Introduction
The development of thyroid cancer is closely related
to a variety of factors, papillary thyroid carcinoma (PTC) ranks
highest in incidence amongst thyroid cancers; epidemiological
surveys have shown that the incidence of PTC has a rising trend,
and that it occurs more frequently in women (1–3). Even
with recent advancements in medical technology, PTC is difficult to
diagnose at an early stage because of its insidious onset devoid of
clinical symptoms, leading to its poor prognosis at the time of
diagnosis.
A common treatment for PTC includes surgical
resection followed by radioactive 131I therapy and other
chemotherapeutic drugs (4–6). Molecular biology research has made
microRNAs (miRNAs) a hotspot in the medical and life sciences, and
a large number of studies have shown that miRNAs are closely
related to the development and progression of multiple cancers
(7,8). A recent study found that the
overexpression of miR-146a can significantly increase the incidence
of ovarian cancer, and that clinical treatment with a drug blocking
miR-146a expression can significantly increase the survival time of
patients (9). Also, the expression
of miR-146b is associated with the occurrence of breast cancer, and
is closely related to the proliferation and migration of breast
cancer cells (10).
The IRAK family proteins participate in the
TLRs/IL-1 signaling pathway, that is involved in intracellular
signal control and the inflammatory response. The IRAK1 protein is
closely related to the development of inflammation and tumors.
However, there are no reports on the relationship between the
expression of miR-146a and miR-146b in PTC and whether IRAK1
protein is involved in their regulation.
In this study, the expression levels of miR-146a and
miR-146b in PTC tissues were quantified, and statistical analyses
were performed looking for possible associations to clinical and
pathological features and prognosis of patients. Additionally,
siRNAs were used to transfect TPC-1 cells in order to knock down
their expression levels of miR-146a and miR-146b and compare their
proliferation and migration abilities.
Materials and methods
Subjects and biological samples
The samples (73 in total) in this study were
selected from PTC tumors removed by surgery and confirmed by
pathology from patients admitted to Yidu Central Hospital of
Weifang from September 2013 to September 2015. The patients ages
ranged from 38 to 67 years, there were 34 males and 39 females; all
patients were cleared from other severe diseases and all signed
informed consent forms. All patients presented complete clinical
and pathological data. Cancer-free tumor adjacent tissue samples
(at least 5 cm away from tumor) were also examined as negative
controls. All patients underwent standard treatment and were
followed up for 1-year after diagnosis. Samples of cancer and
adjacent tissue from each patient were stored in liquid nitrogen.
The study was approved by the Ethics Committee of Yidu Central
Hospital of Weifang.
Semi-quantitative RT-PCR detection of
the expression of miR-146a and miR-146b in cancer tissues
Tissue samples were taken out of the liquid nitrogen
storage and thawed. The total RNA was extracted from each sample
according to the instructions on the TRIzol kit (Invitrogen,
Carlsbad, CA, USA). The quality of the resulting RNA samples was
confirmed by agarose gel electrophoresis. The gels showed clear and
distinct 28S, 18S and 5S bands with the brightness of the 28S band
being about twice that of the 18S, indicating that the RNA was
intact and could be used for subsequent experiments. Then, cDNA was
produced using a reverse transcription kit (Invitrogen), and the
expression levels of miR-146a and miR-146b in cancer and adjacent
tissues were detected by semi-quantitative PCR using a SYBR
ExScript™ RT-PCR kit (Takara Bio, Shiga, Japan), using β-actin as
internal reference gene. The PCR reaction conditions included 35
cycles of 95°C for 30 sec for denaturation, 64°C for 25 sec for
annealing, and 72°C for 30 sec for extension. Primers were
synthesized by Tiangen Biotech Co., Ltd. (Beijing, China) and the
sequences are shown in Table I.
After the amplification, agarose gel electrophoresis was performed
and the gel was observed using an UV imaging system. Each RT-PCR
experiment was repeated at least three times for statistical
analysis.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Gene | Sequence |
|---|
| miR-146a | F:
5-ATCCACCTTGACGATGCTTTAC-3 |
|
| R:
5-TTCAGATGTTCTAAGCCTACGG-3 |
| miR-146b | F:
5-TGGCCCTCGTAGCCTTGAGGAC-3 |
|
| R:
5-CCAGTGCTGCAGGGTCCGAGGT-3 |
| β-actin | F:
5-GATGATTGGCATGGCTTT-3 |
|
| R:
5-CACCTTCCGTTCCAGTTT-3′ |
Construction of miR-146a and miR-146b
low expression cell lines
miR146a-siRNA, miR146b-siRNA and its corresponding
control sequences were designed and synthesized by Toyobo Co., Ltd.
(Osaka, Japan). Proliferating TPC-1 cells (Chinese Academy of
Sciences Shanghai Cell Bank) were transfected with the synthesized
siRNAs. After 48 h, total RNA was extracted from the cells and cDNA
was obtained by reverse transcription, the expression of miR-146a
and miR-146b was detected by semi-quantitative RT-PCR. Successfully
transfected cells were placed in incubator and cultured at 37°C, 5%
CO2 for subsequent experiments. The expression levels of
miR-146a and miR-146b were low in the miRNA-transfected cells but
not so in the ones transfected with negative control siRNAs.
Detection of the effect of low
expression of miR-146a and miR-146b on TPC-1 cells
MTT assays for proliferation of thyroid papillary
carcinoma cells
The MTT assay was used to compare the proliferation
of the different siRNA-transfected TPC-1 cells. MTT (Sigma-Aldrich,
St. Louis, MO, USA) was prepared at 5 mg/ml. Ninety-six-well plates
with 3×104 cells/well were incubated for 48 h before
adding MTT. Then, after 4 h of incubation, the medium was
discarded. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was added to
each well and the absorbance values were measured at 570 nm using a
microplate reader (Eppendorf, Hamburg, Germany).
Transwell migration assays for transfected
papillary thyroid carcinoma cells
Transwell assays were performed to investigate the
effect of low expression of miR-146a and miR-146b on the migration
of papillary thyroid carcinoma cells. After 24-h starvation, the
cells were adjusted to a concentration of 5×105 cells/ml
and added to the Transwell chamber. The number of cells passing
through the chamber was calculated under a microscope (Olympus,
Tokyo, Japan) after dyeing and fixing (10).
Detection of the expression level of IRAK1
protein by western blot analysis
The samples from the papillary thyroid carcinoma
tissues and adjacent healthy tissues were taken out of the liquid
nitrogen. The tissue was cut with scissors, and the cells were
homogenized in lysis buffer. After centrifugation 2,600 × g for 5
min, the supernatant was transferred to a fresh tube to save the
soluble protein. A Protein Quantification kit (Millipore,
Billerica, MA, USA) was used to quantify the extracted protein, and
the protein samples of similar concentration were prepared. The
samples were loaded onto SDS-PAGE and then transferred to a western
blot membrane. After a standard transfer, the membranes were
blocked and washed before adding the rabbit anti-human IRAK1
polyantibody (dilution, 1:1,000; cat. no. 06-872; Millipore) for
overnight incubation at 4°C. Next morning, the membranes were
washed 3 times with TBST before adding the goat anti-rabbit
peroxidase polyantibody (dilution, 1:5,000; cat. no. A0545;
Sigma-Aldrich, St. Louis, MO, USA) and incubating at room
temperature for 2 h. The membranes were then triple washed with
TBST. Finally, the chemiluminescent signals were visualized and the
target protein bands were scanned for analysis. The expression of
β-actin was used as an internal reference (rabbit anti-human
monoantibody; 1:5,000; cat. no. A5060; Sigma-Aldrich); the sheep
serum for blocking from Jackson ImmunoResearch Laboratories, Inc.
(West Grove, PA, USA).
Statistical analysis
The data in this study were expressed as mean ±
standard deviation values. The SPSS 19.0 software (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analyses. The
measurement data were analyzed using the t-test. Comparisons
between groups of enumeration data were analyzed by the
χ2 test. The homogeneity of variance test was performed,
if the variance was homogeneous, the comparison between two was
conducted using the Bonferronic method, if the variance was not
homogeneous, then the Welchs method was adopted. Additionally, the
Dunnett's T3 method was used for multiple comparisons, and the
Pearson correlation statistical analysis for correlation analysis.
A P<0.05 for any given difference was considered as
statistically significant.
Results
miR-146a and miR-146b expression
levels in PTC patients
The expression levels of miR-146a and miR-146b in
cancer and adjacent healthy tissues of PTC patients (73 samples
each) were detected by semi-quantitative RT-PCR. The results showed
that the relative expression of miR-146a and miR-146b in cancer
tissues was significantly higher than in the healthy tissues
(P<0.01), as shown in Fig.
1.
Relationship between the expression
levels of miR-146a and miR-146b and the clinicopathological
features and prognosis of patients
The expression levels of miR-146a and miR-146b in
the tissues of papillary thyroid carcinoma detected by ELISA were
recorded to find any possible correlations with patients age, tumor
size, clinical stage or other clinical features, and the results
are shown on Table II. The
relative expression of miR-146a and miR-146b in peripheral blood of
PTC patients does not correlate with age, GA125 or FIGO staging
(P>0.05). However, the relative expression correlates with the
presence of lymph node metastasis and cancer recurrence
(P<0.05). The expression levels were used to classify the data
in groups with either high, normal or low expression levels of
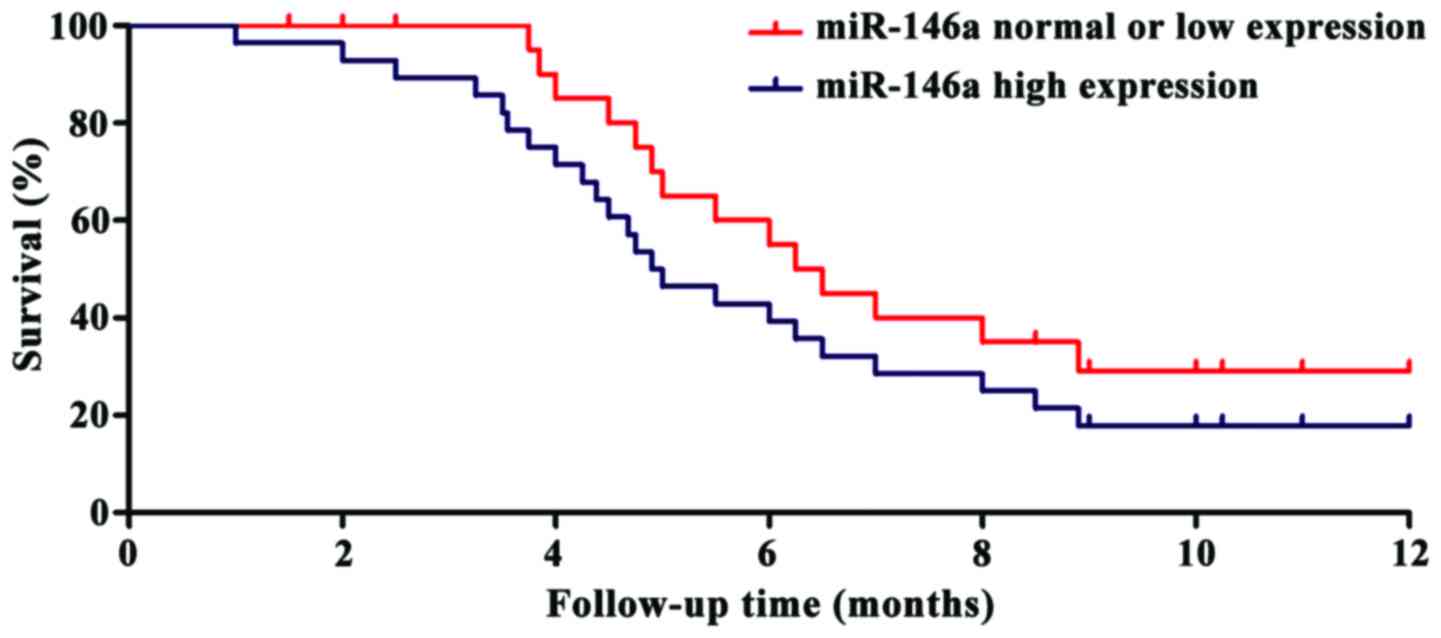
miR-146a and miR-146b. During the follow-up year, it was observed
that the survival time of the patients with high expression of
miR-146a and miR-146b was significantly shorter than that of the
patients in the normal or low expression groups (P<0.01). The
survival curves can be seen in Figs.
2 and 3.
 | Table II.Relationship between the expression of
miR-146a and miR-146b and different clinical features of PTC
patients. |
Table II.
Relationship between the expression of
miR-146a and miR-146b and different clinical features of PTC
patients.
| Item | n | miR-146a
expression | P-value | miR-146b
expression | P-value |
|---|
| Age (years) |
|
| 0.0692 |
| 0.0782 |
| ≤50 | 37 | 5.98±1.33 |
| 3.53±1.08 |
|
|
<50 | 36 | 6.16±1.28 |
| 3.85±1.12 |
|
| GA125 (U/ml) |
|
| 0.0619 |
| 0.0873 |
| ≤35 | 43 | 5.87±1.08 |
| 5.63±1.62 |
|
|
>35 | 30 | 6.16±1.22 |
| 5.57±1.29 |
|
| FIGO staging |
|
| 0.0538 |
| 0.0686 |
| Stage
I–II | 28 | 6.29±1.39 |
| 6.08±1.19 |
|
| Stage
III–IV | 45 | 6.89±1.87 |
| 5.95±2.01 |
|
| Lymphatic
metastasis |
|
| 0.0078 |
| 0.0097 |
| Yes | 36 | 6.76±2.01 |
| 6.73±1.78 |
|
| No | 37 | 3.58±1.96 |
| 2.17±1.05 |
|
| Recurrence |
|
| 0.0086 |
| 0.0176 |
| Yes | 17 | 5.97±1.86 |
| 5.32±1.25 |
|
| No | 56 | 2.38±1.29 |
| 3.17±1.03 |
|
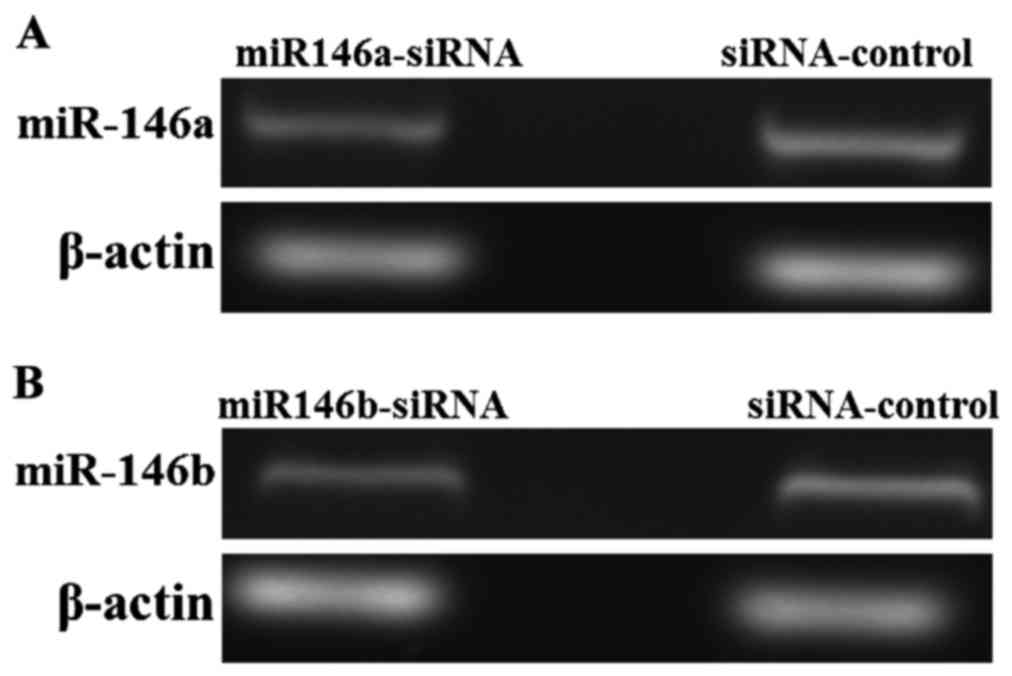
Construction of miR-146a and miR-146b
low expression TPC-1 cell lines
The expression of miR-146a and miR-146b in thyroid
papillary carcinoma cells TPC-1 were downregulated using a standard
siRNA method. Next, total RNA was extracted and the expression was
detected with semi-quantitative PCR after reverse transcription.
The results are shown in Fig. 4.
Compared with the cells transfected with siRNA-control, the
expression of miR-146a in the miR146a-siRNA transfected cells
decreased to 17.82±3.42% (P<0.01). The expression of miR-146b in
the miR146b-siRNA cells decreased 23.67±5.83% (P<0.01),
indicating that the miR-146a and miR-146b knock-down TPC-1 cell
lines were successfully constructed.
Effects of low expression of miR-146a
and miR-146b on cell proliferation
The MTT assay was used to determine proliferation of
the knock-down cell lines, as shown in Fig. 5. The numbers of cells in the
miR146a-siRNA and the miR146b-siRNA group were significantly lower
than the number of cells in the siRNA-control group
(P<0.01).
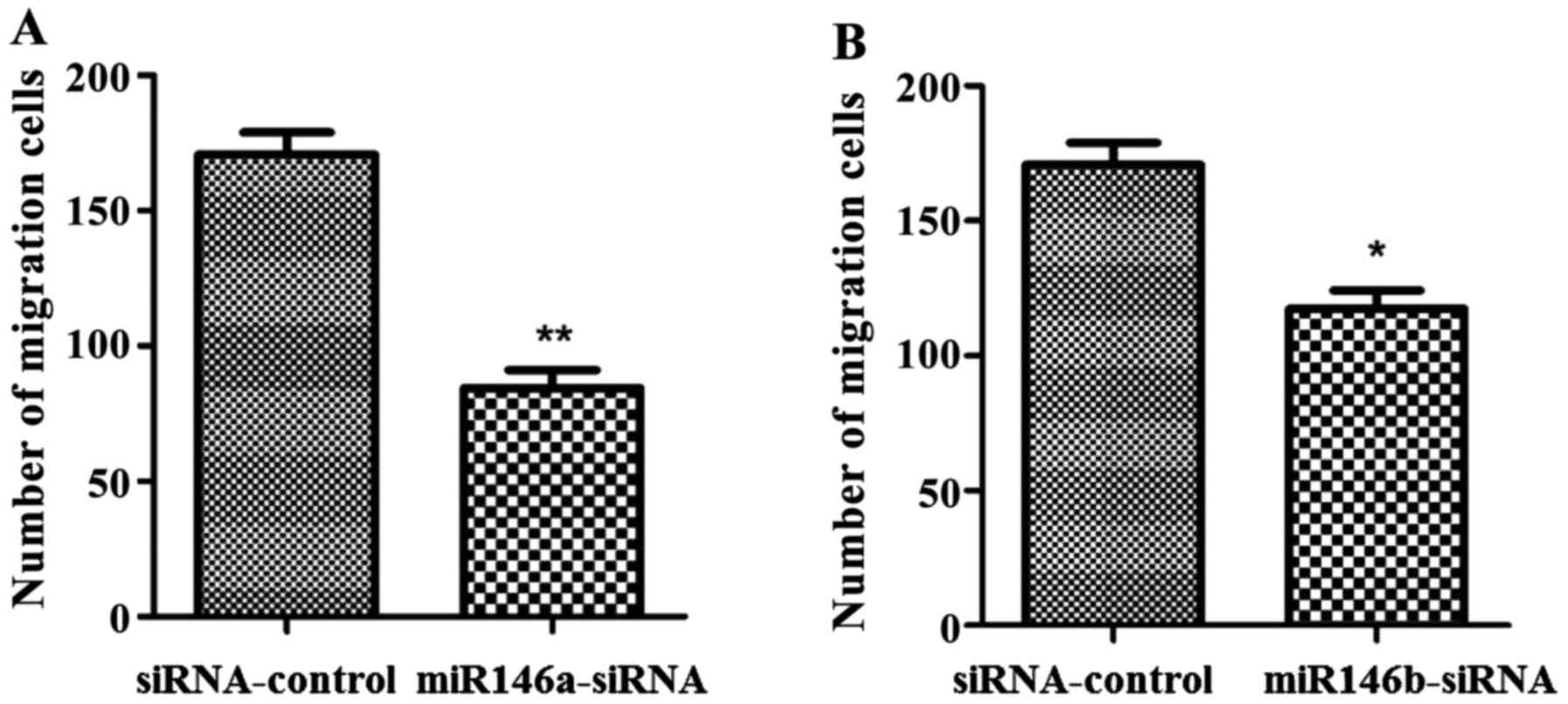
Effects of low expression of miR-146a
and miR-146b on cell migration
The Transwell assay was used to assess cell
migration on the miR-146a and miR-146b knock-down cell lines, the
results are shown in Fig. 6. The
migration ability of cells in the miR146a-siRNA and miR146b-siRNA
groups was significantly lower than the migration ability of cells
in the siRNA-control group.
Detection of the expression of IRAK1
protein by western blot analysis
In order to investigate the effect of miR-146a and
miR-146b on the expression of IRAK1 protein, the expression levels
of IRAK1 protein in cancer and healthy adjacent tissues of patients
with papillary thyroid carcinoma was detected by western blot
analysis. The results are shown in Fig.
7. Compared with the normal tissues, the expression level of
IRAK1 protein in the cancer tissues was significantly decreased
(P<0.01).
Discussion
The regulation of miRNAs on human pathological and
physiological processes is done in the body mainly through the
expression of proteins during and after gene transcription. miRNAs
can induce abnormal metabolism of tumor cells by influencing their
transcription and translation processes (11,12).
High expression of miRNAs causing abnormal proliferation and
migration of tumor cells can induce cancer, however in other cases
the role of miRNAs can be tumor suppression (13). In studies of miRNAs, it has been
observed that miR-146a expression is significantly higher in a
variety of tumor cells than in normal tissues, and miR-146a is
mostly involved in inflammation (14–16). A
study found that breast cancer cells expressing high levels of
miR-146a were more prone to distant metastasis than tumor cells
with low expression levels of miR-146a (17). Other researchers using an animal
sarcoma model also found that miR-146a can affect the proliferation
and migration of tumor cells, and that proliferation and migration
were significantly decreased after knockout of miR-146a (18). At present, there are relatively few
studies on miR-146b. A research group found evidence for a role of
miR-146b on the pathogenesis of colon cancer, where the possibility
of colon cancer in miR-146b overexpression patients is
significantly increased (19).
In this study, it was found that the expression
levels of miR-146a and miR-146b were closely related to the
occurrence of PTC. This indicates that regulating the expression of
miR-146a and miR-146b may be a valid therapeutic strategy against
cancer. The prognoses of patients with normal or low expression of
miR-146a and miR-146b were significantly better than those of
patients with high expression levels. The study of the relationship
between the expression of miR-146a and miR-146b and PTC
clinicopathological features showed that the expressions of
miR-146a and miR-146b was closely related to the metastasis and
recurrence of PTC (P<0.01). miR-146a and miR-146b may lead to
PTC recurrence and metastasis by affecting the proliferation and
migration ability of papillary thyroid cancer cells. At present,
there is evidence that miR-146a and miR-146b are mainly involved in
inflammation processes (19).
Therefore, the regulation of inflammation by these two genes may
also affect the progress of PTC. Western blot analysis showed that
miR-146a and miR-146b could significantly increase the expression
of IRAK1. Other researchers found that miR-146b can upregulate
IRAK1 expression and plays a role in the inflammatory response in
ovarian cancer (20). However, the
mechanisms by which miR-146a and miR-146b regulate the expression
of IRAK1 protein and their effects on the occurrence and prognosis
of PTC is not clear at present.
In summary, miR-146a and miR-146b were highly
expressed in PTC cancer tissues; and the prognoses of patients were
related to the levels of expression of both miRNAs in cancer cells.
The relative expressions of miR-146a and miR-146b should be useful
as molecular markers for PTC screening, and the miRNAs could
provide new targets for the clinical treatment against PTC.
References
|
1
|
Lai XJ, Zhang B, Jiang YX, Li JC, Zhao RN,
Yang X, Zhang Q, Zhang XY, Li WB and Zhu SL: High risk of lateral
nodal metastasis in lateral solitary solid papillary thyroid
cancer. Ultrasound Med Biol. 42:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu TR, Su X, Qiu WS, Chen WC, Men QQ, Zou
L, Li ZQ, Fu XY and Yang AK: Thyroid-stimulating hormone receptor
affects metastasis and prognosis in papillary thyroid carcinoma.
Eur Rev Med Pharmacol Sci. 20:3582–3591. 2016.PubMed/NCBI
|
|
3
|
Wojakowska A, Chekan M, Marczak Ł,
Polanski K, Lange D, Pietrowska M and Widlak P: Detection of
metabolites discriminating subtypes of thyroid cancer: Molecular
profiling of FFPE samples using the GC/MS approach. Mol Cell
Endocrinol. 417:149–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan A, Stewart CJ, Garrett KL, Rye M and
Cohen PA: Novel BRAF and KRAS mutations in papillary thyroid
carcinoma arising in struma ovarii. Endocr Pathol. 26:296–301.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soylu L, Aydin OU, Ozbas S, Bilezikci B,
Ilgan S, Gursoy A and Kocak S: The impact of the multifocality and
subtypes of papillary thyroid carcinoma on central compartment
lymph node metastasis. Eur Rev Med Pharmacol Sci. 20:3972–3979.
2016.PubMed/NCBI
|
|
6
|
Kamaya A, Tahvildari AM, Patel BN,
Willmann JK, Jeffrey RB and Desser TS: Sonographic detection of
extracapsular extension in papillary thyroid cancer. J Ultrasound
Med. 34:285–297. 2015. View Article : Google Scholar
|
|
7
|
Li B, Yang XX, Wang D and Ji HK:
MicroRNA-138 inhibits proliferation of cervical cancer cells by
targeting c-Met. Eur Rev Med Pharmacol Sci. 20:1109–1114.
2016.PubMed/NCBI
|
|
8
|
Shu XL, Fan CB, Long B, Zhou X and Wang Y:
The anti-cancer effects of cisplatin on hepatic cancer are
associated with modulation of miRNA-21 and miRNA-122 expression.
Eur Rev Med Pharmacol Sci. 20:4459–4465. 2016.PubMed/NCBI
|
|
9
|
Sharma N, Verma R, Kumawat KL, Basu A and
Singh SK: miR-146a suppresses cellular immune response during
Japanese encephalitis virus JaOArS982 strain infection in human
microglial cells. J Neuroinflammation. 12:302015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Ansari MM and Aboussekhra A:
miR-146b-5p mediates p16-dependent repression of IL-6 and
suppresses paracrine procarcinogenic effects of breast stromal
fibroblasts. Oncotarget. 6:30006–30016. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafiee-Pour HA, Behpour M and Keshavarz M:
A novel label-free electrochemical miRNA biosensor using methylene
blue as redox indicator: Application to breast cancer biomarker
miRNA-21. Biosens Bioelectron. 77:202–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esposito CL, Catuogno S and de Franciscis
V: Aptamer-MiRNA conjugates for cancer cell-targeted delivery.
Methods Mol Biol. 1364:197–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Williams J, Smith F, Kumar S, Vijayan M
and Reddy PH: Are microRNAs true sensors of ageing and cellular
senescence? Ageing Res Rev. 17:435–447. 2016.
|
|
14
|
Wei Q, Lei R and Hu G: Roles of miR-182 in
sensory organ development and cancer. Thorac Cancer. 6:2–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi Z, Johnson JJ, Jiang R, Liu Y and
Stack MS: Decrease of miR-146a is associated with the
aggressiveness of human oral squamous cell carcinoma. Arch Oral
Biol. 60:1416–1427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Xu B, Li S, Zhang B, Mao P, Qian B,
Guo L and Ni P: Association of SNPs in miR-146a, miR-196a2, and
miR-499 with the risk of endometrial/ovarian cancer. Acta Biochim
Biophys Sin (Shanghai). 47:564–566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stückrath I, Rack B, Janni W, Jäger B,
Pantel K and Schwarzenbach H: Aberrant plasma levels of circulating
miR-16, miR-107, miR-130a and miR-146a are associated with lymph
node metastasis and receptor status of breast cancer patients.
Oncotarget. 6:13387–13401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang WJ, Wang H, Tong QX, Jie SH, Yang DL
and Peng C: nvolvement of TLR2-MyD88 in abnormal expression of
miR-146a in peripheral blood monocytes of patients with chronic
hepatitis C. J Huazhong Univ Sci Technolog Med Sci. 35:266–271.
2015. View Article : Google Scholar
|
|
19
|
Chen L, Dai YM, Ji CB, Yang L, Shi CM, Xu
GF, Pang LX, Huang FY, Zhang CM and Guo XR: MiR-146b is a regulator
of human visceral preadipocyte proliferation and differentiation
and its expression is altered in human obesity. Mol Cell
Endocrinol. 393:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Xu J, Li H, Sun C, Yu L, Li Y, Shi
C and Zhou X: miR-146b-5p functions as a tumor suppressor by
targeting TRAF6 and predicts the prognosis of human gliomas. J Cell
Physiol. 231:328–335. 2015.PubMed/NCBI
|