Introduction
As a consequence of immune tolerance, primary liver
cancer patients usually do not have a good response to tumor cells
(1). Exogenous immunization aims to
disrupt such intolerance and enhance the immune response (1). However, the therapeutic outcomes of
most existing immunotherapeutic approaches on hepatocellular
carcinoma (HCC) usually are not satisfactory, mainly due to poor
immunogenicity, the phenomenon of immune suppression and the
interference of regulatory T cells (2–5).
As a novel approach of antitumor immunotherapy,
epitope peptide vaccine has been developed rapidly in recent years.
Compared with other vaccines, peptide vaccine has significant
advantages such as simple composition, targeted immune response and
exemption of pathological epitopes (6,7).
However, its smaller molecular weight and simple structure often
lead to a decreased immunogenicity and an unsatisfactory immune
response, especially the humoral immune response (7,8). To
resolve this problem, the multiple antigenic polypeptide (MAP)
design has been suggested in recent years, which simulates the
natural epitope structure well and eliminates the carrier protein
(9). Theoretically, the MAP design
can arouse humoral immunity in vivo, inducing highly
specific polyclonal antibodies.
Besides the structural design, it is also essential
to choose an ideal tumor associate antigen (TAA) as the therapeutic
target for epitope peptide vaccines. As a promising TAA in
immunotherapeutic areas, heparanase (HPSE) has been investigated in
recent years (10). The HPSE
precursor protein has a molecular weight of ~65 kDa. It is a
heterodimer consisting of two subunits, with a molecular weight of
50 and 8 kDa. The former weight represents its mature activated
form (10). HPSE is currently the
only endoglycosidase identified that can specifically degrade the
heparan sulfate (HS) side chain of heparan sulfate proteoglycans
(HSPG) in the ECM or at BM, resulting in the destruction of ECM or
BM, release of multiple types of cytokines such as bFGF and VEGF
and facilitation of malignant angiogenesis and tumor growth
(11–13). HPSE is overexpressed in most tumors,
including in HCC, and plays a key role in cancer growth and
metastasis (14). While HPSE is
expressed at a relatively low level in mammalian lymphoid organs,
leukocytes and platelets, it is hardly expressed in other normal
tissues. Therefore, HPSE may be regarded as an important TAA as
well as a target molecule in antitumor treatment (15,16).
Certain HPSE inhibitors such as antisense nucleic acid, siRNA and
antibodies against its 50 kDa large subunits may effectively
suppress the growth and metastasis of malignant tumors (17–22).
However, evident defects of these inhibitors, such as heterology,
being easily degradable, having a short aging time and poor
maneuverability in clinical treatment were also identified
(17,19).
Based on human HPSE protein structure and its
predicted B-cell epitopes via bioinformatics, we designed and
synthesized the MAP vaccine, and validated that it induced specific
anti-MAP polyclonal antibodies in vivo (23,24).
In this study, to investigate the immunotherapeutic effect on human
HCC growth, specific antibodies induced by the self-synthesized MAP
were administered to tumor-bearing BALB/c nude mice through passive
immunity. Our results suggest that the synthesized HPSE B-cell
epitope-based MAP vaccine effectively limited HCC growth in
vivo, by virtue of its anti-MAP polyclonal antibodies. Our
study provides theoretical evidence for additional studies on the
MAP vaccine composed of HPSE B-cell epitopes in the treatment of
HCC.
Materials and methods
Experimental animals and cells
Four-week-old pathogen-free male BALB/c nude mice
(weighing 20±2 g, SPF grade, certificate no. SCXK20130198) were
purchased from the Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). White-hair-black-eye (WHBY) rabbits, derived
from Japanese big-ear white rabbits, were provided by Animal
Experimental Center of Zhejiang Chinese Medical University
(Hangzhou, China). Animals were kept at the Animal Research Center
of Zhejiang Chinese Medical University and provided with water and
food ad libitum. The animal experiments were approved by the
Ethics Committee of the Zhejiang Chinese Medical University.
Invasive manipulations were performed under sodium pentobarbital
anesthesia, and all efforts were made to minimize suffering. The
HCC cell line HCC97-H (HPSE-positive) was purchased from the Liver
Cancer Institute of Zhongshan Hospital (Shanghai, China),
maintained at our laboratory under conditions of 37°C and 5%
CO2, and was routinely cultured in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with penicillin (100 U/ml),
streptomycin (100 μg/ml) and 10% fetal bovine serum (FBS).
Preparation of HPSE B-cell epitope-based
MAP vaccine
The MAP vaccine composed of the B-cell epitope of
HPSE was synthesized, purified, and identified as described
previously (23,24). Briefly, according to the amino acid
sequence of human HPSE, peptide ‘HCTNTDNPRYKEGDL’ (279–293) of HPSE
was selected as the dominant B-cell epitope by DNAStar software and
BcePred online predication tool. The MAP vaccine was constructed by
fusing 8 copies of epitope peptide ‘HCTNTDNPRYKEGDL’ to 5 copies of
lysine (K) by the Chinese Peptide Co. (Hangzhou, China). The
schematic drawing of the self-designed MAP structure is provided in
Fig. 1. The synthesized MAP was
purified using reverse-phase high-performance liquid chromatography
(HPLC) on a Vydac C18 column. The purity of the MAP was confirmed
by analytic HPLC.
Harvest of specific anti-MAP
antiserum
WHBY rabbits were immunized with the MAP vaccine,
and its antiserum was isolated and identified according to the
procedure we described previously (24). Briefly, eight-branched self-designed
MAP was used to actively immunize WHBY rabbit intravenously four
times, with an interval of 2 weeks. Freund’s complete adjuvant was
used in the prime immunization, while Freund’s incomplete adjuvant
(both from Sigma-Aldrich, St. Louis, MO, USA) and Th linear peptide
(Chinese Peptide Co.) were used to boost the immunization. Serum
samples were taken before the first immunization and 10 days after
each manipulation, 5 times in total. For each sample, a
standardized indirect ELISA assay was performed to mensurate the
titers of polyclonal anti-MAP antibodies. After the entire
immunizing procedure, WHBY rabbits were sacrificed and abundant
antisera were harvested. The polyclonal antibodies against MAP
vaccine contained in the antiserum were purified by caprylic
acid/ammonium sulfate (CA-AS) precipitation, which is a classical,
efficient and low-cost purification method for humoral antibodies
(25). The concentration of the
specific anti-MAP antibodies was determined by the Coomassie
Brilliant Blue (Boster Biotechnology, China) assay as mentioned in
our previous studies (24).
Binding affinity test
The ELISA assay was carried out to evaluate the
affinity between the synthesized MAP and commercialized HPSE
antibody (Takara Bio, Inc., Tokyo, Japan). Briefly, 96-well ELISA
plates were coated with MAP at the concentration of 2 μg/100 μl and
4 μg/100 μl, respectively and maintained overnight at 4°C. After
blocking using FBS, 0.05 μg/100 μl/well of the commercialized HPSE
antibodies were added, while unimmunized rabbit serum with a
dilution of 1:5,000 was used as a negative control. The results
were indicated by the mean OD value of triple wells.
Western blot assay and protein
electrophoresis
To identify the specificity of the anti-MAP
antibodies, western blot analysis and protein electrophoresis were
performed according to the manufacturer’s instructions (KangChen
Bio-Tech Inc., Shanghai, China). Specific immunoreactive bands
became evident when the specific antibodies contained in the
antiserum reacted with the HPSE of HCC97-H cells. Briefly, HCC97-H
cells (4×107) were lysed and proteins were extracted
offhandedly. An SDS-PAGE was produced. The commercialized rabbit
anti-human HPSE antibody (InSight Biopharmaceuticals Ltd., Rehovot,
Israel) with a dilution of 1:200, or the purified rabbit anti-MAP
antiserum with a dilution of 1:3,000 served as the primary
antibody. The unimmunized rabbit serum was used as a negative
control. Immunoreactive bands were demonstrated using
chemiluminescence.
HPSE activity suppression test
To evaluate the enzymatic activity of HPSE, the
specific anti-MAP polyclonal antibodies contained in the rabbit
antiserum were cultured together with HCC97-H cells in
vitro. The HPSE activity was determined by detecting the amount
of remaining HS substrate after digestion of the enzyme-substrate
reaction. HPSE activity was calculated by measuring the absorbance
at 450 nm, which indicates the content of HS not being degraded by
HPSE. The test was conducted as indicated in the user manual of the
HPSE activity kit (Takara Bio, Inc.). Briefly, 1.0 U/well of HPSE
Standard was added to a 96-well plate and incubated at a final
concentration of 0 μg/ml (blank control), 50 and 100 μg/ml of
anti-MAP antibodies, or 100 μg/ml of unimmunized rabbit IgG as a
negative control in the nutrient medium for 1 h at 37°C.
Subsequently, HCC97-H cells (7×104/well) were cultured
in a 24-well plate for 48 h, then treated with MAP-induced specific
antibodies at the final concentration of 0 μg/ml (blank control),
50 and 100 μg/ml, respectively, or 100 μg/ml of unimmunized rabbit
IgG as a negative control in culture medium for 1 h at 37°C. The
supernatant of the nutrient medium was scraped out to measure the
HPSE activity to evaluate the inhibitive effect of the anti-MAP
antibodies on the HPSE enzymatic activity of HCC97-H cells.
Establishment of tumor-bearing murine
model
The human HCC97-H cells (1×107/mouse) at
logarithmic growth period were inoculated into the right flank of
10 BALB/c nude mice. Approximately six weeks later, the HCC97-H
cells in five mice developed into solid tumors of ~1
cm3, which were entirely gouged out subsequently and
were immediately immersed into normal saline. Connective tissues
were wrinkled and the lumps were sheared into ~2 mm3
sections. The sections were subcutaneously implanted into the
dorsal skin in 30 BALB/c nude mice, which were randomly divided
into the small-dose immunizing (SDI) group, large-dose immunizing
(LDI) group, and the control group, with 10 mice in each group.
Four weeks later (before the beginning of passive immunization), B
ultrasonography equipped with a water bag was performed to measure
the implanted tumor sizes, ensuring there were no significant
differences in size among the three groups. The mean tumor volume
was measured and calculated according to the formula:
v=ab2/2 (‘a’ is the maximum diameter of the tumor, and
‘b’ is the vertical diameter of the maximum diameter).
Passive immunization
After the HCC-bearing murine models were established
(measured by B ultrasonography), MAP vaccine-derived polyclonal
antibodies at the volume of 0.2 ml were administered to the mice
through the caudal vein on the SDI and LDI groups with the antibody
dose of 5 and 10 mg/kg, respectively, twice in total with an
interval of two weeks. For the control group, 0.2 ml unimmunized
rabbit serum with a dilution of 1:100 was intravenously
administered. B ultrasonography was implemented three weeks later
to measure the HCC tumor volume, using the formula mentioned above.
Six weeks after the passive immunization, HCC-bearing nude mice
were sacrificed. The hypodermic xenograft was entirely gouged out,
and its size was measured using a vernier caliper.
Immunohistochemical evaluation
At the end of the experiment, HCC-bearing nude mice
were sacrificed. Histological sections of the hepatoma were
manufactured and immunohistochemically evaluated for VEGF, bFGF and
CD34 according to the manufacturer’s instructions (Biotech Co.,
Ltd., Shanghai, China). Staining intensity of VEGF and bFGF was
semiquantitatively evaluated according to a previously described
method (24). Briefly, the
determination of immunohistochemical results was blindly assessed
by two senior pathologists. It was carried out firstly under low
power lens (x100) to select the ‘densely stained area’, then 500
tumor cells were counted within five visual fields at a
magnification of ×200. The scoring standards of immunohistochemical
staining were based on the coloring of the cancer cells and on the
percentage of positive cells. Scoring for color was: no staining,
0; light yellow, 1; brown, 2; and dark brown, 3. Scoring for
percentage was: negative, 0; the percentage of positive cells ≤10%,
1; 11–50%, 2; and ≥51%, 3. The intensity of VEGF or bFGF expression
was denoted by the product of cancer cell staining intensity and
positive cell percentage: 0–2, −; 3–4, +; 5–7, ++; and 8–9,
+++.
CD34 immunostaining was used to represent mean
vessel density (MVD), which was present in the endothelial cells of
microvessels and could be stained as brown or brownish yellow. In
this study, MVD was evaluated according to Weider’s methodology
(26): a high vascular density area
was selected under low-power objective (x100), and the number of
vascular stained by CD34 were counted in three visual fields under
high-power microscope (x400), and the average value was regarded as
the MVD value of the tumor.
ELISA assay of serum VEGF and bFGF
Blood samples were taken from the murine orbits
prior to euthanasia. Serum VEGF and bFGF concentrations were
assessed by ELISA assay, according to the manufacturer’s
instructions (Cusabio, Wuhan, China). Briefly, 100 μl standard,
blank or sample was plated in triplicate per well in a 96-well
plate and incubated for 2 h at 37°C. Biotin-antibody working
solution, horseradish peroxidase (HRP)-avidin working solution and
3,3′,5,5′-tetramethylbenzidine (TMB) were subsequently added.
Substrate was added to each well in turn and incubated in different
environmental conditions. Stop solution was then added to each well
when the first four wells containing the highest concentration of
standards developed an obvious blue color. The optical density of
each ELISA well was indicated by a microplate reader set to 450 nm.
Accordingly, the VEGF or bFGF value was calculated on the basis of
the ‘standard curve’ drawn by the professional soft ‘CurveExert
1.4’.
Statistical analysis
Experimental results were presented as mean ±
standard deviation (SD). Data were analyzed by the Student’s t-test
or one-way ANOVA analysis. Statistical significance was defined as
P<0.05. All statistical analyses were performed by SPSS 11.5
software (SPSS Inc., Chicago, IL, USA).
Results
HPSE B-cell epitope-based MAP
immunization induced high titers of MAP-specific IgG
antibodies
The synthesized MAP composed of HPSE B-cell epitopes
was already identified in our previous studies, and the purity of
the MAP polypeptides was >95% determined by purification through
HPLC (23,24). To evaluate humoral immune responses
induced by MAP based on HPSE B-cell epitopes, WHBY rabbits were
vaccinated with 200 μg of self-synthesized MAP plus Freund’s
adjuvant and Th linear peptide as mentioned in Materials and
methods, and MAP-specific IgG antibodies were detected in rabbit
serum samples by standard indirect ELISA. The synthesized MAP
induced strong MAP-specific IgG antibody responses, with the titer
of 1:103 1 week following the first immunization, then
the titer reached ~1:104 prior to boost, and reached the
highest peak over 1:105 2 weeks following the boost
immunization (Fig. 2). By contrast,
only the background level of antibody responses was detected in the
rabbit serum immunized with Th linear peptide alone.
The synthesized MAP had high-binding
affinity with commercialized HPSE antibody
Binding affinity between commercialized HPSE
antibody and the synthesized MAP polypeptides was assessed by
indirect ELISA assay. The coated HPSE B-cell epitope-based MAP of
different concentrations reacted with the commercialized HPSE
antibody (1:5,000 dilution) or the unimmunized rabbit serum
(negative control, 1:5,000 dilution), respectively. The results
showed that the synthesized MAP had high-binding affinity with the
commercialized HPSE antibody, compared with the negative control
(Fig. 3).
Anti-MAP antibodies bound with 65- and
50-kDa HPSE protein
Protein extracted from HCC97-H cells was utilized to
react with the anti-MAP polyclonal antibodies. The commercialized
polyclonal rabbit anti-human HPSE antibody and the unimmunized
rabbit serum were used as a positive and negative control,
respectively. Electrophoresis and chemiluminescence showed a clear
band of ~50 kDa and a visible band of 65 kDa in the column of the
positive control. In the anti-MAP antiserum column, 65- and 50-kDa
bands were markedly exhibited (Fig
4). Nevertheless, no band was observed in both the
corresponding locations in the negative control column. According
to the instructions of the commercialized antibody, protein of the
65- and 50-kDa was most probably the precursor of HPSE protein of
HCC97-H cells and its large subunit, respectively.
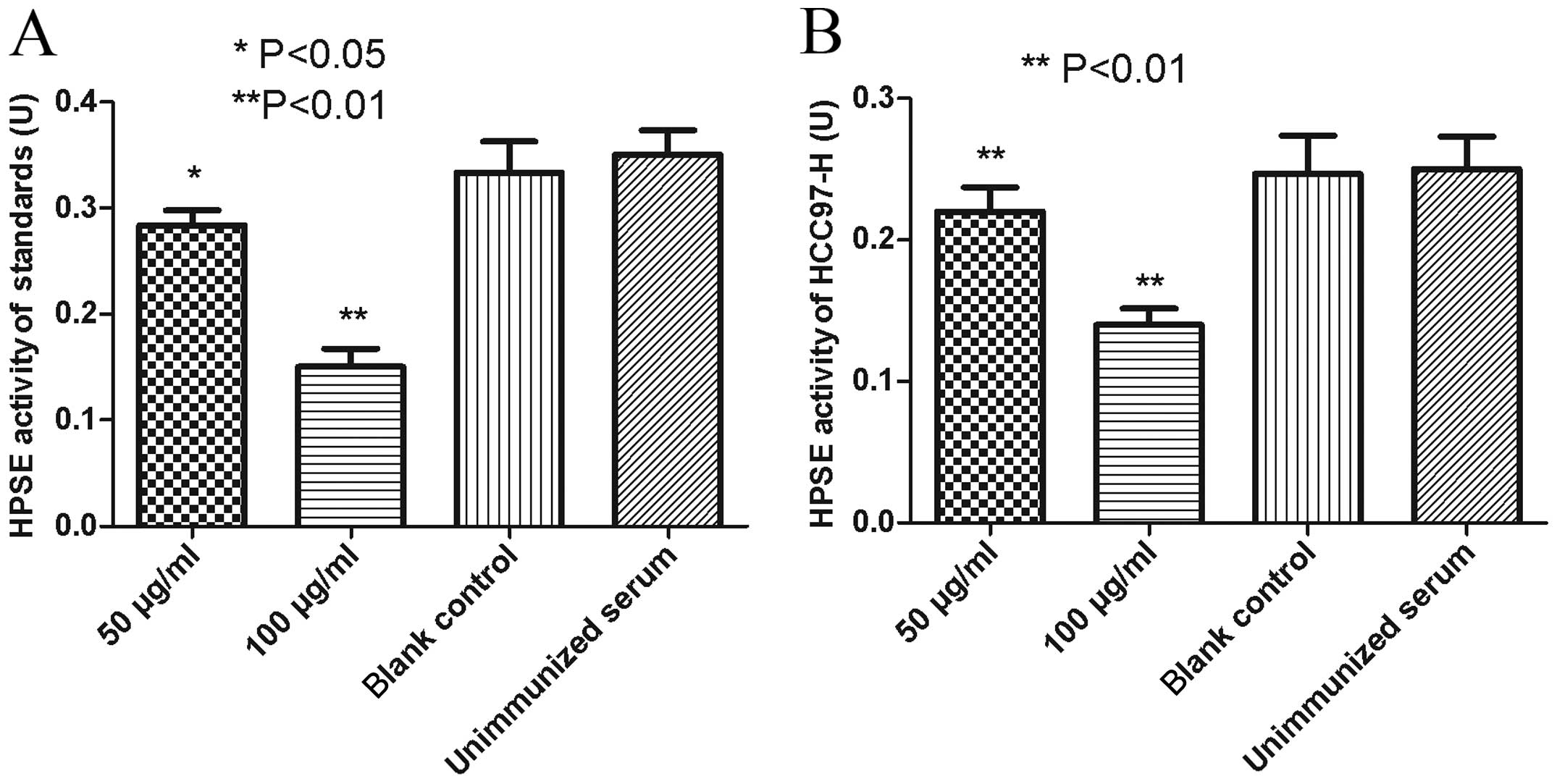
Anti-MAP polyclonal antibodies elicited
potent inhibitory effect on HPSE activity
When the HPSE standards were incubated with anti-MAP
antibodies at a concentration of 100 μg/ml, HPSE activity decreased
by 56.3%, compared with the blank control group or the negative
control group (P<0.01). After the standards were treated with a
final concentration of 50 μg/ml anti-MAP antibodies, a decrease of
17.8% on HPSE enzymatic activity was detected (P<0.05). No
impact on the HPSE activity was observed under the treatment with a
final anti-MAP antibody concentration of 0 μg/ml (blank control),
compared with the negative control (P>0.05) (Fig. 5A).
To assess the impact on HPSE secreted by malignant
HCC97-H cells, anti-MAP antibodies contained in the immunized
rabbit antiserum were cultured with the HCC97-H cell line. After
being incubated with anti-MAP antibodies at a final concentration
of 100 and 50 μg/ml, the HPSE activity of the culture supernatant
decreased by 42.9 and 11.6%, respectively, compared with the blank
control or the negative control (P<0.01). The supernatant HPSE
activity was not significantly altered following culture with the
anti-MAP antibody of 0 μg/ml (blank control), compared with the
negative control (P>0.05) (Fig.
5B).
Xenograft volume four weeks post-HCC
implanting
The HCC-implanting operation was performed
successfully and no mouse died during and after the surgery. B
ultrasonography equipped with a water bag was implemented at the
end of the 4th week after the surgical process. A representative
ultrasonic image is provided in Fig.
6A. According to the outcomes of B ultrasonic measurement and
the formula mentioned in Materials and methods, the mean tumor
volumes of the SDI, LDI and control groups were 45.3±6.8, 46.7±4.2
and 44.9±5.1 mm3, respectively, and there was no
statistical difference among the three groups (P>0.05) (Fig. 6B).
HPSE B-cell epitope-based MAP-limited HCC
growth in mice
After the tumor-bearing models were validated by B
ultrasonography, MAP vaccine-derived polyclonal antibodies were
injected to the mice in the SDI and LDI groups with different
doses. For the control group, diluted unimmunized rabbit serum was
administered. All the mice survived until the end of the
experiment. Three weeks after passive immunization, B
ultrasonography showed the mean tumor volume of the SDI, LDI and
control groups to be 967±88, 592±126 and 1,460±233 mm3,
respectively. Significant differences between two groups were
observed (P<0.05) (Fig. 7A). Six
weeks after passive immunization, the mean tumor volume of the SDI,
LDI and control groups were 2,270±415, 1,175±291 and 3,188±566
mm3, respectively, as determined by vernier caliper. The
mean tumor volume in the SDI or LDI group was significantly
reduced, as compared with the control group (P<0.01) (Fig. 7B).
 | Figure 7Hepatocellular carcinoma (HCC) volume
three and six weeks after passive immunization. (A) Three weeks
after passive immunization, B ultrasonography showed the mean tumor
volume of the small-dose immunizing (SDI), large-dose immunizing
(LDI) and control groups to be 967±58, 592±96 and 1,460±103
mm3, respectively, with significant differences between
two groups (*P<0.05). (B) Six weeks after passive
immunization, the mean tumor volumes of the SDI, LDI and control
groups were 2,270±515, 1,175±591 and 3,188±366 mm3,
respectively, as determined by vernier caliper. The mean tumor
volume in the SDI or LDI group were significantly reduced, as
compared with the control group (**P<0.01). |
Immunohistochemical evaluation of VEGF,
bFGF and CD34
All tumor-bearing mice survived until the end of the
experiment (six weeks after the first passive immunization).
Histological HCC sections were manufactured, and
immunohistochemical stainings of VEGF, bFGF and CD34 were
performed. According to the scoring standard mentioned above, the
expression of VEGF or bFGF in the SDI, LDI and control groups was
++, + and +++, respectively (Table
I). The mean value of MVD (represented by CD34 immunostaining)
in the SDI, LDI and control groups was 21.64±5.79/field,
13.48±4.31/field and 29.67±5.83/field, respectively. There were
significant differences among the groups (P<0.05) (Table I). Representative
immunohistochemical images are shown in Fig. 8.
 | Figure 8Immunohistochemical images of
micro-vessel density (MVD), VEGF and bFGF. At the end of the
experiment (six weeks after the first passive immunization),
histological sections of hepatocellular carcinoma (HCC) were
manufactured, and immunohistochemistry of VEGF, bFGF and CD34 was
performed. (A–C) Representative MVD immunohistochemical stainings
of the large-dose immunizing (LDI), small-dose immunizing (SDI) and
control groups, respectively (x400). (D–F) Representative VEGF
immunostainings of the LDI, SDI and control groups, respectively
(x400). (G–I) Representative bFGF immunohistochemical images of the
LDI, SDI and control groups, respectively (x400). |
 | Table IImmunohistochemical evaluation of
VEGF, bFGF and CD34. |
Table I
Immunohistochemical evaluation of
VEGF, bFGF and CD34.
| Groups | VEGF | bFGF | MVD |
|---|
| SDI | ++ | ++ |
21.64±5.79/field |
| LDI | + | + |
13.48±4.31/field |
| Control | +++ | +++ |
29.67±5.83/field |
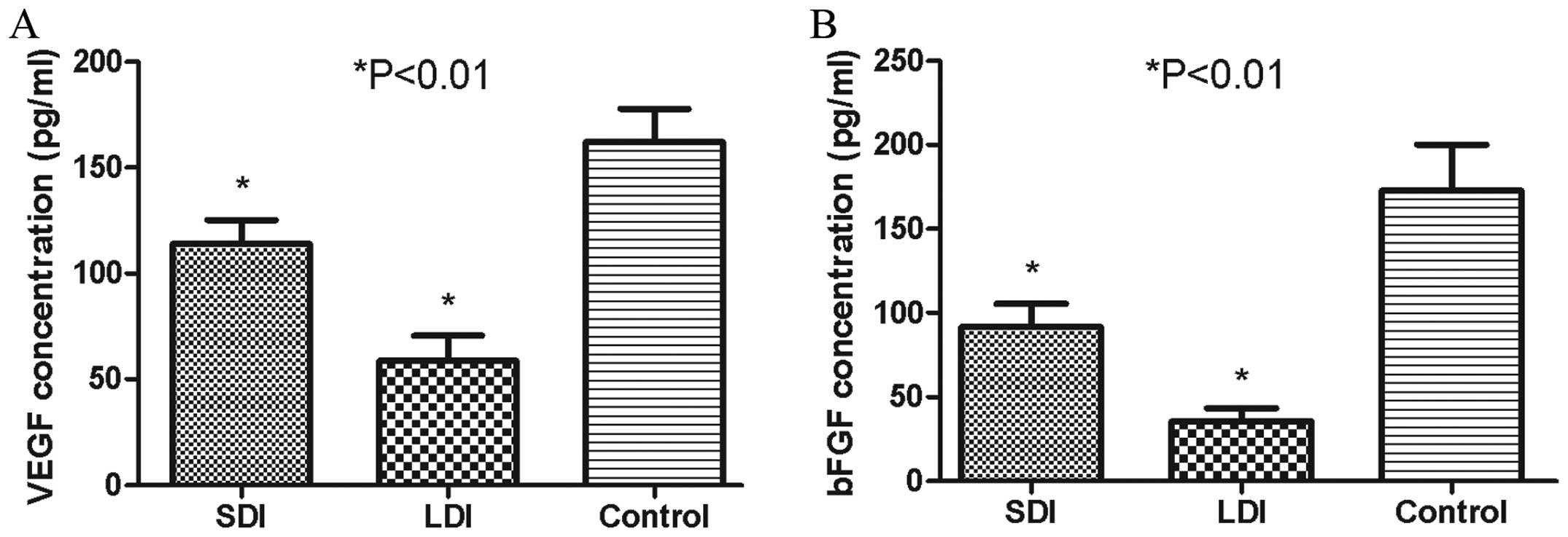
The anti-MAP antibody reduced serum
concentrations of VEGF and bFGF
Serum VEGF and bFGF levels were evaluated by ELISA,
which assisted the in vivo malignant cascades of HCC and
were released mostly by HPSE enzymolysis (10,12).
The mean VEGF concentrations in the SDI, LDI and control groups
were 113.85±19.48, 58.81±20.26 and 161.90±27.41 pg/ml, respectively
(Fig. 9A), while the bFGF levels in
the corresponding groups were 91.80±23.59, 35.47±13.32 and
172.83±47.32 pg/ml, respectively (Fig.
9B). The mean concentration of VEGF or bFGF in the SDI or LDI
group was much lower than that in the control group (P<0.01).
The serum level of VEGF or bFGF in the LDI group was also markedly
lower than that in the SDI group (P<0.01), which showed a
certain dose-dependent effect.
Discussion
Although there have been advances in preventive and
therapeutic approaches, HCC remains one of the major causes of
mortality worldwide. Over-growth, invasion and metastasis remain
the major bewilderments in curing HCC patients, thus effective
therapy to combat hepatoma is yet to be established (27,28).
To identify a complementary approach in the HCC therapeutic area of
study, immunotherapy has been under investigation during the past
few years. It utilizes the immune system to recognize and cope with
tumor cells and has shown encouraging results in certain human
clinical trials (4). The
fundamental purpose of such therapy is to manufacture vaccines that
elicit potent anti-growth or anti-metastatic immune responses
without side effects.
The central issue in the development of HCC
immunotherapeutic strategies is the identification of relevant TAA
capable of mediating antitumor effects via a competent immune
system. Investigators have advocated that the ideal TAA should be:
i) unique, distinctly different than in normal cells, ii)
constitutively expressed during the cell cycle and, iii) the
constitutive expression is essential for cell survival (29). Currently, a number of TAA have been
identified and described (30). The
appearance of antigen-loss or antigen-alteration mutations in tumor
cells in response to immune pressure has also been highlighted and
well described (31,32). To circumvent this issue, a class of
TAA termed Universal Tumor Antigens (UTAs) has been suggested that
is hypothesized to induce antitumor immunity against a wide range
of tumor types, and to have critical functional roles in tumor
growth and development (33).
Previous studies have been performed to determine promising UTAs
prior to the identification of HPSE (34).
Unlike other TAA, HPSE is found to be highly
expressed in most mammalian malignant tumors, and its expression
has been associated with tumor growth, metastasis and angiogenesis
(11). Some tumor cells can
downregulate or mutate the TAA expression to evade immune
surveillance (35). However, due to
the crucial role of HPSE in tumor progression, the downregulation
or mutation of its expression as a means of immune escape may
itself have deleterious effects on the proliferation and metastasis
of tumor cells. Tregs usually accumulate at the tumor site, where
they suppress the function of effective lymphocytes and inhibit
tumor growth (36). Notably, Tregs
against HPSE were not found in patients with certain malignancies
(37). HPSE-targeted immunotherapy
is thus expected to be prolonged and more efficient owing to the
absence of suppressive cells. Additionally, HPSE has been the sole
endoglycosidase capable of vitiating the ECM and BM, by splitting
the HS chain of glycosaminoglycan (10–16).
As a consequence, activation of HPSE plays a key role in growth and
invasion that enables tumor cells to break through the ECM and BE
barriers, releasing multiple types of cytokine and causing the
formation of new malignant vessels and the over-growth of tumors
(10–16,38).
Some studies have demonstrated that certain HPSE inhibitors, such
as polysaccharides, siRNA, and polypeptide antibodies, had the
potency of suppressing tumor growth, invasion, metastasis and
angiogenesis (13,17–24).
Based on the above results, HPSE may be regarded as a promising and
crucial target for antitumor immunological studies.
The first group of immunogenic nonapeptide epitopes
of the HPSE amino acid sequence was previously identified by
Sommerfeldt et al (16)
approximately 10 years ago (26).
In previous studies, we screened and identified that peptide
fragment 279–293 was a dominant B-cell epitope of HPSE, and
accordingly synthesized the MAP vaccine adopting the 8-branched
design (23,24). In this study, we evaluated its
immunological effects on hindering HCC growth in tumor-bearing nude
mice.
The establishment of tumor-bearing murine models was
a crucial process in this study. B ultrasonography was therefore
carried out to ensure there were no significant differences in
xenograft size among the three groups prior to administration, and
the ultrasonic outcomes presented a fine uniformity in the tumor
load in the SDI, LDI and control groups. To assess the anti-growth
potency of the self-synthesized MAP composed of B-cell epitopes of
HPSE, we administrered the tumor-bearing nude mice with the
purified antiserum containing the anti-MAP polyclonal antibodies.
As early as in 1981, this passive immunizing method applied on
immunodeficient genetically engineered mice was approved as
practical and safe by Katz et al (39), as long as the volume of heterogenic
antiserum used in the immunization was ≤0.2 ml.
To determine the binding domain of HPSE protein
reacting with the anti-MAP polyclonal antibodies contained in the
immunized rabbit serum, western blot analysis with electrophoresis
was conducted after the antiserum was isolated and purified. As a
result, two clear bands located at ~65 and 50 kDa were presented by
chemiluminescence in the anti-MAP antiserum column, while no
visible band was observed in the corresponding locations in the
negative control column. The above findings indicate the specific
antibodies induced by the synthesized MAP were probably bound with
the dominant epitopes of the HPSE precursor protein and its large
subunit monomer. It has been previously reported that HPSE
polypeptide antibodies derived from the epitopes contained in the
region of N-terminus of the 50-kDa large subunit may effectively
block HPSE, reduce the amount of HPSE or slash the enzymatic
activity (40). However, in this
study and former studies (23,24),
the HPSE activity of HCC97-H cells was evidently also decreased
when reacted with anti-MAP polyclonal antibodies induced by the
self-designed MAP composed of B-cell eipitopes (279–293 of the
large subunit) of HPSE, which was not present in the N-terminus of
the 50 kDa large subunit.
To verify the anti-growth potency of the synthesized
MAP, the specific anti-MAP antibodies induced by the HPSE B-cell
epitope-based vaccine were intravenously immunized to the murine
models bearing HCC xenografts, while the unimmunized rabbit serum
was administrered to the control group. Three or six weeks after
passive immunization, size-monitoring results demonstrated that HCC
volumes in the SDI and LDI groups were significantly smaller than
that in the control group. There were also significant differences
between the SDI and LDI groups, which suggested that the specific
antibodies induced by the self-synthesized MAP vaccine could
markedly and dose-dependently limit the over-growth of HCC in
mice.
The enzymolysis of HPSE could split HS in HSPG,
release and activate HS-linking cytokines such as bFGF and VEGF,
which are fundamental positive regulators of angiogenesis,
stimulating the proliferation of endothelial cells and enhancing
vascular permeability (41).
Findings of previous studies have reported that VEGF and bFGF were
closely correlated with HCC growth, angiogenesis and probably
prognosis (42,43). In this experiment, the content of
VEGF and bFGF was semi-quantitatively assessed by calculating the
percentage of positively immunostained tumor cells, and was
quantitatively analyzed by ELISA. Immunohistochemistry demonstrated
that the expression of VEGF or bFGF in the SDI and LDI groups was
much lower than that in the control group, and VEGF and bFGF
expressed significantly higher in the LDI than in the SDI group.
ELISA results showed that the serous concentration of VEGF and bFGF
in the LDI and SDI groups were significantly lower than that in the
control group. The VEGF and bFGF levels in the LDI group were also
significantly lower than that in the SDI group. To assess the
impact of synthesized HPSE B-cell epitope-based MAP on the
angiogenesis of HCC, we semi-quantitatively counted MVD in the
immunochemical sections, which was deemed as the gold standard of
angiogenesis and may be presented by CD34 immunostaining (44). The result showed that the MVD value
in the LDI or SDI group was much smaller than that in the control
group, and there was also a significant difference between the SDI
and LDI groups. Based on the above findings, we hypothesize that
the specific polyclonal antibodies induced by self-synthesized MAP
composed of HPSE B-cell epitopes were able to inhibit the release
and expression of VEGF and bFGF, reduce MVD, and therefore suppress
the malignant angiogenesis and over-growth. Moreover, similar to
the findings of Xu et al (45), we identified a certain positive
correlation between VEGF/bFGF levels and MVD. In this correlation,
the stronger the expression of VEGF or bFGF, the higher the
MVD.
In conclusion, our experiment suggests that the
self-designed MAP vaccine composed of B-cell epitopes of human HPSE
is capable of limiting human HCC growth in mice, which is probably
induced by suppressing HPSE activity and tumor-associated
angiogenesis, by virtue of its specific anti-MAP polyclonal
antibodies. This study provides theoretical evidence for further
study of the synthesized HPSE MAP vaccine in treating HCC.
Acknowledgements
This study was supported by the Zhejiang Medicine
and Public Health Research Program (no. 2013KYA014) and National
Natural Science Foundation of China (81400682). The authors would
like to thank Professor HouQuan Tao for his excellent technical
assistance.
References
|
1
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rabinovich GA, Gabrilovich D and Sotomayor
EM: Immunosuppressive strategies that are mediated by tumor cells.
Annu Rev Immunol. 25:267–296. 2007. View Article : Google Scholar
|
|
3
|
Ostrand-Rosenberg S: Immune surveillance:
a balance between protumor and antitumor immunity. Curr Opin Genet
Dev. 18:11–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borghaei H, Smith MR and Campbell KS:
Immunotherapy of cancer. Eur J Pharmacol. 625:41–54. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zarour HM and Ferrone S: Cancer
immunotherapy: Progress and challenges in the clinical setting. Eur
J Immunol. 41:1510–1515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van der Burg SH, Bijker MS, Welters MJ, et
al: Improved peptide vaccine strategies, creating synthetic
artificial infections to maximize immune efficacy. Adv Drug Deliv
Rev. 58:916–930. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bijker MS, Melief CJ, Offringa R and van
der Burg SH: Design and development of synthetic peptide vaccines:
past, present and future. Expert Rev Vaccines. 6:591–603. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brunsvig PF, Kyte JA, Kersten C, et al:
Telomerase peptide vaccination in NSCLC: a phase II trial in stage
III patients vaccinated after chemoradiotherapy and an 8-year
update on a phase I/II trial. Clin Cancer Res. 17:6847–6857. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joshi VG, Dighe VD, Thakuria D, et al:
Multiple antigenic peptide (MAP): a synthetic peptide dendrimer for
diagnostic, antiviral and vaccine strategies for emerging and
re-emerging viral diseases. Indian J Virol. 24:312–320. 2013.
View Article : Google Scholar :
|
|
10
|
Vlodavsky I, Ilan N, Naggi A and Casu B:
Heparanase: structure, biological functions, and inhibition by
heparin-derived mimetics of heparin sulfate. Curr Pharm Des.
13:2057–2073. 2007. View Article : Google Scholar
|
|
11
|
Vlodavsky I, Elkin M, Abboud-Jarrous G, et
al: Heparanase: one molecule with multiple functions in cancer
progression. Connect Tissue Res. 49:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roy M and Marchetti D: Cell surface
heparan sulfate released by heparanase promotes melanoma cell
migration and angiogenesis. J Cell Biochem. 106:200–209. 2009.
View Article : Google Scholar :
|
|
13
|
Nasser NJ, Avivi A, Shafat I, et al:
Alternatively spliced Spalax heparanase inhibits extracellular
matrix degradation, tumor growth, and metastasis. Proc Natl Acad
Sci USA. 106:2253–2258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vlodavsky I, Goldshmidt O, Zcharia E, et
al: Mammalian heparanase: involvement in cancer metastasis,
angiogenesis and normal development. Semin Cancer Biol. 12:121–129.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McKenzie EA: Heparanase: a target for drug
discovery in cancer and inflammation. Br J Pharmacol. 151:1–14.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sommerfeldt N, Beckhove P, Ge Y, et al:
Heparanase: a new metastasis-associated antigen recognized in
breast cancer patients by spontaneously induced memory T
lymphocytes. Cancer Res. 66:7716–7723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miao HQ, Liu H, Navarro E, et al:
Development of heparanase inhibitors for anti-cancer therapy. Curr
Med Chem. 13:2101–2111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang XD, Wan Y, Chen L, et al:
H-2Kb-restricted CTL epitopes from mouse heparanase elicit an
antitumor immune response in vivo. Cancer Res. 68:1529–1537. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang XJ, Yuan L, Hu J, et al:
Phosphomannopentaose sulfate (PI-88) suppresses angiogenesis by
downregulating heparanase and vascular endothelial growth factor in
an oxygen-induced retinal neovascularization animal model. Mol Vis.
18:1649–1657. 2012.PubMed/NCBI
|
|
20
|
Dredge K, Hammond E, Handley P, et al:
PG545, a dual heparanase and angiogenesis inhibitor, induces potent
anti-tumour and anti-metastatic efficacy in preclinical models. Br
J Cancer. 104:635–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He X, Brenchley PE, Jayson GC, et al:
Hypoxia increases heparanase-dependent tumor cell invasion, which
can be inhibited by antiheparanase antibodies. Cancer Res.
64:3928–3933. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borsig L, Vlodavsky I, Ishai-Michaeli R,
et al: Sulfated hexasaccharides attenuate metastasis by inhibition
of P-selectin and heparanase. Neoplasia. 13:445–452.
2011.PubMed/NCBI
|
|
23
|
Du L, Wang HJ, Yang JM, et al: T-helper
epitope peptide improves immunological effects of the B cell
epitopes of human heparanase protein. Chin J Microbiol Immunol.
28:869–872. 2008.
|
|
24
|
Yang JM, Wang HJ, Du L, et al: Screening
and identification of novel B cell epitopes in human heparanase and
their anti-invasion property for hepatocellular carcinoma. Cancer
Immunol Immunother. 58:1387–1396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Cai M, Wang X and Li X: One simple
and efficient method for purification of IgG McAb from mice
ascites: caprylic acid/ammonium sulfate precipitation. Hua Xi Yi Ke
Da Xue Xue Bao. 30:455–456. 1999.(In Chinese).
|
|
26
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
27
|
Bridges JF, Dong L, Gallego G, et al:
Prioritizing strategies for comprehensive liver cancer control in
Asia: a conjoint analysis. BMC Health Serv Res. 12:3762012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aravalli RN, Cressman EN, Steer CJ, et al:
Cellular and molecular mechanisms of hepatocellular carcinoma: an
update. Arch Toxicol. 87:227–247. 2013. View Article : Google Scholar
|
|
29
|
Darzynkiewicz Z: Will cancer immunotherapy
fail? Scientist. 20:142006.
|
|
30
|
Kratky W, Reis e Sousa C, Oxenius A and
Spörri R: Direct activation of antigen-presenting cells is required
for CD8+ T-cell priming and tumor vaccination. Proc Natl
Acad Sci USA. 108:17414–17419. 2011. View Article : Google Scholar
|
|
31
|
Koop A, Sellami N, Adam-Klages S, et al:
Down-regulation of the cancer/testis antigen 45 (CT45) is
associated with altered tumor cell morphology, adhesion and
migration. Cell Commun Signal. 11:412013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weldon JE, Xiang L, Zhang J, et al: A
recombinant immunotoxin against the tumor-associated antigen
mesothelin reengineered for high activity, low off-target toxicity,
and reduced antigenicity. Mol Cancer Ther. 12:48–57. 2013.
View Article : Google Scholar :
|
|
33
|
Vonderheide RH: Universal tumor antigens
for cancer vaccination: targeting telomerase for immunoprevention.
Discov Med. 7:103–108. 2007.PubMed/NCBI
|
|
34
|
Zhang YF, Tang XD, Gao JH, et al:
Heparanase: a universal immunotherapeutic target in human cancers.
Drug Discov Today. 16:412–417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palena C and Schlom J: Vaccines against
human carcinomas: strategies to improve antitumor immune responses.
J Biomed Biotechnol. 2010:3806972010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bonertz A, Weitz J, Pietsch DH, et al:
Antigen-specific Tregs control T cell responses against a limited
repertoire of tumor antigens in patients with colorectal carcinoma.
J Clin Invest. 119:3311–3321. 2009.PubMed/NCBI
|
|
38
|
Ilan N, Elkin M, Vlodavsky I, et al:
Regulation, function and clinical significance of heparanase in
cancer metastasis and angiogenesis. Int J Biochem Cell Biol.
38:2018–2039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katz M, Lynn M, Solotorovsky M, et al:
Serological and biological activities of anti-Haemophilus
influenzae ribosomal serum. Infect Immun. 31:1125–1131.
1981.PubMed/NCBI
|
|
40
|
Levy-Adam F, Abboud-Jarrous G, Guerrini M,
et al: Identification and characterization of heparin/heparan
sulfate binding domains of the endoglycosidase heparanase. J Biol
Chem. 280:20457–20466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fidler IJ and Ellis LM: The implications
of angiogenesis for the biology and therapy of cancer metastasis.
Cell. 79:185–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
An FQ, Matsuda M, Fujii H and Matsumoto Y:
Expression of vascular endothelial growth factor in surgical
specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol.
126:153–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Poon RT, Ng IO, Lau C, et al: Serum
vascular endothelial growth factor predicts venous invasion in
hepatocellular carcinoma: a prospective study. Ann Surg.
233:227–235. 2011. View Article : Google Scholar
|
|
44
|
Yang P, Yuan W, He J, et al:
Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular
carcinoma: implications for tumor progression and prognosis.
Hepatol Res. 39:1169–1177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu YZ, Zhu Y, Shen ZJ, et al: Significance
of heparanase-1 and vascular endothelial growth factor in
adrenocortical carcinoma angiogenesis: potential for therapy.
Endocrine. 40:445–451. 2011. View Article : Google Scholar : PubMed/NCBI
|