Introduction
Major risk factors of head and neck squamous cell
carcinoma (HNSCC) are environmental factors, typified by tobacco
and alcohol abuse, although the carcinogenesis of a number of
malignancies, particularly in oropharyngeal sites, is associated
with human papillomavirus (HPV) infection. The number of oral and
pharyngeal cancers considered to be caused by HPV infection is
increasing in the USA (1). Although
oncogenic HPV was detected in 25.9% of all HNSCCs worldwide
(2), high-risk HPV type 16 DNA was
detected in up to 72% in oropharyngeal sites and the infection rate
of non-smokers with oropharyngeal squamous cell carcinoma (OPSCC)
is 15-fold higher than that of smokers (3,4). Some
reports have shown that the prognosis for patients with HPV-induced
HNSCC is more favorable than that for patients with HNSCC caused by
environmental factors (5–10). In general, the more
poorly-differentiated a tumor, the more aggressive its behavior
(11) Paradoxically, most
HPV-positive HNSCCs are histologically graded as poorly
differentiated in spite of their favorable clinical outcomes.
E-cadherin is a cell-cell adhesion molecule and a
representative molecular marker of epithelial cells. The loss of
E-cadherin expression is a distinctive event in
epithelial-mesenchymal transition (EMT), which is a process
involving a high degree of cellular plasticity and a large number
of distinct genetic and epigenetic alterations, as well as the
conversion of differentiated epithelial cells into poorly
differentiated, migratory and invasive mesenchymal cells (12–14).
E-cadherin is often heterogeneously expressed in HNSCCs. In oral
SCCs, E-cadherin is highly expressed at the centre of the tumor,
with the expression gradually decreasing toward the invasive front
(15,16). Vimentin is highly expressed in
mesenchymal cells and is generally used as the molecular marker to
identify cancer cells undergoing EMT (17).
In the present study, we investigated the expression
of the EMT markers and analyzed their correlation with HPV status
and prognosis in order to clarify the reason for the paradoxical
treatment response of HPV-positive OPSCCs.
Patients and methods
The study group comprised 79 Japanese patients who
were diagnosed with OPSCC and treated at Hokkaido University
Hospital, Japan, between 1998 and 2010. The main clinical
characteristics of the patients are shown in Table I. The subjects included 69 men and
10 women with a mean age of 62.9 years (range, 40–85 years).
Demographic and clinicopathological data, including age, gender,
smoking history, tumor stage and clinical outcomes, were obtained
from the patient charts. All patients were treated with surgery,
radiotherapy or chemoradiotherapy. Sixteen patients were treated
with surgery, 15 were treated using radiotherapy at a total dose of
65–70 Gy, with 50 Gy received concurrently with chemoradiotherapy
(39 of these were treated with concomitant platin-based
chemoradiotherapy and 11 were treated with concomitant docetaxel
radiotherapy). Median follow-up time was 54 months for surviving
patients, and 43 months when deceased patients were included. The
present study was approved by the Institutional Review Board of
Hokkaido University Hospital (013-0271).
 | Table IClinicopathological features of all 79
patients. |
Table I
Clinicopathological features of all 79
patients.
| Patients
N=79 | HPV-pos
N=23 | HPV-neg
N=56 | P-value |
|---|
| Gender | | | | NS |
| Male | 69 | 18 | 51 | |
| Female | 10 | 5 | 5 | |
| Age, years (median,
63) | | | | NS |
| <63 | 34 | 13 | 21 | |
| ≥63 | 45 | 10 | 35 | |
| Grade | | | | 0.0074a |
| Well | 14 | 0 | 14 | |
| Moderate | 49 | 17 | 32 | |
| Poor | 15 | 6 | 9 | |
| Unknown | 1 | 0 | 1 | |
| TNM stage | | | | 0.0162b |
| I | 7 | 1 | 6 | |
| II | 11 | 0 | 11 | |
| III | 22 | 7 | 15 | |
| IV | 39 | 15 | 24 | |
| Primary tumor (T
class) | | | | NSc |
| 1 | 11 | 3 | 8 | |
| 2 | 31 | 9 | 22 | |
| 3 | 23 | 8 | 15 | |
| 4 | 11 | 1 | 10 | |
| X | 4 | 2 | 2 | |
| Lymph nodes (N
class) | | | | 0.0212d |
| 0 | 31 | 4 | 27 | |
| 1 | 12 | 4 | 8 | |
| 2a | 2 | 1 | 1 | |
| 2b | 21 | 11 | 10 | |
| 2c | 10 | 2 | 8 | |
| 3 | 4 | 1 | 3 | |
| Overall survival at
5 years (%) | 60.2 | 82.7 | 48.3 | 0.0013 |
HPV typing by multiplex polymerase chain
reaction (PCR)
DNA was extracted from five 10-μm sections of
paraffin-embedded pre-treatment tissue obtained from biopsies.
Overall, 16 different HPV genotypes (HPV-6, HPV-11, HPV-16, HPV-18,
HPV-30, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52,
HPV-56, HPV-58, HPV-59 and HPV-66) were detected by multiplex PCR.
Mutiplex PCR was conducted as described by Nishiwaki et al
(18). In brief,
HPV-genotype-specific primers were designed on the basis of
multiplex-sequence alignments. PCRs were performed with a multiplex
PCR kit (Qiagen Inc., Valencia, CA, USA), according to the
manufacturer’s instructions, with minor modifications. The
HPV-genotypes in the samples were identified by amplicon size.
Statistical analysis
Factors associated with HPV status including gender;
age; tumor subsites; tumor, node, metastasis (TNM) status; primary
tumor size and involvement of lymph nodes were analyzed by
cross-tabulations using the two-tailed Fisher’s exact test.
Statistical significance was set at p<0.05. Overall survival
curves were calculated using the Kaplan-Meier method. Survival was
calculated from the date of treatment initiation until either
mortality or the last date on which the patient was known to be
alive. The statistical significance of differences between survival
times was determined by the log-rank test in univariate analyses at
a significance level of p<0.05.
Immunohistochemistry
Paraffin-embedded tumor specimens from the primary
sites were obtained from all the patients. These specimens were cut
into 4-mm sections. They were then deparaffinized in xylene,
dehydrated through graded alcohol and placed in 0.1% hydrogen
peroxide to quench any endogenous peroxidase activity. Following
3-times of microwave pretreatment in a citrate buffer (pH 6.0) for
5 min at 750 W, these sections were treated with a 10% normal
rabbit serum for 30 min to prevent non-specific binding of the
antibody. The slides were then incubated with the specific
monoclonal antibody to human E-cadherin or vimentin in a humid
chamber at 37.8°C overnight. The sections were then incubated with
a biotin-labeled rabbit anti-mouse secondary antibody [Histofine
SAB-PO (M) kit; Nichirei, Tokyo, Japan] for 30 min at 37.8°C,
followed by reaction with a streptavidin-biotin horseradish
peroxidase complex. We observed the reaction products by immersing
the slides in a freshly prepared diaminobenzidine solution for 10
min and counterstaining them with hematoxylin before dehydration
and mounting. We determined the percentage of vimentin tumor cells
by counting the number of brown-stained tumor cells in the most
highly stained area of each slide. The vimentin index was
calculated from the ratio of the number of positively stained tumor
cells to the total number of tumor cells per section. Vimentin
expression in mesenchymal cells other than tumor cells (blood
vessels, stromal tissues) was excluded by observation of
hematoxylin and eosin (H&E)-stained slides. We determined that
tumor tissue with a vimentin index ≥20% was positive. For
E-cadherin, we graded the staining intensity as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. The proportion of
positive cells was categorized as follows: 0, <1%; 1, 2–40%; 2,
40–80%; 3, >80%. The two scores were then multiplied and the
final immunostaining score was determined; a score of 0 was
regarded as negative, 2 and 3 as weakly positive, 3 and 4 as
moderately positive, and 6–9 as strongly positive. This scoring
system was based on one described in a previous report (16). The heterogeneity of E-cadherin
expression was estimated by the presence of strongly stained cancer
cell nests adjacent to negative or weakly stained cancer cell
nests. Furthermore, we repeated the immunostaining thrice and
confirmed the results to avoid counting irregular staining. All
specimens were examined by two observers (H.H. and T.M.)
Results
Patients and treatment
HPV status and clinicopathological
features
In total, 23 of the 79 OPSCC tissue samples were
found to be HPV-positive. Among them, 20 patients (87%) were
positive for HPV-16, two (8.7%) were positive for HPV-18 and one
(4.3%) was positive for HPV-58. No patient presented with dual
infections.
The correlations between HPV status and
clinicopathological features are shown in Table I. Gender, age and T class were not
correlated with HPV status. HPV-positive patients presented
significantly more often with lymph-node metastasis compared to
HPV-negative patients (p=0.0212). The HPV-positive OPSCCs were
significantly more often histopathologically graded as moderately
or poorly differentiated (p=0.007). Kaplan-Meier analysis showed
that HPV-positive patients had an improved 5-year overall survival
rate compared with HPV-negative patients (82.7 vs. 48.3%,
respectively; p=0.0013, log-rank test; Fig. 1).
Expression of E-cadherin and vimentin
in OPSCC and its correlation to clinicopathological features
Immunohistochemical staining for E-cadherin and
vimentin was performed in 79 OPSCC tissue samples. Intensity of the
staining was confirmed by staining the normal pharyngeal
epithelium. E-cadherin expression was observed mainly at the basal
and spinosum layers and gradually disappeared in cells that had
undergone keratinization (Fig. 2A).
Representative weak and strong staining for E-cadherin is shown in
Fig. 2B and C. Strong and moderate
E-cadherin staining was observed in 43/56 HPV-positive tumors
(76.7%) and in 10/23 (43.4%) HPV-negative tumors (p=0.007)
(Table IIA). High and low
E-cadherin expression significantly correlated with all 5-year
survival rates in 79 OSCC patients (81.8% in high E-cadherin
expression vs. 53.2% in low, respectively; p=0.032, log-rank test;
Fig. 3A). However, E-cadherin
expression did not significantly correlate with 5-year survival
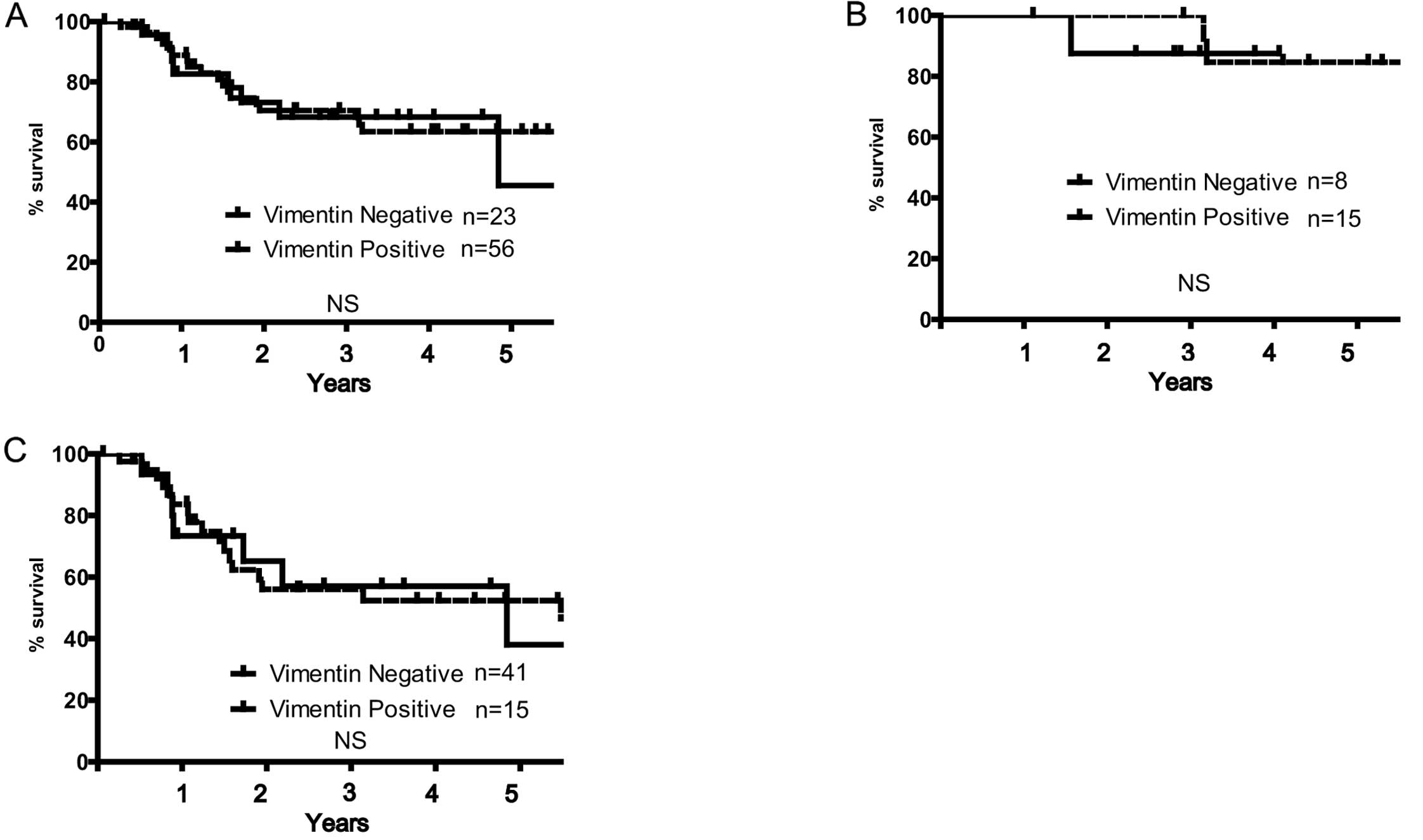
rate either in HPV-positive or -negative OSCC patients (Fig. 3B and C). Positive rate of vimentin
expression was 29.1% in all OSCC patients. A typical image of
positive vimentin expression is shown in Fig. 2D. Vimentin expression rate was not
associated with any clinicopathological features and HPV status
(Table IIB). Although we expected
vimentin expression to play a counterpart of E-cadherin as the
representative marker of mesenchymal phenotype, there was no
inverse correlation between E-cadherin and vimentin expression.
Therefore, there was no statistical difference in the 5-year
survival rates of the patients with HPV-positive and -negative OSCC
with regard to vimentin expression (Fig. 4A–C).
 | Table IIE-cadherin and vimentin
expression. |
Table II
E-cadherin and vimentin
expression.
| A, E-cadherin
expression |
|---|
|
|---|
| | E-cadherin
expression | |
|---|
| |
| |
|---|
| Status | n | Negative | Weak | Moderate | Strong | P-value |
|---|
| HPV status | | | | | | 0.007 |
| Positive | 23 | 3 | 10 | 5 | 5 | |
| Negative | 56 | 6 | 7 | 14 | 29 | |
|
| B, Vimentin
expression |
|
| | Vimentin
expression | | | |
| |
| | | |
| Status | n | Negative | Positive | P-value | | |
|
| HPV status | | | | NS | | |
| Positive | 23 | 15 | 8 | | | |
| Negative | 56 | 41 | 15 | | | |
Heterogeneity of E-cadherin expression was found in
23 samples. Typical heterogeneous E-cadherin expression is shown in
Fig. 5. HPV-negative patients
presented significantly greater heterogeneity of E-cadherin
expression compared to HPV-positive patients (p=0.0349) (Table III). Fig. 5A and B shows that patients with a
heterogeneous expression of E-cadherin had significantly higher
5-year survival rates compared to the patients with homogeneous
E-cadherin expression (Fig.
5C).
 | Table IIIHeterogeneity of E-cadherin
expression. |
Table III
Heterogeneity of E-cadherin
expression.
| E-cadherin
expression | |
|---|
|
| |
|---|
| Status | Heterogeneous | Homogeneous | P-value |
|---|
| HPV status | | | 0.0349 |
| Positive | 3 | 20 | |
| Negative | 20 | 36 | |
Discussion
The phenotype of HPV-positive HNSCC is completely
different from that of HPV-negative HNSCC, both clinically and
pathologically (11). Several
molecular studies have also indicated that HPV-positive tumors do
not show the same frequent oncogenic molecular alternations which
characterize HPV-negative HNSCC, such as mutations of p53,
mutations and deletions in p16INK4a, overexpression of
cyclin D1 or increased EGFR copy numbers (19–24).
Loss of E-cadherin expression significantly
correlated with an increased risk of distant metastasis in HNSCC
(15,25). Meta-analysis of existing studies
also showed a correlation between low E-cadherin expression and
poor prognosis of HNSCC (16),
although some studies showed no correlation between these factors
(26). Our results, which reveal a
more favorable prognosis in patients with OPSCC with low E-cadherin
expression, showed an opposite outcome from previous studies as our
investigation was limited to oropharynx sites and E-cadherin
expression is significantly lower in HPV-positive OPSCC than in
HPV-negative cases. The HPV-positive rate in OPSCC is higher than
that in other sites, thus they should not be analyzed as the same
type of tumor (27). Although most
previous reports on E-cadherin expression in HNSCC included all
HNSCC sites, it is better to distinguish between HPV-positive and
-negative cases for analysis if they include OPSCC. The most
frequent subtype of tonsillar HPV detected in the present study was
HPV16, a high-risk subtype of HPV for OPSCC, which was in agreement
with the results of previous studies (28,29).
Several reports have shown that HPV16-E6/E7 regulate E-cadherin
expression. E-cadherin expression in the epithelium was shown to be
reduced during HPV16 infection, which is associated with the
depletion of Langerhans cells at the site of the infection
(30,31). A recent study showed that
HPV16-E6/E7 regulated E-cadherin expression and induced ZEB1, an
EMT-activating transcriptional factor (32). These studies suggested HPV infection
induces EMT, yet they did not demonstrate the reason for the
favorable clinical outcome in cases of HPV-positive HNSCC.
Intratumor heterogeneity has been shown genetically
and is associated with the prognosis and drug resistance in
patients with cancer (33). In
HNSCC, Mroz and Rocco (34)
developed a new system for measuring genetic heterogeneity using
next-generation sequencing data, and showed that high genetic
heterogeneity is associated with tumor progression and poor
treatment outcome. Furthermore, they reported that HPV-positive
tumor tissues showed significantly greater intratumor homogeneity
than did HPV-negative tissues, which is in agreement with our data
(34,35). From these results, a favorable
clinical response in cases of HPV-positive HNSCC can be reasoned by
their low heterogeneity. No quantitative evaluation of
heterogeneity based on EMT has been established. In general, HNSCC
biopsy samples are small and we see merely the tip of the whole
tumor. Accurate analysis of heterogeneity requires whole tumor
specimens both from the primary and the metastasis sites. We
expected vimentin expression to play a role as a counterpart to
E-cadherin, but vimentin expression was low, even in
E-cadherin-negative cancer tissues. The vimentin expression rate is
inherently low in SCC; it may not be an appropriate marker in
opposition to E-cadherin. N-cadherin and fibronectin have also been
used as markers for mesenchymal cells, yet the molecular expression
of these compounds is not sufficiently sensitive to reflect
mesenchymal phenotype in HNSCC (15,36).
The results of the present study indicate that
HPV-positive tumors tend to lose an epithelial phenotype and were
found to be homogeneous based on EMT analysis. This may be the
reason for the paradoxical favorable clinical outcome in
HPV-positive patients. Future development of an estimation system
for intratumor heterogeneity based on EMT may have important
applications to clinical decision-making and tailor-made therapy in
cases of HNSCC.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Kreimer AR, Clifford GM, Boyle P and
Franceschi S: Human papillomavirus types in head and neck squamous
cell carcinomas worldwide: a systematic review. Cancer Epidemiol
Biomarkers Prev. 14:467–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lindel K, Beer KT, Laissue J, Greiner RH
and Aebersold DM: Human papillomavirus positive squamous cell
carcinoma of the oropharynx: a radiosensitive subgroup of head and
neck carcinoma. Cancer. 92:805–813. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
D’Souza G, Kreimer AR, Viscidi R, et al:
Case-control study of human papillomavirus and oropharyngeal
cancer. N Engl J Med. 356:1944–1956. 2007.PubMed/NCBI
|
|
5
|
Kumar B, Cordell KG, Lee JS, et al: EGFR,
p16, HPV titer, Bcl-xL and p53, sex, and smoking as indicators of
response to therapy and survival in oropharyngeal cancer. J Clin
Oncol. 26:3128–3137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Worden FP, Kumar B, Lee JS, et al:
Chemoselection as a strategy for organ preservation in advanced
oropharynx cancer: response and survival positively associated with
HPV16 copy number. J Clin Oncol. 26:3138–3146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Licitra L, Perrone F, Bossi P, et al:
High-risk human papillomavirus affects prognosis in patients with
surgically treated oropharyngeal squamous cell carcinoma. J Clin
Oncol. 24:5630–5636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weinberger PM, Yu Z, Haffty BG, et al:
Molecular classification identifies a subset of human
papillomavirus-associated oropharyngeal cancers with favorable
prognosis. J Clin Oncol. 24:736–747. 2006. View Article : Google Scholar
|
|
9
|
Fakhry C, Westra WH, Li S, et al: Improved
survival of patients with human papillomavirus-positive head and
neck squamous cell carcinoma in a prospective clinical trial. J
Natl Cancer Inst. 100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizumachi T, Kano S, Sakashita T, et al:
Improved survival of Japanese patients with human
papillomavirus-positive oropharyngeal squamous cell carcinoma. Int
J Clin Oncol. 18:824–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Westra WH: The morphologic profile of
HPV-related head and neck squamous carcinoma: implications for
diagnosis, prognosis, and clinical management. Head Neck Pathol.
6(Suppl 1): S48–S54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalluri R: EMT: when epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashimoto T, Soeno Y, Maeda G, et al:
Progression of oral squamous cell carcinoma accompanied with
reduced E-cadherin expression but not cadherin switch. PLoS One.
7:e478992012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Z, Ge J, Sun Y, et al: Is E-cadherin
immunoexpression a prognostic factor for head and neck squamous
cell carcinoma (HNSCC)? A systematic review and meta-analysis. Oral
Oncol. 48:761–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishiwaki M, Yamamoto T, Tone S, et al:
Genotyping of human papillomaviruses by a novel one-step typing
method with multiplex PCR and clinical applications. J Clin
Microbiol. 46:1161–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olshan AF, Weissler MC, Pei H, et al:
Alterations of the p16 gene in head and neck cancer: frequency and
association with p53, PRAD-1 and HPV. Oncogene. 14:811–818. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Braakhuis BJ, Snijders PJ, Keune WJ, et
al: Genetic patterns in head and neck cancers that contain or lack
transcriptionally active human papillomavirus. J Natl Cancer Inst.
96:998–1006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haraf DJ, Nodzenski E, Brachman D, et al:
Human papilloma virus and p53 in head and neck cancer: clinical
correlates and survival. Clin Cancer Res. 2:755–762.
1996.PubMed/NCBI
|
|
22
|
Perrone F, Suardi S, Pastore E, et al:
Molecular and cytogenetic subgroups of oropharyngeal squamous cell
carcinoma. Clin Cancer Res. 12:6643–6651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kessis TD, Slebos RJ, Nelson WG, et al:
Human papillomavirus 16 E6 expression disrupts the p53-mediated
cellular response to DNA damage. Proc Natl Acad Sci USA.
90:3988–3992. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koch WM, Lango M, Sewell D, Zahurak M and
Sidransky D: Head and neck cancer in nonsmokers: a distinct
clinical and molecular entity. Laryngoscope. 109:1544–1551. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nijkamp MM, Span PN, Hoogsteen IJ, van der
Kogel AJ, Kaanders JH and Bussink J: Expression of E-cadherin and
vimentin correlates with metastasis formation in head and neck
squamous cell carcinoma patients. Radiother Oncol. 99:344–348.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andrews NA, Jones AS, Helliwell TR and
Kinsella AR: Expression of the E-cadherin-catenin cell adhesion
complex in primary squamous cell carcinomas of the head and neck
and their nodal metastases. Br J Cancer. 75:1474–1480. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baumann JL, Cohen S, Evjen AN, et al:
Human papillomavirus in early laryngeal carcinoma. Laryngoscope.
119:1531–1537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kreimer AR, Alberg AJ, Daniel R, et al:
Oral human papillomavirus infection in adults is associated with
sexual behavior and HIV serostatus. J Infect Dis. 189:686–698.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Syrjänen S: Human papillomavirus (HPV) in
head and neck cancer. J Clin Virol. 32(Suppl 1): S59–S66. 2005.
|
|
30
|
Vessey CJ, Wilding J, Folarin N, et al:
Altered expression and function of E-cadherin in cervical
intraepithelial neoplasia and invasive squamous cell carcinoma. J
Pathol. 176:151–159. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matthews K, Leong CM, Baxter L, et al:
Depletion of Langerhans cells in human papillomavirus type
16-infected skin is associated with E6-mediated down regulation of
E-cadherin. J Virol. 77:8378–8385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung YS, Kato I and Kim HR: A novel
function of HPV16-E6/E7 in epithelial-mesenchymal transition.
Biochem Biophys Res Commun. 435:339–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gerlinger M, Rowan AJ, Horswell S, et al:
Intratumor heterogeneity and branched evolution revealed by
multiregion sequencing. N Engl J Med. 366:883–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mroz EA and Rocco JW: MATH, a novel
measure of intratumor genetic heterogeneity, is high in
poor-outcome classes of head and neck squamous cell carcinoma. Oral
Oncol. 49:211–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mroz EA and Rocco JW: Gene expression
analysis as a tool in early-stage oral cancer management. J Clin
Oncol. 30:4053–4055. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|