Introduction
China is facing a health crisis caused by cancer
with nearly 2 million cancer-related deaths and more over 3 million
cancer cases diagnosed annually (1). Unfortunately, tumor metastasis is one
of the main causes of mortality among patients with malignant
tumors (2). Previous research
concerning tumor metastasis has focused on the adhesion and
migratory ability of cancer cells themselves. However, an
increasing number of studies show that the tumor microenvironment
plays a vitally important role in the progression of cancer,
especially in tumor metastasis (3).
As a complex integrated system, the tumor microenvironment consists
of fibroblasts, adipocytes, immune cells, inflammatory cells,
endothelial cells and extracellular matrix (4). In the tumor microenvironment, the
fibroblast is a specific cell type located in the stroma of cancer,
namely cancer-associated fibroblast cells (CAFs). Accumulating
evidence indicates that CAFs support the proliferation, migration
and chemotherapy resistance of cancer cells by producing
extracellular matrix, secreting cytokines, and activating signaling
pathways in cancer cells (5,6). The
adipocyte, another mesenchymal cell, stores energy and also
secretes various adipokines and cytokines to promote tumor
development. During the interaction with the tumor
microenvironment, adipocytes may dedifferentiate into
pre-adipocytes or cancer-associated adipocytes (CAAs) which can
stimulate the migration and invasion of cancer cells (7,8).
Obviously, fibroblasts and adipocytes as representative mesenchymal
cells can be considered as targets for cancer therapy.
The cytoskeleton is essential for directional cell
motility and migration. Actin cytoskeleton is the major player in
cell motility and locomotion in most eukaryotic cells. Meanwhile,
non-muscle myosin II (NMII) is a member of the myosin II subfamily,
namely actin-based myosin motor protein, and NMIIA is a member of
the NMII subclass (9). Previous
evidence has demonstrated that NMIIA functions in cell adhesion,
motility and migration, especially in cancer cell migration
(10–14). Defects or low expression of NMIIA
can promote cancer cell motility and migration via lamellipodia
extension, decrease in focal adhesion, and upregulation of
paxillin/p-paxillin (15–17). Thus, NMIIA may be a significant
potential anti-metastatic target in cancer with high metastatic
potential.
DT-13, the saponin monomer 13 of dwarf lilyturf
tuber, is isolated from Ophiopogon japonicus (Thunb.)
Ker-Gaul and is widely used in traditional Chinese medicine (TCM).
DT-13 has been found to exhibit antitumor effects, especially
anti-metastatic effects in vitro and in vivo
(18–21). Our previous research showed that
DT-13 could exhibit anti-metastatic effects directly by regulating
NMIIA especially in lung cancer 95D cells (22). However, it is unknown whether DT-13
can inhibit cell migration by regulating NMIIA in cancer cells
indirectly in the umor microenvironment. In the present study, we
revealed the indirect metastatic inhibitory effect of DT-13 by
regulating NMIIA in cancer cells through establishing a CAF-like
myofibroblastic phenotype model by exposure to hypoxia, as in
previous reports (23,24). In addition, we investigated the
indirect metastatic inhibitory effect by establishing a chemically
induced adipocyte model from 3T3-L1 cell line differentiation which
is a classical method avoiding lack of appropriate adipocytes
(25,26).
Materials and methods
Cell culture
The human lung fetal cell line MRC-5 was cultured in
minimum essential media (MEM; Gibco). Mouse embryonic fibroblast
3T3-L1 cell line was cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco). Human lung cancer cell line, 95D (with high
metastatic potential) was cultured in RPMI-1640 medium (Gibco). All
the cells were obtained from the Cell Bank of the Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The media were supplemented with 10% fetal
bovine serum (FBS; Gibco), 100 U/ml penicillin and 100 U/ml
streptomycin. The cells were incubated in a humidified atmosphere
with 95% air and 5% CO2 at 37°C.
Drugs and reagents
DT-13 powder was kindly provided by Professor Yu
Boyang (China Pharmaceutical University). We purchased
anti-paxilin, anti-phospho-paxiliin, anti-FAK, anti-phospho-FAK,
anti-c-raf, anti-phospho-c-raf, anti-ERK, anti-phospho-ERK,
anti-HIF-1α, anti-NMIIA and FITC-conjugated secondary antibodies
from Cell Signaling Technology. Anti-α-SMA was from Abcam.
Methylisobutylxanthine (IBMX) and insulin (bovine) were purchased
from Sigma. Dexamethasone was from G-Biosciences.
Conditioned medium (CM) preparation
The normoxic conditioned medium (Nor-CM) and hypoxic
conditioned medium (Hypo-CM) were harvested from the condition of
normoxic/hypoxic MRC-5 cells or adipocytes, respectively. Then the
conditioned medium was cleared through centrifugation. The CM was
stored at −80°C without repeated freezing and thawing.
To obtain Hypo-CM (DT-13) from DT-13-treated hypoxic
MRC-5 cells or adipocytes, the MRC-5 cells or adipocytes were
treated with DT-13 (0.1, 1, and 10 µM) and cultured in
normoxic condition with medium. Twelve hours later, the used medium
was removed and the cells were washed twice with PBS. Subsequently,
the cells were cultured with fresh medium for additional 12 h in a
hypoxic condition. Then the Hypo-CM (DT-13) was harvested as
described above.
Migration assays
Wound-healing assay
Cells were seeded onto 12-well plates and incubated
in complete medium to 100% confluency for the experiment. Wounds
were created in the plates using a pipette tip. Then the cells were
washed twice with PBS, replaced with fresh serum-free media and
treated with CM or/and reagent at specified concentrations for
another 12 h. Subsequently, cell migration following each treatment
was observed via photo-micrograph.
Transwell migration assay
Cell migration was determined in Transwell chambers
(8-µm pore size; Corning, Corning, NY, USA). Briefly, the CM
was added to 24-well plates (the lower chamber), while the cells
were seeded into the upper chamber with serum-free medium. After 12
h, the cells migrated through the membrane and adhered to the
underside of the membrane. Subsequently, the migrated cells were
stained with crystal violet and counted via photomicrograph at ×400
magnification.
Invasion assay
Cancer cell invasion was assayed in Transwell
chambers (8-µm pore size; Corning). The membrane of the
upper chamber was coated with 30 µg of Matrigel (Sigma) for
2 h. After 12 h, the cells invaded through the Matrigel and
membrane to the underside of the chamber membrane. Subsequently,
the migrated cells were stained by crystal violet and counted via
photomicrograph at ×400 magnification.
Immunofluorescent staining
Cells were seeded on coverslips in 24-well plates
and cultured to 50–60% confluency for the experiment. After 12 h,
the coverslips were washed with PBS, and then fixed in 4%
paraformaldehyde for 20 min at room temperature. Next, the cells
were washed and incubated with the antibody for 12 h at 4°C. In a
dark place, the cells were then incubated with a secondary antibody
for 1 h at room temperature and with Hoechst 33342 for 30 min.
Subsequently, the processed cells were mounted, and the results
were analyzed via fluorescence photomicrograph by an inverted
microscope (Olympus IX-71; Olympus, Japan).
Western blot analysis
Total cell lysates were extracted from the cultured
cells with protease inhibitors. The proteins were fractionated by
8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electroblotted onto PVDF membranes (Millipore, USA).
Blocking buffer was used for blocking and the membranes were
incubated with primary antibodies for 12 h at 4°C. Then, it was
probed with relative secondary antibody for 1 h at room
temperature. Subsequently, the expression of the target
immunoreactive proteins was examined by Immobilon Western
Chemiluminescent HRP substrate (Millipore).
Statistical analysis
The results are expressed as the mean ± standard
deviation (SD). GraphPad Prism 5.0 and the Student's t-test were
used to determine the level of significance. A p-value of <0.05
was considered to indicate a statistically significant result.
Results
Establishment of the tumor
microenvironment model through exposure to hypoxia and chemically
induced differentiation
Activation of the α-SMA gene is an essential feature
during the conversion of fibroblasts into myofibroblasts.
Therefore, we investigated the expression of α-SMA protein in
hypoxic MRC-5 cells, to characterize the shift of cultured
fibroblasts. After hypoxia for 12 h, the expression of α-SMA was
significantly upregulated, suggesting that hypoxic MRC-5 cells were
analogous to CAFs (Fig. 1A) as
previously reported (23,24). In addition, HIF-1α was confirmed to
be the major transcriptional factor in response to hypoxia.
Therefore, the upregulated level of HIF-1α also demonstrated the
response of MRC-5 cells to hypoxia (Fig. 1A). The cell proliferation assay
showed that the CM from the hypoxic fibroblasts had no effect on
cancer cell proliferation (Fig.
1B).
Meanwhile, the chemically induced adipocyte model
was established according to published protocols by response to a
mixture of insulin, dexamethasone and IBMX. After the 8-day
procedure (25), the mature
adipocytes were detected by Nile Red O staining (Fig. 1C), and then hypoxia for 12 h. The
cell proliferation assay showed that the CM from hypoxic adipocytes
did not affect cancer cell proliferation (Fig. 1D). Obviously, the model which was
established through exposure to hypoxia and chemically induced
differentiation was analogous to CAFs and adipocytes, respectively,
to some extent. In addition, CM is an appropriate mediator to
explore the crosstalk between two cell types. Therefore, the model
made it possible for us to evaluate the migration induced by the
tumor microenvironment.
The conditioned medium from the tumor
microenvironment model promotes migration and invasion
In the present study, wound-healing and Transwell
chamber assays were used to examine the invasion and migration
induced by Hypo-CM (hypoxic-conditioned medium). After 95D cells
were treated with Hypo-CM from the fibroblasts for 12 h, the
migration distance increased compared with the Norm-CM group
(Fig. 2A). Meanwhile, a higher
percentage of cancer cells moved across the membrane to the
underside when compared with that noted in the Norm-CM group
(Fig. 2A), after treatment with
Hypo-CM for 12 h. Similarly, Hypo-CM from adipocytes significantly
promoted the migration distance and the migration number of 95D
cells compared with the Norm-CM group, after treatment with Hypo-CM
from adipocytes for 12 h (Fig. 2B).
However, Hypo-CM from the fibroblasts also stimulated the invasion
of the 95D cells (Fig. 2A).
In addition, we investigated the distribution of
NMIIA in cancer cells affected by Hypo-CM from hypoxic fibroblasts
and adipocytes via immunofluorescent staining, to confirm whether
cell migration ability could be improved via regulation of NMIIA in
the cancer cells. Ultimately, the photomicrograph showed that NMIIA
spread to the cytoplasm after treatment with Hypo-CM from the tumor
microenvironment model, while NMIIA closely accumulated around the
nucleus in the control (Fig. 4C).
Further western blot analysis showed that Hypo-CM from the tumor
microenvironment model significantly inhibited NMIIA, confirming
that low expression of NMIIA can promote cancer cell motility and
migration as in previous research (15–17)
(Fig. 2C). Obviously, all these
results indicated that hypoxic fibroblasts and adipocytes were able
to promote 95D migration by regulating NMIIA.
 | Figure 4DT-13 inhibits the migration and
invasion of cancer cells indirectly in the tumor microenvironment
model. (A) Hypo-CM (DT-13) (CM from DT-13-treated hypoxic
fibroblasts) inhibited the migration and invasion of cancer cells
as detected by wound-healing, Transwell migration and Transwell
invasion assays, respectively, indirectly in hypoxic fibroblast
microenvironment. (B) Hypo-CM (DT-13) (CM from DT-13-treated
hypoxic adipocytes) inhibited the migration of cancer cells as
detected by Transwell migration assay. (C) Hypo-CM (DT-13) (CM from
DT-13-treated hypoxic fibroblasts or adipocytes) inhibited the
spread of NMIIA toward the cytoplasm by IF analysis. The cancer
cells were treated with Nor-CM, Hypo-CM or Hypo-CM (DT-13),
respectively, for 12 h. Data are expressed as mean ± SD,
***P<0.001, **P<0.01, compared with the
Nor-CM; ###P<0.001, ##P<0.01, compared
with the Hypo-CM. |
NMIIA regulates the migration and
invasion of cancer cells in the tumor microenvironment model
Downregulation of NMIIA can promote cancer cell
motility and migration via lamellipodia extension, decrease in
focal adhesions, and upregulation of paxillin/p-paxillin. In
addition, blebbistatin, an inhibitor of non-muscle myosin II, can
promote cell migration by inhibiting NMIIA but has no effect on the
proliferation of cancer cells (27). After concomitant treatment with
Hypo-CM from hypoxic fibroblasts and blebbistatin for 12 h, the
cell migration was improved, compared with that treated only with
Hypo-CM or Norm-CM via wound-healing and Transwell chamber assays
(Fig. 3A). However, the cell
invasion was slightly reduced compared with the Hypo-CM group, but
significantly improved in comparison with the Norm-CM group
(Fig. 3A). Meanwhile, the Transwell
migration assay also confirmed that the inhibition of NMIIA could
promote 95D cell migration in the condition of hypoxic adipocytes
(Fig. 3B). In brief, the above
results suggested that NMIIA plays a role in cell motility and
migration in the hypoxic tumor microenvironment; thus, indicating
that NMIIA is a significant potential target for
anti-metastasis.
 | Figure 3NMIIA regulates the migration and
invasion of cancer cells. (A) Blebbistatin further promoted cancer
cell migration and invasion as detected by wound-healing, Transwell
migration and Transwell invasion assays, respectively, indirectly
in the hypoxic fibroblast microenvironment. (B) Blebbistatin
further promoted cancer cell migration as detected by Transwell
migration assay indirectly in the hypoxic adipocyte
microenvironment. The cancer cells were treated with Nor-CM,
Hypo-CM or/and blebbistatin (+B), respectively, for 12 h. Data are
expressed as mean ± SD, ***P<0.001,
**P<0.01, compared with the Nor-CM;
###P<0.001, ##P<0.01, compared with the
Hypo-CM. Nor-CM, normoxic conditioned medium; Hypo-CM, hypoxic
conditioned medium. |
DT-13 inhibits the migration and invasion
of cancer cells indirectly in the tumor microenvironment model
DT-13 has been found to exhibit an anti-metastatic
effect in vitro and in vivo. As a natural traditional
Chinese medicine, it inhibits cell migration via multi-levels,
different pathways and multiple targets. Our previous research
showed that DT-13 could exhibit antitumor effects directly by
regulating NMIIA in cancer cells (22). Firstly, the cell proliferation assay
showed that DT-13 had no effect on the proliferation of MRC-5 cells
(data not shown). However, the Hypo-CM (DT-13) from hypoxic
fibroblasts reduced cell migration and invasion significantly
(Fig. 4A), but without a
proliferation effect on the cancer cells (data not shown).
Meanwhile, the Transwell migration assay also confirmed that DT-13
could inhibit 95D cell migration in the condition of hypoxic
adipocytes (Fig. 4B). Furthermore,
the immunofluorescent staining showed that DT-13 could reverse the
Hypo-CM-induced spread of NMIIA to the cytoplasm both in the
condition of hypoxic fibroblasts and adipocytes (Fig. 4C). Therefore, all these results
indicated that DT-13 inhibits cell migration and invasion by
regulating NMIIA in cancer cells indirectly in the tumor
microenvironment model.
DT-13 inhibits cell migration by
regulating NMIIA in cancer cells indirectly in the tumor
microenvironment model
NMIIA can regulate the migration and invasion of
cancer cells in the hypoxic tumor microenvironment. Meanwhile,
DT-13 inhibits metastasis indirectly by hypoxic fibroblasts and
adipocytes. Therefore, western blot analysis was used to
investigate whether NMIIA and downstream proteins could be affected
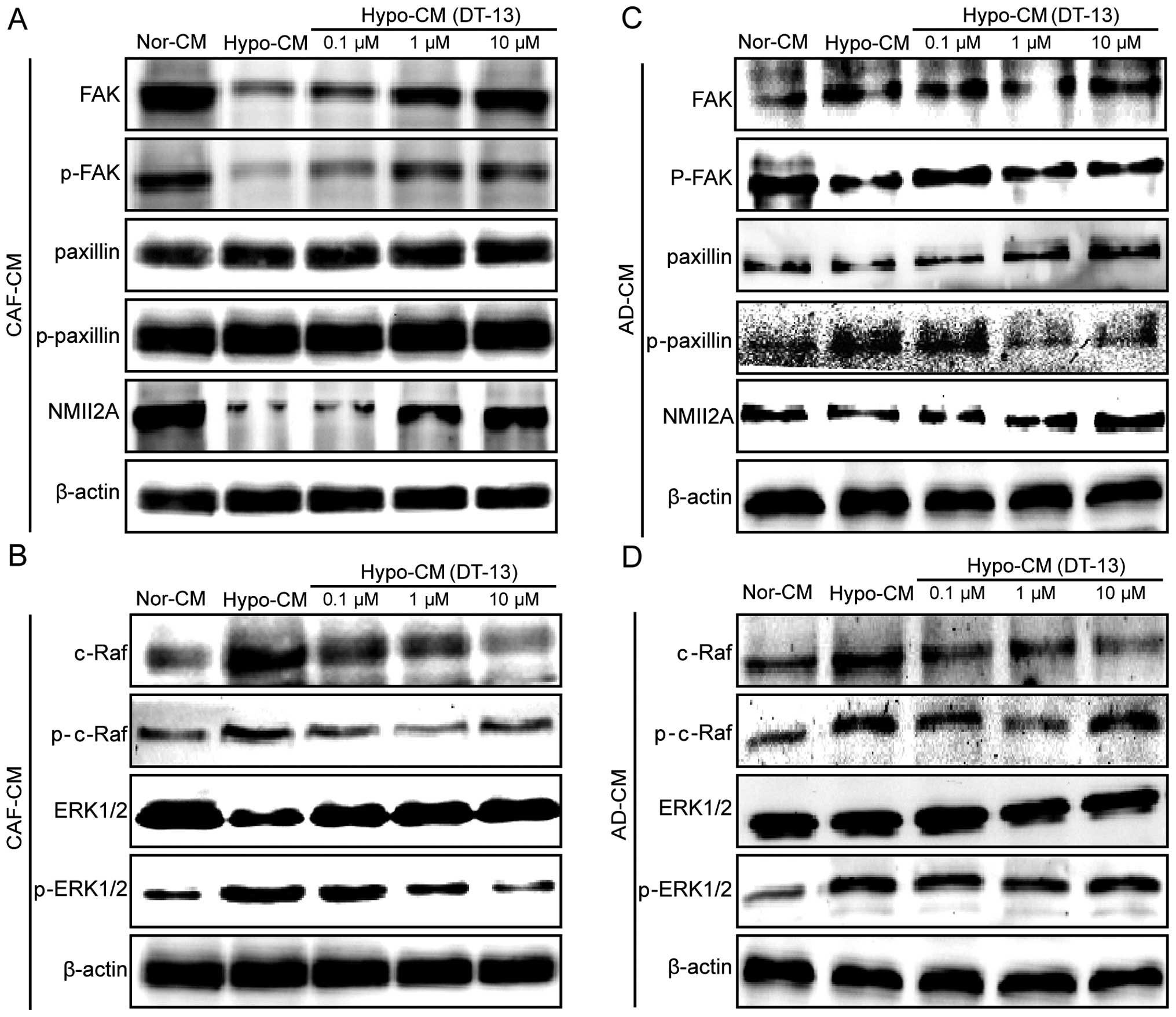
by Hypo-CM (DT-13). Notably, Hypo-CM (DT-13) upregulated NMIIA,
while its expression was inhibited by Hypo-CM. p-FAK was inhibited
by Hypo-CM, but was significantly upregulated by Hypo-CM (DT-13)
compared with the Hypo-CM group, under conditions of hypoxic
fibroblasts and adipocytes (Fig. 5A
and C). Paxillin and p-paxillin had no change in the hypoxic
fibroblast microenvironment, while Hypo-CM (DT-13) significantly
inhibited p-paxillin induced by Hypo-CM from the adipocytes
(Fig. 5A and C). Obviously, these
data suggest that DT-13 inhibits cell migration by regulating NMIIA
in cancer cells indirectly in a hypoxic tumor microenvironment.
Next, we ascertained whether the regulatory pathway
of NMIIA in cancer cells was affected by Hypo-CM (DT-13). Western
blot analysis found that p-c-Raf and p-ERK1/2 were upregulated by
Hypo-CM, while the phosphorylation levels of both were greatly
inhibited by Hypo-CM (DT-13) (Fig.
5B and D), indicating that DT-13 regulates NMIIA via the
Raf-ERK1/2 signaling pathway in cancer cells after Hypo-CM (DT-13)
treatment.
Discussion
The tumor microenvironment has been increasingly
confirmed as a key factor in multiple stages of cancer progression
(2). Thereby, more therapeutic
strategies are purposefully designed to not only target cancer
cells but also regulate the tumor microenvironment (5). Notably, DT-13 has been found to
exhibit antitumor effects, especially anti-metastasis in
vitro and in vivo. Moreover, our research showed that
DT-13 can exhibit anti-metastasis effects directly by regulating
NMIIA especially in lung cancer cells 95D (22). Therefore, it is not known whether
DT-13 can also inhibit cell migration by regulating NMIIA in cancer
cells indirectly in the tumor microenvironment.
Fibroblasts and adipocytes are two most
representative mesenchymal cells in the tumor microenvironment
(4). We established a CAF-like
myofibroblastic phenotype model and a chemically induced adipocyte
model as previously reported (23–25),
revealing the effects of the tumor microenvironment on cancer cells
to some extent. In this model, the conditioned medium from the
tumor microenvironment model significantly promoted 95D cell
migration and regulated the expression of NMIIA. Then, we confirmed
the hypothesis that the tumor microenvironment regulates NMIIA in
cancer cells and facilitates migration by using the non-muscle
myosin II inhibitor, blebbistatin, in further experiments.
Importantly, this is the first report to the best of our knowledge
that the tumor microenvironment can promote cancer cell migration
by regulating the expression of NMIIA. Finally, DT-13 was found to
inhibit cell migration by regulating NMIIA and its downstream
proteins in cancer cells indirectly in the tumor microenvironment
model. Further results showed that DT-13 exhibited anti-migratory
effects via the raf/ERK1/2 signal pathway. Consequently, the study
confirmed that DT-13 significantly inhibited 95D cell migration
in vitro, showing potential anti-metastatic effects on lung
cancer and the scientific basis for drug development.
Mesenchymal cells include CAFs, adipocytes, immune
cells, inflammatory cells and endothelial cells. T cells, B cells,
macrophages and mast cells are immune cells which can also promote
immune escape, tumor growth and metastasis (28). Inflammatory cells can secrete
excessive amounts of inflammatory cytokines such as IL-6, IL-10,
TGF-β, EGF to stimulate invasion, migration, proliferation, to
enhance other mesenchymal cells inducing metastasis (29). Under the condition of tumor
angiogenesis factors, endothelial cells can establish tumor
vasculature to support cancer metastasis and proliferation,
aggravating tumor malignancy (30).
In brief, immune cells, inflammatory cells, endothelial cells and
other mesenchymal cells compose the complex tumor microenvironment
with CAFs and adipocytes. Together they promote cancer cell
migration and invasion.
NMIIA is a significant potential target for
anti-metastasis in highly metastatic cancer (31). DT-13 has been found to exhibit an
anti-metastatic effect directly by regulating NMIIA in 95D cells.
In the present study, the data suggested that DT-13 can also
inhibit 95D cell migration by regulating the mechanism of cancer
cells indirectly by hypoxic fibroblasts and adipocytes. Whether
DT-13 can inhibit cancer cell migration indirectly through immune
cells, inflammatory cells or endothelial cells warrants further
investigation. The filament assembly of NMIIA is selectively
regulated by certain S100 proteins such as S100A4 (the small
Ca2+-binding protein) which can enhance cell migration
in certain types of cancer (32,33).
Thus, further research will determine the effect of DT-13 on
S100A4. Moreover, subsequent experiments should confirm the in
vitro mechanism by establishment of orthotopic tumor models and
observation by PET/CT (34).
Although metastases are responsible for the majority
of cancer-related deaths, there are few therapeutic approaches that
specifically target metastasis. On the basis of these findings, the
tumor microenvironment is further confirmed as a novel strategy by
which to inhibit tumor metastases. Importantly, DT-13 may be a
potential anti-metastatic drug for clinical application.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China for Youth (no. 81102853), the National
Natural Science Foundation of China (no. 81573456) and the National
High-Tech Research and Development Projects (863) (no.
2014AA022208).
References
|
1
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H,
Zou X and He J: Annual report on status of cancer in China, 2010.
Chin J Cancer Res. 26:48–58. 2014.PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leonardi GC, Candido S, Cervello M,
Nicolosi D, Raiti F, Travali S, Spandidos DA and Libra M: The tumor
microenvironment in hepatocellular carcinoma (Review). Int J Oncol.
40:1733–1747. 2012.PubMed/NCBI
|
|
4
|
Semenza GL: The hypoxic tumor
microenvironment: A driving force for breast cancer progression.
Biochim Biophys Acta. 1863:382–391. 2016. View Article : Google Scholar
|
|
5
|
De Wever O, Van Bockstal M, Mareel M,
Hendrix A and Bracke M: Carcinoma-associated fibroblasts provide
operational flexibility in metastasis. Semin Cancer Biol. 25:33–46.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Routray S, Sunkavali A and Bari KA:
Carcinoma-associated fibroblasts, its implication in head and neck
squamous cell carcinoma: A mini review. Oral Dis. 20:246–253. 2014.
View Article : Google Scholar
|
|
7
|
Ali AT, Hochfeld WE, Myburgh R and Pepper
MS: Adipocyte and adipogenesis. Eur J Cell Biol. 92:229–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bochet L, Meulle A, Imbert S, Salles B,
Valet P and Muller C: Cancer-associated adipocytes promotes breast
tumor radioresistance. Biochem Biophys Res Commun. 411:102–106.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiu HC, Chang TY, Huang CT, Chao YS and
Hsu JT: EGFR and myosin II inhibitors cooperate to suppress
EGFR-T790M-mutant NSCLC cells. Mol Oncol. 6:299–310. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gupton SL and Waterman-Storer CM:
Spatiotemporal feedback between actomyosin and focal-adhesion
systems optimizes rapid cell migration. Cell. 125:1361–1374. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Even-Ram S, Doyle AD, Conti MA, Matsumoto
K, Adelstein RS and Yamada KM: Myosin IIA regulates cell motility
and actomyosin-microtubule crosstalk. Nat Cell Biol. 9:299–309.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hosono Y, Usukura J, Yamaguchi T,
Yanagisawa K, Suzuki M and Takahashi T: MYBPH inhibits NM IIA
assembly via direct interaction with NMHC IIA and reduces cell
motility. Biochem Biophys Res Commun. 428:173–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH and Adelstein RS: LPA(1)-induced
migration requires nonmuscle myosin II light chain phosphorylation
in breast cancer cells. J Cell Physiol. 226:2881–2893. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vicente-Manzanares M, Ma X, Adelstein RS
and Horwitz AR: Non-muscle myosin II takes centre stage in cell
adhesion and migration. Nat Rev Mol Cell Biol. 10:778–790. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jacobelli J, Friedman RS, Conti MA,
Lennon-Dumenil AM, Piel M, Sorensen CM, Adelstein RS and Krummel
MF: Confinement-optimized three-dimensional T cell amoeboid
motility is modulated via myosin IIA-regulated adhesions. Nat
Immunol. 11:953–961. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka C, Ito S, Nishio N, Kodera Y,
Sakurai H, Suzuki H, Nakao A and Isobe K: GADD34 suppresses wound
healing by upregulating expression of myosin IIA. Transgenic Res.
19:637–645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Betapudi V: Myosin II motor proteins with
different functions determine the fate of lamellipodia extension
during cell spreading. PLoS One. 5:e85602010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Liu J, Kou J, Yu J and Yu B:
DT-13 suppresses MDA-MB-435 cell adhesion and invasion by
inhibiting MMP-2/9 via the p38 MAPK pathway. Mol Med Rep.
6:1121–1125. 2012.PubMed/NCBI
|
|
19
|
Zhao R, Sun L, Lin S, Bai X, Yu B, Yuan S
and Zhang L: The saponin monomer of dwarf lilyturf tuber, DT-13,
inhibits angiogenesis under hypoxia and normoxia via
multi-targeting activity. Oncol Rep. 29:1379–1386. 2013.PubMed/NCBI
|
|
20
|
Ren-Ping Z, Sen-Sen L, Yuan ST, Yu BY, Bai
XS, Sun L and Zhang LY: DT-13, a saponin of dwarf lilyturf tuber,
exhibits anti-cancer activity by down-regulating C-C chemokine
receptor type 5 and vascular endothelial growth factor in
MDA-MB-435 cells. Chin J Nat Med. 12:24–29. 2014.PubMed/NCBI
|
|
21
|
Lin SS, Fan W, Sun L, Li FF, Zhao RP,
Zhang LY, Yu BY and Yuan ST: The saponin DT-13 inhibits gastric
cancer cell migration through down-regulation of CCR5-CCL5 axis.
Chin J Nat Med. 12:833–840. 2014.PubMed/NCBI
|
|
22
|
Wei X, Lin S, Liu Y, Zhao R, Ghulam JK, Du
H, Mao T, Yu B, Yuan S and Sun L: DT-13 attenuates lung cancer
metastasis via directly regulating NMIIA activity under hypoxia
condition. Oncol Rep. In press.
|
|
23
|
Fang D, Sun L, Lin S, Zhou L, Su N, Yuan S
and Yu B: Vinorelbine inhibits angiogenesis and 95D migration via
reducing hypoxic fibroblast stromal cell-derived factor 1
secretion. Exp Biol Med (Maywood). 237:1045–1055. 2012. View Article : Google Scholar
|
|
24
|
Zhou L, Sun L, Lin S, Fang D, Zhao R, Zhu
J, Liu J, Chen L, Shi W, Yuan S, et al: Inhibition of angiogenic
activity of hypoxic fibroblast cell line MRC-5 in vitro by
topotecan. Med Oncol. Nov 9–2010.Epub ahead of print.
|
|
25
|
Choi KC, Lee SY, Yoo HJ, Ryu OH, Lee KW,
Kim SM, Baik SH and Choi KM: Effect of PPAR-delta agonist on the
expression of visfatin, adiponectin, and resistin in rat adipose
tissue and 3T3-L1 adipocytes. Biochem Biophys Res Commun.
357:62–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gernapudi R, Yao Y, Zhang Y, Wolfson B,
Roy S, Duru N, Eades G, Yang P and Zhou Q: Targeting exosomes from
preadipocytes inhibits preadipocyte to cancer stem cell signaling
in early-stage breast cancer. Breast Cancer Res Treat. 150:685–695.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jean L, Majumdar D, Shi M, Hinkle LE,
Diggins NL, Ao M, Broussard JA, Evans JC, Choma DP and Webb DJ:
Activation of Rac by Asef2 promotes myosin II-dependent
contractility to inhibit cell migration on type I collagen. J Cell
Sci. 126:5585–5597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Semov A, Moreno MJ, Onichtchenko A,
Abulrob A, Ball M, Ekiel I, Pietrzynski G, Stanimirovic D and
Alakhov V: Metastasis-associated protein S100A4 induces
angiogenesis through interaction with Annexin II and accelerated
plasmin formation. J Biol Chem. 280:20833–20841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Z, Wang S and Zhao RC: The roles of
mesenchymal stem cells in tumor inflammatory microenvironment. J
Hematol Oncol. 7:142014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du H, Shi H, Chen D, Zhou Y and Che G:
Cross-talk between endothelial and tumor cells via basic fibroblast
growth factor and vascular endothelial growth factor signaling
promotes lung cancer growth and angiogenesis. Oncol Lett.
9:1089–1094. 2015.PubMed/NCBI
|
|
31
|
Derycke L, Stove C, Vercoutter-Edouart AS,
De Wever O, Dollé L, Colpaert N, Depypere H, Michalski JC and
Bracke M: The role of non-muscle myosin IIA in aggregation and
invasion of human MCF-7 breast cancer cells. Int J Dev Biol.
55:835–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elliott PR, Irvine AF, Jung HS, Tozawa K,
Pastok MW, Picone R, Badyal SK, Basran J, Rudland PS, Barraclough
R, et al: Asymmetric mode of Ca2+-S100A4 interaction
with nonmuscle myosin IIA generates nanomolar affinity required for
filament remodeling. Structure. 20:654–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bowers RR, Manevich Y, Townsend DM and Tew
KD: Sulfiredoxin redox-sensitive interaction with S100A4 and
non-muscle myosin IIA regulates cancer cell motility. Biochemistry.
51:7740–7754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takahashi O, Komaki R, Smith PD,
Jürgensmeier JM, Ryan A, Bekele BN, Wistuba II, Jacoby JJ,
Korshunova MV, Biernacka A, et al: Combined MEK and VEGFR
inhibition in orthotopic human lung cancer models results in
enhanced inhibition of tumor angiogenesis, growth, and metastasis.
Clin Cancer Res. 18:1641–1654. 2012. View Article : Google Scholar : PubMed/NCBI
|