Introduction
Testicular germ cell tumors (TGCTs) represent the
most common type of solid malignancy among young men between the
ages of 20 and 40 (1). TGCTs are
highly curable malignancies, with current cure rates exceeding
70–80%, even in patients with disseminated disease. However, there
are patients with metastatic testicular cancer who do not achieve a
durable complete remission with cisplatin-based chemotherapy.
Surgical resection of residual masses is necessary to achieve
long-term disease control in these patients. The presence of
teratoma components in these residual masses has important clinical
consequences. Teratoma in the retroperitoneum may grow, which is a
structure characterized by enlarging metastatic masses without
marker elevation, termed growing teratoma syndrome. Alternatively,
patients may develop high-grade malignant components, including
sarcomas and primitive neuroectodermal tumors (2,3). In
addition, only 20–25% of the patients progressing/relapsing after
first-line chemotherapy can be cured by standard dose or high-dose
chemotherapy with autologous hematopoietic stem-cell rescue
(4–6). Patients who fail to achieve remission
following conventional therapy have an extremely poor prognosis and
the vast majority of them will eventually die of the disease
(7–9). Therefore, a better understanding of
resistance to chemotherapy together with developing novel
therapeutic strategies based on the molecular and genetic
characteristics of the tumor is required (7).
In order to achieve an effective prediction of
clinical efficacy of new approaches in cancer drug development,
more realistic models reflecting the heterogeneity of patients are
required. Established cancer cell lines and monocellular layers of
tumor cells cultivated in vitro, as well as mouse xenografts
derived from those tumor cells have been used as the standard
toolkit in preclinical drug evaluation and biomarker
identification. However, despite several advantages, recent data
suggests that the cells grown in culture and xenografts derived
from these expanded cells adapted to growth in artificial culture
conditions have principally diverged from the primary tumors from
which they were derived (10–12).
Patient-derived xenografts (PDXs) are tumor models
based on transplanting surgically resected tumor tissue samples
from donors directly into immunodeficient mice. In comparison with
cell line culture, PDXs maintain more similarities to the parental
tumors and therefore serve as a more realistic preclinical model
with strong predictive power in the translation of new anti-cancer
agents to clinical practice (13,14).
PDXs retain histology as well as the gene expression profiles of
their donor tumors (15,16). The correlation between PDX tumors
and clinical response to chemotherapy has also been described
(17). In order to investigate
treatment failure in patients with TGCT, there has been increasing
interest in the establishment of PDX models that realistically
model the disease and have the potential to predict clinical
outcomes for novel therapeutic strategies (18). Several research groups have
attempted to establish PDX models derived from testicular cancer.
Abraham et al xenografted human non-seminomatous germ cell
tumors into SCID mice (19). The
study carried out by Castillo-Avila et al established
orthotopic models for cisplatin sensitive non-seminomas, as well as
for cisplatin-resistant choriocarcinoma variants induced in mice.
Notably, only non-seminomatous tumors (predominantly
choriocarcinomas, embryonal carcinoma, yolk sac tumor or mixed
TGCTs) were successfully implanted in mice. No pure seminomas that
presented as primary tumors have been grown as xenografts (20). To the best of our knowledge, only
the study by de Vries et al established a PDX model from a
metastatic lesion of a patient with refractory testicular cancer
(21). The engraftment of tissue
samples obtained from the metastatic lesions of patients with
progressive disease after standard cisplatin-based chemotherapy
treatment represents an ideal model system for studying mechanisms
implicated in resistance to cisplatin, as well as in clarification
of the mechanisms involved in growing teratoma syndrome.
In the present study, we aimed to establish and
characterize testicular cancer patient-derived xenografts, from
retroperitoneal lymph nodes infiltrated with TGCTs after
cisplatin-based chemotherapy administration. We present our data
demonstrating that two successfully implanted patient samples
diverged from the original tumor histology and in vivo
passage resulted in terminal differentiation into lymphoma and
plasmocytoma. Consequently, these samples could not be serially
propagated in vivo.
Materials and methods
Study patients
As a part of an ongoing translational study
(protocol IZLO1; chair, M. Mego) TGCT patients who underwent
retroperitoneal lymph node dissection between April 2015 and August
2015 at the National Cancer Institute of Slovakia were enrolled.
Patients with concurrent malignancies other than non-melanoma skin
cancer in the previous 5 years were excluded from the study. Data
regarding age, tumor histological subtype, clinical stage,
histological subtype and number of metastatic lesions, previous
chemotherapy were recorded for all patients. The Institutional
Review Board of the National Cancer Institute (Bratislava,
Slovakia) approved the present study and all patients provided
written informed consent.
Tissue sampling
Clinical samples for the PDX tumor model were
obtained immediately after resection and transferred into RPMI-1640
culture medium on wet ice for engraftment within 24 h. Tumor tissue
collected from each patient was separated into three sections under
sterile conditions. One section was cryopreserved in liquid
nitrogen and stored at −80°C; the second was fixed in 10% neutral
buffered formalin solution and embedded in paraffin for
histopathological analysis of the implanted tumor; and the third
portion of the tumor (representing F0 generation) was used for
engraftment in SCID beige male mice. Baseline characteristics of
donor patients are provided in Table
I.
 | Table I.The baseline characteristics of donor
patients enrolled in establishment of the testicular cancer-derived
xenograft model. |
Table I.
The baseline characteristics of donor
patients enrolled in establishment of the testicular cancer-derived
xenograft model.
|
|
|
|
|
|
|
|
| F1 mouse |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Age | Sex | Chemotherapy | Histological
subtype of TGCT | Stage of TGCT | Site of mts | Histology of RPLN
mts | Strain | Implanted site | Duration time in F1
(days) | Histology |
|---|
| TGCT_001 | 46 | Male | BEP, VIP, HDC | Seminoma | III.A | RPLN mts | Seminoma | SCID | Subcutaneous | 64,80 | DLBCL |
| TGCT_002 | 30 | Male | BEP | Nonseminoma EC,
YST, TER, SEM components | II.B | RPLN mts |
Teratocarcinoma | SCID | Subcutaneous | 56,116 | Plasmocytoma |
| TGCT_003 | 45 | Male | BEP, 2nd line
TIP | Seminoma | I.A | RPLN mts | Seminoma | SCID | Subcutaneous | NA | No growth |
Establishment of the patient
tumor-derived xenografts
Fresh tumor tissue was mechanically dissociated into
small pieces in 0.5 ml RPMI-1640 cultivation media. Suspension was
collected into a sterile syringe and passed through an 18G needle
three times. The final volume was determined and an equivalent
volume of ECM (ECM Gel from Engelbreth-Holm-Swarm murine sarcoma;
cat. no. 1270; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
added on ice.
Six to eight weeks old SCID beige male mice (CD17
Cg-Prkdscid Lystbg/Crl) were bilaterally subcutaneously injected
with 150–200 µl suspension. The mice were housed under controlled
environmental conditions (24±2°C, relative humidity 40–60% and a
12-h light/dark cycle). Animals had free access to food and water
ad libitum and were regularly monitored for their weight,
the presence of any pathological conditions and tumor growth. Tumor
volume was determined based on the caliper measurements of two
perpendicular diameters and calculated according to the following
formula: Volume=length × width2/2. Animals were
sacrificed when any of the tumor diameters exceeded 10 mm or tumor
xenografts exhibited signs of necrosis and bleeding. Tumor
xenografts were collected for further analysis. Each tumor was
divided into two: One section was fixed in buffered formalin and
embedded in paraffin for histological and immunohistochemical
analyses and the other was submerged in PBS for preparation of the
suspension as described above. An aliquot from the suspension was
used for DNA isolation.
Animal experiments were performed in the approved
animal facility (license number SK PC 14011), as approved by the
Institutional Ethics Committee and by the national competence
authority (State Veterinary and Food Administration of the Slovak
Republic; registration no. Ro 3108/14-221). Experiments were
performed in compliance with the Directive 2010/63/EU of the
European Parliament and the European Council and the Regulation
377/2012 on the protection of animals used for scientific
purposes.
Histological and immunohistochemical
evaluation of PDX tumors
Histological examinations were based on paraffin
sections using H&E, Giemsa and PAS staining and Gomori's silver
impregnation staining method to determine the growth pattern and
tumor cell histopathology.
For determination of the tumor phenotype, acomplex
panel approach was used. This initially involved the detection of
i) lymphoid B-cell markers (PAX5, CD20, CD138, kappa and lambda Ig
light chains) and T-cell markers (CD3, CD5), as well as ii)
placental alkaline phosphatase (PLAP) and cytokeratins (using
AE1/AE3, CK7 and CK20 antibodies) in both further documented PDX
tumors. Following this, more detailed phenotypic analysis to
contribute to the diagnosis of both cases was performed, including
evaluation of the expression of antigens Bcl-2, BCL6, CD10,
MUM1/IRF-4, CD30, CD15, CD56, as well as latent membrane protein
(LMP) Epstein-Barr virus. Deparaffinization, rehydration and target
retrieval with the Target Retrieval Solution High pH (pH 9.0; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) or EDTA (pH 9.0)
at 96°C was performed in the PT Link (Dako PT100; Dako; Agilent
Technologies, Inc.). Slides were subsequently processed on the
Autostainer Link 48 (Dako AS480; Dako; Agilent Technologies, Inc.)
using an automated staining protocol validated for the individual
antibodies, or mechanically using kit EnVision FLEX (Agilent
Technologies, Inc.), High pH (Link) in agreement with the
manufacturer's recommendations. Reagents utilized in addition to
the mentioned components included a FLEX antibody diluent, FLEX
wash buffer and a hematoxylin counterstain (Dako; Agilent
Technologies, Inc.). IHC-stained slides were mounted in
non-aqueous, permanent mounting media.
DNA extraction and qPCR analysis
The ISOLATE II FFPE RNA/DNA kit (Bioline, London,
UK) was used for the isolation and purification of genomic DNA from
formalin-fixed paraffin-embedded tissue samples. We proceeded
according to the manufacturer's instructions. The isolated genomic
DNA was analyzed by qPCR. Reactions were performed using a Maxima
probe qPCR Master Mix (Fermentas; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). qPCR was carried out with a CFX96 thermocycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the
following thermocycling parameters: Pre-treatment at 50°C 2 min,
initial denaturation at 95°C for 10 min, followed by 50 cycles at
95°C for 20 sec and 60°C for 1 min. The plate was then read. Final
annealing was peformed at 60°C for 10 min followed by a cooling
step to 7°C. The specific primers and probes used in duplex qPCR
are described in Table II. The
mouse Rapsyn (receptor-associated protein at the synapse) gene was
used as an internal control. The quantity of the PCR product was
calculated by the Cq value (22).
The positive control DNA was isolated from human breast cancer
cells MDA-MB-231 (ATCC® HTB-26™; ATCC, Manassas, VA,
USA). EGFP-expressing MDA-MB-231 were kindly provided by Dr
Miroslava Matuskova (23). DNA
isolated from the mouse cell line NIH/3T3 (ATCC®
CRL-1658™; ATCC) was used as a negative control. Evaluation of
human specificity of β-globin amplification was demonstrated using
a 2-fold dilution for 100 to 0 ng human gDNA per assay in PCR grade
water, and in 0 to 100 ng murine gDNA per assay. The quantity of
human gDNA was calculated according to the calibration curve
prepared as stated above. The amount of human β-globin was related
to 150 ng total DNA.
 | Table II.Sequences of primers used in this
study. |
Table II.
Sequences of primers used in this
study.
| RAPSYN sense |
|
5′-ACAATGCCATCAACCTTAGC−3′ |
| RAPSYN
antisense |
|
5′-GTGAGTGAGGCAGGTTCATT-3′ |
| RAPSYN probe | 3′-BHQ-1
5′-JOE |
5′-AGAATTATCTGACCCACCCATCCTGC-3′ |
| β-globin sense |
|
5′-CTAAGCCAGTGCCAGAAGAG-3′ |
| β-globin
antisense |
|
5′-CTCTGCCCTGACTTTTATGC-3′ |
| β-globin probe | 3′-BHQ-1
5′-FAM |
5′-ACGGCTGTCATCACTTAGACCTCACC-3′ |
Results
Baseline characteristics
Tumorspecimens from retroperitoneal lymph nodes
infiltrated with TGCTs from three testicular cancer patients were
engrafted into SCID mice in order to establish a PDX model. The
summary of donor patient characteristics is presented in Table I. The median age of patients
enrolled in this study was 45 years (range, 30–46 years). Histology
of the primary tumors determined the following subtypes: Seminomas
(cases TGCT_001 and TGCT_003) and non-seminoma with embryonal
carcinoma, yolk sac tumor, immature teratoma and seminoma
components (case TGCT_002). All patients were pretreated with
cisplatin-based chemotherapy. In one patient, a retroperitoneal
lymph node biopsy was performed prior to starting second line
chemotherapy (case TGCT_001) to histologically confirm disease
recurrence, while other patients underwent retroperitoneal lymph
node dissection after finishing first line chemotherapy (case
TGCT_002) and salvage chemotherapy (case TGCT_003). The histology
of post chemotherapy retroperitoneal lymph node dissection
specimens from cases TGCT_001 and TGCT_003 representing F0
generation determined that the histological subtype was correlated
with primary tumor histology (immunohistochemical positivity for
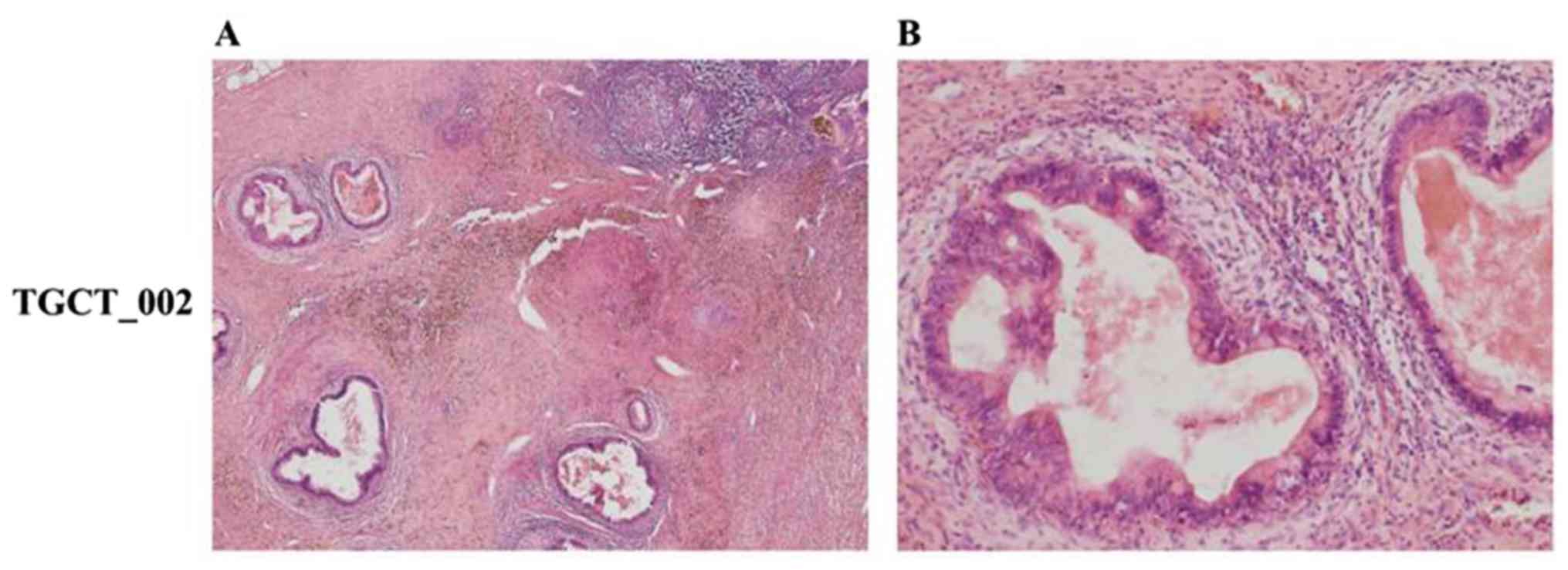
PLAP and CD117 and negative for CD30), while in case TGCT_002, RPLN
metastasis was derived only from a teratocarcinoma component
(immunohistochemical positivity for CK20, CEA, CDX2 and negative
for CK7) (Figs. 1 and 2).
Establishment of PDX models
To establish PDX models from fresh tumor tissue
samples, they were mixed with ECM gel and subcutaneously engrafted
into F1 mice. A total of 3 retroperitoneal lymph node metastases
tissue sample were xenografted. Since these tissue samples were
residual tumors in patients who underwent cisplatin-based
chemotherapy, we supposed that they also contained tumor cells
resistant to cisplatin. The baseline histological and clinical
characteristics of the successfully implanted tissue samples of
patients are summarized in Table I.
In all cases, the post-chemotherapy treatment tissue samples
representing F0 generation were grafted. Growing xenografts were
established from the two out of the three inoculated patient
samples. The first sample, designated TGCT_001, originated from a
retroperitoneal metastatic seminoma and produced four tumor
xenografts. These were subsequently injected into F2 mice as
schematically depicted in Fig. 3A.
The first two tumors were dissociated and injected 64 days after
the experiment start date, and produced 3 tumors out of 4
inoculates. The other two tumors were dissociated and injected 80
days post-implantation, but did not produce any tumors. In contrast
to the first injection, when exponential growth was observed in all
four tumors after 40–55 days post-injection, the second injection
produced only one exponentially growing xenograft (Fig. 3B and C). The other two injections
produced palpable persistent tumor masses of stable volume.
Subsequent inoculation of the suspension derived from these three
xenografts did not produce any tumors in the next in vivo
passage.
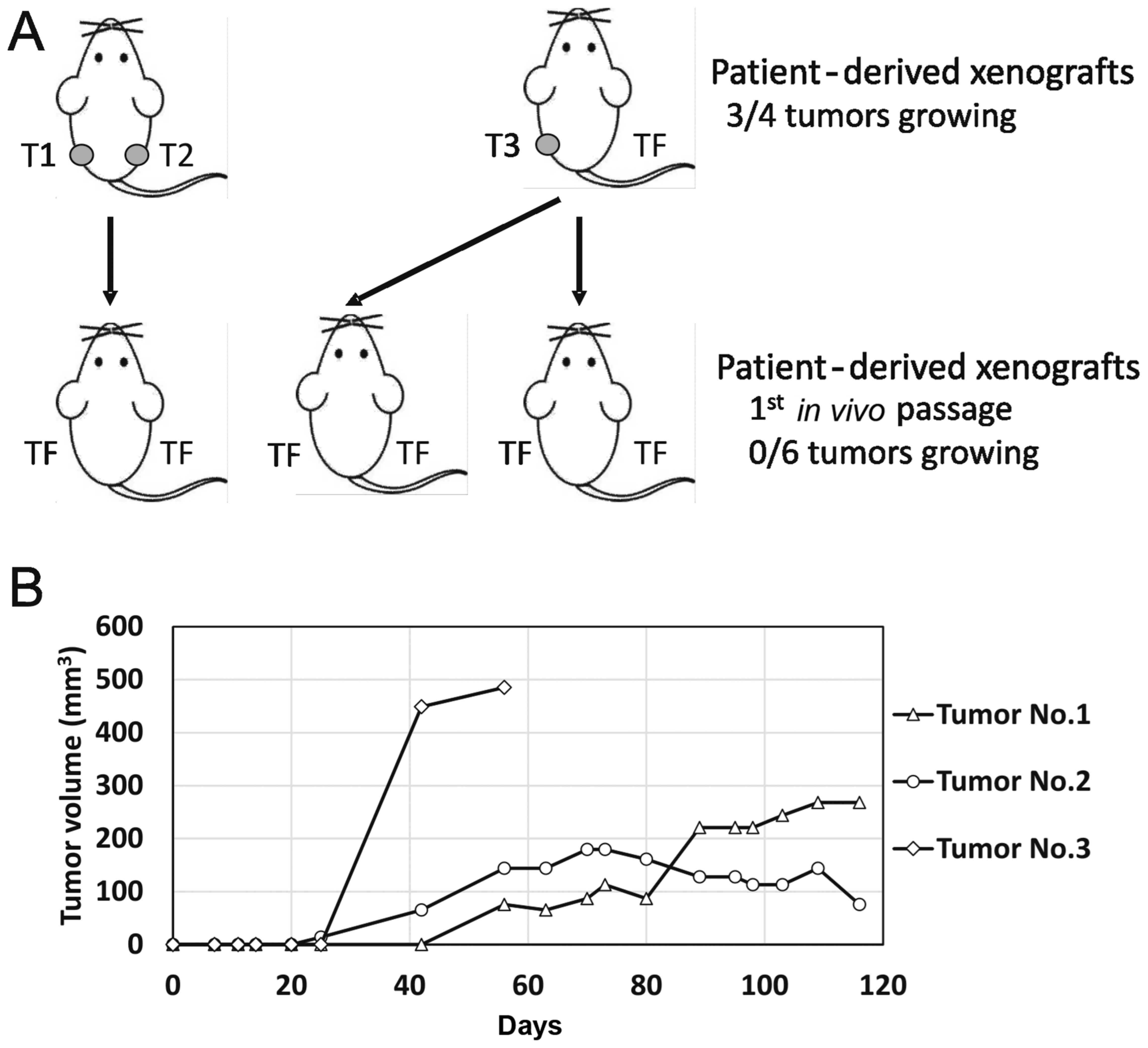
The second sample, designated TGCT_002, originated
from a retroperitoneal metastatic mixed germ-cell tumor with
embryonal carcinoma, yolk sac, teratoma and seminoma components.
This sample produced three tumor xenografts. Exponentially growing
tumorsweresubsequently injected into F2 mice 56 days after the
experiment start date, as schematically depicted in Fig. 4A. The two other tumors were
dissociated and injected 116 days post-implantation, but none of
the inoculations produced any tumor. We observed exponential growth
25 days post-injection in this case, in one tumor only (Fig. 4B). The other two injections produced
palpable persistent tumor masses that changed volume at an
extremely slow rate. Subsequent inoculation of the suspension
derived from these three xenografts did not produce any tumors in
the next in vivo passage.
Molecular analysis confirmed the presence of human
specific β-globin sequences in both successfully engrafted samples
(Fig. 5).
Histological and immunohistochemical
analysis of PDX tumors
Histological and immunohistochemical analysis of
established PDX tumors demonstrated that the original histology was
not maintained in any of the cases. Both successfully engrafted
tumor tissues obtained from F1 mice displayed a lymphocytic
dominant pattern, regardless of the tumor histology of patients
(seminoma vs. mixed germ cell tumor). These cases were analyzed to
determine their detailed histological and immunohistochemical
features.
The histological analysis of case no. TGCT_001
exhibited diffuse proliferation of prevailing medium-sized lymphoid
mononuclear blasts of centroblastic and immunoblastic morphology,
with few intermingled and dispersed multinucleated giant blasts
resembling Sternberg-Reed cells. Irrespective of the tumor cell
size, all cells expressed immunohistochemical positivity for PAX5,
CD20, CD30 (positivity appeared in >70% of the mononuclear
blasts and multinucleated giant cells), Bcl-2 and MUM1/IRF-4
together with a high proliferation rate (Ki-67 index, ~70%). By
contrast, they were negative for CD3, CD5, CD15, BCL6, CD10, CD138,
PLAP and cytokeratins. Twenty to thirty percent of tumor cells
co-expressed p53 protein and LMP, and ~40% of tumor cells also had
Myc protein nuclear positivity (Fig.
6). According to all these results, we may conclude that PDX
tumor no. TGCT_001 represented an anaplastic variant of a
CD20+/CD30+ diffuse large B-cell
lymphoma.
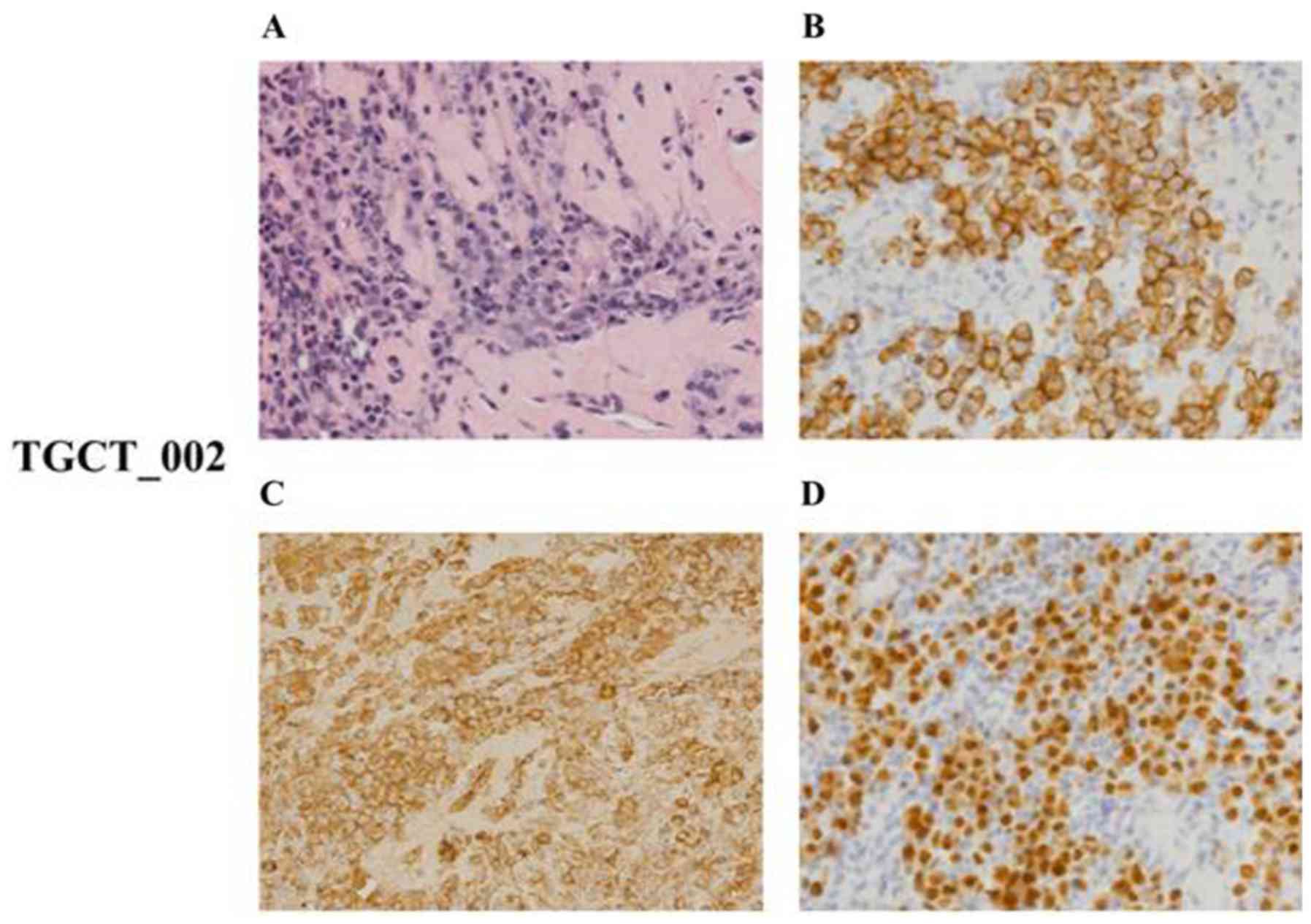
The case no. TGCT_002 was characterized by diffuse
infiltration of uniform cells with the morphology of mature,
predominantly mononuclear plasma cells of Marschalko-type. These
cells presented with an immunohistochemical phenotype of clonal
plasmacytic proliferation with cytoplasmic Ig of l light chain
type, were positive for CD138 and MUM1/IRF-4, and negative for
PAX5, CD20, CD3, CD56 and CK20 (Fig.
7). The Ki-67 proliferation index was low (<5%). The
described morphological and phenotypic patterns allowed us to
conclude that the analyzed tissue sample was infiltrated with tumor
cells corresponding to the plasmocytoma of grade 1 expressing l
light chain restriction.
Discussion
PDX models that realistically reflect patient
heterogeneity and retain tumor biology represent a novel approach
in the development of new treatment strategies. PDXs may also shed
light on the pathways underlying the response of cancer cells to
different treatment modalities, and enable the development of
optimized combination therapies to overcome therapeutic
resistance.
The aim of the present study was to establish and
characterize TGCT patient-derived xenografts. These originated from
retroperitoneal lymph node metastases infiltrated with TGCTs
following previous cisplatin-based chemotherapy, in order to
analyze novel treatment options for cisplatin-resistant testicular
tumors. To the best of our knowledge, only a limited number of
studies have investigated this field of TGCTs. The engraftment
efficacy of primary xenotransplants in our patient cohort was 67%.
Several studies have analyzed factors associated with the
successful engraftment of PDX tumors. It was demonstrated that PDX
models derived from metastatic lesions have a higher engraftment
rate, in comparison to PDXs derived from primary tumors (24,25).
In addition, a reduction of ischemic time and overall procedure
time appearto be significant experimental parameters affecting
successful implantation (12).
Likewise, implantation in the same organ as the original tumor
(orthotopic implantation), where the tumor develops in the same
anatomical microenvironment, may also affect the success of PDX
model establishment (26).
Furthermore, using ECM as well as severe immunocompromised SCID
mice has been demonstrated to improve successful engraftment in
various cancer types (27–29). However, another study revealed that
these factors, together with preoperative chemotherapy, did not
have any significant influence on engraftment (12). The elapsed time for the successful
engraftment in F1 animals was 40–55 days in the first case, and
25–40 days in the other case. In both cases, histological
characteristics of the primary tumor were not retained, and
lymphoma transformation was observed.
In clinical practice, the surgical resection of
residual lymph nodal masses infiltrated with TGCTs after primary
chemotherapy is usually performed. Histologically, these surgical
specimens may contain mature teratoma in ~30–40% of cases, fibrosis
or necrosis in 48% of cases and cells in 10–20% of the patients
(30–32). These findings are consistent with
our results, where a surgical specimen obtained from the
retroperitoneal lymph node metastasis of patient TGCT_002 was
histologically characterized as teratocarcinoma. Notably, the study
published by Brandli et al reported that the fibrous stromal
cells adjacent to residual metastatic mature teratoma in lymph node
specimens after chemotherapy have genetic abnormalities, similar to
adjacent metastatic teratoma. These results indicated that both
tumor components are derived from the same element of the original
germ cell tumor or the same progenitor cell (33). Based on these data, we suggest that
the transformation of the TGCTs PDX samples toward
plasmocytoma/lymphoma observed in our study may be explained by the
presence of totipotent EC as a common progenitor cell. The
differentiation of seminoma cell line TCam2 towards EC-like cells
was observed by Nettersheim et al, following the
transplantation of TCam2 cells into the seminiferous tubules of
germ cell deficient (busulfan-treated) nude mice. The upregulation
of SOX2 marker and downregulation of SOX17 expression was also
reported (34). In addition, a
similar transformation of seminoma cells towards embryonal
carcinoma cells was observed in our laboratory following
engraftment of TCam2 cells into SCID mice.
Although it is generally known that NOD/SCID mice
demonstrate a high incidence of thymic lymphomas with age (35), we excluded this possibility since
qPCR analysis confirmed the presence of human β-globin-specific
sequences in both successfully engrafted samples. However, Eppstein
Barr virus (EBV)-related B-cell lymphomas have been observed in up
to 68% of PDX tumor models derived from several types of cancer
(36,37). In the study by Chen et al,
the engraftment of primary human hepatocellular carcinoma (HCC)
tumor fragments or bulk tumor cell suspensions into immunodeficient
mice resulted in lymphoid neoplasms, rather than HCC in 11 of the
21 established xenografts (38).
Therefore, it was hypothesized that human solid tumor xenografts in
immunodeficient mice are vulnerable to lymphomagenesis associated
with TILs (tumor-infiltrating lymphocytes). TILs have been
demonstrated likewise to be an important biomarker with prognostic
and predictive features in different types of cancer. The
increasing risk of relapse associated with low numbers of TILs has
also been demonstrated in stage I seminomas (39). Cumulative data from murine studies
has determined that myeloid lineage leukocytes, including
tumor-associated macrophages, dendritic cells and myeloid-derived
suppressor cells, are important factors in shaping the
microenvironment via the factors they produce, towards either an
immunostimulatory antitumor milieu or a wound healing
tumor-promoting microenvironment (40). Therefore, a potential explanation
for the lymphoma transformation observed in PDXs in our study may
include TILs from the patient-originated tumors, which are
transformed under new microenviroment conditions after their
engraftment into immunodeficient recipient mice. It has been
suggested that this potentially confounding process may be
prevented by excluding leucocytes from the source tissue; for
example, by treating mice with rituximab (a monoclonal antibody
against B-cells) (41). However,
there is no effective solution to prevent lymphoma transformation
in PDXs at present (12).
In summary, we revealed that the PDX tumors in
immunodeficient mice, derived from patients with lymph node
metastases infiltrated with TGCTs (seminoma and teratocarcinoma)
after previous cisplatin-based chemotherapy, transformed into
lymphoma and subsequently into plasmocytoma. Lymphomagenesis
represents new challenge in the establishment and serial
propagation of PDX models derived from patients with testicular
cancer.
Acknowledgements
We would like to acknowledge our collaborators
Miroslava Matuskova, Roman Bohovic and Svetlana Miklikova from the
Laboratory of Molecular Oncology, Cancer Research Institute,
Biomedical Research Center of the Slovak Academy of Sciences,
Bratislava, Slovakia for excellent technical assistance and animal
maintenance.
Funding
The present study was supported by the Slovak
Research and Development Agency [APVV-0016-11, APVV-15-0086] and
The Slovak Scientific Grant Agency [VEGA 1/0043/18].
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KK, LK and MM designed the study, analyzed the data,
and wrote the manuscript. KK, LK, SS and LT performed the research.
ZK participated in the molecular analysis of tested samples. LK
prepared Figs. 3 and 4. MC, PP, JM and MM provided clinical
data. DP provided tissue samples. DM and LP performed the
histological and immunohistochemical evaluations. LK and MM revised
the manuscript and supervised the study. All authors discussed the
results and reviewed the manuscript critically for important
intellectual content. All the authors provided their final approval
of the version to be published and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The Institutional Review Board of the National
Cancer Institute, Bratislava, Slovakia (protocol IZLO1, chair: M.
Mego) approved the present study and all patients provided written
informed consent. Animal experiments were performed in the approved
animal facility (license no. SK PC 14011) as approved by the
Institutional Ethics Committee and by the national competence
authority (State Veterinary and Food Administration of the Slovak
Republic, registration no. Ro 3108/14-221) in compliance with the
Directive 2010/63/EU of the European Parliament and the European
Council and the Regulation 377/2012 on the protection of animals
used for scientific purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Einhorn LH: Treatment of testicularcancer:
A new and improved model. J Clin Oncol. 8:1777–1781. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Einhorn LH: General Motors Research
Prizewinners Laureates Lectures. Charles F. Kettering Prize.
Clinical trials in testicular cancer. Cancer. 71:3182–3184.
1993.PubMed/NCBI
|
|
3
|
Motzer RJ, Amsterdam A, Prieto V,
Sheinfeld J, Murty VV, Mazumdar M, Bosl GJ, Chaganti RS and Reuter
VE: Teratoma with malignant transformation: Diverse malignant
histologies arising in men with germ cell tumors. J Urol.
159:133–138. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Sheinfeld J, Mazumdar M, Bains
M, Mariani T, Bacik J, Bajorin D and Bosl GJ: Paclitaxel,
ifosfamide, and cisplatin second-line therapy for patients with
relapsed testicular germ cell cancer. J Clin Oncol. 18:2413–2418.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mardiak J, Salek T, Sycova-Mila Z,
Obertova J, Hlavata Z, Mego M, Reckova M and Koza I: Paclitaxel
plus ifosfamide and cisplatin in second-line treatment of germ cell
tumors: A phase II study. Neoplasma. 52:497–501. 2005.PubMed/NCBI
|
|
6
|
De Giorgi U, Rosti G, Papiani G and
Marangolo M: The status of high-dose chemotherapy with
hematopoietic stem cell transplantation in germ cell tumor
patients. Haematologica. 87:95–104. 2002.PubMed/NCBI
|
|
7
|
Feldman DR, Patil S, Trinos MJ, Carousso
M, Ginsberg MS, Sheinfeld J, Bajorin DF, Bosl GJ and Motzer RJ:
Progression-free and overall survival in patients with
relapsed/refractory germ cell tumors treated with single-agent
chemotherapy: Endpoints for clinical trial design. Cancer.
118:981–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oechsle K, Kollmannsberger C, Honecker F,
Mayer F, Waller CF, Hartmann JT, Boehlke I and Bokemeyer C; and
German Testicular Cancer Study Group, : Long-term survival after
treatment with gemcitabine and oxaliplatin with and without
paclitaxel plus secondary surgery in patients with cisplatin-
refractory and/or multiply relapsed germ cell tumors. Eur Urol.
60:850–855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mego M, Svetlovska D, Miskovska V,
Obertova J, Palacka P, Rajec J, Sycova-Mila Z, Chovanec M,
Rejlekova K, Zuzák P, et al: Phase II study of everolimus in
refractory testicular germ cell tumors. Urol Oncol. 34(122):
e17–e22. 2016.
|
|
10
|
Daniel VC, Marchionni L, Hierman JS,
Rhodes JT, Devereux WL, Rudin CM, Yung R, Parmigiani G, Dorsch M,
Peacock CD and Watkins DN: A primary xenograft model of small-cell
lung cancer reveals irreversible changes in gene expression imposed
by culture in vitro. Cancer Res. 69:3364–3373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siolas D and Hannon GJ: Patient derived
tumor xenografts: Transforming clinical samples into mouse models.
Cancer Res. 73:5315–5319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi YY, Lee JE, Kim H, Sim MH, Kim KK,
Lee G, Kim HI, An JY, Hyung WJ, Kim B, et al: Establishment and
characterization of patient-derived xenografts as paraclinical
models for gastric cancer. Sci Rep. 6:221722016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fichtner I, Slisow W, Gill J, Becker M,
Elbe B, Hilebrand T and Bibby M: Anticancer drug response and
expression of molecular markers in early-passage xenotransplanted
colon carcinomas. Eur J Cancer. 40:298–307. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rubio-Viqueira B and Hidalgo M: Direct in
vivo xenograft tumor model for predicting chemotherapeutic drug
response in cancer patients. Clin Pharmacol Ther. 85:217–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DeRose YS, Wang G, Lin YC, Bernard PS,
Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, et al:
Tumor grafts derived from women with breast cancer authentically
reflect tumor pathology, growth, metastasis and disease outcomes.
Nat Med. 17:1514–1520. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao X, Liu Z, Yu L, Zhang Y, Baxter P,
Voicu H, Gurusiddappa S, Luan J, Su JM, Leung HC and Li XN: Global
gene expression profiling confirms the molecular fidelity of
primary tumor-based orthotopic xenograft mouse models of
medulloblastoma. Neuro Oncol. 14:574–583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Houghton JA, Houghton PJ and Green AA:
Chemotherapy of childhood rhabdomyosarcomas growing as xenografts
in immune-deprived mice. Cancer Res. 42:535–539. 1982.PubMed/NCBI
|
|
18
|
Talmadge JE, Singh RK, Fidler IJ and Raz
A: Murine models to evaluate novel and conventional therapeutic
strategies for cancer. Am J Pathol. 170:793–804. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abraham D, Abri S, Hofmann M, Höltl W and
Aharinejad S: Low dose carboplatin combined with angiostatic agents
prevents metastasis in human testicular germ cell tumor xenografts.
J Urol. 170:1388–1393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castillo-Avila W, Piulats JM, Del Muro
Garcia X, Vidal A, Condom E, Casanovas O, Mora J, Germà JR, Capellà
G, Villanueva A and Viñals F: Sunitinib inhibits tumor growth and
synergizes with cisplatin in orthotopic models of
cisplatin-sensitive and cisplatin-resistant human testicular germ
cell tumors. Clin Cancer Res. 15:3384–3395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Vries G, Rosas-Plaza X, van Vugt MA,
Suurmeijer A, Gietema JA and de Jong S: Development of testicular
cancer patient derived xenograft models to test combination
therapies targeting PI3K/Akt and MDM2. Cancer Res. 77 Suppl
13:38612017. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matuskova M, Kozovska Z, Toro L,
Durinikova E, Tyciakova S, Cierna Z, Bohovic R and Kucerova L:
Combined enzyme/prodrug treatment by genetically engineered AT-MSC
exerts synergy and inhibits growth of MDA-MB-231 induced lung
metastases. J Exp Clin Cancer Res. 34:332015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Némati F, Sastre-Garau X, Laurent C,
Couturier J, Mariani P, Desjardins L, Piperno-Neumann S, Lantz O,
Asselain B, Plancher C, et al: Establishment and characterization
of a panel of human uveal melanoma xenografts derived from primary
and/or metastatic tumors. Clin Cancer Res. 16:2352–2362. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sivanand S, Peña-Llopis S, Zhao H,
Kucejova B, Spence P, Pavia-Jimenez A, Yamasaki T, McBride DJ,
Gilen J, Wolff NC, et al: A validated tumor graft model reveals
activity of dovitinib against renal cell carcinoma. Sci Transl Med.
4:137ra752012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Fu X and Hoffman RM: A new
patient-like metastatic model of human lung cancer constructed
orthotopically with intact tissue via thoracotomy in
immunodeficient mice. Int J Cancer. 51:992–995. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shuhendler AJ, Prasad P, Cai P, Hui KK,
Henderson JT, Rauth AM and Wu XY: Matrigel alters the
pathophysiology of orthotopic human breast adenocarcinoma
xenografts with implications for nanomedicine evaluation.
Nanomedicine. 9:795–805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Read M, Liu D, Duong CP, Cullinane C,
Murray WK, Fennell CM, Shortt J, Westerman D, Burton P, Clemons NJ
and Phillips WA: Intramuscular transplantation improves engraftment
rates for esophageal patient-derived tumor xenografts. Ann Surg
Oncol. 23:305–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujii E, Suzuki M, Matsubara K, Watanabe
M, Chen YJ, Adachi K, Ohnishi Y, Tanigawa M, Tsuchiya M and Tamaoki
N: Establishment and characterization of in vivo human tumor models
in the NOD/SCID/gamma(c)(null) mouse. Pathol Int. 58:559–567. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krege S, Beyer J, Souchon R, Albers P,
Albrecht W, Algaba F, Bamberg M, Bodrogi I, Bokemeyer C,
Cavallin-Ståhl E, et al: European consensus conference on diagnosis
and treatment of germ cell cancer: A report of these condmeeting of
the european germ cell cancer consensus group (EGCCCG): Part I. Eur
Urol. 53:478–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beck SD and Foster RS: Long-term outcome
of retroperitoneal lymph node dissection in the management of
testis cancer. World J Urol. 24:267–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oldenburg J, Alfsen GC, Lien HH, Aas N,
Waehre H and Fossa SD: Postchemotherapy retroperitoneal surgery
remains necessary in patients with nonseminomatous testicular
cancer and minimal residual tumor masses. J Clin Oncol.
21:3310–3317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brandli DW, Ulbright TM, Foster RS,
Cummings OW, Zhang S, Sweeney CJ, Eble JN and Cheng L: Stroma
adjacent to metastatic mature teratoma after chemotherapy for
testicular germ cell tumors is derived from the same progenitor
cells as the teratoma. Cancer Res. 63:6063–6068. 2003.PubMed/NCBI
|
|
34
|
Nettersheim D, Westernstroer B, Gillis
AJM, Looijenga LHJ, Schlatt S and Schorle H: Chanages in the
microenviroment affect differentiation of the seminoma cell line
TCam-2 and infusion into the mouse testis provides a model for the
study CIS. 7th Copenhagen Workshop on CIS-Testis and Germ Cell
Cancer Abstracts: abs. 24:2010.
|
|
35
|
Prochazka M, Gaskins HR, Shultz LD and
Leiter EH: The nonobese diabetic scid mouse: Model for spontaneous
thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci
USA. 89:3290–3294. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Liu Y, Wang X, Tang Z, Li S, Hu
Y, Zong X, Wu X, Bu Z, Wu A, et al: The extent of inflammatory
infiltration in primary cancer tissues is associated with
lymphomagenesis in immunodeficient mice. Sci Rep. 5:94472015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
John T, Yanagawa N, Kohler D, Craddock KJ,
Bandarchi-Chamkhaleh B, Pintilie M, Sykes J, To C, Li M, Panchal D,
et al: Characterization of lymphomas developing in immunodeficient
mice implanted with primary human non-small cell lung cancer. J
Thorac Oncol. 7:1101–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen K, Ahmed S, Adeyi O, Dick JE and
Ghanekar A: Humansolid tumor xenografts in immunodeficient mice are
vulnerable to lymphomagenesis associated with epstein-barr virus.
PLoS One. 7:e392942012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parker C, Milosevic M, Panzarella T,
Banerjee D, Jewett M, Catton C, Tew-George B, Gospodarowicz M and
Warde P: The prognostic significance of the tumour infiltrating
lymphocyte count in stage I testicular seminoma managed by
surveillance. Eur J Cancer. 38:2014–2019. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
international TILs working group 2014. Ann Oncol. 26:259–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fujii E, Kato A, Chen YJ, Matsubara K,
Ohnishi Y and Suzuki M: Characterization of EBV-related
lymphoproliferative lesions arising in donor lymphocytes of
transplanted human tumor tissues in the NOG mouse. Exp Anim.
63:289–296. 2014. View Article : Google Scholar : PubMed/NCBI
|