Introduction
Retinoblastoma (RB) is a pediatric retinal tumor and
the most common primary intraocular childhood malignancy (1–3), with an
incidence of ~1 in 15,000 live births (4). The occurrence and development of tumors
seriously damages the function of the retina. In addition, the
current effective treatment methods for RB, including local
chemotherapy and radiotherapy, can lead to further retinal damage
(5). Recent progress in RB diagnosis
and treatment has pushed the goal of RB management from life-saving
to eye and vision preservation. Numerous types of neuron exist in
the retina, including ganglion cells, bipolar cells, horizontal
cells, and photoreceptor cells. Light is collected by these neurons
and transformed to electric signals that are transmitted to the
brain, leading to the phenomenon of vision. Thus, ideal therapeutic
agents for the adjuvant therapy of RB should possess the following
properties: Inhibition of tumor cells, proliferation and protection
of neurons against damage from excitotoxicity induced by tumor
cells, and radiotherapy.
Chuanxiong has been used in Chinese traditional
medicine for more than 2,000 years. Its bioactive component,
tetramethylpyrazine (TMP), was extracted from Chuanxiong in 1973
(6). According to data from the China
Food and Drug Administration (CFDA), there are 132 pharmaceutical
factories that produce TMP injections or tablets in China (7). TMP has been widely used in the clinic to
treat ischemia, cerebral infarction, degenerative diseases, and so
on, albeit there are mild side effects (8–11).
Furthermore, a paper published by our research group identified
that TMP exerts neuroprotective effects, and revealed a mechanism
underpinning TMP-mediated treatment that involved the inhibition of
C-X-C chemokine receptor type 4 (CXCR4) expression in cerebral
neurocytes and glioma cells (12).
Accumulating evidence has confirmed that TMP can significantly
attenuate chemotherapeutic multidrug resistance, and is able to
inhibit the proliferation and metastasis of various types of cancer
cells, including melanoma cells, lung cancer and gastric carcinoma
cells (13–18). More importantly, in our previous
study, it was shown that TMP can significantly inhibit RB cell
growth, and that CXCR4 is the target gene (19).
The chemokine receptor CXCR4 belongs to a large
superfamily of G protein-coupled receptors with a 7-transmembrane
spanning structure. CXCR4 is widely expressed in numerous types of
cancerous tissues, including lung, kidney, breast, and retinal
tumors, and is considered to serve a pivotal role in a number of
biological processes that promote cancer growth and spreading,
including angiogenesis, invasion, locomotion, extravasation,
directional migration, homing, and cell survival (20–26).
Therefore, CXCR4 has been shown to be a prognostic marker in
various types of cancer, including leukemia, breast cancer, and
prostate cancer (27–29). In addition, certain eye diseases have
been shown to be associated with abnormal activation of CXCR4, such
as primary open-angle glaucoma, angiogenesis, and eye inflammation
(30,31). In our previous study, it was shown
that CXCR4 is overexpressed in RB cells, and is a target gene of
TMP to inhibit the growth of RB cells (19). Nevertheless, the mechanism underlying
the regulation of CXCR4 expression by TMP in RB cells is not well
defined.
The regulation of transcription is a vital process
in all living organisms, and has a strong impact on gene
expression. CXCR4 transcription is controlled by various
mechanisms, depending on the cell type. In oral cancer cells,
Krüppel-like factor 2 (KLF2) was shown to reduce CXCR4 promoter
activity as a negative regulator of CXCR4 expression (32). In breast cancer cells, nuclear
factor-κB (NF-κB) could directly bind to the CXCR4 promoter region
(from −66 to +7 bp) and positively regulate CXCR4 expression, thus
promoting tumor migration and metastasis (33). Our previous study also demonstrated
that NF-κB and Nrf-1 transcriptionally co-regulate CXCR4 in corneal
neovascularization (34). In
addition, the transcription factors c-myc, Yin Yang 1 (YY1),
specificity protein 1 (SP1), and hypoxia-inducible factor-1α
(HIF-1α) have also been reported to be involved in the
transcriptional regulation of CXCR4 (35–37).
However, the transcription factors regulating CXCR4 expression by
TMP in RB cells remain unidentified.
Therefore, the aim of the present study was to
investigate the possible transcriptional mechanism by which TMP
mediates the downregulation of CXCR4 in RB cells in vitro
and in vivo. The results obtained will lead to the
identification of novel potential targets for the treatment of RB,
and provide evidence for the clinical application of TMP in
adjuvant therapy of RB.
Materials and methods
Ethics statement
Thirty-two female nude mice (4–5 weeks old),
weighing between 16 and 20 g, were obtained from the Laboratory
Animal Center, Sun Yat-sen University (Guangzhou, China). All
experimental procedures were performed in accordance with the ARVO
Statements for the Use of Animals in Ophthalmic and Vision
Research, and were approved through the Institutional Animal
Ethical Committee of Zhongshan Ophthalmic Center, Sun Yat-sen
University (permit no. 2014-007). All animals were maintained in a
room (temperature: 20–26°C, atmosphere: 40–70%) with a light
schedule of alternating 12 h periods of light and dark, and
received adequate amounts of food and water freely.
Cell culture
Cells of the human WERI-Rb1 RB cell line were
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and maintained in Invitrogen®
RPMI-1640 medium (Thermo Fisher Scientific, Inc, Waltham, MA, USA)
supplemented with 10% FBS containing 100 U/ml penicillin and 100
µg/ml streptomycin in a humidified atmosphere of 5% CO2
at 37°C. Primary mouse retinal neurocytes were cultured as
described previously (38). For
co-culture assay, Transwell 12-well plates (0.4 µm pore size; BD
Biosciences, Bedford, MA, USA) were used. Approximately
5×104 primary mouse retinal neurocytes were cultured for
6 days in the lower chamber filled with 1 ml medium, and
subsequently 200 µl WERI-Rb1 cell suspension (7.5×105
cells/ml) was seeded in the upper chamber. Cells were maintained in
RPMI-1640 and DMEM medium (1:1) supplemented with 10% FBS. For the
cell density assay, the WERI-Rb1 cells were seeded in 6-well plates
and maintained in 2 ml Complete™ RPMI-1640 medium at different
densities (1×105, 2.5×105,
5.0×105, 7.5×105, and 106
cells/ml) for 24 h; subsequently, cell proteins were extracted for
performing western blot assays. TMP was purchased from Sigma (now a
brand of Merck KGaA; Darmstadt, Germany) and dissolved in component
solvent (DMSO: Saline=1:1) to an appropriate concentration. The
component solvent was applied as a control in all experiments.
Immunohistofluorescence assay
Cultured primary retinal neurocytes or WERI-Rb1
cells were fixed with ice-cold 4% paraformaldehyde for 15 min. For
mono-staining (of Map-2 or CXCR4), the fixed cells were blocked
with 10% normal goat serum for 30 min. For double-staining (CXCR4
and Nrf-1), the fixed cells were incubated with 0.1% Triton X-100
for 10 min, and then blocked with 10% normal goat serum for 30 min.
Subsequently, cells were incubated overnight at 4°C with primary
antibodies against Map-2 (1:100; cat. no. BM1243, Boster Biological
Technology, Ltd., Wuhan, China), CXCR4 (1:100; cat. no. ab2074,
Abcam, Cambridge, UK) and Nrf-1 (1:100; cat. no. sc-23624, Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Alexa Fluor 555
anti-rabbit lgG (1:500; cat. no. 4413, Cell Signaling Technology,
Inc., Dallas, TX, USA) and Alexa Fluor 488 anti-goat lgG (1:500;
cat. no. A-11055, Thermo Fisher Scientific, Inc.) were used as
secondary antibodies, and nuclei were stained with DAPI. Images
were captured using fluorescence microscopy (Leica Microsystems,
Wetzlar, Germany; original magnification, ×100).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA from WERI-Rb1 cells following treatment
with TMP was isolated with Invitrogen®
TRIzol® reagent (Thermo Fisher Scientific, Inc.). The
DNA contaminants in total RNA isolates were eliminated by treatment
with DNAse I for 30 min at 37°C. One microgram of total RNA was
subjected to reverse transcription using a PrimeScript™ RT Reagent
kit (Takara Biotechnology Co., Ltd., Dalian, China) following the
manufacturer's protocol. Expression levels of CXCR4 and Nrf-1 were
measured by RT-qPCR with the Roche LightCycler® 480
System (Roche, Indianapolis, IN, USA). The PCR program was as
follows: 94°C for 5 min, followed by 40 cycles of 94°C for 30 sec,
60°C for 30 sec, and 72°C for 30 sec. The following primer pairs
were used: CXCR4, 5′-CTTATCCTGCCTGGTATTGTC-3′ (forward) and
5′-CAATGTAGTAAGGCAGCCAAC-3′ (reverse); Nrf-1,
5′-GGAATTCCCATGGAGGAACACGGAGTGAC-3′ (forward) and
5′-CGGGATCCCGTTATTTCCTTTTCAGTTGCTG-3′; (reverse); and β-actin,
5′-TCACCCACACTGTGCCCAT-3′ (forward) and 5′-TCTTTAATGTCACGCACGATT-3′
(reverse). Relative target gene expression levels (measured against
β-actin) were calculated using the 2ΔΔCq method
(39).
Western blot assay
Western blotting assays were performed according to
a standard protocol. Briefly, whole proteins were extracted by
using a radioimmunoprecipitation (RIPA) lysate kit (Beyotime
Institute of Biotechnology, Jiangsu, China). The protein
concentration was determined using the bicinchoninic acid (BCA)
method. Equal amounts of protein (30 µg/well) were separated on an
8% sodium dodecyl sulfate (SDS)-polyacrylamide gel by
electrophoresis, which were subsequently electrophoretically
transferred to a polyvinylidene difluoride membrane. The membranes
were blocked with 5% non-fat milk for 1 h at room temperature, then
incubated with primary antibodies. The following primary antibodies
were used: CXCR4 (1:500; cat. no. ab2074, Abcam), Nrf-1 (1:1,000;
cat. no. ab175932, Abcam), YY1 (1:300; cat. no. sc-1703×, Santa
Cruz Biotechnology, Inc.), KLF2 (1:300; cat. no. ab203591, Abcam),
SP1 (1:300; cat. no. BA1402, Boster Biological Technology, Ltd.),
NF-kB1 (1:500; cat. no. BA1297, Boster Biological Technology, Ltd.)
and GAPDH (1:1,000; cat. no. 10494-1-AP, ProteinTech Group, Inc.,
Chicago, IL, USA). GAPDH served as the loading control. Protein
bands were detected using an enhanced chemiluminescence detection
system (EMD Millipore, Billerica, MA, USA).
Cell Counting Kit-8 (CCK-8) cell
viability assay
The effect of TMP on the cell viability of retinal
neurocytes and WERI-Rb1 cells in a co-culture system was measured
using a CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Following treatment with 200 µM TMP or vehicle
control for the relevant time periods (24, 48 or 72 h), WERI-Rb1
cells were transferred to new wells. Subsequently, CCK-8 reagent
was added to each well, followed by incubation at 37°C for an
additional 1 h. The absorbance of the product was measured at 450
nm using a fluorescence plate reader (Power Wave XS; BioTek China,
Beijing, China). Cell viability was calculated by determining the
optical density ratio of a treated culture over an untreated
control.
RNA interference
The siRNA sequences used for targeted silencing of
Nrf-1 and control sequences were as follows: Human Nrf-1 siRNA:
5′-CGTTAGATGAATATACTAC-3′ and the control, GGUUUGGCUGGGGUGUUAUdTdT.
The oligos were purchased from Guangzhou RiboBio (Guangzhou,
China). WERI-Rb1 cells (2×106 cells in 60 mm dishes) in
good condition underwent lipidmediated transfection using
Invitrogen® Lipofectamine™ RNAiMAX (Thermo Fisher
Scientific, Inc.), as recommended by the manufacturer. The mRNA and
protein expression levels of Nrf1 and CXCR4 were measured by
RT-qPCR or western blotting assays, respectively, at 24 or 48 h
after transfection.
Reporter and plasmid construction
A fragment spanning from −1,981 to +80 bp that
included the CXCR4 promoter sequence was produced by PCR with the
forward primer, 5′-GGGGTACCCCACACAATTCTGAATCCTGCCT-3′, and the
common reverse primer, 5′-CCGCTCGAGCGGTCCAGATGCGGTGGCTACTG-3′. This
fragment was fused to the promoter-less firefly luciferase gene of
pGL3-Basic vector (Promega Corporation, Madison, WI, USA) to
generate the constructed plasmid, named ‘PGL3-hCXCR4-pro’.
PGL3-hCXCR4-pro was derived from the pGL3-Basic vector (Promega
Corporation), in which human CXCR4 cDNA was inserted into
pGL3-Basic vector using the restrictive digestion sites,
KpnI and XhoI.
CXCR4 promoter-reporter assay
WERI-Rb1 cells (2×106 cells in 60 mm
dishes) were transfected using Invitrogen®
Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The transfected plasmids contained
2 µg expression plasmid PGL3-hCXCR4-pro or pGL3-Basic vectors, and
100 ng Renilla luciferase reporter plasmid, pCMV-RL (Promega
Corporation). The pCMV-RL plasmid encoding Renilla
luciferase was included in all the samples to monitor transfection
efficiency. At 24 h post-transfection, the levels of firefly and
Renilla luciferase activity were measured sequentially from
a single sample using the Dual-Glo Luciferase Assay system (Promega
Corporation). The levels of firefly luciferase activity were
normalized against Renilla luciferase activity.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed using the ChIP assay kit
(Upstate Cell Signaling Solutions, Lake Placid, NY, USA), according
to the manufacturer's protocol. Approximately 5×105
cells/ml were used for each assay. WERI-Rb1 cells were cross-linked
by the addition of 1% formaldehyde for 10 min at room temperature,
and the reaction was terminated upon the addition of glycine (final
concentration, 0.125 M). Subsequently, cells were collected and
incubated in 600 µl SDS lysis buffer containing protease inhibitors
(2 µg/ml leupeptin, 2 µg/ml aprotinin, and 2 mM PMSF) for 10 min on
ice. The samples were sonicated to yield fragments of chromatin of
~0.5 kb in length on ice (the duration of the sonication process
was 8 min (6 sec ON-12 sec OFF) and the power was set at 400 W).
After sonication, the lysate was centrifuged at 16,800 g for 10 min
at 4°C. The supernatant was diluted in ChIP dilution buffer that
included the protease inhibitors. A total 5% of the supernatant was
saved as input DNA, and primary rabbit antibody Nrf-1 (Santa Cruz
Biotechnology, Inc.) or rabbit normal IgG (Merck KGaA) was added to
the supernatant and incubated overnight at 4°C with rotation.
Following incubation with protein A-agarose, the immune complexes
were eluted with elution buffer. A part of the captured
immune-complex was subjected to western blotting analysis to detect
whether the captured chromatins contained Nrf-1. Cross-linking was
reversed by heating at 65°C overnight. RNA was subsequently
degraded with RNase A for 30 min, whereas protein was degraded with
proteinase K for 2 h. DNA was purified using an Ez-ChIP™
polypropylene spin column (Merck Millipore), and subjected to PCR
amplification using the following primers for H-CXCR4-CHIP:
5′-GACCACCCGCAAACAGCAGG-3 (sense) and 5′-GCAGCCAACAAACTGAAGTTTC-3′
(antisense).
Murine xenograft model of
retinoblastoma
All experimental procedures were approved by the
Ethical Committee of Zhongshan Ophthalmic Center, Sun Yat-sen
University (permit no. 2014-007). Female athymic nude mice aged 4–5
weeks old were obtained from the Laboratory Animal Center, Sun
Yat-sen University. They were maintained in a standard environment
with filter tops. After the WERI-Rb1 cells had been resuspended in
PBS, the mice were anesthetized with 3% isoflurane delivered by
mask, and 1×105 cells were injected into the vitreous
cavity of the right eye using a Hamilton needle. All surgical
procedures were performed under sterile conditions with a
dissecting microscope; the left eyes were used as untreated
controls. The eyes were observed on a weekly basis for tumor
development. At 2 weeks after the mice had been xenotransplanted
intravitreally with WERI-Rb1 cells, fundus photographs (Phoenix
MicronIV™; Phoenix Research Labs, Pleasanton, CA, USA) were taken
for all animals. All animals were subsequently randomly divided
into two groups (n=16 mice in each group). Mice were anesthetized
with 3% isoflurane delivered by mask, and each treatment group then
received vitreous injections with TMP at a final dose of 200 µM,
whereas the control group received vitreous injections with
balanced salt solution. At 48 h following vitreous injection, the
mice were euthanized by carbon dioxide inhalation (flow rate: 2–3
l/min) in a 10 l sealed box, and death of the mice was verified
through several indicators, including absence of a heartbeat,
respiratory arrest, and rigor mortis. The globes (n=4 for each
group) were enucleated and fixed in 4% paraformaldehyde for 12 h.
The eyeballs were subsequently processed into paraffin-embedded
sections, and sequential meridian sections (6 µm thick) were
created and stained with hematoxylin and eosin (H&E). To detect
the mRNA and protein levels of Nrf-1 and CXCR4 following TPM
treatment, tumor tissue was collected and analyzed by RT-qPCR and
western blotting, respectively, after vitreous injection with TPM
for 48 h (n=6 for each group).
Statistical analyses
All experiments were performed at least three times.
Data are expressed as the mean ± SD. Differences between mean
values were evaluated using two-tailed Student's t-test (for 2
groups) or analysis of variance (ANOVA; >2 groups). SPSS 21.0
software (IBM Corp., Armonk, NY, USA) was used for all statistical
analyses. P<0.05 was considered to indicate a statistically
significant value.
Results
TMP significantly promotes retinal
neurocyte survival and suppresses WERI-Rb1 cell growth in
co-culture systems
To assay the bioactivity of TMP on primary retinal
neurocytes and WERI-Rb1 cells, a co-culture system was used to
mimic RB physiological conditions. First, the primary rat retinal
neurocytes were cultured 1 day after birth, and Map-2 staining was
performed to verify the identity of these cells. As denoted by the
green coloration in Fig. 1A, the
majority of the cells were Map-2-positive cells. Subsequently, the
primary retinal neurocytes were co-cultured with WERI-Rb1 cells,
and treated with TMP or vehicle. At different time points following
treatment, the cell viability of neurocytes and tumor cells were
measured by CCK-8 assay. Our data showed that the viability of
WERI-Rb1 cells was significantly inhibited following TMP treatment
compared with the controls (at 24 h: Control, 1; and TMP,
0.82±0.04; at 48 h: Control, 1; TMP, 0.77±0.04; and at 72 h:
Control, 1; TMP, 0.70±0.08) (P<0.05; Fig. 1B), results which were consistent with
our previous study (19).
Simultaneously, the viability of retinal neurocytes was
significantly enhanced by TMP compared with controls (at 24 h:
Control, 1; TMP, 1.10±0.04; at 48 h: Control, 1; TMP, 1.23±0.10;
and at 72 h: Control, 1; TMP, 1.35±0.04) (P<0.05; Fig. 1C). These results indicated that TMP
may serve as an ideal therapeutic agent for the adjuvant treatment
of RB.
TMP downregulates CXCR4 expression in
WERIRb1 cells
CXCR4 fulfills a critical role in fundamental
aspects of cancer, including proliferation, migration, invasion,
and metastases (20–26). CXCR4 was found to be strongly
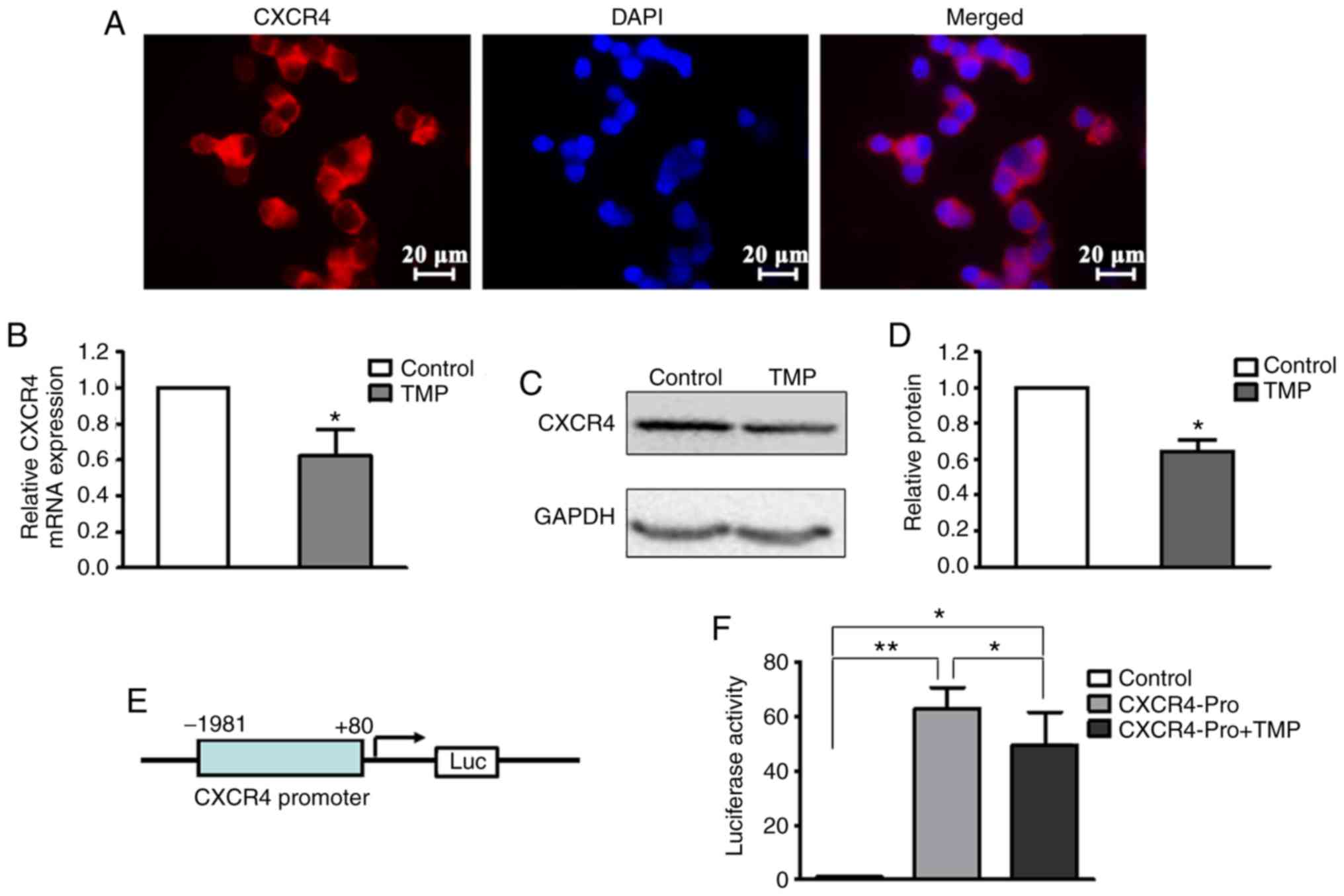
expressed on the nuclear membrane of WERI-Rb1 cells (Fig. 2A). At 24 h after treatment with 200 µM
TMP, total RNA and whole-cell lysates were extracted for RT-qPCR
and western blot analyses, respectively. As shown in Fig. 2B, the level of CXCR4 mRNA in WERI-Rb1
cells following TMP treatment was significantly decreased compared
with the control (control, 1; TMP, 0.63±0.14; P<0.05). The
western blot assay also revealed that TMP treatment led to a
notable attenuation of the CXCR4 protein level in WERI-Rb1 cells
compared with the control (Fig. 2C).
The relative quantification of CXCR4 expression is shown as a
histogram in Fig. 2D (control, 1;
TMP, 0.64±0.06; P<0.05).
To examine whether TMP regulated CXCR4 expression in
WERI-Rb1 cells at the transcriptional level, a firefly luciferase
reporter was constructed using the predicted CXCR4 promoter region
(−1,981 to +80 bp) (Fig. 2E).
Luciferase assay revealed that TMP significantly inhibited CXCR4
reporter activity (Control, 1; CXCR4Pro, 62.90±7.79; and CXCR4-Pro
+ TMP, 49.67±11.96) (P<0.05; P<0.001; Fig. 2F). These results indicated that TMP
downregulates CXCR4 expression in WERI-Rb1 cells via inhibition of
its transcription.
The transcription factor Nrf-1 is
downregulated by TMP in WERI-Rb1 cells
To identify the transcription factors regulated by
TMP in WERI-Rb1 cells, several reported transcription factors
(i.e., KLF2, SP1, Nrf-1, YY1, and NF-κB1) that bind to the CXCR4
promoter were analyzed. After treatment with TMP for 24 or 48 h,
whole-cell lysates of WERI-Rb1 were analyzed by western blotting.
As shown in Fig. 3A, Nrf-1 expression
was markedly downregulated in accordance with the expression of
CXCR4 in WERI-Rb1 cells; however, the expression levels of KLF2,
SP1, YY1, and NF-κB1 in WERI-Rb1 cells were not significantly
altered following TMP treatment. The relative expression levels
were then quantified by densitometry and normalized against GAPDH
levels. As shown in Fig. 3B, Nrf-1
expression was significantly decreased by 27.62±7.62 and
33.65±9.52%, respectively, at 24 and 48 h following TMP treatment.
Similarly, CXCR4 expression was decreased by 35.87±8.89 and
45.40±11.75%, respectively. For the expression of the other
transcription factors, no significant differences were observed
between the control group and the TMP-treated group (P>0.05).
These results indicated that Nrf-1 may be involved in the
transcriptional regulation of CXCR4 by TMP in WERI-Rb1 cells.
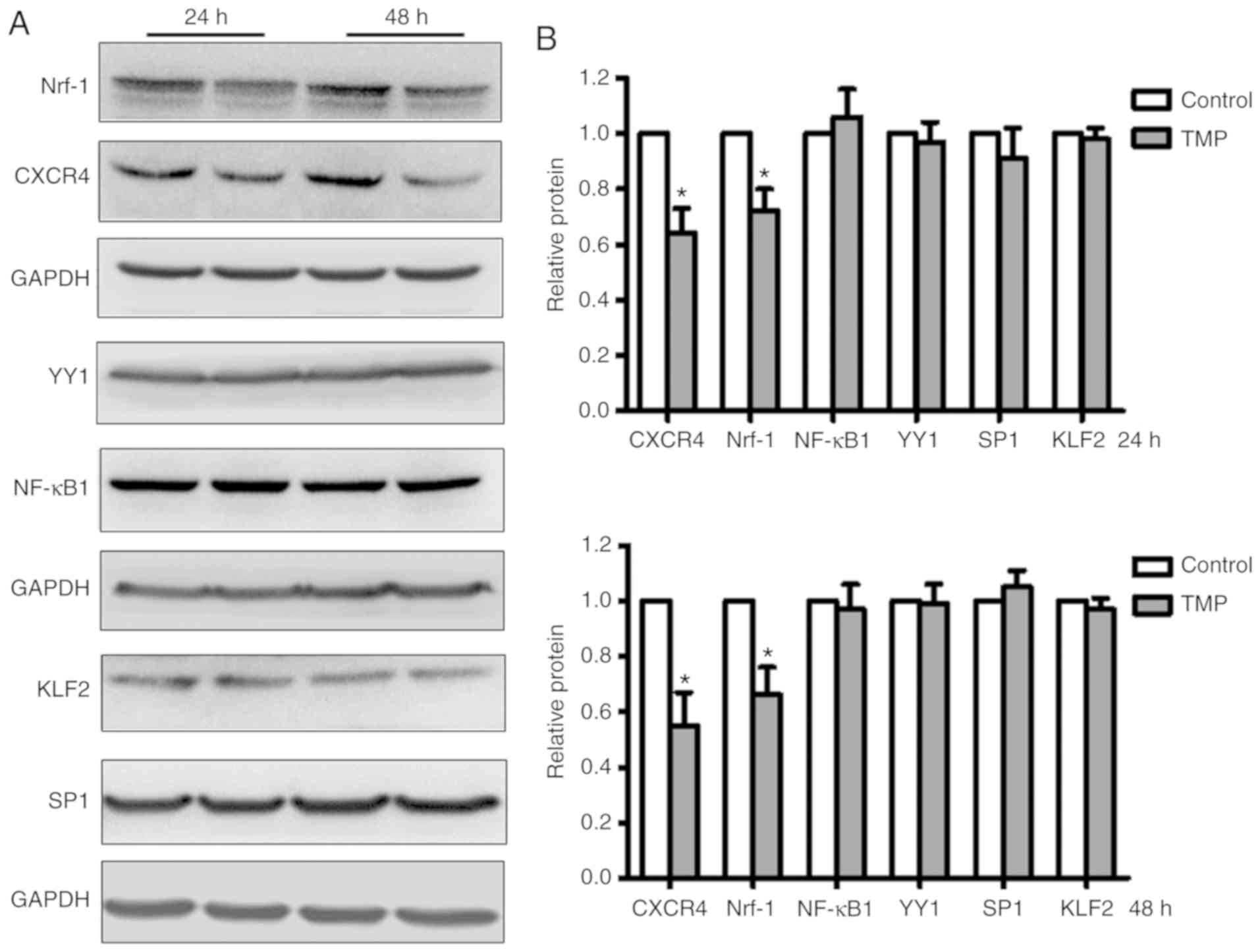 | Figure 3.The transcription factor Nrf-1 is
downregulated in WERI-Rb1 cells following TMP treatment. (A)
Several CXCR4-associated transcription factors (KLF2, SP1, Nrf-1,
YY1, NF-κB1) were analyzed by western blotting in WERI-Rb1 cells
after TMP treatment for 24 or 48 h. (B) Relative quantification of
protein expression levels revealed that the expression levels of
Nrf-1 and CXCR4 were significantly decreased; however, the
expression levels of KLF2, SP1, YY1, and NF-κB1 were not
significantly altered in WERI-Rb1 cells following treatment with
TMP (200 µM) for 24 or 48 h. *P<0.05 comparing TMP treatment
with the control. TMP, tetramethylpyrazine; CXCR4, C-X-C chemokine
receptor type 4; Nrf-1, nuclear respiratory factor-1; YY1, Yin Yang
1; KLF2, Krüppel-like Factor 2; SP1, specificity protein 1; NF-κB1,
nuclear factor-κB subunit 1. |
Nrf-1 directly binds to the CXCR4
promoter and positively regulates the expression of CXCR4 in
WERI-Rb1 cells
To confirm whether Nrf-1 directly binds the CXCR4
promoter in WERI-Rb1 cells, ChIP assays were performed. Cells were
sonicated to break up the chromatin molecules into ~0.5 kb
fragments (Fig. 4A), followed by
incubation with a rabbit Nrf-1 antibody or normal rabbit IgG. A
portion of each immunoprecipitation reaction was subjected to
western blot assay. Nrf-1 was shown to be readily detectable in the
samples incubated with Nrf-1 antibody, but not normal rabbit IgG
(Fig. 4B). In addition, the
precipitated DNA was subjected to PCR amplification using primers
designed to amplify a 200 bp fragment of the CXCR4 promoter region
flanking the ATF site. As shown in Fig.
4C, a 200 bp band was only detected in DNA incubated with
anti-Nrf-1 and the input DNA. These results indicated that Nrf-1
directly binds to the promoter region of CXCR4.
To further verify that Nrf-1 exerts a crucial role
in the regulation of CXCR4 in WERI-Rb1 cells, siRNA transfection
was performed to silence Nrf-1 expression. As shown in Fig. 4D, the mRNA expression of CXCR4 was
significantly decreased after Nrf-1 silencing. Western blot
analysis also showed that silencing Nrf-1 notably inhibited CXCR4
protein expression in WERI-Rb1 cells (Fig. 4E). The relative quantification of
Nrf-1 and CXCR4 protein expression is presented in histogram form
in Fig. 4F. The protein expression of
Nrf-1 was decreased by 42.19±11.46% after siRNA silencing.
Accordingly, CXCR4 was also decreased by 35.42±11.98% (P<0.05).
These data further suggested that Nrf-1 is involved in the positive
transcriptional regulation of CXCR4 in WERI-Rb1 cells.
Nrf-1 and CXCR4 expression are
increased in accordance with cell density
Our previous study demonstrated that CXCR4
downregulation by TMP is associated with cell density in WERI-Rb1
cells (19). Therefore, in the
present study, the protein levels of Nrf-1 and CXCR4 following
culture (24 h) of WERI-Rb1 cells plated at different densities
(1×105, 2.5×105, 5.0×105,
7.5×105, and 106 cells/ml) were measured. To
verify the expression location of Nrf-1 and CXCR4, and that Nrf-1
and CXCR4 are co-expressed in WERI-Rb1 cells, double
immunohistofluorescence staining was performed. The results
demonstrated that Nrf-1 was expressed in the nucleus, and that
CXCR4 was expressed predominantly on the nuclear membrane, with
moderate staining evident in the cytoplasm (Fig. 5A). Western blot analysis demonstrated
that Nrf-1 and CXCR4 protein expression was increased in accordance
with cell density (Fig. 5B). The
relative expression of Nrf-1 and CXCR4 in WERI-Rb1 cells was also
quantified by densitometry. The average ratio of Nrf-1 or CXCR4 to
GAPDH in WERI-Rb1 cells at the lowest cell density investigated
(1×105 cells/ml) was defined as 1. As shown in Fig. 5C, relative quantification revealed
that Nrf-1 in WERI-Rb1 cells was significantly upregulated with
increasing cell density, compared with cells at the lowest density
(1×105 cells/ml, 1; 2.5×105 cells/ml,
1.30±0.05; 5×105 cells/ml, 1.58±0.14; 7.5×105
cells/ml, 1.82±0.10; and 106 cells/ml, 2.04±0.10)
(P<0.05). The expression of CXCR4 was also significantly
increased in cells, in essentially a stepwise manner, with
increasing cell density (1×105 cells/ml, 1;
2.5×105 cells/ml, 1.26±0.10; 5×105 cells/ml,
1.47±0.21; 7.5×105 cells/ml, 1.83±0.09; and
106 cells/ml, 2.09±0.05) (P<0.05; Fig. 5D). These results demonstrated that the
expression pattern of Nrf-1 is similar to that of CXCR4, further
indicating that the expression of CXCR4 in WERI-Rb1 cells is
regulated by the transcription factor Nrf-1.
 | Figure 5.Nrf-1 and CXCR4 expression are
increased in accordance with cell density. (A)
Immunohistofluorescence double staining showed that Nrf-1 (green)
is expressed in the nuclei of WERI-Rb1 cells and CXCR4 (red) is
expressed mainly on the nuclear membrane, with moderate staining in
the cytoplasm. Scale bars, 20 µm. (B) Western blot analysis showed
that Nrf-1 and CXCR4 expression increased with cell density
(1.0×105, 2.5×105, 5.0×105,
7.5×105, and 106 cells/ml). (C and D)
Relative quantification of protein expression also revealed that
Nrf-1 and CXCR4 levels increased in WERI-Rb1 cells concomitantly
with an increase in cell density (i.e., 2.5×105,
5.0×105, 7.5×105, and 106
cells/ml) compared with the Nrf-1 and CXCR4 levels at the lowest
density (i.e., 1×105 cells/ml). *P<0.05 compared with
the lowest density (i.e., 1×105 cells/ml). Nrf-1,
nuclear respiratory factor-1; TMP, tetramethylpyrazine; CXCR4,
C-X-C chemokine receptor type 4. |
Nrf-1 and CXCR4 are downregulated by
TMP in vivo
Based on the in vitro results, an RB
orthotopic xenotransplantation model was established to validate
the TMP-mediated inhibition of CXCR4 by the transcription factor
Nrf-1. Fundus photographs were taken for all animals aged over 2
weeks. Fig. 6A shows fundus
photographs of normal mice eyes (left panel) and mice eyes with RB
(center panel; the white arrowheads indicate RB tumors); H&E
staining revealed that the cell mass of the RB is located in the
vitreous cavity (right panel; the white arrowheads indicate RB
tumors). Subsequently, the mice were divided into two groups
receiving either vitreous injection of TMP, or vehicle. At 48 h
following treatment, total RNA and protein lysates of RB were
obtained for RT-qPCR and western blot analyses. As shown in
Fig. 6B, the mRNA expression levels
of both Nfr-1 and CXCR4 were significantly decreased in
retinoblastoma treated with TMP, compared with controls (for Nrf-1:
Control, 1; TMP-treated, 0.23±0.06; for CXCR4: Control, 1;
TMP-treated, 0.47±0.22) (P<0.05). Similarly, the altered Nfr-1
and CXCR4 protein expression levels were consistent with the
changes in mRNA levels (Fig. 6C). The
relative quantification of Nrf-1 and CXCR4 protein expression is
presented in histogram form in Fig.
6D (for Nrf-1: Control, 1; TMP-treated, 0.66±0.08; for CXCR4:
Control, 1; TMP-treated, 0.40±0.04) (P<0.05). These data further
demonstrated that CXCR4 is downregulated by TMP through Nrf-1 in
vivo.
Discussion
TMP is commonly used in the clinic to treat vascular
diseases and neurodegenerative diseases with mild side effects
(8–11). According to our previous study, TMP
possesses a remarkable anti-RB effect (19); however, its underlying molecular
mechanism has not been fully elucidated. Currently, TMP is only
used in certain Oriental countries, including China and Korea.
Therefore, clarifying the anti-RB mechanism of TMP will extend its
clinical therapeutic application in medical practice.
In the present study, RB cells and a retinal
neurocyte co-culture system were used to mimic the RB ocular
physiological environment, and the data obtained revealed that TMP
is able to significantly inhibit the viability of WERIRb1 cells and
to promote retinal neurocyte survival (Fig. 1). Furthermore, accumulating evidence
has confirmed that combining TMP with other treatments could
significantly attenuate the multidrug resistance of chemotherapy
(15–17). Therefore, TMP may be an ideal chemical
for the adjuvant treatment of RB. CXCR4 is the target gene of TMP,
which acts as an inhibitor of RB cell growth. In addition to the
downregulation of mRNA and protein expression of CXCR4 by TMP, the
present study also disclosed that the activity of the CXCR4
promoter was significantly reduced following TMP treatment in
WERI-Rb1 cells, suggesting that TMP inhibits CXCR4 expression in
WERI-Rb1 cells via transcriptional regulatory mechanisms.
The regulation of transcription is a vital process
in all living organisms, which exerts a strong impact on gene
expression. Previous studies in various cell lines have reported
that the CXCR4 promoter is regulated by several transcription
factors, including KLF2, SP1, Nrf-1, YY1, NF-κB1, and so on
(32–36). In the present study, however, TMP was
only found to mediate the downregulation of Nrf-1. In addition,
ChIP assays confirmed that Nrf-1 directly bound to the CXCR4
promoter region, and silencing Nrf-1 notably decreased CXCR4
expression. Furthermore, Nrf-1 and CXCR4 expression were
downregulated by TMP in an RB model. These results strongly suggest
that TMP downregulates the transcription of CXCR4 via Nrf-1 in
WERI-Rb1 cells.
A previously published study by our research group
demonstrated that NF-κB and Nrf-1 transcriptionally co-regulate
CXCR4 expression in human umbilical vein endothelial cells and the
alkali-burn cornea (34). However, in
the present study, TMP was found not to affect NF-κB1 expression in
WERI-Rb1 cells. Therefore, these results suggested that CXCR4 is
regulated by different transcriptional factors in different cells.
In addition, a previous study also demonstrated that Nrf-1
expression correlates with that of CXCR4 during retinal development
(40). CXCR4 and Nrf-1 are expressed
in the postnatal rat retina, and are silenced together in the adult
rat retina. Furthermore, in the oxygen-induced retinopathy rat
model, retinal hypoxia was able to concurrently induce the
upregulation of CXCR4 and Nrf-1 expression. Therefore, the
transcriptional mechanism of CXCR4 in WERI-Rb1 cells may be similar
to that found in normal retinal tissue. RB is a malignant
intraocular tumor derived from the retina; therefore, it is
possible to speculate that transcription factors of CXCR4 are
specific to host tissue.
In our previous study, it was shown that TMP
downregulation of CXCR4 in WERI-Rb1 cells is sensitive to cell
density (19). Interestingly, Nrf-1
and CXCR4 expression were found to be significantly increased in
parallel with cell density in the present study. Nrf-1, a member of
the basic leucine-zipper family of proteins (41,42), was
first identified as a transcription factor that regulates
mitochondria-associated genes, including p43, CREB, p53, and Stat3
(43,44). A further study also showed that Nrf-1
is able to mediate reactive oxygen species (ROS) and play a vital
role in regulating cell metabolism and respiration (45). Therefore, these studies suggest that
increasing the cell density induces the upregulation of Nrf-1,
which subsequently promotes the transcription of CXCR4 in
retinoblastoma cells.
In addition, previous studies demonstrated that
Nrf-1 is involved in neural ischemic diseases. For example, the
mRNA content of Nrf-1 rapidly increased 3–6 h following an
ischemic-tolerant state (46). Nrf-1
was significantly upregulated in the retina of an
ischemia-reperfusion surgery model, and decreased after treatment
with lithium, a neuroprotective reagent (38). TMP has been used in patients with
neural diseases, and our research group previously verified that
TMP is able to downregulate CXCR4 expression in cerebral
neurocytes, which inhibits increases in somatic Ca2+ and
decreases glutamate release to protect neurons (12). Therefore, it is possible to speculate
that TMP exerts a neuroprotective action by inhibiting Nrf-1
expression, with the subsequent decrease in CXCR4 expression.
In addition, Nrf-1 is known to regulate the
expression of other genes, and our previous study demonstrated that
Nrf-1 binds the promoter of ligase IV, which regulates DNA repair
in retinal neurocytes (38).
Therefore, in WERI-Rb1 cells, the Nrf-1-CXCR4 pathway might be one
of several complicated signaling pathways that respond to TMP.
Further investigation of this subject is required in the
future.
In conclusion, the results obtained in the present
study have helped to further elucidate the anti-RB molecular
mechanism of TMP. The transcription factor Nrf-1 directly binds to
the promoter sequence of CXCR4, and TMP downregulates CXCR4
expression at the transcriptional level via Nrf-1, thereby
inhibiting the growth of WERI-Rb1 cells. Our study has identified
novel potential targets for the treatment of RB, and provides
evidence for the clinical application of TMP in adjuvant therapy of
retinoblastoma.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (project nos. 81470626 and
81670848).
Availability of data and materials
The datasets used and/or analyzed during the current
study would be available from the corresponding author upon
reasonable request.
Authors' contributions
NW and YY designed the study, performed the
experiments, collected and analyzed the data, and wrote the
manuscript. NY, YW, XH, and JQ performed experiments and analyzed
the data. SC, JZ, XC, CW, and MY assisted in collecting data and
assembling the figures. JG assisted in designing the study, and
provided professional suggestions. KY and JZ designed the study,
analyzed data, and wrote the manuscript. NW and JZ revised the
manuscript in terms of its important intellectual content. All
authors read and approved the manuscript, and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the ARVO statements for the Use of Animals in
Ophthalmic and Vision Research, and were approved through the
Institutional Animal Ethical Committee of Zhongshan Ophthalmic
Center, Ethical Committee of Zhongshan Ophthalmic Center, Sun
Yat-Sen University (permit no. 2014-007).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chintagumpala M, ChevezBarrios P, Paysse
EA, Plon SE and Hurwitz R: Retinoblastoma: Review of current
management. Oncologist. 12:1237–1246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Houston SK, Murray TG, Wolfe SQ and
Fernandes CE: Current update on retinoblastoma. Int Ophthalmol
Clin. 51:77–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devesa SS: The incidence of
retinoblastoma. Am J Ophthalmol. 80:263–265. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamboli A, Podgor MJ and Horm JW: The
incidence of retinoblastoma in the United States: 1974 through
1985. Arch Ophthalmol. 108:128–132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Francis JH, Brodie SE, Marr B, Zabor EC,
MondesireCrump I and Abramson DH: Efficacy and toxicity of
intravitreous chemotherapy for retinoblastoma: Fouryear experience.
Ophthalmology. 124:488–495. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beijing Pharmaceutical Industry Research
Institute, . Active ingredient research of Chuanxiong: I.
Extraction, isolation and structural identification of
ligustrazine. Natl Med J Chin. 7:420–421. 1977.(In Chinese).
|
|
7
|
China Food and Drug Administration.
http://app1.sfda.gov.cn/datasearch/face3/base.jsp?tableId=25&tableName=TABLE25&title=%B9%FA%B2%FA%D2%A9%C6%B7&bcId=124356560303886909015737447882June
22–2019
|
|
8
|
You JM, Zhang ZG, Lin C and Ji Y: Ischemic
stroke and the regulation of syndrome of traditional Chinese
medicine compound efficacy TMP combined. Chin Archives of Tradit
Chin Med. 12:2666–2668. 2010.(In Chinese).
|
|
9
|
Tang Z, Wang S and Lin Y: Progress in
protective effects of tetramethylpyrazine on diabetes complications
in nervous system and possible mechanisms. Chin J Pharmacol
Toxicol. 25:114–118. 2011.(In Chinese).
|
|
10
|
Chen Y and Liu M: Systemic evaluation of
Security of ligustrazine for treatment of cerebral infarction. Chin
J Clin Rehab. 07:1299–1301. 2004.(In Chinese).
|
|
11
|
Yang XG and Jiang C: Ligustrazine as a
salvage agent for patients with relapsed or refractory
non-Hodgkin's lymphoma. Chin Med J (Engl). 123:3206–3211. 2010.(In
Chinese). PubMed/NCBI
|
|
12
|
Chen Z, Pan X, Georgakilas AG, Chen P, Hu
H, Yang Y, Tian S, Xia L, Zhang J, Cai X, et al:
Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits
glioma by down regulating chemokine receptor CXCR4 expression.
Cancer Lett. 336:281–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu J and Li Q: Effect of
tetramethypyrazine on the proliferation and apoptosis of lung
cancer cell line of a549 and its mechanism. Med J Wuhan Univ.
3:50–51. 2010.(In Chinese).
|
|
14
|
Lu X, Wang Z and Chen J:
Tetramethylpyrazine induced expression of iNOS and NO to inhibit
lung metastasis of melanoma bearing mice. Chin J Mod Med.
16:3208–3210. 2006.(In Chinese).
|
|
15
|
Hua Z and Zhou Y: TMP inhibits the
proliferation of gastric carcinoma cells and endothelial cells. J
Changchun Univ Tradit Chin Med. 25:20–22. 2009.(In Chinese).
|
|
16
|
Wang S, Lei T and Zhang M: The reversal
effect and its mechanisms of Tetramethylpyrazine on multidrug
resistance in human bladder cancer. PLoS One. 11:e01577592016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Liu X, Zuo T, Liu Y and Zhang JH:
Tetramethylpyrazine reverses multidrug resistance in breast cancer
cells through regulating the expression and function of
P-glycoprotein. Med Oncol. 29:534–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XB, Wang SS, Zhang QF, Liu M, Li HL,
Liu Y, Wang JN, Zheng F, Guo LY and Xiang JZ: Inhibition of
tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug
resistant human hepatocellular carcinoma cells. Oncol Rep.
23:211–215. 2010.PubMed/NCBI
|
|
19
|
Wu N, Xu L, Yang Y, Yu N, Zhang Z, Chen P,
Zhang J, Tang M, Yuan M, Ge J, et al: Tetramethylpyrazinemediated
regulation of CXCR4 in retinoblastoma is sensitive to cell density.
Mol Med Rep. 15:2481–2488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su L, Zhang J, Xu H, Wang Y, Chu Y, Liu R
and Xiong S: Differential expression of CXCR4 is associated with
the metastatic potential of human non-small cell lung cancer cells.
Clin Cancer Res. 11:8273–8280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith MC, Luker KE, Garbow JR, Prior JL,
Jackson E, PiwnicaWorms D and Luker GD: CXCR4 regulates growth of
both primary and metastatic breast cancer. Cancer Res.
64:8604–8612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schrader AJ, Lechner O, Templin M, Dittmar
KE, Machtens S, Mengel M, ProbstKepper M, Franzke A, Wollensak T,
Gatzlaff P, et al: CXCR4/CXCL12 expression and signalling in kidney
cancer. Br J Cancer. 86:1250–1256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang Z, Brooks J, Willard M, Liang K,
Yoon Y, Kang S and Shim H: CXCR4/CXCL12 axis promotes VEGFmediated
tumor angiogenesis through Akt signaling pathway. Biochem Biophys
Res Commun. 359:716–722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Larsen PH, Hao C and Yong VW:
CXCR4 is a major chemokine receptor on glioma cells and mediates
their survival. J Biol Chem. 277:49481–49487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin X, Liu Z, Zhu P, Wang Y, Ren Q, Chen H
and Xu J: CXCL12/CXCR4 promotes proliferation, migration, and
invasion of adamantinomatous craniopharyngiomas via PI3K/AKT signal
pathway. J Cell Biochem. 120:9724–9736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung SJ, Kim CI, Park CH, Chang HS, Kim
BH, Choi MS and Jung HR: Correlation between chemokine receptor
CXCR4 expression and prognostic factors in patients with prostate
cancer. Korean J Urol. 52:607–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spoo AC, Lübbert M, Wierda WG and Burger
JA: CXCR4 is a prognostic marker in acute myelogenous leukemia.
Blood. 109:786–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Ni C, Chen W, Wu P, Wang Z, Yin
J, Huang J and Qiu F: Expression of CXCR4 and breast cancer
prognosis: A systematic review and meta-analysis. BMC Cancer.
14:492014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu N, Zhang Z, Chen P, Zhong Y, Cai X, Hu
H, Yang Y, Zhang J, Li K, Ge J, et al: Tetramethylpyrazine (TMP),
an active ingredient of chinese herb medicine Chuanxiong,
attenuates the degeneration of trabecular meshwork through
SDF1/CXCR4 axis. PLoS One. 10:e01330552015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai X, Chen Z, Pan X, Xia L, Chen P, Yang
Y, Hu H, Zhang J, Li K, Ge J, et al: Inhibition of angiogenesis,
fibrosis and thrombosis by tetramethylpyrazine: Mechanisms
contributing to the SDF-1/CXCR4 axis. PLoS One. 9:e881762014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uchida D, Onoue T, Begum NM, Kuribayashi
N, Tomizuka Y, Tamatani T, Nagai H and Miyamoto Y: Vesnarinone
downregulates CXCR4 expression via upregulation of Kruppel-like
factor 2 in oral cancer cells. Mol Cancer. 8:622009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Helbig G, Christopherson KW II,
Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE and
Nakshatri H: NFkappaB promotes breast cancer cell migration and
metastasis by inducing the expression of the chemokine receptor
CXCR4. J Biol Chem. 278:21631–21638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang MJ, Yang Y, Yu JZ, Qiu J, Chen P, Wu
YH, Wang QY, Xu ZJ, Ge J, Yu K and Zhuang J: Tetramethylpyrazine in
a murine alkaliburn model blocks NFκB/NRF1/CXCR4-signaling-induced
corneal neovascularization. Invest Ophth Vis Sci. 59:2133–2141.
2018. View Article : Google Scholar
|
|
35
|
Moriuchi M, Moriuchi H, Margolis DM and
Fauci AS: USF/c-Myc enhances, while Yin-Yang 1 suppresses, the
promoter activity of CXCR4, a coreceptor for HIV-1 entry. J
Immunol. 162:5986–5992. 1999.PubMed/NCBI
|
|
36
|
Wegner SA, Ehrenberg PK, Chang G, Dayhoff
DE, Sleeker AL and Michael NL: Genomic organization and functional
characterization of the chemokine receptor CXCR4, a major entry
co-receptor for human immunodeficiency virus type 1. J Biol Chem.
273:4754–4760. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schioppa T, Uranchimeg B, Saccani A,
Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni
M, Vago L, et al: Regulation of the chemokine receptor CXCR4 by
hypoxia. J Exp Med. 198:1391–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Y, Wu N, Tian S, Li F, Hu H, Chen P,
Cai X, Xu L, Zhang J, Chen Z, et al: Lithium promotes DNA stability
and survival of ischemic retinal neurocytes by upregulating DNA
ligase IV. Cell Death Dis. 7:e24732016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen P, Cai XX, Yang Y, Chen Z, Qiu J, Yu
N, Tang MJ, Wang QY, Ge J, Yu K and Zhuang J: Nuclear respiratory
factor-1 (NRF-1) regulates transcription of the CXC receptor 4
(CXCR4) in the rat retina. Invest Ophth Vis Sci. 58:4662–4669.
2017. View Article : Google Scholar
|
|
41
|
Chan JY, Kwong M, Lu R, Chang J, Wang B,
Yen TS and Kan YW: Targeted disruption of the ubiquitous CNC-bZIP
transcription factor, Nrf-1, results in anemia and embryonic
lethality in mice. EMBO J. 17:1779–1787. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chan JY, Cheung MC, Moi P, Chan K and Kan
YW: Chromosomal localization of the human NF-E2 family of bZIP
transcription factors by fluorescence in situ hybridization. Hum
Genet. 95:265–269. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
LeighBrown S, Enriquez JA and Odom DT:
Nuclear transcription factors in mammalian mitochondria. Genome
Biol. 11:2152010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huo L and Scarpulla RC: Mitochondrial DNA
instability and peri-implantation lethality associated with
targeted disruption of nuclear respiratory factor 1 in mice. Mol
Cell Biol. 21:644–654. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang J, Gu JY, Chen ZS, Xing KC and Sun
B: Astragalus polysaccharide suppresses palmitate-induced apoptosis
in human cardiac myocytes: The role of Nrf1 and antioxidant
response. Int J Clin Exp Pathol. 8:2515–2524. 2015.PubMed/NCBI
|
|
46
|
Stetler RA, Leak RK, Yin W, Zhang LL, Wang
SP, Gao YQ and Chen J: Mitochondrial biogenesis contributes to
ischemic neuroprotection afforded by LPS pre-conditioning. J
Neurochem. 123 (Suppl):S125–S137. 2012. View Article : Google Scholar
|