Introduction
Osteosarcoma (OS) is the most common primary bone
tumor in children and young adults characterized by osteoid
production and osteoblastic formation (1). Considerable improvement in the
prognosis of patients has been observed due to the development of
various adjuvant chemotherapies (2). However, the complete effectiveness of
these chemotherapies is controversial as 80% of the patients would
eventually developed metastatic disease. Thus, the outcome remains
unsatisfactory for these patients (3). Therefore, the identification of
effector molecules and signaling pathways that exhibits a close
relationship with tumor progression and metastasis in order to
improve the existing OS treatment, is crucial.
MicroRNAs (miRNAs) are small (22–24 nucleotides)
non-coding RNAs emerging as promising diagnostic and prognostic
tools of malignant neoplasm (4).
miRNA is crucial in the regulation of diverse target mRNAs at the
level of mRNA degradation or translation (5). It has been reported that biological
activities of various miRNAs contribute to invasion and metastasis
in OS (6). Emerging evidence
indicates that miR-26b is downregulated in breast cancer (7), nasopharyngeal carcinoma (8), colorectal cancer (9), hepatocellular carcinoma (10) and in OS (6). However, the biological effect of
miR-26b in OS tumorigenesis and metastasis remains to be
elucidated. Therefore, it is of utmost significance to investigate
the mechanism of miR-26b in OS.
Metabolic reprogramming accompanied by persistent
aerobic glycolysis and deregulated mitochondrial function promotes
OS progression and metastasis (11,12).
6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatases (PFKFBs)
possess bifunctional enzymatic activities that regulate a high
glycolytic flux by controling the intracellular concentration of
fructose-2,6-bisphosphate (F2,6BP), a potent allosteric regulator
of 6-phosphofructo-1-kinase (PFK-1) (13). PFKFB3 is one of the four isoforms of
the PFKFB family, the protein expression of which is elevated in
most types of tumor, including OS (14), suggesting its contribution to
attaining glycolytic phenotype by several malignancies (11).
In the present study, we demonstrated that restoring
the expression of miR-26b in the human U2OS OS cell line caused
profound suppression of the proliferation, migration, invasion,
cell cycle arrest and induction of apoptosis. Furthermore,
computational prediction and experimental confirmation suggested
that the tumor suppressive effects of miR-26b in OS cells were
mediated through the inhibition of PFKFB3. Our results showed that
an ectopic expression of miR-26b significantly suppressed the
expression of PFKFB3 and glycolytic activity indicated by a
decrease in extracellular lactate, ATP levels and glucose
consumption. These results emphasized the antiproliferative and
anti-metabolic role of miR-26b in OS cells and provided a basis to
therapeutically intervene in metastasis of OS patients by targeting
miRNA expression.
Materials and methods
Cell culture and transfection
Human U2OS OS cells were obtained from ATCC
(Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% heat-inactivated fetal bovine
serum (FBS), streptomycin (100 lg/ml) and penicillin (100 U/ml)
(Sigma-Aldrich, St. Louis, MO, USA). Cell cultures were maintained
in 5% CO2 atmosphere at 37°C in a humidified incubator.
The hsa-miR-26b mimics (Pre-miR miRNA Precursor Product, AM17100)
and its scramble mimics (Pre-miR Negative Control, AM17111) were
purchased from Applied Biosystems (Foster City, CA, USA). PFKFB3
and control siRNA were purchased from Dharmacon (Austin, TX, USA).
Transfection of the oligonucleotides was performed using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. After 6 h the original medium was
replaced with fresh medium.
Cell proliferation assay
The cell proliferation assay was determined by Cell
Counting Kit-8 assay (Dojindo, Kumamoto, Japan), a redox assay
similar to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) according to the manufacturer’s instructions. The
proliferation level was determined at 0, 24, 48, 72 and 96 h after
transfection. CCK-8 solution (10 μl) was added to each well,
followed by incubation for 2 h at 37°C. The absorbance at 450 nm
was determined by a multiplate reader (Lambda Bio-20; Beckman
Coulter, Inc., Brea, CA, USA). The cell proliferation assay was
carried out six times.
Cell cycle analysis
Transfected OS cells were collected and fixed in 75%
ethanol at −20°C for 16 h. For the cell cycle analysis, the cells
were collected, washed twice in cold phosphate-buffered saline
(PBS) and stained with propidium iodide (PI) (Invitrogen), then
examined with a BD FACSCalibur flow cytometer (BD Biosciences, San
Jose, CA, USA). DNA histograms were analyzed with ModFit software
(BD Biosciences). The experiments were repeated in triplicate.
Apoptosis analysis
The percentage of apoptotic cells was determined by
Annexin V-FITC and PI staining (BD Pharmingen, San Diego, CA, USA)
according to the manufacturer’s instructions. The apoptotic
morphology was determined by 4,6-diamidino-2-phenylindole (DAPI)
staining. Control or miRNA transfected cells were stained with DAPI
(1 μg/μl; Sigma-Aldrich) to visualize apoptotic cells with
fragmented or condensed nuclei. At least five visual fields were
observed under a fluorescence microscope for each sample (Nikon
Eclipse 80i; Nikon, Tokyo, Japan).
Wound-healing assay
After transfections, the cells were seeded in 6-well
plates at 2×105 cells/well. After 24 h, the cells were
washed with PBS and linear scratch wounds were created using a
sterile 200 μl pipette tip. The cells were then washed three times
with PBS and incubated in DMEM medium containing 5% FBS. Migration
at the wound site was observed under an inverted microscope and
images were captured at 0 and 24 h. The percentage of wound closure
was analyzed using ImageJ (version 1.44 software; NCBI).
Experiments were performed in triplicate.
Cell invasion assay
U2OS cells were transfected with the miR-26b mimics,
PFKFB3-siRNA or negative control (NC), cultivated for 24 h, and
transferred at the top of Matrigel-coated chambers (24-well insert,
8-μm pore size; BD Biosciences) in a serum-free DMEM. Medium
containing 10% fetal calf serum was added to the lower chamber as a
chemoattractant. After incubation for 48 h non-invaded cells were
removed from the upper well with cotton swabs while the invaded
cells were subjected to H&E staining, and photographed
(magnification, ×200) in five independent fields for each well.
Each test was repeated in triplicate.
Gelatin zymography
This experiment was conducted to detect MMP-2 and
MMP-9 enzyme activity. The medium without the serum was collected
from the transfected cells after 24 h. MMP activity was measured by
SDS-PAGE under non-reducing conditions (the gel contained 1%
gelatin and 30% acrylamide). Following electrophoresis, gels were
incubated in renaturation buffer (2.5% Triton X-100 and 5 mM
CaCl2) for 30 min at room temperature. After that the
gel was equilibrated in zymogram-developing buffer (50 mM Tris, 200
mM NaCl, 10 mM CaCl2, pH 7.5) for 30 min at room
temperature and then incubated at 37°C overnight. MMP activity was
visualized by staining with Coommasie Blue R-250
(Sigma-Aldrich).
Western blot analysis
Western blotting was performed according to the
standard methods as previously described (11) using anti-VEGF, anti-MMP-9,
anti-MMP-2, anti-cyclinD1, anti-p27 (Cell Signaling, Danvers, MA,
USA), anti-LDHA, anti-GLUT-1 antibodies (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and anti-PFKFB3 (Proteintech, Chicago,
IL, USA). The membranes were stripped and reprobed with
anti-β-actin antibody (Sigma-Aldrich) as a loading control. The
experiments were repeated three times.
Measurement of secreted VEGF protein
The level of VEGF in the cell culture supernatant
was measured with a commercially available ELISA kit (DVE00;
R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions.
Quantitative RT-PCR
Total RNA and miRNA was extracted using TRIzol
reagent (Invitrogen) and miRNeasy Mini kits (Qiagen, Valencia, CA,
USA) according to the manufacturer’s instructions. Total RNA (2 μg)
was reverse transcribed with Omniscript Reverse Transcriptase
(Qiagen) in a 20-μl reaction mixture using oligo-d(T)12–18 primer.
RT-PCR was carried out with the Platinum PCR SuperMix (Invitrogen).
Quantitative RT-PCR (RT-qPCR) for PFKFB3 mRNA was performed using a
SYBR-Green kit (Roche Diagnostics, Mannheim, Germany) and Light
Cycler 480 System II (Roche). The primers used for the specific
amplification for human PFKFB3 were: mRNA:
5′-GATGCCCTTCAGGAAAGCCT-3′ and 5′-TTGAACACTTTTGTGGGGACGC-3′ (NCBI
reference sequence: NM_001145443.1) and 18S: 5′-GTAACCCGTTGA
ACCCCATT-3′ and 5′-CC ATCCAATCGGTAGTAGCG-3′ (NCBI reference
sequence: M10098). The specificity of each PCR product was assessed
by the melting curve analysis and agarose gel electrophoresis. The
expression of miR-26b was determined by RT-qPCR using TaqMan
MicroRNA Assay kits (Life Technologies, Carlsbad, CA, USA).
Expression level of 18S was used as internal control for mRNAs, and
the U6 level was regarded as an internal miRNA control.
Vectors and luciferase reporter
assays
The 3′UTR of human PFKFB3, which contains a miR-26b
binding site, was PCR-amplified from genomic DNA and cloned
downstream of Renilla luciferase in psiCHECK-2 vector
(Promega, Madison, WI, USA). Mutation in 3′UTR of PFKFB3
gene was generated using the QuikChange Site-Directed Mutagenesis
kit (Stratagene, Santa Clara, CA, USA). U2OS cells
(1×105) were plated on 24-well plates. After 24 h, the
cells were co-transfected with 100 ng reporter plasmid and 100
nmol/l of miR-26 mimic or scramble mimic using Lipofectamine 2000.
A luciferase reporter construct containing the miR-26b consensus
target sequence served as positive control (PC). After 48 h, the
cells were lysed using passive lysis buffer and luciferase
activities were measured with the Dual-Luciferase Assay kit
(Promega). Renilla luciferase signal was normalized to the
firefly luciferase signal. Three independent experiments were
performed in triplicate.
Measurement of lactate and ATP
production
U2OS cells were treated with or without miR-26b or
scramble mimics (100 nm) in serum-free medium for 24 h. Culture
medium (50 ml) was collected from each sample and measured for
lactate concentration using a lactate ELISA kit (BioVision,
Mountain View, CA, USA) according to the manufacturer’s
instructions. While intracellular levels of ATP in the indicated
groups were measured using s CellTiter-Glo® Luminescent
Assay (Promega), the values were normalized to the scrambled
control.
Measurement of glucose uptake, oxygen
consumption and mitochondrial membrane potential (MMP)
For glucose uptake, 1×105 cells were
stained with fluorescent glucose analogue
6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose
(6-NBDG; 50 μM; Invitrogen) for 20 min, washed with PBS and
analyzed by flow cytometry. O2 consumption was measured
using an XF24 Extracellular Flux Analyzer (Seahorse Biosciences,
Billerica, MA, USA) as per the manufacturer’s instructions. The
experiments were repeated three times in triplicate. The data were
expressed in pMol/min. Flow cytometry was utilized to measure
alterations in MMP using cationic fluorescent dye
1,1′,3,3′-tetraethylbenzamidazolocarbocyanin iodide (JC-1;
Invitrogen). After completion of the indicated treatment time
points, the cells were loaded with 2 μM JC-1 in HBSS at 37°C for 15
min. The mean ratios of red vs. green fluorescent signal
intensities were measured in three independent experiments.
Statistical analysis
Results were presented as the means ± SD (standard
deviation) unless otherwise specified. Statistical analyses were
performed by one-way ANOVA followed by Newman-Keuls post-hoc
comparison test. P≤0.05 was considered to indicate statistically
significant differences (GraphPad Prism 5; GraphPad software, Inc.,
San Diego, CA, USA).
Results
Induced expression of miR-26b inhibited
OS cell proliferation and cell cycle progression
Findings of a recent study demonstrated significant
downregulation of miR-26b in human OS samples (6). However, the effect of miR-26b on OS
has not been fully elucidated. Thus, we examined the mechanistic
role of miR-26b in OS cells. We investigated the functional
significance of miR-26b on growth, cell cycle progression and
apoptosis in U2OS cells using miRNA mimics or scramble mimics. Our
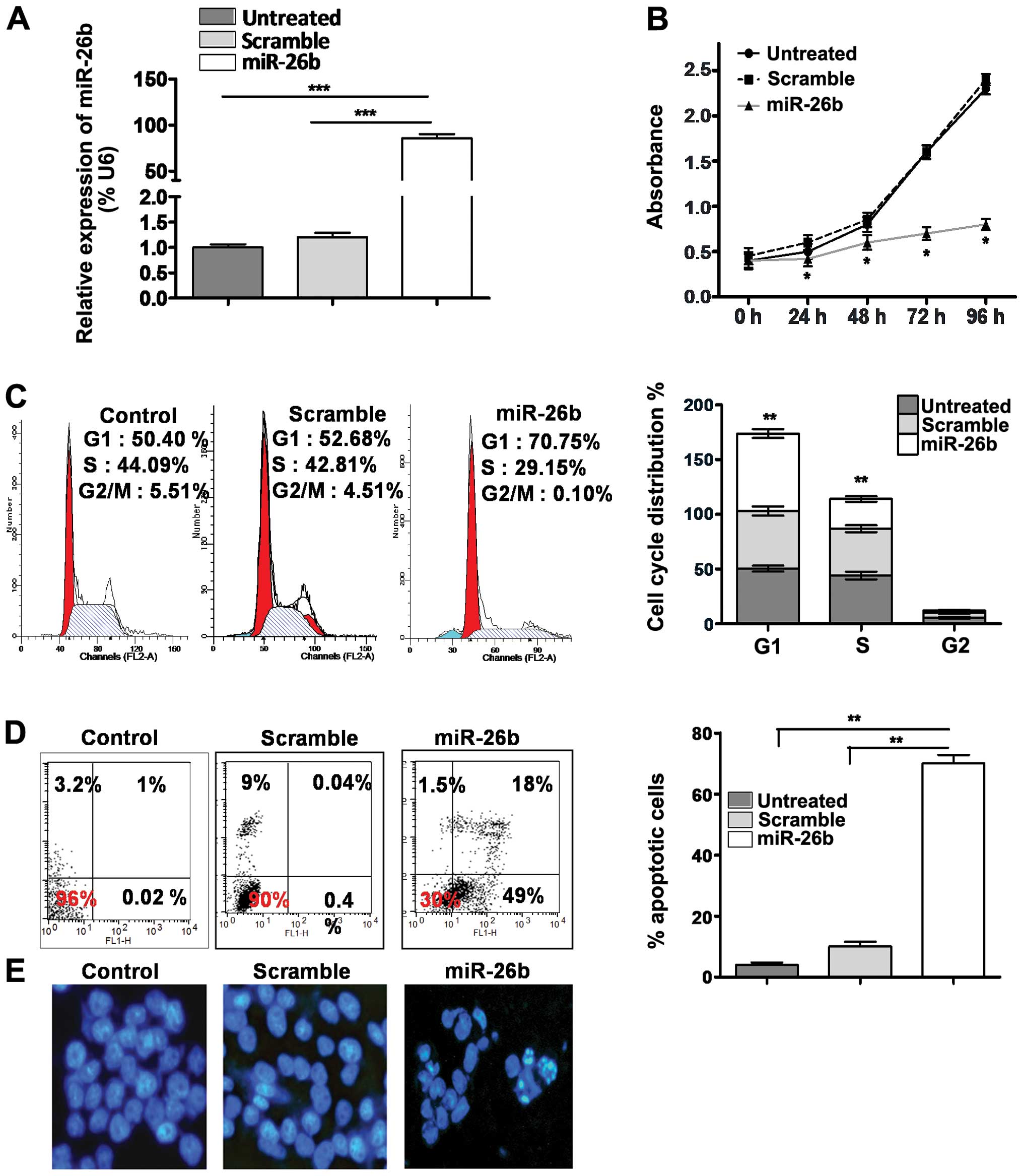
data showed a significant increase of miR-26b levels (~85-folds) in
miR-26b-transfected cells compared to that of scramble or parental
cells (P<0.001) (Fig. 1A). Then
we compared the proliferation and cell cycle changes after miR-26b
overexpression in OS cells. Our results showed that the ectopic
expression of miR-26b suppressed cell proliferation in a
time-dependent manner (P<0.05) with a subsequent elevation of
the percentage of G1-phase cells and reduction of S-phase cells (by
20%±5) (P<0.01), respectively (Fig.
1B and C). Furthermore, treatment with miR-26b resulted in the
increased induction of apoptotic cells as demonstrated by Annexin
V-FITC Apoptosis Detection kit (P<0.01) (Fig. 1D). In addition miR-26b mimic
enhanced chromatin condensation and the formation of apoptotic
bodies identifiable by staining cells with Hoechst 3342 fluorescent
dye (Fig. 1E). Taken together,
these results indicated that miR-26b efficiently inhibited cell
proliferation and cell cycle progression, and induced apoptosis
in vitro, thus manifesting the tumor suppressive role of
miR-26b in OS cells.
Effect of miR-26b on the migration and
invasion of OS cells
miR-26b has been reported to be closely associated
with invasiveness and metastasis in malignancy (15). Therefore, we studied the effects of
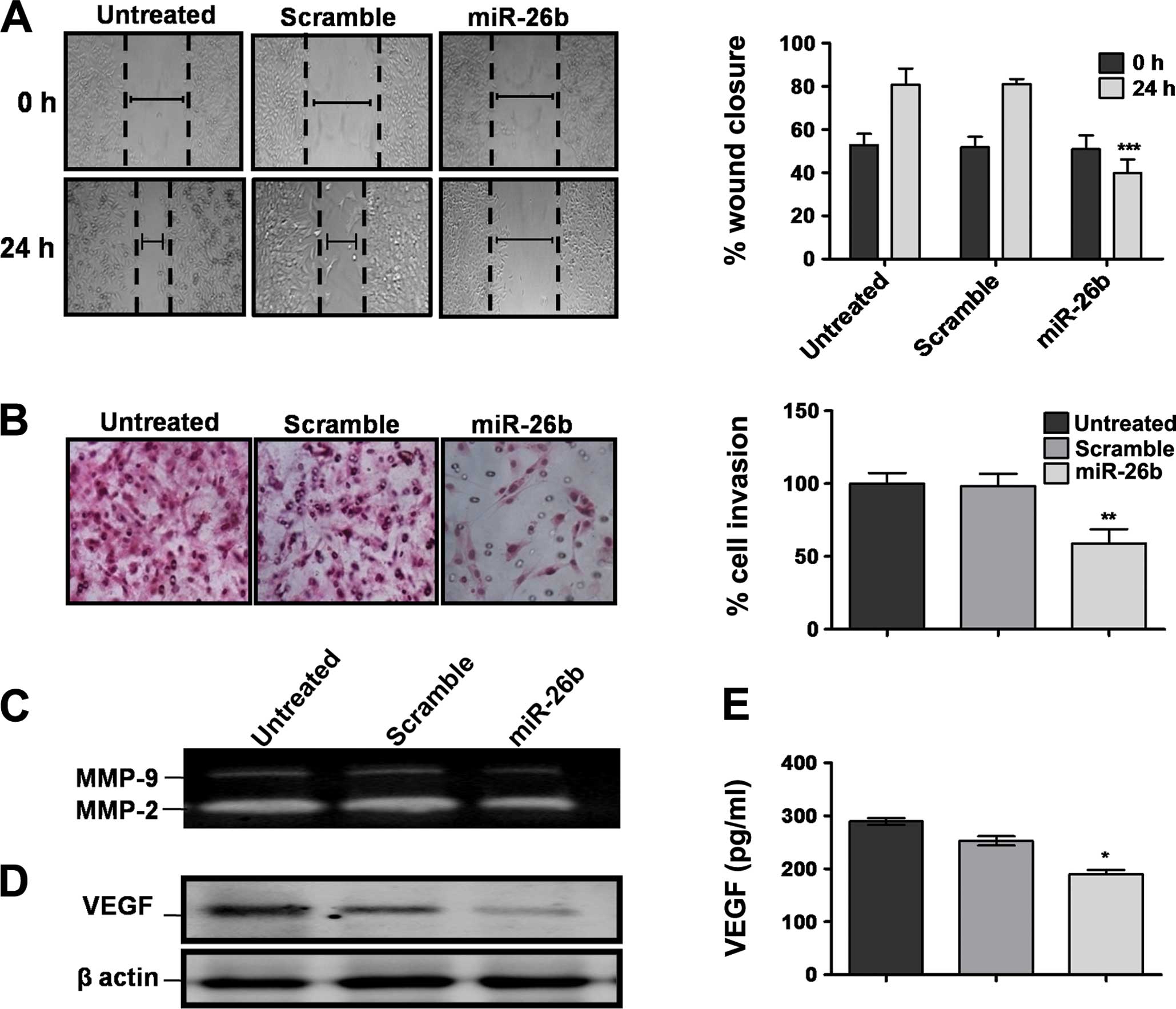
miR-26b in OS cell migration and invasion. We found that both
invading and migrating miR-26b overexpressed U2OS cells were
greatly decreased (Fig. 2A and B)
allowing only 40–50% of cells to migrate or invade respectively,
compared to those with scrambled or untreated cells (>80%).
Since MMP-2 and MMP-9 play a critical role in tumor cell
invasiveness (16), we examined the
effect of miR-26b on the enzyme activities of MMP-2 and MMP-9 by
employing gelatin zymography. The gelatinolytic activities of MMP-2
and MMP-9 were found to be reduced in conditioned media of U2OS
cells, suggesting a reduction in cell invasion by overexpressed
miR-26b (Fig. 2C). Simultaneously,
we investigated VEGF protein expression levels and found that the
miR-26b-transfected group significantly downregulated VEGF
expression and VEGF secretion in U2OS conditioned media compared to
that of scrambled or untreated groups (P<0.05) (Fig. 2D and E).
miRNA-26b targeted PFKFB3 and suppressed
the expression of LDH-A, GLUT-1, MMP-9, MMP-2 and cyclin D1
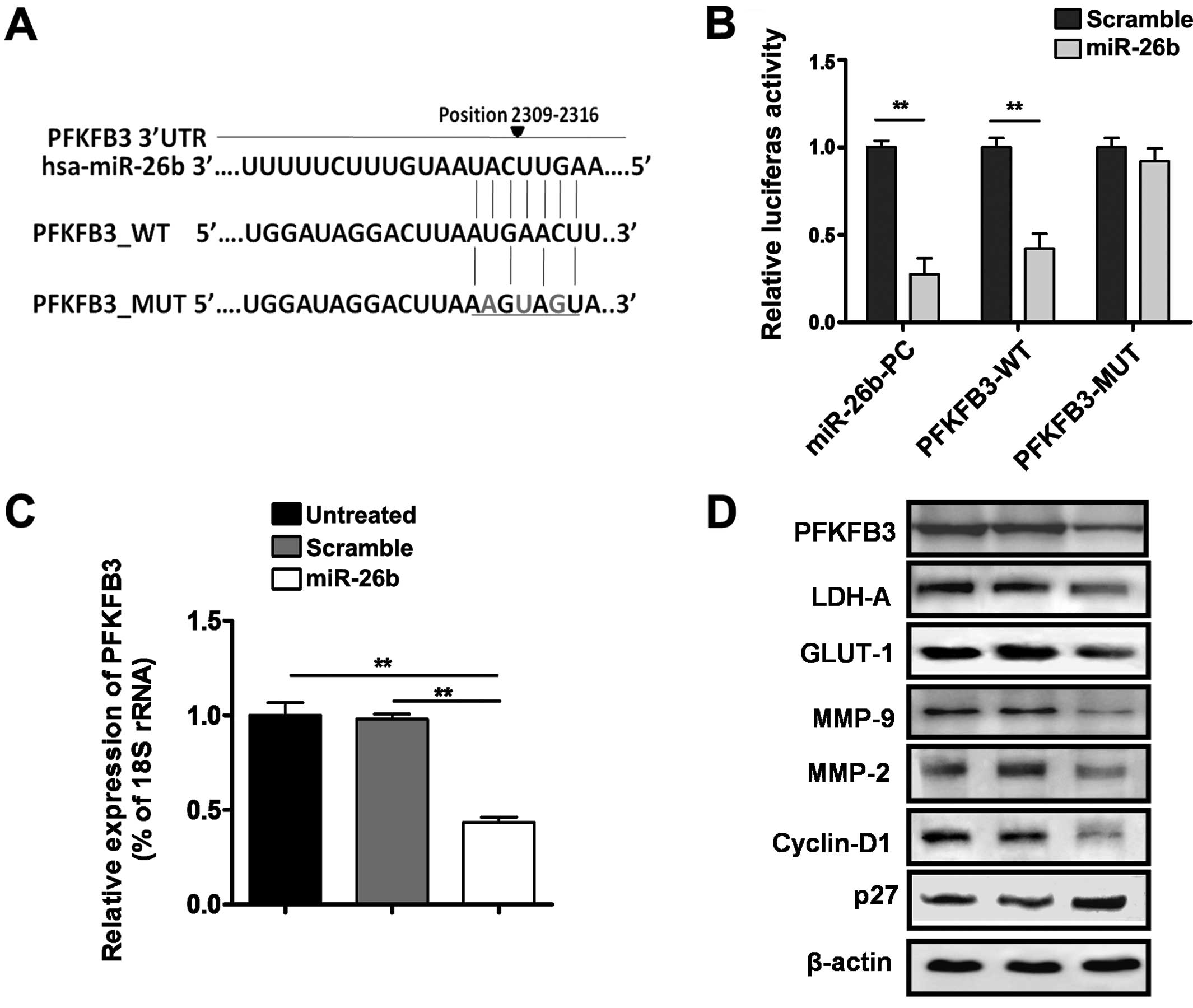
To determine the molecular mechanism of tumor
progression triggered by miR-26b in OS, putative target genes of
miR-26b were searched using Targetscan 6.2. PFKFB3 was predicted to
be the target of miR-26b (Fig. 3A).
The luciferase assay showed that miR-26b significantly suppressed
wild-type (WT) 3′UTR (~5-fold) but not the mutant (MUT) 3′UTR of
PFKFB3 reporter activity in U2OS cells (P<0.01) (Fig. 3B). In addition, overexpression of
miR-26b downregulated PFKFB3 mRNA and protein levels (P<0.01),
suggesting the post-transcriptional regulation of PFKFB3
gene expression by miR-26b (Fig. 3C and
D). To examine the mechanism of how miR-26b influences the
glycolysis and invasive phenotype of OS cells, we measured the
expression of LDHA, GLUT-1, MMP-9 and MMP-2 using western blot
analysis. The results showed that overexpression of miR-26b
decreased the expression of LDH-A, GLUT-1, MMP-9 and MMP-2 in U2OS
cells. Moreover, the expression of G1-S regulatory proteins was
analyzed demonstrating G0/G1 cell cycle arrest by down-regulation
of cyclin D1 and elevation of p27 levels in the same experimental
conditions.
Ectopic expression of miR-26b inhibits
glycolytic phenotype in OS cells
Induction of aerobic glycolysis in OS cells
accomplishes survival advantage and invasiveness accompanied by
enhanced glucose uptake, glycolytic flux into lactate and reduced
mitochondrial function (11,17).
miRNAs were shown to closely regulate cancer-associated glycolytic
pathways (18). Our findings showed
that miR-26b mediated the significant downregulation of PFKFB3
levels, the enzyme of which is known to regulate high glycolytic
flux in cancer cells (19).
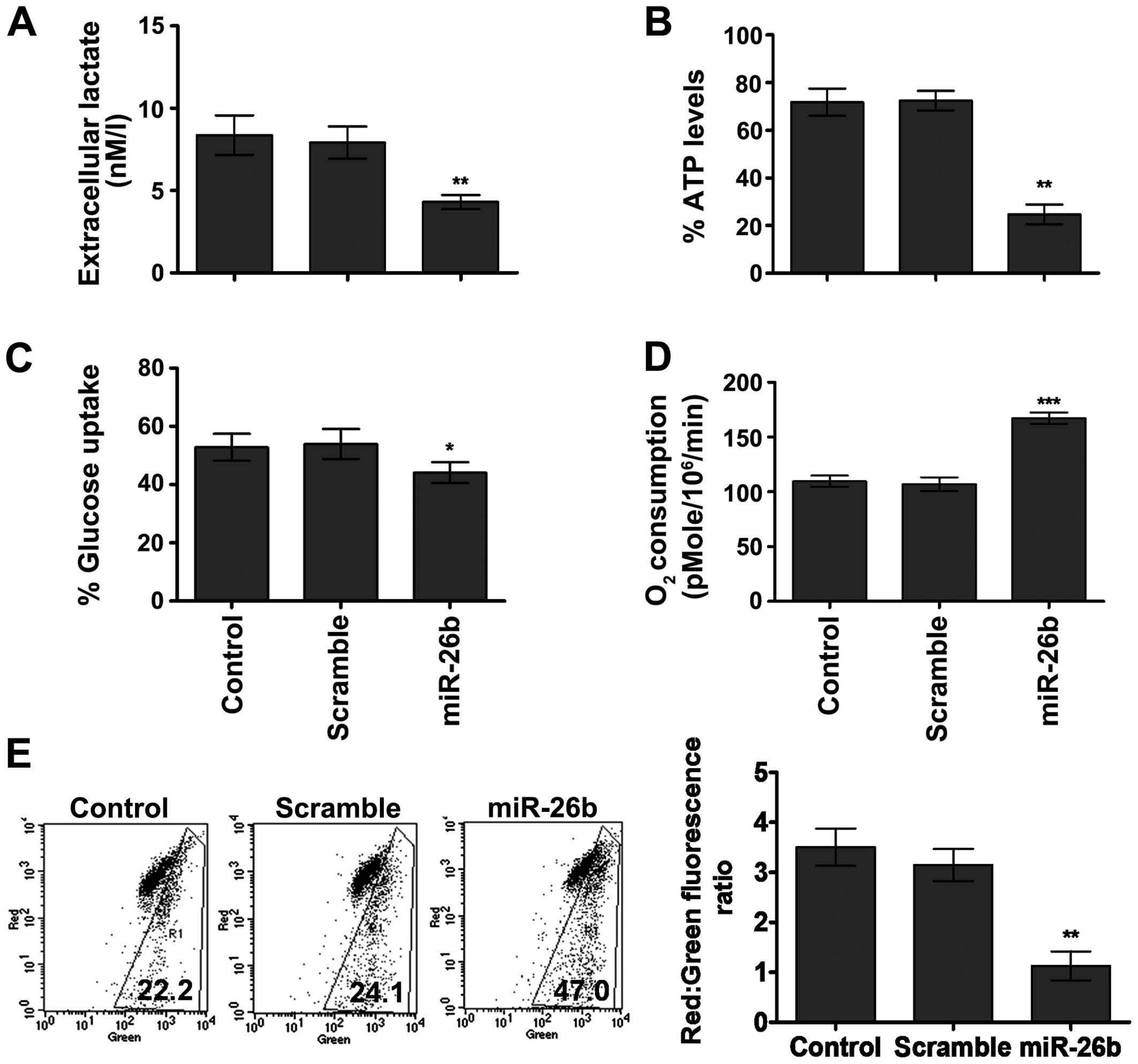
Therefore, we observed the effects of miR-26b on various glycolytic
parameters. The results indicated that cell expressing miR-26b
mimics induced a decrease in extracellular lactate (P<0.01), ATP
levels (P<0.01) and glucose consumption (P<0.05) in U2OS
cells (Fig. 4A–C). Furthermore,
suppression of the glycolytic phenotype was accompanied by a
metabolic shift towards mitochondrial respiration indicated by an
increase in the oxygen consumption rate (P<0.001) (Fig. 4D). In addition, our results showed a
significant decrease in the mitochondrial membrane potential in
cells overexpressing miR-26b compared to that of scramble or
untreated cells (P<0.01) (Fig.
4E). Taken together, these results suggested that miR-26b the
induced metabolic shift away from the glycolytic pathway and
thereby may be responsible for the suppression of migration and
invasion in OS cells.
PFKFB3 knockdown inhibited cell
proliferation and cell cycle progression in OS cells
The tumor progressive role of PFKFB3 has been
demonstrated in several types of cancer by elevation of the high
glycolytic rate (19). Our results
have shown miR-26b arbitrated antitumor and anti-metabolic effects
by potent inhibition of the PFKFB3 gene with subsequent
inhibition of the glycolytic pathway. However, the growth
inhibitory effects mediated by silencing of PFKFB3 in OS have not
been studied previously. In line of these observations, we
hypothesized that the direct suppression of PFKFB3 may have an
inhibitory effect on cell population growth in OS cells. To
confirm, we silenced the expression of PFKFB3 by RNA interference
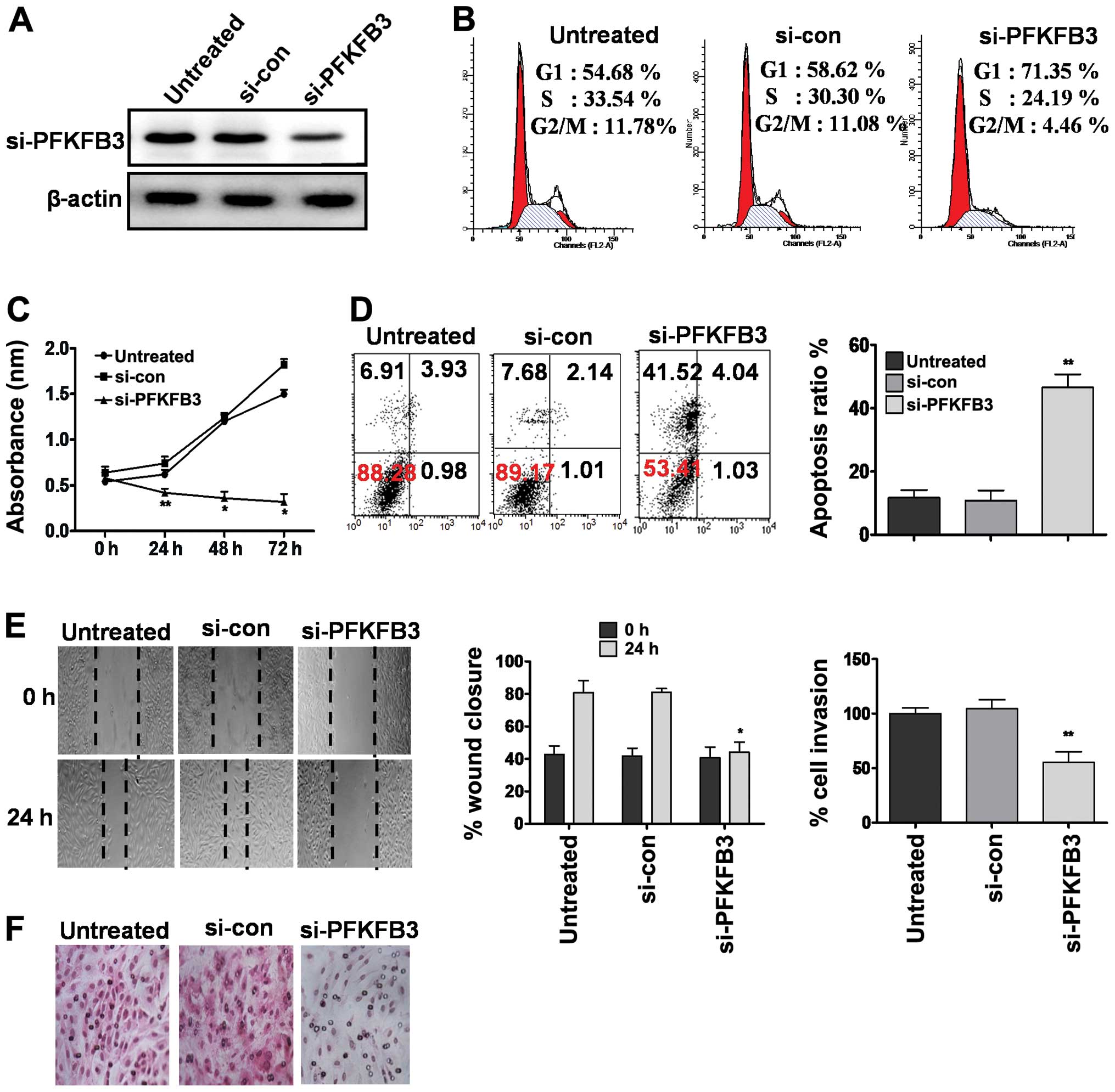
directed against the PFKFB3 gene (Fig. 5A). Results showed that inhibition of
PFKFB3 expression markedly arrested cell cycle progression
(Fig. 5B), reduced cell
proliferation (Fig. 5C) and induced
apoptosis (Fig. 5D), accompanied by
impaired cell migration (Fig. 5E)
and cell invasion (Fig. 5F). Taken
together, these findings suggested that PFKFB3 induces OS
progression and aggressiveness. Overexpression of miR-26b mediated
suppression of cell proliferation and invasion were partially
attributed to the inhibition of PFKFB3 gene expression.
Discussion
miRNAs are short non-coding RNAs that
post-transcriptionally modify gene expression in eukaryotic cells.
Expression of single miRNA modulates many cell functions by
regulating the expression of a wide array of genes (20). The differential expression of miRNA
between normal and tumor tissues has been previously identified.
This emphasizes the tumor-promoting role of dysregulated miRNA
expression in cancer cells (20).
Therefore, understanding the functional mechanism of miRNAs by
identification of its putative molecular targets is important.
A recent study has demonstrated the miRNA expression
signature associated with OS-characterizing pathogenesis, clinical
metastasis and chemotherapy response (6). Expression profiling from 18 pretreated
OS tumors were compared to 12 normal bone tissues. RT-qPCR
confirmed differential expression of miRNAs with substantial
downregulation of miR-26b in OS patient samples compared to healthy
individuals (6). However, the
precise molecular mechanism mediated by miR-26b in OS remains
unclear. To the best of our knowledge, in the present study, we
performed for the first time functional assays to provide insight
into the biological role played by miR-26b in OS. Few recent
studies have reported the miR-26b downregulation in other types of
cancer including breast cancer (15), hepatocellular (21), nasopharyngeal (8) and lung carcinoma, and glioma (22,23).
Mechanistically, miR-26b has shown to be tumor suppressive by
modulating a diverse array of cellular and molecular responses in
cancer cells. The putative molecular targets of miR-26b-mediated
antiproliferative and apoptogenic response includes NFkB, USP9X in
HCC (24,25), EphA2 in glioma (23), PTGS2, SLC7A11 in breast cancer
(26,27) and SODD in melanoma (28).
We have shown that reinstating the expression of
miR-26b inhibited OS cell proliferation, cell cycle progression and
apoptosis induction. OS encounters treatment failure due to
metastasis and chemoresistance, while the underlying mechanism
remains unclear. Therefore, we investigated the role of miR-26b on
OS cell migration and invasion. The results showed that
overexpressed miR-26b significantly suppressed the migration and
invasive ability of U2OS cells in vitro. Previous studies
have demonstrated the enhanced expression of matrix
metalloproteinases (MMPs) such as MMP-2 and MMP-9 in promoting OS
cell metastasis by degrading components of the basement membrane
and epimatrix (16). In addition,
an increased expression of vascular endothelial growth factor
(VEGF) has been associated with increasing malignant potential of
OS cells (29). We found that the
ectopic expression of miR-26b significantly downregulated MMP-2 and
MMP-9 enzymatic activities and VEGF expression levels. Thus, our
results suggest the tumor suppressive role of miR-26b, which
concurs with other studies (23–26)
and provided a basis for identification of a promising therapeutic
agent for OS.
Few recent studies have demonstrated the unique
defects in mitochondrial oxidative phosphorylation in OS cells
resulting in metabolic shift towards the glycolytic pathway
(11). The high prevalence of
glycolysis and dysregulation of oxidative metabolism, known as the
Warburg effect, provides survival advantage and aggressive
phenotype in OS cells (11,17,30).
Moreover, the administration of 2-deoxy-d-glucoe (2-DG), a potent
glycolytic inhibitor has been shown to sensitize OS cell growth and
bioenergetic functions demonstrated by reduced cell viability and
intracellular ATP levels, indicating glycolytic dependency of OS
cells (31). Another study has
demonstrated the anti-metastatic effects of glycolytic inhibition
via 2-DG in the highly metastatic DLM8-luc-M1 OS cell line
accompanied by cytoskeletal rearrangements and inhibition of
cathepsin expression (17).
Therefore, the renewed interest to characterize the metabolic
vulnerabilities in OS has led to examination of the underlying
genes including their products and microRNAs, which may provide a
novel avenue for metastatic prevention.
In line of these evidences, we identified PFKFB-3 as
a direct functional target of miR-26b using a prediction program.
Computational analysis revealed binding sites for the miR-26b seed
sequence at 3′UTR of PFKFB3. Furthermore, reinstatement of miR-26b
expression led to the decrease in luciferase activity of wild-type
PFKFB3 3′UTR in U2OS cells whereas the site-directed mutation
attenuated miR-26b regulation. In addition, results from the
RT-qPCR and protein expression analysis indicated that the ectopic
expression of miR-26b suppressed mRNA and protein levels of PFKFB3
simultaneously. Taken together, these results suggest that miR-26b
regulated the expression of PFKFB3 by directly targeting 3′UTR of
PFKFB3 in OS cells.
PFKFB3 is a key regulator of high glycolytic flux in
various types of cancer compared to normal tissues (19), regulated by HIF1α, PTEN and Akt
(32–34). Notably, PFKFB3 has drawn particular
interest in the pharmaceutical industry since mRNA and protein
levels of PFKFB3 are upregulated in most tumor types (19). PFKFB3 regulates the synthesis of
fructose 2,6-bisphosphate (F26BP) which is an activator of
6-phosphofructo-1-kinase (PFK-1), a key step of glycolysis.
Notably, genomic deletion of PFKFB3 suppressed glucose metabolism
and tumor growth in vitro and in vivo (35) rendering this enzyme a potential
target for anticancer therapy. Previously a small molecule
competitive inhibitor of PFKFB3 was identified, known as
3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO). 3PO induced
the suppression of glucose metabolism and antitumor activity in
several human cancer cell lines in vitro and xenograft
tumors in vivo (36) but the
efficacy of 3PO was compromised due to metabolic resistance and its
pharmacokinetic profile, which renders it inappropriate for
evaluation in human subjects. Recently, the functional involvement
of PFKFB3 in the regulation of CDK1 activity has been shown where
siRNA-mediated silencing of PFKFB3 caused cell cycle arrest at
G0/G1 accompanied by increased apoptosis in HeLa cells (37). These studies signify that
re-establishment for the development of novel molecular inhibitors
of PFKFB3 is necessary. miRNA-mediated regulation of altered
metabolic pathways in various types of cancer has been identified
(18). Emerging evidence has proven
that miRNAs are significant regulators of glucose metabolism
although miRNA predictions have yet to be verified. Based on
microarray- and NGS-comprised validation methods, miRNAs including
hsa-miR-26b-5p and hsa-miR-330-3p are predicted to obtain putative
binding sites at 3′UTR of PFKFB3 (Linkouts: TargetScan 5.1,
MicroCosm, microRNAMap 2.0) although the functional validation
remained to be investigated.
As demonstrated, PFKFB3 is a crucial component of
aerobic glycolysis in cancer cells (38) including OS (14). We investigated the effects of
miR-26b on molecules modulating glycolysis, invasion and cell cycle
progression in OS cells. The results showed that the overexpression
of miR-26b significantly downregulated the protein expression of
PFKFB3, lactate dehydrogenase (LDHA), glucose transporter (GLUT-1),
MMP-9, MMP-2 and cyclin D1 while p27 levels were upregulated. The
results were consistent with the decrease of glycolytic phenotype
demonstrated by a decrease in extracellular lactate, ATP levels and
glucose consumption in OS cells. By contrast, we observed an
elevated oxygen consumption rate accompanied by loss of
mitochondrial membrane potential. These results indicate that
miR-26b substantially downregulated glycolytic activity but
enhanced mitochondrial respiration without a concominant increase
in energy production suggesting participation of miR-26b in the
possession of glycolytic dependency by OS cells. In addition,
antitumor effects induced by miR-26b were mediated through the
downregulation of PFKFB3 permitting the inactivation of the
glycolytic pathway.
In summary, our findings have demonstrated the tumor
suppressive effects of miR-26b in OS cells through suppression of
the glycolytic pathway by targeting PFKFB3 gene. However,
restoration of miR-26b decreased the expression of LDHA, GLUT1,
MMP-9, MMP-2 and cyclin D1 leading to the attenuation of glycolytic
activity, cell proliferation, migration, invasion and apoptosis
induction in vitro. In addition, genomic inhibition of
PFKFB3 alone induced significant antiproliferative effects in OS
cells. These results show a new approach in the molecular therapy
of OS by the overexpression of miR-26b, having the potential to
manifest substantial tumor suppressive effects by targeting altered
metabolism in cancer cells. The findings suggest further
investigation of the miR-26b as a promising biomarker and
therapeutic target in OS treatment.
Acknowledgements
This study was supported by the Science and
Technology Planning Project of Zhejiang Province (no.
2012B031834032), and the Zhejiang Natural Science Foundation (no.
S24230400143).
References
|
1
|
Geller DS and Gorlick R: Osteosarcoma: a
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z and Cai H, Lin L, Tang M and Cai H:
Upregulated expression of microRNA-214 is linked to tumor
progression and adverse prognosis in pediatric osteosarcoma.
Pediatr Blood Cancer. 61:206–210. 2014. View Article : Google Scholar
|
|
5
|
Ambros V: MicroRNA pathways in flies and
worms: growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jones KB, Salah Z, Del Mare S, et al:
miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Li X, Kong X, Luo Q, Zhang J and
Fang L: MiRNA-26b inhibits cellular proliferation by targeting CDK8
in breast cancer. Int J Clin Exp Med. 7:558–565. 2014.PubMed/NCBI
|
|
8
|
Ji Y, He Y, Liu L and Zhong X: MiRNA-26b
regulates the expression of cyclooxygenase-2 in
desferrioxamine-treated CNE cells. FEBS Lett. 584:961–967. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Tong J and Huang G: Nicotinamide
phosphoribosyl transferase (Nampt) is a target of microRNA-26b in
colorectal cancer cells. PLoS One. 8:e699632013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gramantieri L, Fornari F, Callegari E, et
al: MicroRNA involvement in hepatocellular carcinoma. J Cell Mol
Med. 12:2189–2204. 2008. View Article : Google Scholar
|
|
11
|
Shapovalov Y, Hoffman D, Zuch D, de Mesy
Bentley KL and Eliseev RA: Mitochondrial dysfunction in cancer
cells due to aberrant mitochondrial replication. J Biol Chem.
286:22331–22338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giang AH, Raymond T, Brookes P, et al:
Mitochondrial dysfunction and permeability transition in
osteosarcoma cells showing the Warburg effect. J Biol Chem.
288:33303–33311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Schaftingen E, Hue L and Hers HG:
Fructose 2,6-bisphosphate, the probably structure of the glucose-
and glucagon-sensitive stimulator of phosphofructokinase. Biochem
J. 192:897–901. 1980.PubMed/NCBI
|
|
14
|
Zawacka-Pankau J, Grinkevich VV, Hünten S,
et al: Inhibition of glycolytic enzymes mediated by
pharmacologically activated p53: targeting Warburg effect to fight
cancer. J Biol Chem. 286:41600–41615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verghese ET, Drury R, Green CA, et al:
MiR-26b is downregulated in carcinoma-associated fibroblasts from
ER-positive breast cancers leading to enhanced cell migration and
invasion. J Pathol. 231:388–399. 2013.PubMed/NCBI
|
|
16
|
Liao CL, Lin JH, Lien JC, et al: The crude
extract of Corni Fructus inhibits the migration and invasion of U-2
OS human osteosarcoma cells through the inhibition of matrix
metalloproteinase-2/-9 by MAPK signaling. Environ Toxicol. Aug
19–2013.(Epub ahead of print). PubMed/NCBI
|
|
17
|
Sottnik JL, Lori JC, Rose BJ and Thamm DH:
Glycolysis inhibition by 2-deoxy-d-glucose reverts the metastatic
phenotype in vitro and in vivo. Clin Exp Metastasis. 28:865–875.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh PK, Mehla K, Hollingsworth MA and
Johnson KR: Regulation of aerobic glycolysis by microRNAs in
cancer. Mol Cell Pharmacol. 3:125–134. 2011.PubMed/NCBI
|
|
19
|
Atsumi T, Chesney J, Metz C, et al: High
expression of inducible
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2;
PFKFB3) in human cancers. Cancer Res. 62:5881–5887. 2002.PubMed/NCBI
|
|
20
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji J, Shi J, Budhu A, et al: MicroRNA
expression, survival, and response to interferon in liver cancer. N
Engl J Med. 361:1437–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arora H, Qureshi R, Park AK and Park WY:
Coordinated regulation of ATF2 by miR-26b in γ-irradiated lung
cancer cells. PLoS One. 6:e238022011. View Article : Google Scholar
|
|
23
|
Wu N, Zhao X, Liu M, et al: Role of
microRNA-26b in glioma development and its mediated regulation on
EphA2. PLoS One. 6:e162642011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao N, Wang R, Zhou L, Zhu Y, Gong J and
Zhuang SM: MicroRNA-26b suppresses the NF-κB signaling and enhances
the chemosensitivity of hepatocellular carcinoma cells by targeting
TAK1 and TAB3. Mol Cancer. 13:352014. View Article : Google Scholar
|
|
25
|
Shen G, Lin Y, Yang X, Zhang J, Xu Z and
Jia H: MicroRNA-26b inhibits epithelial-mesenchymal transition in
hepatocellular carcinoma by targeting USP9X. BMC Cancer.
14:3932014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Kong X, Zhang J, Luo Q, Li X and
Fang L: MiRNA-26b inhibits proliferation by targeting PTGS2 in
breast cancer. Cancer Cell Int. 13:72013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu XX, Li XJ, Zhang B, et al:
MicroRNA-26b is underexpressed in human breast cancer and induces
cell apoptosis by targeting SLC7A11. FEBS Lett. 585:1363–1367.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang B, Liu XX, He JR, et al:
Pathologically decreased miR-26a antagonizes apoptosis and
facilitates carcinogenesis by targeting MTDH and EZH2 in breast
cancer. Carcinogenesis. 32:2–9. 2011. View Article : Google Scholar
|
|
29
|
Ohba T, Cates JM, Cole HA, et al:
Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells
associates with aggressive osteosarcoma. Mol Cancer Res.
12:1100–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palorini R, Votta G, Balestrieri C, et al:
Energy metabolism characterization of a novel cancer stem cell-like
line 3AB-OS. J Cell Biochem. 115:368–379. 2014. View Article : Google Scholar
|
|
31
|
Issaq SH, Teicher BA and Monks A:
Bioenergetic properties of human sarcoma cells help define
sensitivity to metabolic inhibitors. Cell Cycle. 13:1152–1161.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Minchenko A, Leshchinsky I, Opentanova I,
et al: Hypoxia-inducible factor-1-mediated expression of the
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3)
gene. Its possible role in the Warburg effect. J Biol Chem.
277:6183–6187. 2002. View Article : Google Scholar
|
|
33
|
Garcia-Cao I, Song MS, Hobbs RM, et al:
Systemic elevation of PTEN induces a tumor-suppressive metabolic
state. Cell. 149:49–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manes NP and El-Maghrabi MR: The kinase
activity of human brain
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase is regulated
via inhibition by phosphoenolpyruvate. Arch Biochem Biophys.
438:125–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Telang S, Yalcin A, Clem AL, et al: Ras
transformation requires metabolic control by
6-phosphofructo-2-kinase. Oncogene. 25:7225–7234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clem B, Telang S, Clem A, et al:
Small-molecule inhibition of 6-phosphofructo-2-kinase activity
suppresses glycolytic flux and tumor growth. Mol Cancer Ther.
7:110–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yalcin A, Clem BF, Imbert-Fernandez Y, et
al: 6-Phosphofructo-2-kinase (PFKFB3) promotes cell cycle
progression and suppresses apoptosis via Cdk1-mediated
phosphorylation of p27. Cell Death Dis. 5:e13372014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Almeida A, Bolaños JP and Moncada S: E3
ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by
linking glycolysis to cell proliferation. Proc Natl Acad Sci USA.
107:738–741. 2010. View Article : Google Scholar : PubMed/NCBI
|