Introduction
De novo metastatic breast cancer (dnMBC),
namely MBC at the initial diagnosis, comprises 5–10% of all newly
diagnosed breast cancer cases worldwide (1–4).
Compared with recurrent MBC, dnMBC is associated with a notably
improved 5-year disease specific survival (DSS) rate (44 vs. 21%)
and an improved 5-year DSS over time (55% in 2005–2010 vs. 28% in
1990–1998) (5). With the
improvement of diagnostic and intervention methods in the field of
breast cancer, the median overall survival (OS) of patients with
dnMBC has also improved over time (6). The 3-year OS rate of patients with
dnMBC increased from 38.7% in the 1990s to 70.1% in 2010–2014 in
candidates diagnosed in Korea (7).
The 10-year OS rate has been shown to be ~13% (4).
Historically, dnMBC was regarded as incurable and
systemic strategy remained the cornerstone of treatment. Local
treatment of the primary tumor (PT) has been reserved as a
palliative treatment to control symptoms, such as skin ulcerations,
bleeding and pain (8). However, an
emerging collection of retrospective studies determined that
complete PT removal was beneficial for the survival of patients
with dnMBC (3,7–13).
Bhoo-Pathy et al (14)
revealed that in an Asian setting, a higher proportion of patients
with dnMBC underwent PT surgery with free margins in recent years.
Data from Europe and the USA have indicated that ~40% of patients
with dnMBC underwent local radiotherapy (LRT) (2,8,15,16).
These findings have been questioned due to study limitations, such
as selection bias, stage migration and the retrospective nature.
Previous prospective trials have provided conflicting results
(17–19). The negative findings presented in
certain prospective trials may be the result of the underuse of
systemic therapy according to current principles (19). A number of meta-analyses have
indicated that surgical excision of the PT leads to a survival
advantage in the dnMBC population (20–23). A
recent meta-analysis, including 42 studies and 216,066 patients
with dnMBC, documented that all forms of LRT reduced the mortality
rate by 31.8% (24). Modern
systemic therapy strategies grant patients with dnMBC a longer
survival and an improved control of metastatic lesions, which
encourages clinicians to explore the use of LRT in the treatment of
dnMBC (25,26). The present review aimed to identify
the optimal means and timing of LRT for the treatment of dnMBC and
the population most likely to benefit from LRT, thus providing an
important reference for clinical practice.
Heterogeneity of dnMBC
dnMBC is highly heterogeneous
This heterogeneity is manifested in different
molecular types, gene signatures, metastatic patterns and
demographic variables, and these factors interrelate, leading to
different outcomes between individuals (27). In a previous study on 7,986 patients
with dnMBC from the SEER database from the years 2010–2016, hormone
receptor (HR)+/human epidermal growth factor
(HER)2− was the most common subtype (57.6%), followed by
HR+/HER2+ (19.0%), triple-negative breast
cancer (TNBC; 13.6%) and HR−/HER2+ (9.8%)
(27). In addition, 50.3% of dnMBC
tumors were poorly differentiated or undifferentiated (27). The prognosis of patients with de
novo invasive lobular carcinoma may be worse than that of those
with de novo invasive ductal carcinoma (28). The majority of patients with dnMBC
suffer from regional lymph node metastasis, suggesting that
regional involvement may precede distant metastasis (29). The most common distant metastatic
site is bone, accounting for 73.4% of sites, while the brain is the
least common disseminated organ (6.9%) (27). Different subtypes of dnMBC
demonstrate specific predilection in distant metastasis. A previous
study demonstrated that luminal tumors were more likely to
metastasize to bone [P=0.02; bone vs. other organs, including the
liver, lungs and central nervous system (CNS)] (30). The upregulated HER2 subtype harbored
a liver-homing feature (P=0.0004; liver vs. other sites), while
TNBC subtypes were inclined to metastasize to the lungs (P=0.01;
lung vs. other sites) (30).
dnMBC differs from relapsed breast
cancer (31)
During adjuvant systemic treatment, more
treatment-resistant tumor clones develop, resulting in disease
progression. Therefore, refractory MBC generally harbors a more
dismal prognosis than dnMBC (27,32–36).
Following active treatment, some patients with dnMBC can obtain a
long survival time (37).
Clinicopathologic parameters defining
the prognosis of patients with dnMBC
The Epidemio-Strategy-Medical-Economical (ESME)-MBC
data platform (NCT03275311) included 16,702 patients with dnMBC
from January 1, 2008 to December 31, 2014 (38). Of these patients, the median OS
rates of patients with the HR+/HER2−
(n=9,907), HER2+ (n=2,861) and TNBC (n=2,317) subtypes
were 42.12, 44.91 and 14.52 months, respectively. Elbaiomy et
al (39) found that the TNBC
subtype of dnMBC highly expressed breast cancer stem cell (BCSC)
markers (CD44+ and CD24−), which predicted a
poor treatment response, and shorter OS and progression-free
survival (PFS) times. In the future, BCSC marker expression may be
used to tailor the treatment strategy for patients with dnMBC, and
BCSC target therapy may be a promising strategy (39). Dawood et al (40) reported that the number of patients
with dnMBC surviving >2 years increased over time. A younger
age, lower tumor grade, HR positivity, non-inflammatory disease and
definitive primary resection contributed to this trend. As
previously demonstrated, compared with patients with dnMBC aged
40–59 years, those aged <40 years had a longer survival time,
particularly those with the TNBC subtype (41). An uninsured status and older age
have also been shown to be associated with early mortality in
patients with dnMBC (42).
Immediate breast reconstruction has also been shown to not affect
survival outcomes when compared with mastectomy in patients with
dnMBC (43). In 2002, Khan et
al (44) performed a
comprehensive retrospective study of 16,023 patients with dnMBC in
the duration of 1990–1993 using the National Cancer Database. Their
findings revealed that four parameters determined the prognosis of
patients with dnMBC, including surgical removal of the PT, the
administration of systemic treatment, metastatic burden and tumor
type (visceral or soft tissue). The retrospective study by Soares
et al (45) demonstrated
that breast surgery (hazard ratio, 0.45) and initial CNS metastasis
(hazard ratio, 3.09) were independent prognostic factors for
patients with dnMBC. In 2006, Babiera et al (11) analyzed 224 patients with dnMBC and
revealed that only one metastatic site and no HER2 gene
amplification indicated a more favorable OS, while estrogen
receptor (ER) positivity and PT resection were closely linked to an
improved metastatic PFS (MPFS). Similarly, a previous multivariate
analysis of prognostic factors and OS in 395 dnMBC cohorts
performed by Blanchard et al (46) found that definitive primary surgery,
HR positivity and a low number of metastases were associated with
an improved prognosis. King et al (47) re-evaluated the data of dnMBC cohorts
enrolled in the Translational Breast Cancer Research Consortium
(TBCRC) 013 program and screened out 101 patients with 21-gene
recurrence score (RS) results. In the
ER+/HER2− (n=69) subgroup, a 21-gene low risk
(RS <18) score was a strong indicator of an improved 2-year OS
and time to first progression (47). The metastatic pattern has a profound
impact on the prognosis of patients with dnMBC. The OS times of
visceral, nodal and bone-only metastases are approximately <6
months, 18 months and 3–4 years, respectively (48). Lin et al (49) determined that the allocation and
number of metastatic sites were independent prognostic factors for
dnMBC. Therefore, they proposed the subdivision of the M1 stage
criteria in a heterogeneous setting: i) M1a, a single metastatic
site excluding the liver and brain; ii) M1b, multiple metastatic
sites excluding the liver and brain, or liver only involvement; and
iii) M1c, liver plus other metastatic sites, or brain only
involvement. The M1a subtype benefited the most from PT resection,
while the upstaging of M1 was related to a higher benefit from
chemotherapy alone (49). In 2020,
Plichta et al (50) proposed
a novel dnMBC staging system for prognosis. The proposed recursive
partitioning analysis including anatomical and biological factors
to refine prognostic estimates. The distant metastatic site number
and ER status were identified as the first and second
stratification points, respectively, and the HER2 status, clinical
T stage and tumor grade were incorporated for further divisions.
Subsequently, dnMBC was classified into three stages: IVA, IVB and
IVC, with a 3-year OS rate of >50, 30–50 and <30%,
respectively (50).
Genomic mutations in dnMBC
The genomic analysis of dnMBC was previously
conducted to reveal the drivers contributing to the early onset of
metastasis (51). In
HR+/HER2− dnMBC, PIK3CA mutations were
enriched (41.9%), indicating that PI3K inhibitors may play a
critical role in the treatment of this subset. Additionally,
KMTD2 and SETD2 mutations were more common, while
TP53 and BRCA1 were less common in
HR+/HER2− dnMBC, compared with recurrent MBC.
In TNBC dnMBC, MYB amplification was prevalent and high
tumor mutation burden predicted a better prognosis (51). PTEN mutations were more frequent in
dnMBC, which are closely associated with immune evasion (52). As previously demosntrated, 11.8% of
patients with dnMBC harbored a BRCA2 mutation, indicating
that BRCA germline testing was necessary (53).
In dnMBC, with the aim of optimizing disease
monitoring, active image evaluation, such as positron emission
tomography-computed tomography scanning and magnetic resonance
imaging, for CNS screening and tumor marker surveillance is
encouraged (54).
Timing of LRT for dnMBC
Initial LRT for dnMBC
Thomas et al (2) conducted the largest retrospective
study of dnMBC (n=21,372) using the SEER database spanning 24 years
(1988–2011). Patients undergoing surgery as initial therapy
(n=8,330) harbored a significantly longer median OS time than those
who did not (n=13,042) (28 vs. 19 months) (2). Additionally, MF 07–01, the first
randomized trial, also verified the OS benefit gained by upfront
LRT in dnMBC (17). A total of 274
candidates were randomized into two groups: The upfront LRT
followed by systemic treatment group (n=138), and the systemic
therapy only group (n=136). The LRT arm demonstrated a notable OS
advantage (median OS time, 46 vs. 37 months, P=0.005; 5-year OS
rate, 41.6 vs. 24.4%). Unplanned subgroup analyses found that
patients with the ER/PR+ (P=0.008) and HER2−
(P=0.01) subtypes, an age <55 years (P=0.007) and solitary
bone-only metastases (P=0.04) benefited the most from LRT (17). Despite more favorable features in
the LRT arm, such as a higher proportion of ER/PR+ (85.5
vs. 71.8%: P<0.05) and lower rates of TNBC (7.3 vs. 17.4%;
P<0.05), the MF 07–01 trial highlighted that in a well-selected
population of dnMBC, the upfront LRT may lead to a survival benefit
(17). In the study by Shien et
al (55), 344 patients with
dnMBC were divided into two groups: The local surgery (n=160) and
no local surgery (n=184) groups. A total of 150 (94%) patients in
the surgery group had primarily local surgery. Following a median
follow-up time of 33 months, the local surgery group attained a
superior OS time compared with the no surgery group (median OS
time, 27 vs. 22 months; P=0.049), notably in younger individuals
(<50 years old) (median OS time, 35 vs. 24 months: P=0.021)
(55).
In 2017, Barinoff et al (56) re-analyzed two prospective trials
conducted in Germany with patients with MBC administered targeted
therapy (ML16684: Started in 2000, trastuzumab for HER2+
patients; ML21165: Started in 2007, bevacizumab for
HER2− patients). Among the 570 patients with dnMBC (405
from ML16684 and 165 from ML21165), 2 patients had no data on local
resection, 426 patients underwent upfront breast surgery and 142
patients did not undergo breast surgery (56). The surgery cohort had less patients
with T4 staging and patients with a lower metastatic tumor burden,
but more patients with N3 staging. Other characteristics, such as
HR and HER2 status, Eastern Cooperative Oncology Group (ECOG) score
and prior palliative management, were equally distributed in the
surgery and no surgery cohorts. However, upfront primary surgery
failed to bring a benefit for either PFS (surgery vs. no surgery,
13.55 vs. 11.76 months; P=0.18) or OS (surgery vs. no surgery, 34.1
vs. 31.7 months; P=0.23). Nonetheless, in the subgroup without
visceral metastasis, the removal of the PT manifested an overt OS
time advantage (45.7 vs. 27.2 months; P=0.026) (56).
On the contrary, the ABCSG-28 POSYTIVE trial, a
prospective randomized study aiming to evaluate the effect of
upfront surgery in untreated patients with dnMBC, drew the opposite
conclusion (18). In 2011–2015, 90
untreated candidates with dnMBC were randomly assigned into two
groups: The upfront PT surgery followed by systemic therapy (group
A, n=45) and systemic therapy only (group B, n=45) groups. Upfront
surgery failed to bring any survival benefit (group A vs. B; median
survival time, 34.6 vs. 54.8 months, P=0.267; time to distant
progression, 13.9 vs. 29.0 months, P=0.0668) (18). This trial was strengthened by a
well-balanced design regarding critical prognostic factors, but was
criticized for insufficient recruitment and early closing of the
study, which underpowered the conclusion (18).
Upfront systemic therapy for
dnMBC
Co et al (57) and Kwong and Co (58) conducted a retrospective study on 172
patients with dnMBC in Hong Kong between 2007–2016. Following
systemic intervention for 8–10 months, resection of the PT was
conducted in 91 good responders, who gained a notably improved
5-year OS rate (43.9 vs. 33.9%; P=0.026) (57,58)
Additionally, Rao et al (59) analyzed the optimal time for surgery
for patients with stage IV breast cancer initially with a complete
PT excision. In their study, 75 patients were divided into three
groups according to the interval between the diagnosis of dnMBC and
surgery of the PT: Group 1, 0–2.9 months (n=47); group 2, 3–8.9
months (n=14); and group 3, ≥9.0 months (n=14). Notably, group 2
had the longest MPFS (P=0.0008), despite not having an OS
superiority (P=0.12), particularly in patients with a single
metastatic site and negative margin (59). A rational explanation for this was
that systemic chemotherapy was completed within 3–9 months after
diagnosis and those good responders benefited the most from
curative surgical management. However, this conclusion was limited
by the small sample size and retrospective nature of the study
(59).
Lane et al (8) conducted a large-scale retrospective
study using the American College of Surgeons National Cancer
Database for the years 2003–2012. In total, 24,015 patients with
dnMBC who were alive 1 year after diagnosis were enrolled in their
study. The candidates were divided into three groups according to
the treatment sequence: The systemic treatment only (n=13,505), the
upfront surgery before systemic treatment (n=4,552) and the surgery
following systemic treatment (n=5,958) groups. The median OS times
of these groups were 37.5, 49.4 and 52.8 months, respectively
(8). Surgery, whenever it was
performed, was closely associated with an improved prognosis,
compared with systemic therapy alone (P<0.001). Furthermore,
surgery following systemic intervention appeared to prolong the
median OS time, compared with the surgery prior to systemic
treatment group (8).
For aggressive subtypes of dnMBC, upfront surgery
may lead to deleterious consequences. Patients with multiple
visceral metastases benefit from first-line systemic treatment
other than initial surgery (2). In
2015, the results of the TATA trial, an open-labelled randomized
study, were published (19). A
total of 350 patients with dnMBC in India responsive to first-line
systemic treatment (336 chemotherapy and 14 endocrine) were
randomly divided into two groups: The LRT (n=173) and no LRT
(n=177) groups. Following a median follow-up time of 23 months, LRT
to the PT failed to confer any survival benefit (19). The median OS times and the 2-year OS
rates in the LRT and no LRT groups were 19.2 vs. 20.5 months
(P=0.79) and 41.9 vs. 43.0%, respectively. The LRT modality also
significantly improved the locoregional PFS time (median not
attained vs. 18.2 months; P<0.0001), whereas it was detrimental
to the distant PFS time (median, 11.3 vs. 19.8 months; P=0.012)
(19). That trial was criticized
due to insufficient systemic treatment according to the current
guidelines. A total of 107 patients (31%) were HER2+,
but only 9 patients (8%) underwent anti-HER2 therapy. In addition,
only a few patients were administered taxane-based chemotherapy.
Moreover, the median OS time in the TATA trial was much shorter
than that reported by others such as Lane et al (8) and Pons-Tostivint et al
(16), partly due to the
undertreated systemic therapy and later diagnosis of symptoms.
Collectively, this underpowered the conclusions made, particularly
in the era of a constantly updated systemic strategy.
Pons-Tostivint et al (16) published their latest results on
4,276 patients with dnMBC in the NCT03275311 trial in 2018. A total
of 1,706 patients underwent LRT, while 2,570 patients did not. The
median duration between MBC diagnosis and LRT was 5.7 months.
Compared with the TATA trial, all the patients in the NCT03275311
trial received modern systemic therapy (16). In total, 94% of the HER2+
cohort was administered trastuzumab and 90% of the HR+
candidates were administered endocrine therapy. LRT attained a
superior OS time in patients with the
HR+/HER2− (61.6 vs. 45.9 months; P<0.001)
and HER2+ (77.2 vs. 52.6 months; P=0.008) subtypes, but
had no effect on OS in the TNBC population. Additionally, in the
visceral metastatic subset, LRT also reduced the risk of mortality
(16). In a previous retrospective
study on 622 patients with dnMBC performed by Cady et al
(60), primary surgery (n=234)
indicated a survival benefit compared with without surgery (n=388;
P<0.0001). For the bone metastasis subset (n=255), the survival
advantage was overt in the primary surgery group (P<0.0001), but
this benefit was reduced after case-matching (n=168; P=0.0003),
which reflected the selection bias. Similarly, in the visceral
metastatic subgroup (n=159), the survival superiority conferred by
primary resection was significant (P=0.0008), but this is not the
case after case-matching (n=100; P=0.09) (60). With regards to the order of systemic
treatment and primary surgery, it was found that 90% of patients
who survived for 2-years were administered chemotherapy initially.
However, this survival benefit was not present in the cohort of
patients who received surgery immediately after systemic therapy,
suggesting that patients with dnMBC who respond robustly to upfront
systemic intervention are most likely to benefit from primary
surgery (60).
NCT03870919 has been launched to evaluate the OS of
combined modality with the CDK4/6 inhibitor, palbociclib, plus
letrozole and the most adapted LRT in the treatment of patients
with naïve HR+/HER2− dnMBC. The ECOG 2108
(NCT01242800) study was designed to clarify the effects of local
treatment in patients with stage IV breast cancer. In the 2020 ASCO
meeting, the results of the long-awaited ECOG 2108 study were
presented in which 390 patients with dnMBC were enrolled, of which
256 patients who responded to initial systemic therapy were
randomized to either undergo LCT (surgery and radiation, n=125)
followed by systemic therapy or continue with systemic treatment
(n=131) (61). However, early LCT
failed to bring any survival benefit (neither OS nor PFS). Without
LRT, a 2.5-fold higher risk of local failure was posed. Therefore,
the ECOG-ACRIN E2108 trial indicated that LRT to the PT should not
be recommended to patients with dnMBC for the purpose of survival
extension and only reserved for those with well-controlled systemic
disease and a progressive PT (61).
However, these results were limited for the following reasons: i)
Multivariate analysis was not conducted; ii) the inclusion
criterion was PT surgery with free margin. However, in the LRT
group (n=125), only 109 patients underwent surgery and only 87
(80%) patients had R0 resection; and iii) the T4 and/or N2/3
patients accounted for 48% of the cohort. The effect of the high
proportion of patients with a locally advanced status needs to be
clarified (61).
Retrospective analyses indicated that for aggressive
subtypes, such as multiple visceral metastases or those with heavy
tumor burden, upfront LRT was detrimental. Timely and effective
systemic treatment to make metastatic lesions controllable and
asymptomatic followed by definitive LRT may infer the greatest
survival benefit (62,63).
Optimal means of LRT
Surgery vs. surgery plus RT
The role of adjuvant RT has been explored by a
series of studies. In the study by Altundag (64), 118 patients with dnMBC administered
LRT were further divided into three groups: RT only, 2 (1%)
patients; surgery only, 54 (29%) patients; surgery followed by RT,
132 (70%) patients. Post-operative RT provided a significant
survival advantage (64–66). The 5-year OS and PFS rates in the
three groups were 0, 22 and 56% (P<0.001) and 0, 3 and 27%
(P=0.001), respectively (66).
Additionally, Kim et al (67) used the SEER database to address this
issue. Following propensity-score matching, the addition of RT
further improved the cancer-specific survival (CSS) of candidates
who lived >6 months. The study by Arciero et al (25) also favored the addition of RT to
surgery of the PT in dnMBC. In the multivariate analysis of the OS
of patients with dnMBC who underwent surgery, the administration of
RT was an independent factor of an improved outcome (P<0.001)
(25).
By contrast, in the analyses performed by Lane et
al (8), the addition of RT had
no association with OS (P=0.83), which was revealed by an adjusted
multivariate OS model. However, the majority of patients did not
receive RT (59.75%) in this cohort, and the RT administered was not
only confined to the PT area (breast, chest or regional lymph
nodes; 17.89%), but also included the spine (9.19%) and extremity
bones, including the pelvis (4.36%). Therefore, the conclusion of
that study could not truly represent the value of RT to the PT
region (8).
PT and distant metastatic site
resection vs. PT resection vs. distant metastases removal
Tan et al (68) conducted a population-based study
using the SEER database for the years 2004–2008. A total of 10,441
patients with MBC were divided into four subsets: i) R0 resection
(both resection of primary and metastatic sites), 272 (2.61%)
patients; ii) PT removal only, 4,025 (38.55%) patients; iii)
metastatic lesion resection only, 409 (3.92%) patients; and iv) no
surgery, 5,735 (54.93%) patients. The median OS times of the
patients in the four groups were 51, 43, 31 and 21 months,
respectively (P<0.001) (68).
Furthermore, the HR status influenced the survival benefit from
surgery. For the HR+ cohort, the median OS times and
5-year OS rates for R0 resection, primary resection, metastases
resection and no resection were 66, 52, 38 and 28 months and 54.1,
44.9, 31.7 and 22.0%, respectively (P<0.001). However, for the
HR− cohort, the median OS times and 5-year OS rates for
R0 resection, primary resection, metastases resection and no
resection were 18, 24, 12 and 12 months and 26.7, 25.0, 6.8 and
11.8%, respectively (68). In the
HR+ cohort, the R0 resection group attained a further
survival benefit compared with the primary resection group
(P<0.001), and the same was observed for the metastases
resection group compared with the no resection group (68). However, this additional survival
advantage disappeared in the HR− cohort. These findings
indicated that HR+ patients with dnMBC may attain more
survival benefits from a more aggressive LRT approach (68).
The addition of post-operative RT to the PT region
following surgery may lead to an improved outcome of patients with
dnMBC. For HR+ patients with dnMBC, distant metastatic
site resection could also lead to an improved survival outcome.
Population most likely to benefit from
LRT
Bone-only metastases
In 2006, Rapiti et al (10) conducted a study including 300
patients with dnMBC recorded at the Geneva Cancer Registry between
1977–1996. After adjusting for other cancer prognostic confounding
factors, complete resection of the PT reduced the risk of breast
cancer-specific mortality by 40% (P=0.049) (10). This benefit was particularly evident
for patients with dnMBC and bone metastasis only. The metastatic
pattern was a vital covariable that determined the prognosis of
dnMBC (10). Wang et al
(69) retrospectively analyzed
8,142 patients with dnMBC from 2010–2015 using the SEER database.
By comparing the surgical (n=1,891) and non-surgical groups
(n=6,251), they noted that surgery provided a survival advantage in
those with a bone-only metastasis or with multiple metastases
including the bone (P<0.05), but a survival inferiority in those
with multiple metastases only in visceral organs (P<0.05)
(69). The aforementioned MF 07-01
study, revealed that the solitary bone-only metastatic subset
benefited from LRT (17). However,
the other three well-known randomized trials, TATA (19), TBCRC 013 (47) and POSYTIVE (18), failed to identify the superiority
brought by surgery. Notably, the proportion of bone-only metastasis
in the TATA (19), TBCRC 013
(47), POSYTIVE (18) and MF 07-01 (17) studies was 28, 37, 38 and 46%,
respectively. The highest ratio of patients with bone-only
metastasis was found in the MF 07-01 study, which largely
determined the positive results of the study (17).
Oligometastatic disease or low
metastatic burden
Barinoff et al (56) found that the number of involved
sites was closely associated with PFS and OS in the dnMBC cohort.
Multivariate analysis determined that <3 metastatic sites led to
an improved PFS and OS in patients with dnMBC (P=0.0005 and
P=0.00051, respectively). Xiong et al (3) retrospectively reviewed 313 patients
with dnMBC from January, 2006 to April, 2013 at the Sun Yet-sen
University Cancer Center. Patients who underwent local surgery
(n=188) had a notable median OS time advantage compared with those
who did not undergo surgery (n=125) (78 vs. 37 months; P=0.002).
Stratified survival analysis revealed that bone metastasis plus a
PT <5 cm, ≤3 metastatic sites, or soft tissue metastasis
predicted a benefit from local removal (3). Bafford et al (9) analyzed the impact of staging for
breast surgical removal in patients with dnMBC. The 147 patients
enrolled were divided into two groups: The surgery (n=61) and
non-surgery (n=86) groups. After adjusting for age, number of
metastatic sites, ER/Her2neu status and the use of chemotherapy,
trastuzumab and endocrine therapy, the surgery group gained a
significantly improved OS time compared with the non-surgery cohort
(4.13 vs. 2.36 years; P=0.003). However, the number of metastases
in the non-surgery group was markedly higher than in the surgery
group (≥3, 84 vs. 41%) (9).
Notably, in the surgery group, 36 patients underwent surgery before
the diagnosis of metastatic disease. The 25 patients diagnosed with
stage IV pre-operatively had a very similar OS time to those
without surgery (2.4 vs. 2.36 years), while the patients undergoing
surgery before metastatic diagnosis had a notably improved OS time
(4.05 years) (9). This may be
explained by the fact that those who underwent resection of the
intact PT before a stage IV diagnosis were typically asymptomatic
and with a limited metastatic burden, which contributed to a
relatively optimal outcome (9).
Previously, Lin et al (49)
identified 8,582 patients with dnMBC from 2010–2014 using the SEER
database, and further subdivided M1 into three groups. Patients
with an M1a status (meaning a single metastatic site, except the
liver and brain) gained the most benefit from PT surgery.
HR+
In a retrospective study conducted by Tan et
al (68), resection of both the
primary and metastatic lesions for stage IV BC led to the optimal
survival of patients with HR+ breast cancer. In a single
institutional analysis of 111 patients with dnMBC conducted by
Samiee et al (70),
HR+ and surgical removal both indicated a better
prognosis (P<0.001 and P=0.041, respectively), using
multivariate analysis. The ESME-MBC study demonstrated a median OS
time of 42.12 months for newly diagnosed
HR+/HER2− patients with dnMBC (38). In another study, HR positivity
predicted a favorable outcome in the surgical group independently,
following multivariate analyses. ER positivity was found to be the
only good prognostic predictor in surgical patients with dnMBC
(57).
Favorable profile and relatively long
survival
Rashaan et al (71) demonstrated that a younger age
(P=0.03) and an absence of complications (P=0.03) were independent
prognostic factors for improved survival in the dnMBC cohort
receiving surgery. In the retrospective study by Shien et al
(55), PT surgery conferred an OS
advantage in young candidates (P=0.021), but not in older
candidates (>51 years old, P=0.665). Blanchard et al
(46) also demonstrated that the
subpopulation of relatively long survivors of dnMBC gained more
benefit from definitive surgery. For those patients who underwent
surgery and survived for >2 years (n=119), survival was
significantly improved compared with no primary surgery (n=51;
P=0.0037) (46).
Prediction scoring model
Yoo et al (7)
developed and validated a survival prediction-scoring model using
the Korean Breast Cancer Registry database, aiming to identify the
subset of long-term survivors of dnMBC who underwent surgery of the
PT. Their study excluded those who received resection of metastatic
foci. The 2,232 enrolled patients were divided into three groups,
according to the surgery type: Surgery (n=1,541), non-surgery
(n=588) and partial surgery (n=103; only breast or axillary
removal) groups. The surgery cohort had a significantly improved
median survival time compared with the non-surgery group (53 vs. 31
months; P<0.001) (7). There was
no significant difference with respect to the median OS time
between the non-surgery and partial surgery cohorts (37 vs. 31
months; P=0.113). The OS prediction-scoring model for patients with
dnMBC undergoing surgery of the PT was established based on the
following seven factors: T stage, tumor grade, lymphovascular
invasion (LVI), ER expression, and the levels of Ki-67, CA 153 and
alkaline phosphatase (7). The
patients in the surgery group were further grouped into four
subsets: Subsets with a score of 0–3, 4–5, 6–7 or 8–10, with
clearly defined 3-year survival rate outcomes (87.3%, score 0–3;
68.4%, score 4–5; 48.2%, score 6–7; 35.3%, score 8–10). The group
with a score 0–3 had a significant advantage as regards OS,
demonstrated both in the discovery cohort (87.3%; P<0.001) and
in the validated cohort (85.9%; P<0.001). This tool provided a
method to identify the candidates with dnMBC who would benefit the
most from primary surgery (7).
Since dnMBC is highly heterogeneous, multiple
factors, including the status of the patient, tumor pathology,
biology and metastatic pattern, may help to determine the cohorts
most likely to benefit from LRT. A younger age and a good general
condition without complications, a smaller tumor, a lower tumor
grade, no LVI, HR positivity, bone-only metastasis and low
metastatic burden have been found to be associated with an improved
outcome. In the future, a gene signature of dnMBC may help to
identify the patients who will benefit the most from LRT.
Additionally, an improvement in systemic intervention and new drugs
may achieve the complete remission of metastatic lesions, which
will result in more candidates who will benefit from definitive
LRT. In clinical practice, the flow diagram with therapeutic
options presented in Fig. 1 may aid
in decision-making when treating patients with dnMBC.
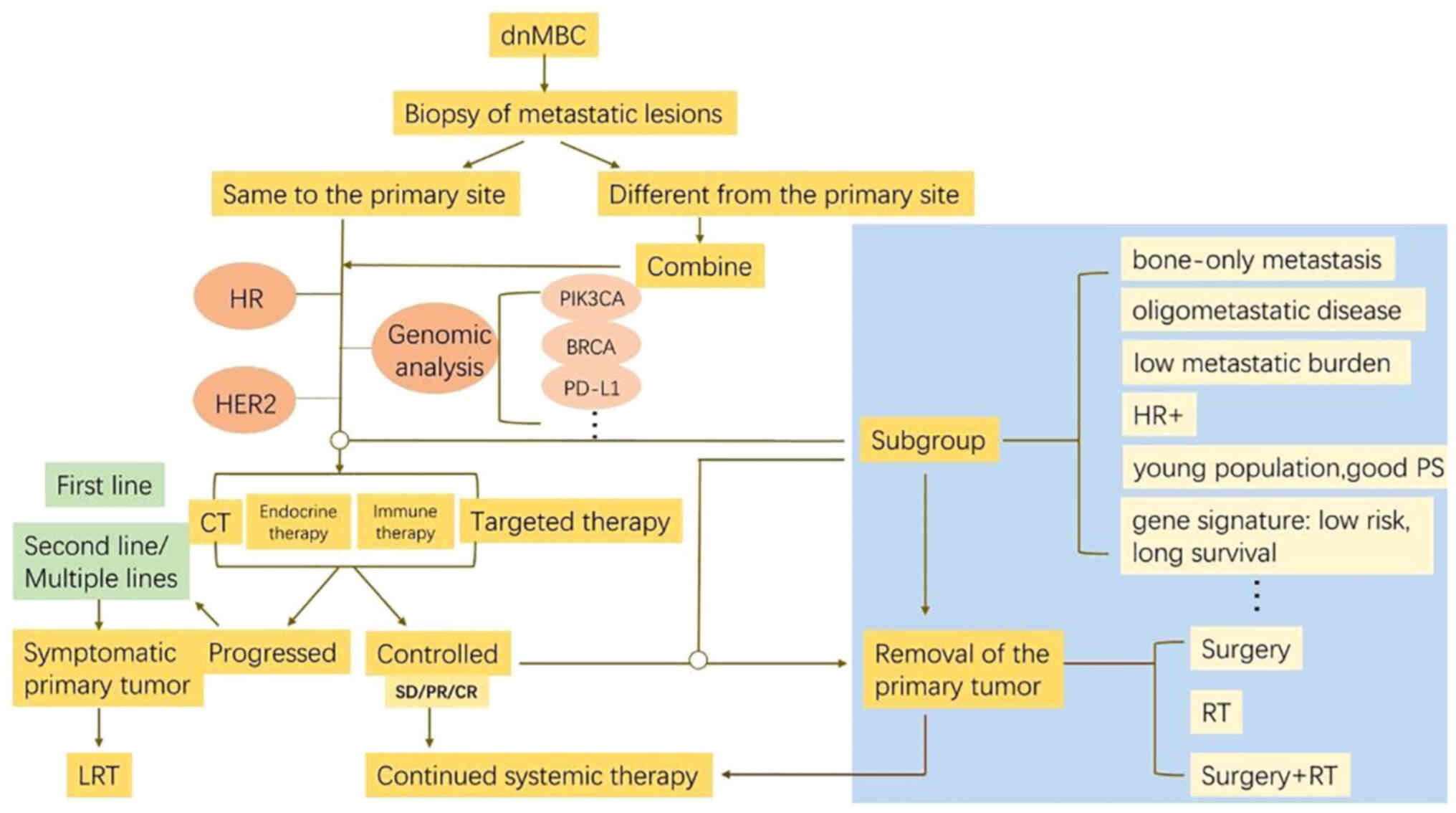 | Figure 1.Flow diagram with therapeutic options
for patients with dnMBC. When treating patients with dnMBC, the
molecular typing, including HR, HER2 and Ki-67 expression, and
genomic analysis of both the primary and metastatic tumors are
fundamental. Upfront systemic strategies are tailored according to
the tumor biology and status of the patient. For good respondents
to upfront systemic therapy and subgroup of favorable profiles,
radical LRT including PT surgery followed by RT and resection of
distant metastases will be recommended. LRT should also be
administered if PT is still symptomatic. Therefore, the treatment
paradigm for dnMBC may change from ‘palliative LRT’ into ‘curative
LRT’ in highly selected entity with careful evaluation. dnMBC,
de novo metastatic breast cancer; HR, hormone receptor;
HER2, human epidermal growth factor receptor 2; LRT, local
radiotherapy; PT, primary tumor; PD-L1, programmed cell death
ligand 1; RT, radiotherapy. |
Mechanisms through which LRT benefits
patients with dnMBC
The crosstalk between the primary
tumor and metastatic foci
Previously, some studies proposed that resection of
the PT would promote the progression of distant metastasis, partly
mediated by the angiogenesis rebounds and the growth factor surge
responding to the surgical wounding (72,73).
It was suggested that the PT secreted angiogenesis inhibitors, such
as angiostatin, controlling the growth of metastatic lesions
(74). PT removal would therefore
alter the growth kinetics of the residual tumor (75). However, these theories resulted from
an animal model with an unclear clinical translational implication
(76).
The metastasis of a tumor is comprehensive,
involving multiple factors and associations. Under certain
incentives, such as hypoxia, tumor cells develop
epithelial-interstitial transformation and increase their invasive
ability (77). Accordingly, the
interactions with stromal cells in the surrounding microenvironment
degrade, restructuring the extracellular matrix and allowing tumor
cells to invade the basement membrane and enter blood vessels. In
this process, there are approximately three mechanisms involved.
Firstly, the PT will secrete relevant cytokines, such as TGF-β,
VEGF, matrix metalloproteinases and the remodeling enzyme, lysyl
oxidase, and promote the migration of tumor cells. Secondly,
endocrine signaling from the primary foci recruits mesenchymal stem
cells (MSCs) from the bone marrow into the microenvironment of the
primary foci to promote metastasis (78–80).
Thirdly, pro-inflammatory and pro-angiogenic factors secreted by
the primary foci, such as TNF-α, IL-8 and VEGF (78,81–83),
which increase angiogenesis and vascular permeability of the
primary foci, facilitate the invasion of blood vessels by tumor
cells. Circulating tumor cells (CTCs) form clusters and migrate in
the bloodstream (84–86). Various cytokines, chemokines and
growth factors secreted by the primary foci and MSCs recruited from
the bone marrow also enter the target organ in advance, which
shapes the targeted microenvironment and forms a pre-metastatic
niche to welcome the arrival of the CTCs (83,87–92).
Organ specificity during metastasis is regulated by chemokines,
exosomes, integrins and other factors, such as chemokine receptors
(87–89,93–97).
If CXCR4 is highly expressed by the PT, the bone, lungs and brain
are prone to metastasis due to the extensive expression of CXCR4
ligand-CXCL12. The new metastasis may originate from the PT or from
an existing metastasis (98–100).
Crosstalk exists between the primary and secondary tumor. The PT
secretes highly effective immunosuppressive molecules, such as
TGF-β, VEGF, IL-10, inflammatory factors (such as IL-6 and IL-8)
and angiogenesis inhibitors (such as angiostatin and endostatin),
to regulate growth of the secondary tumor (Fig. 2) (78,81–83).
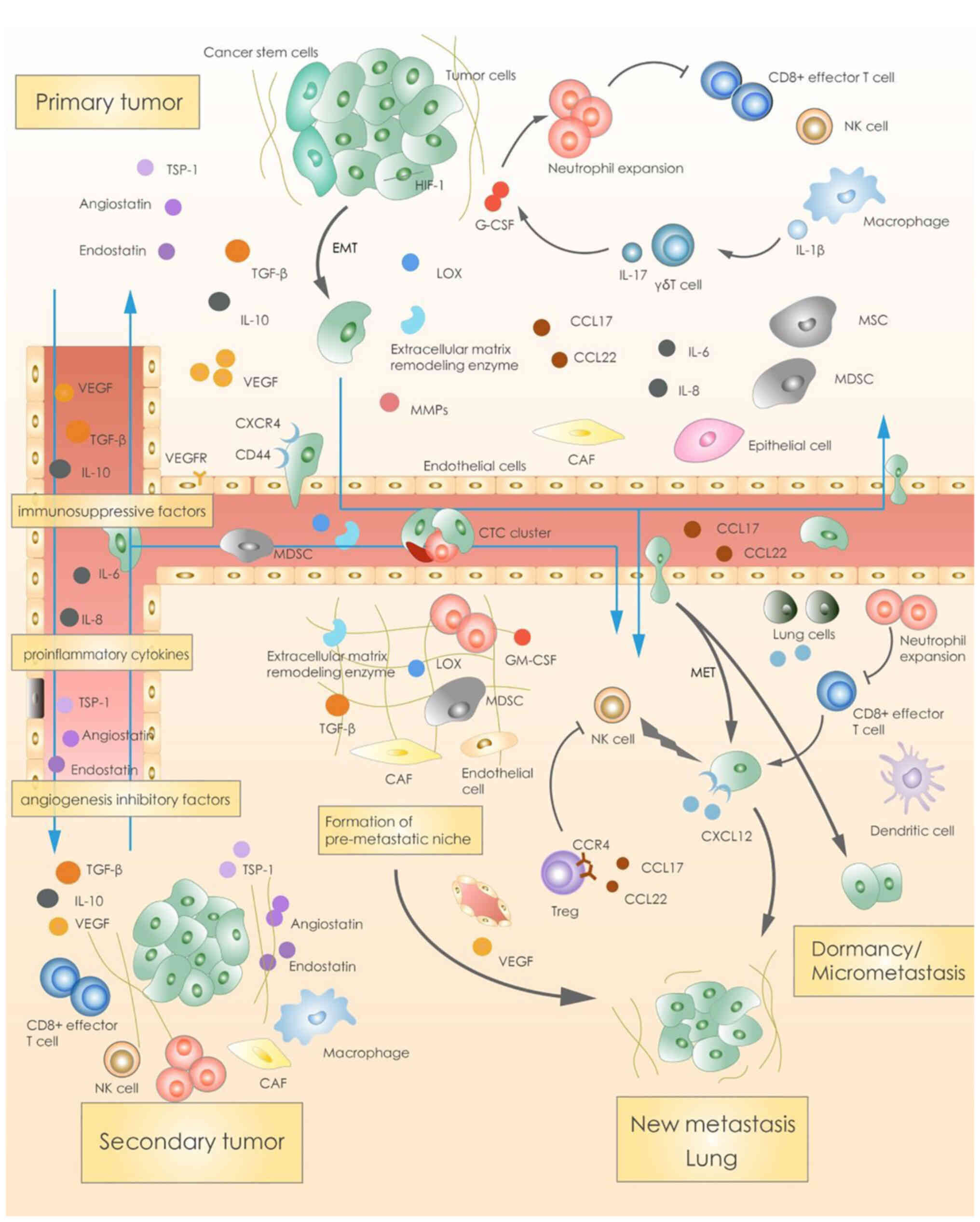 | Figure 2.Crosstalk between the primary and
secondary/metastatic tumor. The primary tumor secretes relevant
cytokines and promote the migration of tumor cells. Endocrine
signaling from the primary foci recruits MSCs from the bone marrow
into the microenvironment of the primary foci to promote
metastasis. Pro-inflammatory and pro-angiogenic factors secreted by
the primary foci, which increase angiogenesis and vascular
permeability of the primary foci, facilitate invasion of blood
vessels by tumor cells. Circulating tumor cells form clusters and
migrate into the bloodstream. Organ specificity during metastasis
is regulated by this crosstalk between the primary and
secondary/metastatic tumor. MSCs, mesenchymal stem cells; TSP-1,
thrombospondin 1; EMT, epithelial-mesenchymal transition; HIF-1,
hypoxia-inducible factor-1; MMPs, matrix metalloproteinases; G-CSF,
granulocyte colony-stimulating factor; CAF, cancer-associated
fibroblast; GM-CSF, granulocyte macrophage colony-stimulating
factor; MET, mesenchymal-epithelial transition. |
Control of the PT decreases tumor
burden and eliminates the source of dissemination
Total tumor burden has been revealed to be closely
linked with survival (44,101). The level of CTCs is also an
independent prognostic factor for the PFS and OS of patients with
MBC (102,103). Similarly, the PT of dnMBC serves
as another metastatic site or source of dissemination (10,13,44).
Resection of the PT can block the continued production of CTCs,
reduce subsequent metastasis and improve prognosis (46–49).
In the study conducted by Rapiti et al (10), surgery of the PT with a positive
margin failed to improve survival of patients with dnMBC compared
with patients who did not undergo surgery. The 5-year CSS rates of
patients who underwent primary surgery with negative margins,
positive margins, an unknown margin status or who did not undergo
surgery were 27, 16, 12 and 12%, respectively (P=0.0002) (10). Yoo et al (7) conducted a retrospective study on dnMBC
with a larger sample size. Patients were further categorized into
three groups according to the following: The surgery (n=1,541),
partial surgery (n=103, no definite resection of the PT) and
non-surgery (n=588) groups. The definite surgery cohort
demonstrated a significant OS time advantage compared with the
non-surgery group (53 vs. 31 months; P<0.001), which was not
observed in the partial surgery group (37 vs. 31 months; P=0.113)
(7). Additionally, Hazard et
al (104) verified that the
chest wall status had a direct effect on the OS of patients with
dnMBC. Compared with a symptomatic chest wall, the presence of a
free or asymptomatic status could notably decrease the hazard ratio
of mortality (hazard ratio, 0.415). Multivariate analysis further
supported that primary surgery markedly reduced the probability of
uncontrolled chest wall status (104). Furthermore, MSCs recruited by the
PT from the bone marrow release paracrine signals, such as C-C
motif ligand 5, which interact with C-C motif receptor 5 on cancer
cells, induce the metastasis of primary cancer cells and maintain
the metastatic phenotype of cancer cells (79). Cancer stem cells (CSCs) in the PT,
are able to self-renew, differentiate and activate, and are the
main source of continuous dissemination and metastasis (52). These findings highlight that the
complete clearance of the PT, eradicating the source of metastasis,
may translate into a survival benefit (101).
Enhancing the sensitivity to
therapy
Maximal tumor debulking markedly increases drug
accessibility (101). Notably,
necrotic, hypoxic and avascular tumors exhibit resistance to
chemotherapy or RT. Hazard et al (104) demonstrated that surgery improved
the local control (S vs. NS: 36/44 82% vs. 20/59 34%, P=0.001) and
prolonged time to first progression (P=0.015) in dnMBC. In total,
18.2% (8/44) patients in the surgery group also experienced cancer
relapse following LRT (104). The
removal of these tumors could limit the emergence of
treatment-resistant cells, resulting in an increased sensitivity to
treatment (12,105). Additionally, CSCs are resistant to
common anticancer strategies. Eradiation of the PT will therefore
improve treatment efficacy (54–60,62).
Positive immunomodulation
The tumor evolution of some dnMBC patients is
related to immune factors and benefit most from removal of the PT
(2). In a 4T1-bearing mammary
carcinoma mouse model, both antigen specific T-cell proliferation
and antibody responses were markedly suppressed compared with
tumor-free mice. In patients with BC, the levels of cytokines
produced by CD4+ and CD8+ T cells, including
IL-2, IFN-γ, TNF-α and IL-4, were markedly decreased compared with
the healthy population (63,64).
Tumor cells can synthesize and secrete various immunosuppressive
factors, such as TGF-β and VEGF, which inhibit effector cells
(65). Myeloid-derived suppressor
cells play a vital role in immune suppression in patients with BC
(66,67). Removal of the PT helps to restore
both antibody and cell-mediated immune responses, which manifests
as an activated macrophage and dendritic cell function, even in the
presence of metastatic disease (100). Moreover, the PT harbors a certain
humoral resistance that does not belong to or at least develops
slowly in the metastatic lesions. The eradication of this dependent
relationship existing between the primary and metastatic foci by PT
removal causes the disseminated lesions to be more vulnerable to
therapy (106).
Conclusions and future perspectives
dnMBC has long been considered incurable, which has
been challenged with the new understanding of tumor biology and the
advent of novel systemic strategies. The heterogeneity of dnMBC
leads to difficulties in assessing the suitability of candidates,
and the optimal time and means for LRT. The results from published
(Table I) and ongoing trials
(Table II) have demonstrated that
initial effective systemic management to control the metastatic
lesions followed by definitive LRT are most likely to improve the
OS of patients with dnMBC. The updated results of the long-awaited
ECOG-ACRIN E2108 trial revealed that early LRT did not attain any
survival advantage (OS or PFS) for patients with dnMBC, but did
markedly decrease the local failure risk (61). Thus, patients with dnMBC with a
satisfactory control of distant metastases and a progressive local
tumor may benefit from LRT. In clinical practice, when treating
patients with dnMBC, the molecular typing, including HR, HER2 and
Ki-67 expression, and genomic analysis of both the primary and
metastatic tumors are fundamental. Upfront systemic strategies,
including chemotherapy, endocrine therapy, immunotherapy and
targeted therapy are tailored according to the tumor biology and
status of the patient. For good respondents (stable disease/partial
remission/complete remission) and subgroups with favorable
profiles, such as bone-only metastasis, oligometastatic disease or
a low metastatic burden, HR+, young patients without any
complications, and a gene signature with a low risk and long
survival, will be recommended radical LRT, including PT surgery
followed by RT and the resection of distant metastases. For
patients with progressive disease, second line or multiple systemic
strategies can be administered. LRT should be administered if the
PT is still symptomatic. Therefore, the treatment paradigm for
dnMBC may change from ‘palliative LRT’ to ‘curative LRT’ in the
most suitable population, which translates into a survival
advantage.
 | Table I.Published studies on LRT for
dnMBC. |
Table I.
Published studies on LRT for
dnMBC.
| Positive
results |
|---|
|
|---|
|
Author(s)/trial | Year of
publication/PMID | Country | Study nature | Design | Outcome | Limitation | (Refs.) |
|---|
| Khan et
al | 2002/PMID:
12407345 | USA | Retrospective | Non-surgery
(n=6,861); surgery (n=9,162); partial mastectomy (PM)(n=3,513);
total mastectomy ™ (n=5,649) | Mean survival:
Non-surgery, 19.3 months; PM, 26.9 months; TM, 31.9 months. 3-Year
survival: Non-surgery, 17.3%; PM, 27.7%; TM, 31.8% | Patients with 1
metastatic site more likely to undergo mastectomy | (44) |
| Rapiti et
al | 2006/PMID:
16702580 | Switzerland | Retrospective | Surgery (n=127);
mastectomy (n=87); tumorectomy (n=40); margin-(n=61); margin +
(n=33); margin unknown (n=33); non-surgery (n=173) | Primary tumor
complete resection (margin -) reduced 40% breast cancer specific
mortality risk compared with those without surgery (P=0.049) and
this benefit wasparti-cularly obvious in the subgroup of bone
metastasis only (P=0.001) | Retrospective
nature despite of adjusting for other main factors of prognosis and
avoiding selection bias using subgroup analyses | (10) |
| Babiera et
al | 2006/PMID:
16614878 | USA | Retrospective | Non-surgery
(n=142); surgery (n=82); segmental mastectomy (n=39); mastectomy
(n=43) | After adjusting,
surgery group: A trend for improved OS (P=0.12); improved
metastatic PFS (P=0.0007) | Baseline unbalance:
Surgery group; younger, lower tumor burden, more HER2+,
more liver metastases, more chemotherapy as initial systemic
therapy | (11) |
| Fields et
al | 2007/PMID:
17687611 | USA | Retrospective | Surgery (n=187);
non-surgery (n=222) | After adjusting:
Median OS: S vs. NS (31.9 vs. 15.4 months, P<0.0001) | Baseline unbalance:
Surgery group; younger, smallerprimary tumor and lower proportion
of bone metastasis | (13) |
| Gnerlich et
al | 2007/PMID:
17522944 | USA | Retrospective | Surgery (n=4,578);
non-surgery (n=5,156) | Median OS: S vs. NS
(36 vs. 21 months, P<0.001, alive; 18 vs. 7 months, P<0.001,
did not survive). | Surgery: Younger,
diagnosed earlier, more likely to be Caucasian, married, <5 cm,
grade III, HR+ | (12) |
| Blanchard et
al | 2008/PMID:
18438108 | USA | Retrospective | Surgery (n=242);
non-surgery (n=153) | Median OS: S vs. NS
(27.1 vs. 16.8 months, P<0.0001). | Selection biases;
margin status unclear in most cases | (46) |
| Cady et
al | 2008/PMID:
18726129 | USA | Retrospective | Surgery (n=234);
non-surgery (n=388) | OS: S vs. NS
(P<0.0001). | Selection bias | (60) |
| Hazard et
al | 2008/PMID:
18780312 | USA | Retrospective | Surgery (n=47): 26
diagnosed IV post-operatively; 24 systemic therapy first;
non-surgery (n=64) | Local control: S
vs. NS: 36/44 82% vs. 20/59 34%, P=0.001; time to first progression
(TTFP): S: prolonged, P=0.015 | S: Younger
(P=0.033); more HR− (P=0.0005); more RT (P<0.001);
included those with metastasis within 6 months of diagnosis | (104) |
| Ruiterkamp et
al | 2009/PMID:
19398188 | The
Netherlands | Retrospective | Surgery (n=288);
non-surgery (n=440) | S vs. NS: (median
OS: 31 vs. 14 months, 5-year OS rate: 24.5% vs. 13.1%,
P<0.0001). | S: Younger, smaller
T, less likely to have visceral metastases or co-morbidity, more
often with RT/systemic therapy | (107) |
| Bafford et
al | 2009/PMID:
18581232 | USA | Retrospective | Surgery (n=61);
n=36, surgery before the diagnosis of metastatic disease; n=25,
diagnosed with IV stage pre-operatively; non-surgery (n=86) | After adjusting:
OS: S vs. NS (4.13 vs. 2.36 years, P=0.003) | 36 Patients
underwent surgery before the diagnosis of metastatic disease; stage
migration | (9) |
| Shien et
al | 2009/PMID:
19212646 | Japan | Retrospective | Surgery (n=160);
94%: Local surgery as primary therapy, no post-operative RT;
non-surgery (n=184) | OS of patients with
primary tumor resection improved (P=0.049); younger patients
(<50 years, P=0.023), and with soft tissue or bone metastases
(P=0.013) | Surgery group:
Younger, earlier in the study period, more bone/soft metastasis,
more hormonal therapy | (55) |
| Leung et
al | 2010/PMID:
19375721 | USA | Retrospective | Surgery (n=52,
33%); non-surgery(n=105, 67%) | Median OS: Surgery,
25 months; non-surgery, 13 months; Wilcoxon test (P=0.004) and
log-rank test (P=0.06) | Retrospective
study: Unrecognized biases | (108) |
| Pathy et
al | 2011/PMID:
21858791 | Malaysia | Retrospective | Surgery (n=139),
non-surgery (n=236) | Surgery vs.
non-surgery group:2-year OS% (46.3 vs. 21.2%) | Selection
biases | (109) |
| Rashaan et
al | 2012/PMID:
22032912 | The
Netherlands | Retrospective | Surgery (n=59),
non-surgery (n=112) | Younger patients
(P=0.03) and individuals without comorbidities (P=0.03) that
underwent surgery had an improved survival | Surgery group had a
younger age, clinical T stage and no medication use | (71) |
| Samiee et
al | 2012/PMID:
22876156 | Canada | Retrospective | Surgery (n=48, 29
had surgery before metastatic diagnosis); non-surgical group
(n=63) | Surgery vs.
non-surgery group: OS (49 vs. 33 months,P=0.01);symptomatic local
progression (14 vs. 44%, P<0.001). | Surgical group:
Less likely to present with T4 (23 vs. 35%), N3 (8 vs. 19%) and
visceral metastasis (67 vs. 73%) | (70) |
| Bertaut et
al | 2015/PMID:
25757548 | France | Retrospective | Total: 232; surgery
(n=92); non-surgery (n=139); missing data (n=1) | Surgery of PT:
Improved survival (relative excess rate = 0.43; 95% CI:
0.28–0.68) | 18 (40%)
HER2+ patients did not receive trastuzumab | (110) |
| Tan et
al | 2016/PMID:
27542240 | USA | Retrospective | 272 (2.61%) R0
resection (both primary and metastatic sites resection); n=4,025
(38.55%), primary tumor removal only; n=409 (3.92%), metastatic
lesion resection only; n=5,735 (54.93%), no surgery | The median OS of
the four groups was 51, 43, 31 and 21 months (P<0.001).
HR+: The median OS and 5-year OS rates for R0 resection,
pri-mary resection, metastases resection and no resection were 66,
52, 38, 28 months, and 54.1, 44.9, 31.7 and 22.0%, respectively
(P<0.001). HR−: The median OS and 5-year OS rates for
R0 resection, primary resection, meta-stases resection and no
resection were 18, 24, 12, 12 months, and 26.7, 25.0, 6.8 and
11.8%, respectively. The R0 resection and the primary tumor
resection both markedly prolonged the OS compared with the no
resection group, but the metastatic group did not have this
benefit. R0 resection failed to attain survival advantage compared
with the group of primary tumor resection only (P=0.691) | Several important
factors including performance status, number of metastatic sites,
HER2 status, endocrine therapy, chemotherapy and the size of the
metastases were not accessible | (68) |
| Thomas et
al | 2016/PMID:
26629881 | USA | Retrospective | Surgery (n=8,330),
non-surgery (n=13,042) | Surgery vs.
non-surgery group: Median OS (28 vs. 19 months) | Excluded a large
group of patients who received radiation as the initial course of
therapy | (2) |
| Barinoff et
al | 2017/PMID:
28735068 | Germany | Retrospective | Upfront breast
surgery (n=426); no surgery (n=142) | Surgery vs. no
surgery: PFS (13.6 vs. 11.8 months, P=0.18) or OS (34.1 vs. 31.7
months, P=0.23); OS (45.7 vs. 27.2 months, P=0.026); subgroup
without visceral metastasis | Unbalanced baseline
characteristics: Surgery (less T4 and lower metastatic tumor burden
but more N3) | (56) |
| Yoo et
al | 2017/PMID:
28573447 | Korea | Retrospective | Surgery (n=1,541),
non-surgery (n=588) partial surgery group (n=103, only breast or
axillary removal) | Surgery vs.
non-surgery group: OS (53 vs. 31 months, P<0.001); non-surgery
vs. partial surgery cohorts (37 vs. 31 months, P=0.113) | Data on the
metastatic sites were unavailable; unbalanced baseline
characteristics: Surgery (smaller tumor, less axillary nodal
involvement, lower grade and Ki-67, ductal carcinoma and clinical
factors suggesting a lower tumor burden) | (7) |
| Soran et
al | 2018/PMID:
29777404 | Turkey | Prospective
randomized multicenter, phase III | LRT followed by ST
(n=138) ST alone (n=136) | LRT vs. no LRT;
hazard of mortality, 34% lower; median OS, 46 vs. 37 months
(P=0.005); 5-year survival rate, 41.6 vs. 24.4%; suitable
candidates: (ER)/(PR)(+) (P=0.01), (HER2)(−) (P=0.01), <55 years
(P=0.007), solitary bone-only metastases (P=0.04) | Unbalanced baseline
characteristics: LRT, higher rates of ER/PR+(85.5% vs.
71.8%; P=0.01) and lower rates of triple-negative tumors (7.3 vs.
17.4%; P=0.01) | (17) |
| Xiong et al
(3) | 2018/PMID:
30200932 | China | Retrospective | Surgery (n=188),
non-surgery (n=125) | Surgery vs.
non-surgery group: Median OS (78 vs. 37 months, P=0.002) | Single center;
selection bias |
|
| Pons-Tostivint
et al | 2019/PMID:
30539492 | France | Retrospective | LRT (n=1,706);
surgery (26%); RT (31%); S + R, 43%; no LRT (n=2,570) | LRT, improved OS;
subgroup analysis: LRT with improved median OS in
HR+/HER2− (61.6 vs. 45.9 months, P<0.001),
HER2+ (77.2 vs. 52.6 months, P=0.008), but not in TNBC,
and mortality risk reduction in visceral metastatic patients
(P<0.001) | Details of surgical
margin and RT, PS were unavailable The duration of systemic therapy
was longer in LRT (16.4 vs.7.8 months) | (16) |
| Lane et
al | 2019/PMID:
29227346 | USA | Retrospective | Systemic treatment
alone (n=13,505), upfront surgery prior to systemic treatment
(n=4,552) and surgery after systemic treatment (n=5,958) | The median OS of
the three groups was 37.5, 49.4 and 52.8 months, respectively.
Surgery, whenever it was performed, was closely associated with an
improved prognosis compared with systemic therapy alone
(P<0.001) | Following
multivariable adjustment, the group which underwent systemic
therapy prior to surgery was more likely to undergo mastectomy | (8) |
| Arciero et
al | 2019/PMID:
31087448 | USA | Retrospective | Surgical group
(n=5,202) and non-surgical group (n=6,492) | Propensity score
matching revealed an obvious survival benefit in the surgical
cohort, regardless of the administration of systemic intervention
(P<0.001) | Surgical arm:
Smaller tumor (T1) and higher burden of nodes (N2-3) | (25) |
| Co et
al | 2019/PMID:
30825858 | Hong Kong, SAR | Retrospective | LRT (n=91) No LRT
(n=81) | LRT vs. no LRT;
median OS, 55 vs. 40 months; 2-year OS, 84.5 vs. 73.7%; 5-year OS,
43.9 vs. 33.9% (P=0.026) | Surgical group:
Significantly younger | (57) |
| Lopez-Tarruella
et al | 2019/PMID:
31882586 | Spain | Retrospective | Surgery, 44.5%; no
surgery, 55.5% | Surgery vs. no
surgery: OS, 39.6 vs. 22.4 months (P<0.0001) | Surgery group:
Younger, smaller tumors, more bone and oligometastatic disease,
less visceral metastasis | (111) |
| Pons-Tostivint
et al | 2020/PMID:
31931289 | France | Retrospective | n=1,965; n=891 LRT
including 41.1% (n=366) exclusive radiotherapy, 13.7% (n=122)
exclusive surgery, 45.2% (n=403) bimodality therapy (surgery +
radiotherapy) | Following
propensity score matching, exclusive radiotherapy, surgery and
bimodality therapy: Significantly improved progression-free
survival in multivariable analysis | Selection
biases | (112) |
|
| Negative
results |
|
| Fitzal et al
ABCSG-28 POSYTIVE | 2019/PMID:
29697452 | Austria | Prospective,
randomized, phase III | Upfront surgery of
the primary tumor then systemic therapy (Arm A n=45) or primary
systemic therapy (Arm B, n=45) | A vs. B: Median OS,
34.6 vs. 54.8 months (P=0.267); time to distant progression, 13.9
vs. 29.0 months (P=0.0668) | Poor recruitment,
early closing | (18) |
| Badwe et al
TATA NCT00193778 | 2015/PMID:
26363985 | India | Prospective
randomized | First-line systemic
therapy: LRT (n=173); no LRT (n=177) | LRT vs. no LRT:
Median OS, 19.2 vs. 20.5 months (P=0.79); 2-year OS: 41.9 vs.
43.0%; median locoregional progression-free survival, not attained
vs. 18.2 months, P<0.0001; distant progression-free survival:
11.3 vs. 19.8 months, P=0.012 | Later diagnosis,
systemically undertreated | (19) |
| Rosche et
al | 2011/PMID:
22104157 | Germany | Retrospective | Surgery (n=35),
non-surgery (n=26), surgery of the primary tumor performed 0–19
months after dnMBC diagnosis; 20/35 in surgery, 7/26 in non-surgery
received RT | Surgery vs. no
surgery: OS and progression-free survival, no difference | Surgery group: Good
profile including younger age, only one metastatic lesion (P=0.01)
and performed RT more frequently (P=0.04) | (113) |
 | Table II.Important clinical trials of LRT for
dnMBC. |
Table II.
Important clinical trials of LRT for
dnMBC.
| Trial ID | Name | Country | Intervention
model | Start sate | Estimated primary
completion date | Estimated
studycompletion date | Arm | Primary outcome
measure | (Refs.) |
|---|
| NCT03870919 | Locoregional
Treatment and Palbociclib in de Novo, Treatment Naive, Stage
IV ER+, HER2-Breast Cancer Patients (PALATINE) | France | Single group
assignment | October 23,
2019 | October 23,
2023 | October 23,
2027 | Palbociclib + LRT:
Palbociclib + letrozole for 24–26 weeks, then the most adapted LRT
(surgery with or without RT, or RT). Palbociclib continued until
progression | OS |
|
| ECOG 2108
NCT01242800 | Early Surgery or
Standard Palliative Therapy in Treating Patients With Stage IV
Breast Cancer | Eastern Cooperative
Oncology Group | Randomized | February 8,
2011 | June 30, 2022 | December 21,
2022 | Arm I: Standard
palliative therapy (RT/surgery/both), to address symptoms; Arm II:
Breast-conserving therapy (BCT) or total mastectomy (R0 surgery) +
adjuvant RT | OS | (61) |
| NCT00941759 | Analysis of Surgery
in Patients Presenting With Stage IV Breast Cancer | USA | Observational | July, 2009 | July, 2021 | July, 2024 | Known or suspected
Stage IV disease and an intact primary: Research core needle biopsy
of the primary tumor/original diagnostic biopsy material/diagnostic
biopsy of a metastatic site/ blood draw; unsuspected metastatic
disease W/I 3 months of primary b: blood sample/paraffin tissue
from the prior surgical procedure and diagnostic biopsy of a
metastatic site/fresh frozen tissue | Response to
first-line therapy, frequency and proportion of surgical
referral |
|
| NCT00193778 | Assessing Impact of
Loco-regional Treatment on Survival in Metastatic Breast Cancer at
Presentation | India | Randomized | February, 2005 | March, 2020 | March, 2020 | Arm I: Standard
loco-regional treatment i.e., surgery (modified radical mastectomy
(MRM)/simple SMAC/BCT) +/- RT; Arm II: Not receive any
loco-regional treatment | OS | (19) |
| NCT02089100 | Trial of
Superiority of Stereotactic Body Radiation Therapy in Patients With
Breast Cancer (STEREO-SEIN) | France | Randomized | February, 2014 | February, 2020 | February, 2023 | ARM1: SBRT to all
metastases + systemic therapy ARM2: No specific treatment to the
oligometastatic site other than palliation | PFS |
|
| NCT02364557 | Testing Whether
Treating Breast Cancer Metastases With Surgery or High-Dose
Radiation Improves Survival | USA | Randomized | December 24,
2014 | December 31,
2022 | December 20,
2026 | Arm 1 (standard of
care): Systemic therapy; Arm2: Stereotactic radiosurgery, surgery +
systemic therapy | PFS, OS |
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82073475).
Availability of data and materials
Not applicable.
Authors' contributions
BL was a major contributor to the writing of the
manuscript and HL revised it critically. ML revised the manuscript,
reviewed the literature and gave the final approval of the version
to be submitted. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas A, Khan SA, Chrischilles EA and
Schroeder MC: Initial surgery and survival in stage IV breast
cancer in the United States, 1988–2011. JAMA Surg. 151:424–431.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong Z, Deng G, Wang J, Li X, Xie X,
Shuang Z and Wang X: Could local surgery improve survival in de
novo stage IV breast cancer? BMC Cancer. 18:8852018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eng LG, Dawood S, Sopik V, Haaland B, Tan
PS, Bhoo-Pathy N, Warner E, Iqbal J, Narod SA and Dent R: Ten-year
survival in women with primary stage IV breast cancer. Breast
Cancer Res Treat. 160:145–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malmgren JA, Mayer M, Atwood MK and Kaplan
HG: Differential presentation and survival of de novo and recurrent
metastatic breast cancer over time: 1990–2010. Breast Cancer Res
Treat. 167:579–590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao L, Chu L, Wang LI, Moy L, Brammer M,
Song C, Green M, Kurian AW, Gomez SL and Clarke CA: Occurrence and
outcome of de novo metastatic breast cancer by subtype in a large,
diverse population. Cancer Causes Control. 27:1127–1138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoo TK, Chae BJ, Kim SJ, Lee J, Yoon TI,
Lee SJ, Park HY, Park HK, Eom YH, Kim HS, et al: Identifying
long-term survivors among metastatic breast cancer patients
undergoing primary tumor surgery. Breast Cancer Res Treat.
165:109–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lane WO, Thomas SM, Blitzblau RC, Plichta
JK, Rosenberger LH, Fayanju OM, Hyslop T, Hwang ES and Greenup RA:
Surgical resection of the primary tumor in women with de novo stage
IV breast cancer: Contemporary practice patterns and survival
analysis. Ann Surg. 269:537–544. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bafford AC, Burstein HJ, Barkley CR, Smith
BL, Lipsitz S, Iglehart JD, Winer EP and Golshan M: Breast surgery
in stage IV breast cancer: Impact of staging and patient selection
on overall survival. Breast Cancer Res Treat. 115:7–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rapiti E, Verkooijen HM, Vlastos G,
Fioretta G, Neyroud-Caspar I, Sappino AP, Chappuis PO and Bouchardy
C: Complete excision of primary breast tumor improves survival of
patients with metastatic breast cancer at diagnosis. J Clin Oncol.
24:2743–2749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Babiera GV, Rao R, Feng L, Meric-Bernstam
F, Kuerer HM, Singletary SE, Hunt KK, Ross MI, Gwyn KM, Feig BW, et
al: Effect of primary tumor extirpation in breast cancer patients
who present with stage IV disease and an intact primary tumor. Ann
Surg Oncol. 13:776–782. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gnerlich J, Jeffe DB, Deshpande AD, Beers
C, Zander C and Margenthaler JA: Surgical removal of the primary
tumor increases overall survival in patients with metastatic breast
cancer: Analysis of the 1988–2003 SEER data. Ann Surg Oncol.
14:2187–2194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fields RC, Jeffe DB, Trinkaus K, Zhang Q,
Arthur C, Aft R, Dietz JR, Eberlein TJ, Gillanders WE and
Margenthaler JA: Surgical resection of the primary tumor is
associated with increased long-term survival in patients with stage
IV breast cancer after controlling for site of metastasis. Ann Surg
Oncol. 14:3345–3351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhoo-Pathy N, Verkooijen HM, Tan EY, Miao
H, Taib NA, Brand JS, Dent RA, See MH, Subramaniam S, Chan P, et
al: Trends in presentation, management and survival of patients
with de novo metastatic breast cancer in a Southeast Asian setting.
Sci Rep. 5:162522015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pons-Tostivint E, Alouani E, Kirova Y,
Dalenc F and Vaysse C: Is there a role for locoregional treatment
of the primary tumor in de novo metastatic breast cancer in the era
of tailored therapies?: Evidences, unresolved questions and a
practical algorithm. Crit Rev Oncol Hematol. 157:1031462021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pons-Tostivint E, Kirova Y, Lusque A,
Campone M, Geffrelot J, Mazouni C, Mailliez A, Pasquier D,
Madranges N, Firmin N, et al: Survival impact of locoregional
treatment of the primary tumor in de novo metastatic breast cancers
in a large multicentric cohort study: A propensity score-matched
analysis. Ann Surg Oncol. 26:356–365. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soran A, Ozmen V, Ozbas S, Karanlik H,
Muslumanoglu M, Igci A, Canturk Z, Utkan Z, Ozaslan C, Evrensel T,
et al: Randomized trial comparing resection of primary tumor with
no surgery in stage IV breast cancer at presentation: Protocol
MF07-01. Ann Surg Oncol. 25:3141–3149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fitzal F, Bjelic-Radisic V, Knauer M,
Steger G, Hubalek M, Balic M, Singer C, Bartsch R, Schrenk P,
Soelkner L, et al: Impact of breast surgery in primary metastasized
breast cancer: Outcomes of the prospective randomized phase III
ABCSG-28 POSYTIVE trial. Ann Surg. 269:1163–1169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badwe R, Hawaldar R, Nair N, Kaushik R,
Parmar V, Siddique S, Budrukkar A, Mittra I and Gupta S:
Locoregional treatment versus no treatment of the primary tumour in
metastatic breast cancer: An open-label randomised controlled
trial. Lancet Oncol. 16:1380–1388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris E, Barry M and Kell MR:
Meta-analysis to determine if surgical resection of the primary
tumour in the setting of stage IV breast cancer impacts on
survival. Ann Surg Oncol. 20:2828–2834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petrelli F and Barni S: Surgery of primary
tumors in stage IV breast cancer: An updated meta-analysis of
published studies with meta-regression. Med Oncol. 29:3282–3290.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao W, Zou Y, Zheng S, Hu X, Liu P and
Xie X, Yu P, Tang H and Xie X: Primary tumor resection in stage IV
breast cancer: A systematic review and meta-analysis. Eur J Surg
Oncol. 44:1504–1512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Headon H, Wazir U, Kasem A and Mokbel K:
Surgical treatment of the primary tumour improves the overall
survival in patients with metastatic breast cancer: A systematic
review and meta-analysis. Mol Clin Oncol. 4:863–867. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gera R, Chehade HELH, Wazir U, Tayeh S,
Kasem A and Mokbel K: Locoregional therapy of the primary tumour in
de novo stage IV breast cancer in 216 066 patients: A
meta-analysis. Sci Rep. 10:29522020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arciero C, Liu Y, Gillespie T and Subhedar
P: Surgery and survival in patients with stage IV breast cancer.
Breast J. 25:644–653. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cortesi L, Toss A, Cirilli C, Marcheselli
L, Braghiroli B, Sebastiani F and Federico M: Twenty-years
experience with de novo metastatic breast cancer. Int J Cancer.
137:1417–1426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao W, Wu L, Zhao A, Zhang M, Tian Q,
Shen Y, Wang F, Wang B, Wang L, Chen L, et al: A nomogram for
predicting survival in patients with de novo metastatic breast
cancer: A population-based study. BMC Cancer. 20:9822020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di Meglio A, Freedman RA, Lin NU, Barry
WT, Metzger-Filho O, Keating NL, King TA, Sertoli MR, Boccardo F,
Winer EP and Vaz-Luis I: Time trends in incidence rates and
survival of newly diagnosed stage IV breast cancer by tumor
histology: A population-based analysis. Breast Cancer Res Treat.
157:587–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bitencourt A, Rossi Saccarelli C, Morris
EA, Flynn J, Zhang Z, Khan A, Gillespie E, Cahlon O, Mueller B,
Cuaron JJ, et al: Regional lymph node involvement among patients
with de novo metastatic breast cancer. JAMA Netw Open.
3:e20187902020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen T, Siegal GP and Wei S:
Clinicopathologic factors associated with de novo metastatic breast
cancer. Pathol Res Pract. 212:1167–1173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen T, Gao C, Zhang K, Siegal GP and Wei
S: Prognostic outcomes in advanced breast cancer: The
metastasis-free interval is important. Hum Pathol. 70:70–76. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lobbezoo DJA, van Kampen RJW, Voogd AC,
Dercksen MW, van den Berkmortel F, Smilde TJ, van de Wouw AJ,
Peters FP, van Riel JMGH, Peters NAJB, et al: Prognosis of
metastatic breast cancer: Are there differences between patients
with de novo and recurrent metastatic breast cancer? Br J Cancer.
112:1445–1251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dawood S, Broglio K, Ensor J, Hortobagyi
GN and Giordano SH: Survival differences among women with de novo
stage IV and relapsed breast cancer. Ann Oncol. 21:2169–2174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yardley DA, Kaufman PA, Brufsky A, Yood
MU, Rugo H, Mayer M, Quah C, Yoo B and Tripathy D: Treatment
patterns and clinical outcomes for patients with de novo versus
recurrent HER2-positive metastatic breast cancer. Breast Cancer Res
Treat. 145:725–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lao C, Kuper-Hommel M, Elwood M, Campbell
I, Edwards M and Lawrenson R: Characteristics and survival of de
novo and recurrent metastatic breast cancer in New Zealand. Breast
Cancer. 28:387–397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
den Brok WD, Speers CH, Gondara L, Baxter
E, Tyldesley SK and Lohrisch CA: Survival with metastatic breast
cancer based on initial presentation, de novo versus relapsed.
Breast Cancer Res Treat. 161:549–556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nieder C, Mannsåker B and Haukland E:
Exceptional responses to standard therapy in a patient with
metastatic HER2-positive breast cancer. Cureus.
9:e14122017.PubMed/NCBI
|
|
38
|
Gobbini E, Ezzalfani M, Dieras V, Bachelot
T, Brain E, Debled M, Jacot W, Mouret-Reynier MA, Goncalves A,
Dalenc F, et al: Time trends of overall survival among metastatic
breast cancer patients in the real-life ESME cohort. Eur J Cancer.
96:17–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elbaiomy MA, Akl T, Atwan N, Elsayed AA,
Elzaafarany M and Shamaa S: Clinical impact of breast cancer stem
cells in metastatic breast cancer patients. J Oncol.
2020:25617262020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dawood S, Haaland B, Albaracin C, Gupta S,
Cortes J, Sim YY and Dent RA: Is the proportion of patients
diagnosed with synchronous stage IV breast cancer who survive more
than two years increasing over time? Oncology. 89:79–87. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ogiya R, Sagara Y, Niikura N and Freedman
RA: Impact of subtype on survival of young patients with stage IV
breast cancer. Clin Breast Cancer. 19:200–207.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vaz-Luis I, Lin NU, Keating NL, Barry WT,
Winer EP and Freedman RA: Factors associated with early mortality
among patients with de novo metastatic breast cancer: A
population-based study. Oncologist. 22:386–393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen H, Zhang M, Wang M, Zhang P, Bai F
and Wu K: Immediate breast reconstruction in de novo metastatic
breast cancer: An analysis of 563 cases based on the SEER database.
Clin Breast Cancer. 19:e135–e141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khan SA, Stewart AK and Morrow M: Does
aggressive local therapy improve survival in metastatic breast
cancer? Surgery. 132:620–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Soares LR, Freitas-Junior R, Curado MP,
Paulinelli RR, Martins E and Oliveira JC: Low overall survival in
women with de novo metastatic breast cancer: Does this reflect
tumor biology or a lack of access to health care? JCO Glob Oncol.
6:679–687. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Blanchard DK, Shetty PB, Hilsenbeck SG and
Elledge RM: Association of surgery with improved survival in stage
IV breast cancer patients. Ann Surg. 247:732–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
King TA, Lyman JP, Gonen M, Voci A, De
Brot M, Boafo C, Sing AP, Hwang ES, Alvarado MD, Liu MC, et al:
Prognostic impact of 21-gene recurrence score in patients with
stage IV breast cancer: TBCRC 013. J Clin Oncol. 34:2359–2365.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carmichael AR, Anderson EDC, Chetty U and
Dixon JM: Does local surgery have a role in the management of stage
IV breast cancer? Eur J Surg Oncol. 29:17–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin C, Wu J, Ding S, Goh C, Andriani L, Lu
S, Shen K and Zhu L: Subdivision of M1 stage for de novo metastatic
breast cancer to better predict prognosis and response to primary
tumor surgery. J Natl Compr Canc Netw. 17:1521–1528. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Plichta JK, Thomas SM, Sergesketter AR,
Greenup RA, Rosenberger LH, Fayanju OM, Kimmick G, Force J, Hyslop
T and Hwang ES: A novel staging system for de novo metastatic
breast cancer refines prognostic estimates. Ann Surg. 275:784–792.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Garrido-Castro AC, Spurr LF, Hughes ME, Li
YY, Cherniack AD, Kumari P, Lloyd MR, Bychkovsky B, Barroso-Sousa
R, Di Lascio S, et al: Genomic characterization of de novo
metastatic breast cancer. Clin Cancer Res. 27:1105–1118. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Seltzer S, Corrigan M and O'Reilly S: The
clinicomolecular landscape of de novo versus relapsed stage IV
metastatic breast cancer. Exp Mol Pathol. 114:1044042020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
McKenzie HS, Maishman T, Simmonds P,
Durcan L; POSH Steering Group, ; Eccles D and Copson E: Survival
and disease characteristics of de novo versus recurrent metastatic
breast cancer in a cohort of young patients. Br J Cancer.
122:1618–1629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Di Meglio A, Lin NU, Freedman RA, Barry
WT, Winer EP and Vaz-Luis I: Patterns of utilization of imaging
studies and serum tumor markers among patients with de novo
metastatic breast cancer. J Natl Compr Canc Netw. 15:316–324. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shien T, Kinoshita T, Shimizu C, Hojo T,
Taira N, Doihara H and Akashi-Tanaka S: Primary tumor resection
improves the survival of younger patients with metastatic breast
cancer. Oncol Rep. 21:827–832. 2009.PubMed/NCBI
|
|
56
|
Barinoff J, Schmidt M, Schneeweiss A,
Schoenegg W, Thill M, Keitel S, Lattrich CR, Hinke A, Kutscheidt A
and Jackisch C: Primary metastatic breast cancer in the era of
targeted therapy-prognostic impact and the role of breast tumour
surgery. Eur J Cancer. 83:116–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Co M, Ng J and Kwong A: De-novo metastatic
breast cancers with or without primary tumor resection-A 10-year
study. Cancer Treat Res Commun. 19:1001182019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kwong A and Co M: Long term survival study
of de-novo metastatic breast cancers with or without primary tumour
resection. Cancer Treat Res Commun. 20:1001482019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rao R, Feng L, Kuerer HM, Singletary SE,
Bedrosian I, Hunt KK, Ross MI, Hortobagyi GN, Feig BW, Ames FC and
Babiera GV: Timing of surgical intervention for the intact primary
in stage IV breast cancer patients. Ann Surg Oncol. 15:1696–1702.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cady B, Nathan NR, Michaelson JS, Golshan
M and Smith BL: Matched pair analyses of stage IV breast cancer
with or without resection of primary breast site. Ann Surg Oncol.
15:3384–3395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Soran A, Özbaş S, Doğan L, Sezgin E, Özmen
V, Beriwal S and Brufsky A: Loco-regional treatment for intact
primary tumor in patient with de novo metastatic breast cancer;
comments and concerns of ECOG-ACRIN 2108 trial. Eur J Breast
Health. 16:158–159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Poggio F, Lambertini M and de Azambuja E:
Surgery of the primary tumour in patients presenting with de novo
metastatic breast cancer: to do or not to do? ESMO Open.
3:e0003242018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Teshome M: Role of operative management in
stage IV breast cancer. Surg Clin North Am. 98:859–868. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Altundag K: Questions about the primary
tumor resection for de-novo metastatic breast cancers. Cancer Treat
Res Commun. 20:1001312019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Altundag K: Predictive role of
loco-regional radiotherapy among metastatic breast cancer patients
who had undergone primary tumor surgery. Breast Cancer Res Treat.
167:3032018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gultekin M, Yazici O, Eren G, Yuce D,
Aksoy S, Ozisik Y, Guler N, Yazici G, Hurmuz P, Yildiz F, et al:
Impact of locoregional treatment on survival in patients presented
with metastatic breast carcinoma. Breast. 23:775–783. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim YJ, Jung SY and Kim K: Survival
benefit of radiotherapy after surgery in de novo stage IV breast
cancer: A population-based propensity-score matched analysis. Sci
Rep. 9:85272019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tan Y, Li X, Chen H, Hu Y, Jiang M, Fu J,
Yuan Y and Ding K: Hormone receptor status may impact the survival
benefit of surgery in stage IV breast cancer: A population-based
study. Oncotarget. 7:70991–71000. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang K, Shi Y, Li ZY, Xiao YL, Li J, Zhang
X and Li HY: Metastatic pattern discriminates survival benefit of
primary surgery for de novo stage IV breast cancer: A real-world
observational study. Eur J Surg Oncol. 45:1364–1372. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Samiee S, Berardi P, Bouganim N,
Vandermeer L, Arnaout A, Dent S, Mirsky D, Chasen M, Caudrelier JM
and Clemons M: Excision of the primary tumour in patients with
metastatic breast cancer: A clinical dilemma. Curr Oncol.
19:e270–e279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rashaan ZM, Bastiaannet E, Portielje JE,
van de Water W, van der Velde S, Ernst MF, van de Velde CJ and
Liefers GJ: Surgery in metastatic breast cancer: Patients with a
favorable profile seem to have the most benefit from surgery. Eur J
Surg Oncol. 38:52–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Retsky M, Bonadonna G, Demicheli R,
Folkman J, Hrushesky W and Valagussa P: Hypothesis: Induced
angiogenesis after surgery in premenopausal node-positive breast
cancer patients is a major underlying reason why adjuvant
chemotherapy works particularly well for those patients. Breast
Cancer Res. 6:R372–E374. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hofer SO, Molema G, Hermens RA, Wanebo HJ,
Reichner JS and Hoekstra HJ: The effect of surgical wounding on
tumour development. Eur J Surg Oncol. 25:231–243. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
O'Reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: A novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gunduz N, Fisher B and Saffer EA: Effect
of surgical removal on the growth and kinetics of residual tumor.
Cancer Res. 39:3861–3865. 1979.PubMed/NCBI
|
|
76
|
Rashid OM and Takabe K: Does removal of
the primary tumor in metastatic breast cancer improve survival? J
Womens Health (Larchmt). 23:184–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cacho-Díaz B, García-Botello DR,
Wegman-Ostrosky T, Reyes-Soto G, Ortiz-Sánchez E and
Herrera-Montalvo LA: Tumor microenvironment differences between
primary tumor and brain metastases. J Transl Med. 18:12020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Khodari W, Sedrati A, Naisse I, Bosc R and
Belkacemi Y; AROME (Association of Radiotherapy and Oncology of the
Mediterranean arEa; www.aromecancer.org), : Impact of loco-regional
treatment on metastatic breast cancer outcome: A review. Crit Rev
Oncol Hematol. 87:69–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Trovato R, Canè S, Petrova V, Sartoris S,
Ugel S and De Sanctis F: The engagement between MDSCs and
metastases: Partners in crime. Front Oncol. 10:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Moserle L, Amadori A and Indraccolo S: The
angiogenic switch: Implications in the regulation of tumor
dormancy. Curr Mol Med. 9:935–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kazerounian S and Lawler J: Integration of
pro- and anti-angiogenic signals by endothelial cells. J Cell
Commun Signal. 12:171–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kozłowski J, Kozłowska A and Kocki J:
Breast cancer metastasis-insight into selected molecular mechanisms
of the phenomenon. Postepy Hig Med Dosw (Online). 69:447–451. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nguyen DH and Truong PT: A debate on
locoregional treatment of the primary tumor in patients presenting
with stage IV breast cancer. Expert Rev Anticancer Ther.
11:1913–1922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pantel K and Speicher MR: The biology of
circulating tumor cells. Oncogene. 35:1216–1224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang WC and Zhang XF, Peng J, Li XF, Wang
AL, Bie YQ, Shi LH, Lin MB and Zhang XF: Survival mechanisms and
influence factors of circulating tumor cells. Biomed Res Int.
2018:63047012018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Becker A, Thakur BK, Weiss JM, Kim HS,
Peinado H and Lyden D: Extracellular vesicles in cancer:
Cell-to-cell mediators of metastasis. Cancer Cell. 30:836–848.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dos Anjos Pultz B, Andrés Cordero da Luz
F, Socorro Faria S, Peixoto Ferreira de Souza L, Cristina Brígido
Tavares P, Alonso Goulart V, Fontes W, Ricardo Goulart L and José
Barbosa Silva M: The multifaceted role of extracellular vesicles in
metastasis: Priming the soil for seeding. Int J Cancer.
140:2397–2407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wortzel I, Dror S, Kenific CM and Lyden D:
Exosome-mediated metastasis: Communication from a distance. Dev
Cell. 49:347–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wong CC, Gilkes DM, Zhang H, Chen J, Wei
H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, et al:
Hypoxia-inducible factor 1 is a master regulator of breast cancer
metastatic niche formation. Proc Natl Acad Sci USA.
108:16369–16374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Noguchi M, Nakano Y, Noguchi M, Ohno Y and
Kosaka T: Local therapy and survival in breast cancer with distant
metastases. J Surg Oncol. 105:104–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yousefi M, Nosrati R, Salmaninejad A,
Dehghani S, Shahryari A and Saberi A: Organ-specific metastasis of
breast cancer: Molecular and cellular mechanisms underlying lung
metastasis. Cell Oncol (Dordr). 41:123–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sun S and Qiu XS: Cancer stem cells and
tumor metastasis. J Cancer Res Ther. 9 (Suppl):S150–S152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Marcuzzi E, Angioni R, Molon B and Calì B:
chemokines and chemokine receptors: Orchestrating tumor
metastasization. Int J Mol Sci. 20:962018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
O'Hayre M, Salanga CL, Handel TM and Allen
SJ: Chemokines and cancer: Migration, intracellular signalling and
intercellular communication in the microenvironment. Biochem J.
409:635–649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ma R, Feng Y, Lin S, Chen J, Lin H, Liang
X, Zheng H and Cai X: Mechanisms involved in breast cancer liver
metastasis. J Transl Med. 13:642015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Seguin L, Desgrosellier JS, Weis SM and
Cheresh DA: Integrins and cancer: Regulators of cancer stemness,
metastasis, and drug resistance. Trends Cell Biol. 25:234–240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Naxerova K and Jain RK: Using tumour
phylogenetics to identify the roots of metastasis in humans. Nat
Rev Clin Oncol. 12:258–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Norton L and Massagué J: Is cancer a
disease of self-seeding? Nat Med. 12:875–878. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Danna EA, Sinha P, Gilbert M, Clements VK,
Pulaski BA and Ostrand-Rosenberg S: Surgical removal of primary
tumor reverses tumor-induced immunosuppression despite the presence
of metastatic disease. Cancer Res. 64:2205–2211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Criscitiello C, Giuliano M, Curigliano G,
De Laurentiis M, Arpino G, Carlomagno N, De Placido S, Golshan M
and Santangelo M: Surgery of the primary tumor in de novo
metastatic breast cancer: To do or not to do? Eur J Surg Oncol.
41:1288–1292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jiang ZF, Cristofanilli M, Shao ZM, Tong
ZS, Song EW, Wang XJ, Liao N, Hu XC, Liu Y, Wang Y, et al:
Circulating tumor cells predict progression-free and overall
survival in Chinese patients with metastatic breast cancer,
HER2-positive or triple-negative (CBCSG004): A multicenter,
double-blind, prospective trial. Ann Oncol. 24:2766–2772. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LWMM and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hazard HW, Gorla SR, Scholtens D, Kiel K,
Gradishar WJ and Khan SA: Surgical resection of the primary tumor,
chest wall control, and survival in women with metastatic breast
cancer. Cancer. 113:2011–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dauplat J, Le Bouëdec G, Pomel C and
Scherer C: Cytoreductive surgery for advanced stages of ovarian
cancer. Semin Surg Oncol. 19:42–48. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dooley WC: A surgical indication in
incurable breast cancer. Ann Surg Oncol. 13:759–760. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ruiterkamp J, Ernst MF, van de Poll-Franse
LV, Bosscha K, Tjan-Heijnen VC and Voogd AC: Surgical resection of
the primary tumour is associated with improved survival in patients
with distant metastatic breast cancer at diagnosis. Eur J Surg
Oncol. 35:1146–1151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Leung AM, Vu HN, Nguyen KA, Thacker LR and
Bear HD: Effects of surgical excision on survival of patients with
stage IV breast cancer. J Surg Res. 161:83–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Pathy NB, Verkooijen HM, Taib NA, Hartman
M and Yip CH: Impact of breast surgery on survival in women
presenting with metastatic breast cancer. Br J Surg. 98:1566–1572.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bertaut A, Mounier M, Desmoulins I, Guiu
S, Beltjens F, Darut-Jouve A, Ponnelle T, Arnould L and Arveux P:
Stage IV breast cancer: A population-based study about prognostic
factors according to HER2 and HR status. Eur J Cancer Care (Engl).
24:920–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lopez-Tarruella S, Escudero MJ, Pollan M,
Martín M, Jara C, Bermejo B, Guerrero-Zotano A, García-Saenz J,
Santaballa A, Alba E, et al: Survival impact of primary tumor
resection in de novo metastatic breast cancer patients (GEICAM/El
Alamo Registry). Sci Rep. 9:200812019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pons-Tostivint E, Kirova Y, Lusque A,
Campone M, Geffrelot J, Rivera S, Mailliez A, Pasquier D, Madranges
N, Firmin N, et al: Radiation therapy to the primary tumor for de
novo metastatic breast cancer and overall survival in a
retrospective multicenter cohort analysis. Radiother Oncol.
145:109–116. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rosche M, Regierer AC,
Schwarzlose-Schwarck S, Weigel A, Bangemann N, Schefe JH, Scholz
CW, Possinger K and Eucker J: Primary tumor excision in stage IV
breast cancer at diagnosis without influence on survival: A
retrospective analysis and review of the literature. Onkologie.
34:607–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
















