Introduction
Photodynamic therapy (PDT) is an anticancer
treatment involving a photosensitizer and a light source resulting
in the production of reactive oxygen species (ROS) within affected
tissues. PDT is an efficient inducer of apoptosis both in
vitro and in vivo. It induces autophagy and apoptosis in
skin cancer cells by activating MAPK (1). In a previous study, treatment with
hematoporphyrin (HP) and PDT (HP-PDT) induced autophagy by
increasing the levels of autophagy-related proteins such as LC3II
and ATG5 and by inactivating mTOR (2). Recently, we reported that PDT
stimulated the apoptosis of FaDu cells by cleaving PARP-1 and
increasing caspase activity (2). To
enhance the effects of PDT, it is important to maintain a balance
between apoptosis and autophagy. Moreover, use of PDT may result in
PDT resistance depending on the photosensitizer, light dose, and
cell types used (3). Several
studies have suggested that drug dose and its exposure density
induce PDT resistance (4).
Mechanisms underlying resistance induced by a photosensitizer are
thought to be the same as those underlying drug resistance and may
be related to different uptake or efflux rates, altered
intracellular trafficking of the photosensitizer, and inactivation
of the photosensitizer (3). In
addition, alterations of proteins related to cell death and
survival determine the anticancer effects of PDT (3). PDT induces oxidative stress. This
transiently increases the expression of downstream early response
genes and stress genes such as those encoding heat shock proteins
(HSP, glucose-regulating proteins, and heme oxygenase in mammalian
cells) (5,6). Mechanisms underlying PDT-induced cell
death and PDT resistance are related to cell survival induced by
the combined action or interaction (or both) of cell death pathways
(7,8).
Defective apoptosis and autophagy are believed to
play a crucial role in the sensitivity of cells to PDT (9). PDT can induce both apoptosis and
autophagy. Autophagy enhances the survival of cells showing low
levels of photodamage (10), thus
serving as a pro-survival response by recycling damaged organelles.
In contrast, autophagy induces the death of cells treated with
high-dose PDT (11,12). Some studies have shown that PDT
limits autophagy and apoptosis (13). Autophagy maintains a balance between
cell death and PDT resistance during PDT (14). However, the roles of HSPs in
PDT-induced autophagy are unclear.
HSPs act as intracellular chaperones of other
proteins. They prevent unwanted protein aggregation and stabilize
partially unfolded proteins, thus helping cells to recover from
PDT-induced damage (15). Some of
these signals act as mediators or promoters of apoptosis, while
some signals act as stress responses that promote the repair of or
increase the resistance to PDT-induced damage (16). Effects of PDT may be determined by
balancing death and survival for cancer therapy resistance
mechanisms (17,18). In previous study, HSP70 was found to
regulate the apoptosis of chemoresistant cells by inhibiting
pro-apoptotic proteins (19,20).
We suggest that HSP70 expression is related to other proteins which
regulate cell death and resistance in PDT. Regulation of HSP27
expression resulted in degradation of apoptotic protein and a delay
in PDT-induced apoptosis by autophagy (21,22).
Regulation of HSP27 expression may be one of the markers in PDT
resistance and cancer therapy in oral cancer cells.
The aim of this study was to investigate the
mechanisms which control PDT resistance during PDT-induced cell
death by regulating the expression of HSPs. Thus, this study
investigated the effects of PDT on HSP expression to control PDT
resistance.
Materials and methods
Chemicals and reagents
HP,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
acridine orange, 3-methyladenine (3-MA), and
3,4-dihydro-5[4-(1-piperindinyl)butoxy]-1(2H)-isoquinoline (DPQ)
were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human oral cancer FaDu cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Grand Island,
NY, USA) supplemented with antibiotics and antimycotics (100 U/ml
penicillin, 0.1 mg/ml streptomycin, and 0.25 mg/ml amphotericin B)
(antibiotics and antimycotics; Gibco-BRL) and 10% heat-inactivated
fetal bovine serum (FBS; Hyclone, Logan, UT, USA) at 37°C in a
humidified atmosphere of 5% CO2 and 95% air.
Photosensitizer and irradiation
treatments
Cells were treated with HP at varying concentrations
in the range of 0–6 µM. HP-treated cells were then
irradiated using a light-emitting diode (LED; 4.5
mW/cm2) for 15 min. Effects of PDT on FaDu cells were
investigated after 24 h. To determine the effect of an autophagy
inhibitor, FaDu cells were treated with 5 mM 3-MA and HP at 2 and 4
µM for 24 h. The cells were treated again with 3-MA along
with irradiation for 24 h. After irradiation, the PDT-treated cells
were incubated at 37°C in a humidified atmosphere of 5%
CO2 and 95% air. To determine the effect of an apoptosis
inhibitor, FaDu cells were treated with 20 µM DPQ in the
same way as the autophagy inhibitor.
Induction of HP-PDT resistance
PDT-induced variants of FaDu cells were modified as
previously reported (23). Briefly,
FaDu cells cultured in 100-mm dishes were incubated with 2
µM HP in medium for 24 h, followed by a 15-min irradiation
using an LED (4.5 mW/cm2). Surviving cells were
recovered by a 24-h incubation in complete medium. After recovery,
the PDT-treated cells were exposed to HP-PDT. The cells that
survived the second cycle of PDT were repeatedly (15 times) exposed
to PDT before the isolation of PDT-resistant cells. The surviving
PDT-resistant cells were then treated 15 times with HP-PDT.
Cytotoxicity assay
Cytotoxicity was determined by measuring the
reduction of MTT to insoluble formazan. FaDu cells were cultured
(1×105 cells/ml) in 96-well plates for 24 h. HP was
added to the culture medium, and the cells were incubated for 24 h.
Light irradiation was performed for 15 min. After irradiation,
fresh medium was added, and the cells were incubated for 24 h. The
cells were then incubated in PBS containing 30 ml MTT at 37°C for 3
h. The formazan produced was solubilized by adding 50 ml dimethyl
sulfoxide. Optical density was measured at 570 nm by using an
ELx800uv ELISA reader (Bio-Tek Instruments, Winooski, VT, USA).
Western blotting
Proteins from the cell cultures were extracted using
lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM
EDTA, 1% NP-40, 1 mM phenylmethanesulfonyl fluoride, and 1%
protease inhibitor cocktail. Next, 100 µg of the protein
extract was mixed with a sample buffer, and proteins present in the
extract were separated by performing sodium dodecyl
sulfate-polyacrylamide gel electrophoresis on a 10% gel. The
proteins were transferred onto a membrane, and non-specific binding
sites on the membrane were blocked using 2% non-fat milk. Next, the
membranes were incubated overnight with primary antibodies against
HSP27, HSP70, HSP90, and GAPDH (1:500 dilution; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and with primary antibodies
against LC3II and PARP-1 (1:1,000; Cell Signaling Technology,
Beverly, MA, USA) in a blocking solution at 4°C. After washing with
1X TBS-T, the membranes were incubated with HRP-conjugated
anti-mouse or anti-rabbit antibodies for 2 h at room temperature.
The membranes were then washed 4 times with TBS-T (5 min/wash), and
the immunoblotted proteins were visualized using enhanced
chemiluminescence (Santa Cruz Biotechnology), according to the
manufacturer's instructions.
siRNA transfection
FaDu cells were grown to 70% confluency in
antibiotic-free DMEM supplemented with 0.2% FBS. The cells were
then transfected with siRNAs against genes encoding HSP27 or HSP70
(L-005269-00 or L-005168-00, respectively) or with siControl
non-targeting siRNA #1 (all from Dharmacon, Lafayette, CO, USA) for
24 h. Transfection was performed using HiPerFect transfection
reagent (Qiagen, Chicago, IL, USA) to facilitate the uptake of
siRNAs into the cells, according to the manufacturer's protocol. At
48 h after PDT, the cells were collected and used for further
analysis.
Autophagosome staining
FaDu cells were seeded in 4-well plates and were
grown to 70% confluency. The cells were then irradiated and treated
with 2 or 4 µM HP for 24 h. At designated times, the cells
were incubated with 1 µg/ml acridine orange (Molecular
Probes, Eugene, OR, USA) in serum-free medium for 15 min. After
incubation, acridine orange was removed and the cells were observed
under a confocal fluorescence microscope (Carl Zeiss, Oberkochen,
Germany). The cytoplasm and nucleus of the stained cells appeared
bright green, and the acidic autophagic vacuoles appeared bright
red.
TUNEL assay
TUNEL assay (DeadEnd™ Fluorometric TUNEL system;
Promega, Madison, WI, USA) was performed to detect the breaks in
DNA strands. Cells were fixed with 4% methanol-free formaldehyde
and were permeabilized with 0.2% Triton X-100. The cells were then
incubated with 100 µl reaction mixture containing
biotinylated nucleotide mix and recombinant terminal
deoxynucleotidyl transferase (rTdT) in equilibration buffer at 37°C
for 1 h. Next, the cells were incubated in 2X SSC at room
temperature. After washing, the cells were mounted with
Vectashield® including DAPI to stain the nuclei. The
reaction without the rTdT enzyme was used as a negative control.
The stained cells were analyzed using a confocal microscope (Carl
Zeiss).
Statistical analysis
Data are expressed as mean ± standard deviation. All
of the experiments were performed 3 times. Differences between the
groups were evaluated using Student's t-test and Tukey's multiple
comparison test by using GraphPad software version 5 (GraphPad
Software, San Diego, CA, USA). Null hypotheses of no difference
were rejected if p-values were <0.05.
Results
Alteration of HSP expression in
PDT-treated head and neck cancer cells
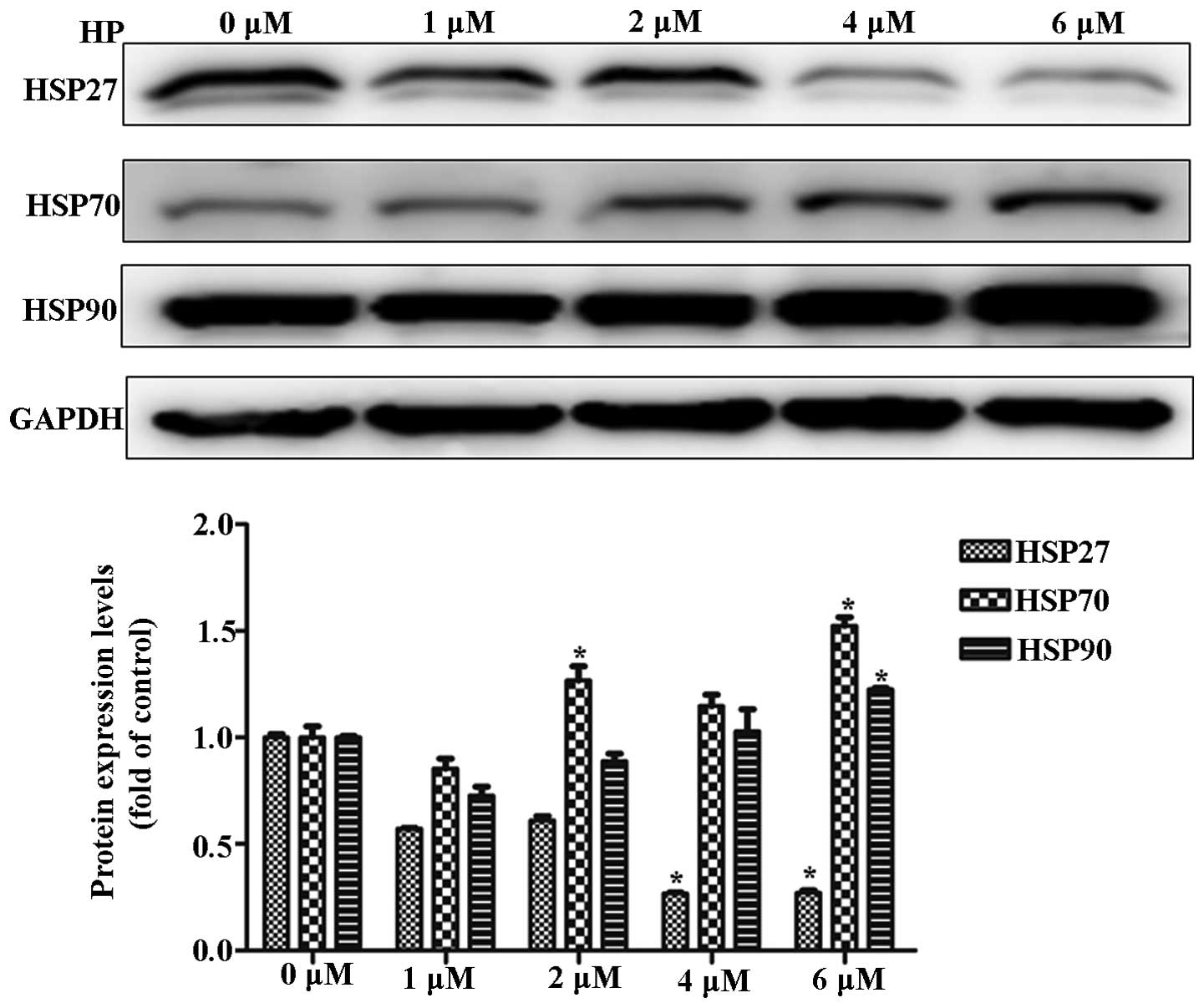
To investigate the alteration in HSP expression
during PDT, cancer cells were irradiated at 625 nm after HP
treatment. HP treatment decreased the expression of HSP27 and
increased the expression of HSP70 in a dose-dependent manner. These
results suggested a negative correlation between HSP27 and HSP70
expression during HP-PDT (Fig.
1).
Effects of HSP expression on PDT-induced
cell death and autophagy
In a previous study, PDT induced apoptosis as well
as autophagy (2). Therefore, we
investigated the alteration in HSP expression by treating FaDu
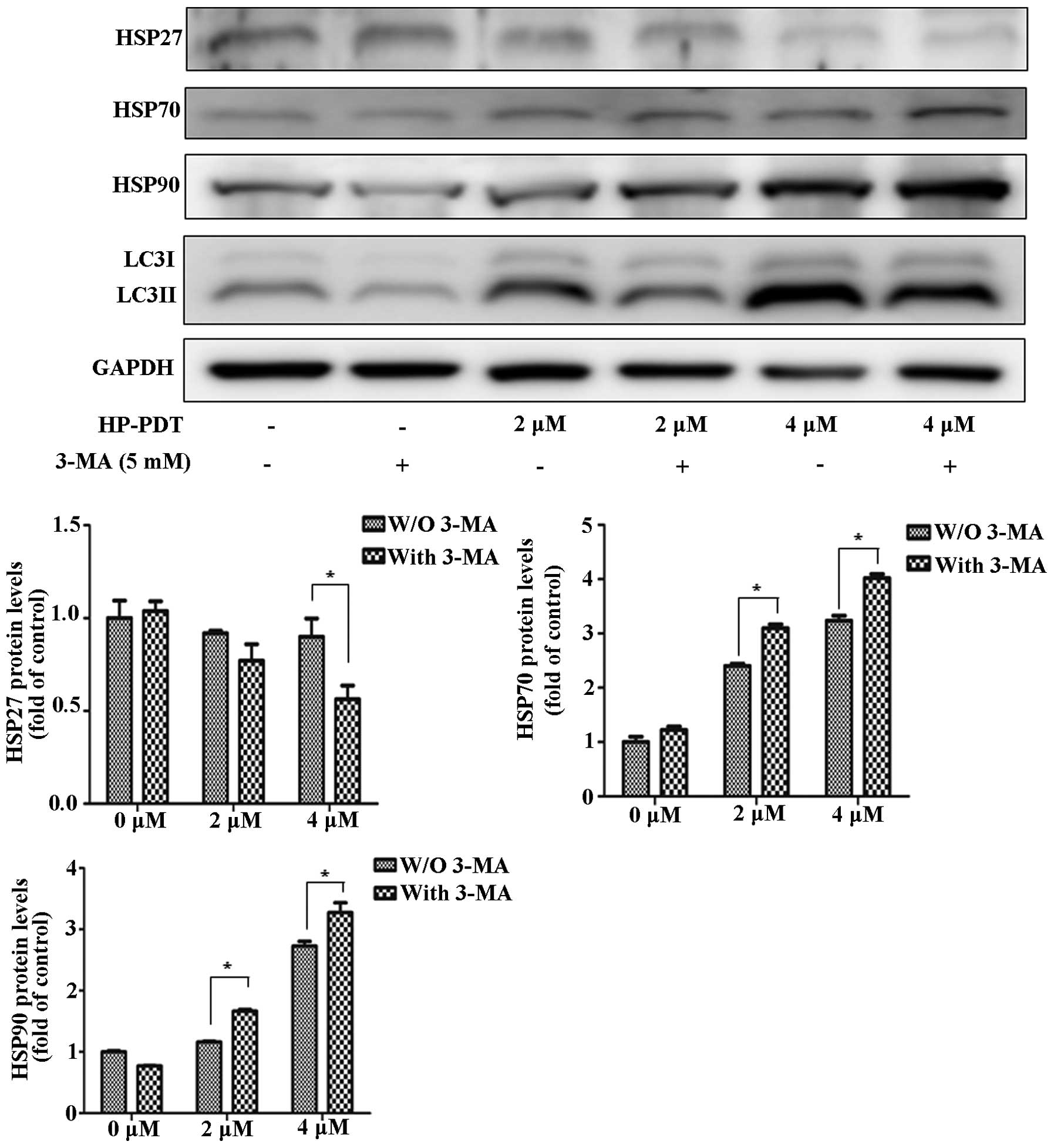
cells with an autophagy inhibitor (Fig.
2). Treatment with the combination of 3-MA and PDT decreased
HSP27 expression compared with PDT alone. In contrast, treatment
with 3-MA and PDT increased HSP70 and HSP90 expression. In a
previous study, we found that PDT induced apoptosis by PARP-1
(2). We suggested that PARP-1
dysfunction was related to PDT resistance by changes in HSP
expression. To investigate the effect of a PARP-1 inhibitor on HSP
expression, FaDu cells were treated with or without the PARP-1
inhibitor DPQ during PDT (Fig. 3).
Inhibition of apoptosis by the PARP-1 inhibitor increased the
expression of HSP27 during PDT. At 4 µM, HSP70 and HSP90
were decreased as 20 µM DPQ was administered. Therefore, we
concluded that apoptotic and autophagic signaling regulated HSP
expression. The autophagy inhibitor showed maximized effects with
HSP expression levels in PDT. DPQ as a PARP-1 inhibitor reversed
the HSP expression levels in PDT.
Effects of HSP downregulation on PDT
resistance
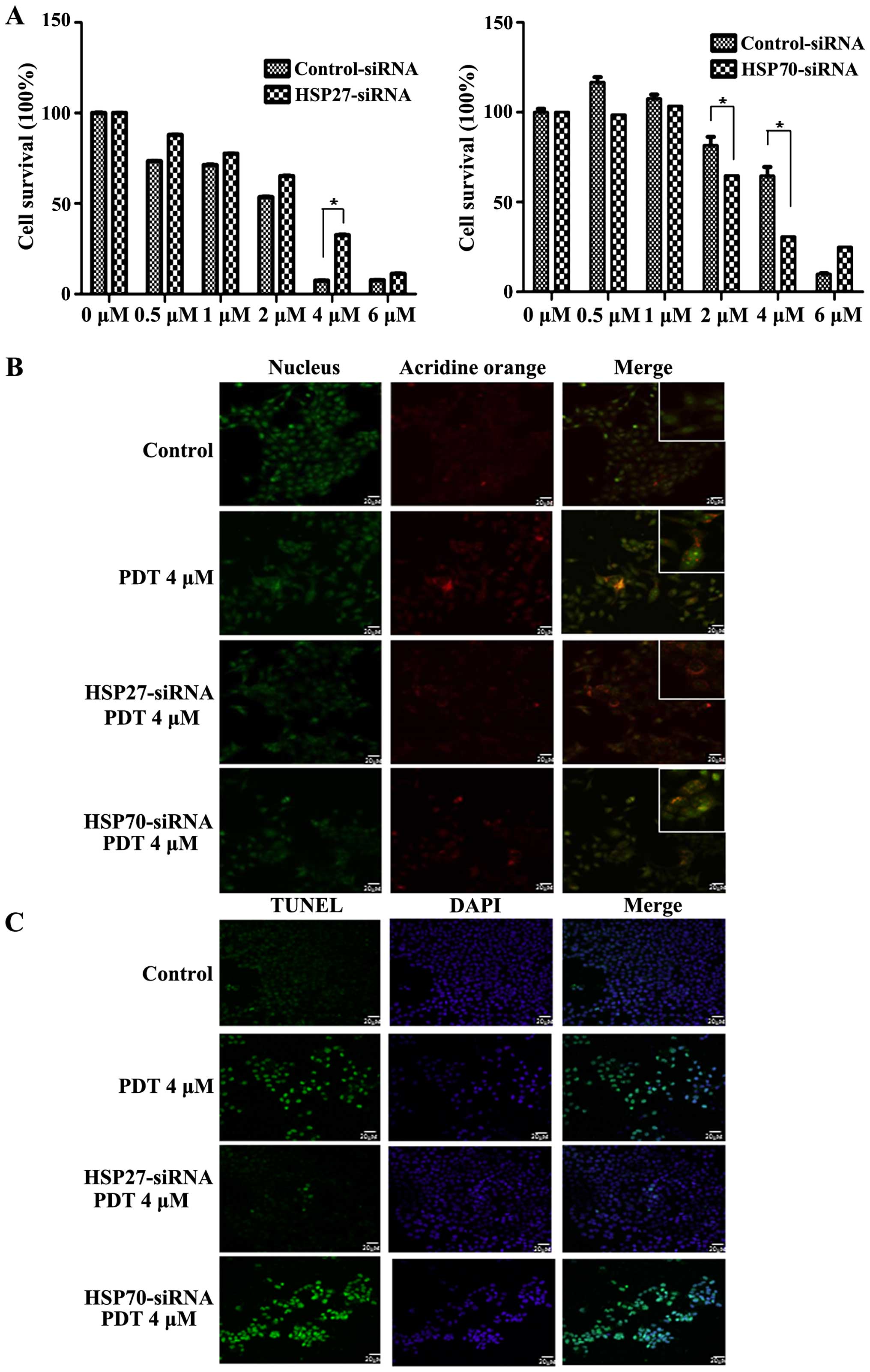
To evaluate the effects of HSP downregulation on PDT
resistance, FaDu cells were transfected with siRNAs against genes
encoding HSP27 and HSP70. Treatment with 4 µM PDT increased
the survival of cells transfected with the siRNA against the gene
encoding HSP27 in a dose-dependent manner, indicating that
downregulation of HSP27 increased PDT resistance (Fig. 4A left panel). In contrast, PDT
decreased the survival of cells transfected with siRNA against the
gene encoding HSP70, indicating that downregulation of HSP70
induced cell death during PDT (Fig.
4A right panel). To confirm the regulation of cell death by
HSPs, cells were stained with autophagic and apoptotic markers. PDT
induced both autophagy and apoptosis in a dose-dependent manner.
Positive autophagic staining by acridine orange staining was
observed at 2 and 4 µM (Fig.
4B). The number of autophagic cells peaked after treatment with
2 µM PDT, and the number of cells transfected with siRNA
against the gene encoding HSP27 peaked after treatment with 4
µM PDT (Fig. 4B). Cells
transfected with siRNA against the gene encoding HSP70 and not
treated with PDT showed autophagy (Fig.
4B). Downregulation of HSP70 did not affect PDT-induced
autophagy. We also examined the effects of HSP27 and HSP70
downregulation on apoptosis during PDT. Downregulation of HSP27
decreased the apoptosis of cells while downregulation of HSP70
increased the apoptosis of cells during PDT compared with that of
the non-transfected cells (Fig.
4C). These results indicated that PDT induced apoptosis along
with autophagy. In addition, these results indicated that
downregulation of HSP27 stimulated autophagy while that of HSP70
induced apoptosis. Thus, these results revealed that HSP27 and
HSP70 regulated PDT resistance.
Effects of HSP27 and HSP70 downregulation
on cell death proteins
To observe the alteration in cell death proteins
after HSP downregulation, we determined the differential expression
of LC3 and PARP-1. In the cells transfected with siRNA against the
gene encoding HSP27, treatment with 4 µM PDT induced LC3II
conversion; however, this was not observed in the non-transfected
cells (Fig. 5A). Moreover, HSP27
downregulation decreased HSP70 expression. However, PDT cleaved
PARP-1 regardless of the transfection of siRNA against the gene
encoding HSP27. In cells showing HSP70 downregulation, PDT
increased the expression of LC3II. PARP-1 cleavage increased after
HSP70 downregulation. HSP27 expression had no effect on the cells
transfected with siRNA against HSP70 (Fig. 5B). These results indicated that
HSP27 mediated the autophagic signal in association with LC3II and
that HSP70 regulated PARP-1, which was associated with
apoptosis.
Alteration in apoptotic protein
expression during PDT resistance
To investigate the alteration in the expression of
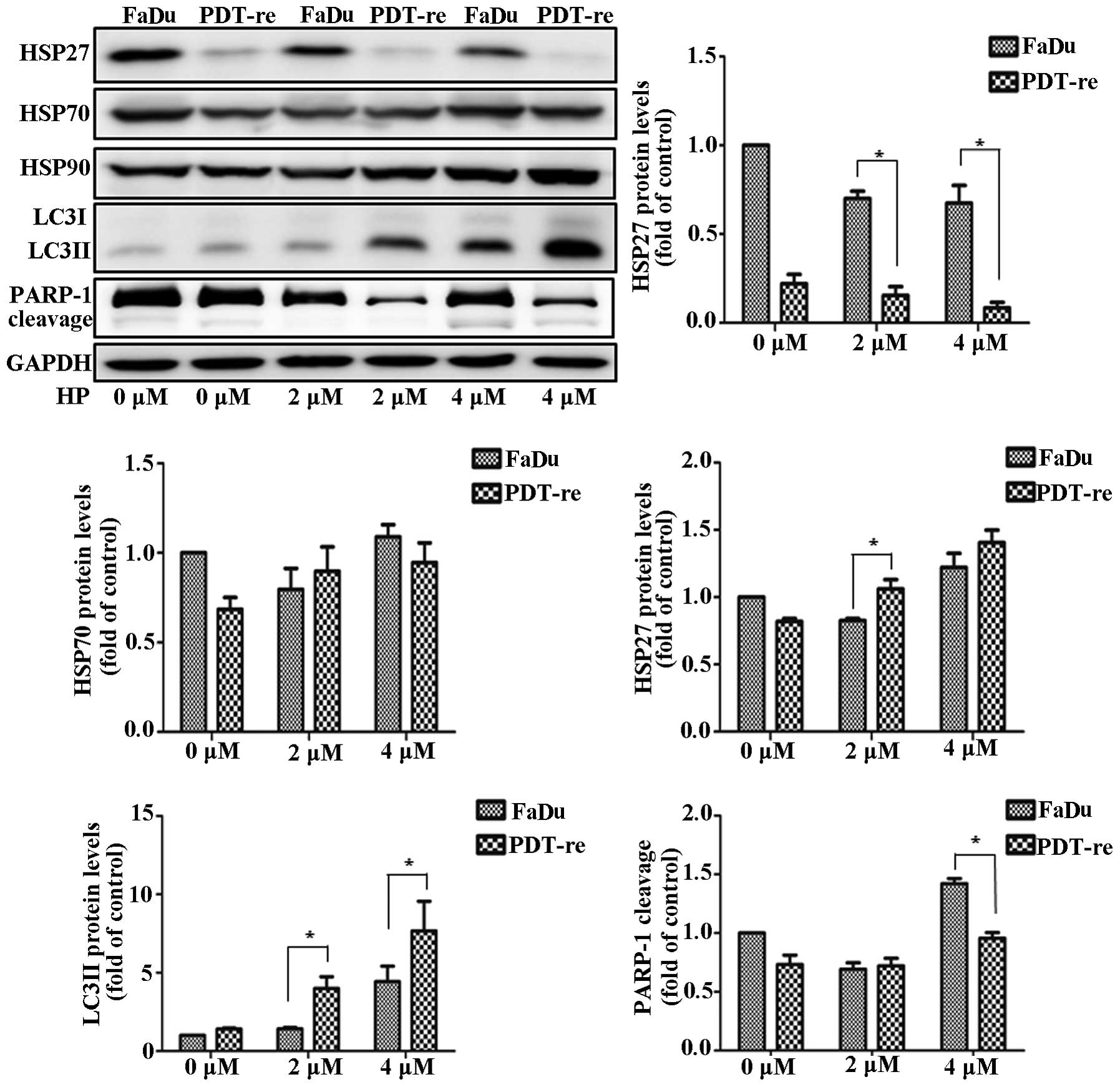
cell death proteins during PDT resistance, we obtained
PDT-resistant cells that remained alive after repeated PDT. Cell
lysates were immunoblotted using antibodies against LC3, HSPs, and
PARP-1. Since PDT-resistant cells showed decreased HSP27
expression, we concluded that PDT inhibited HSP27 expression
(Fig. 6). HSP70 levels were
unchanged in the PDT-resistant and non-resistant cells. HSP90
expression was slightly higher in the PDT-resistant cells than in
the PDT-treated FaDu cells. Remarkably, LC3II levels increased
after PDT in the PDT-resistant cells. Downregulation of HSP27
increased the level of active LC3II, which was associated with
autophagy. This result indicated that HSP27 plays a key role in PDT
resistance and cell death by controlling autophagy. PDT induced the
cleavage of PARP-1 in FaDu cells. Furthermore, PARP-1 expression
and cleavage were lower after PDT in PDT-resistant cells than in
the non-resistant cells. Repeated PDT downregulated HSP27 in the
PDT-resistant cells which in turn increased PDT resistance through
autophagy. Moreover, PDT sensing was decreased in PDT-resistant
cells. These results indicated that HSP27 downregulation regulated
PDT resistance through autophagy and apoptosis.
Discussion
Regulation of HSP expression levels may
play critical roles in cancer therapy
PDT has destructive power with reactive oxygen
species generated by light irradiation on a photosensitizer,
followed by accumulated cancerous cells. Effect of PDT is regulated
by HSPs, i.e., by the upregulation of HSP70 and downregulation of
HSP27. The potent cytoprotective and folding properties of HSPs are
associated with oncogenesis since high expression of HSPs
facilitates tumor growth and survival (23). In these patients, low HSP27
expression was correlated with non-responsiveness to the
chemotherapy regimen, which was in contrast to that observed in
patients with breast cancer (23).
This is one of the few studies, which showed that low HSP27
expression was correlated with a negative outcome in patients with
cancer.
Downregulation of HSP27 affects
PDT-induced cell death
The present study suggested that alteration of HSP
expression by PDT plays a critical role in tumor cell death and PDT
resistance. As shown in Fig. 1, PDT
decreased the expression of HSP27 and increased the expression of
HSP70 and HSP90. Moreover, downregulation of HSP27 induced PDT
resistance by activating autophagy (Fig. 4). HSP27 (encoded by HSPB1) is
usually overexpressed in patients with breast cancer and affects
the disease outcome and sensitivity of these patients to
chemotherapy and radiotherapy (24,25). A
recent study reported that histone deacetylase 6, transcription
factor STAT2, and pro-caspase-3 were degraded in human cancer cells
in which HSP27 was downregulated by siRNA transfection, suggesting
that these are target proteins of HSP27 (21). A decrease in HSP27 expression by PDT
may exert protective effects against PDT-induced cell death in oral
cancer cells. HSP27 knockdown induced autophagy and attenuated
PDT-induced apoptosis of oral cancer cells (22). In the present study, we confirmed
that downregulation of HSP27 expression induced autophagy through
LC3II in PDT-resistant cells (Fig.
6). We also found that HSP27 participated in cancer cell
survival. These results elucidated the mechanisms involved in PDT
resistance.
HSP70 plays a fundamental role in
protecting cells against PDT
Kayama et al reported the role of HSP70 in
the molecular interplay between pro-survival and pro-apoptotic
pathways after photoreceptor stress (26). In the present study, PDT induced the
overexpression of HSP70 and HSP90 (Fig.
1). HSP70 upregulation prevents photoreceptors from undergoing
immediate apoptosis. Downregulation of HSP70 by siRNA increased
PDT-induced apoptosis through PARP-1 cleavage (Fig. 5). Furthermore, HSP70 prevented PDT
resistance and inhibited the activation of apoptotic pathways.
Transient knockdown of HSP70 expression was found to potentiate
apoptosis, and its overexpression prevented OSU-03012-induced
increase in cytotoxicity and autophagy (27). However, the role of HSP70 in
ROS-induced autophagy remains known. In our study, downregulation
of HSP70 inhibited autophagy by decreasing LC3II and induced
apoptosis by cleaving PARP-1 (Fig.
5). Upregulation of PAPR-1 and the PARP-1 inhibition which
blocks the cleaved form induced PDT resistance against PDT. HSP70
regulated PARP-1 activation. Therefore, we suggest that
downregulation of HSP70 may inhibit PDT resistance by activating
PARP-1.
Autophagy is related to PDT resistance
through HSP27 downregulation
PDT concurrently induces both autophagic and
apoptotic pathways in cancer cells (2,13).
Autophagy acts as both a tumor suppressor and a promoter. Autophagy
may delay apoptosis and be activated in response to PDT
contributing to cell resistance (28,29).
Therefore, inhibitors of autophagy may re-sensitize resistant
cancer cells to anticancer therapies. In the present study, PDT
decreased HSP27 expression, which was associated with autophagy
induction by LC3II after PDT resistance (Fig. 6). It has been suggested that
regulation of autophagy may play a critical role in maintaining a
balance between PDT resistance and PDT-induced apoptosis.
Therefore, it appears that regulation of autophagic signals may
re-sensitize PDT-resistant cells to PDT. HSP70 exerted protective
effects against PDT in oral cancer cells. Downregulation of HSP70
induced both apoptosis and autophagy. Moreover, HSP70 sensitized
oral cancer cells to ROS stress but had no effect on PDT-resistant
cells treated with PDT (Fig. 6).
Therefore, autophagy induction by HSP27 decreased PDT sensitivity
and PDT resistance. Downregulation of HSP70 increased PDT
sensitivity and mediated PDT-induced apoptosis. Wei et al
reported that autophagy is associated with PDT resistance in
colorectal cancer stem-like cells (30). Autophagy and apoptosis may be
triggered by common signals, resulting in combined autophagy and
apoptosis. Autophagic and apoptotic signals may also complement
each other. Several molecules regulate the crosstalk between
apoptotic and autophagic pathways; however, the exact molecules are
unknown. Our study suggests that a balance between autophagy and
apoptosis that is regulated by HSPs may be responsible for the
effects of PDT.
Taken together, PDT resistance occurs due to
crosstalk between autophagic and apoptotic pathways in oral cancer
cells. In the present study, PDT altered the expression of HSP27
and HSP70. Downregulation of HSP27 was associated with autophagy,
which promoted the survival of cancer cells. HSP70 was inhibited
during PDT-induced apoptosis as shown in our study and by others.
Therefore, HSP27 and HSP70 may play a critical role in the
cross-regulation of PDT-induced autophagy and apoptosis during
cancer treatment.
Acknowledgments
This research was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIP) (2011-0030759) and the Korean Health Technology R&D
Project, Ministry of Health & Welfare, Republic of Korea
(A121228).
References
|
1
|
Yoon HE, Oh SH, Kim SA, Yoon JH and Ahn
SG: Pheophorbide a-mediated photodynamic therapy induces autophagy
and apoptosis via the activation of MAPKs in human skin cancer
cells. Oncol Rep. 31:137–144. 2014.
|
|
2
|
Kim J, Lim W, Kim S, Jeon S, Hui Z, Ni K,
Kim C, Im Y, Choi H and Kim O: Photodynamic therapy (PDT)
resistance by PARP1 regulation on PDT-induced apoptosis with
autophagy in head and neck cancer cells. J Oral Pathol Med.
43:675–684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Casas A, Di Venosa G, Hasan T and Batlle
Al: Mechanisms of resistance to photodynamic therapy. Curr Med
Chem. 18:2486–2515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Casas A, Perotti C, Ortel B, Di Venosa G,
Saccoliti M, Batlle A and Hasan T: Tumor cell lines resistant to
ALA-mediated photodynamic therapy and possible tools to target
surviving cells. Int J Oncol. 29:397–405. 2006.PubMed/NCBI
|
|
5
|
Gomer CJ, Ferrario A, Rucker N, Wong S and
Lee AS: Glucose regulated protein induction and cellular resistance
to oxidative stress mediated by porphyrin photosensitization.
Cancer Res. 51:6574–6579. 1991.PubMed/NCBI
|
|
6
|
Luna MC and Gomer CJ: Isolation and
initial characterization of mouse tumor cells resistant to
porphyrin-mediated photodynamic therapy. Cancer Res. 51:4243–4249.
1991.PubMed/NCBI
|
|
7
|
Tong Z, Singh G, Valerie K and Rainbow AJ:
Activation of the stress-activated JNK and p38 MAP kinases in human
cells by Photofrin-mediated photodynamic therapy. J Photochem
Photobiol B. 71:77–85. 2003. View Article : Google Scholar
|
|
8
|
Oleinick NL and Evans HH: The photobiology
of photodynamic therapy: Cellular targets and mechanisms. Radiat
Res. 150(Suppl 5): S146–S156. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue LY, Chiu SM and Oleinick NL: Atg7
deficiency increases resistance of MCF-7 human breast cancer cells
to photodynamic therapy. Autophagy. 6:248–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kessel D and Oleinick NL: Initiation of
autophagy by photodynamic therapy. Methods Enzymol. 453:1–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kessel D and Arroyo AS: Apoptotic and
autophagic responses to Bcl-2 inhibition and photodamage. Photochem
Photobiol Sci. 6:1290–1295. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kessel D and Oleinick NL: Photodynamic
therapy and cell death pathways. Methods Mol Biol. 635:35–46. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kessel D, Vicente MG and Reiners JJ Jr:
Initiation of apoptosis and autophagy by photodynamic therapy.
Lasers Surg Med. 38:482–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inguscio V, Panzarini E and Dini L:
Autophagy contributes to the death/survival balance in cancer
photodynamic therapy. Cells. 1:464–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Almeida RD, Manadas BJ, Carvalho AP and
Duarte CB: Intracellular signaling mechanisms in photodynamic
therapy. Biochim Biophys Acta. 1704:59–86. 2004.PubMed/NCBI
|
|
16
|
Oleinick NL, Morris RL and Belichenko I:
The role of apoptosis in response to photodynamic therapy: What,
where, why, and how. Photochem Photobiol Sci. 1:1–21. 2002.
View Article : Google Scholar
|
|
17
|
Nonaka M, Ikeda H and Inokuchi T:
Inhibitory effect of heat shock protein 70 on apoptosis induced by
photodynamic therapy in vitro. Photochem Photobiol. 79:94–98. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferrario A, Rucker N, Wong S, Luna M and
Gomer CJ: Survivin, a member of the inhibitor of apoptosis family,
is induced by photodynamic therapy and is a target for improving
treatment response. Cancer Res. 67:4989–4995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X, Wang J, Zhou Y, Wang Y, Wang S and
Zhang W: Hsp70 promotes chemoresistance by blocking Bax
mitochondrial translocation in ovarian cancer cells. Cancer Lett.
321:137–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grivicich I, Regner A, Zanoni C, Correa
LP, Jotz GP, Henriques JA, Schwartsmann G and da Rocha AB: Hsp70
response to 5-fluorouracil treatment in human colon cancer cell
lines. Int J Colorectal Dis. 22:1201–1208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gibert B, Eckel B, Fasquelle L, Moulin M,
Bouhallier F, Gonin V, Mellier G, Simon S, Kretz-Remy C, Arrigo AP,
et al: Knock down of heat shock protein 27 (HspB1) induces
degradation of several putative client proteins. PLoS One.
7:e297192012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumano M, Furukawa J, Shiota M, Zardan A,
Zhang F, Beraldi E, Wiedmann RM, Fazli L, Zoubeidi A and Gleave ME:
Cotargeting stress-activated Hsp27 and autophagy as a combinatorial
strategy to amplify endoplasmic reticular stress in prostate
cancer. Mol Cancer Ther. 11:1661–1671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vidyasagar A, Wilson NA and Djamali A:
Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic
target. Fibrogenesis Tissue Repair. 5:72012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vargas-Roig LM, Gago FE, Tello O, Aznar JC
and Ciocca DR: Heat shock protein expression and drug resistance in
breast cancer patients treated with induction chemotherapy. Int J
Cancer. 79:468–475. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciocca DR and Calderwood SK: Heat shock
proteins in cancer: Diagnostic, prognostic, predictive, and
treatment implications. Cell Stress Chaperones. 10:86–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kayama M, Nakazawa T, Thanos A, Morizane
Y, Murakami Y, Theodoropoulou S, Abe T, Vavvas D and Miller JW:
Heat shock protein 70 (HSP70) is critical for the photoreceptor
stress response after retinal detachment via modulating
anti-apoptotic Akt kinase. Am J Pathol. 178:1080–1091. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park MA, Yacoub A, Rahmani M, Zhang G,
Hart L, Hagan MP, Calderwood SK, Sherman MY, Koumenis C, Spiegel S,
et al: OSU-03012 stimulates PKR-like endoplasmic
reticulum-dependent increases in 70-kDa heat shock protein
expression, attenuating its lethal actions in transformed cells.
Mol Pharmacol. 73:1168–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dewaele M, Martinet W, Rubio N, Verfaillie
T, de Witte PA, Piette J and Agostinis P: Autophagy pathways
activated in response to PDT contribute to cell resistance against
ROS damage. J Cell Mol Med. 15:1402–1414. 2011. View Article : Google Scholar
|
|
29
|
Bauvy C, Gane P, Arico S, Codogno P and
Ogier-Denis E: Autophagy delays sulindac sulfide-induced apoptosis
in the human intestinal colon cancer cell line HT-29. Exp Cell Res.
268:139–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei MF, Chen MW, Chen KC, Lou PJ, Lin SY,
Hung SC, Hsiao M, Yao CJ and Shieh MJ: Autophagy promotes
resistance to photodynamic therapy-induced apoptosis selectively in
colorectal cancer stem-like cells. Autophagy. 10:1179–1192. 2014.
View Article : Google Scholar : PubMed/NCBI
|