Introduction
Lung cancer is the most common cause of
cancer-related mortality worldwide. The primary reason for the
difficulty in treating lung cancer is that it is mostly identified
at a very late stage. Lymphogenous or hematogenous metastasis
occurs in early stage (1). The
majority of patients with advanced non-small cell lung cancer
(NSCLC) are treated with combination therapy including a
platinum-based compound (2). The
major barriers limiting the use and efficacy of cisplatin (CDDP)
are toxicity and resistance (3).
Therefore, finding new therapeutic agents is of great clinical
interest in lung cancer research. Preclinical tumor models are a
fundamental component of study and design of new regimens for
cancer treatment (4). Considerable
efforts have been made to develop more clinically relevant models
by using orthotopic implantation models. An organ-specific site
presumably provides tumor cells with the most appropriate
environment for local growth and metastasis. Orthotopic models are
likely to provide more relevant pharmacokinetic and
pharmacodynamics information than subcutaneous models (5). We established an orthotopic implanted
SCID mouse model of lung cancer without thoracotomy (1,6–11). Our
model was simple, easy and reproducible. Many models can be
produced at once. The main downside of the orthotopic model is that
tumor size or volume changes are more difficult to continuously
monitor reproducibility, except at necropsy (5,12).
Thus, small animal imaging, which is a non-invasive and repeatable
method for monitoring the course of disease under therapy, has
become increasingly important (13,14).
One disadvantage of using anatomical imaging techniques such as
computed tomography (CT) and magnetic resonance imaging (MRI) in
monitoring the tumor size as a response to treatment is the amount
of time it requires before a volume response becomes evident.
Therefore, new biological measurements suggest using functional
fluorodeoxyglucose (FDG) PET for measurement of treatment effects
(15). FDG is internalized into
cells, where it is subsequently phosphorylated by the enzyme
hexokinase. Thus, the accumulation of FDG-6-phosphate observed in
PET scans is correlated with cellular glucose metabolism (16). Neoplastic cells usually exhibit
increased anaerobic metabolism, which is associated with trapping
FDG within the cells (17).
Furthermore, the therapeutic response of tumor cells is generally
correlated with their metabolic changes and these appear earlier
than conventional morphological changes. Thus, functional PET
images can document therapeutic success earlier than morphological
techniques such as CT and MRI (18,19).
In the present study, we evaluated the utility of FDG positron
emission tomography-computed tomography (PET-CT) to non-invasively
and repeatedly monitor the inhibitory effect of CDDP on lung cancer
in an orthotopic SCID mouse model, in order to establish a standard
model for examining novel regimens in lung cancer treatment.
Materials and methods
Animal model
Male SCID mice (CB-17/Icr-scidJc1; Clea Japan, Inc.,
Tokyo, Japan) 6–8 weeks of age were used in the present study, and
were maintained in the Laboratory for Animal Experiments. The
protocols of all animal experiments were approved by the
Institutional Animal Care and Use Committee of the University of
Tokushima, School of Medicine, and were carried out according to
their guidelines.
Cells line
Human NSCLC cell lines Ma44 were kindly provided by
Dr N. Masuda and Dr Y. Takada (Osaka Prefecture Habikino Hospital,
Osaka, Japan). An Ma44-3 cell line was cloned in our laboratory
using the limiting dilution method. This cell line was cultured in
RPMI-1640 (Sigma Chemical Co., St. Louis, MO, USA) with 10% heat
inactivated fetal bovine serum (BioWhittaker, Walkersville, MD,
USA) and maintained at 37°C in a humidified incubator with 5%
CO2 in air.
Orthotopic intrapulmonary
implantation
As in our previous studies (1,7,8), the
mice were fully anesthetized by ether inhalation, and placed in the
right lateral decubitus with the four limbs restrained. A 1 cm
transverse incision was made on the left lateral skin just below
the inferior border of the scapula of the SCID mouse. Muscles were
separated from the ribs by sharp dissection, and intercostal
muscles were exposed. The left lung was visible through the
intercostal muscles. A 30-gauge needle was inserted ~5 mm into the
lung through the intercostal muscle, and an inoculum of
2×106 tumor cells/ml with 400 mg/ml Matrigel
(Collaborative Biomedical Products, Bedford, Canada) was then
dispersed into the left lung in a final volume of 10 μl
(2×104 cells) medium. The procedure required ~1 min for
completion and was easily performed. The skin incision was closed
with 3-0 silk. In validation of in vivo FDG uptake,
orthotopic implantation was carried out in the left lung of the
mice. In monitoring of in vivo response of CDDP and control
group, the implantation was performed in the right lung to clearly
delineate tumor uptake from heart uptake.
Micro PET and micro CT imaging
The mice were injected intravenously with 10.0±0.3
(means ± SD) MBq FDG. Mice were fasted overnight before each FDG
PET scan (20). One hour after
tracer injection, mice were anesthetized with 3% sevofluran (Abbott
Scandinavia AB, Solna, Sweden) mixed with 35% O2 in
N2 and fixed on a bed in the presence of three fiducial
markers allowing fusion of PET and CT images. A PET scan was
acquired using a micro PET Focus 120 followed by a micro CT scan
acquired with a micro CATWII system (both from Siemens Medical
Solutions, Malvern, PA, USA), as previously described (21). PET data were arranged into sinograms
and subsequently reconstructed with the maximum a posteriori (MAP)
reconstruction algorithm. The pixel size was 0.866 × 0.866 × 0.796
mm and in the center field of view the resolution was 1.2 mm
full-width-at-half-maximum. PET and micro CT images were fused in
the Inveon software (Siemens Medical Solutions). Before fusion,
regions of interest (ROIs) were drawn on the CT images manually by
qualitative assessment covering the whole tumors and subsequently
tumor volume and tracer uptake assessed by standard uptake value
(SUV) was generated by summation of voxels within the tomographic
planes. SUV was calculated according to the formula
(CT*W)/Dinj, where CT is tissue
radioactivity concentration, W is weight of the animal and
Dinj is injected dose. SUV max was calculated from the
voxel with the highest tracer concentration.
Histologic evaluation
SCID mice used for validation were sacrificed on day
10, the control group on day 21 and the CDDP group on day 28 after
implantation by ether inhalation and cervical dislocation. Major
organs (bilateral lungs, heart and mediastinal tissues) were
removed, fixed in 10% formalin and embedded in paraffin. Five
micrometer histologic sections were made from the lung and
mediastinal tissues at 3-mm intervals. Paraffin sections stained
with hematoxylin and eosin were examined with a microscope.
Experimental design
In vivo validation of FDG PET-CT
uptake
In vivo uptake of FDG in human lung cancer in
the orthotopic SCID mouse model was evaluated by injecting Ma44-3
cell line into the left lungs of 6 SCID mice. Tumor volume and SUV
max were calculated for all mice with FDG PET-CT (3 on day 9 and 3
on day 10) after implantation. All SCID mice were sacrificed at 10
days after implantation for histopathologic analysis.
Response monitoring
In vivo response monitoring was carried out
by injecting Ma44-3 cell line into the right lungs of 6 SCID mice,
which were divided into 2 groups; the control group (n=3) and the
treatment group (n=3). Treatment mice were intraperitoneally
injected with CDDP (7 mg/kg body weight) on day 6 after
implantation. FDG PET-CT was made on day 6, 8, 13 and 25 after
implantation. Tumor volume and SUV max were calculated for all
mice. The body weight of all mice was measured weekly to monitor
the toxicity of CDDP. Control group mice were sacrificed at day 21,
CDDP group mice were sacrificed at day 28 after implantation for
histopathologic analysis.
Statistical analysis
Comparison of tumor volume and SUV max between
control group and CDDP group was calculated using unpaired
Student’s t-test. Correlation between SUV max, tumor volume and
tumor length was calculated using linear regression. P<0.05 was
considered to indicate a statistically significant difference.
Results
In vivo validation of FDG PET-CT
uptake
CT images clearly delineated tumors growing in SCID
mice. Fusion of CT and PET images allowed the high uptake areas of
PET scan to be matched with corresponding anatomical structure.
Intense FDG uptake was demonstrated in the tumor in the left lung.
Tumor volume, SUV max and tumor length of the sacrificed mice were:
tumor volume, 24.3±9.3 mm3; SUV max, 1.2±0.3 and tumor
length, 2.9±0.87 mm, as shown in Table
I.
 | Table ITumor volume, SUV and tumor length of
6 mice in validation of FDG uptake. |
Table I
Tumor volume, SUV and tumor length of
6 mice in validation of FDG uptake.
| Mice | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Tumor volume (CT,
mm3) | 24.40 | 9.30 | 14.60 | 33.80 | 33.90 | 25.60 |
| SUV max | 1.10 | 0.75 | 1.28 | 1.37 | 1.62 | 1.33 |
| Tumor length
(specimen, mm) | 2.50 | 1.90 | 2.50 | 3.40 | 4.40 | 2.90 |
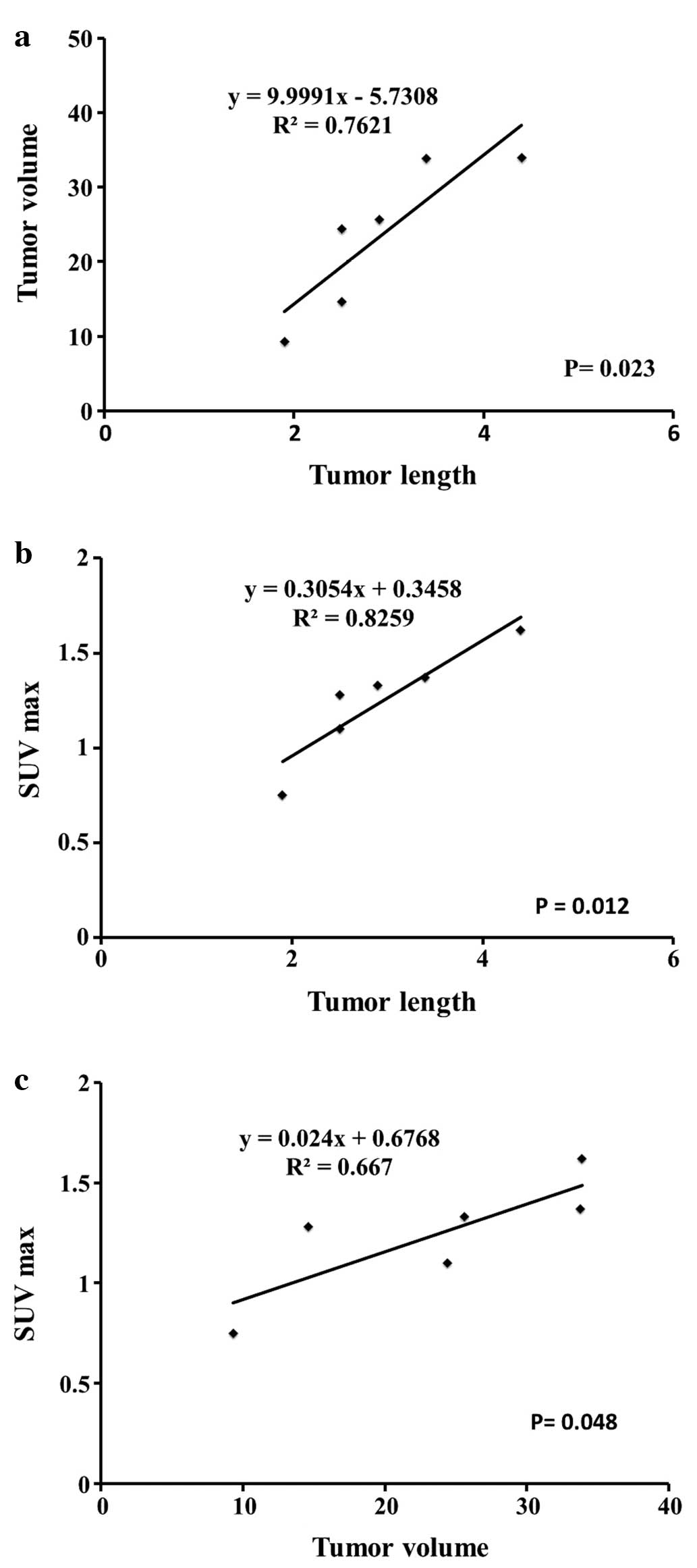
Both tumor volume and SUV max were significantly
correlated with postmortem tumor length measured in specimens of 6
mice (r2=0.7621, P=0.023 and r2=0.8259,
P=0.012, respectively), as shown in Fig. 1A and B. Tumor volume and SUV max of
6 mice were significantly correlated (r2=0.6669,
P=0.048) as shown in Fig. 1C.
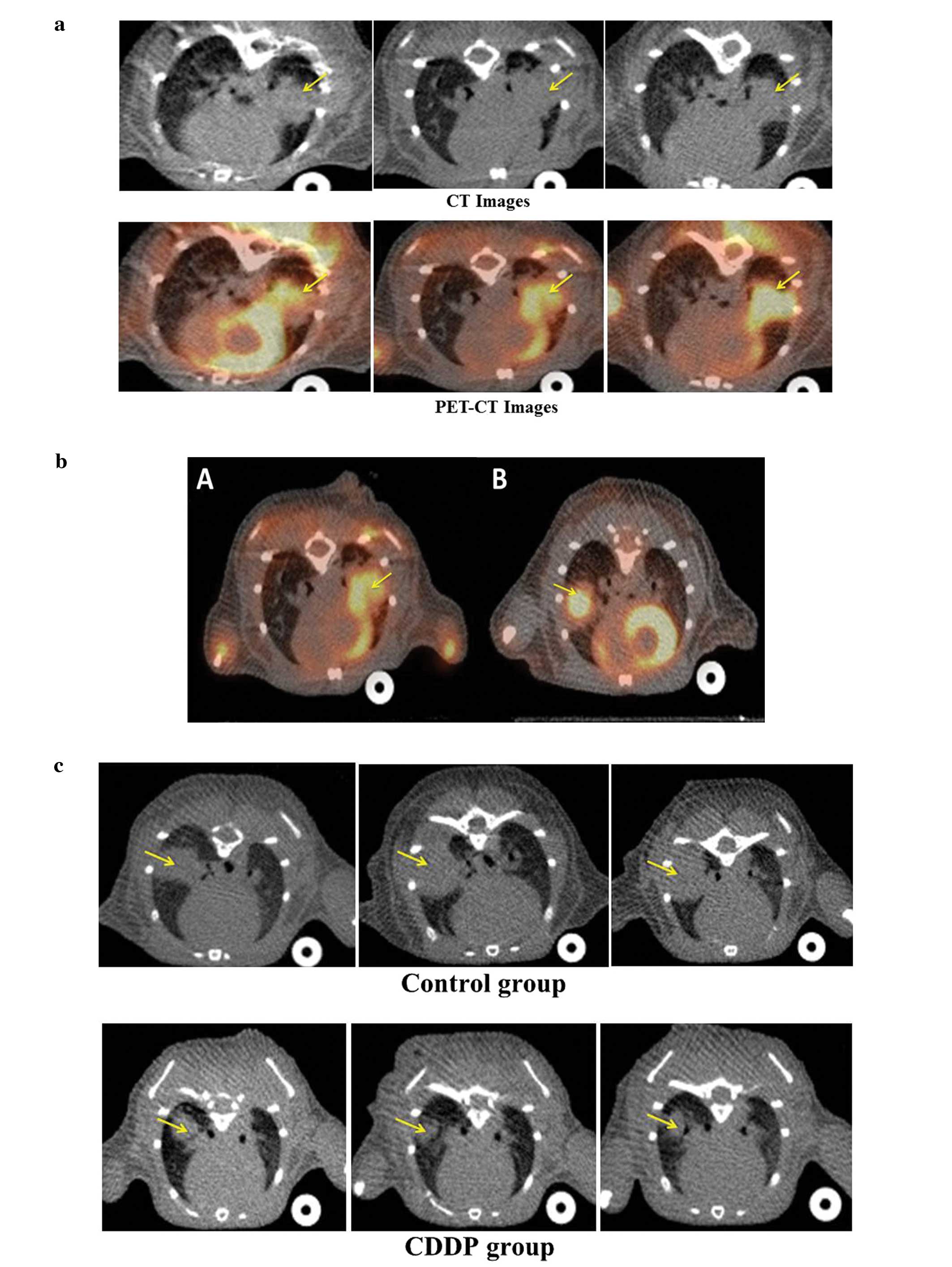
Representative CT, PET-CT images are shown in Fig. 2A.
As FDG uptake of tumor in the left lung was
overlapped by physiologic FDG uptake of the left ventricle,
measuring a precise FDG uptake of tumor in the left lung was
slightly difficult. We sought to change the implantation to the
right side. The FDG uptake of tumor in the right lung was not
overlapped by that of the left ventricle as shown in Fig. 2B.
Response monitoring
Representative CT, PET-CT images of the control and
the CDDP group are shown in Fig. 2C and
D. Tumor volume, SUV max and tumor length of CDDP and control
mice are shown in Table II.
 | Table IITumor volume, SUV and tumor length of
implanted mice in CDDP and control group. |
Table II
Tumor volume, SUV and tumor length of
implanted mice in CDDP and control group.
| CDDP group | Control group |
|---|
| Tumor volume | M3 | M5 | M6 | M4 | M7 | M11 |
| Baseline | 6.5 | 2.9 | 4.2 | 3.7 | 5.3 | 3.2 |
| 8 Days | 7.1 | 3.1 | 6.1 | 8.6 | 14.3 | 6.1 |
| 13 Days | 10 | 3.2 | 7.1 | 25.6 | 66 | 69.8 |
| SUV max | M3 | M5 | M6 | M4 | M7 | M11 |
| Baseline | 0.552 | 0.397 | 0.43 | 0.47 | 0.49 | 0.46 |
| 8 Days | 0.483 | 0.383 | 0.543 | 0.642 | 0.675 | 0.606 |
| 13 Days | 0.621 | 0.355 | 0.521 | 1.102 | 1.206 | 1.391 |
| Tumor length | 8.8 | 7.6 | 6.5 | 7.7 | Friable tumor | 11.2 |
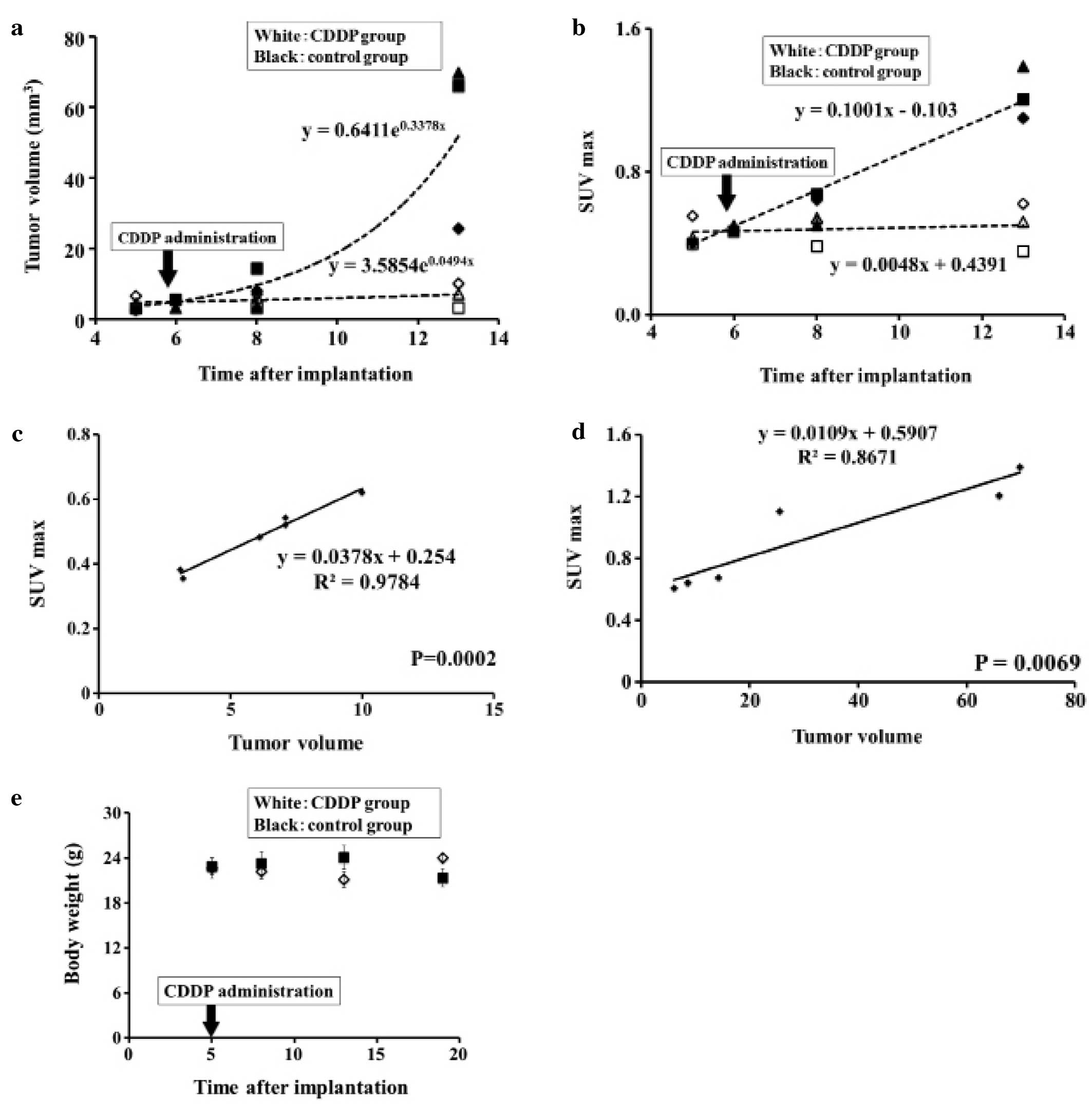
Tumor volume
Tumor volumes of the CDDP group were lower than the
control group on day 8 (5.43±2.08 vs. 9.6±4.2 mm3)
although the difference was insignificant. Tumor volumes of the
CDDP group were significantly lower than the control group on day
13 (6.76±3.4 vs. 53.8±24.4 mm3, P=0.03). Both groups
were quite similar on baseline (4.53±1.8 vs. 4.06±1.09
mm3) as shown in Fig.
3A.
SUV max
SUV max of the CDDP group was significantly lower
than that of the control group on day 8 (0.469±0.08 vs. 0.641±0.03,
P=0.02) and on day 13 (0.499±0.13 vs. 1.233±0.14, P=0.003). Both
groups were quite similar on baseline (0.456±0.08 vs. 0.474±0.015)
as shown in Fig. 3B. SUV max of the
CDDP group was elevated on day 25 reaching 1.696±0.12 (data not
shown).
Correlation between SUV max and tumor
volume measured by CT images
SUV max of the CDDP group was significantly
correlated with tumor volume measured by CT (r2=0.9784,
P=0.0002) as shown in Fig. 3C. SUV
max of the control group was significantly correlated with tumor
volume measured by CT (r2=0.8671, P=0.0069) as shown in
Fig. 3D.
Correlation between SUV max and tumor
length measured in specimens
SUV max of the CDDP group was significantly
correlated with tumor length measured in specimens (P=0.0093). In
the control group, one mouse died before being sacrificed and tumor
tissue was too friable to prepare slide. The number of mice became
too few to test for significant correlation.
Body weight
There were no marked changes in body weight between
the CDDP group and the control group until day 8 after
implantation. The body weight of the mice in the CDDP group on day
13 was significantly lower than that in the control group (21.133
vs. 24.067 g, P=0.017). However, the body weight of the mice in the
CDDP group on day 19 was significantly higher than that in the
control group (24.00 vs. 21.350 g, P=0.018) as shown in Fig. 3E.
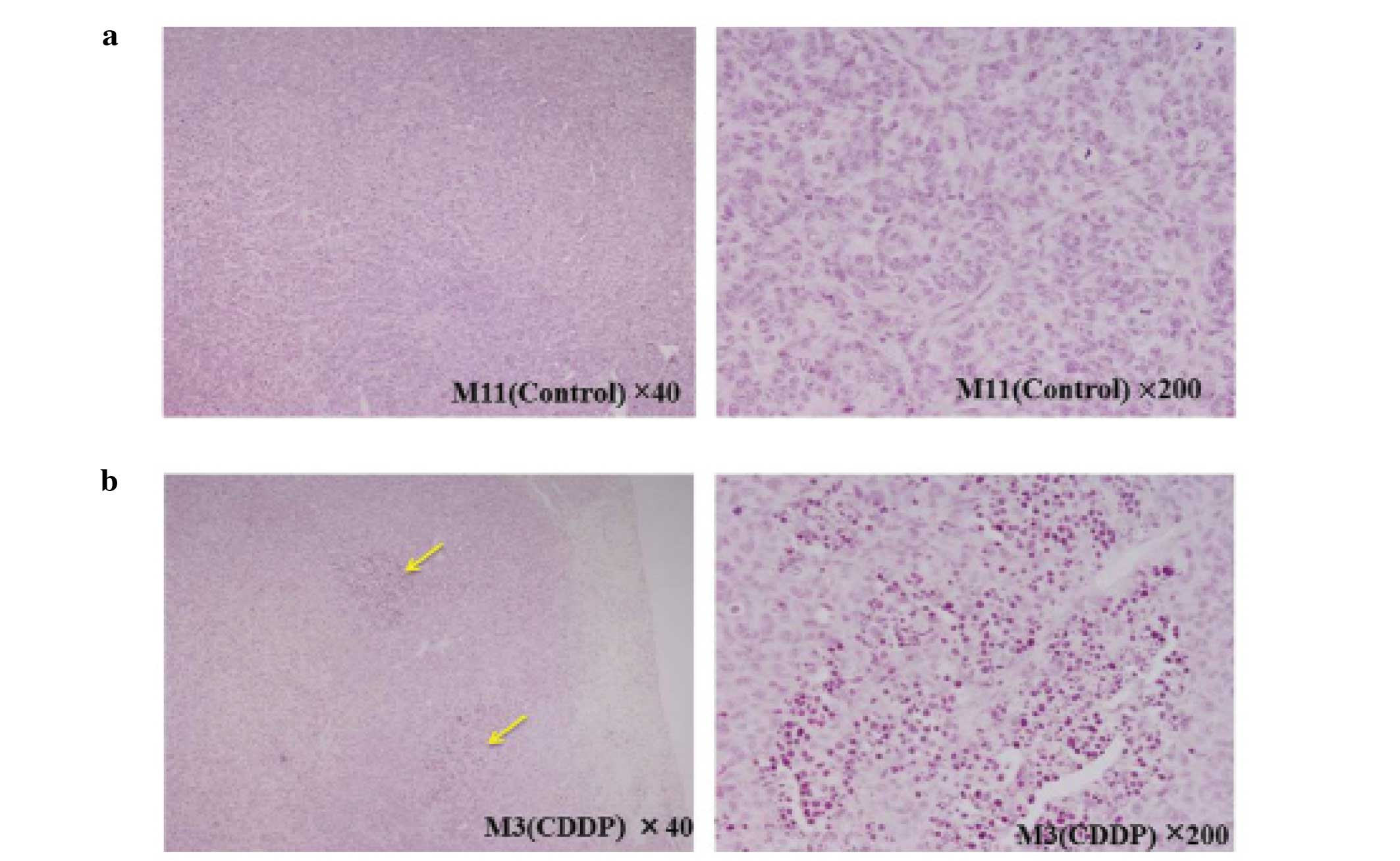
Histopathology
Tumors were histopathologically confirmed in the
lungs of all mice. In the CDDP group specimens, we observed some
regions where most of the tumor cells showed nuclear pycnosis
consistent with necrosis. However, there was no such region in the
control group (Fig. 4A and B). The
tumor length measured in specimens for the CDDP group (sacrificed
on day 28) was 1.696±0.15, the tumor length measured in specimens
for the control group (sacrificed on day 21) was 1.24±0.14, one
mouse of the control group died before being sacrificed and the
tumor was too friable to be prepared for histopathological
slides.
Discussion
In 1889, Paget postulated that an organ-specific
site presumably provides tumor cells with the most appropriate
environment for local growth and metastasis ‘seed and soil’ theory
(22). Considerable effort has been
made to develop more clinically relevant models by the use of
orthotopic implantation models (5).
We have established an orthotopic lung cancer model (1,6–11). Our
model was simple, easy and reproducible. Many models can be
produced at once. The main downside of this model is that tumor
size or tumor volume changes are more difficult to continuously
monitor reproducibility, except at necropsy (5,12).
Thus, to overcome this shortcoming we used small animal imaging,
which is a non-invasive and repeatable method for monitoring the
course of disease under therapy.
The present study demonstrated the feasibility of
using FDG PET-CT to evaluate repeatedly and non-invasively tumor
progression in an orthotopic lung cancer model. Validation of in
vivo FDG uptake in the orthotopic lung cancer model revealed
that FDG uptake predicted tumor volume and size. Both tumor volume
and SUV max were significantly correlated with postmortem tumor
length measured in specimens (P=0.023, P=0.012). Tumor volume and
SUV max were significantly correlated (P=0.048). SUV max was more
sensitive than tumor volume in prediction of tumor size. Therefore,
the present study confirmed that FDG PET-CT is a valuable and
reliable tool in staging progression of lung cancer in an
orthotopic model without needing to sacrifice more mice. Many
studies reported that combined PET-CT allows the acquisition of
functional PET images and morphological CT images, thus the areas
of tracer uptake can be better localized to the corresponding
anatomical structure, resulting in improved sensitivity and
specificity in tumor imaging (23–25).
The present study demonstrated the reproducibility
of FDG PET-CT in monitoring repeatedly and non-invasively the
inhibitory effect of CDDP on tumor growth in an orthotopic model
without the need to sacrifice more mice. SUV max of the CDDP group
was significantly lower than the control group on day 8 (P=0.02)
and on day 13 (P=0.003). Tumor volume of the CDDP group was lower
than the control group on day 8 and significantly lower on day 13
(P=0.03). Changes in FDG signal appeared earlier than changes in
tumor volume by CT; significant difference in SUV max between CDDP
group and control group on day 8 (P=0.02) appeared earlier than
tumor volume that showed no significant difference on day 8, and
that was consistent with the general concept that metabolic changes
appear earlier than morphological changes (26,27).
Thus, the present study may overcome the drawbacks of tumor volume
changes which occur late after therapy impeding quick decision in
case of non-response. In the treatment group, there was no
regression in tumor volume, but static or minor elevation very
close to baseline. As we used CDDP as a single agent it will be
very difficult to cause tumor shrinkage. Therefore, it may exert
tumor stasis rather than tumor shrinkage. Several novel anticancer
drugs are cytostatic and do not necessarily lead to reduction of
tumor volume, but of viable tumor tissue (28). Identification of the effect with
drugs exerting tumor stasis can be difficult, as the conventional
anatomical imaging modalities CT and MRI measure treatment effect
by assessing changes in tumor size. A tumor stasis effect of
anticancer treatment can consequently be missed by these anatomical
imaging modules. Therefore, identification of biological biomarkers
is of great value in treatment regimens involving tumoristatic
compounds (29). In the present
study, FDG uptake changes following treatment predicted changes in
tumor volume and size supporting the potential use of SUV max as a
predictive parameter of tumor response to chemotherapy and a
sensitive marker for tumor staging in preclinical trials.
SUV max of the CDDP group increased to 1.69±0.12 on
day 25; we can explain the increase in SUV max in our results by
our use of CDDP as monotherapy and it may be less effective and
does not inhibit glucose uptake in the most aggressive parts of the
tumor. CDDP monotherapy can maintain reduction in FDG uptake until
day 13 after implantation but cannot maintain reduction until day
25. This may be due to the proliferation of CDDP-resistant cells
after most sensitive cells were killed during the early therapeutic
response.
The right side implantation was better in the
discrimination of tumor uptake from the heart uptake than the left
side. SUV max of the right side was significantly correlated with
tumor volume (P=0.0002, P=0.0069) compared with that in the left
side (P=0.048).
In our model, we detected CDDP toxicity by measuring
mouse body weight; the body weight of the CDDP group on day 13 was
significantly lower than that of the control group due to CDDP
toxicity (P=0.017). However, the body weight of the CDDP group on
day 19 was significantly higher than that of the control group
(P=0.018) due to cachexia associated with advanced tumor.
In conclusion, the present study provided additional
support for using FDG PET-CT in the detection of tumor progression
and therapeutic response of lung cancer in an orthotopic model
non-invasively and repeatedly. Furthermore, our design overcame the
drawbacks of orthotopic models and traditional anatomical imaging
modalities.
Acknowledgements
This study was supported by the Grants-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology (24659634).
Abbreviations:
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
FDG
|
fluorodeoxyglucose
|
|
CDDP
|
cisplatin
|
|
NSCLC
|
non-small cell lung cancer
|
|
MRI
|
magnetic resonance imaging
|
|
SUV max
|
maximal standardized uptake value
|
References
|
1
|
Fujino H, Kondo K, Ishikura H, et al:
Matrix metalloproteinase inhibitor MMI-166 inhibits lymphogenous
metastasis in an orthotopically implanted model of lung cancer. Mol
Cancer Ther. 4:1409–1416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oliver TG, Mercer KL, Sayles LC, et al:
Chronic cisplatin treatment promotes enhanced damage repair and
tumor progression in a mouse model of lung cancer. Genes Dev.
24:837–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Francia G, Cruz-Munoz W, Man S, et al:
Mouse models of advanced spontaneous metastasis for experimental
therapeutics. Nat Rev Cancer. 11:135–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyoshi T, Kondo K, Ishikura H, et al:
SCID mouse lymphogenous metastatic model of human lung cancer
constructed using orthotopic inoculation of cancer cells.
Anticancer Res. 20:161–163. 2000.PubMed/NCBI
|
|
7
|
Ishikura H, Kondo K, Miyoshi T, et al:
Artificial lymphogenous metastatic model using orthotopic
implantation of human lung cancer. Ann Thorac Surg. 69:1691–1695.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishikura H, Kondo K, Miyoshi T, et al:
Suppression of mediastinal metastasis by uracil-tegafur or
cis-diamminedichloroplatinum(II) using a lymphogenous
metastatic model in a human lung cancer cell line. Clin Cancer Res.
7:4202–4208. 2001.PubMed/NCBI
|
|
9
|
Fujino H, Kondo K, Miyoshi T, et al:
Establishment of patient-like SCID mouse model by orthotopically
implanting primary cultured cells from surgically-resected lung
cancer tissues. Oncol Rep. 10:1709–1715. 2003.PubMed/NCBI
|
|
10
|
Ishikura H, Kondo K, Miyoshi T, et al:
Green fluorescent protein expression and visualization of
mediastinal lymph node metastasis of human lung cancer cell line
using orthotopic implantation. Anticancer Res. 24:719–723.
2004.
|
|
11
|
Kondo K, Fujino H, Miyoshi T, et al:
Orthotopically implanted SCID mouse model of human lung cancer
suitable for investigating metastatic potential and anticancer drug
effects. Oncol Rep. 12:991–999. 2004.PubMed/NCBI
|
|
12
|
Zhang X and Wu J: Establishing of the
transplanted animal models for human lung cancer. JNMU. 23:1–5.
2009.
|
|
13
|
Myers R: The biological application of
small animal PET imaging. Nucl Med Biol. 28:585–593. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chatziioannou AF: Molecular imaging of
small animals with dedicated PET tomographs. Eur J Nucl Med Mol
Imaging. 29:98–114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wahl RL, Jacene H, Kasamon Y, et al: From
RECIST to PERCIST: evolving considerations for PET response
criteria in solid tumors. J Nucl Med. 50(Suppl 1): 122S–150S. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pauwels EK, Sturm EJ, Bombardieri E, et
al: Positron-emission tomography with
[18F]fluorodeoxyglucose. Part I Biochemical uptake
mechanism and its implication for clinical studies. J Cancer Res
Clin Oncol. 126:549–559. 2000.
|
|
17
|
Kostakoglu L, Hardoff R, Mirtcheva R and
Goldsmith SJ: PET-CT fusion imaging in differentiating physiologic
from pathologic FDG uptake. Radiographics. 24:1411–1431. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dose Schwarz J, Bader M, Jenicke L, et al:
Early prediction of response to chemotherapy in metastatic breast
cancer using sequential 18F-FDG PET. J Nucl Med.
46:1144–1150. 2005.PubMed/NCBI
|
|
19
|
von Falck C, Maecker B, Schirg E, et al:
Post transplant lymphoproliferative disease in pediatric solid
organ transplant patients: a possible role for
[18F]-FDG-PET(/CT) in initial staging and therapy
monitoring. Eur J Radiol. 63:427–435. 2007.PubMed/NCBI
|
|
20
|
Fueger BJ, Czernin J, Hildebrandt I, et
al: Impact of animal handling on the results of 18F-FDG
PET studies in mice. J Nucl Med. 47:999–1006. 2006.PubMed/NCBI
|
|
21
|
Jensen MM, Erichsen KD, Björkling F, et
al: Early detection of response to experimental chemotherapeutic
Top216 with [18F]FLT and [18F]FDG PET in
human ovary cancer xenografts in mice. PLoS One.
5:e129652010.PubMed/NCBI
|
|
22
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 133:571–573. 1889.
View Article : Google Scholar
|
|
23
|
Beyer T, Townsend DW and Blodgett TM:
Dual-modality PET/CT tomography for clinical oncology. Q J Nucl
Med. 46:24–34. 2002.PubMed/NCBI
|
|
24
|
Ell PJ and von Schulthess GK: PET/CT: a
new road map. Eur J Nucl Med Mol Imaging. 29:719–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tatsumi M, Nakamoto Y, Traughber B, et al:
Initial experience in small animal tumor imaging with a clinical
positron emission tomography/computed tomography scanner using
2-[F-18]fluoro-2-deoxy-D-glucose. Cancer Res. 63:6252–6257.
2003.PubMed/NCBI
|
|
26
|
Kawada K, Murakami K, Sato T, et al:
Prospective study of positron emission tomography for evaluation of
the activity of lapatinib, a dual inhibitor of the ErbB1 and ErbB2
tyrosine kinases, in patients with advanced tumors. Jpn J Clin
Oncol. 37:44–48. 2007. View Article : Google Scholar
|
|
27
|
Kostakoglu L and Goldsmith SJ:
18F-FDG PET evaluation of the response to therapy for
lymphoma and for breast, lung, and colorectal carcinoma. J Nucl
Med. 44:224–239. 2003.
|
|
28
|
Barthel H, Cleij MC, Collingridge DR, et
al: 3′-Deoxy-3′-[18F]fluorothymidine as a new marker for
monitoring tumor response to antiproliferative therapy in vivo with
positron emission tomography. Cancer Res. 63:3791–3798. 2003.
|
|
29
|
Stimson L and La Thangue NB: Biomarkers
for predicting clinical responses to HDAC inhibitors. Cancer Lett.
280:177–183. 2009. View Article : Google Scholar : PubMed/NCBI
|