Introduction
Non-small cell lung cancer (NSCLC), capturing almost
85% of lung cancers, is one of the leading causes of cancer-related
mortality all over the world (1).
Although chemotherapy is currently considered a valuable treatment
strategy for cancer therapy, the efficacy of it on patients with
advanced lung cancer is extremely limited because of drug
resistance and toxicity (2). This
frustrating fact renders lung cancer research and non-toxic
therapeutic drugs and new intervention targets as urgent to explore
and to provide more clinical benefits for lung cancer therapy.
Programmed cell death (PCD), known to be a crucial
process that has an influential role in development,
differentiation, cellular homeostasis, elimination of undesired and
malignant cells, including apoptosis, autophagic death and
necrosis, is an important target in cancer chemotherapy. Apoptosis,
namely type I PCD, featured by cell shrinkage, chromatin
condensation and fragmentation followed by the formation of
apoptotic bodies containing intact cytoplasmic organelles or
fragments of the nucleus, is a major cytotoxic mechanism of
anticancer agents (3). Autophagy, a
catabolic process for the degradation and recycling of
macromolecules and organelles which can be activated during stress
conditions is considered as a survival mechanism induced in adverse
conditions to maintain cell integrity, or conversely, as an
alternative cell death pathway (namely type II PCD) (4). Beclin1 is necessary in the formation
of autophagic vesicles (AVs), and its level can reflect whether
autophagy occurs. During the autophagy process, the cytoplasmic
form of microtubule-associated protein light chain 3 (LC3-I, 16
kDa) is processed to its membrane associated form LC3-II (14 kDa)
and recruited to the autophagosomes, simultaneously p62 will
continue to be consumed. Thus, the Beclin1, LC3-II/LC3-I, and p62
are regarded as a hallmark used to evaluate the level of autophagy.
Recent studies have pointed towards a complex interplay between
apoptosis and autophagy involved in the process of cell death
because they can occur simultaneously, sequentially, or exclusively
depend on cellular exposure environment and the levels of stress
involved (4–6). Accumulated evidence suggests that the
two different modes of cell death may be triggered by the common
upstream signals, which affect the development and therapy of
cancer, such as p53, Bcl-2 and PI3K/Akt/mTOR pathway (5–8).
The PI3K/Akt/mTOR pathway plays an important role in
cell proliferation, cell metabolism, angiogenesis, cell cycle
progression, apoptosis and autophagy, representing one of the major
survival pathways that is dysregulated in various types of human
cancer, and contributing to cancer pathogenesis and therapy
resistance. In most malignancies this pathway is constitutively
active leading to inhibition of PCD and promotion of cell survival
(9). So, inhibition of
PI3K/Akt/mTOR signaling pathway may be of immense potential in
causing cell death associated with apoptosis and/or autophagy.
However, the detailed mechanisms of different anticancer drug
treatments, especially the natural drugs, all of which may involve
different PCDs to a certain extent, are still rarely
understood.
Natural substances are the most reliable resources
for the therapy of cancer. Curcumin, a kind of liposoluble
polyphenol pigment extracted from rhizome of curcuma, having a
broad range of pharmacological effects such as anti-inflammatory,
anticoagulant, hypolipidemic, antioxidant, free radical scavenging,
and anti-atherosclerosis. Clinical trials have shown curcumin as a
dietary constituent with demonstrated anti-carcinogenic capability,
which is safe and well tolerated in humans (10,11).
Literature points to the fact that curcumin is capable of hindering
the growth of multiple cancer lines in vitro and in
vivo through effect on many different signaling pathways
(12–14) indicating its potential clinic
application in cancer control. A recent report highlighted that
curcumin-induced cytotoxicity is attributable to apoptosis but not
autophagy in human lung adenocarcinoma cells (15). In another study, it was shown that
turmeric toxicity associated with autophagy degradation of
anti-apoptosis in A431 epidermoid cancer cells (16). Based on our early studies, the
results have been suggested that the promotion of lung cancer cell
autophagy activity induced by curcumin even inducing autophagic
death is a potential tumor treatment (17). However, the effect of apoptosis is a
legacy question. No consensus has been reached yet for the
interpretation of the effects of curcumin on the underlying
mechanisms and the role of curcumin in inducing various PCDs in
human lung cancer cells remains to be defined. Therefore, studies
on the two modes of PCD related to curcumin as well as exploration
of regulating function of PI3K/Akt/mTOR, which is an essential
factor in the determination of the overall fate of tumor cells,
will help us to further understand their roles in tumorigenesis and
provide new ideas for the treatment of lung cancer.
In the present study, the antitumor activity of
curcumin especially its underlying molecular mechanism of action
with apoptosis and autophagy were investigated in NSCLC A549
cells.
Materials and methods
Materials
The NSCLC A549 cells were obtained from the First
Affiliated Hospital of Xi'an Jiaotong University as a gift. The
curcumin, MTT, rapamycin, and LY294002 were purchased from Sigma
(San Francisco, CA, USA). Roswell Park Memorial Institute culture
medium (RPMI-1640) and penicillin-streptomycin were purchased from
Hyclone Co. (Logan, USA). Fetal bovine serum (FBS; Biological
Industries, Kibbutz Beit-Haemek, Israel). Dimethyl sulphoxide
(DMSO) was purchased from Amresco (Houston, TX, USA). The Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit was purchased from 7Sea Pharmatech Co., Ltd.
(Shanghai, China). TRIzol reagent was purchased from Life
Technologies (Carlsbad, CA, USA). The Revert Aid First Strand cDNA
Synthesis kit was purchased from Thermo Fisher Scientific (Waltham,
MA, USA). FastStart Universal SYBR Green Master (Rox) was purchased
from Roche (Basel, Switzerland). The Pierce bicinchoninic acid
(BCA) protein assay kit was purchased from Merck (Darmstadt,
Germany). The skim milk was purchased from Wandashan Dairy Co.,
Ltd. (Heilongjiang, China). Goat anti-rabbit IgG-HRP, Akt (60 kDa),
p-Akt (60 kDa), mTOR (289 kDa), p-mTOR (289 kDa), GAPDH (38 kDa)
antibodies were purchased from Abcam (Cambridge, MA, USA). The
antibodies against Beclin1 (60 kDa), LC3 (LC3-II, 14 kDa and LC3-I,
16 kDa) and p62 (62 kDa) were purchased from Cell Signaling
Technology Inc. (Beverly, MA, USA).
Curcumin, rapamycin, and LY294002 were dissolved
into micro-DMSO stock solution and then RPMI-1640 culture medium
was added to the desired concentration, waiting to be used. DMSO is
a control for the entire study at a final concentration of
<0.1%.
Methods
Cell culture
A549 cells were cultured in RPMI-1640 medium
supplemented with 10% (v/v) FBS, 1% penicillin-streptomycin and
specifically maintained in a 5% CO2, 95% air humidified
incubator at 37°C. Taking the logarithmic growth phase of the A549
cells for following tests and the cells were seeded and adhered on
petri dishes for 24 h before starting the treatment. A549 cells
were exposed to curcumin at the indicated concentrations and time
periods in each experiment. The rapamycin and LY294002 were widely
used as blockers of mTOR and PI3K/Akt, respectively. When
co-cultured with curcumin the cells were pre-treated with rapamycin
of 40 µM or LY294002 of 20 µM for 3 h based on a large number of
references (6,18) and our pre-experimental results.
Cell viability assay
The cytotoxic activity of different stimulus in A549
cells was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. A549 cells were seeded in 96-well plates at a density of
5×104 cells/well for 24 h before challenged by different
concentrations of curcumin for 24, 48, 72 and 96 h. Or the cells
were pretreated with 40 µM rapamycin or 20 µM LY294002 for 3 h and
subsequently with or without 40 µM curcumin for 48 h. Each 96-well
plate was set up with control (cells-only) and zero-adjustment
(medium only). Additionally, then washed once and incubated with 20
µl MTT (5 mg/ml) at 37°C for 4 h. Carefully aspirating the liquid,
the purple formazan crystals were dissolved in 150 µl DMSO. Shaking
5 min, the absorbance of each well was read with the microplate
reader (Infinire M200; Tecan Group Ltd., Mannedorf, Switzerland) at
570 nm. Assays were performed in triplicate on three independent
experiments. Cell viability rate (%) = (experimental group OD-zero
adjustment group OD)/(control group OD-zero adjustment group OD)
×100%.
Apoptosis assay
The Annexin V-FITC/PI detection kit was used for the
determination of cell apoptosis. A549 cells (4×105
cells/well) were seeded in 6-well tissue culture plates followed by
exposure with curcumin (0–40 µM) as well as co-incubation with
curcumin (40 µM) and rapamycin (40 µM) or curcumin (40 µM) and
LY294002 (20 µM) for 48 h at 37°C and then were collected, washed
once with cold phosphate-buffered saline (PBS), then re-suspended
in 400 µl binding buffer at a concentration of 1×106
cells/ml. After 5 µl of Annexin V-FITC was added, the cells were
incubated for 15 min at room temperature in the dark. Then 10 µl of
PI (propidium iodide) was added and the cells were incubated for 5
min at 4°C in the dark. The rate of apoptosis was immediately
analyzed by a flow cytometer (Guava® easy Cyte HT; Merck
Millipore, Billerica, MA, USA). Annexin V-FITC-positive cells were
considered to be undergoing apoptosis and those negative for FITC
were considered to be alive.
Monodansylcadaverine (MDC)
labeling
Formating and promoting the AVs is one of the
characters of autophagy (19). To
detect autophagy, MDC labeling was performed, which could infer the
activation of autophagy from the changes in fluorescent particles.
Collecting the logarithmic growth phase A549 cells seeded at
3×104 cells per well in 24-well culture plates and
treated with 0 µM curcumin (control), 40 µM curcumin,
rapamycin+curcumin (40 µM), LY294002+curcumin (40 µM) respectively.
MDC (50 µM) was added to living cells 48 h after different
treatments. The cells were then incubated for 15 min at 37°C and 5%
CO2 in the dark, washed twice with PBS, and the
anti-quencher was added. Visualizing and imaging quickly with UV
excitation by an inverted fluorescence microscope (Nikon Eclipse
Ti; Nikon, Tokyo, Japan). In order to quantify MDC staining, the
relative MDC fluorescence intensity of the different treatment
groups were measured by the Image-Pro Plus. The experiment was
repeated three times.
Quantitative real-time PCR
The A549 cells (2×106 cells/well) were
seeded in 6-well tissue culture plates followed by exposure to
curcumin (0–40 µM) or co-incubation with curcumin (40 µM) and
rapamycin (40 µM) or curcumin (40 µM) and LY294002 (20 µM) for 48
h. Then total RNA was isolated with Trizol reagent from A549 cells
and 2 µg of the total RNA was reverse-transcribed into cDNA with
the RevertAid First Strand cDNA Synthesis kit according to the
manufacturer's instructions. Quantitative real-time PCR (qPCR) was
performed with a FastStart Universal SYBR Green Master (Rox) kit.
Each sample was run in triplicate in final volume of 25 µl
containing 2.5 µl first-strand cDNA, 1 µl (7.5 µM) of each primer
(purchased from Oke Dingsheng Biological Technology Co., Ltd.,
Beijing, China), 12.5 µl of 2X Fast Start Universal SYBR Green
Master (ROX) and 8 µl distilled water. Cycling parameters of the
qPCR were as follows: 1 cycle at 95°C for 10 min, followed by 40
cycles at 95°C for 15 sec, 60°C for 60 sec in the Step One Plus
fluorescence quantitative PCR instrument (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). After the
reaction, the results were obtained by ΔΔCT method. Primer
sequences were: Akt forward, 5′-CAAGTCCTTGCTTTCAGGGC-3′ and
reverse, 5′-ATACCTGGTGTCAGTCTCCGA-3′ (184-bp product); mTOR
forward, 5′-AACCTCCTCCCCTCCAATGA-3′ and reverse,
5′-CTCACGGAGAACCAGGACAG-3′ (186-bp product); GAPDH forward,
5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′ (496-bp product). Amplification of
housekeeping gene GAPDH was taken as an endogenous control.
Western blot analysis
A549 cells were seeded in culture dish at a density
of 2×106 cells/well and then incubated with or without
curcumin (0–40 µM, 48 h) in the presence or absence of various
inhibitors (40 µM rapamycin or 20 µM LY294002). The cells were
lysed in the radio-immunoprecipitation assay buffer (RIPA).
Proteins were quantified using a BCA protein assay kit. Equal
amount (20 µg) of protein samples were subjected to 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
further transferred to polyvinylidene fluoride (PVDF) membranes
which were soaked with methanol for 30 sec. After blocking with 5%
bovine serum albumin for 1 h at room temperature and probed with
the indicated antibodies. The membrane was washed thrice with TBST
and incubated with goat anti-rabbit IgG conjugated to HRP. The
membranes were visualized using the electrochemiluminescence and
quantitated using the Image-Pro Plus6.0 software. Equal loading was
assessed by GAPDH as internal control for western blotting.
Statistical analysis
SPSS18.0 statistical software was used for data
analysis. The data are presented as mean ± SEM. The one-way ANOVA
were used to analyze data. Comparisons between groups were done
using the Dunnett's t-test (*P<0.05, **P<0.01 or
#P<0.05, ##P<0.01).
Results
Curcumin exhibits antiproliferative
activity against human lung cancer A549 cells
In the first place, the toxicity of curcumin
treatment at concentration ranging from 0–40 µM for different time
period (24, 48, 72 and 96 h) was assayed by using an MTT test on
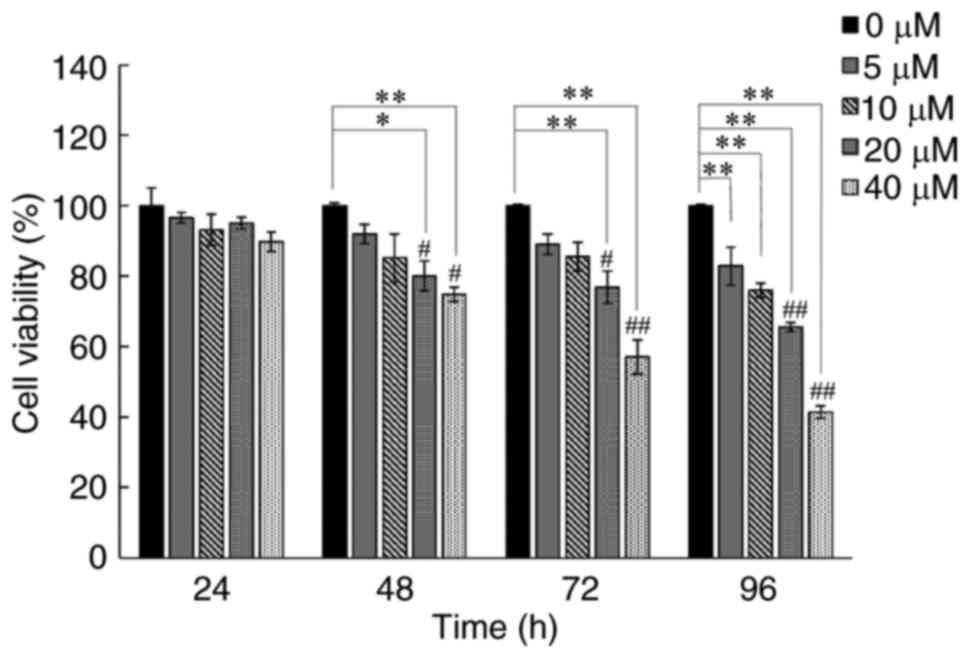
human lung cancer A549 cells. As is shown in Fig. 1 the growth inhibition of curcumin on
the cells was manifested in a dose- and time-dependent manner. Of
note, A549 cell viability could be obviously reduced with an
incubation period for 48 h at 20 and 40 µM of curcumin consistent
with our previous findings (17)
selected as an effective condition for the subsequent studies to
elucidate the underling molecular mechanisms of curcumin.
Apoptotic cell death is induced after
treatment with curcumin in A549 cells
Next, Annexin V-FITC and PI staining were used to
examine whether this inhibitory effect of curcumin on cell ability
was related to the induction of apoptosis. Apoptotic or necrotic
cells were detected after treated with curcumin of increasing
concentrations for 48 h. The apoptosis phenomenon was observed in
cells exposed to different doses of curcumin, whereas necrotic
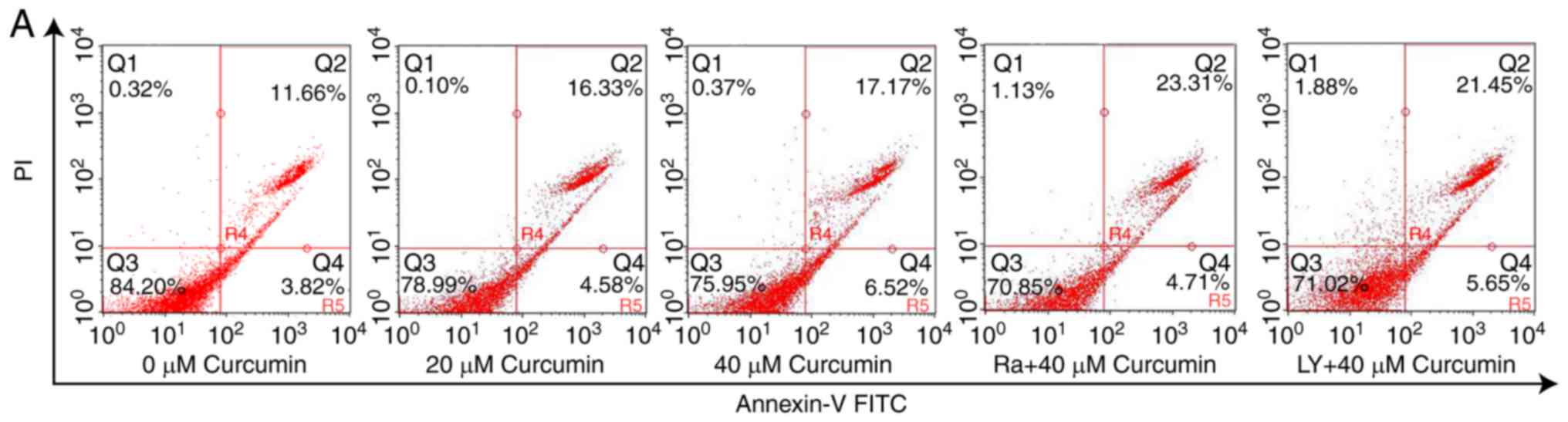
population was almost negligible. As shown in Fig. 2, the apoptotic (Annexin V-positive)
cell populations (early and late apoptosis) were increased to 20.91
and 23.68% after treatment of A549 cells with 20 and 40 µM
curcumin, respectively, with a 1.35- and 1.53-fold increase,
respectively, compared with control cells. These data suggested
that curcumin resulted in a dose-dependent increase in the
apoptotic cell death in human lung cancer A549 cells when used for
48 h. Taken together, these results revealed its central role in
regulating apoptosis mediated by curcumin.
Curcumin induces autophagy in A549
cells
Autophagy, one of the most studied fields in cancer
biology, has been verified by using a variety of methods. MDC is a
luminescent dye that can be absorbed by cells and displayed on AVs.
The activation of autophagy was inferred from changes in
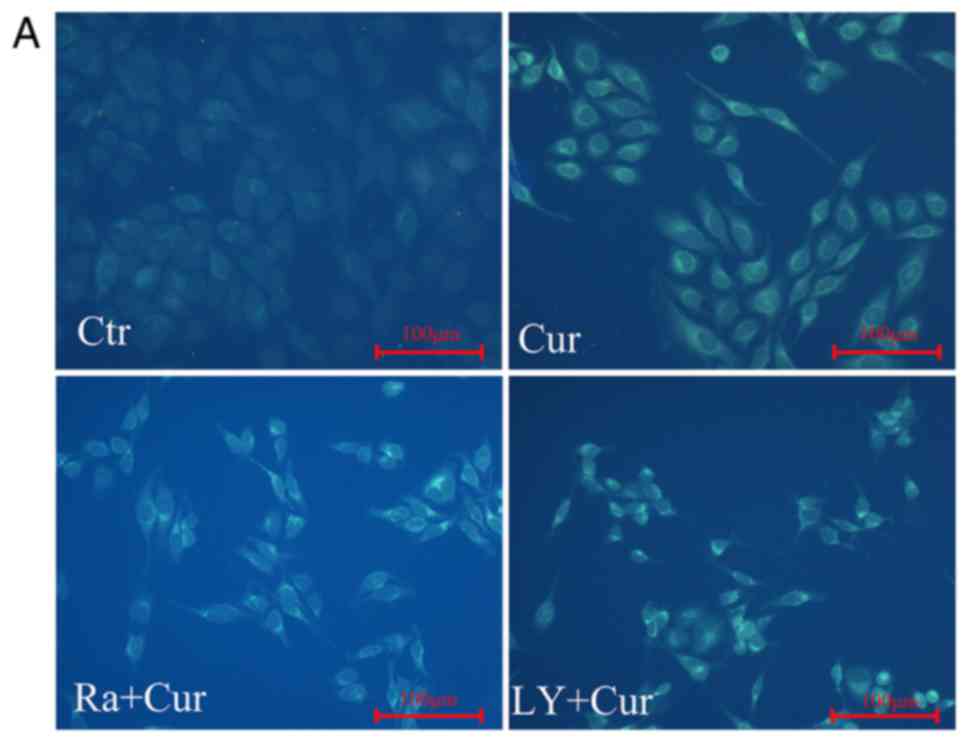
fluorescent particles infected with MDC first. After curcumin
treatment MDC staining showed that the relative fluorescence
intensity and density of cells was significantly increased compared
with control cells, indicating the occurrence of autophagy
(Fig. 3A and B). This was the
result expected and consistent with our previous study that
curcumin enhanced autophagy in a dose-dependent manner in A549
cells (17).
Taking into account the non-specificity of MDC
staining, the protein levels of autophagy markers Beclin1 (60 kDa),
LC3-II (14 kDa), LC3-I (16 kDa), and p62 (62 kDa) were detected by
western blotting. As shown in Fig.
3C, curcumin administration dramatically increased Beclin1 and
LC3-II expression, decreasing p62 protein level. As expected, the
ratio of LC3-II/LC3-I was enhanced (Fig. 3C and D) in a dose-dependent manner.
All the results above provided real evidence for curcumin-induced
autophagy of A549 cells.
Curcumin blocks the PI3K/Akt/mTOR
signal transduction pathway
Thus far our results revealed that curcumin could
substantially induce both apoptosis and autophagy. Given the
critical role of PI3K/Akt/mTOR pathway in controlling cell
survival/death in cancer cells, including lung cancer cells
(20–22), we investigated whether curcumin
induced apoptosis and autophagy via the inhibition of PI3K/Akt/mTOR
signaling pathway.
To determine the role of this way, two critical
molecules, Akt and mTOR, were examined. The expression levels of
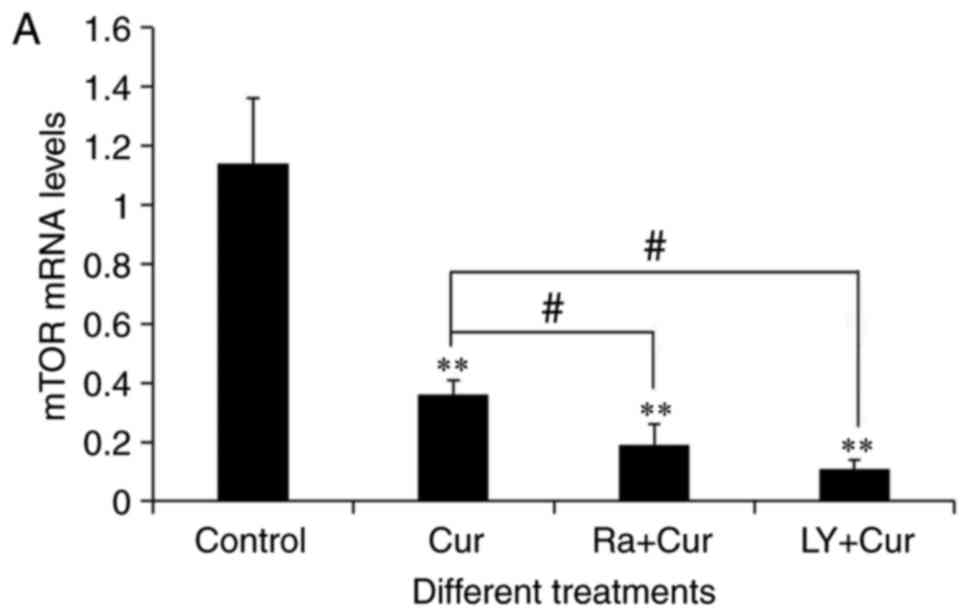
Akt and mTOR mRNA was detected in A549 cells by qRT-PCR (Fig. 4A and B). As expected, compared with
the control group (1.14±0.22), 40 µM of curcumin treated A549 cells
48 h, greatly reduced the transcription level of mTOR (0.36±0.05)
(P<0.01), as indicated in Fig.
4A. As shown in Fig. 4B, the
expression of Akt mRNA in curcumin group (0.44±0.09) was
significantly lower than that in control (1.04±0.09) (P<0.01).
In order to further ascertain and identify the role of mTOR and Akt
in the curcumin-modulation PI3K/Akt/mTOR signaling in A549 cells,
the effect of rapamycin and LY294002 were detected. The rapamycin
and LY294002 were widely used as inhibitors of mTOR and PI3K/Akt,
respectively (23,24). Similar to the effect of curcumin,
there was a more significant inhibitory effect on mTOR and Akt mRNA
levels when it combined with rapamycin or LY294002 compared with
curcumin treatment alone (Fig. 4A and
B).
Eukaryotic gene expression and regulation is
complex, mRNA levels can not fully represent the expression of
protein. Thus, the protein expression of important signaling
molecules such as p-Akt, Akt, p-mTOR, and mTOR was determined by
western blotting. As shown in Fig.
4C, dose-dependent inhition of Akt phosphorylation and mTOR
phosphorylation were observed when A549 cells were treated with
curcumin for 48 h. To be more intuitive, the inhibition of
PI3K/Akt/mTOR pathway was judged by the ratio of p-Akt to total Akt
as well as p-mTOR to total mTOR, which was consistent with the
above results (Fig. 4D). These data
together imply that the cytotoxicity of curcumin may be related to
the PI3K/Akt/mTOR pathway inhibition.
Pre-treatment with rapamycin or
LY294002 enhances curcumin-induced apoptosis and autophagy with
PI3K/Akt/mTOR pathway suppression, reducing cell viability in A549
cells
Above observations prompted to infer that
downregulation of PI3K/Akt/mTOR might have a crucial role in
curcumin-induced cytotoxicity due to apoptosis and autophagy.
Therefore, the cell viability was examined by MTT. To determine
that the curcumin-induced apoptosis and autophagy occurred via the
PI3K/Akt/mTOR signaling pathway, we employed mTOR blocker rapamycin
and PI3K/Akt inhibitor LY294002. The MTT assay showed that
inactivation of PI3K/Akt or inactivation of mTOR tremendously
sensitized A549 cells toward cytotoxicity of curcumin (Fig. 5A). The level of curcumin-induced
cell death was enhanced when the PI3K/Akt/mTOR signal transduction
pathway was blocked, which may be related to the induction of
PCDs.
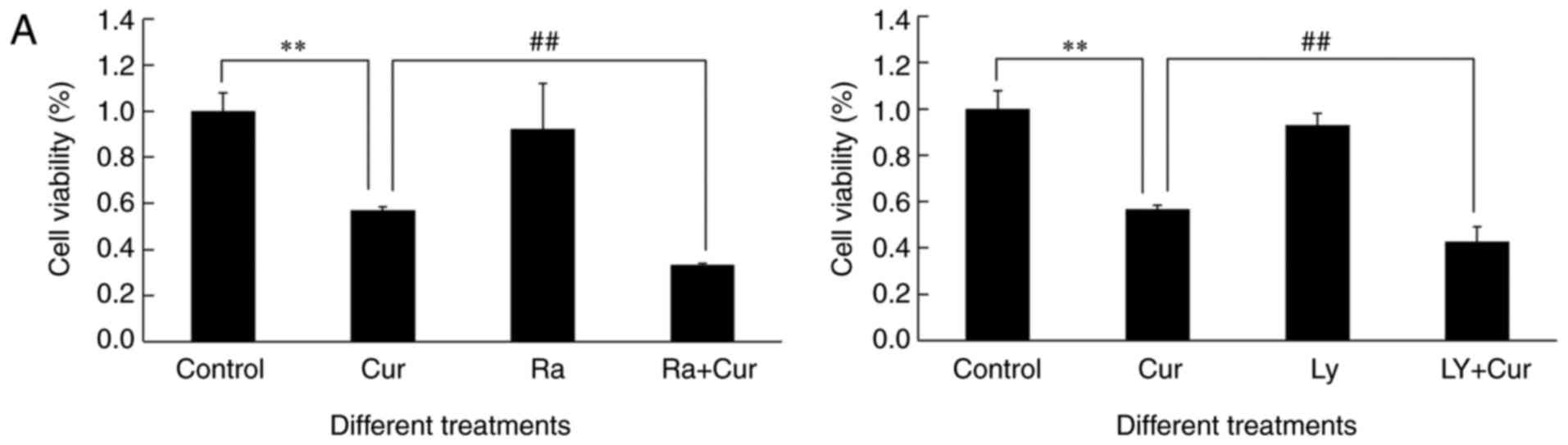 | Figure 5.Pre-treatment with rapamycin or
LY294002 enhances curcumin-induced apoptosis and autophagy with the
PI3K/Akt/mTOR pathway being suppressed, reducing cell viability in
A549 cells. A549 cells were pre-treated with rapamycin (Ra) of 40
µM or LY294002 (LY) of 20 µM for 3 h, followed by treatment with or
without curcumin (Cur) for an additional 48 h. Control is
concentration 0 µM of curcumin. (A) The cell viability was measured
by MTT assay. The level of curcumin-induced cell death was enhanced
when pre-treated with rapamycin or LY294002, compared with curcumin
alone group. (B) Bar graph indicates the percentage of apoptotic
cell populations (early and late apoptosis) determined by Annexin
V-FITC/PI double staining and flow cytometry suggesting that
curcumin resulted in a dose-dependent increase in apoptotic cell
death in A549 cells. (C and D) Proteins of total and
phosphorylation of mTOR as well as LC3-II and LC3-I were detected
by western blot assay. GAPDH was used as loading control. Bar
graphs indicate the ratio of p-mTOR/mTOR and LC3-II/LC3-I was
decreased and enhanced, respectively in curcumin, rapamycin, and
ra+Cur groups as compared with control group. (E and F) Proteins of
total and phosphorylation of Akt as well as LC3-II and LC3-I were
detected by western blot assay. GAPDH was used as loading control.
Bar graphs indicate the ratio of p-Akt/Akt and LC3-II/LC3-I was
decreased and enhanced, respectively, in curcumin, LY294002, and
LY+Cur groups as compared with control group. Data are presented as
mean ± SEM, as three separate experiments performed in triplicate.
* or #, P-values <0.05 and ** or ##,
P-values <0.01. |
As shown in Figs. 2A
and 5B, the Annexin V-FITC/PI
double stained assays revealed that the cell pre-treatment with
rapamycin of 40 µM or LY294002 of 20 µM resulted in a significantly
greater number of apoptotic (Annexin V-positive) cells than
treatment with curcumin alone. MDC labeling analyses were done to
detect the acidic vacuoles. Similarly, fluorescence microscopy
shows significant increase in the number of MDC-labeled vacuoles
(Fig. 3A and B) in cells exposed to
rapamycin or LY294002 co-cultured with curcumin compared with
curcumin treatment alone. Compared to curcumin alone, the ratio of
LC3-II/LC3-I was significantly enhanced (Fig. 5C and D) in a dose-dependent manner
when cells were pretreated with rapamycin, resulting in consistent
results when curcumin was co-cultured with LY294002 (Fig. 5E and F), suggesting autophagy was
clearly induced. The above results illustrated that combined
treatment of PI3K/Akt or mTOR blockers significantly enhanced
apoptosis and autophagy, accompanied by the inhibition of
PI3K/Akt/mTOR pathway can be derived from the ratio of p-mTOR to
total mTOR (Fig. 5C and D) as well
as p-Akt to total Akt (Fig. 5E and
F) downgrade. These data might support the idea that
cytotoxicity derived from apoptosis and autophagy in A549 cells by
curcumin proceeds through PI3K/Akt/mTOR inhibition. Thus, our
findings unequivocally substantiated the fact that PI3K/Akt/mTOR is
involved in the regulation of both apoptosis and autophagy induced
by curcumin. Inhibition of mTOR promoted the development of both
autophagy and apoptosis.
The present study collectively showed that curcumin
has a similar function as the PI3K/Akt/mTOR pathway inhibitor
resulting in varying degrees of reduction of the Akt
phosphorylation and mTOR phosphorylation as well as mRNA
expression. From the present results, our data indeed identified
that curcumin exerts cytotoxic effect on A549 cells by inhibiting
the PI3K/Akt/mTOR pathway to promote apoptosis and autophagy
induced, indicating that PI3K/Akt/mTOR signal transduction pathway
is a key pathway involved in the role of curcumin in lung cancer,
and might be also the main target of curcumin.
Discussion
It is known that tumor is a disease in which cell
proliferation and death are imbalanced and the lung cancer is the
most prevalent malignant tumor. Recently, many natural plant agents
possessing availability and relatively low toxicity have been
reported to treat cancer by inhibiting cell proliferation (25). One of the liposoluble polyphenol
pigments, curcumin, has previously been reported to have effects on
suppressing the growth of a variety of cancer lines (12–14).
This sequence of events is strongly supported by the results of the
present study which disseminated evidence that induction of
multiple modes of cell death and potential mechanisms in the
context of cytotoxicity induced by curcumin in human lung cancer
A549 cells.
The anti-proliferation of curcumin in human lung
adenocarcinoma A549 cell line was confirmed, showing that curcumin
is a potent inhibitor of lung cancer cells in vitro. Also,
evidence was obtained that treatment with curcumin at different
concentration and time range selectively decreased the
proliferation of the cells in a dose- and time-dependent manner
(Fig. 1). Nevertheless, we
endeavoured to further explore the molecular process underlying
curcumin induced cell death.
Various forms of PCD are increasingly involved in
anticancer treatment, and the complex interplay among them is
critical to the ultimate fate of proliferating cells. Whether
curcumin possesses the ability to induce multiple PCDs including
apoptosis and autophagy in human lung cancer A549 cells, has
aroused our interest. Induction of apoptosis, namely type I PCD,
was a highly desirable characteristic for screening of
chemotherapeutic drugs (6). Our
results showed that curcumin proved to be a potent inducer of
apoptosis in A549 cells as evidenced by an dose-dependent increase
in Annexin V-positive cell populations (Fig. 2). Next, the events governing the
autophagy process was studied. To elucidate the autophagy (namely
type II PCD) induction by curcumin in A549 cells, MDC labeling
analysis was done to detect the AVs suggesting an enhanced increase
of the fluorescent structures compared with control. Evidence has
been captured that curcumin administration dramatically increased
Beclin1 and LC3-II expression, decreased p62 protein level,
indicating that autophagy was induced (Fig. 3).
In recent years, an increasing number of research
shows that autophagy may play a dual role in tumors, likely this
could be a survival or cell death mechanism. Focusing on our
earlier study on the role of curcumin-induced autophagy in lung
cancer A549 cells, it was demonstrated that autophagy was the
antitumor mechanism of curcumin rather than a protective mechanism
of the A549 cells itself when treated with curcumin (17). Based on the above results, it was
determined that curcumin-exposure within a limited range could
induced apoptosis and autophagic death in A549 cells. These data
clearly expressed the contribution of curcumin-induced apoptosis
and autophagy in the inhibition of A549 cell growth. Recent studies
have pointed towards a complex interplay between apoptosis and
autophagy involved in the process of cell death because they may
act independently of one another or may function as partners in a
synchronized manner (5,6,26). In
the debate on the intimate relationship between apoptosis and
autophagy in different tumor cells, we considered the possibility
that curcumin could inhibit the growth of human lung cancer A549
cells by concomitantly inducing apoptosis and autophagy, supporting
the view of some scholars (27).
However, the mechanism involved in curcumin-induced apoptosis and
autophagy of A549 cells should be further explored and elucidated
urgently.
Accumulated evidence suggests that apoptosis and
autophagy share some common signaling pathways, such as p53, Bcl-2
and PI3K/Akt/mTOR pathway (5–8,28,29).
In view of the important role played by PI3K/Akt/mTOR signaling in
cell growth, studies have shown that downregulation of this pathway
caused cell death associated with apoptosis and/or autophagy
(30,31). Two key molecules, mTOR and Akt, were
detected in order to determine the role of this pathway in the
curcumin-treated human lung adenocarcinoma A549 cells. The present
results demonstrated that the Akt phosphorylation and mTOR
phosphorylation as well as the mRNA expression were significantly
decreased after curcumin treatment (Fig. 4), thus indicating that curcumin
blocked the PI3K/Akt/mTOR signal transduction pathways in human
lung cancer A549 cells. In addition, the PI3K/Akt-specific
inhibitor LY294002 and mTOR blocker rapamycin were used in order to
further determine curcumin-induced apoptosis and autophagy by
PI3K/Akt/mTOR signaling pathway. Some previous studies have showed
that mTOR regulates apoptosis by phosphorylation of Bax to disrupt
the Bad binding to Bcl-XL, and/or Blc-2 in different cancer cells
(32). It should also be pointed
out that mTOR, emerging as a key negative regulator of autophagy,
promotes autophagy induction by dephosphorylation of Atg1, Atg13 to
promote autophagosome formation (33). Our results are consistent with the
above studies that p-mTOR downregulation attributed to the
consequent inhibition of the PI3K/Akt/mTOR pathway, and might act
as an important factor in cross talk between curcumin-induced
apoptosis and autophagy.
In our study, more significant inhibition of
PI3K/Akt/mTOR pathway occurred in the co-culture of curcumin and
LY294002 or curcumin and rapamycin (Fig. 5). It is worth mentioning that either
LY294002 or rapamycin pre-treatment not only enhanced the
apoptosis-inducing activity (Fig.
5B) and anticancer efficacy of curcumin (Fig. 5A) but also potentiated autophagy
induction in A549 cells in comparison with curcumin treatment alone
(Fig. 5C-F). All these data further
support the idea that apoptosis and autophagy activation by
curcumin proceeds through PI3K/Akt/mTOR inhibition. Briefly, the
induction of apoptosis and autophagy causing cell death after
curcumin exposure is closely linked to the inhibition of
PI3K/Akt/mTOR demonstrating this pathway plays a pivotal role in
curcumin treatment of A549 cells. However, there is no doubt that
the mechanism of inducing PCD is very complex, and the fate of
tumor cells is not determined by only one pathway. Therefore, it is
the direction of our future research to explore and elucidate the
complex relationship between curcumin-induced apoptosis and
autophagy as well as more detailed mechanisms in lung cancer
cells.
In conclusion, the A549 cell growth inhibitory
effect of curcumin was studied. Specifically, curcumin-induced
apoptosis may act as autophagy partner to induce cell death,
contributing to curcumin toxicity via inhibiting PI3K/Akt/mTOR
pathway which has been further deciphered in human lung cancer A549
cells. The results of the study indicates curcumin may be
considered as a candidate agent targeting PCD in clinical practice
and inhibiting the PI3K/Akt/mTOR signaling pathway which has been
suggested as a potential therapeutic target in NSCLC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81172598).
References
|
1
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim WK, Pyee Y, Chung HJ, Park HJ, Hong
JY, Son KH and Lee SK: Antitumor activity of spicatoside a by
modulation of autophagy and apoptosis in human colorectal cancer
cells. J Nat Prod. 79:1097–1104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ge J, Liu Y, Li Q, Guo X, Gu L, Ma ZG and
Zhu YP: Resveratrol induces apoptosis and autophagy in T-cell acute
lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating
p38-MAPK. Biomed Environ Sci. 26:902–911. 2013.PubMed/NCBI
|
|
6
|
Kumar D, Das B, Sen R, Kundu P, Manna A,
Sarkar A, Chowdhury C, Chatterjee M and Das P: Andrographolide
analogue induces apoptosis and autophagy mediated cell death in
U937 cells by inhibition of PI3K/Akt/mTOR pathway. PLoS One.
10:e01396572015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri
S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K,
et al: Caffeine induces apoptosis by enhancement of autophagy via
PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 7:176–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou ZW, Li XX, He ZX, Pan ST, Yang Y,
Zhang X, Chow K, Yang T, Qiu JX, Zhou Q, et al: Induction of
apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated
pathways by plumbagin in human prostate cancer cells. Drug Des
Devel Ther. 9:1511–1554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gills JJ, Lopiccolo J and Dennis PA:
Nelfinavir, a new anti-cancer drug with pleiotropic effects and
many paths to autophagy. Autophagy. 4:107–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gupta SC, Patchva S and Aggarwal BB:
Therapeutic roles of curcumin: Lessons learned from clinical
trials. AAPS J. 15:195–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou GZ, Cao FK, Chang JM, Sun GC and Chen
XB: Mechanism of curcumin analog MHMD-induced cell death in A549
lung cancer cells. Eur Rev Med Pharmacol Sci. 18:3134–3138.
2014.PubMed/NCBI
|
|
12
|
Chang CC, Fu CF, Yang WT, Chen TY and Hsu
YC: The cellular uptake and cytotoxic effect of curcuminoids on
breast cancer cells. Taiwan J Obstet Gynecol. 51:368–374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu D, You M, Xu Y, Li F, Zhang D, Li X
and Hou Y: Inhibition of curcumin on myeloid-derived suppressor
cells is requisite for controlling lung cancer. Int
Immunopharmacol. 39:265–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watson JL, Greenshields A, Hill R, Hilchie
A, Lee PW, Giacomantonio CA and Hoskin DW: Curcumin-induced
apoptosis in ovarian carcinoma cells is p53-independent and
involves p38 mitogen-activated protein kinase activation and
downregulation of Bcl-2 and survivin expression and Akt signaling.
Mol Carcinog. 49:13–24. 2010.PubMed/NCBI
|
|
15
|
Zhang J, Zhang T, Ti X, Shi J, Wu C, Ren X
and Yin H: Curcumin promotes apoptosis in A549/DDP
multidrug-resistant human lung adenocarcinoma cells through an
miRNA signaling pathway. Biochem Biophys Res Commun. 399:1–6. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thongrakard V, Titone R, Follo C, Morani
F, Suksamrarn A, Tencomnao T and Isidoro C: Turmeric toxicity in
A431 epidermoid cancer cells associates with autophagy degradation
of anti-apoptotic and anti-autophagic p53 mutant. Phytother Res.
28:1761–1769. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W,
Yang D, Yang A and Yu Y: Curcumin induced autophagy anticancer
effects on human lung adenocarcinoma cell line A549. Oncol Lett.
14:2775–2782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiao D, Wang J, Lu W, Tang X, Chen J, Mou
H and Chen QY: Curcumin inhibited HGF-induced EMT and angiogenesis
through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways
in lung cancer. Mol Ther Oncolytics. 3:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Zhou T, Sun H and Huang B: Study
on the relationship between autophagy and apoptosis in a549 cells
induced by curcumin analogue EF24. Chin J Cell Biol. 34:590–596.
2012.
|
|
20
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:e8752013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Graupera M and Potente M: Regulation of
angiogenesis by PI3K signaling networks. Exp Cell Res.
319:1348–1355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huo R, Wang L, Liu P, Zhao Y, Zhang C, Bai
B, Liu X, Shi C, Wei S and Zhang H: Cabazitaxel-induced autophagy
via the PI3K/Akt/mTOR pathway contributes to A549 cell death. Mol
Med Rep. 14:3013–3020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang LB, Cao L, Yin XF, Yasen M, Yishake
M, Dong J and Li XL: Activation of autophagy via Ca(2+)-dependent
AMPK/mTOR pathway in rat notochordal cells is a cellular adaptation
under hyperosmotic stress. Cell Cycle. 14:867–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A,
Cao HH, Tian HY, Fung KP, Kurihara H, Pan JX and Ye WC:
Arenobufagin, a natural bufadienolide from toad venom, induces
apoptosis and autophagy in human hepatocellular carcinoma cells
through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis.
34:1331–1342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R
and Darwiche N: Cell death mechanisms of plant-derived anticancer
drugs: Beyond apoptosis. Apoptosis. 20:1531–1562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai JP, Lee CH, Ying TH, Lin CL, Lin CL,
Hsueh JT and Hsieh YH: Licochalcone A induces autophagy through
PI3K/Akt/mTOR inactivation and autophagy suppression enhances
Licochalcone A-induced apoptosis of human cervical cancer cells.
Oncotarget. 6:28851–28866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar S, Guru SK, Pathania AS, Manda S,
Kumar A, Bharate SB, Vishwakarma RA, Malik F and Bhushan S:
Fascaplysin induces caspase mediated crosstalk between apoptosis
and autophagy through the inhibition of PI3K/AKT/mTOR signaling
cascade in human leukemia HL-60 cells. J Cell Biochem. 116:985–997.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Turcotte S and Giaccia AJ: Targeting
cancer cells through autophagy for anticancer therapy. Current Opin
Cell Biol. 22:246–251. 2010. View Article : Google Scholar
|
|
29
|
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J,
Zeng J, Zhang T, Wu H, Chen L, et al: Quercetin induces protective
autophagy in gastric cancer cells: Involvement of Akt-mTOR- and
hypoxia-induced factor 1alpha-mediated signaling. Autophagy.
7:966–978. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Wang F, Zhou ZW, Xia HC, Wang XY,
Yang YX, He ZX, Sun T and Zhou SF: Alisertib induces G2/M arrest,
apoptosis, and autophagy via PI3K/Akt/mTOR- and p38 MAPK-mediated
pathways in human glioblastoma cells. Am J Transl Res. 9:845–873.
2017.PubMed/NCBI
|
|
31
|
Wang F, Mao Y, You Q, Hua D and Cai D:
Piperlongumine induces apoptosis and autophagy in human lung cancer
cells through inhibition of PI3K/Akt/mTOR pathway. Int J
Immunopathol Pharmacol. 28:362–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tirado OM, Mateo-Lozano S and Notario V:
Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the
Bax: Bcl-xL ratio through concurrent mechanisms dependent and
independent of its mTOR inhibitory activity. Oncogene.
24:3348–3357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bilir A, Erguven M, Oktem G, Ozdemir A,
Uslu A, Aktas E and Bonavida B: Potentiation of cytotoxicity by
combination of imatinib and chlorimipramine in glioma. Int J Oncol.
32:829–839. 2008.PubMed/NCBI
|