Introduction
Neuroblastoma, one of the most common extracranial
solid tumors, accounts for ~10% of all childhood cancers (1). Moreover, neuroblastoma is the most
frequently diagnosed neoplasm during infancy (1). It is known that neuroblastoma
originates from embryonal neural crest cells that play an important
role in sympathetic nervous system development (2). Neuroblastoma is a very heterogeneous
and incurable tumor, ranging from the presentation of spontaneously
regressing growth to aggressive malignant potential (3,4). It
was reported that the clinical outcome of neuroblastoma is closely
correlated with patient age, tumor stage and histological
classification (5,6). Although huge advances have been
achieved in neuroblastoma treatment, such as surgery and
chemotherapy, its morbidity and mortality remain at a high level
(4,5). Thus far, the elusive molecular
mechanism underlying the genesis and progression of neuroblastoma
remain unclear.
Overexpression of the catalytic subunit (RRM2) of
ribonucleotide reductase is involved in the modification of
gemcitabine metabolism, and thus induces inherent or acquired
resistance to chemotherapeutic agents such as gemcitabine (7). Ribonucleotide reductase (RR) catalyzes
the inhibition of ribonucleotides yielding deoxyribonucleotides,
and is a rate-limiting enzyme for DNA synthesis (8). Transcriptional regulation is the main
mechanism in controlling the enzymatic activity of RR (9). RRM2 expression at the mRNA and protein
levels were found to be increased 9- and 2-fold in the
gemcitabine-resistant cell line KB-Gem, respectively (10). In addition, it was demonstrated that
RRM2 expression levels in tumors are a potential predictive
indicator of treatment responsiveness to chemotherapeutic agents
(11). Knockdown of RRM2 (12) or treatment with flavopiridol (a
cyclin-dependent kinase inhibitor) (13) rescued the sensitivity of cancer
cells to chemotherapeutic agents. Flavopiridol promotes cell
apoptosis by gemcitabine in human pancreatic, gastric and colon
cancer cells, which may be associated with inhibition of the RRM2
protein (13).
Although the RRM2 levels in various tumor types have
been investigated, little is known concerning the level and role of
RRM2 in neuroblastoma. In the present study, we assessed the RRM2
levels in human neuroblastoma tissues and matched adjacent
non-cancerous tissues and the correlation between the RRM2 levels
in neurobastoma and various clinicopathological characteristics. In
addition, the effect of chemotherapy on RRM2 expression, and the
role of RRM2 in the biological functions of neuroblastoma cells
were also explored.
Materials and methods
Patients and specimens
Neuroblastoma specimens were collected from 67
children (including 29 males and 38 females, ranging in age from 1
month to 13 years with a median age of 5.16 years) with primary
neuroblastoma during surgical operation at the Department of
Pediatric Surgery, Weifang People's Hospital (Weifang, China)
between September 2014 and August 2016. The pairs of neuroblastoma
and matched adjacent non-cancerous tissues were collected from the
site >5 cm away from the primary site. The present study was
approved by the Ethics Review Committee of Weifang People's
Hospital and signed informed consent was obtained from all
patients. Clinical staging of neuroblastoma was assessed according
to the International Neuroblastoma Staging System by two
independent pathologic examinations (14). Among the 67 cases of neuroblastoma,
40 cases had not received preoperative treatment including 27 cases
of stage I and II and 13 cases of stage III and IV. The other 27
cases received the same chemotherapy before surgery. All specimens
were snap frozen in liquid nitrogen and stored at −80°C until use.
The clinicopathological characteristics of all 67 neuroblastoma
patients are summarized in Table
I.
 | Table I.Correlation between RRM2 and
clinicopathological parameters of the neuroblastoma patients. |
Table I.
Correlation between RRM2 and
clinicopathological parameters of the neuroblastoma patients.
| Characteristics | No. of cases | Expression of RRM2
(fold) | P-value |
|---|
| Sex |
| Male | 29 | 2.512±0.104 |
|
|
Female | 38 | 2.934±0.197 | 0.190 |
| Age (years) |
| ≥5 | 22 | 2.856±0.290 |
|
|
<5 | 45 | 2.016±0.245 | 0.542 |
| TNM stage |
| I+II | 27 | 1.462±0.187 |
|
|
III+IV | 40 | 2.872±0.231 | 0.016 |
| Histology |
|
Favorable | 36 | 2.216±0.105 |
|
|
Unfavorable | 31 | 3.010±0.321 | 0.672 |
| Preoperative
chemotherapy |
| Yes | 27 | 1.25±0.405 |
|
| No | 40 | 2.421±0.165 | 0.011 |
Cell culture and transfection
The neuroblastoma cell line SH-5Y5Y and human neural
stem cell line N7800-200 were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). SH-5Y5Y cell and
N7800-200 were maintained in DMEM/F12 supplementing with 10% fetal
bovine serum (FBS) (both from Gibco, Carlsbad, CA, USA) at 37°C, in
a humidified 5% CO2 atmosphere. For transfection of RRM2
siRNA, the RRM2 siRNAs (siRNA-1, 5′-GCGAUUUAGCCAAGAAGUUCA-3′;
siRNA-2, 5′-GCGAUUUAGCCAAGAAGUUTT-3′; siRNA-3,
5′-GGGAUUAAACAGUCCUUUATT-3′; mixed) and negative control (NC) siRNA
(5′-UAGCGACUAAACACAUCAAUU-3′) were constructed. Cell transfection
was performed using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions. After 48 h, the
knockdown effects of RRM2 expression were confirmed by qRT-PCR.
qRT-PCR
Quantitative real-time PCR (qRT-PCR) was performed
using an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster
City, CA, USA) with SYBR Premix (Takara Bio, Otsu, Japan). Primer
pairs used for real-time PCR analysis of RRM2 were
5′-CACGGAGCCGAAAACTAAAGC-3′ and 5′-TCTGCCTTCTTATACATCTGCCA-3′. The
PCR reaction was performed in conditions: Initiation 30 sec at
95°C, amplification 5 sec of 40 cycles at 95°C and 34 sec at 60°C.
The experiments were performed in triplicate. Data was normalized
to GAPDH using the ∆∆Ct method.
Western blotting
Protein was extracted from tissues using RIPA
(15). After centrifugation at
12,000 × g 10 min, protein was collected and its concentration was
measured by an enhanced BCA protein assay kit (Beyotime Institute
of Biotechnology, Haimen, China). Protein (30 µg) of each sample
was separated by 10% SDS-PAGE, and then was transferred onto PVDF
membranes (Millipore, Billerica, MA, USA). After being blocked in
5% non-fat milk for 1 h, the membranes were then incubated with the
primary antibody anti-RRM2 (1:1,000; Abcam Biotechnology,
Cambridge, UK) overnight at 4°C. Then, horseradish peroxidase
(HRP)-conjugated secondary antibodies were used to incubate the
membranes for 1 h at room temperature. Anti-GAPDH (1:1,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used as an
internal control. The blots were visualized using an enhanced ECL
detection system (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and data were analyzed using ImageJ software (NIH, Bethesda, MD,
USA).
Cell proliferation
Cells (400/well) were placed in a 6-well plate in
triplicate. After transfection, the effect of RRM2 on SH-5Y5Y cell
proliferation was performed using the Cell Counting Kit-8 (CCK-8;
Beyotime Institute of Biotechnology). In brief, at various time
points (1, 2, 3 and 4 days), CCK-8 (10 µl) was added to each well
at 37°C for 1.5 h. Then, the cells were harvest and the absorbance
at 450 nm was detected by a microplate spectrophotometer.
Cell apoptosis and cell cycle
Cell cycle and cell apoptosis were measured using
flow cytometry. In brief, after transfection, cells were washed
with PBS, trypsinized and resuspended in ice-cold PBS. After
centrifugation at 300 × g, 5 min, at 4°C, the cells were fixed and
permeabilized by 70% ethanol at −20°C. After incubation with a
propidium iodide (PI) staining solution (50 µg/ml PI and 100 µg/ml
RNase A in PBS), in the dark for 30 min, the PI fluorescence was
measured using flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). The percentages of cells in the G0/G1, S and G2 phases were
analyzed using ModFit software (BD Biosciences).
The harvested cells also underwent apoptosis
detection. Cell apoptosis was performed using flow cytometry with
the Annexin V-FITC apoptosis detection kit (Sigma, St. Louis, MO,
USA). FITC(+) and PI(−) cells represent early apoptotic cells, and
FITC(+) and PI(+) cells represent late apoptotic cells. Cell
apoptosis was confirmed using Hoechst 33342 staining.
Statistical analysis
Data are presented as mean ± SD from at least three
independent experiments. Differences were compared using SPSS 15.0
statistical software (SPSS, Inc., Chicago, IL, USA) with the
Student's t-test and one-way analysis of variance (ANOVA). P-value
<0.05 was indicative of statistical significance.
Results
Upregulation of RRM2 in neuroblastoma
tissues
RRM2 levels in all 67 pairs of neuroblastoma and
adjacent non-cancerous tissues were detected using qRT-PCR and
western blotting. Among the 40 patients that did not receive
preoperative treatment, RRM2 mRNA expression in the neuroblastoma
tissues was significant higher than that noted in the non-cancerous
tissues (P<0.01) (Fig. 1A). The
RRM2 protein levels in neuroblastoma tissues were also higher than
levels in the non-cancerous tissues (Fig. 1B).
Correlation between RRM2 and
clinicopathological characteristics
The RRM2 mRNA level was significantly associated
with the clinical stage of the neuroblastoma patients. The RRM2
mRNA expression in stage III and IV neuroblastoma tissues was
significant higher than that in stage I and II tissues (P=0.016)
(Table I). There was no significant
association between RRM2 mRNA expression and sex, age and
histological classification (Table
I).
Effect of chemotherapy on RRM2
expression
We investigated the RRM2 expression level after
chemotherapy. Results showed that in stage III and IV neuroblastoma
tissues, the chemotherapy subgroup (27 cases) expressed lower RRM2
than the preoperative non-chemotherapy subgroup (13 cases)
(P=0.011) (Table I).
RRM2 expression in SH-5Y5Y cells
We detected RRM2 mRNA expression in SH-5Y5Y and
N7800-200 cells by qRT-PCR. RRM2 mRNA expression in the SH-5Y5Y
cells was significant higher than that in the N7800-200 cells
(Fig. 2A). The RRM2 protein level
was measured by western blotting. The RRM2 protein level in SH-5Y5Y
cells was also significant higher than that in the N7800-200 cells
(Fig. 2B).
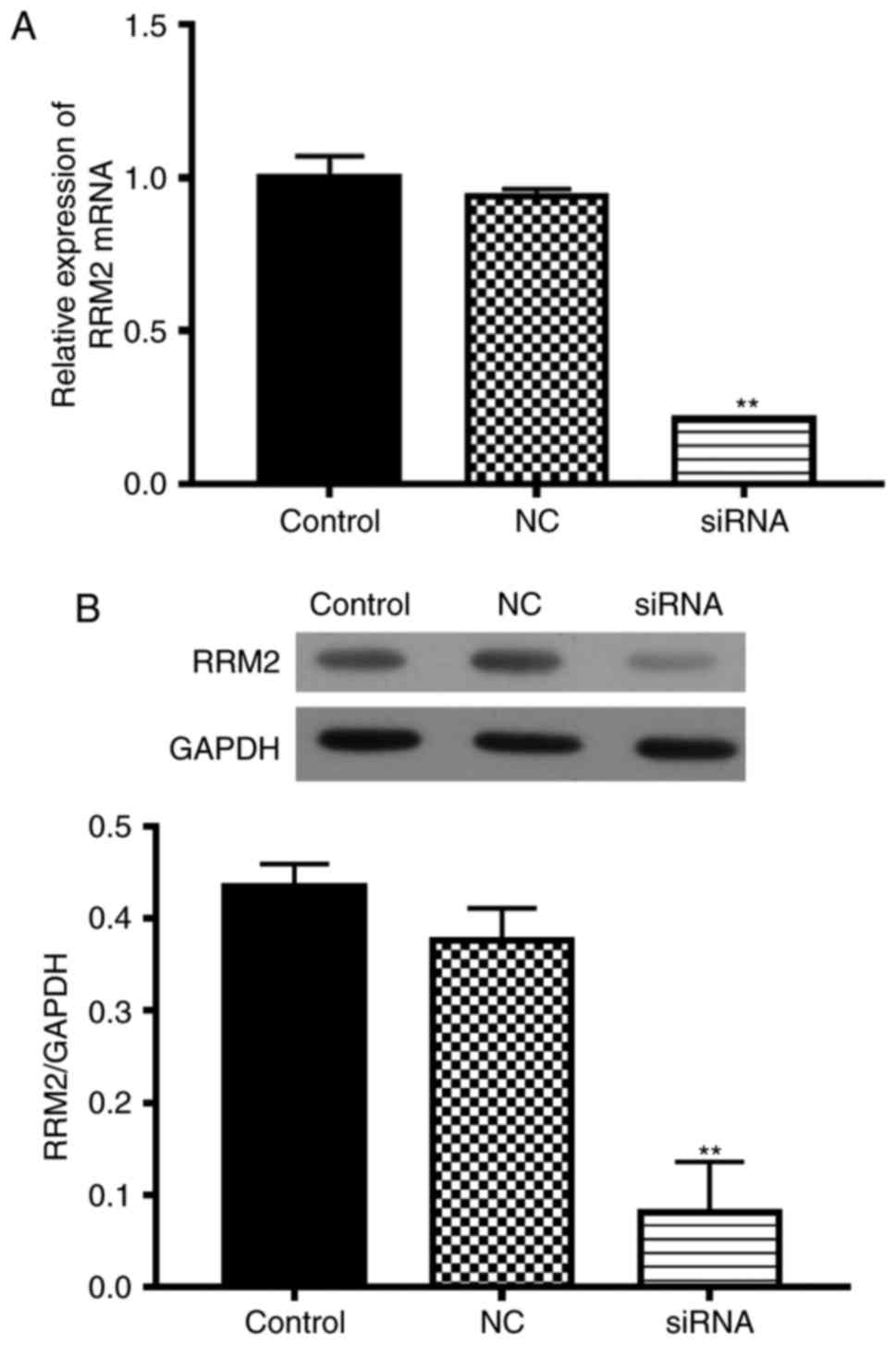
RRM2 siRNA inhibits the viability of
SH-5Y5Y cells
To investigate the function of RRM2 in SH-5Y5Y
cells, we transfected cells with RRM2-siRNA, with non-functional
siRNA as the negative control (NC). RRM2 mRNA expression in the
RRM2-siRNA transfected cells was less than that in the wild-type
group (Fig. 3A). The RRM2 protein
level was also inhibited by RRM2 siRNA (Fig. 3B).
The cell viability of the transfected cells was
assessed by CCK-8 assay (Fig. 4).
At 72 h, cell viability was significantly inhibited by RRM2 siRNA
compared with the control and NC groups (P<0.05).
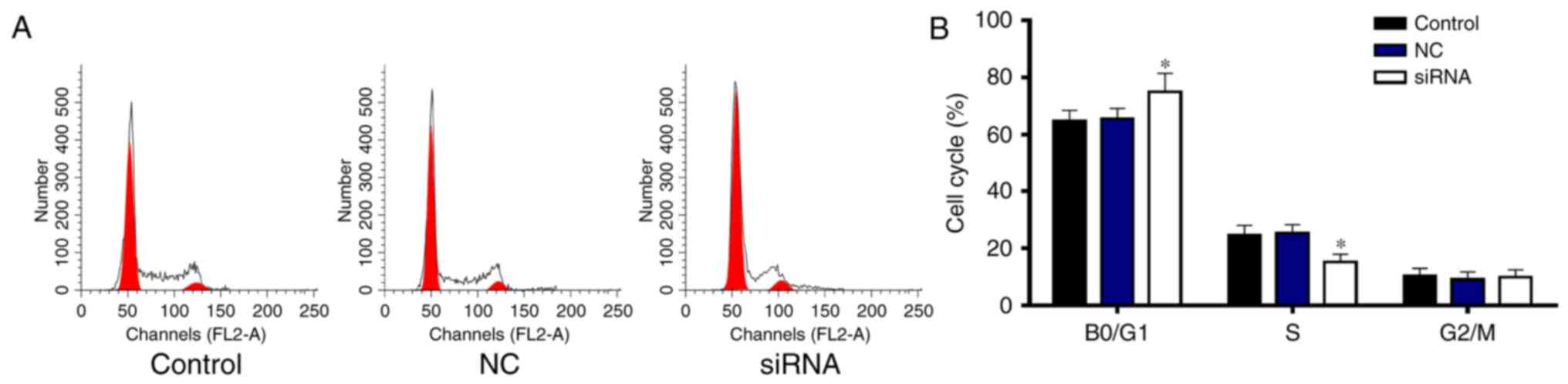
RRM2 siRNA induces cell cycle arrest
in the G0/G1 phase
To determine whether RRM2 siRNA decreases the cell
viability by decreasing cell proliferation, the cell cycle was
performed by flow cytometry (Fig.
5). The percentage of cells in the G0/G1 phase was
significantly increased by RRM2 siRNA compared with the percentage
in the control and NC groups (P<0.05), while the proportion of
cells in the S phase was significantly decreased (Fig. 5A and B). Thus, RRM2 siRNA induced
cell arrest in the G0/G1 phase.
RRM2 siRNA induces cell apoptosis in
SH-5Y5Y cells
To further determine whether RRM2 siRNA decreases
the cell number by promoting cell apoptosis, cell apoptosis was
evaluated using flow cytometry and Hoechst 33342 staining (Fig. 6). The early and late apoptosis rates
were significantly increased by RRM2 siRNA compared with the
control and NC groups (Fig. 6A and
B) (P<0.05). Similar to the flow cytometry results, Hoechst
33342 staining showed that RRM2 siRNA promoted cell apoptosis
(Fig. 6C). Thus, RRM2 siRNA
promoted cell apoptosis in the SH-5Y5Y cells.
Discussion
Ribonucleotide reductase M2 (RRM2), a rate-limiting
enzyme for DNA synthesis and repair (16,17),
was found to be highly expressed in various diseases including
gestational trophoblastic disease, breast, pancreatic and
gallbladder cancer, which is related to the growth, invasion and
chemoresistance of malignant tumors (11,18–20).
In the present study, RRM2 was detected in 67 pairs of
neuroblastoma tissues and adjacent non-cancerous tissues. When
patients did not receive preoperative treatment, RRM2 expression at
the mRNA and protein levels was significant higher in neuroblastoma
tissues than that in the adjacent non-cancerous tissues.
It was previously demonstrated that RRM2 is
overexpressed in gastric carcinoma tissues compared to that noted
in normal gastric mucosa, and it was associated with sex, depth of
invasion, EB virus infection, but not with age, tumor size,
histological type and lymph node metastasis (21). The high expression of RRM2 in tumor
specimens from patients with bladder cancer suggests that RRM2 may
be an indicator and potential target of early diagnosis and
treatment of bladder cancer (22).
In the present study, a high level of RRM2 was significantly
associated with the clinical stage in patients with neuroblastoma.
The RRM2 mRNA expression in stage III and IV tissues was
significant higher than that noted in stage I and II tissues. RRM2
mRNA expression was not associated with sex, age, and histological
classification. Thus, RRM2 may a diagnostic indicator and a
therapeutic target for neuroblastoma.
It was demonstrated that drug resistance in tumor
cells is associated with a prolonged DNA replication phase, and
that downregulation of RRM2 expression can increase cell apoptosis
induced by chemotherapeutic agents (23). In the present study, tissues from
the chemotherapy subgroup had suppressed RRM2 levels in stage III
and IV tumors, compared with the preoperative non-chemotherapy
subgroup, indicating the RRM2 may be associated with chemotherapy.
The effects of the reduction or blocking of RRM2 expression levels
on the proliferation and apoptosis of SH-5Y5Y cells exposed to
chemotherapy drugs may be evaluated in the future.
A high level of RRM2 is closely correlated with
increased resistance to chemotherapy in cancer cells, and the
reduction or blocking of the RRM2 expression levels by various
techniques can improve the sensitivity to chemotherapeutic agents
in cancer cells (12,24). At present, the method used for
suppression of the expression level of RRM2 mainly includes
antisense oligonucleotides or specific drugs (25,26).
Recently, studies have reported that blocking RRM2 expression by
RNAi may be a new strategy for the gene therapy of malignant tumors
(27,28).
Studies have found that cancer cells with a high
RRM2 expression level have induced VEGF mRNA expression, which then
confers cancer cells with increased growth, invasion and metastasis
characteristics, and thus a poor prognosis (18). RRM2 serves as a rate-limiting enzyme
for DNA synthesis, and its role is closely related to cell survival
(29,30). We detected the RRM2 levels in
SH-5Y5Y and N7800-200 cells. The results found that the RRM2 mRNA
and protein levels in the SH-5Y5Y cells were significant higher
than levels in the N7800-200 cells, confirming the overexpression
of RRM2 in neuroblastoma cells. To explore the biological role of
RRM2 in SH-5Y5Y cells, we transfected cells with RRM2-siRNA or
negative control non-functional siRNA (NC). The cell viability was
significantly inhibited by RRM2 siRNA compared with the control and
NC groups. RRM2 siRNA induced cell cycle arrest in the G0/G1 phase,
suggesting that RRM2 siRNA decreased the cell number by decreasing
proliferation. RRM2 siRNA promoted cell apoptosis in the SH-5Y5Y
cells, suggesting that RRM2 siRNA also decreased the cell number by
promoting cell apoptosis. The research showed that there is a
direct correlation between RRM2 and tumor biological behavior. Its
high expression has clinical value for early diagnosis and
treatment of malignant tumors. Therefore RRM2 is expected to become
a new index for malignant tumor diagnosis and prognostic
evaluation.
In conclusion, the RRM2 level in neuroblastoma
tissues was found to be correlated with clinical stage, and its
overexpression was suppressed by chemotherapy. Knockdown of RRM2
decreased cell viability, induced cell cycle arrest in the G0/G
phase and promoted cell apoptosis. Our findings suggest that RRM2
may play a vital role in the progression of neuroblastoma and could
be a promising therapeutic target.
References
|
1
|
Matthay KK, Maris JM, Schleiermacher G,
Nakagawara A, Mackall CL, Diller L and Weiss WA: Neuroblastoma. Nat
Rev Dis Primers. 2:160782016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marshall GM, Carter DR, Cheung BB, Liu T,
Mateos MK, Meyerowitz JG and Weiss WA: The prenatal origins of
cancer. Nat Rev Cancer. 14:277–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi Y, Sipp D and Enomoto H: Tissue
interactions in neural crest cell development and disease. Science.
341:860–863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheung NK and Dyer MA: Neuroblastoma:
Developmental biology, cancer genomics and immunotherapy. Nat Rev
Cancer. 13:397–411. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bergman AM, Pinedo HM and Peters GJ:
Determinants of resistance to 2′,2′-difluorodeoxycytidine
(gemcitabine). Drug Resist Updat. 5:19–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewis CS, Voelkel-Johnson C and Smith CD:
Suppression of c-Myc and RRM2 expression in pancreatic cancer cells
by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget.
7:60181–60192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eriksson S and Martin DW Jr:
Ribonucleotide reductase in cultured mouse lymphoma cells. Cell
cycle-dependent variation in the activity of subunit protein M2. J
Biol Chem. 256:9436–9440. 1981.PubMed/NCBI
|
|
10
|
Goan YG, Zhou B, Hu E, Mi S and Yen Y:
Overexpression of ribonucleotide reductase as a mechanism of
resistance to 2,2-difluorodeoxycytidine in the human KB cancer cell
line. Cancer Res. 59:4204–4207. 1999.PubMed/NCBI
|
|
11
|
Itoi T, Sofuni A, Fukushima N, Itokawa F,
Tsuchiya T, Kurihara T, Moriyasu F, Tsuchida A and Kasuya K:
Ribonucleotide reductase subunit M2 mRNA expression in pretreatment
biopsies obtained from unresectable pancreatic carcinomas. J
Gastroenterol. 42:389–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: RNA interference targeting the M2 subunit of
ribonucleotide reductase enhances pancreatic adenocarcinoma
chemosensitivity to gemcitabine. Oncogene. 23:1539–1548. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung CP, Motwani MV and Schwartz GK:
Flavopiridol increases sensitization to gemcitabine in human
gastrointestinal cancer cell lines and correlates with
down-regulation of ribonucleotide reductase M2 subunit. Clin Cancer
Res. 7:2527–2536. 2001.PubMed/NCBI
|
|
14
|
Brodeur GM, Pritchard J, Berthold F,
Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE,
Favrot M, Hedborg F, et al: Revisions of the international criteria
for neuroblastoma diagnosis, staging and response to treatment.
Prog Clin Biol Res. 385:363–369. 1993.
|
|
15
|
Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang
Y, Feng T, Wu J and Liu X: Sphingosine-1-phosphate induced
epithelial-mesenchymal transition of hepatocellular carcinoma via
an MMP-7/syndecan-1/TGF-β autocrine loop. Oncotarget.
7:63324–63337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reece SY and Seyedsayamdost MR: Long-range
proton-coupled electron transfer in the Escherichia coli class Ia
ribonucleotide reductase. Essays Biochem. 61:281–292. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pai CC and Kearsey SE: A critical balance:
dNTPs and the maintenance of genome stability. Genes. 8:pii: E57.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui JQ, Shi YF, Zhou HJ and Li JQ: The
changes of gene expression profiles in hydatidiform mole and
choriocarcinoma with hyperplasia of trophoblasts. Int J Gynecol
Cancer. 14:984–997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okamura H, Kamei T, Sakuma N, Hanai N and
Ishihara T: Ribonucleotide reductase immunoreactivity in
adenocarcinoma cells and malignant or reactive mesothelial cells in
serous effusions. Acta Cytol. 47:209–215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Juhasz A, Vassilakos A, Chew HK, Gandara D
and Yen Y: Analysis of ribonucleotide reductase M2 mRNA levels in
patient samples after GTI-2040 antisense drug treatment. Oncol Rep.
15:1299–1304. 2006.PubMed/NCBI
|
|
21
|
Morikawa T, Hino R, Uozaki H, Maeda D,
Ushiku T, Shinozaki A, Sakatani T and Fukayama M: Expression of
ribonucleotide reductase M2 subunit in gastric cancer and effects
of RRM2 inhibition in vitro. Hum Pathol. 41:1742–1748. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morikawa T, Maeda D, Kume H, Homma Y and
Fukayama M: Ribonucleotide reductase M2 subunit is a novel
diagnostic marker and a potential therapeutic target in bladder
cancer. Histopathology. 57:885–892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mannargudi MB and Deb S: Clinical
pharmacology and clinical trials of ribonucleotide reductase
inhibitors: Is it a viable cancer therapy? J Cancer Res Clin Oncol.
143:1499–1529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duxbury MS, Ito H, Benoit E, Zinner MJ,
Ashley SW and Whang EE: Retrovirally mediated RNA interference
targeting the M2 subunit of ribonucleotide reductase: A novel
therapeutic strategy in pancreatic cancer. Surgery. 136:261–269.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujita H, Ohuchida K, Mizumoto K, Itaba S,
Ito T, Nakata K, Yu J, Kayashima T, Souzaki R, Tajiri T, et al:
Gene expression levels as predictive markers of outcome in
pancreatic cancer after gemcitabine-based adjuvant chemotherapy.
Neoplasia. 12:807–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Souglakos J, Boukovinas I, Taron M, Mendez
P, Mavroudis D, Tripaki M, Hatzidaki D, Koutsopoulos A,
Stathopoulos E, Georgoulias V and Rosell R: Ribonucleotide
reductase subunits M1 and M2 mRNA expression levels and clinical
outcome of lung adenocarcinoma patients treated with
docetaxel/gemcitabine. Br J Cancer. 98:1710–1715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moorthy NS, Cerqueira NM, Ramos MJ and
Fernandes PA: Development of ribonucleotide reductase inhibitors: A
review on structure activity relationships. Mini Rev Med Chem.
13:1862–1872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cerqueira NM, Pereira S, Fernandes PA and
Ramos MJ: Overview of ribonucleotide reductase inhibitors: An
appealing target in anti-tumour therapy. Curr Med Chem.
12:1283–1294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giannattasio M and Branzei D: S-phase
checkpoint regulations that preserve replication and chromosome
integrity upon dNTP depletion. Cell Mol Life Sci. 74:2361–2380.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dyawanapelly S, Kumar A and Chourasia MK:
Lessons learned from gemcitabine: Impact of therapeutic carrier
systems and Gemcitabine's drug conjugates on cancer therapy. Crit
Rev Ther Drug Carrier Syst. 34:63–96. 2017. View Article : Google Scholar : PubMed/NCBI
|