB38 than those with mutation IDH1 gene (Fig. 4). The expression difference is shown
in Fig. 5.
RAB38 was significantly associated with
cell migration
Through Pearson correlation analysis, the
significant, positively correlated genes of the data from CGGA
(pink marked genes in Fig. 4) were
used for GO analysis. The top 10 GO terms listed in Table II show that RAB38 was associated
with cell migration. Using a P-value of <0.001, >40 biologic
processes mainly correlated to cell migration were significantly
enriched in the GBMs samples with RAB38 high expression.
 | Table IIGene sets increased in glioblastoma
samples with RAB38 overexpression. |
Table II
Gene sets increased in glioblastoma
samples with RAB38 overexpression.
| Name | Count | Fold-enrichment | P-value | FDR |
|---|
|
GO:0005576~extracellular region | 762 | 1.391647 | 2.22E-30 | 3.35E-27 |
| GO:0031226~intrinsic
to plasma membrane | 487 | 1.471373 | 1.07E-24 | 1.61E-21 |
| GO:0005887~integral
to plasma membrane | 475 | 1.467734 | 8.33E-24 | 1.26E-20 |
| GO:0005886~plasma
membrane | 1248 | 1.212935 | 1.04E-21 | 1.57E-18 |
| hsa04080:Neuroactive
ligand-receptor interaction | 140 | 2.012199 | 2.96E-21 | 3.68E-18 |
| GO:0031224~intrinsic
to membrane | 1711 | 1.1451 | 8.49E-19 | 1.28E-15 |
| GO:0007166~cell
surface receptor linked signal transduction | 651 | 1.317325 | 1.72E-18 | 3.29E-15 |
| GO:0016021~integral
to membrane | 1648 | 1.142082 | 1.46E-16 | 1.67E-13 |
| GO:0044459~plasma
membrane part | 753 | 1.254731 | 2.47E-15 | 3.67E-12 |
|
GO:0044421~extracellular region part | 370 | 1.414818 | 2.59E-15 | 3.85E-12 |
|
GO:0005576~extracellular region | 762 | 1.391647 | 2.22E-30 | 3.35E-27 |
| GO:0031226~intrinsic
to plasma membrane | 487 | 1.471373 | 1.07E-24 | 1.61E-21 |
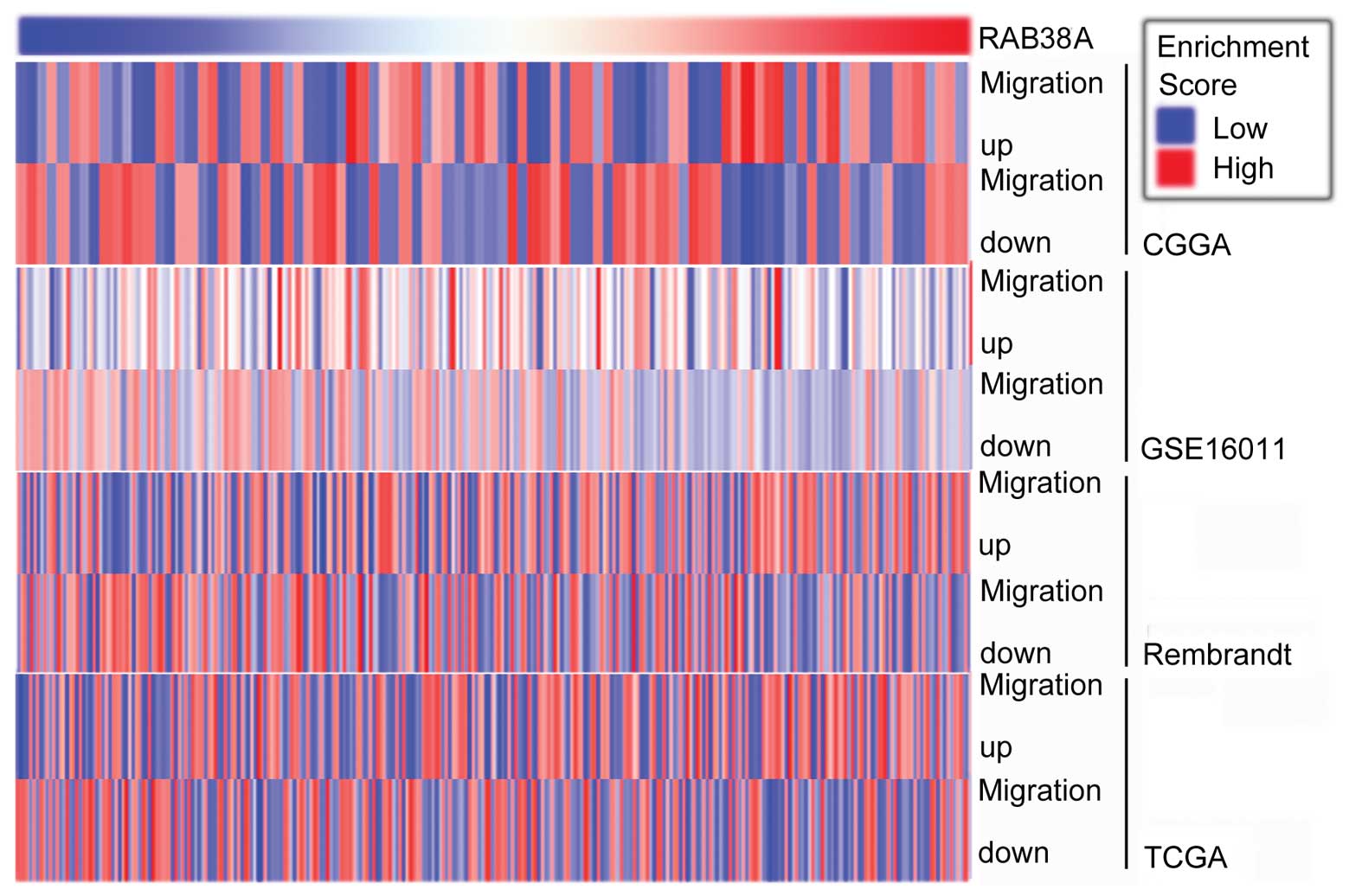
Correlation between the migration genes
and RAB38
Because RAB38 had a tight association with migration
in GO analysis, we performed GSVA with RAB38 expression (Fig. 6). Genes up- and downregulated in
migration were positively and negatively associated with RAB38
expression in CGGA and the other 3 validated datasets.
Discussion
In the present study, we found that mRNA level of
RAB38 shows high expression in HGGs of CGGA, this was further
validated in two published datasets. Protein level of RAB38 was in
accordance with mRNA detected by IHC. Prognostic analysis confirmed
that high expression of RAB38 indicates short survival time in
HGGs. GO and GSVA showed RAB38 was correlated with cell migration.
This is the first description of RAB38 in glioma.
Glioma is a diffusely infiltrating brain tumor, with
characteristic invasive patterns. The invasiveness is a major cause
of therapeutic failure, especially in GBM (15–17).
Applying the high throughput technologies, we can explore the
significant genes with aberrant expression in tumors and establish
their functions.
RAB GTPases mediate vesicle formation and vesicle
movement along with actin and tubulin and membrane fusion (18). This process is involved in cell
migration with lamellipodial formation. For specific recognition, a
vesicle and its target are coded by a unique compliment of RAB
GTPases (19). Thus, it is
important to understand the intracellular signaling of RAB proteins
in various cells to find new molecular approaches to cancer
biology. Some RAB proteins have been reported to be necessary for
the adhesion and migration of cancer cells. RAB5 and RAB21, are
associated with subunits of α-integrin, altering their endosomal
traffic and subcellular localizations (20). RAB27a and RAB27b were involved in
exocytosis of endocrine cells and were associated with the invasive
and metastatic potential of breast cancer. There are only few
reports on RAB GTPases in glioma (9,10).
RAB38 is a member of the RAB superfamily, its
mutation is associated with platelet dense granule storage pool
disease (1,21). The effect of RAB38 in tumors has not
been reported before. In the present study, we found that RAB38 was
highly expressed in HGGs compared to LGGs in mRNA microarray of
CGGA (Fig. 1). The different
expression levels were validated by microarray data from two other
published datasets. The protein level of RAB38 detected by IHC
resulted from an independent group of patients. From the above
results, we can conclude that RAB38 was a potential marker for
grading of gliomas. In the CGGA dataset, significant RAB38
positively correlated genes were analyzed by GO, the result showed
RAB38 functions mainly in promoting migration (Table II). GSVA, of the 4 datasets, also
showed supporting results.
The contribution of grade progression and survival
rate of tumors are very important for the clinical assessment.
Improved knowledge of the downstream mediators of RAB38 effects in
cancer cells may allow dissection of the different RAB38-induced
phenotypes and thereby generation of specific targeted therapies.
Analysis of the prognosis in CGGA and 3 other datasets found that
patients with higher expression of RAB38 showed a significantly
worse prognosis in gliomas. Hence, RAB38 was shown to be have
important effects and to have potential as a prognostic marker and
a therapeutic target. We also found the preference of expression of
RAB38 in mesenchymal subtype, G3 subtype or IDH1 mutation patients,
which was in concordance with the worst prognosis of high RAB38
expression patients.
Collectively, RAB38 expression was increased in
HGGs, mesenchymal and G3 subtype and wild-type IDH1. High RAB38
expression confered the worst prognosis in anaplastic gliomas and
glioblastomas. RAB38 is a novel, potential biomarker and
therapeutic target.
Acknowledgements
We would like to thank Dr Susan Furness for her
critical reading and Professor Y. Chen (Beijing Sanbo Brain
Hospital) for IHC technical support. This research was supported by
grants from the National High Technology Research and Development
Program (no.2012AA02A508), the International Science and Technology
Cooperation Program (no. 2012DFA30470), the National 973 Program
(no.2011CB707804) and the National Natural Science Foundation of
China (no. 81201993).
References
|
1
|
Osanai K and Voelker DR: Analysis and
expression of Rab38 in oculocutaneous lung disease. Methods
Enzymol. 438:203–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan W, Zhang W, You G, et al: Molecular
classification of gliomas based on whole genome gene expression: a
systematic report of 225 samples from the Chinese Glioma
Cooperative Group. Neuro Oncol. 14:1432–1440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao Z, Zhang C, Yan W, et al: BMP4, a
strong better prognosis predictor, has a subtype preference and
cell development association in gliomas. J Transl Med. 11:1002013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Recchi C and Seabra MC: Novel functions
for Rab GTPases in multiple aspects of tumour progression. Biochem
Soc Trans. 40:1398–1403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He H, Dai F, Yu L, et al: Identification
and characterization of nine novel human small GTPases showing
variable expressions in liver cancer tissues. Gene Expr.
10:231–242. 2002.PubMed/NCBI
|
|
6
|
Shimada K, Uzawa K, Kato M, et al:
Aberrant expression of RAB1A in human tongue cancer. Br J Cancer.
92:1915–1921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Culine S, Honore N, Closson V, et al: A
small GTP-binding protein is frequently overexpressed in peripheral
blood mononuclear cells from patients with solid tumours. Eur J
Cancer. 30A:670–674. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao R, Wang Y, Lubet RA and You M:
Differentially expressed genes associated with mouse lung tumor
progression. Oncogene. 21:5814–5821. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Zhou Y and Zhu K: Inhibition of
glioma cell lysosome exocytosis inhibits glioma invasion. PLoS One.
7:e459102012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Overmeyer JH, Young AM, Bhanot H and
Maltese WA: A chalcone-related small molecule that induces
methuosis, a novel form of non-apoptotic cell death, in
glioblastoma cells. Mol Cancer. 10:692011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verhaak RG, Hoadley KA, Purdom E, et al:
Integrated genomic analysis identifies clinically relevant subtypes
of glioblastoma characterized by abnormalities in PDGFRA, IDH1,
EGFR, and NF1. Cancer Cell. 17:98–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gravendeel LA, Kouwenhoven MC, Gevaert O,
et al: Intrinsic gene expression profiles of gliomas are a better
predictor of survival than histology. Cancer Res. 69:9065–9072.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanzelmann S, Castelo R and Guinney J:
GSVA: gene set variation analysis for microarray and RNA-Seq data.
BMC Bioinformatics. 14:72013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
R Core Team. A language and enviroment for
statistical computing. http://www.R-project.org.
R Foundation for Statistical Conputing; 2012, Vienna, Austria:
|
|
15
|
Senft C, Priester M, Polacin M, et al:
Inhibition of the JAK-2/STAT3 signaling pathway impedes the
migratory and invasive potential of human glioblastoma cells. J
Neurooncol. 101:393–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Chen L, Bao Z, et al: Inhibition
of STAT3 reverses alkylator resistance through modulation of the
AKT and β-catenin signaling pathways. Oncol Rep. 26:1173–1180.
2011.PubMed/NCBI
|
|
18
|
Stenmark H and Olkkonen VM: The Rab GTPase
family. Genome Biol. 2:Reviews 30072001.PubMed/NCBI
|
|
19
|
Barr FA: Rab GTPase function in Golgi
trafficking. Semin Cell Dev Biol. 20:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Subramani D and Alahari SK:
Integrin-mediated function of Rab GTPases in cancer progression.
Mol Cancer. 9:3122010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ninkovic I, White JG, Rangel-Filho A and
Datta YH: The role of Rab38 in platelet dense granule defects. J
Thromb Haemost. 6:2143–2151. 2008. View Article : Google Scholar : PubMed/NCBI
|