Introduction
Gastric cancer (GC) is one of the most common
malignancies and the second leading cause of cancer-related deaths
worldwide. It accounted for ~723,000 cancer-associated deaths (~10%
of the total recorded deaths from cancer) in 2012 worlwide
(1). In underdeveloped regions of
Asia, Eastern Europe and South America, there is a high incidence
of GC. In China, the morbidity and mortality of GC is increasing
with population size, prompting an urgent need to develop more
effective treatments and identify novel therapeutic targets for GC
(2,3). Although the pathogenesis of GC is not
fully understood, it is thought that immunomodulatory disorders,
such as persistent inflammation, play a significant role in its
promotion (4). Anti-inflammatory
molecules may therefore, be potential candidates for cancer
treatment.
TNFAIP8-like 2 (TIPE2) is a member of the tumor
necrosis factor-a inducible protein-8 (TNFAIP8) family. Recent
studies have revealed that TIPE2 is a negative immune regulator
that is selectively expressed in immune organs and cells, and
serves an important role in the maintenance of human physiological
and immunological homeostasis (5).
TIPE2 knockout in mice induced inflammation in multiple organs.
TIPE2 was found to execute its negative regulatory role by binding
to caspase-8 and thus, inhibiting activating protein-1 and nuclear
factor-κB (NF-κB) stimulation in macrophages (5,6).
TIPE2 has been reported to be a negative regulator
of the human immune response, as demonstrated by its downregulation
in peripheral blood mononuclear cells (PBMCs) obtained from
patients with systemic lupus erythematosus (7) and chronic hepatitis B (8). In addition to its role as a negative
regulator of the immune response, TIPE2 is expressed in various
epithelial cell types, including esophageal and cervical squamous
epithelial cells, transitional epithelial cells of the bladder and
ureter, and glandular epithelial cells of the stomach, colon and
appendix (9,10), thus indicating an activity beyond
immune cell regulation.
It has been reported that TIPE2 levels are reduced
in hepatocellular cancer (HCC) cells (11), and TIPE2 overexpression decreased
tumor growth and metastasis in a xenograft mouse model bearing a
human HCC cell line. Sun et al (11) reported that the expression of TIPE2
was either completely suppressed or significantly decreased in
human liver cancer. Zhu et al found that adenovirus-directed
expression of TIPE2 suppressed GC growth via induction of apoptosis
and inhibition of AKT and ERK1/2 signaling in AGS and HGC-27 GC
cells (12). In addition, TIPE2
promoted a p27-associated signaling cascade that decreased GC cell
proliferation (13). A biochemical
characterization study of TIPE2, conducted by Cao et al,
revealed that TIPE2 binds to RAC1 to reduce its activity and
inhibit the activation of MMP9 and Upa, thereby suppressing
metastasis (14). In contrast, Li
et al reported that TIPE2 was overexpressed in colon cancer
tissues (15), suggesting that the
function of TIPE2 may vary depending on the type of cancer
cells.
The function of TIPE2 in GC remains unclear. In the
present study, we aimed to identify the role of TIPE2 in GC cell
migration and proliferation. To characterize the functional
consequence of TIPE2 downregulation in GC cells, we generated a
TIPE2-silenced gastric cell line.
As gastric carcinoma has been reported to be related
with epithelial inflammation, we used LPS to stimulate GC cells and
mimic the inflammatory process observed during tumorigenesis. In
TIPE2-silenced GC cell lines, cell death was reduced following
stimulation with LPS, but not in unstimulated cells. In the present
study, a model for the role of TIPE2 in GC development is presented
and discussed. We aimed to explain how TIPE2 inhibited tumor growth
via proliferation, apoptosis and inflammatory pathways.
Materials and methods
Patients
For RNA detection, 42 tumor samples were collected
from GC patients at the Zhongshan Hospital of Xiamen University
between January 2014 and January 2015. This cohort was comprised of
7 females and 35 males, ranging from 43 to 88 years old. For
immunohistochemistry detection, 63 tumor samples were collected
from GC patients at the Zhongshan Hospital of Xiamen University
between January 2013 and January 2015. Written informed consent for
the study was provided by all participants. The study was approved
by the Medical Ethics Committee of Zhongshan Hospital of Xiamen
University.
Cell culture
Human BGC823 and SGC7901 GC cells were purchased
from the Chinese Academy of Medical Sciences (Shanghai, China).
BGC823 cells were maintained in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone Laboratories; GE Healthcare Life
Sciences, Logan, UT, USA), 100 U/ml penicillin and 100 U/ml
streptomycin in a humidified atmosphere with 5% CO2 at
37°C.
Establishment of a
TIPE2-overexpressing GC cell line
The TIPE2-overexpression plasmid was constructed by
cloning human TIPE2 cDNA into a GV218 lentivirus vector. Briefly,
total cellular RNA was purified using an RNA extraction kit
(Tiangen Biotech Co., Ltd., Beijing, China) and the full-length
coding sequence (CDS) of TIPE2 was amplified via reverse
transcription-PCR (RT-PCR). The first-strand cDNA was synthesized
using a Reverse Transcription kit (Tiangen Biotech Co., Ltd. PCR
was performed using cDNA as a template with the following
TIPE2-specific primer pair: TIPE2-AgeI-F,
5′-GAGGATCCCCGGGTACCGGTCGCCACCATGGAGTCCTTCAGCTC-3′ and TIPE2-Age
I-R, 5′-TCACCATGGTGGCGACCGGGCTCAGAGCTTCCCTTC-3′. The fragments were
sub-cloned into a GV218 lentivirus vector and verified by DNA
sequencing. Pack virus according to Lenti-Easy Packaging system
(Shanghai GeneChem, Co., Ltd., Shanghai, China). BGC823 and SGC7901
cells were transfected and selected with ٢ µg/ml puromycin.
Separated cell clones were confirmed via western blot analysis and
stored for further experiments.
Cell viability assay
Cell viability was evaluated using a CCK-8 kit
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's instructions. Briefly, cells were seeded into
96-well plates at 1×104 cells/well. After culturing for
the indicated time periods, CCK-8 solution was added to each well
and incubated for 1 h. We determined the absorbance for each well
at a wavelength of 450 nm using a microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). Experiments for each
time-point were performed in quadruplicate and three times
independently.
Scratch assay
Cell migration was evaluated using a scratch assay.
Briefly, we marked the bottom of each 6-well plate with a
horizontal line as a reference point for image acquisition. BGC823
cells were seeded into 6-well plates and cultured to 90%
confluence. Cells were scratched using a 10-µl pipette tip to
create a cell-free area and washed gently with PBS three times to
remove detached cells. Plates were then incubated for 48 h in
Dulbecco's modified Eagle medium (DMEM; HyClone Laboratories; GE
Healthcare Life Sciences) containing 4% FBS. Cell-free areas were
imaged, and the gap distance was quantitatively calculated using
ImageJ 1.46r software (National Institutes of Health, Bethesda, MD,
USA).
Real-time reverse
transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted using an RNA
Extraction kit (Tiangen Biotech Co., Ltd.). First-strand cDNA was
synthesized using a Reverse Transcription kit (Tiangen Biotech Co.,
Ltd.) and subjected to real-time PCR analysis. PCR was performed in
triplicate with an ABI Step One Plus Real-Time PCR (Applied
Biosystems; Thermo Fisher Scientific, Inc.) under the following
conditions: 95°C for 2 min followed by 40 cycles at 95°C for 10
sec, 60°C for 30 sec and 72°C for 30 sec, with a final extension at
72°C for 10 min as previously described (16,17).
Sequences of the gene-specific primers (sense and antisense,
respectively) were: 5′-TCTTCCAGCCTTCCTTCCT-3′ and
5′-AGCACTGTGTTGGCGTACAG-3′ for β-actin; and
5′-CACCGCAATGGCTCCTTT-3′ and 5′-CACCAACTCTAGCAGCACATC-3′ for
TIPE2.
Western blot analysis
Cells were harvested and lysed in RIPA buffer [1%
Triton, 0.1% SDS, 0.5% deoxycholate, 1 mM EDTA, 20 mM Tris (pH
7.4), 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4,
and 0.1 mM phenylmethyl sulfonyl fluoride] on ice for 30 min. We
collected the supernatants via centrifugation and detected the
concentrations of proteins using a BCA Assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Equal amounts of the prepared protein
were separated by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride (PVDF) (Millipore, Billerica, MA, USA) membranes. The PVDF
membranes were blocked in 5% non-fat milk in TBST at room
temperature for 1 h, and then incubated overnight at 4°C with
primary antibodies against the following proteins: TIPE2 (1:1,000;
rabbit ployclonal; cat. no. 15940–1-AP; ProteinTech, Group, Inc.,
Chicago, IL, USA), β-actin (1:1,000; mouse monoclonal; cat. no.
sc-58673; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), AKT
(1:1,000; mouse monoclonal; cat. no. sc-377457; Santa Cruz
Biotechnology), p-AKT (1:1,000; mouse monoclonal; cat. no.
sc-52940; Santa Cruz Biotechnology), CDK1 (1:1,000; mouse
monoclonal; cat. no. 9116), p-CDK1 (1:1,000; rabbit monoclonal;
cat. no. 4539), cyclin B1 (1:2,000; rabbit polyclonal; cat. no.
4138), Bcl-2 (1:1,000; mouse monoclonal; cat. no. 15071), cleaved
caspase-3 (1:1,000; rabbit ployclonal; cat. no. 9661), cleaved
caspase-9 (1:1,000; rabbit monoclonal; cat. no. 20750), IκBα
(1:1,000; rabbit monoclonal; cat. no. 4812), p-IKK (1:1,000; rabbit
monoclonal; cat. no. 2697), p-p65 (1:1,000; rabbit monoclonal; cat.
no. 3033), vimentin (1:1,000; rabbit monoclonal; cat. no. 5741),
N-cadherin (1:1,000; rabbit monoclonal; cat. no. 13116), E-cadherin
(1:1,000; mouse monoclonal; cat. no. 14472; all were from Cell
Signaling Technology, Inc., Beverly, MA, USA). The membranes were
washed three times with TBST, and then were incubated with an
HRP-conjugated secondary antibody (1:10,000; goat ployclonal; cat.
no. LP31460; Xiamen Lulong Biotech Co., Ltd., Xiamen, China) for 1
h at 37°C. Immunoreactive products were visualized using an
enhanced chemiluminescence system (GE Healthcare Life Sciences,
Little Chalfont, UK).
Immunohistochemistry (IHC)
Paraffin-embedded tissue sections were
deparaffinized with xylene and gradually rehydrated. Each section
was added to a solution of 0.3% hydrogen peroxide and 10% methanol
for 10 min at room temperature to block the activity of endogenous
peroxidase. Goat serum (Invitrogen; Thermo Fisher Scientific, Inc.)
was used to block non-specific staining. Sections were incubated
with an anti-TIPE2 antibody (1:1,000; mouse ployclonal; cat. no.
H00079626-B01P; Abnova, Taipei, Taiwan) overnight at 4°C. After
washing with PBS, secondary staining was performed with an
HRP-conjugated secondary antibody (1:10,000; goat ployclonal; cat.
no. LP31460; Xiamen Lulong Biotech Co., Ltd.). Immunoreactivity was
detected using a freshly prepared DAB solution (Fuzhou Maixin
Biotech. Co., Ltd., Fuzhou, China), followed by counterstaining
with hematoxylin and dehydration in graded concentrations of
alcohol and dimethyl benzene. The sections were observed using
optical microscopy (Olympus BX51; Olympus Corp., Tokyo, Japan).
Flow cytometric analysis
The effects of TIPE2 on the cell cycle were detected
via flow cytometric analysis. Briefly, cells were trypsinized with
EDTA-free trypsin and centrifuged to obtain a cell pellet,
following which the supernatant was discarded and the cells were
washed with ice-cold PBS. Cells were re-suspended and fixed in 70%
pre-cooled ethanol at 4°C overnight. Ethanol-fixed cells were
centrifuged, the supernatant was discarded, and the cells were
washed three times with PBS to remove residual ethanol. Cells were
re-suspended in 1 ml staining solution [50 µg/ml propidium iodide
(PI)] containing 10 µg/ml RNase A and stained for 15 min at room
temperature. Stained cells were analyzed by flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA). Cell cycle distribution was
analyzed using ModFit v.3.0 software (Verity Software House,
Topsham, ME, USA). The effect of TIPE2 on cell apoptosis was
determined by flow cytometry using an FITC-Annexin V Apoptosis
Detection kit (BD Biosciences). Briefly, ~1×105 cells
were resuspended in 100 µl binding buffer, to which 5 µl
FITC-Annexin V and 10 µl PI were added. Cells were gently vortexed
followed by incubation for 15 min at room temperature, in the dark.
Finally, we added 400 µl binding buffer to each tube and analyzed
the solutions via flow cytometry within 1 h.
Detection of caspase activity
The activity of caspase-3, caspase-8 and caspase-9
was analyzed using Caspase-Glo®3/7 Assay System,
Caspase-Glo®8 Assay System and Caspase-Glo®9
Assay System (Promega, Madison, WI, USA) according to the
manufacturer's instructions. Briefly, 96-well plates containing
cells were removed from the incubator and the plates were allowed
to equilibrate to room temperature. Caspase-Glo® reagent
(100 µl) was added to each well of a white-walled 96-well plate
containing 100 µl of blank or cells in culture medium. The contents
of the wells were gently mixed and incubated at room temperature
for 30 min. Finally, the luminescence of each sample was assessed
on a plate-reading luminometer (PerkinElemer EnVision;
PerkinElemer, Waltham, MA, USA) as directed by the manufacturer's
instructions.
ELISA
Levels of the inflammatory cytokines IL-1β (cat. no.
BRK0181; BioRike, Changsha, China), IL-6 (cat. no. BRK0049;
BioRike) and TNF-α (cat. no. BRK0122; BioRike) were detected using
an ELISA assay. After treatment with LPS (20 ng/ml) for 24 h, the
supernatants were collected and the IL-1β, IL-6 and TNF-α standard
protein samples were added to the wells, which were then sealed and
incubated at room temperature for 1 h. Following incubation, the
wells were washed 4 times with a buffer, following which a
biotin-conjugated detection antibody was added to each well and
incubated for 1 h at room temperature. The wells were subsequently
washed another 4 times, before streptavidin-conjugated HRP was
added and incubated for 30 min. Finally, the wells were washed 4
times, and the chromogenic substrate TMB was added to develop the
color. The absorbance was read at 405 nm.
Promoter reporters and dual-luciferase
assays
SGC7901/vector and SGC7901/TIPE2 cells were
transfected with pGL4-NF-κB-Luc (Promega) and pRL-TK-vector
(Promega) using the Lipofectin reagent (Life Technologies, Inc.,
Gaithersburg, MD, USA). After 24 h of transfection, cells were
treated with or without LPS (10 ng/ml) for 12 h. The cells were
then harvested in passive lysis buffer (Promega). Firefly
luciferase and Renilla luciferase activities were quantified
using the Dual-Luciferase Assay System (Promega). Changes in
firefly luciferase activity were calculated relative to the
Renilla luciferase.
Statistical analysis
Experimental data are presented as the mean ±
standard deviation (SD). Differential expression of the TIPE2
protein between the GC and adjacent non-cancerous tissues was
analyzed using the Chi-square test. The Student's t-test was used
to compare differences between groups, which were then analyzed
using SPSS v.11.0 software (SPSS, Inc., Chicago, IL, USA). A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of TIPE2 in gastric
carcinoma and normal gastric mucosa
The TIPE2 protein was first discovered in 2008 by
Sun et al (University of Pennsylvania) in the neurological
tissues of patients with experimental autoimmune encephalomyelitis.
The protein was revealed to play an important regulatory role in
human immune homeostasis (5). To
detect differences in TIPE2 expression between GC and adjacent
normal gastric mucosae, we collected paired tissue samples from 42
patients with GC who were admitted to Zhongshan Hospital of Xiamen
University between January 2014 and January 2015. Total RNA was
extracted and the expression of TIPE2 mRNA was detected by RT-PCR
in gastric carcinoma and normal gastric mucosa tissues. As shown in
Fig. 1A and B, the mRNA expression
of TIPE2 was significantly lower in GC tissues than in normal
gastric mucosa (P<0.05).
In order to assess the relationship between TIPE2
expression and GC tissues, we used immunohistochemistry to detect
the expression of TIPE2 in tumor tissue samples collected from 63
patients with GC at the Zhongshan Hospital of Xiamen University
from January 2013 to January 2015. The results showed that TIPE2
staining ranged from light-brown to brown in the normal gastric
mucosae, while TIPE2 staining was negative or relatively weak in GC
cells. Further analysis of the 63 tissue pairs revealed that TIPE2
was expressed in 69.84% (44/63) of the adjacent tissues, which was
significantly higher than the 15.87% (10/63) of GC tissues
(P<0.05) (Fig. 1C; Table I). These results indicated that
TIPE2 expression was reduced in GC, and that TIPE2 has a central
role in the development and progression of GC.
 | Table I.TIPE2 protein expression in GC and
adjacent tissues. |
Table I.
TIPE2 protein expression in GC and
adjacent tissues.
| Clinical data | Negative (−) | Weakly positive
(+) | Positive (++) | Strongly positive
(+++) | P-value |
|---|
| Normal gastric
mucosa | 0 | 19 | 42 | 2 | <0.05 |
| GC | 4 | 49 | 9 | 1 |
|
Establishment of a stable
TIPE2-overexpressing cell line
In order to explore the function and mechanism of
TIPE2 in GC, the fragment containing a 555-bp CDS of human TIPE2
was successfully cloned (Fig. 2A).
The fragment was subcloned into the GV218 lentiviral vector and
verified by DNA sequencing (data not shown). The vectors were
transfected into the BGC823 GC cell line to generate a
TIPE2-overexpressing stable cell line, named BGC823/TIPE2, and a
control cell line, named BGC823/Vector. TIPE2 overexpression was
detected via western blotting (Fig.
2B).
TIPE2 inhibits GC cell
proliferation
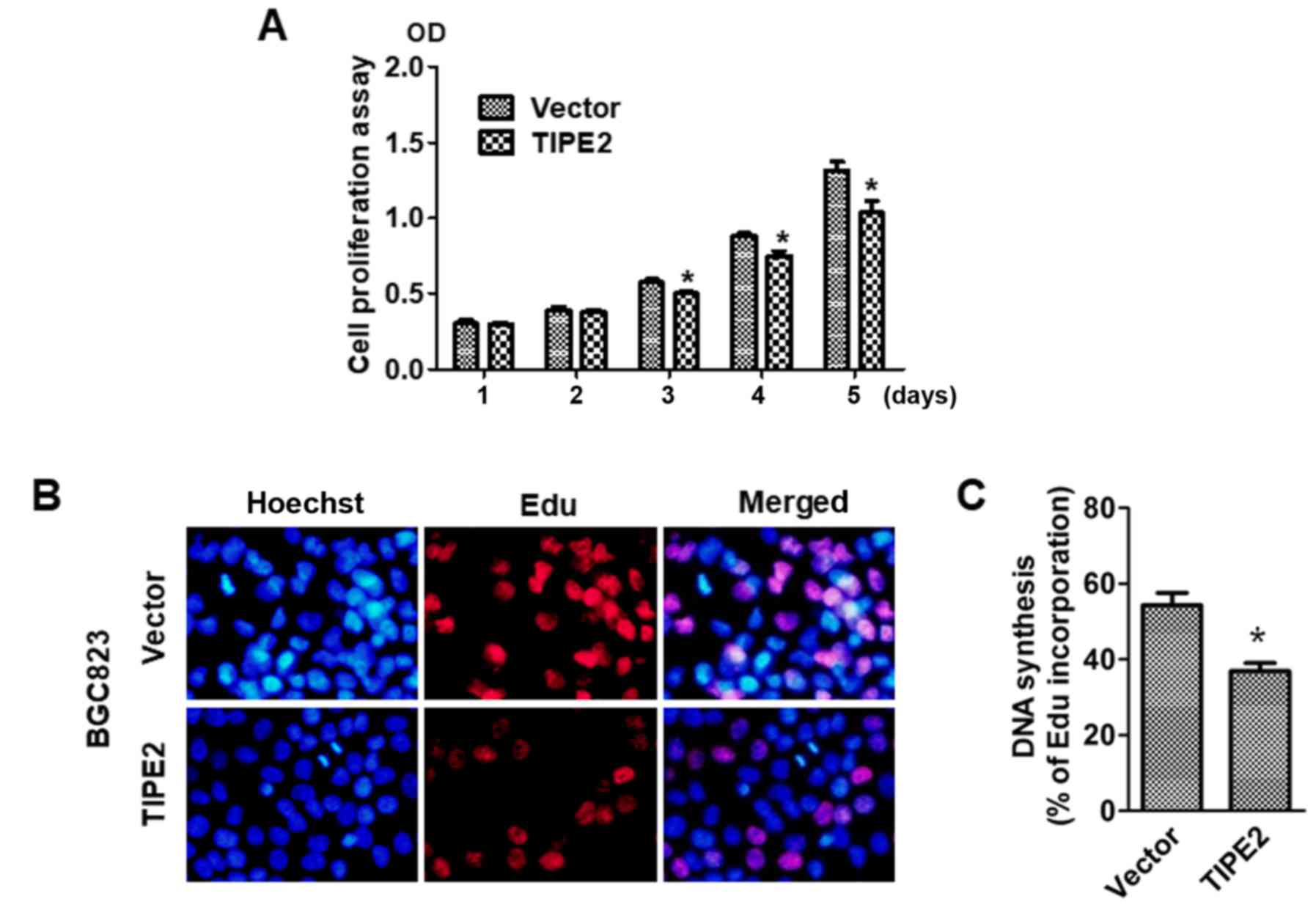
To explore the effect of TIPE2 on GC cell growth,
the viability of BGC823/Vector and BGC823/TIPE2 cells were assessed
at different time-points (days 1–5) using a CCK-8 assay. Compared
with the vector control group, TIPE2 significantly suppressed
BGC-823 tumor cell growth in vitro on day 3, days 4 and 5
after transfection in a time-dependent manner (P<0.05; Fig. 3A). Furthermore, in vivo
EdU-incorporation assays revealed that TIPE2 caused a marked
reduction in the proportion of BGC-823 human GC cells that
incorporated EdU (P<0.05; Fig. 3B
and C). Collectively, these results indicated that TIPE2
increased DNA synthesis and inhibited the proliferation of GC
cells.
TIPE2 causes G2/M phase cell-cycle
arrest in GC cells
To determine whether TIPE2 inhibited cell-cycle
progression in GC cells, BGC823/Vector and BGC823/TIPE2 cells were
cultured without serum for 12 h and then cultured in normal medium.
The cell cycle distribution was analyzed by flow cytometry after 24
h. The results revealed that the percentage of cells in the S phase
decreased from 33.16 to 27.36% and that the cells of the G2/M phase
increased from 8.53 to 15.23%, following TIPE2 overexpression in
BGC823 cells compared with the control group (Fig. 4A). These results indicated that
TIPE2 affected the cell cycle distribution in GC cells and could
block the cell cycle in the G2/M phase.
In order to further study the specific molecular
mechanism of TIPE2-induced G2/M phase arrest, we detected the
expression of AKT, p-AKT, ERK, p-ERK, CDK1, p-CDK1 and cyclin B1 in
GC cells after TIPE2 overexpression using western blotting. The
results revealed that TIPE2 overexpression could downregulate the
expression of p-AKT, p-ERK, CDK1 and cyclin B1 in GC cells and
upregulate the expression of p-CDK1 protein (Fig. 4B-E). These results indicated that
TIPE2-induced G2/M phase cell cycle arrest was associated with the
inhibition of p-AKT, p-ERK, CDK1 and cyclin B1, as well as the
promotion of p-CDK1 in the GC cell cycle.
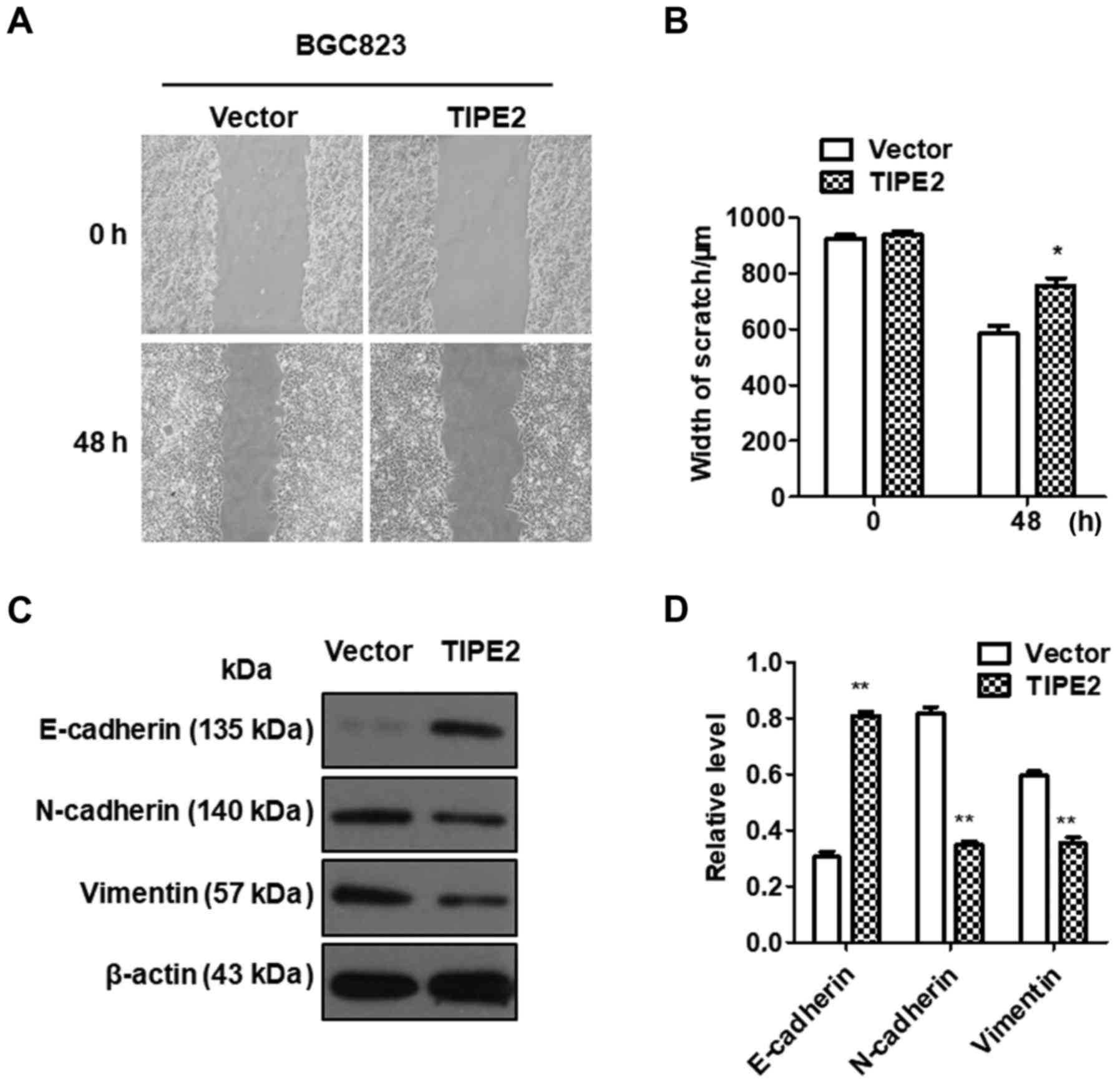
TIPE2 inhibits the migration of GC
cells
Tumor metastasis is an important hallmark of cancer,
resulting in ≤90% of cancer-associated deaths (1). To investigate the effect of TIPE2 on
GC migration in vitro, a scratch-wound assay was performed
using BGC-823/Vector and BGC-823/TIPE2 cells. As displayed in
Fig. 5A and B, TIPE2 overexpression
evidently inhibited the migration of BGC823 GC cells compared with
the control (P<0.05). Our data indicated that TIPE2 negatively
regulated GC cell motility.
Epithelial-mesenchymal transition (EMT) is an
important process by which a malignant tumor can obtain metastatic
ability (18,19). The mechanism by which TIPE2
inhibited tumor migration was determined by assessing the
expression changes in EMT-related factors. Western blot analysis
revealed that the expression of E-cadherin in the
TIPE2-overexpressing group was significantly upregulated compared
with the control group, while the expression of N-cadherin and
vimentin was inhibited, indicating that the effect of TIPE2 on cell
migration may occur via the regulation of the expression of
EMT-related factors in GC (Fig. 5C and
D).
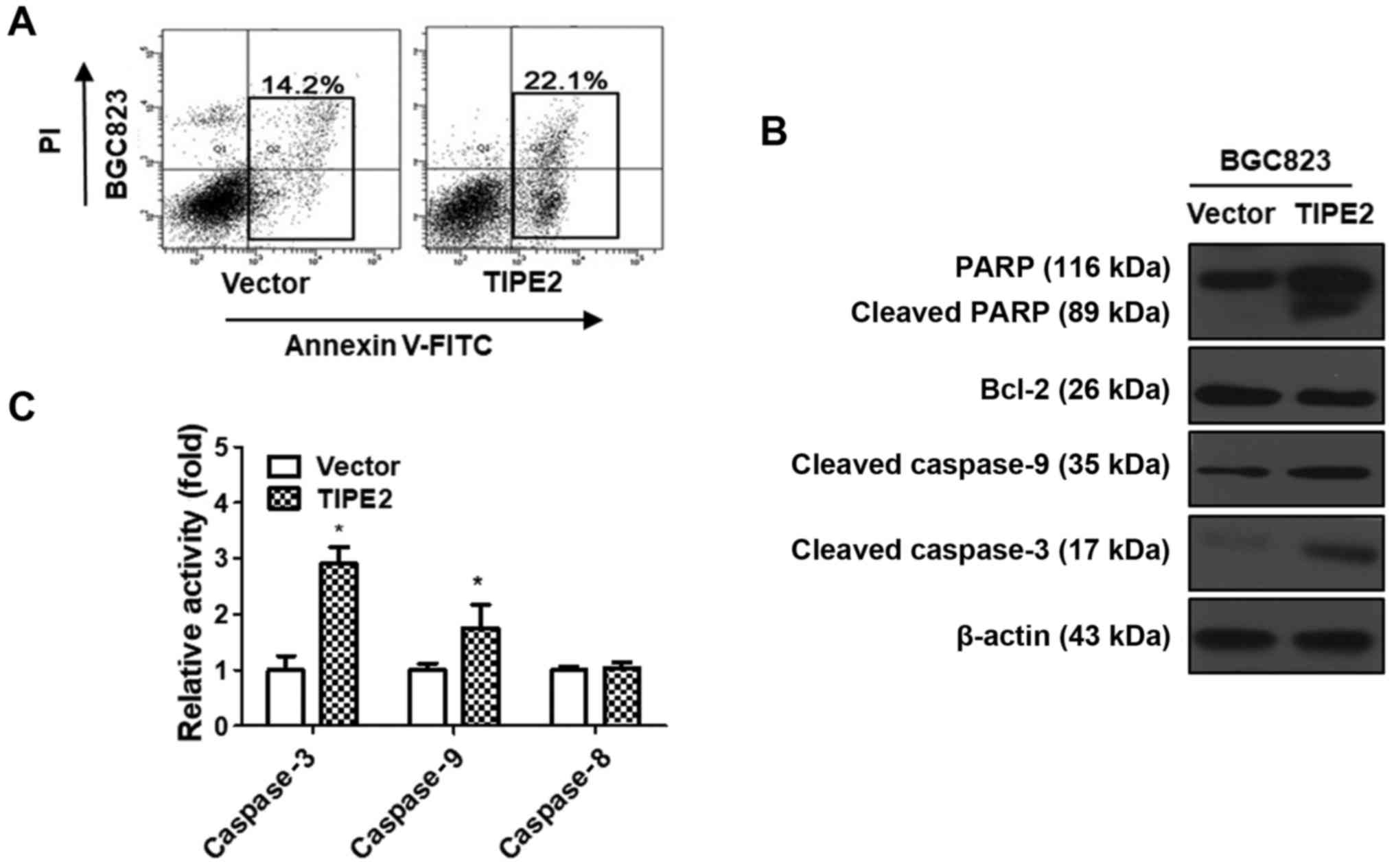
TIPE2 promotes GC cell apoptosis
The growth of cancer cells is affected by two major
factors, namely proliferation and apoptosis. The aforementioned
results indicated that TIPE2 inhibited BGC823 cell proliferation.
However, whether TIPE2 has a potential role on the apoptosis of GC
cells remained under question. Therefore, a TIPE2-overexpressing
vector was constructed and transiently transfected into the BGC823
GC cell line over 48 h. Following this, we conducted flow cytometry
to determine the effect of TIPE2 on BGC823 cell apoptosis. The
results revealed that the ratio of BGC823cells in early-stage and
late-stage apoptosis increased from 14.2 to 22.1% after TIPE2
overexpression (Fig. 6A). These
results indicated that TIPE2 could effectively promote the
apoptosis of BGC823 cells in vitro.
To elucidate the molecular mechanism responsible for
TIPE2-mediated apoptosis, the expression levels of
apoptosis-related proteins such as Bcl-2, caspase-9, caspase-3 and
PARP in TIPE2-transfected and vector-transfected BGC823 cells were
detected by western blot analysis. As displayed in Fig. 6B, TIPE2 overexpression clearly
upregulated cleaved caspase-9, cleaved caspase-3 and cleaved PARP,
as well as downregulated Bcl-2 in BGC823 cells. These results
revealed that TIPE2 promoted the apoptosis of GC cells, possibly
via activating the intrinsic apoptotic pathway.
To confirm whether TIPE2 affected the exogenous
apoptosis pathway, we detected the activity of caspase-3, caspase-8
and caspase-9 in TIPE2 transfected and vector-transfected BGC823
cells. As displayed in Fig. 6C, the
activity of caspase-3 and caspase-9 increased after TIPE2
overexpression, which was consistent with previous western blotting
results. However, there was no difference in the activity of
caspase-8 between TIPE2 transfected and vector transfected BGC823
cells. These results indicated that TIPE2 induced apoptosis through
the endogenous apoptosis pathway.
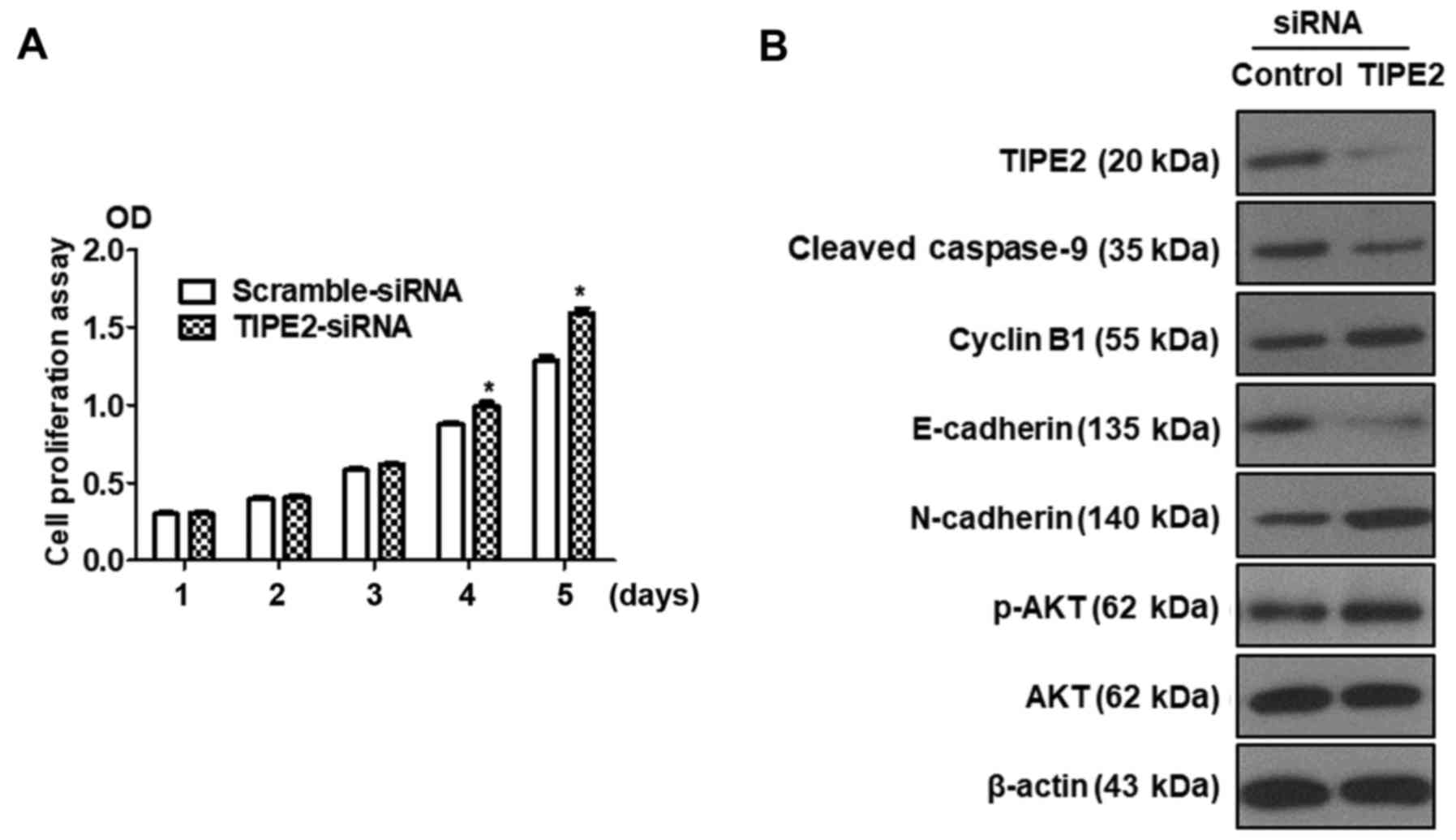
Silencing of TIPE2 promotes GC cell
proliferation and reverses the expression of related proteins
compared with TIPE2 overexpression
Previous results revealed that TIPE2 inhibited the
proliferation of BGC823 cells. Silencing of TIPE2 was performed to
further confirm the effect of TIPE2 on proliferation. BGC823 cells
were transfected with scramble siRNA or TIPE2-siRNA, and the
viability of BGC823 cells was assessed at different time-points
(days 1–5) by CCK-8 assay. As displayed in Fig. 7A, silencing of TIPE2 promoted
BGC-823 tumor cell growth in vitro on days 4 and 5. In
addition, we detected the expression of related proteins by western
blotting. As depicted in Fig. 7B,
the expression of TIPE2 was shut down following transfection with
TIPE2-siRNA, whereas the expression of p-AKT, N-cadherin and cyclin
B1 was upregulated and the expression of E-cadherin and
cleaved-caspase-9 was downregulated. These results were contrary to
the results obtained with TIPE2 overexpression. The aforementioned
results indicated that TIPE2 affected the function of GC cells not
only in proliferation but also in apoptosis, the cell cycle and
migration.
TIPE2 inhibits LPS-induced
inflammation
LPS can activate NF-κB signaling by initiating an
intracellular signaling cascade through the TLR4 pathway (20,21).
By controlling the expression of inflammation-related genes, the
activation of NF-κB plays a crucial role in enhancing the cellular
inflammatory response (21). To
verify the role of TIPE2 in LPS-induced inflammatory responses, we
examined the effect of TIPE2 on NF-κB transcription activity in
SGC7901 cells using a Dual-Luciferase Reporter Gene System
(Promega). The results revealed that NF-κB activity was
significantly enhanced following LPS induction, however the NF-κB
activity increase in the TIPE2-overexpression group was
significantly lower than that observed in the vector control group
(Fig. 8A). Activation of NF-κB
occurs when it is isolated from IκBα, and IκBα degrades after being
phosphorylated. We further detected the expression of IκBα, p-IKK
and p-p65 after LPS induction in SGC7901/vector and SGC7901/TIPE2
cells by western blotting. As displayed in Fig. 8B, the expression of p-IKK and p-p65
increased following LPS induction, but this was attenuated by
TIPE2. The activation of NF-κB induced a large number of
inflammatory factors such as IL-1β, IL-6 and TNF-α. To determine
whether TIPE2 could inhibit the NF-κB signaling pathway, the levels
of IL-1β, IL-6 and TNF-α were detected by ELISA after
LPS-stimulation in the SGC7901/vector and SGC7901/TIPE2 groups. The
results revealed that TIPE2 overexpression could inhibit
LPS-induced expression of the inflammatory cytokines IL-1β, IL-6
and TNF-α (Fig. 8C-E). These
results indicated that TIPE2 could inhibit LPS-induced inflammation
by suppressing the NF-κB signaling pathway. Notably, LPS could
upregulate the expression of TIPE2 in SGC7901/vector cells, but not
in SGC7901/TIPE2 cells (Fig. 8F),
which may be caused by the feedback of LPS-induced
inflammation.
Discussion
The causes of GC are complex and only partially
understood. Thus, many treatments indicated for GC are
controversial. Certain clinical studies have observed that, during
the development of GC, the expression profiles of specific
biomolecules change. Such changes in expression may serve a key
role in tumor progression, including the processes of cell
proliferation (٦,١٠,١٨,٢٢,23), motility (24), adhesion (25), apoptosis and tissue inflammation. As
an inflammatory inhibitor, TIPE2 not only induces cell death, but
also inhibits Ras-induced tumor formation, providing a molecular
bridge from inflammation to cancer. Research has revealed that
TIPE2 is an important negative regulator of inflammation and
carcinogenesis (26). Studies have
revealed that TIPE2 also plays an important role in non-immune
cells, including lung, stomach and liver cells (22,27).
Compared with healthy individuals, it was revealed TIPE2 expression
was significantly reduced in PBMCs from patients with chronic
hepatitis B, chronic hepatitis C and systemic lupus erythematosus
(14,15,27).
In the present study, we proposed a model to
describe the function of TIPE2 in carcinogenesis. In gastric
epithelium, inflammation induced by stimulatory reagents such as
LPS (or pathogens such Helicobacter pylori) leads to
increased cell apoptosis, and TIPE2 is necessary for this process.
This could be a mechanism to eliminate cells that have been exposed
to excessive free radicals generated by inflammation, and therefore
to prevent the damaged cells from surviving and proliferating. Upon
the reduction of TIPE2 expression, however, this process is
inhibited and damaged cells have a better chance of survival and
proliferation, thereby accumulating genomic mutations and
eventually leading to carcinogenesis.
According to our clinical and biochemical data, the
expression of TIPE2 in GC tissues was reduced or absent, and may be
related to the occurrence and development of GC. TIPE2 inhibited
the proliferation of GC cells by inhibiting p-AKT and p-ERK, thus
inhibiting the PI3K-AKT and Ras-Raf-MEK-ERK1/2 signaling pathways.
In addition, TIPE2 induced G2/M-phase arrest by downregulating the
expression of CDK1 and cyclin B1, while upregulating the expression
of p-CDK1. TIPE2 inhibited the expression of Bcl-2 and increased
the levels of cleaved-caspase-9 and cleaved-caspase-3, which
promote endogenous apoptosis. Furthermore, TIPE2 inhibited the
activation of NF-κB induced by LPS, as well as the expression of
IL-1β, IL-6 and TNF-α, reducing LPS-induced cell proliferation.
The invasion and metastasis of tumors as the leading
cause of death in cancer increases not only the suffering of
patients, but also the difficulty of clinical treatment. EMT is an
effective way for epithelial cells to acquire migratory capacity
(18,19), it has become an important pathway
for invasion and metastasis of epithelial cell carcinoma. Various
studies have shown that the development of EMT is accompanied with
changes in markers, including E-cadherin, N-cadherin and vimentin.
In the present study, our results revealed that TIPE2 significantly
inhibited the migration of GC cells accompanied with E-cadherin
upregulation and N-cadherin and vimentin downregulation, thus
indicating a new molecular mechanism by which TIPE2 regulated cell
migration in gastric cells.
In conclusion, we presented TIPE2 as a potential
therapeutic target for GC, and our results revealed that it
functioned by reducing the migration and proliferation of GC
cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants obtained
from the Public Projects of Fujian Province (2016R1034-3).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZQ, GZ and ZL researched idea and study design. ZL,
WL, CX and YF performed data collection. ZQ, GZ, ZL and CX analyzed
and interpreted data. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Written informed consent for the study was provided
by all participants. The study was approved by the Medical Ethics
Committee of the Zhongshan Hospital of Xiamen University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TIPE2
|
tumor necrosis factor-α-induced
protein-8 like-2
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
CDK
|
cyclin-dependent kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
CKI
|
cyclin-dependent kinase inhibitor
|
|
CCK-8
|
Cell Counting Kit-8
|
|
ERK
|
extracellular regulated protein
kinases
|
|
PI
|
propidium iodide
|
|
LPS
|
lipopolysaccharide
|
|
IKK
|
inhibitor of nuclear factor κB
kinase
|
|
DED
|
death effector domain
|
|
MMP-13
|
matrix metalloproteinase-13
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
AP-1
|
activator protein 1
|
|
TNM
|
tumor node metastasis
|
|
PARP
|
poly ADP-ribose polymerase
|
|
H&E
|
hematoxylin-eosin staining
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
PIKK
|
phosphatidylinositol 3-kinase related
kinase
|
|
PS
|
phosphatidylserine
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA: Cancer J Clin.
61:69–90. 2011.PubMed/NCBI
|
|
2
|
Fang JY, Cheng ZH, Chen YX, Lu R, Yang L,
Zhu HY and Lu LG: Expression of Dnmt1, demethylase, MeCP2 and
methylation of tumor-related genes in human GC. World J
Gastroenterol. 10:3394–3398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen CN, Lin JJ, Chen JJ, Lee PH, Yang CY,
Kuo ML, Chang KJ and Hsieh FJ: Gene expression profile predicts
patient survival of GC after surgical resection. J Am Soc Clin
Oncol. 23:7286–7295. 2005. View Article : Google Scholar
|
|
4
|
Nathan C and Ding A: Nonresolving
inflammation. Cell. 140:871–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun HH, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang K, Ren Y, Liu Y, Zhang J and He JJ:
Tumor necrosis factor (TNF)-alpha-induced protein 8-like-2 (TIPE2)
inhibits proliferation and tumorigenesis in breast cancer cells.
Oncol Res. 25:55–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Wang J, Fan C, Li H, Sun H, Gong
S, Chen YH and Shi Y: Crystal structure of TIPE2 provides insights
into immune homeostasis. Nat Struct Mol Biol. 16:89–90. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y,
Qu Z, Cui J, Zhang G, Liang X, et al: Roles of TIPE2 in hepatitis B
virus-induced hepatic inflammation in humans and mice. Mol Immunol.
48:1203–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma
C, Chen YH and Zhang L: The unique expression profile of human
TIPE2 suggests new functions beyond its role in immune regulation.
Mol Immunol. 48:1209–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fayngerts SA, Wang ZJ, Zamani A, Sun H,
Boggs AE, Porturas TP, Xie W, Lin M, Cathopoulis T, Goldsmith JR,
et al: Direction of leukocyte polarization and migration by the
phosphoinositide-transfer protein TIPE2. Nat Immunol. 18:1353–1360.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun H, Zhuang G, Chai L, Wang Z, Johnson
D, Ma Y and Chen YH: TIPE2 controls innate immunity to RNA by
targeting the phosphatidylinositol 3-kinase-Rac pathway. J Immunol.
189:2768–2773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Y, Tao M, Wu J, Meng Y, Xu C, Tian Y,
Zhou X, Xiang J, Zhang H and Xie Y: Adenovirus-directed expression
of TIPE2 suppresses GC growth via induction of apoptosis and
inhibition of AKT and ERK1/2 signaling. Cancer Gene Ther.
23:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Q, Zhao M, Dong T, Zhou C, Peng Y,
Zhou X, Fan B, Ma W, Han M and Liu S: Tumor necrosis
factor-α-induced protein-8 like-2 (TIPE2) upregulates p27 to
decrease gastic cancer cell proliferation. J Cell Biochem.
116:1121–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao XL, Zhang L, Shi YY, Sun Y, Dai S, Guo
C, Zhu F, Wang Q, Wang J, Wang X, et al: Human tumor necrosis
factor (TNF)-alpha-induced protein 8-like 2 suppresses
hepatocellular carcinoma metastasis through inhibiting Rac1. Mol
Cancer. 12:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XM, Su JR, Yan SP, Cheng ZL, Yang TT
and Zhu Q: A novel inflammatory regulator TIPE2 inhibits
TLR4-mediated development of colon cancer via caspase-8. Cancer
Biomarkers. 14:233–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruan H, Zhan YY, Hou J, Xu B, Chen B, Tian
Y, Wu D, Zhao Y, Zhang Y, Chen X, et al: Berberine binds RXRα to
suppress β-catenin signaling in colon cancer cells. Oncogene.
36:6906–6918. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He K, Chen D, Ruan H, Li X, Tong J, Xu X,
Zhang L and Yu J: BRAFV600E-dependent Mcl-1 stabilization leads to
everolimus resistance in colon cancer cells. Oncotarget.
7:47699–47710. 2016.PubMed/NCBI
|
|
18
|
Yao Y, Wang ZC, Liu JX, Ma J, Chen CL,
Deng YK, Liao B, Wang N, Wang H, Ning Q, et al: Increased
expression of TIPE2 in alternatively activated macrophages is
associated with eosinophilic inflammation and disease severity in
chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol.
7:963–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Wang X, Li Y, Sun H, Wan L, Wang X,
Zhang L, Fang Z and Wei Z: The decreased expression of TIPE2
protein in the decidua of patients with missed abortion and
possible significance. Reprod Biol Endocrinol. 15:682017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Zhang Y, Li F, Zhu X and Huang L:
Negative immune regulatory molecule tipe2 for treating sle mice
through regulating macrophage subtype. Chongqing Med. 2017.
|
|
21
|
Zhang Y, Mei S, Zhou Y, Yang D, Pan T,
Chen Z and Wang Q: TIPE2 negatively regulates mycoplasma
pneumonia-triggered immune response via MAPK signaling pathway. Sci
Rep. 7:133192017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu RL, Fan TT, Geng WW, Chen YHH, Ruan QG
and Zhang C: Negative immune regulator TIPE2 promotes M2 macrophage
differentiation through the activation of PI3K-AKT signaling
pathway. PLoS One. 12:e01706662017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi-Bai Z, Rui-Min L, Ying-Chuan S, Jie Z,
Chao J, Can-Hua Y, Xi C and Wen-Wei Q: TIPE2 expression is
increased in peripheral blood mononuclear cells from patients with
rheumatoid arthritis. Oncotarget. 8:87472–87479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang JS, Wang SS, Fang J, Xu Y, Tong L,
Ye X and Zhou W: Stable silencing of TIPE2 reduced the Poly
I:C-induced apoptosis in THP-1 cells. Mol Med Rep. 16:6313–6319.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Liu L, Cao S, Zhu Y and Mei Q:
Gene delivery of TIPE2 inhibits breast cancer development and
metastasis via CD8+ T and NK cell-mediated antitumor
responses. Mol Immunol. 85:230–237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang G, Zhang W, Lou Y, Xi W, Cui J, Geng
M, Zhu F, Chen YH and Liu S: TIPE2 deficiency accelerates neointima
formation by downregulating smooth muscle cell differentiation.
Cell Cycle. 12:501–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kong L, Liu K, Zhang YZ, Jin M, Wu BR,
Wang WZ, Li W, Nan YM and Chen YH: Downregulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
chronic hepatitis C. Hepato Int. 7:844–849. 2013. View Article : Google Scholar
|