Introduction
Breast cancer occurs with a high incidence in women
(1,2) and has an extremely high mortality rate
as it has a high likelihood of invading almost all organs, causing
metastasis (3). Triple-negative
breast cancer (TNBC) is a subtype of breast cancer characterized by
the lack of estrogen receptor, progesterone receptor, and human
epidermal growth factor receptor 2 expression (4). TNBC cells are highly invasive,
spreading to lymph nodes, which often leads to early relapse with
distant metastasis (5).
The tumor microenvironment is a complex cellular
system that creates an environment in which tumors can become
malignant (6,7). Natural killer (NK) cells within the
tumor microenvironment have been shown to play an important role in
innate immune defense by eliminating tumor cells or in different
contexts, pathogen-infected cells, through the production of
various cytokines (8–10). Accumulating evidence suggests a role
for NK cells in the regulation of cancer metastasis through
microenvironmental and systemic processes such as
immunosurveillance (11). The
function of NK cells depends on the activation or inhibition of
receptors on the cell surface, which determines the release of
cytotoxic granules and pro-inflammatory cytokines (12,13).
Recently, it was shown that NK cells inhibited the migration and
invasion of ovarian carcinoma cells (14). The molecular mechanism for the
inhibitory effect of NK cells on the invasive phenotype of cancer
cells, however, has not been elucidated.
The metastatic spread of tumor cells to distant
locations requires invasion and migration, in which
matrix-degrading activity is involved (15). Matrix metalloproteinases (MMPs)
degrade components of the extracellular matrix (ECM), contributing
to cancer cell invasion and metastases (16,17).
Our laboratory demonstrated a role for MMP-2 and MMP-9 in the
regulation of the invasive phenotype of TNBC cells (18,19).
In addition to MMPs, plasmin can degrade ECM components, either
directly or indirectly through MMP (20,21).
The activated uPA protease cleaves inactive plasminogen to form
enzymatically active plasmin, which in turn cleaves proMMP to
active MMP (22,23). Accordingly, urokinase-type
plasminogen activator (uPA) is involved in multiple physiological
and pathologic processes including cell invasion, wound healing,
tumor growth, and metastasis (24–27).
In the present study, we examined the effect of
NK-92 cells on the invasive phenotype of TNBC cells using an
indirect co-culture system of NK-92 cells and human TNBC cells.
Here we demonstrated an inhibitory effect of NK-92 cells on the
invasiveness of TNBC cells. We further showed that uPA
downregulation was crucial for the NK-induced inhibition of the
invasive phenotype of TNBC cells.
Materials and methods
Cell culture and reagents
MDA-MB-231 cells were purchased from the Korean Cell
Line Bank (KCLB, Seoul, Korea). Human breast carcinoma MDA-MB-231
cells were cultured in RPMI-1640 media (cat. no. 10-041-CVR;
Corning Life Sciences) supplemented with 10% fetal bovine serum
(FBS) and 1% penicillin-streptomycin. Hs578T cells were purchased
from KCLB. Human breast carcinoma Hs578T cells were cultured in
DMEM media (cat. no. SH30243.01; HyClone; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS and 1% penicillin-streptomycin.
MCF-7 cells were purchased from the KCLB. Human breast carcinoma
MCF-7 cells were cultured in EMEM media [cat. no. 30-2003; American
Type Culture Collection (ATCC)] supplemented with 10% FBS, 1%
penicillin-streptomycin, and 0.01 mg/ml insulin. Natural killer
cell line NK-92 cells were obtained from ATCC. NK-92 cells were
cultured in α-MEM (cat. no. 32561-037; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 20% FBS, 1%
penicillin-streptomycin, 0.1 mM 2-mercaptoethanol, and rhIL-2 (200
U/ml). All cells were incubated at 37°C in a humidified atmosphere
with 5% CO2.
Indirect co-culture assay
For the indirect co-culture experiments, Transwell
inserts were used with a pore size of 0.4-µm that can deliver
soluble factors but do not allow cell passage. MDA-MB-231 or Hs578T
cells were seeded at 5×105 cells/6-well plate and
1×106 NK-92 cells were seeded onto the Transwell insert
and the plates were incubated for 48 h.
Immunoblot analysis
Whole-cell lysates were prepared using sodium
dodecyl sulfate (SDS) lysis buffer. Immunoblot analysis was
performed as previously described (28). Primary antibodies to c-Jun (cat. no.
sc-74543; dilution 1:1,000), c-Fos (cat. no. sc-52; dilution
1:1,000), p65 NF-ĸB (cat. no. sc-372; dilution 1:1,000),
phospho-p65 NF-ĸB (cat. no. sc-33020; dilution 1:1,000),
interleukin (IL)-6 (cat. no. sc-28343; dilution 1:1,000), C-C motif
ligand 2 (CCL2) (cat. no. sc-1304; dilution 1:1,000), uPAR (cat.
no. sc-13522; dilution 1:1,000) and mouse anti-goat IgG-HRP (cat.
no. sc-2354; dilution 1:3,000) were purchased from Santa Cruz
Biotechnology, Inc. Anti-ATF-2 (cat. no. 9226; dilution 1:1,000)
and anti-phospho-ATF-2 (cat. no. 9221; dilution 1:1,000) were
purchased from Cell Signaling Technology, Inc. Anti-IL-8 (cat. no.
ab18672; dilution 1:1,000) was purchased from Abcam, Inc. Anti-uPA
(cat. no. MAB1310; dilution 1:1,000) was purchased from R&D
Systems. HRP-conjugated goat anti-mouse (cat. no. 62-6520; dilution
1:3,000) and HRP-conjugated goat anti-rabbit (cat. no. 65-6120;
dilution 1:3,000) were purchased from Invitrogen; Thermo Fisher
Scientific, Inc. The secondary antibody was attached to fit the
primary antibody's origin. The Western Bright ECL kit (Advansta
Inc.) was used for band detection. The relative band intensities
were determined by quantification of each band using the FluorChem™
E (ProteinSimple, Inc.).
Reverse transcriptase (RT)-PCR
assay
RNA was extracted from cells using Trizol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse-transcribed with RT Superscript-III reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.). The primers for uPA
(704 bp) were: 5′-AAAATGCTGTGTGCTGCTGACC-3′ (forward) and
5′-CCCTGCCCTGAAGTCGTTAGTG-3′ (reverse). The primers for IL-10 (500
bp) were: 5′-CTGTGAAAACAAGAGCAAGGC−3′ (forward) and
5′-GAAGCTTCTGTTGGCTCCC-3′ (reverse). The primers for CCL5 (186 bp)
were: 5′-GAGTATTTCTACACCAGTGGCAAG−3′ (forward) and
5′-TCCCGAACCCATTTCTTCTCT−3′ (reverse). The primers for IL-6 (148
bp) were: 5′-ACTCACCTCTTCAGAACGAATTG3′ (forward) and
5′-CCATCTTTGGAAGGTTCAGGTTG-3′ (reverse). The primers for IL-8 (253
bp) were: 5′-GTGGCTCTCTTGGCAGCCTTCCTGAT-3′ (forward) and
5′-TCTCCACAACCCTCTGCACCCAGTTT-3′ (reverse). The primers for CCL2
(143 bp) were: 5′-GATGCAATCAATGCCCCAGTC−3′ (forward) and
5′-TCCTTGGCCACAATGGTCTTG−3′ (reverse). The primers for β-actin (171
bp) were: 5′-ACTCTTCCAGCCTTCCTTC-3′ (forward) and
5′-ATCTCCTTCTGCATCCTGTC-3′ (reverse). The same amount of each
amplified PCR product was loaded onto 1–2% agarose gels. Detection
was confirmed by Gel Doc™ XR+ System (Bio-Rad Laboratories,
Inc.).
In vitro invasion assay
An in vitro invasion assay was performed as
described previously (19). For
indirect co-culture assay, MDA-MB-231 cells (2×104
cells/well) or Hs-578T cells (3×104 cells/well) were
seeded onto the upper compartment of a 24-well Transwell plate. The
cells were cultured with NK-92 cell-conditioned media (CM) and
incubated 48 h. Media conditioned by NK-92 cells were collected at
48 h. The CM was filtered through 0.22-µm pore-size filters and
stored at −70°C.
Human cytokine antibody array
The human cytokine antibody array kit was purchased
from RayBiotech. The cells were cultured in serum-free media for 24
h. Supernatants were collected and centrifuged at 10,000 × g for 10
min to remove cell debris. The human cytokine array membranes were
blocked with blocking buffer for 30 min at room temperature. CM was
incubated with the array membranes for 1.5 h. After washing, the
membranes were incubated with primary antibody for 1.5 h, followed
by additional washes, and incubated with a secondary antibody. The
membrane-bound proteins were detected using ECL detection reagents
(Advansta Inc.). The relative band intensities were quantitated
with an Image Analyzer (ProteinSimple).
Casein-plasminogen zymogram assay
The samples was electrophoresed on 10% SDS-PAGE
containing 5% casein and plasminogen (20 units). After
electrophoresis, the gel was washed three times for 30 min with
2.5% Triton X-100 solution to remove the SDS and restore the
protein. A solution containing 50 mM Tris-HCl buffer (pH 7.6), 5 mM
CaCl2, 0.02% Brij-35 and 0.2% sodium azide was added and
expressed overnight at 37°C. After staining with 0.5% Coomassie
brilliant blue, the band was observed while decolorizing with 10%
acetic acid.
Chromatin immunoprecipitation
(ChIP)
The ChIP assay was performed using a Chromatin
Immunoprecipitation Assay kit (Upstate Biotechnology Inc.)
according to the manufacturer's instructions. The protein-DNA
complexes were immunoprecipitated with c-Jun antibodies. Primers
specific for the c-Jun binding site in the uPA promoter region were
used for DNA amplification as previously described (19).
Ratio of IL-6/uPA and survival
analysis of the TCGA/GTEx dataset
The invasive breast carcinoma (BRCA) cancer data
sets from 1,070 patients in The GEPIA database were used to explore
the correlation of IL-6, uPA, and the IL-6/uPA ratios with survival
time in BRCA patients. The ratio of IL-6/uPA in the TCGA/GTEx
dataset and its association with tumor stage and overall survival
was conducted using the GEPIA database as previously described.
Kaplan-Meier survival plots were obtained using the GEPIA online
tool (http://gepia.cancer-pku.cn).
Statistical analysis
Statistical significance was analyzed by ANOVA using
GraphPad Prism 6 (GraphPad Software, Inc.). Multi-comparison was
performed using Tukey's multiple comparisons test. The data are
shown as the mean ± SD from three independent experiments.
Results
Co-culture with NK-92 cells inhibits
the invasiveness of MDA-MB-231 cells through uPA
downregulation
To investigate the effect of NK-92 cells on the
invasive phenotype of TNBC cells, MDA-MB-231 cells were treated
with CM from NK-92 cells. As shown in Fig. 1A, the invasive phenotype of the
MDA-MB-231 cells was significantly inhibited by NK-92 cell CM. To
identify the proteases involved in the NK cell-induced inhibition
of invasion, reverse transcription (RT)-PCR analysis was used to
detect matrix-degrading enzymes uPA, MMP-1, MMP-2, MMP-3, MMP-8,
MMP-9, and MMP-13 in MDA-MB-231 cells co-cultured with NK-92 cells
(Fig. 1B). The mRNA level of uPA
was dramatically reduced by co-culture with NK-92 cells, while none
of the MMPs were affected. The expression of active uPA (32 kDa)
was significantly reduced by co-culture with NK-92 cells as
evidenced by immunoblot analysis (Fig.
1C). The casein-plasminogen zymogram assay showed that the
matrix-degrading activity of uPA was reduced by co-culture with
NK-92 cells (Fig. 1D). These
results suggest that the NK-92 cells inhibited the invasive
phenotype of MDA-MB-231 cells, possibly via the downregulation of
uPA.
 | Figure 1.Co-culture with NK-92 cells inhibits
the invasive phenotype of MDA-MB-231 cells through uPA
downregulation. (A) An in vitro invasion assay was conducted
on MDA-MB-231 cells and MDA-MB-231 cells treated with the
conditioned media (CM) of NK-92 cells (NK-92 CM) for 48 h (t-test,
**P<0.01, compared with MDA-MB-231 cells without NK-92 CM). (B)
The mRNA levels of MMPs and uPA were detected by RT-PCR analysis in
the MDA-MB-231 cells and cells co-cultured with NK-92 cells for 48
h (t-test, **P<0.01, compared with MDA-MB-231 cells cultured
alone). (C and D) Immunoblot analysis (C) and the
casein-plasminogen zymogram assay (D) were performed (t-test,
*P<0.05 and **P<0.01, compared with MDA-MB-231 cells cultured
alone, respectively). (E) Immunoblot analysis was performed. (F)
ChIP assay was performed using c-Jun primers specific to the AP-1
binding site in the uPA gene promoter. uPA, urokinase-type
plasminogen activator; MMPs, matrix metalloproteinases; ATF-2,
activating transcription factor 2; p, phosphorylated. |
To identify the transcription factor(s) responsible
for NK-92-induced uPA downregulation, we detected the expression of
c-Jun, c-Fos, ATF-2, and p65 NF-ĸB, which are known uPA
transcription factors (29). As
shown in Fig. 1E, c-Jun was reduced
by co-culture with NK-92 cells, while the others were not affected.
The binding of c-Jun to the promoter region of uPA was markedly
inhibited by co-culture with NK-92 cells as evidenced by the ChIP
assay (Fig. 1F). These data
implicate the involvement of c-Jun in the transcriptional
regulation of uPA by NK-92 cells.
Secretion of cytokines CCL5, IL-6,
CCL2, and IL-8 from NK-92 cells is increased by co-culture with
MDA-MB-231 cells
We hypothesized that factor(s) secreted into the CM
of co-cultured cells might play a role in the inhibition of
invasion and downregulation of uPA in MDA-MB-231 cells. To test
this, we conducted a cytokine array and compared the cytokines
secreted from mono-cultured MDA-MB-231 cells to cells co-cultured
with NK-92 cells. Human cytokine antibody array analysis showed
that the levels of IL-6, IL-8, IL-10, and CCL2 and CCL5 were
increased in the CM of co-cultured cells compared to those in the
CM of mono-cultured MDA-MB-231 cells (Fig. 2A). The cytokines increased by
co-culture with NK-92 cells are listed as a table (Fig. 2A, bottom panel).
Next, RT-PCR analysis was conducted to determine
whether these cytokines were secreted from MDA-MB-231 cells or
NK-92 cells upon co-culture. The mRNA levels of IL-6, CCL2, and
IL-8 were increased in MDA-MB-231 cells co-cultured with NK-92
cells (Fig. 2B, left). The mRNA
levels of CCL5, IL-6, CCL2, and IL-8, but not IL-10, were increased
in NK-92 cells upon co-culture with MDA-MB-231 cells (Fig. 2B, right). The mRNA levels of tumor
necrosis factor (TNF)-α and interferon (IFN)-γ were not altered by
co-culture (Fig. S1A and B). In
addition, the expression levels of IL-6, IL-8, and CCL-2 in NK-92
cells were increased by co-culture with MDA-MB-231 cells (Fig. S1C). The secretion of IL-6, IL-8,
and CCL2 from both MDA-MB-231 and NK-92 cells was increased by
co-culture.
IL-6 downregulates uPA in MDA-MB-231
cells
To examine the cytokines involved in NK-92-induced
uPA downregulation, uPA was analyzed in MDA-MB-231 cells treated
individually with CCL5, IL-6, CCL2, or IL-8, whose secretion from
NK-92 cells was increased by co-culture. Treatment with recombinant
human (rh)IL-6 at concentrations of 50 and 100 ng/ml reduced the
uPA mRNA levels in a concentration-dependent manner (Fig. 3A). Neither rhCCL5, nor rhCCL2
affected the level of uPA mRNA. In contrast, rhIL-8 increased the
level of uPA. Treatment with rhIL-6 decreased uPA protein (Fig. 3B) and activity (Fig. 3C) in a concentration-dependent
manner, with a significant inhibition observed at 100 ng/ml. The
binding of c-Jun to the uPA gene promoter was inhibited by
treatment with rhIL-6 at 100 ng/ml as evidenced by the ChIP assay
(Fig. 3D). These results indicate
that a high concentration of rhIL-6 (100 ng/ml) caused a marked
downregulation of uPA in the MDA-MB-231 cells.
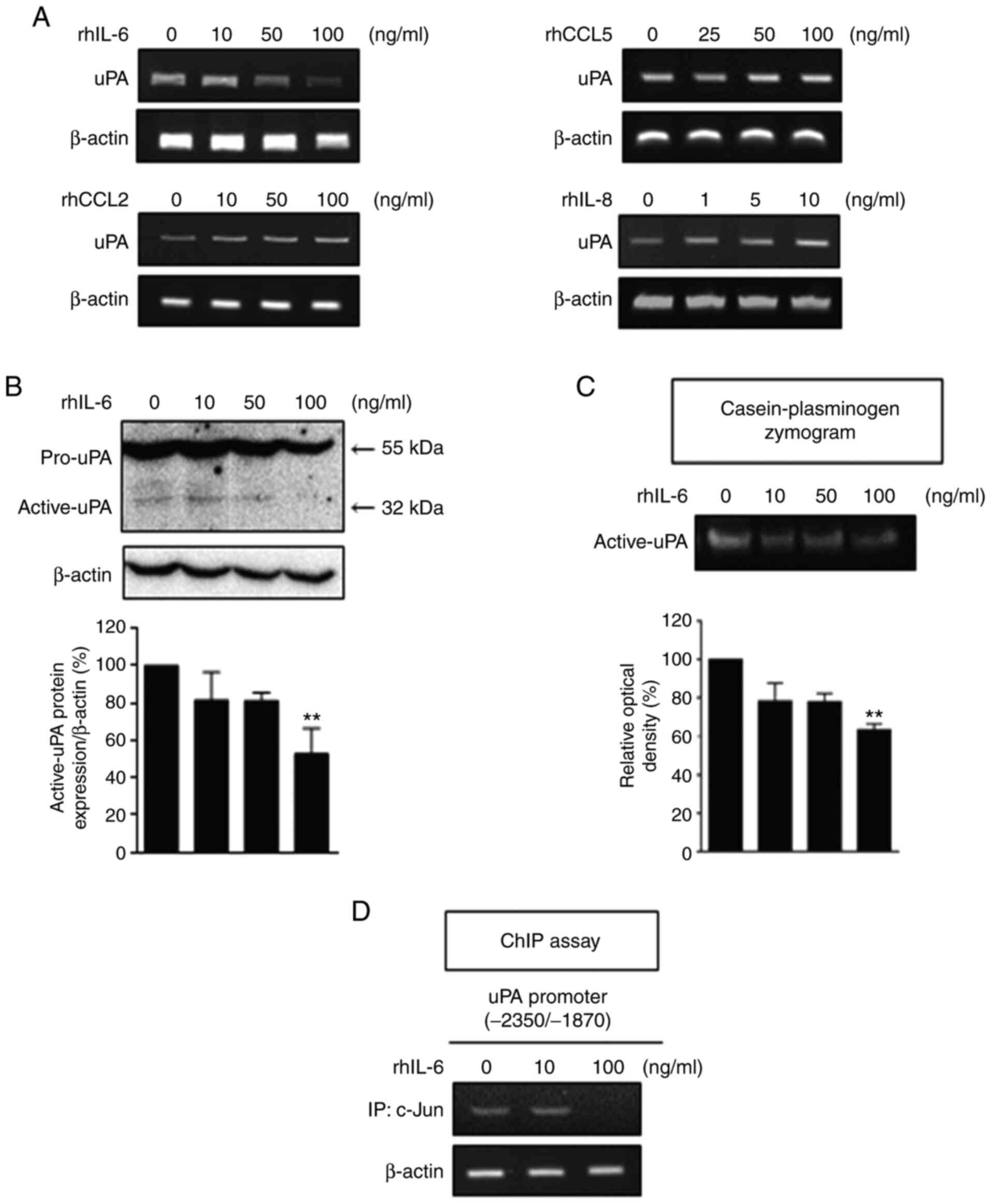 | Figure 3.Expression of uPA is reduced by rhIL-6
in MDA-MB-231 cells. (A) The mRNA levels of uPA in MDA-MB-231 cells
treated with the indicated concentrations of rhIL-6, rhCCL5,
rhCCL2, and rhIL-8 for 48 h were detected by RT-PCR analysis. (B
and C) Immunoblot analysis and the casein-plasminogen zymogram
assay were conducted to detect the protein levels (B) and activity
(C) of uPA in MDA-MB-231 cells treated with various concentrations
of rhIL-6 for 48 h (one-way ANOVA, **P<0.01, compared with
rhIL-6 0 ng/ml, respectively). (D) The ChIP assay was performed to
detect the binding of the indicated proteins to specific regions of
the uPA gene in MDA-MB-231 cells treated with 10 and 100
ng/ml rhIL-6 for 48 h. rh, recombinant human; uPA, urokinase-type
plasminogen activator; IL, interleukin; CCL, C-C motif ligand. |
IL-6 plays a crucial role in uPA
downregulation in TNBC cells
To determine the functional significance of IL-6, we
treated the MDA-MB-231 cells with a neutralizing antibody
(Ab-IL-6). The reduced expression of uPA by co-culture with NK-92
was significantly increased by the neutralization of IL-6 using
Ab-IL-6, both at the mRNA (Fig. 4A)
and the protein level (Fig. 4B), as
shown by RT-PCR and immunoblot analysis, respectively. The
downregulation of uPAR protein levels by co-culture with NK-92 was
recovered by Ab-IL-6 (Fig. 4C).
We further investigated the inhibitory effect of
NK-92 on uPA in another TNBC cell line, the Hs578T cell line. As
shown in Fig. 4D and E, co-culture
with NK-92 cells inhibited the uPA expression in Hs578T TNBC cells.
The reduced uPA mRNA and protein levels by co-culture were
recovered by Ab-IL-6 in the Hs578T cells. However, in the MCF-7
cell line, which is a non-TNBC cell line, the uPA mRNA and protein
levels were not affected by co-culture with NK-92 cells (Fig. S2A and B). These data suggest that
the inhibitory effect of NK-92 on uPA may be specific to TNBC
cells.
In addition, treatment with Ab-IL-6 markedly
reversed the uPA mRNA and protein levels decreased by rhIL-6 (100
ng/ml) (Fig. 5A and B,
respectively). The effect of IL-6 on uPA was further investigated
in Hs578T TNBC cells. As shown in Fig.
5C and D, IL-6 decreased the uPA mRNA and protein levels in
Hs578T TNBC cells. These levels were recovered by Ab-IL-6 in the
Hs578T cells. These results demonstrate that IL-6 secreted by NK-92
cells played a crucial role in the downregulation of uPA in TNBC
cells.
A neutralizing antibody against IL-6
rescues the NK cell-inhibited invasion in TNBC cells
Next, we investigated the role of IL-6 in regulating
the invasive capacity of cells. As shown in Fig. 6A, treatment with Ab-IL-6
significantly restored the MDA-MB-231 cell invasive phenotype that
was inhibited by NK-92 cells. The CM of NK-92 cells also inhibited
the invasive phenotype of Hs578T TNBC cells, and the decreased
invasion was recovered by treatment with Ab-IL-6 (Fig. 6B). In contrast, there was no change
in the invasive phenotype of MCF-7 cells after co-culture with
NK-92 cells (Fig. S2C). These data
suggest a TNBC cell-specific inhibition of the invasive phenotype
by NK-92 cell CM. When MDA-MB-231 cells were treated with rhIL-6 at
100 ng/ml, the invasive phenotype of MDA-MB-231 cells was
significantly inhibited (Fig. 6C).
However, treatment with rhIL-6 at lower concentrations did not
significantly inhibit invasion. These data suggest that the
inhibitory effect of NK-92 cells on TNBC cell invasion may be due
to the increased secretion of IL-6, which inhibits uPA expression
and activation.
The IL-6/uPA ratio is correlated with
the overall survival of breast cancer patients
To examine the clinical relevance of our in
vitro data, we analyzed the correlation of IL-6 and uPA with
the overall survival of breast cancer patients using the Gene
Expression Profiling Interactive Analysis (GEPIA) database
(30). The Kaplan-Meier survival
analysis revealed that neither IL-6 nor uPA (PLAU) were
significantly correlated with overall patient survival (Fig. 6D, left and center, respectively). Of
note, a low IL-6/uPA ratio was significantly associated with the
poor survival of breast cancer patients, compared to a high ratio
(P<0.05) (Fig. 6D, right). The
GEPIA data suggest that the combination of low IL-6 and high uPA
may be crucial for malignant breast cancer, suggesting that the
ratio of IL-6 to uPA is a key factor in determining the overall
survival of breast cancer patients. Taken together, our findings
demonstrated that NK-92 cells downregulated uPA through IL-6,
resulting in the inhibition of the invasive phenotype of TNBC cells
(Fig. 6E).
Discussion
Mounting evidence suggests a role of natural killer
(NK) cells in the regulation of cancer metastasis, primarily
through immunosurveillance by NK cells, which recognizes and kills
metastatic cells (11). In the
present study, we clearly demonstrated that the invasive phenotype
of triple-negative breast cancer (TNBC) cells was significantly
inhibited by co-culture with NK-92 cells. Our results suggest that
NK-92 cells within the tumor microenvironment not only kill cancer
cells via immunosurveillance but can also regulate the metastatic
capability of cancer cells. Consistent with our results, a recent
paper showed that the migration and invasion of ovarian carcinoma
cells were inhibited by NK cells (14).
In an effort to identify matrix-degrading enzymes
that could be involved in NK-inhibited TNBC cell invasion, the
levels of various matrix metalloproteinases (MMPs) were measured,
as well as urokinase-type plasminogen activator (uPA) in MDA-MB-231
cells co-cultured with NK-92 cells. Here, we showed, for the first
time, that NK-92 cells downregulated uPA, which plays a crucial
role in the inhibition of an invasive phenotype of TNBC cells. A
growing body of evidence supports a role for uPA as a prognostic
factor in breast cancer (31). High
levels of uPA significantly correlate with tumor aggressiveness and
poor outcomes in breast cancer (32,33).
These results, in conjunction with our findings, suggest that the
inhibition of uPA may be able to control the aggressive progression
of breast cancer.
NK cell recognition of infected cells or cancer
cells stimulates cytokine secretion (34). Among the cytokines secreted from
NK-92 cells upon co-culture, interleukin (IL)-6 inhibited the
expression and activity of uPA in MDA-MB-231 cells, shown by
RT-PCR, immunoblot, and casein-plasminogen zymogram analysis. The
ChIP assay showed that IL-6 decreased the binding of c-Jun to the
uPA promoter. By using a neutralizing antibody against IL-6, the
inhibitory effect of IL-6 on uPA downregulation and invasion in
TNBC cells was further confirmed.
Treatment with rhIL-6 at a high concentration (100
ng/ml) significantly inhibited the invasive phenotype of MDA-MB-231
cells, implying that the increased secretion of IL-6 by NK-92 cells
exerted an inhibitory effect on invasion through uPA
downregulation. However, in contrast to our results, elevated
levels of IL-6 were shown to be correlated with aggressive tumor
growth in several types of cancer, including nasopharyngeal,
esophageal, and pancreatic cancers (35–37).
The reported tumor-promoting role of IL-6 was due mostly to the
stimulation of tumor cell proliferation and survival through
activation of the PI3K, MEK, and JAK/STAT pathways (38–40).
Of note, uPA downregulation was observed at low concentrations of
IL-6, whereas the invasive phenotype of the TNBC cells was not
inhibited at these concentrations. These results suggest that the
inhibitory effect of IL-6 on tumor cell invasion may only be
achieved at relatively high concentrations. In support of this
hypothesis, the GEPIA analysis showed that a high IL-6/uPA ratio
was more advantageous to the overall survival of breast cancer
patients. Moreover, the apparent discrepancy between these earlier
reports and our findings may also be explained, at least in part,
by the fact that our experimental system was a co-culture system
with TNBC cells and NK-92 cells, and therefore, lacks other
surrounding immune cells, such as T cells and macrophages. The
inhibition of uPA activity by IL-6 may be limited to TNBC cells,
but these results have extraordinary significance for breast cancer
metastasis and breast cancer treatment.
A growing number of studies have attempted to target
the uPA-uPAR system to suppress cancer (41,42).
The present study clearly demonstrates that NK-92 cells inhibited
the invasive phenotype of TNBC cells via the downregulation of uPA.
Based on these findings, we propose that uPA may be a promising
target for therapeutic strategies against TNBC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea (No. 2016R1A6A1A03007648 and
2019R1A2C1009773).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HJ, HJC, ESK and AM conceived and designed the
study. HJ and HJC performed the experiments. HJ, HJC, ESK, HHL, HSC
and AM analyzed and interpreted the data. HJ, HJC and AM
contributed to the manuscript drafting and writing. HJ, HSC and AM
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved. AM supervised
the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bouchardy C, Fioretta G, Verkooijen HM,
Vlastos G, Schaefer P, Delaloye JF, Neyroud-Caspar I, Balmer Majno
S, Wespi Y, Forni M, et al: Recent increase of breast cancer
incidence among women under the age of forty. Br J Cancer.
96:1743–1746. 2007. View Article : Google Scholar
|
|
2
|
Curado MP: Breast cancer in the world:
Incidence and mortality. Salud Publica Mex. 53:372–384. 2011.
|
|
3
|
Redig AJ and McAllister SS: Breast cancer
as a systemic disease: A view of metastasis. J Intern Med.
274:113–126. 2013. View Article : Google Scholar
|
|
4
|
Nakhjavani M, Hardingham JE, Palethorpe
HM, Price TJ and Townsend AR: Druggable molecular targets for the
treatment of triple negative breast cancer. J Breast Cancer.
22:341–361. 2019. View Article : Google Scholar
|
|
5
|
Bayraktar S and Glück S: Molecularly
targeted therapies for metastatic triple-negative breast cancer.
Breast Cancer Res Treat. 138:21–35. 2013. View Article : Google Scholar
|
|
6
|
Kenny PA, Nelson CM and Bissell MJ: The
Ecology of Tumors: By perturbing the microenvironment, wounds and
infection may be key to tumor development. Scientist.
20:302006.
|
|
7
|
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu
Y, Gong Z, Zhang S, Zhou J, Cao K, et al: Role of tumor
microenvironment in tumorigenesis. J Cancer. 8:761–773. 2017.
View Article : Google Scholar
|
|
8
|
Wu J and Lanier LL: Natural killer cells
and cancer. Adv Cancer Res. 90:127–156. 2003. View Article : Google Scholar
|
|
9
|
Cheng M, Chen Y, Xiao W, Sun R and Tian Z:
NK cell-based immunotherapy for malignant diseases. Cell Mol
Immunol. 10:230–252. 2013. View Article : Google Scholar
|
|
10
|
Mandal A and Viswanathan C: Natural killer
cells: In health and disease. Hematol Oncol Stem Cell Ther.
8:47–55. 2015. View Article : Google Scholar
|
|
11
|
López-Soto A, Gonzalez S, Smyth MJ and
Galluzzi L: Control of metastasis by NK cells. Cancer Cell.
32:135–154. 2017. View Article : Google Scholar
|
|
12
|
Guillerey C, Huntington ND and Smyth MJ:
Targeting natural killer cells in cancer immunotherapy. Nat
Immunol. 17:1025–1036. 2016. View
Article : Google Scholar
|
|
13
|
Jiao Y, Huntington ND, Belz GT and Seillet
C: Type 1 innate lymphoid cell biology: Lessons learnt from natural
killer cells. Front Immunol. 7:4262016. View Article : Google Scholar
|
|
14
|
Sun Y, Yao Z, Zhao Z, Xiao H, Xia M, Zhu
X, Jiang X and Sun C: Natural killer cells inhibit metastasis of
ovarian carcinoma cells and show therapeutic effects in a murine
model of ovarian cancer. Exp Ther Med. 16:1071–1078. 2018.
|
|
15
|
Aznavoorian S, Murphy AN,
Stetler-Stevenson WG and Liotta LA: Molecular aspects of tumor cell
invasion and metastasis. Cancer. 71:1368–1383. 1993. View Article : Google Scholar
|
|
16
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar
|
|
17
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar
|
|
18
|
Song H, Ki SH, Kim SG and Moon A:
Activating transcription factor 2 mediates matrix
metalloproteinase-2 transcriptional activation induced by p38 in
breast epithelial cells. Cancer Res. 66:10487–10496. 2006.
View Article : Google Scholar
|
|
19
|
Lee S, Lee E, Ko E, Ham M, Lee HM, Kim ES,
Koh M, Lim HK, Jung J, Park SY and Moon A: Tumor-associated
macrophages secrete CCL2 and induce the invasive phenotype of human
breast epithelial cells through upregulation of ERO1-α and MMP-9.
Cancer Lett. 437:25–34. 2018. View Article : Google Scholar
|
|
20
|
Pepper MS: Role of the matrix
metalloproteinase and plasminogen activator-plasmin systems in
angiogenesis. Arterioscler Thromb Vasc Biol. 21:1104–1117. 2001.
View Article : Google Scholar
|
|
21
|
Kumari S and Malla R: New insight on the
role of plasminogen receptor in cancer progression. Cancer Growth
Metastasis. 8:35–42. 2015. View Article : Google Scholar
|
|
22
|
Lijnen HR: Matrix metalloproteinases and
cellular fibrinolytic activity. Biochemistry (Mosc). 67:92–98.
2002. View Article : Google Scholar
|
|
23
|
Wilkins-Port CE, Higgins SP, Higgins CE,
Kobori-Hotchkiss I and Higgins PJ: Complex regulation of the
pericellular proteolytic microenvironment during tumor progression
and wound repair: Functional interactions between the serine
protease and matrix metalloproteinase cascades. Biochem Res Int.
2012:4543682012. View Article : Google Scholar
|
|
24
|
Holst-Hansen C, Johannessen B,
Høyer-Hansen G, Rømer J, Ellis V and Brünner N: Urokinase-type
plasminogen activation in three human breast cancer cell lines
correlates with their in vitro invasiveness. Clin Exp Metastasis.
14:297–307. 1996.
|
|
25
|
Crippa MP: Urokinase-type plasminogen
activator. Int J Biochem Cell Biol. 39:690–694. 2007. View Article : Google Scholar
|
|
26
|
Mauro CD, Pesapane A, Formisano L, Rosa R,
D'Amato V, Ciciola P, Servetto A, Marciano R, Orsini RC, Monteleone
F, et al: Urokinase-type plasminogen activator receptor (uPAR)
expression enhances invasion and metastasis in RAS mutated tumors.
Sci Rep. 7:93882017. View Article : Google Scholar
|
|
27
|
Mahmood N, Mihalcioiu C and Rabbani SA:
Multifaceted role of the urokinase-type plasminogen activator (uPA)
and its receptor (uPAR): Diagnostic, prognostic, and therapeutic
applications. Front Oncol. 8:242018. View Article : Google Scholar
|
|
28
|
Moon A, Kim MS, Kim TG, Kim SH, Kim HE,
Chen YQ and Kim HR: H-ras, but not N-ras, induces an invasive
phenotype in human breast epithelial cells: A role for MMP-2 in the
H-ras-induced invasive phenotype. Int J Cancer. 85:176–181. 2000.
View Article : Google Scholar
|
|
29
|
Stepanova V, Jayaraman PS, Zaitsev SV,
Lebedeva T, Bdeir K, Kershaw R, Holman KR, Parfyonova YV, Semina
EV, Beloglazova IB, et al: Urokinase-type plasminogen activator
(uPA) promotes angiogenesis by attenuating proline-rich homeodomain
protein (PRH) transcription factor activity and de-repressing
vascular endothelial growth factor (VEGF) receptor expression. J
Biol Chem. 291:15029–15045. 2016. View Article : Google Scholar
|
|
30
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar
|
|
31
|
Lampelj M, Arko D, Cas-Sikosek N, Kavalar
R, Ravnik M, Jezersek-Novakovic B, Dobnik S, Dovnik NF and Takac I:
Urokinase plasminogen activator (uPA) and plasminogen activator
inhibitor type-1 (PAI-1) in breast cancer-correlation with
traditional prognostic factors. Radiol Oncol. 49:357–364. 2015.
View Article : Google Scholar
|
|
32
|
Annecke K, Schmitt M, Euler U, Zerm M,
Paepke D, Paepke S, von Minckwitz G, Thomssen C and Harbeck N: uPA
and PAI-1 in breast cancer: Review of their clinical utility and
current validation in the prospective NNBC-3 trial. Adv Clin Chem.
45:31–45. 2008. View Article : Google Scholar
|
|
33
|
Duffy MJ, McGowan PM, Harbeck N, Thomssen
C and Schmitt M: uPA and PAI-1 as biomarkers in breast cancer:
Validated for clinical use in level-of-evidence-1 studies. Breast
Cancer Res. 16:4282014. View Article : Google Scholar
|
|
34
|
Fauriat C, Long EO, Ljunggren HG and
Bryceson YT: Regulation of human NK-cell cytokine and chemokine
production by target cell recognition. Blood. 115:2167–2176. 2010.
View Article : Google Scholar
|
|
35
|
Sun W, Liu DB, Li W, Zhang LL, Long GX,
Wang JF, Mei Q and Hu GQ: Interleukin-6 promotes the migration and
invasion of nasopharyngeal carcinoma cell lines and upregulates the
expression of MMP-2 and MMP-9. Int J Oncol. 44:1551–1560. 2014.
View Article : Google Scholar
|
|
36
|
Razidlo GL, Burton KM and McNiven MA:
Interleukin-6 promotes pancreatic cancer cell migration by rapidly
activating the small GTPase CDC42. J Biol Chem. 293:11143–11153.
2018. View Article : Google Scholar
|
|
37
|
Gopinathan G, Milagre C, Pearce OM,
Reynolds LE, Hodivala-Dilke K, Leinster DA, Zhong H, Hollingsworth
RE, Thompson R, Whiteford JR and Balkwill F: Interleukin-6
stimulates defective angiogenesis. Cancer Res. 75:3098–3107. 2015.
View Article : Google Scholar
|
|
38
|
Wegiel B, Bjartell A, Culig Z and Persson
JL: Interleukin-6 activates PI3K/Akt pathway and regulates cyclin
A1 to promote prostate cancer cell survival. Int J Cancer.
122:1521–1529. 2008. View Article : Google Scholar
|
|
39
|
Hsu CY, Bristow R, Cha MS, Wang BG, Ho CL,
Kurman RJ, Wang TL and Shih IeM: Characterization of active
mitogen-activated protein kinase in ovarian serous carcinomas. Clin
Cancer Res. 10:6432–6436. 2004. View Article : Google Scholar
|
|
40
|
Leu CM, Wong FH, Chang C, Huang SF and Hu
CP: Interleukin-6 acts as an antiapoptotic factor in human
esophageal carcinoma cells through the activation of both STAT3 and
mitogen-activated protein kinase pathways. Oncogene. 22:7809–7818.
2003. View Article : Google Scholar
|
|
41
|
Ulisse S, Baldini E, Sorrenti S and
D'Armiento M: The urokinase plasminogen activator system: A target
for anti-cancer therapy. Curr Cancer Drug Targets. 9:32–71. 2009.
View Article : Google Scholar
|
|
42
|
Sidenius N and Blasi F: The urokinase
plasminogen activator system in cancer: Recent advances and
implication for prognosis and therapy. Cancer Metastasis Rev.
22:205–222. 2003. View Article : Google Scholar
|




















