Introduction
Breast cancer is one of the malignant tumors with
the highest incidence in females worldwide (1). In the past decade, the incidence of
breast cancer in the Chinese female population has increased by 4%,
with more younger and urban citizens affected (2). With the progresses in clinical
diagnosis and treatment, the survival rate of patients with breast
cancer has dramatically increased in recent years. However,
metastasis and recurrence are still refractory to control, which
influence the survival time and survival rate of breast cancer
patients. Therefore, development of new treatment strategies
against breast cancer as well as identification of the mechanisms
of recurrence and metastasis remain urgent.
It is widely supported that there exists a very
small population of progenitor cells, termed as cancer stem cells
(CSCs), within solid tumors that display self-renewal and
differentiation potential (3–5). These
cells are able to recapitulate the heterogeneity of solid tumors in
immunodeficiency mice (6,7). CSCs are considered to play crucial
roles in tumor development, progression, metastasis and recurrence.
Cell sorting is the earliest available method for isolating and
acquiring stem cells from breast cancer. A group of
ESA+CD44+CD24−/low cells from
human breast cancer were firstly isolated by Al-Hajj et al
(8) in 2003 by cell sorting. It was
demonstrated that 100–200 of such cells were enough to form tumors
in breast tissue of non-obese diabetic/severe combined
immunodeficiency disease (NOD/SCID) mice, thereby putting forward
the hypothesis of the existence of breast CSCs. As breast CSCs
isolated via cell sorting usually display
CD44+CD24−/low phenotypic characteristics
(9–11), it is well feasible to isolate breast
CSCs by using the combination of these surface markers. However,
increased studies show that stem cells from primary breast cancer
with different histological subtypes vary in regards to surface
markers due to their different origins (12), limiting the applicability of one
panel of stem cell surface markers to all types of breast cancers.
In addition, breast cancers with similar histological subtypes
sometimes display heterogeneity in their stem cell populations with
diverse ability to form tumors in immune-deficient mice (12) when sorted out using the same
markers. The rare numbers of CSCs isolated by surface marker-based
cell sorting is another key limitation. The side population (SP)
sorting method through flow cytometry with a 355 nm ultraviolet
excitation device is an alternative method to isolate CSCs owing to
the ability of CSCs to excrete Hoechst 33342 or Rhodamine 123 while
differentiated cells do not (13).
However, the SP technique may be limited by the biological toxicity
of the Hoechst dye as well as the leakage of non-SP cells that
exhibit CSC-like properties (14).
The cell suspension culture method used to enrich
CSCs relies on their ability to form mammospheres in serum-free
culture media with certain growth factors. Reynolds and Weiss
(15) used conditioned medium to
cultivate nerve cells in which spheres formed containing
multi-potential undifferentiated nerve cells; 4–20% of them were
stem cells, while others were progenitor cells in various stages of
differentiation. By using a similar suspension cell culture method,
Dontu et al (16) and Ponti
et al (9) obtained
mammospheres from human breast tissues and breast cancer tissues,
respectively. Mammospheres from normal tissue form functional
ductal alveolar and acinar-like structures in 3D Matrigel culture
system in vitro resembling the entire ductal-acinar
architecture of the mammary tree (17). One thousand cells from mammospheres
of tumor tissues formed tumors in the mammary fat pads of mice
(18). Grimshaw et al
successfully carried out mammosphere formation in suspension
culture containing CD44+CD24+ cells from the
pleural fluid of patients with advanced breast cancer. Implantation
of 5,000 or even fewer mammosphere-derived cells formed tumors in
NOD/SCID mice whereas the same amount of non-sphere cells could not
(19). A serum-free non-adherent
suspension culture system thus facilitates the enrichment of stem
cell-like cells in vitro that maintains the undifferentiated
property, making it possible for subsequent research on breast
CSCs.
With the feasibility to obtain primary CSCs from
primary breast cancer, to determine the drug sensitivity of CSCs
from primary breast cancer in advance may become of great value in
controlling the recurrence and metastasis of breast cancer.
However, to date there are still few clinical studies describing
the drug resistance of primary CSCs. This is partially due to the
difficulty in isolating CSCs from solid breast cancer. Therefore,
to explore new methods for efficient enrichment of breast CSCs is
still worthwhile in this research area.
In the present study, we successfully established a
modified cell suspension culture system by modifying the
combinations of growth factors. The stem cell-like properties of
mammosphere-forming cells obtained were further validated in
vivo by xenograft transplantation. Moreover, drug sensitivity
of the mammosphere-forming cells to first-line chemotherapeutic
drugs against breast cancer was evaluated, which may provide
important clues to the determination of chemotherapy strategy in
the clinic.
Materials and methods
MCF-7 cell line and primary breast cancer
specimen
Human breast cancer cell line MCF-7 was purchased
from Shanghai Cell Bank, Chinese Academy of Sciences. A fresh
specimen of breast cancer was taken from a 42-year-old patient who
had not accepted adjuvant therapy and was delivered to the
laboratory for manipulation within 1 h. Invasive ductal carcinoma
(IDC), ER−, PR− and HER2+ were
pathologically confirmed. Informed consent was obtained from the
patient prior to the specimen acquisition, and this study was
approved by the Ethics Committee of Shanghai Jiao Tong University
School of Medicine.
Preparation of mammosphere-forming cells
from the primary breast cancer
The fresh surgical specimen was rinsed with culture
medium containing penicillin (100 U/ml) and streptomycin (100
µg/ml) and minced with DMEM/F12 medium containing DNase (1
mg/ml), collagenase type IV (1 mg/ml) and 2% fetal bovine serum
(FBS) (all from Invitrogen, Carlsbad, CA, USA) followed by
digestion at 37°C overnight. A single-cell suspension was prepared
and filtered through a 100-mesh stainless steel sieve to remove
incompletely digested tissue debris. Cells were collected by
centrifugation at 800 rpm for 5 min and resuspended in DMEM/F12
complete medium supplemented with 2% FBS, B27 and
insulin-transferrin-selenium cocktail (both from Invitrogen) and
incubated at 37°C with 5% CO2. Fibroblasts were removed
from the culture suspension by repeated adherence method. EGF (20
ng/ml) and bFGF (10 ng/ml) (Invitrogen) were added to DMEM/F12
complete medium for subsequent culture at 37°C with 5%
CO2. Mammospheres were collected on the 13th day.
Preparation and passage of MCF-7
mammosphere-forming cells
Mammosphere formation was performed as previously
described (20). Briefly, MCF-7
cells were treated using PBS buffer containing 0.25% trypsin and
0.02% EDTA and collected by centrifugation at 1,500 rpm for 5 min.
The cells were resuspended in the DMEM/F12 complete medium
containing B27, EGF (20 ng/ml), bFGF (10 ng/ml) and trypsin (5
ng/ml) (Shanghai No.1 Biochemical & Pharmaceutical Co., Ltd,
Shanghai, China) at 2×105/ml and incubated at 37°C with
5% CO2. After 7 days, the mammospheres were collected by
centrifugation at 1,000 rpm for 5 min. To passage the mammosphere
cells, cell pellets were treated with 1 ml PBS-0.25% trypsin-0.02%
EDTA to obtain single cell suspension for subculture of
mammospheres in DMEM/F12 complete medium.
Detection of CD44 and CD24 expression on
mammosphere-forming cells
Mammospheres were digested using PBS containing
0.25% trypsin-0.02% EDTA and washed twice using PBS containing 1%
BSA. A single-cell suspension was incubated with FITC-mouse
anti-human CD44 and PE-mouse anti-human CD24 antibodies (BD
Biosciences, USA) at 4°C for 30 min. After washing twice with PBS
containing 1% BSA, the cells were resuspended in PBS containing 1%
paraformaldehyde solution and acquired through a FACSCalibur flow
cytometer (BD Biosciences). Data analysis was performed by using
CellQuest software (BD Biosciences).
Quantitative real-time PCR
Expression of genes including Nanog, Sox2, Klf4,
Oct4 and mdr1 was detected by semi-quantitative
real-time PCR. Briefly, total RNA was extracted from the
mammosphere cells and subjected to reverse transcription using a
reverse-transcription kit (Ferment, China) for first-strain cDNA
synthesis. Quantitative real-time PCR was performed under
conditions recommended by commercial kits (Takara, Japan). Primers
were designed using Primer Express Software (version 2.0) as shown
in Table I. GAPDH gene was
used as the endogenous control. The reaction was initiated at 50°C
for 2 min and 95°C for 10 min. Forty cycles of two-step PCR (95°C
for 15 sec and 60°C for 60 sec) were performed. Data were collected
and analyzed by using ABI Prism 7500 serial detection system (ABI,
USA). The gene expression levels of each sample were calculated
according to the cycle threshold (CT) value. The relative
expression of target genes was calculated by 2−ΔCt (ΔCt
is the difference of Ct value between the target gene and the
endogenous control).
 | Table IPrimer sequences used in real-time
PCR. |
Table I
Primer sequences used in real-time
PCR.
| Genes | Sequences |
|---|
| Nanog | F:
AGAATAGCAATGGTGTGACGCAGAAGG |
| R:
TCACACGTCTTCAGGTTGCATGTTCAT |
| Oct4 | F:
GACAACAATGAGAACCTTCAGGAGA |
| R:
CTGGCGCCGGTTACAGAACCA |
| Klf4 | F:
GACGCGCTGCTCCCATCTTT |
| R:
TGACTCCGGAGGATGGGTCA |
| Sox2 | F:
ACAACTCGGAGATCAGCA |
| R:
GCAGCGTGTACTTATCCTTC |
| mdr1 | F:
TGCGACAGGAGATAGGCTG |
| R:
GCCAAAATCACAAGGGTTAGCTT |
| GAPDH | F:
GAAGGTCGGAGTCAACGGAT |
| R:
CCTGGAAGATGGTGATGGG |
Histochemistry and cytochemistry
Mammosphere-forming cells from the primary breast
cancer were subjected to paraffin embedding. Hematoxylin and eosin
(H&E) staining was performed as usual. For alkaline phosphatase
(AP) staining, mammosphere-forming cells were fixed with 4% PFA for
2 min and washed twice with PBS. Cells were incubated with PBS-2%
cobalt nitrate for 5 min and PBS-2% ammonium sulfate for 1 min,
followed by rinsing with distilled water. The staining results were
observed under a light microscope. Cells with dark brown staining
were deemed to be undifferentiated cells whereas colorless cells
were differentiated cells.
To determine the breast cancer origin of the
mammosphere-forming cells, breast-specific mammaglobin (MGB1)
expression was evaluated by immunohistochemistry (IHC) assay using
the mouse anti-MGB1 (Dako, Denmark) antibody as the primary
antibody. After incubation with the primary antibody, the slides
were sequentially incubated with biotinylated goat anti-mouse IgG
and ExtrAvidin®-conjugated horseradish peroxidase
(Sigma) at dilutions of 1:200 and 1:30, respectively. The slides
were developed with diaminobenzidine (DAB) (Sigma, St. Louis, MO,
USA) substrate and counterstained with hematoxylin, dehydrated and
mounted. For the negative control in the IHC procedures, PBS with
10% normal mouse serum was used for substitution of primary
antibody.
Xenograft formation of
mammosphere-forming cells in nude mice
Mammosphere-forming cells (103,
104 and 106) from the primary breast cancer
were subcutaneously inoculated in 6-week-old female nude mice.
Sixty days after inoculation, auxiliary lymph nodes were extracted
to prepare paraffin sections for H&E and MGB1 staining. All
animal experiments were approved by the Committee on the Use of
Live Animals of Shanghai Jiao Tong University School of
Medicine.
Apoptosis detection
Mammosphere-forming cells (5×104) from
the primary breast cancer, and MCF-7 cells were incubated with
chemotherapeutic drugs including paclitaxel, doxorubicin,
5-fluorouracil and cisplatin at different concentrations for 24 h
at 37°C with 5% CO2. Annexin V-propidium iodide (PI)
staining (BD Pharmingen, USA) was performed for the detection of
apoptosis after the treatment of the chemotherapeutic drugs
according to the manufacturer's instructions. Briefly, the cells
were collected after drug treatment and resuspended in 500
µl of binding buffer. After addition of 5 µl of
Annexin V-FITC and 5 µl of PI solution, the cells were
incubated at room temperature for 5 min in the dark and acquired
through a FACSCalibur flow cytometer (BD Biosciences). Data
analysis was performed by using CellQuest software (BD
Biosciences).
Statistical analyses
Statistical analysis was performed with SPSS 16.0
software package (SPSS Inc., Chicago, IL, USA). The inter-group
differences were evaluated by one-way ANOVA analysis. p<0.05 was
considered statistically significant.
Results
Preparation and characterization of
mammosphere-forming cells from the primary breast cancer
After the removal of fibroblasts by differential
trypsinization and repetitive adherence, a modified suspension cell
method was carried out to obtain mammosphere-forming cells from a
patient with breast cancer. Freshly isolated cells were cultured in
conditioned medium with a low concentration of serum and growth
factor cocktail. Mammospheres were formed after 7 days and
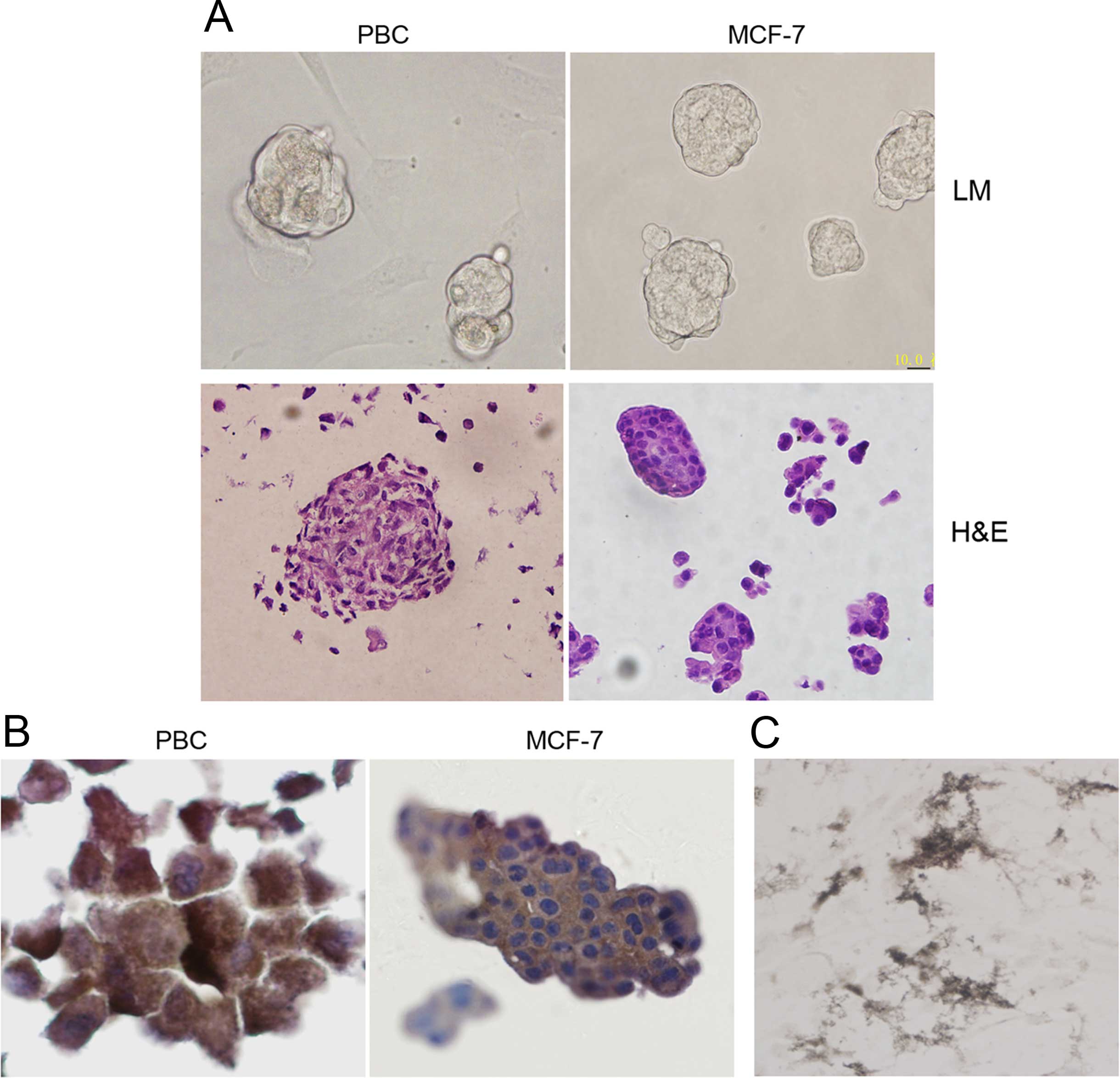
collected on day 14 (Fig. 1A, upper
panel). The ability to form mammospheres remained even after the
passage of mammosphere-forming cells. Histological analysis of
these mammospheres showed that they resembled the mammospheres
derived from breast cancer cell line MCF-7 in conditioned medium in
cell density and size (Fig. 1A,
lower panel). To determine the origin of the mammosphere-forming
cells obtained, we performed labeling for breast-specific markers.
Our results showed that the breast cancer-specific MGB1 staining of
the cells was apparent (Fig. 1B).
In addition, all mammosphere-forming cells exhibited strong
AP-positive staining (Fig. 1C),
which indicated that these cells still retained the tumorigenic
potential of breast cancer. The above results indicated that we
successfully established mammospheres from primary breast cancer
using the modified cell suspension method in the conditioned
medium.
Mammosphere-forming cells from the
primary breast cancer exhibit cancer stem cell-like properties
It is widely suggested that mammosphere-forming
cells generated from conditioned medium possess the CSC-like
properties (21). We next
determined whether the mammosphere-forming cells established from
primary breast cancer displayed CSC-like phenotypes. We first
analyzed the expression of CD44 and CD24, the representative
phenotypes of CSCs, in mammosphere cells by flow cytometry.
Mammosphere-forming cells from the primary breast cancer exhibited
CD44+CD24− characteristics (Fig. 2A), which differed from the
MCF-7-derived mammosphere-forming cells
(CD44loCD24lo).
Sox2, Nanog, Klf4 and Oct4 are the
most commonly used gene markers that are related to stem cell
differentiation (22). We further
analyzed the expression of these four genes in mammosphere-forming
cells from the primary breast cancer. As indicated in Fig. 2B, mammosphere-forming cells
expressed Nanog, Klf4 and Oct4 whereas the expression
level of Sox2 was relatively low (Fig. 2B). The expression level of
Klf4 in the mammosphere-forming cells from primary breast
cancer was comparable to that of the MCF-7-derived
mammosphere-forming cells, while Nanog and Oct4
expression was lower.
According to the above results we deduced that the
mammosphere-forming cells obtained from primary breast cancer
through conditioned culture belonged to a cell population with
CSC-like properties.
Mammosphere-forming cells from the
primary breast cancer maintain proliferative capacity in vitro and
in vivo
With the determination of CSCs-like properties of
the mammosphere-forming cells from the primary breast cancer, we
further evaluated the proliferative capacity of these cells in
vitro and in vivo. When compared with widely used breast
cancer cell line MCF-7, the proliferative rate of cells from the
mammospheres was comparable on day 1, but proliferated rapidly
afterwards. On days 4 and 7, the cellularity of the
mammosphere-forming cell group was significantly higher than that
of the MCF-7 cells (day 7, p=0.0134) (Fig. 3A), which is largely due to the fact
that the mammosphere-forming cells obtained from the primary breast
cancer retained the capacity of long-term survival and
proliferation.
Mammosphere-forming cells (103,
104 and 106) from the primary breast cancer
were inoculated into sub-lethal irradiated nude mice to determine
their tumorigenicity in vivo. Although no apparent neoplasia
was formed after 60 days, axillary lymph nodes were dramatically
enlarged in all mice even when the inoculated cell number was as
low as 103. Histological analysis of the axillary lymph
nodes showed that the structures of the lymph nodes were destroyed
by the compartmentalization of lymphocytes and the extensive
infiltration of tumor cells (Fig.
3B). Consistent with the histological observations, tumor cells
infiltrating into lymph nodes were mostly MGB1-positive, indicating
that these cells were derived from breast cancer (Fig. 3C).
These results indicated that the mammosphere-forming
cells that we obtained from a primary breast cancer maintained the
proliferative capacity in vitro and in vivo, further
verifying their CSC-like characteristics.
Mammosphere-forming cells from the
primary breast cancer are resistant to multiple chemotherapeutic
drugs
Preparation of primary tumor cells largely
facilitates the determination of the chemotherapy strategy through
drug sensitivity assays in the clinic. Considering the potential
roles of CSCs in the recurrence of tumors, drug sensitivity
screening of CSCs from primary breast cancer to chemotherapeutic
drugs may be of great value to control the recurrence of breast
cancers. Benefiting from the expansion of CSC-like
mammosphere-forming cells from the primary breast cancer in our
study, we further performed the drug sensitivity assay by
drug-induced apoptosis detection with four widely used first-line
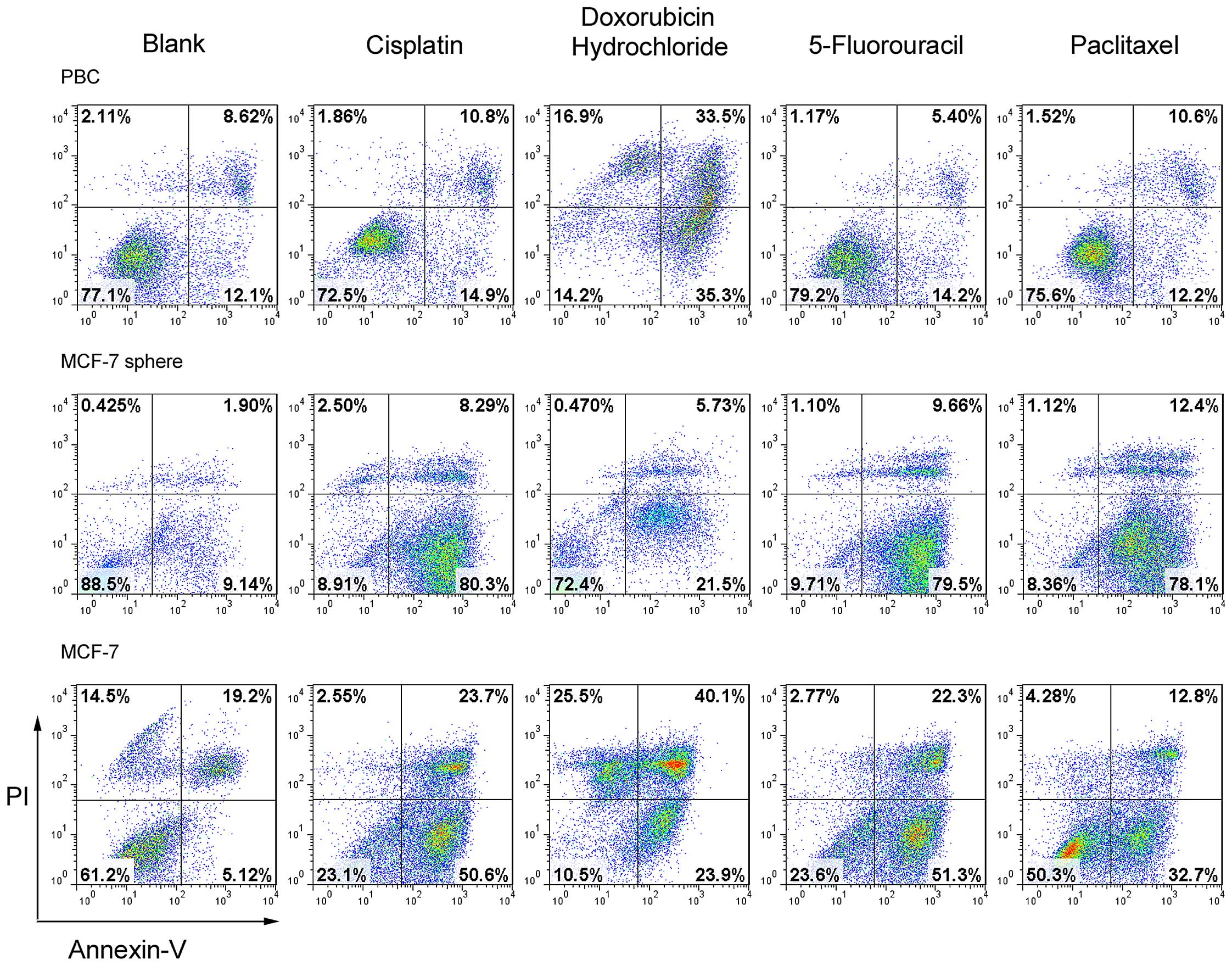
chemotherapeutic drugs. As shown in Fig. 4, the mammosphere-forming cells from
the primary breast cancer exhibited resistance to apoptosis upon
the treatment of three of the four chemotherapeutic drugs tested
(5-fluorouracil, paclitaxel and cisplatin) (Fig. 4) while they were more sensitive to
doxorubicin. However, MCF-7 or MCF-7 mammosphere cells underwent
apoptosis at a much higher frequency upon treatment of these four
drugs at the same concentration. Together with the data from the
proliferation assay, it was deduced that the mammosphere-forming
cells from the primary breast cancer exhibited more resistance to
multiple chemotherapeutic drugs, which may provide useful
information for breast CSC targeted therapy.
Mdr1 expression level in
mammosphere-forming cells from the primary breast cancer is altered
upon chemotherapeutic drug treatment
Increased expression of drug-resistant genes is one
of the important factors contributing to the resistance to
chemotherapeutic agents. In our study, we also analyzed the
expression of the drug-resistant mdr1 gene in three groups
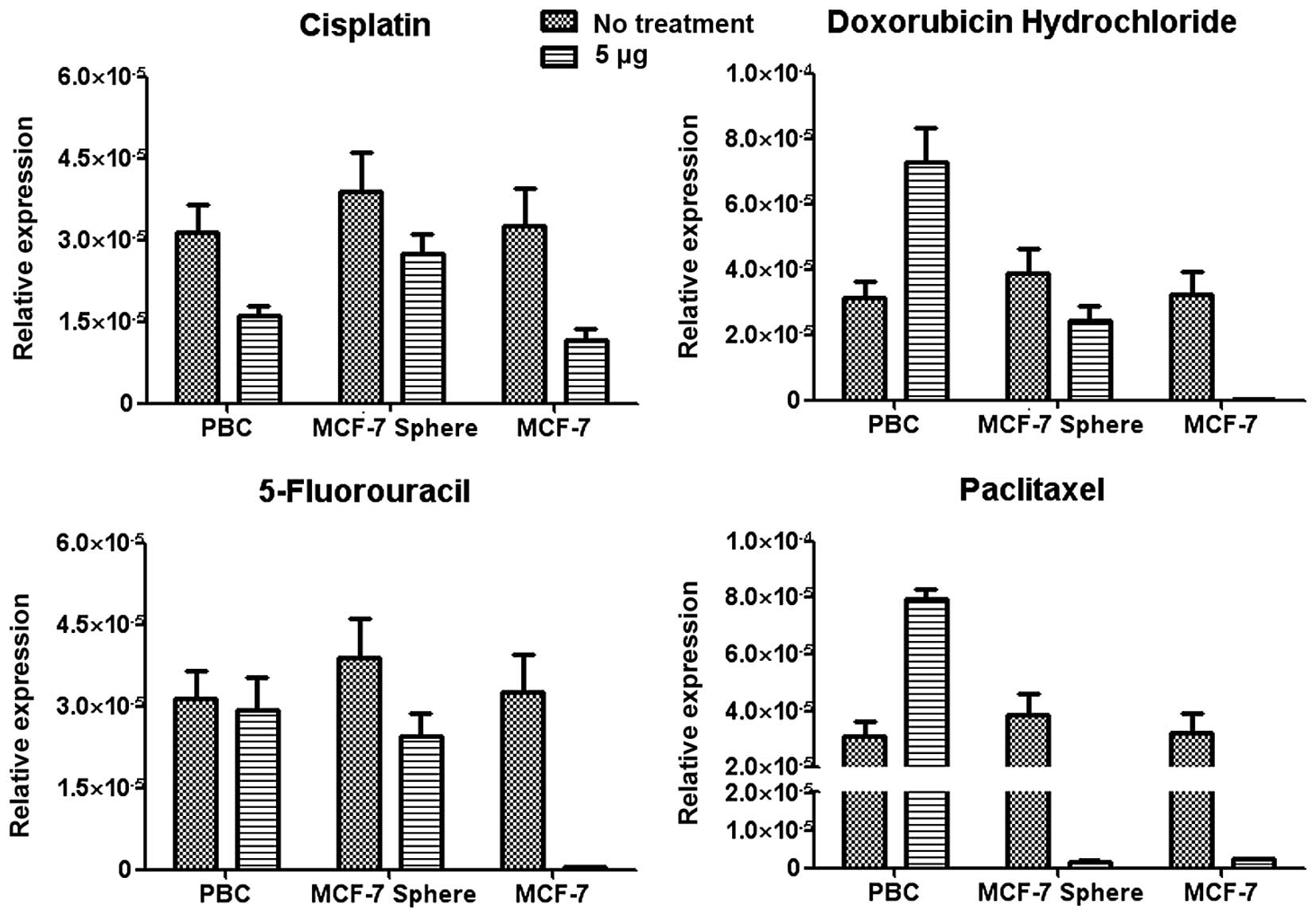
of cells after drug treatment. The mdr1 expression of
mammosphere-forming cells from the primary breast cancer increased
upon 5-fluorouracil and paclitaxel treatment, whereas maintained
upon doxorubicin treatment and decreased upon cisplatin treatment.
On the contrary, the expression of the mdr1 gene in the
MCF-7 or MCF-7-derived mammosphere cells decreased after the
treatment of all four drugs (Fig.
5). As mdr1 is responsible for the efflux of drugs from
inside of the cells, the decrease in mdr1 expression in the
MCF-7 or MCF-7-derived mammosphere-forming cells largely leads to
either increased apoptosis or lower proliferative capacity upon
drug treatment. The increased resistance of mammosphere-forming
cells from the primary breast cancer to the drugs may be partially
attributed to the higher expression of mdr1 expression on
the cell surface, which will shorten the detaining time of the
chemotherapeutic drugs inside the cells and reduce the sensitivity
to these drugs.
Discussion
In the present study, by using a modified cell
suspension culture method, we successfully established
mammosphere-forming cells from a primary breast cancer. These cells
displayed CSC-like phenotypes as well as the resistance to the
efficacy of multiple chemotherapeutic drugs widely used in the
clinic, which may provide important insight into the determination
of chemotherapeutic drugs for patients.
Breast CSCs are reported to be identified by their
capacity to grow in serum-free suspension cultures similar to
neural stem cell-forming aggregates. Enrichment of breast CSCs
through mammosphere culture thus has provided a facilitative in
vitro experimental model for study on breast CSCs. Rappa and
Lorico (23) reported that
mammospheres formed from a breast cancer cell line MA-11 had higher
oncogenic ability than the parent cells. Grimshaw et al
(19) performed suspension culture
with patient pleural fluid and found that, of 27 specimens
investigated, 20 formed mammospheres and had the ability to further
expand and differentiate. However, the difficulty in obtaining a
sufficient quantity of CSCs as well as maintaining the
undifferentiated state of CSCs in an in vitro culture
environment still greatly limits the application of these cells.
Along with the passage of primary breast cancer-derived
mammosphere-forming cells, the sizes of the mammospheres gradually
decreased and so did their reproductive capacity and the formation
rate. In addition, the currently used origins in mammosphere
studies are mostly breast cancer cell lines (24) or metastatic malignant cells from
pleural or ascitic fluid (25,26).
The establishment of mammospheres originating from primary breast
cancer tissues requires further investigation.
In this study, we partially modified the in
vitro culture conditions of embryonic stem cells (27) by adding growth factor cocktails such
as insulin-transferrin-selenium cocktail rather than insulin alone
together with a low concentration of serum. Insulin can improve the
intake of glucose and amino acids and synthesis of fat. Addition of
transferrin and selenium can reduce the toxicity of oxygen free
radicals and peroxides (28). In
addition, bFGF and EGF are both members of the growth factor family
with properties of mitogens, which can promote cell division and
proliferation (29). In
conventional culture system, the presence of serum promotes cell
differentiation while the cell proliferative rate is relatively low
in serum-free medium which becomes the bottleneck in CSC research.
We tried the addition of serum at a low concentration (0.5–2%) with
the significant acceleration of cell proliferation without
influencing the formation of mammospheres. With the modification of
the culture medium, we established and expanded the
mammosphere-forming cells from the primary breast cancer for
further investigation. In fact, it has been previously reported
that the addition of low level serum maintained the
undifferentiated state of stem cells in in vitro
cultivation. Wang et al induced mouse spermatogonial stem
cells to dedifferentiate to oocyte-like cells under a certain
culture condition containing 1% FBS (30).
Previous studies also indicate that the size of the
mammospheres reflects the self-renewal capacity of cancer stem
cells (9,31) and is one of the indicators for the
determination of CSCs. For mammosphere passage in our study,
mammospheres were digested into single cells using trypsin and
subcultured at a concentration of 1,000 cells/ml. Formation of the
2nd-generation mammospheres was observed to be formed with less
time than the 1st generation. Second-generation mammospheres were
observed after 3 days and the mammospheres became stable at day
7–10 with similar morphology to the first generation. From day 10,
the mammosphere centers gradually darkened with decreased
refraction and aging signs without the passage. Therefore, the
ideal passage time was between day 7 and 10 and mammospheres of
primary culture could be subcultured for at least 5
generations.
Cell surface proteins such as CD44 and CD24 are the
most commonly used markers of breast CSCs. In addition, CD20
(32), CD117 (33), CD133 (34–36)
and ALDH1 (37,38) are also prominent markers of CSCs. We
identified the CSC properties of the mammosphere-forming cells with
high CD44 expression and low CD24 expression together with the
breast-origin phenotype such as AP and MGB1-positive staining.
CSC-like mammosphere cells from the primary breast cancer could be
subcultured for at least 5 generations. Single-cell culture by
limited dilution revealed that the mammospheres were gradually
formed by single cells. Additionally, no significant change was
detectable in the mammosphere formation rate along with passage
progression (data not shown). As a control, we also generated
mammospheres originating from the MCF-7 cell line. They displayed a
phenotype such as CD44low and CD24low similar
to previous studies (8,9). Concerning the biological significance,
it has been reported that CD24− cells have higher
oncogenicity than CD24+ cells with increased progenitor
properties (8). Our results, to
some extent, imply that mammospheres obtained from primary breast
cancers may have even higher malignancy, which also corresponds to
the expression of ER/PR/HER2.
In additon to surface markers, mammosphere-forming
cells from the primary breast cancer also exhibited intrinsic stem
cell characteristics with the expression of relevant genes.
Nanog, Oct4, Sox2 and Klf4 are the most widely
studied transcription factors in maintaining self-renewal and
pluripotency of embryonic stem cells with transcriptional
regulation network (39–42). The stem cell phenotypes of human
embryonic cells are maintained by a self-stabilizing network of
transcription factors, such as Nanog, Oct4 and Sox2
(43). These factors positively
regulate genes responsible for the ES cell phenotype while
repressing transcription of genes required for inducing
differentiation. Klf4 has been demonstrated to be expressed
in adult somatic tissues with a higher rate of cell proliferation
(44) and is an upstream regulator
of a feed-forward loop that contains Nanog, Oct4 and
Sox2 (45,46). These factors are also demonstrated
to play important roles in the tumorigenesis of prostate cancer
(47), colorectal cancer (48) and bladder carcinomas (49) and correlate with poor prognosis
(39–42). In mammosphere-forming cells from the
breast cancer, the expression level of Sox2 was low whereas
the expression levels of Klf4, Nanog and Oct4
were detectable. On the contrary, four genes were all highly
expressed in the MCF-7-derived mammospheres.
Drug resistance is an important characteristic of
CSCs (50,51) and is also one of the important
reasons for chemotherapy resistance and the recurrence of tumors.
It has been demonstrated that the key drug-resistance gene,
mdr1, is highly expressed in many CSCs (52). The product of the mdr1 gene
is P-glycoprotein (P-gp) with 1,280 amino acid residues. It was
first isolated from drug-resistant tumor cells (53) and induces multi-drug resistance
depending on its capacity for ATP-dependent transmembrane transport
to carry drugs out of cells. High expression of the mdr1
gene in CSCs decreases the sensitivity of cells to chemotherapeutic
agents which in turn leads to the failure of CSC elimination, while
terminal differentiated tumor cells are cleared due to the low
expression of mdr1. In our study, mammosphere-forming cells
from the primary breast cancer inducibly expressed mdr1 upon
drug treatment (Fig. 5). This
finding may provide useful hints for the selection of
chemotherapeutic drugs.
The significance of our study covers two aspects:
the relationship between mammosphere formation rate with the
malignancy and the drug resistance of mammosphere-forming cells
from primary breast cancer. Concerning the relationship between the
malignant degree of tumors and the formation of mammospheres,
previous studies have indicated that ER+ MCF-7 cells,
whose growth relies on estrogen, have the lowest malignant
potential and mammosphere formation rate; SKBR3 cells with HER2
overexpression have the highest malignant potential and the highest
mammosphere formation rate within the same time period; MDA-MB-231
cells exhibit highest malignant potential with triple-negative
phenotype and require complicated conditions for mammosphere
formation (54). The mammosphere
formation rate of tumor cells may be due to the malignancy of
tumors while the formation rate of in vitro culture
mammospheres of primary tumors may be an important indicator for
prognosis. In this study, the mammosphere formation rate of the
primary breast cancer was high due to optimization of the culture
conditions and possibly also the apparent stem cell-like
characteristics. Such tumors may have even poorer clinical
prognosis. Future studies will assess long-term patient outcome and
investigate the correlation between mammosphere formation with the
cellular characteristics and disease prognosis.
Due to the potential roles of CSCs in the recurrence
of tumors, the establishment of CSCs from primary cancers may
facilitate the determination of the efficacy of chemotherapy
against recurrent tumors. However, to date there are few studies
concerning the significance of drug sensitivity of primary CSCs to
the treatment of recurrent cancers. We detected the drug
sensitivity of the mammosphere-forming cells but still require the
supportive evidence from the clinic. Nevertheless, the present
results obtained may be useful for further treatment if needed.
In conclusion, we successfully obtained
mammosphere-forming cells from a primary breast cancer under a
modified conditioned culture system. The mammosphere-forming cells
displayed CSC-like phenotypes as well as expressed stem
cell-related genes. These cells proliferated in vitro and
displayed lymph node metastasis potential. With regard to the
resistance of mammosphere-forming cells to chemotherapeutic drugs,
the results from this study may provide important clues for the
determination of the most effective individualized chemotherapy for
patients with recurrent tumors.
Acknowledgments
We appreciated Professor Feng-Chun Zhang for the
kind comments on the manuscript. This study was supported by grants
from the China National Basic Research Program (2014CB964704) and
the National Natural Science Foundation of China (nos. 81272328 and
81301858).
References
|
1
|
World Health Organization: Breast Cancer:
Prevention and Control. http://www.who.int/cancer/detection/breast-cancer/en/index.html.
2013
|
|
2
|
Huang ZZ, Chen WQ, Wu CX, Zheng RS, Chen
JG, Yang NN, Wang N, Zhang SW and Zheng Y: Incidence and mortality
of female breast cancer in China - a report from 32 Chinese cancer
registries, 2003–2007. Tumor. 32:435–439. 2012.
|
|
3
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polyak K and Hahn WC: Roots and stems:
stem cells in cancer. Nat Med. 12:296–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
6
|
Ginestier C, Liu S, Diebel ME, Korkaya H,
Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum
D, et al: CXCR1 blockade selectively targets human breast cancer
stem cells in vitro and in xenografts. J Clin Invest. 120:485–497.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarry JE, Murphy K, Perry R, Sanchez PV,
Secreto A, Keefer C, Swider CR, Strzelecki AC, Cavelier C, Récher
C, et al: Human acute myelogenous leukemia stem cells are rare and
heterogeneous when assayed in NOD/SCID/IL2Rγc-deficient mice. J
Clin Invest. 121:384–395. 2011. View
Article : Google Scholar :
|
|
8
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Idowu MO, Kmieciak M, Dumur C, Burton RS,
Grimes MM, Powers CN and Manjili MH:
CD44+/CD24−/low cancer stem/progenitor cells
are more abundant in triple-negative invasive breast carcinoma
phenotype and are associated with poor outcome. Hum Pathol.
43:364–373. 2012. View Article : Google Scholar
|
|
11
|
Bauerschmitz GJ, Ranki T, Kangasniemi L,
Ribacka C, Eriksson M, Porten M, Herrmann I, Ristimäki A, Virkkunen
P, Tarkkanen M, et al: Tissue-specific promoters active in
CD44+CD24−/low breast cancer cells. Cancer
Res. 68:5533–5539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang-Verslues WW, Kuo WH, Chang PH, Pan
CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH, et al:
Multiple lineages of human breast cancer stem/progenitor cells
identified by profiling with stem cell markers. PLoS One.
4:e83772009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tirino V, Desiderio V, Paino F, De Rosa A,
Papaccio F, La Noce M, Laino L, De Francesco F and Papaccio G:
Cancer stem cells in solid tumors: an overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar
|
|
15
|
Reynolds BA and Weiss S: Clonal and
population analyses demonstrate that an EGF-responsive mammalian
embryonic CNS precursor is a stem cell. Dev Biol. 175:1–13. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krause S, Maffini MV, Soto AM and
Sonnenschein C: A novel 3D in vitro culture model to study
stromal-epithelial interactions in the mammary gland. Tissue Eng
Part C Methods. 14:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phuc PVKT, Dong LV, Kiet TD, Giang TT and
Ngoc PK: Isolation and characterization of breast cancer stem cells
from malignant tumours in Vietnamese women. J Cell Anim Biol.
4:163–169. 2010.
|
|
19
|
Grimshaw MJ, Cooper L, Papazisis K,
Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou
J and Burchell JM: Mammosphere culture of metastatic breast cancer
cells enriches for tumorigenic breast cancer cells. Breast Cancer
Res. 10:R522008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shaw FL, Harrison H, Spence K, Ablett MP,
Simões BM, Farnie G and Clarke RB: A detailed mammosphere assay
protocol for the quantification of breast stem cell activity. J
Mammary Gland Biol Neoplasia. 17:111–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dontu G, Al-Hajj M, Abdallah WM, Clarke MF
and Wicha MS: Stem cells in normal breast development and breast
cancer. Cell Prolif. 36(Suppl 1): 59–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ralston A and Rossant J: The genetics of
induced pluripotency. Reproduction. 139:35–44. 2010. View Article : Google Scholar
|
|
23
|
Rappa G and Lorico A: Phenotypic
characterization of mammosphere-forming cells from the human MA-11
breast carcinoma cell line. Exp Cell Res. 316:1576–1586. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holliday DL and Speirs V: Choosing the
right cell line for breast cancer research. Breast Cancer Res.
13:2152011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J, et al: let-7 regulates self
renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent
and-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar
|
|
27
|
Yu Z, Ji P, Cao J, Zhu S, Li Y, Zheng L,
Chen X and Feng L: Dazl promotes germ cell differentiation from
embryonic stem cells. J Mol Cell Biol. 1:93–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raghu HM, Nandi S and Reddy SM: Effect of
insulin, transferrin and selenium and epidermal growth factor on
development of buffalo oocytes to the blastocyst stage in vitro in
serum-free, semidefined media. Vet Rec. 151:260–265. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakaguchi M, Dominko T, Yamauchi N,
Leibfried-Rutledge ML, Nagai T and First NL: Possible mechanism for
acceleration of meiotic progression of bovine follicular oocytes by
growth factors in vitro. Reproduction. 123:135–142. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Cao J, Ji P, Zhang D, Ma L, Dym M,
Yu Z and Feng L: Oocyte-like cells induced from mouse
spermatogonial stem cells. Cell Biosci. 2:272012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu S, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and Bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adhikari AS, Agarwal N, Wood BM, Porretta
C, Ruiz B, Pochampally RR and Iwakuma T: CD117 and Stro-1 identify
osteosarcoma tumor-initiating cells associated with metastasis and
drug resistance. Cancer Res. 70:4602–4612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tirino V, Desiderio V, d'Aquino R, De
Francesco F, Pirozzi G, Graziano A, Galderisi U, Cavaliere C, De
Rosa A, Papaccio G, et al: Detection and characterization of
CD133+ cancer stem cells in human solid tumours. PLoS
One. 3:e34692008. View Article : Google Scholar
|
|
35
|
Tirino V, Desiderio V, Paino F, De Rosa A,
Papaccio F, Fazioli F, Pirozzi G and Papaccio G: Human primary bone
sarcomas contain CD133+ cancer stem cells displaying
high tumorigenicity in vivo. FASEB J. 25:2022–2030. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tirino V, Camerlingo R, Franco R, Malanga
D, La Rocca A, Viglietto G, Rocco G and Pirozzi G: The role of
CD133 in the identification and characterisation of
tumour-initiating cells in non-small-cell lung cancer. Eur J
Cardiothorac Surg. 36:446–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
38
|
Ohi Y, Umekita Y, Yoshioka T, Souda M, Rai
Y, Sagara Y, Sagara Y, Sagara Y and Tanimoto A: Aldehyde
dehydrogenase 1 expression predicts poor prognosis in
triple-negative breast cancer. Histopathology. 59:776–780. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jung M, Peterson H, Chavez L, Kahlem P,
Lehrach H, Vilo J and Adjaye J: A data integration approach to
mapping OCT4 gene regulatory networks operative in embryonic stem
cells and embryonal carcinoma cells. PLoS One. 5:e107092010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lengerke C, Fehm T, Kurth R, Neubauer H,
Scheble V, Müller F, Schneider F, Petersen K, Wallwiener D, Kanz L,
et al: Expression of the embryonic stem cell marker SOX2 in
early-stage breast carcinoma. BMC Cancer. 11:422011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang P, Andrianakos R, Yang Y, Liu C and
Lu W: Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES)
cell differentiation by regulating Nanog gene expression. J Biol
Chem. 285:9180–9189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boyer LA, Lee TI, Cole MF, Johnstone SE,
Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG,
et al: Core transcriptional regulatory circuitry in human embryonic
stem cells. Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Adachi K and Schöler HR: Directing
reprogramming to pluripotency by transcription factors. Curr Opin
Genet Dev. 22:416–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chan KK-K, Zhang J, Chia N-Y, Chan Y-S,
Sim HS, Tan KS, Oh SK, Ng HH and Choo AB: KLF4 and PBX1 directly
regulate NANOG expression in human embryonic stem cells. Stem
Cells. 27:2114–2125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei Z, Yang Y, Zhang P, Andrianakos R,
Hasegawa K, Lyu J, Chen X, Bai G, Liu C, Pera M, et al: Klf4
interacts directly with Oct4 and Sox2 to promote reprogramming.
Stem Cells. 27:2969–2978. 2009.PubMed/NCBI
|
|
47
|
Bae KM, Su Z, Frye C, McClellan S, Allan
RW, Andrejewski JT, Kelley V, Jorgensen M, Steindler DA, Vieweg J,
et al: Expression of pluripotent stem cell reprogramming factors by
prostate tumor initiating cells. J Urol. 183:2045–2053. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin CW, Liao MY, Lin WW, Wang YP, Lu TY
and Wu HC: Epithelial cell adhesion molecule regulates tumor
initiation and tumorigenesis via activating reprogramming factors
and epithelial-mesenchymal transition gene expression in colon
cancer. J Biol Chem. 287:39449–39459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sanchez-Carbayo M, Socci ND, Lozano J,
Saint F and Cordon-Cardo C: Defining molecular profiles of poor
outcome in patients with invasive bladder cancer using
oligonucleotide microarrays. J Clin Oncol. 24:778–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Creighton CJ, Li X, Landis M, Dixon JM,
Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A,
Herschkowitz JI, et al: Residual breast cancers after conventional
therapy display mesenchymal as well as tumor-initiating features.
Proc Natl Acad Sci USA. 106:13820–13825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Donnenberg VS, Meyer EM and Donnenberg AD:
Measurement of multiple drug resistance transporter activity in
putative cancer stem/progenitor cells. Methods Mol Biol.
568:261–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen CJ, Chin JE, Ueda K, Clark DP, Pastan
I, Gottesman MM and Roninson IB: Internal duplication and homology
with bacterial transport proteins in the mdr1 (P-glycoprotein) gene
from multidrug-resistant human cells. Cell. 47:381–389. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang M-z, Zhang F-c and Zhang Y-y:
Influence factors on the formation of mammospheres from breast
cancer stem cells. Beijing Da Xue Xue Bao. 40:500–504. 2008.In
Chinese. PubMed/NCBI
|