Introduction
Gastric cancer (GC) is one of the most common
malignancies around the world (1).
Although its morbidity has been in decline in most developed
countries in recent decades (2), GC
was estimated to be the second cause of cancer-related deaths in
2015 in China (3). Surgery is the
primary method of GC treatment, but it is not suitable for the
majority of patients diagnosed with advanced tumors due to late
diagnosis. Alternatively, chemotherapy is more suitable for these
patients (4). Classical
platinum-based chemotherapies provide limited improvement in
survival rates and possess substantial toxicity (5). In addition, only a small percentage of
patients (~20%) benefit from human epidermal receptor 2
(HER2)-targeted therapy (6–8). Therefore, the development of novel
drugs to treat advanced GC patients is urgent and essential.
In the past few years, numerous natural compounds
extracted from Chinese herbs have been identified to possess
anti-GC activity (9–11). Curcumol, a compound first isolated
from the rhizome of Curcuma by Hiroshi in 1965, has been
demonstrated to be effective in tumor treatment with low
cytotoxicity (12).
Our previous studies revealed that curcumol
inhibited cell proliferation of SPC-A-1 human lung adenocarcinoma
cells in vitro and in vivo (13). Curcumol and other ingredients
isolated from Rhizoma Curcumae have been identified to induce
apoptosis of GC cells and inhibit the proliferation of GC cell
lines (14,15). However, the underlying mechanism has
yet to be fully elucidated.
In the present study, we identified the antitumor
mechanisms of curcumol in GC. We ascertained the antitumor effect
of curcumol on GC cells. We revealed that curcumol induced cell
apoptosis and G2/M cell cycle arrest in GC cells. Moreover, the
results revealed that curcumol upregulated the intracellular
reactive oxygen species (ROS) level and decreased the mitochondrial
membrane potential (MMP). The downregulation of isocitrate
dehydrogenase 1 (IDH1) was also involved in the antitumor activity
of curcumol.
Materials and methods
Chemicals and reagents
Curcumol (racemate, purity ≥96.7%) was donated by
Haimen Desihang Co., Ltd. (Zhejiang, China). Curcumol was dissolved
in absolute ethyl alcohol and diluted in Dulbecco's modified
Eagle's medium (DMEM) culture medium with 1% alcohol to treat
cells. AG-120 (ivosidenib) was purchased from Selleck Chemicals
(Houston, TX, USA). Dimethyl sulfoxide (DMSO) was purchased from
Aladdin Reagent Co. (Los Angeles, CA, USA). Fetal bovine serum
(FBS), DMEM and Trypsin-EDTA were all purchased from Gibco (New
York, NY, USA). Methylthiazolyldiphenyl-tetrazolium bromide (MTT)
was purchased from Gen-View Scientific Inc. (Calimesa, CA,
USA).
Annexin V-FITC Apoptosis Detection kit, Cell Cycle
and Apoptosis Analysis kit, MMP assay kit with JC-1 and Reactive
Oxygen Species Assay kit were purchased from Beyotime Biotechnology
Co., Ltd. (Shanghai, China). A Hoechst 33258 kit was purchased from
Kaiji Biotechnology Co., Ltd. (Jiangsu, China). The antibodies
against IDH1, IDH2 and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were purchased from Proteintech Group, Inc. (Chicago, IL,
USA). PrimeScript™ RT reagent kit with gDNA Eraser (Perfect
Real-Time), SYBR® Premix Ex Taq™ II (Tli RNaseH Plus),
RNAiso Plus and diethyl pyrocarbonate (DEPC)-treated water were
purchased from Takara (Shiga, Japan). The primers of IDH1, IDH2 and
GAPDH were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China).
Cell culture
The human GC cell line MGC-803 and the human
embryonic lung fibroblast cell line MRC-5 were purchased from the
Institute of Biochemistry and Cell Biology, SIBS, of the Chinese
Academy of Sciences (CAS, Shanghai, China). Both cell lines were
cultured in DMEM supplemented with 10% FBS at 37°C in a humidified
atmosphere with 5% CO2.
Assessment of viability
Cell viability was detected by MTT assay. The
MGC-803 and MRC-5 cells were cultured in 96-well plates at a
density of 3×103 cells/100 µl/well. After incubation for
24 h, the cells were treated with: DMEM culture medium, 1% alcohol
(DMEM culture medium with 1% alcohol) and curcumol at
concentrations of 20, 40, 60, 80, 100, and 120 µM or AG-120 at
concentrations of 40, 60, 80 and 100 µM for different time-points,
including 24, 48 and 72 h. NAC was used to detect whether curcumol
inhibited cell proliferation by increasing ROS. The cells were
treated with the control (DMEM culture medium), 1% alcohol (DMEM
culture medium with 1% alcohol), curcumol (80 µM), NAC (5 mM),
curcumol (80 µM) + NAC (5 mM) for 24, 48 and 72 h. Then 10 µl of
0.5 mg/ml MTT were added to each well and the mixture was cultured
at 37°C for an additional 4 h. Subsequently the culture medium was
replaced with 100 µl DMSO to dissolve the formazan crystals. After
shaking for 10 min, the absorbance of each well was determined at
570 nm by a plate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Five replicate wells were designed for each sample. This
experiment was repeated three times and the cell viability was
calculated as follows: cell viability rate (%) = (experimental OD
value - zero set OD value)/(control OD value - zero set OD value) ×
100. The 50% inhibitory concentration (IC50) was
calculated by GraphPad Prism 6 (GraphPad Software Inc., La Jolla,
CA, USA).
Apoptosis assay by Hoechst 33258
The MGC-803 cells in logarithmic growth phase were
cultured in a 6-well plate and treated with curcumol (0, 20, 40,
60, 80 and 100 µM) for 24 h. Then the cells in each well were
stained using Hoechst 33258 and the changes in the nuclei of cells
were observed and photographed with a fluorescence microscope
(Leica Microsystems, Wetzlar, Germany).
Apoptosis assays by Annexin V-FITC/PI
double staining
MGC-803 cells were seeded in 6-well plates at a
density of 1×105 cells/well. After being cultured with
various concentrations (0, 20, 40, 60 and 80 µM) of curcumol for 24
h, the cells were collected and stained using an Annexin V-FITC
Apoptosis Detection kit (including PI) according to the
manufacturer's instructions. Then the cells were analyzed using a
flow cytometer (Beckhman Coulter Inc, Brea, CA, USA).
Cell cycle distribution assay
Similar to the apoptosis assays by Annexin V/PI
staining, the cells treated with curcumol for 24 h were collected.
Then the cells were stained with PI according to the manufacturer's
instructions. The cell cycle distribution was detected a the flow
cytometer and was analyzed using FlowJo software (FlowJo, LLC,
Ashland, OR, USA).
MMP assay
The MMP assay was performed to evaluate the level of
the polarization/depolarization of the mitochondrial membrane using
the JC-1 dye. MGC-803 cells were treated with curcumol (0, 20, 40,
60 and 80 µM) at a density of 1×105 cells/well in a
6-well plate. After 24 h, the cells were collected and stained
using JC-1 according to the manufacturer's instructions. Finally,
the cells were analyzed on a flow cytometer and the results were
analyzed using FlowJo software.
ROS assay
The ROS assay was performed to detect the
intracellular ROS production from the curcumol-treated MGC-803
cells. Briefly, the cells were seeded at a density of
1×105 cells/well in a 6-well plate and incubated for 24
h. The cells were then treated with curcumol (0, 20, 40, 60 and 80
µM, NAC, NAC + 80 µM curcumol) for another 24 h. Then the cells
were loaded with DFCH-DA and harvested for the ROS assay according
to the manufacturer's instructions. Finally, the assay results were
analyzed on a flow cytometer and the data were analyzed using
FlowJo software.
Western blot analysis
MGC-803 cells were cultured in a 6-well plate at a
density of 2×105 cells/well with different curcumol
concentrations, including 0, 20, 40, 60 and 80 µM. AG-120 (80 µM)
treatment was used as a positive control. After 48 h, the cells
were collected in lysis buffer and incubated on ice for 20 min. The
lysates were centrifuged at 4°C for 20 min and then the supernatant
was transferred to a new 1.5-ml test tube. To ensure the equal
loading of protein (40 µg) for each group, protein quantification
(Thermo Fisher Scientific, Waltham, MA, USA) was performed to
detect the total protein. Then the samples were separated by 10%
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and were transferred to polyvinylidene fluoride (PVDF)
membranes. Next the membranes were blocked with 5% skim milk for 1
h at room temperature. Subsequently, the membranes were washed in
TBST and incubated with antibodies of GAPDH (1:1,000 dilution),
IDH1 (1:5,000 dilution) and IDH2 (1:2,500 dilution) overnight at
4°C. On the second day, the membranes were washed in TBST and then
incubated at room temperature for 1 h with the appropriate
secondary antibody (goat anti-mouse IgG for GAPDH and goat
anti-rabbit IgG for IDH1 and IDH2). The bands were visualized using
an enhanced chemiluminescence (ECL) system (Thermo Fisher
Scientific) and Kodak XBT-1 film. Gray value analysis was performed
using ImageJ software (National Institute of Mental Health,
Bethesda, MD, USA). The variation in the gray value was expressed
as fold changes compared to the control in the blot.
RT-qPCR
Total RNA was isolated from the MGC-803 cells
treated with curcumol (0, 20, 40, 60 and 80 µM) using the RNAiso
Plus. The AG-120-treated cells (80 µM) were used as a positive
control.
After incubation at 45°C for 2 min, genomic DNA was
removed in the reaction with a total volume of 10 µl consisting of
2 µl gDNA eraser buffer, 1 µl gDNA eraser, an appropriate amount of
RNA (up to 1 µg) and nuclease-free water. cDNA was synthesized in
the reaction with a total volume of 20 µl consisting of 10 µl of
the reaction liquid of the last step, 4 µl PrimeScript buffer, 1 µl
reverse transcriptase, 1 µl RT Primer Mix and 4 µl nuclease-free
water. The reaction conditions included 37°C for 15 min, followed
by 85°C at 50 sec and ending at 4°C. Real-time RT-PCR was performed
using the Thermal Cycler Real-Time system (Thermo Fisher
Scientific). The reaction was performed at a final volume of 10 µl
containing 5 µl SYBR Premix Ex Taq II, 1 µl of appropriate primer
(10 µM), 1 µl cDNA (50 ng/µl) and nuclease-free water. The cycling
conditions included one cycle at 95°C for 30 sec, followed by 40
cycles of 5 sec at 95°C and 20 sec at 60°C. The sequences of the
primers were as follows: GAPDH forward, 5′-CAGGAGGCATTGCTGATGAT-3′
and reverse, 5′-CAGGAGGCATTGCTGATGAT-3′; IDH1 forward,
5′-ACTTGCACATGACTGGAACG-3′ and reverse, 5′-TCCTGCGGCCTAAACAGTAT-3′;
IDH2 forward, 5′-AGAGTGGAGCCATGACCAAG-3′ and reverse,
5′-TGTCCAGGTTGCTCTTGATG-3′.
Statistical analysis
All of the data are presented as the mean ± standard
deviation (SD). The rate of survival and the IC50 values
were analyzed by GraphPad Prism 6.0 (GraphPad Software Inc.). A
two-tailed independent sample t-test was used to assess the level
of significance between the means. A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
Anti-proliferative activities of
curcumol in MGC-803 cells
Human embryonic lung fibroblast MRC-5 cells treated
with curcumol as a control exhibited light toxicity. AG-120 which
is an inhibitor of IDH1, was used as a positive control in MGC-803
cells. Curcumol inhibited the viability of MGC-803 cells with an
IC50 value of 114.60±3.55 µM at 24 h, 66.36±1.26 µM at
48 h and 29.00±1.14 µM at 72 h. The viability of MGC-803 cells was
suppressed by curcumol in a time- and concentration-dependent
manner (Fig. 1A). Fig. 1B revealed that curcumol was slightly
effective on MRC-5 cells, thus signifying that curcumol has light
toxicity on non-tumor cells. AG-120 inhibited the viability of
MGC-803 cells with an IC50 value of 58.19±1.14 µM at 24
h, 75.40±1.04 µM at 48 h and 67.24±1.14 µM at 72 h. As time
progressed, the antitumor effect of curcumol improved compared to
the AG-120 treatment. This group demonstrated that the MGC-803
cells were also suppressed by AG-120, however the potency was lower
than that of curcumol at low concentrations (Fig. 1C). In addition, NAC was capable of
reversing the inhibitory effect of curcumol on cell proliferation
(Fig. 1D).
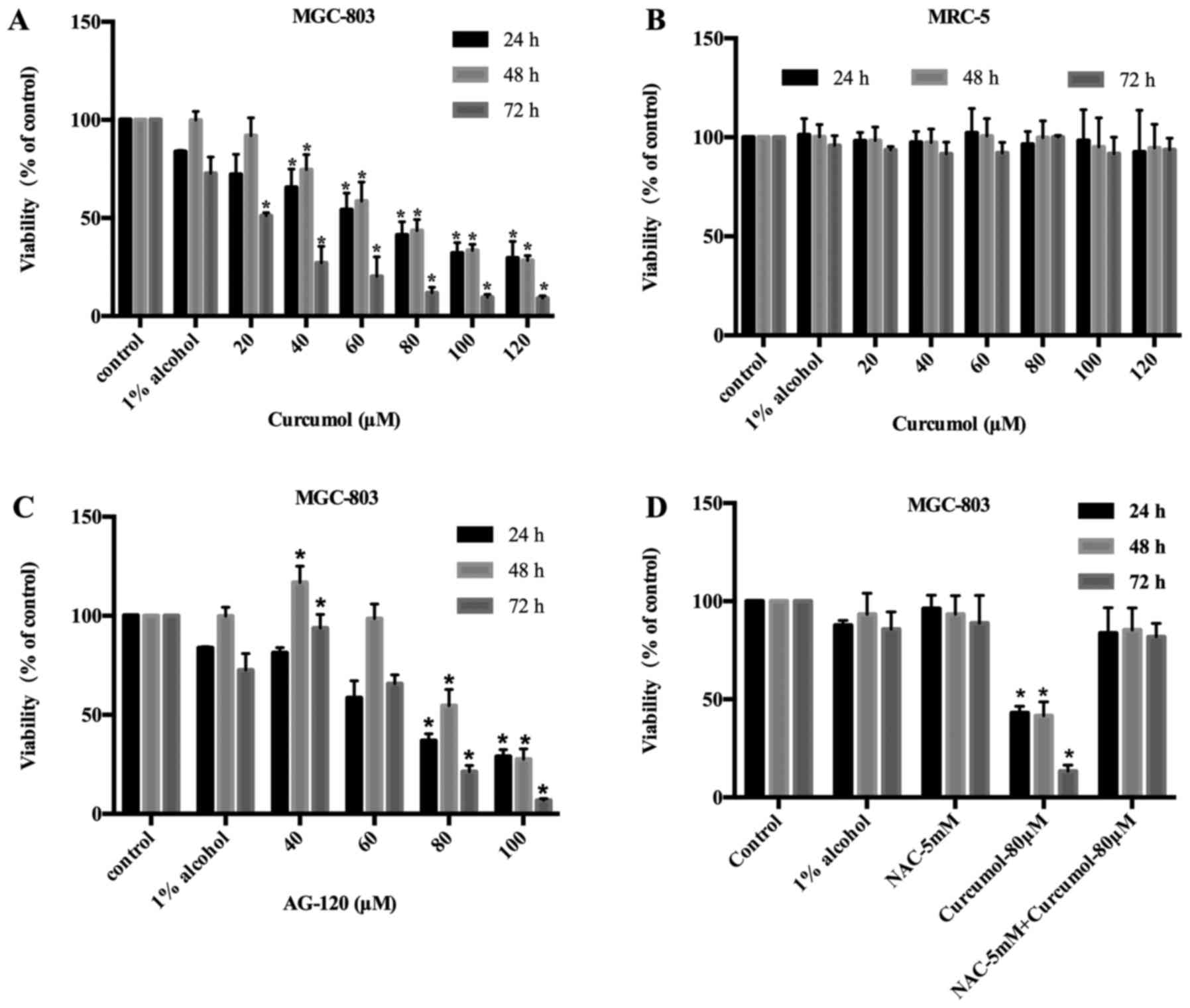 | Figure 1.Curcumol inhibits the viability of GC
cells MGC-803 and human embryonic lung fibroblast cells MRC-5. (A
and B) MGC-803 and MRC-5 cells were treated with the control (DMEM
culture), 1% alcohol (DMEM culture medium with 1% alcohol) and
curcumol (20, 40, 60, 80, 100 and 120 µM) for 24, 48 or 72 h. (C)
MGC-803 cells were treated with the control (DMEM culture), 1%
alcohol (DMEM culture medium with 1% alcohol) and AG-120 (40, 60,
80 and 100 µM) for 24, 48 or 72 h. (D) MGC-803 cells were treated
with the control (DMEM culture), 1% alcohol (DMEM culture medium
with 1% alcohol), curcumol (80 µM), NAC (5 mM), curcumol (80 µM) +
NAC (5 mM) for 24, 48 and 72 h. The MTT assay was performed to
determine cell viability and values are expressed as the mean ± SD
of three separate experiments. *p<0.05 vs. control. GC, gastric
cancer. |
Curcumol induces apoptosis of MGC-803
cells
To assess the level of apoptosis in the MGC-803
cells treated with curcumol, we performed Hoechst 33258
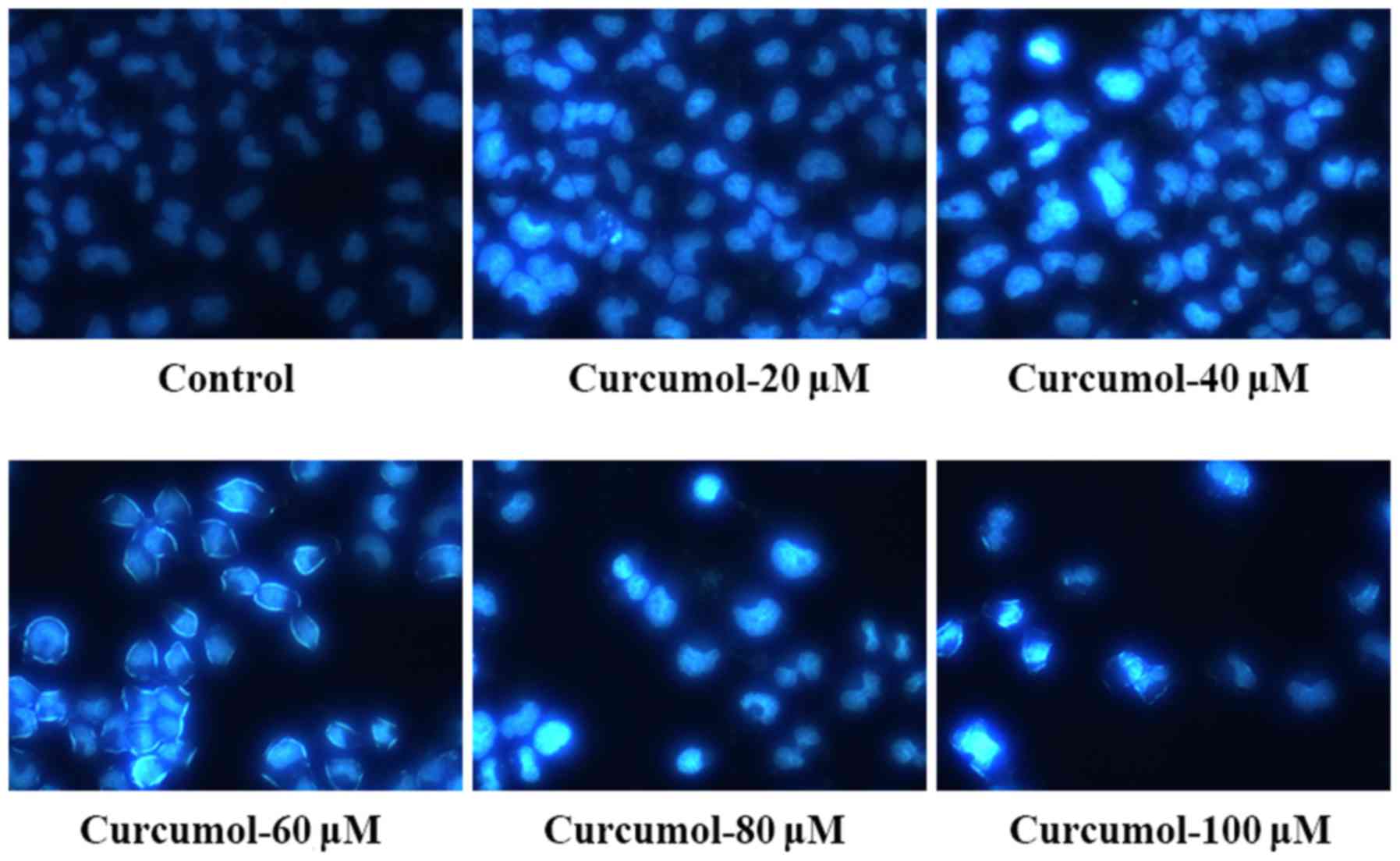
fluorescence staining following curcumol treatment. The apoptotic
morphological changes of MGC-803 cells treated with curcumol for 24
h were observed and compared to those of the control cells treated
with DMEM and 1% ethyl alcohol. Curcumol at 40, 60, 80 and 100 µM
induced significant morphological changes in the MGC-803 cells,
including a decrease in cell volume, intercellular junction
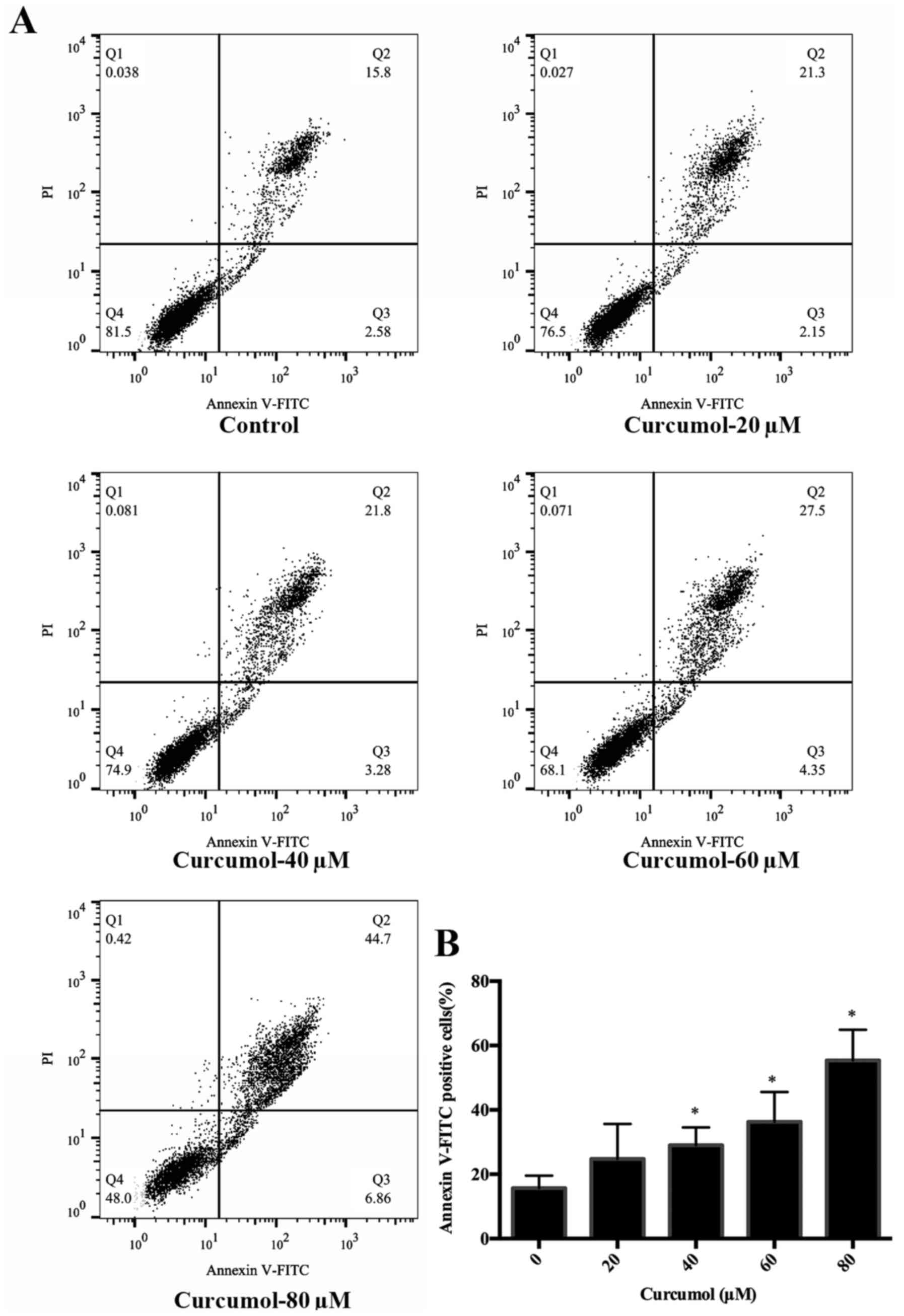
disappearance and formation of apoptotic bodies (Fig. 2). Annexin V-FITC/PI double staining
followed by FACS analysis confirmed the dose-dependent
apoptosis-inducing effect of curcumol. The results revealed a
dose-dependent increase in the apoptosis rate for cells treated
with 40, 60 and 80 µM of curcumol compared to the control cells
(Fig. 3).
Curcumol induces cell cycle arrest at
the G2/M phase of MGC-803 cells
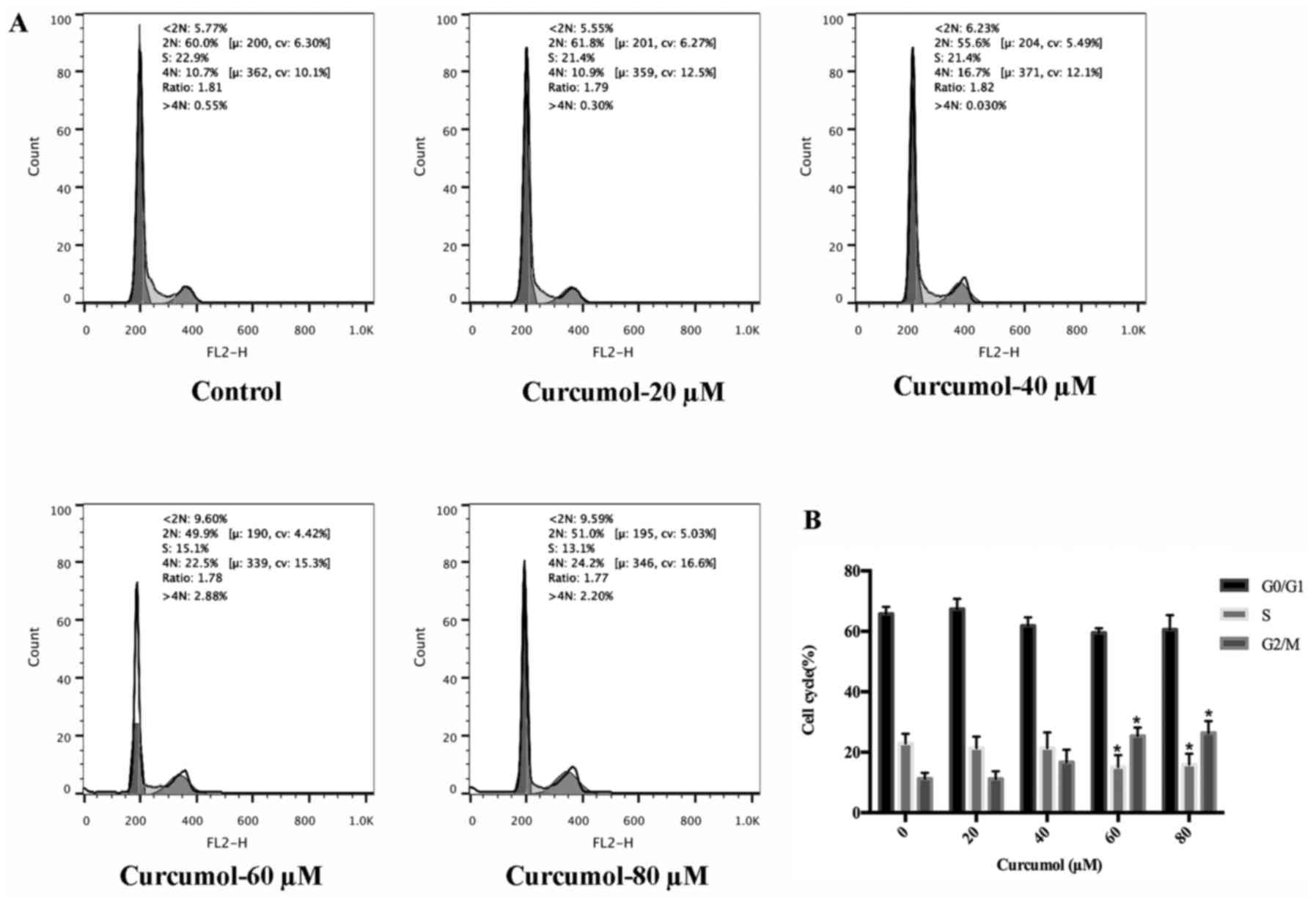
After curcumol was confirmed to inhibit MGC-803 cell
growth, we next examined whether the growth inhibitory effect of
curcumol was related to cell cycle arrest. PI staining followed by
FACS analysis demonstrated the effect of curcumol on cell cycle
progression in MGC-803 cells. MGC-803 cells treated with curcumol
at 60 and 80 µM resulted in a small accumulation of cells in the
G2/M phase and a decrease in the S-phase cell population. This
result revealed a G2/M phase cell cycle arrest of gastric cells
upon exposure to curcumol (Fig. 4).
However the cycle arrest of curcumol was not statistically
significant.
Curcumol decreases the MMP of MGC-803
cells
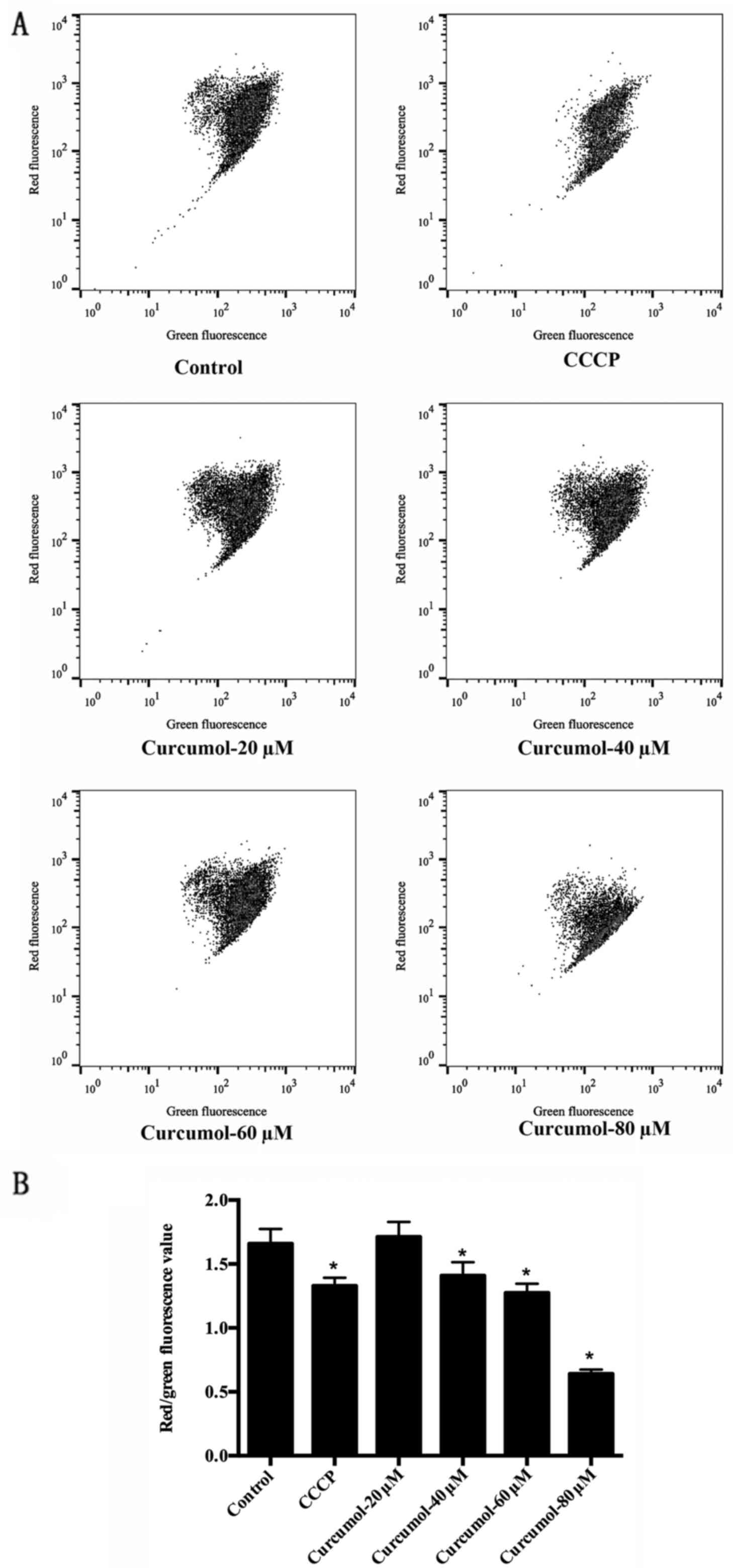
An MMP decrease is an early manifestation of
apoptosis. As an additional verification of the effects of
curcumol, we evaluated the MMP of MGC-803 cells after 24 h of
curcumol treatment. Most of the negative control cells were stained
red by JC-1, indicating an intact mitochondrial membrane. In
contrast, cells treated with curcumol exhibited increasing amounts
of green fluorescence. The effect was more obvious with 80-µM
treatment. Analysis of the red/green fluorescence light density
ratio revealed that the decrease was dose-dependent and significant
for the 40, 60 and 80 µM concentrations of curcumol. CCCP-treated
cells were used as a positive control (Fig. 5).
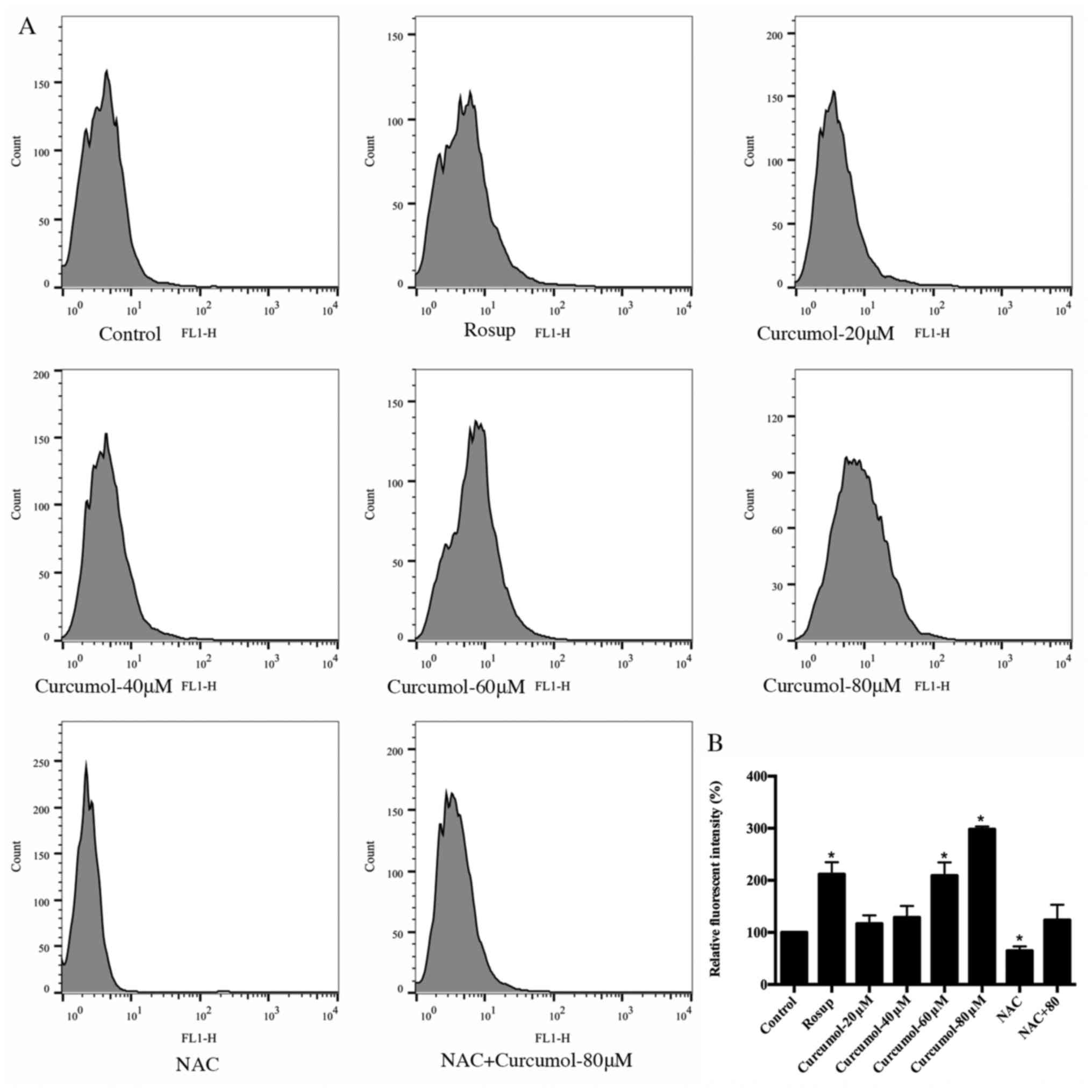
Curcumol increases the levels of ROS
in MGC-803 cells
It has been confirmed that excess ROS promotes cell
death and curcumol upregulates ROS to induce apoptosis in tumors
(16). Thus, we further examined
the effects of curcumol on ROS production in MGC-803 cells. The
level of ROS in the MGC-803 cells was assessed using the DCFH-DA
probe and flow cytometry in the FL1-H channel (Fig. 6A). The result revealed that the
level of ROS increased significantly after treatment with curcumol
for 24 h at concentrations of 60 or 80 µM (p<0.05). NAC was
capable of decreasing the increase in ROS caused by curcumol. Rosup
treated cells were used as a positive control (Fig. 6B).
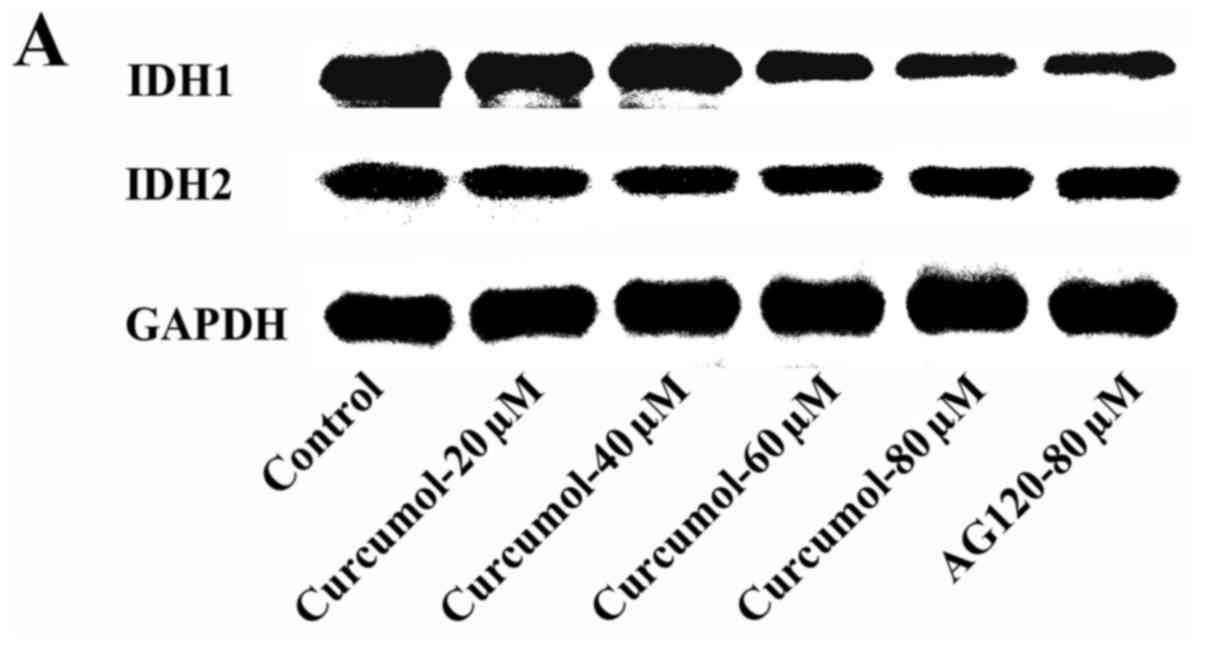
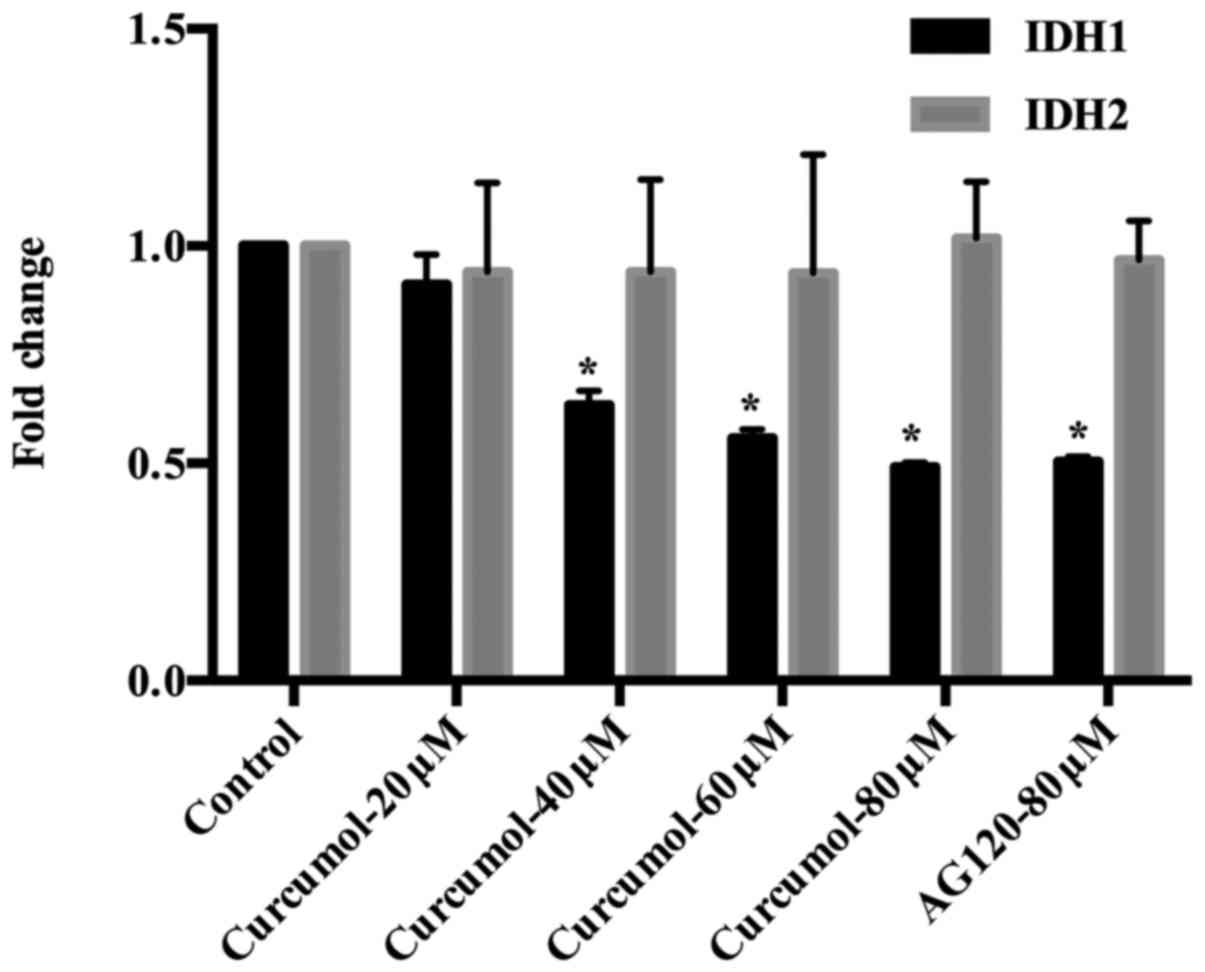
Curcumol downregulates IDH1
expression
Our previous research revealed that curcumol
increased the levels of ROS to promote cell death. However a study
demonstrated that IDH1-dependent reductive carboxylation mitigated
the intracellular ROS level and protected tumor cells from ROS
damage. In order to explore the molecular mechanism through which
curcumol inhibits cell proliferation, the expression levels of IDH1
and IDH2 were determined by western blotting. The expression levels
of IDH1 were downregulated after cells were treated with 60 or 80
µM of curcumol, but the expression levels of IDH2 were not altered
by curcumol. AG-120 (80 µM) treatment was used as a positive
control (Fig. 7). The result of
RT-qPCR confirmed the same tendency in the gene expression of IDH1
and IDH2. In contrast to the negative control group, the expression
level of IDH1 was significantly decreased after 48 h of treatment
with curcumol at 40, 60 and 80 µM (Fig.
8). The results may reveal a new molecular mechanism in gastric
cell apoptosis induced by curcumol.
Discussion
Previous studies have demonstrated that the extract
of Radix Curcumae and curcumol derivatives were effective against
various types of cancer (12,16–19).
However, research on curcumol was limited, especially considering
its anti-proliferation mechanism in GC. In the present study, we
found that curcumol suppressed the proliferation of GC MGC-803
cells in a dose-dependent manner, however slight toxicity was
observed in the non-tumor cells. Although, it would have been
preferable to use normal gastric cells as a control to reflect the
selectivity of curcumol to GC cells, due to laboratory conditions,
we could only use MRC-5 cells as a normal cell control. The human
embryonic lung fibroblast MRC-5 cell line is considered to be
representative of normal human cells. It is derived from normal
lung tissue of a 14-week-old male fetus. Both fibroblasts and
gastric smooth muscle cells were differentiated from the embryonic
mesoderm. MRC-5 cells are often used to test the toxicity of drugs
on normal cells (20). Not only
normal lung cells but also other normal cells that were
differentiated from the embryonic mesoderm could use MRC-5 cells as
a control to detect the toxicity of normal cells treated by
curcumol.
Furthermore, the induction of apoptosis in human GC
MGC-803 cells treated with curcumol was also assessed using H33258
staining, Annexin V/PI double staining and MMP assay. This
induction may be related to the downregulation of IDH1 by curcumol.
AG-120 is an orally available inhibitor of IDH1 with potential
antineoplastic activity. After observation that curcumol had an
inhibitory effect on IDH1, we compared the inhibitory effects of
AG-120 and curcumol on MGC-803 cells. The results revealed that the
antitumor effect of curcumol was superior to that of AG-120,
suggesting that curcumol may still have other anticancer
targets.
For the past few years, accumulating targeted
molecules that may be used for the treatment of GC have been
discovered, including HER2, vascular endothelial growth factor
(VEGF) (21),
phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3, one of
the glycolytic enzymes) (22), the
nuclear factor-κB (NF-κB) signaling pathway (23), Wnt signaling (24), cyclooxygenase-2 (COX-2) and
prostaglandin E2 (PGE2) (25).
However these targeted molecules are only suitable for certain GC
patients. Thus, it is essential to explore new targets.
Increasing evidence has revealed that inducing
cancer cell apoptosis is an effective way to treat tumors (5). Previously, ROS was regarded as a cause
in the induction and promotion of cancer (26,27).
However a study revealed that mitochondrial ROS induces apoptosis
through the intrinsic pathway (28). Moreover, there are numerous studies
demonstrating that drugs induce apoptosis along with the loss of
MMP and the increase of ROS generation (29–38).
Furthermore, in recent years photodynamic therapy (PDT) is
characterized by generation of ROS to treat cancer via the cell
apoptotic pathway (39,40). In the present study, we also
demonstrated that curcumol treatment decreased MMP levels and
increased ROS levels in the cells. NAC is a commonly used ROS
inhibitor and the ROS assay results revealed that it could
antagonize the increase of ROS induced by curcumol. An MTT assay
revealed that NAC could decrease the inhibitory effect of MGC-803
cells induced by curcumol, thus suggesting that curcumol may also
play a role in inhibiting the proliferation of MGC-803 cells by
increasing intracellular ROS.
The isocitrate dehydrogenase (IDH) family is
comprised of key functional metabolic enzymes in the Krebs cycle
that catalyze the conversion of isocitrate to α-ketoglutarate
(α-KG) (41). It is harmful to
malignant cells when intracellular ROS increases. However an
IDH1-dependent reductive carboxylation mitigated intracellular ROS
levels and protected cancer cells from ROS damage. Both IDH1 in the
cytosol and IDH2 in the mitochondria are necessary, as deletion of
either enhances ROS generation in mitochondria and inhibits cell
proliferation (42). A study
demonstrating that downregulation of IDH2 and TET family enzymes
was likely one of the mechanisms underlying 5-hmC loss in melanoma
(43). In addition, the level of
IDH2 in GC MGC-803 cells was significantly lower than that in
adjacent normal tissues (41).
Therefore, IDH1 and IDH2 expression in the GC
MGC-803 cells was evaluated using western blotting and RT-qPCR in
our study. The results revealed that the IDH1 expression level was
lower in curcumol-treated cells compared with the control cells
treated by DMEM with 1% ethyl alcohol in the GC MGC-803 cells.
However the IDH2 expression level was not altered. Curcumol
inhibited GC MGC-803 cell proliferation by downregulating IDH1,
which enhanced intracellular ROS.
AG-120 as an inhibitor of IDH1, was reported to have
a promising effect at the initial treatment of acute myelogenous
leukemia (AML) patients harboring IDH1 mutant in a phase I clinical
trial (44). Our results
demonstrated that AG-120 suppressed proliferation of GC MGC-803
cells, however less efficiently than curcumol.
The Curcuma extract exhibited anticancer
activity in GC as was reported by Shi et al (45) who drew the conclusion that zedoary
oil inhibited AGS and MGC-803 cell proliferation. In addition, they
also found that zedoary oil inhibited AGS cells through cell cycle
arrest and cell apoptosis promotion. Furthermore, they demonstrated
that low concentrations of zedoary oil were less inhibitory toward
normal gastric epithelial GES-1 cells. Their research was very
detailed and it helped us with our research on curcumol. A single
component and a clear structure is the future direction of new drug
research and development. Curcumol is a component of zedoary oil.
Based on the research on zedoary oil, we explored the
pharmacodynamics and mechanism of curcumol in MGC-803 cells, and
provided some new evidence for the development of a new drug.
To summarize, curcumol not only inhibited the
proliferation but also increased the intracellular ROS level in
MGC-803 cells. In addition this may be the way that curcumol
affected cell apoptosis in MGC-803 cells. Moreover, there is some
evidence demonstrating that the cell cycle arrest in the G2/M phase
and the decrease of MMP was induced by curcumol in MGC-803 cells.
IDH1 may be a promising target to treat GC in the future. In
conclusion, curcumol may be a potential drug used in the treatment
of GC with multiple targets. The study of the curcumol mechanism in
cancer treatment will offer new targets, which may be helpful in
the development of new antineoplastic drugs.
Acknowledgements
This study was supported by the Science and
Technology Planning Project of Guangdong Province, China (no.
2015A020211028). We would like to thank Dr Min Wei for the critical
reading and the editing of the manuscript.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hudler P: Challenges of deciphering
gastric cancer heterogeneity. World J Gastroenterol.
21:10510–10527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu W, Yang Z and Lu N: Molecular targeted
therapy for the treatment of gastric cancer. J Exp Clin Cancer Res.
35:12016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ko YC, Lien JC, Liu HC, Hsu SC, Ji BC,
Yang MD, Hsu WH and Chung JG: Demethoxycurcumin induces the
apoptosis of human lung cancer NCI-H460 cells through the
mitochondrial-dependent pathway. Oncol Rep. 33:2429–2437. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomez-Martín C, Lopez-Rios F, Aparicio J,
Barriuso J, García-Carbonero R, Pazo R, Rivera F, Salgado M, Salud
A, Vázquez-Sequeiros E, et al: A critical review of HER2-positive
gastric cancer evaluation and treatment: From trastuzumab, and
beyond. Cancer Lett. 351:30–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Batran SE, Ducreux M and Ohtsu A: mTOR
as a therapeutic target in patients with gastric cancer. Int J
Cancer. 130:491–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang K, Cui J, Xi H, Bian S, Ma L, Shen
W, Li J, Wang N, Wei B and Chen L: Serum HER2 is a potential
surrogate for tissue HER2 status in gastric cancer: A systematic
review and meta-analysis. PLoS One. 10:e01363222015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu JS, He SC, Zhang ZL, Chen R, Fan L,
Qiu GL, Chang S, Li L and Che XM: Anticancer effects of β-elemene
in gastric cancer cells and its potential underlying proteins: A
proteomic study. Oncol Rep. 32:2635–2647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai XZ, Huang WY, Qiao Y, Du SY, Chen Y,
Chen D, Yu S, Che RC, Liu N and Jiang Y: Inhibitory effects of
curcumin on gastric cancer cells: A proteomic study of molecular
targets. Phytomedicine. 20:495–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai LJ, Song SP, Lu B and Meng LN:
Reversal effect of curcuma wenyujin extract on SGC-7901/VCR induced
subcutaneous transplanted tumor in nude mice and its effect on the
expression of P-glycoprotein. Zhongguo Zhong Xi Yi Jie He Za Zhi.
34:1347–1353. 2014.(In Chinese). PubMed/NCBI
|
|
12
|
Guo P, Wang YW, Weng BX, Li XK, Yang SL
and Ye FQ: Synthesis, anti-tumor activity, and structure-activity
relationships of curcumol derivatives. J Asian Nat Prod Res.
16:53–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang QL, Guo JQ, Wang QY, Lin HS, Yang ZP,
Peng T, Pan XD, Liu B, Wang SJ and Zang LQ: Curcumol induces
apoptosis in SPC-A-1 human lung adenocarcinoma cells and displays
anti-neoplastic effects in tumor bearing mice. Asian Pac J Cancer
Prev. 16:2307–2312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao C, Ding Z, Liang B, Chen N and Cheng
D: Study on the effects of curcumin on angiogenesis. Zhong Yao Cai.
26:499–502. 2003.(In Chinese). PubMed/NCBI
|
|
15
|
Lu JJ, Dang YY, Huang M, Xu WS, Chen XP
and Wang YT: Anti-cancer properties of terpenoids isolated from
Rhizoma Curcumae - a review. J Ethnopharmacol. 143:406–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thani Abdullah NA, Sallis B, Nuttall R,
Schubert FR, Ahsan M, Davies D, Purewal S, Cooper A and Rooprai HK:
Induction of apoptosis and reduction of MMP gene expression in the
U373 cell line by polyphenolics in Aronia melanocarpa and by
curcumin. Oncol Rep. 28:1435–1442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan H, Liang Y, Jiang B, Li X, Xun H, Sun
J, He W, Lau HT and Ma X: Curcumin inhibits intracellular fatty
acid synthase and induces apoptosis in human breast cancer
MDA-MB-231 cells. Oncol Rep. 35:2651–2656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du Q, Hu B, An HM, Shen KP, Xu L, Deng S
and Wei MM: Synergistic anticancer effects of curcumin and
resveratrol in Hepa1-6 hepatocellular carcinoma cells. Oncol Rep.
29:1851–1858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao G, Han X, Zheng S, Li Z, Sha Y, Ni J,
Sun Z, Qiao S and Song Z: Curcumin induces autophagy, inhibits
proliferation and invasion by downregulating AKT/mTOR signaling
pathway in human melanoma cells. Oncol Rep. 35:1065–1074. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park EH, Bae WY, Eom SJ, Kim KT and Paik
HD: Improved antioxidative and cytotoxic activities of chamomile
(Matricaria chamomilla) florets fermented by
Lactobacillus plantarum KCCM 11613P. J Zhejiang Univ Sci B.
18:152–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narita Y and Muro K: Challenges in
molecular targeted therapy for gastric cancer: Considerations for
efficacy and safety. Expert Opin Drug Saf. 16:319–327. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han J, Meng Q, Xi Q, Wang H and Wu G:
PFKFB3 was overexpressed in gastric cancer patients and promoted
the proliferation and migration of gastric cancer cells. Cancer
Biomark. 18:249–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang Y, Hu W, Bai E, Zheng H, Liu Z, Wu J,
Jin R, Zhao C and Liang G: Curcumin sensitizes human gastric cancer
cells to 5-fluorouracil through inhibition of the NFκB
survival-signaling pathway. Onco Targets Ther. 9:7373–7384. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo X, Zhang L, Fan Y, Zhang D, Qin L,
Dong S and Li G: Oxysterol binding protein-related protein 8
inhibits gastric cancer growth through induction of ER stress,
inhibition of Wnt signaling and activation of apoptosis. Oncol Res.
25:799–808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Echizen K, Hirose O, Maeda Y and Oshima M:
Inflammation in gastric cancer: Interplay of the
COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways.
Cancer Sci. 107:391–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
DeNicola GM, Karreth FA, Humpton TJ,
Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES,
et al: Oncogene-induced Nrf2 transcription promotes ROS
detoxification and tumorigenesis. Nature. 475:106–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ishikawa K, Takenaga K, Akimoto M,
Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y and Hayashi
J: ROS-generating mitochondrial DNA mutations can regulate tumor
cell metastasis. Science. 320:661–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yee C, Yang W and Hekimi S: The intrinsic
apoptosis pathway mediates the pro-longevity response to
mitochondrial ROS in C. elegans. Cell. 157:897–909. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao X, Xu L, Zheng L, Yin L, Qi Y, Han X,
Xu Y and Peng J: Potent effects of dioscin against gastric cancer
in vitro and in vivo. Phytomedicine. 23:274–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang SQ, Wang C, Wang JW, Yang DX, Wang R,
Wang CJ, Li HJ, Shi HG, Ke Y and Liu HM: Geridonin, a novel
derivative of oridonin, inhibits proliferation of MGC 803 cells
both in vitro and in vivo through elevating the intracellular ROS.
J Pharm Pharmacol. 69:213–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi XJ, Yu B, Wang JW, Qi PP, Tang K,
Huang X and Liu HM: Structurally novel steroidal spirooxindole
by241 potently inhibits tumor growth mainly through ROS-mediated
mechanisms. Sci Rep. 6:316072016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Wei L, Zhang H, Dai Q, Li Z, Yu B,
Guo Q and Lu N: FV-429 induced apoptosis through ROS-mediated ERK2
nuclear translocation and p53 activation in gastric cancer cells. J
Cell Biochem. 116:1624–1637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang XC, Jin L, Wang M, Liang D, Chen ZF,
Zhang Y, Pan YM and Wang HS: Design, synthesis and in vitro
evaluation of novel dehydroabietic acid derivatives containing a
dipeptide moiety as potential anticancer agents. Eur J Med Chem.
89:370–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye MY, Yao GY, Pan YM, Liao ZX, Zhang Y
and Wang HS: Synthesis and antitumor activities of novel
α-aminophosphonate derivatives containing an alizarin moiety. Eur J
Med Chem. 83:116–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li JF, Huang RZ, Yao GY, Ye MY, Wang HS,
Pan YM and Xiao JT: Synthesis and biological evaluation of novel
aniline-derived asiatic acid derivatives as potential anticancer
agents. Eur J Med Chem. 86:175–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou Y, Tian L, Long L, Quan M, Liu F and
Cao J: Casticin potentiates TRAIL-induced apoptosis of gastric
cancer cells through endoplasmic reticulum stress. PLoS One.
8:e588552013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu C, Wang C, Han T, Zhou X, Guo S and
Zhang J: Insight into the cellular internalization and cytotoxicity
of graphene quantum dots. Adv Healthc Mater. 2:1613–1619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qian X, Li J, Ding J, Wang Z, Duan L and
Hu G: Glibenclamide exerts an antitumor activity through reactive
oxygen species-c-jun NH2-terminal kinase pathway in human gastric
cancer cell line MGC-803. Biochem Pharmacol. 76:1705–1715. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen CW, Chan YC, Hsiao M and Liu RS:
Plasmon-enhanced photodynamic cancer therapy by upconversion
nanoparticles conjugated with Au nanorods. ACS Appl Mater
Interfaces. 8:32108–32119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu L, Fu L, Jing T, Ruan Z and Yan L:
pH-triggered polypeptides nanoparticles for efficient BODIPY
imaging-guided near infrared photodynamic therapy. ACS Appl Mater
Interfaces. 8:8980–8990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chou NH, Tsai CY, Tu YT, Wang KC, Kang CH,
Chang PM, Li GC, Lam HC, Liu SI and Tsai KW: Isocitrate
dehydrogenase 2 dysfunction contributes to 5-hydroxymethylcytosine
depletion in gastric cancer cells. Anticancer Res. 36:3983–3990.
2016.PubMed/NCBI
|
|
42
|
Jiang L, Shestov AA, Swain P, Yang C,
Parker SJ, Wang QA, Terada LS, Adams ND, McCabe MT, Pietrak B, et
al: Reductive carboxylation supports redox homeostasis during
anchorage-independent growth. Nature. 532:255–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lian CG, Xu Y, Ceol C, Wu F, Larson A,
Dresser K, Xu W, Tan L, Hu Y, Zhan Q, et al: Loss of
5-hydroxymethylcytosine is an epigenetic hallmark of melanoma.
Cell. 150:1135–1146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
IDH1 inhibitor shows promising early
results. Cancer Discov. 5:42015.
|
|
45
|
Shi H, Tan B, Ji G, Lu L, Cao A, Shi S and
Xie J: Zedoary oil (Ezhu You) inhibits proliferation of AGS
cells. Chin Med. 8:132013. View Article : Google Scholar : PubMed/NCBI
|