Introduction
Nearly 2.0 million new cancer cases are predicted to
occur in 2024 in the USA (1). With
increasing patient numbers, medical and caregiving costs over the
next 30 years are projected to reach a staggering 25 trillion US
dollars globally (2). To improve
the long-term prognosis of cancer patients using existing treatment
modalities optimally, it is essential to consider factors such as
age, physical condition, tumor location, staging and treatment
outcomes comprehensively (3).
Despite the efficacy of conventional treatments such as
chemotherapy in cancer therapy, challenges remain, including
precision issues, high costs and severe side effects. For specific
tumor types, such as brain tumors or those near the genitourinary
tract, surgical complications often make radiotherapy the preferred
option (4,5).
Radiotherapy directly impacts the DNA of tumor cells
or generates highly reactive oxygen radicals, leading to cell
mutations and cell death for therapeutic purposes (6). Although this approach has a long
history in clinical practice and relatively mature technology, it
still encounters challenges, including tissue damage and treatment
failures. The shift from general to precision medicine and the
application of radiation research findings in clinical practice
have been bottlenecks in the field (7). Emerging in vitro models hold
the potential to address these challenges.
Research models associated with radiotherapy
predominantly comprise cell and animal models. Cell models are
advantageous for cell culture, genetic research and high-throughput
screening, but are limited in their ability to replicate cell-cell
interactions and in vivo microenvironments over extended
periods. This limitation results in an incomplete representation of
primary tumors, thus raising concerns about the accuracy of
experimental data (8). By contrast,
animal models, including genetically engineered mice and
patient-derived xenograft mice, offer clinical relevance, genetic
stability, tumor heterogeneity and complete internal environmental
systems, encompassing neural control and immune responses (9). Gene-editing techniques can be used to
investigate the association between specific tumor mutation genes,
such as tumor protein p53 (10) or
ATM serine/threonine kinase (11),
and radiotherapy. However, some oncogenes, such as lncGRS-1, are
highly conserved in primates but absent in rodents, making
traditional mouse models unsuitable for subsequent experimental
research (12). Similarly, genes
such as APC regulator of WNT signaling pathway in colorectal cancer
or KRAS proto-oncogene GTPase in pancreatic cancer cannot be
replicated in animal models due to the insidious onset, high degree
of malignancy and rapid progression. Once found, most cases are at
an advanced stage (13,14). Additionally, the differences in
genetics, anatomy and physiology reduce the reproducibility of
animal models and fail to accurately represent the development of
human organs and systems. This is particularly evident in the study
of rare diseases such as fragile X syndrome (15), where animal experiments have proven
ineffective. For castration-resistant prostate cancer that does not
rely on the androgen receptor signaling mechanism, animal models
have not established yet (16), and
ethical concerns, such as reducing the number of animals used,
cannot be ignored (17).
Three-dimensional organoid models overcome these limitations,
offering advantages in accuracy and efficiency, and promoting the
transition of basic research into clinical applications (18,19).
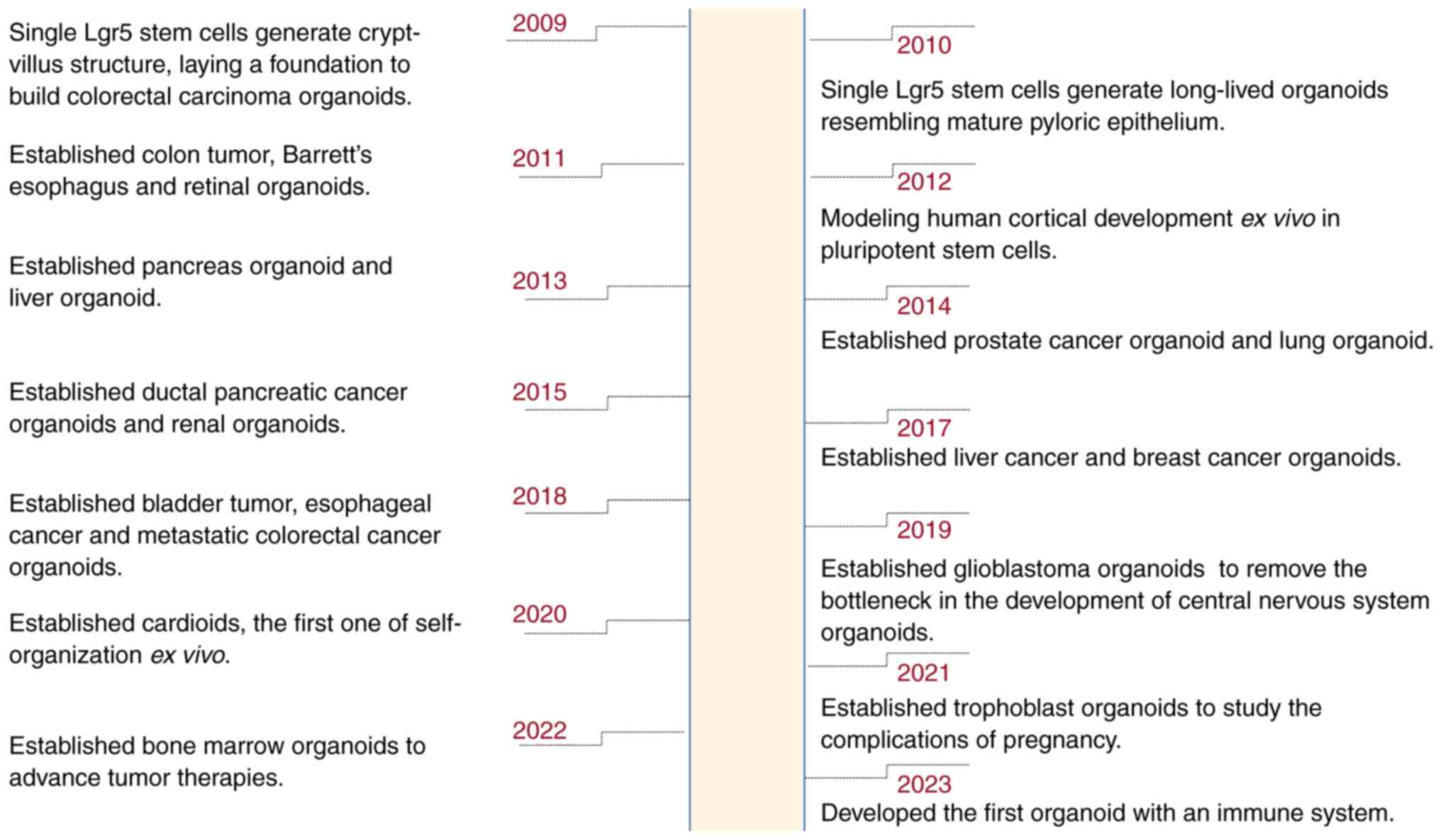
Organoid technology, recognized as the Life Science
Method of the Year by ‘Nature Methods’, constructs multicellular,
self-organizing, three-dimensional structures using stem cells,
such as embryonic stem cells, induced pluripotent stem cells
(iPSCs) or tissue-resident stem cells/progenitor cells (18–20).
Tumor organoids are created by in vitro three-dimensional
culturing of tumor stem cells, allowing them to self-assemble into
‘minimal systems’ that closely mimic the morphology and function of
corresponding in vivo tumor tissues. Categorization of
organoids based on distinct germ layers includes: i) the ectoderm,
which mainly encompasses the nervous system, with glioblastoma
organoids (21) predominantly in
radiotherapy, ii) the mesoderm, comprising organoids from the
urogenital system, notably breast cancer (22) and renal organoids (23), with the former widely used in
high-throughput drugs screenings, mechanistic explorations and
pharmacological assessments, and the latter primarily focusing on
post-transplantation functionality; and iii) the endoderm,
primarily encompassing gastrointestinal tract organoids (24), known for their early development
stages, standardized cultivation techniques and extensive
applications in radiotherapy. Additionally, the rapidly advancing
field of organoid technology has introduced new forms such as
cardioids (25), trophoblasts
(26) and bone marrow organoids
(27). In addressing prior
challenges associated with the lack of immune components, Bouffi
et al (28) made a
significant breakthrough in 2023 by developing an intestinal
organoid that incorporates an immune system (Fig. 1).
In contrast to models focused on single tumor types,
biobanks provide a comprehensive view of various pathological
stages of tumors. For instance, the gastrointestinal tumor organoid
(GITO) biobank (24) spans stages
ranging from inflammation and erosion to ulcers, atrophy,
intestinal metaplasia, hyperplasia, carcinogenesis and even
metastasis. These biobanks are particularly effective in
integrating genomic and functional data from clinical sources,
predominantly derived from patient-derived tumor subtypes, to
identify potential therapeutic targets, thus playing a pivotal role
in bridging basic research with clinical applications. Currently,
an array of organoid biobanks is being established, catering to
cancers such as prostate (16),
bladder (5), liver (29) and pancreatic (14) cancer, among others. These biobanks
differ in terms of tissue sources, cellular composition,
cultivation methods, the duration of generation and maintenance,
and their number and applications. More crucially, the role of
biobanks as a repository is fundamental in precision medicine
(Table I) (30–38).
 | Table I.Overview of various tumor organoid
biobanks (2011–2022). |
Table I.
Overview of various tumor organoid
biobanks (2011–2022).
| Tumor tissue | Cell
composition | Culture
methods |
Generated/maintaining time | Organoid
numbers | Applications | Publication
year | (Refs.) |
|---|
| Colon | Colonic cancer
epithelial | Submerged
culture | 10 days/within 3
months | 22 | Developing a
technology to culture the gastrointestinal tract | 2011 | (30) |
| Prostate | Prostate cancer
biopsy specimen/circulating tumor cells | Submerged
culture | 3 days-3
weeks/>6 months | 6 | Investigating
relevant genetic and pharmacological studies | 2014 | (16) |
| Posterior
tongue | Adult taste stem
cells/progenitor cells | Induced pluripotent
stem cells | 2-3 days/1
month | 43 | Generating the
functional taste bud model from stem cells | 2014 | (31) |
| Pancreatic
duct | Human PDA
endoscopic needle biopsies | Submerged
culture | 3 days-2 weeks/up
to 1 months | 19 | Investigating PDA
pathogenesis and identifying molecular pathways with disease
progression | 2015 | (14) |
| Fallopian tube | Gynecological
tissue specimens | Submerged
culture | >16 months | 7 | Investigating the
signaling routes | 2015 | (32) |
| Liver | Patient-derived
liver tumor tissue of surgical resection samples | Submerged
culture | Around 1 year | 8 | Identifying
biomarker and screening drug | 2017 | (29) |
| Endometrium | Human adult stem
cell | Submerged
culture | >6 months | 25 | Study diseases
(such as endometriosis and endometrial cancer) and the physiology
of early gestation | 2017 | (33) |
| Gastrointestinal
tract | Patient-derived
biopsies of gastrointestinal cancers | Submerged
culture | >3 months | 63 | Implementing in
personalized medicine | 2018 | (24) |
| Breast | Patient tissue
underwent lumpectomy | Submerged
culture | >4 months | 95 | Discovering drug
and cancer mechanism | 2018 | (22) |
| Bladder | Patient-derived
biopsies of bladder cancers | Submerged
culture | >7 weeks | 12 | Studying tumor
evolution and treatment response | 2018 | (5) |
| Esophageal | Esophageal
adenocarcinoma tissue samples of esophagectomy | Submerged
culture | >6 months | 10 | Screening drugs and
studying tumor clonality | 2018 | (34) |
| Epidermis | Murine
keratinocytes | Induced adult
epidermal stem cells | >7 months | 23 | Studying the
biology of skin diseases | 2019 | (35) |
| Lung | Lung cancer tissues
of surgically resected/a small biopsy tissue | Submerged
culture | Around 4
weeks/>2 months | 39 | Predicting
patient-specific drug responses | 2019 | (36) |
| Renal | Surgical
specimens | Submerged
culture | >120 days | 10 | Improving
therapeutic treatments | 2019 | (23) |
| Biliary tract | Biliary tract
carcinoma of patients | Submerged
culture | >1 year | 6 | Screening drugs as
potential therapeutic agents | 2019 | (37) |
| Glioblastoma | Patient-derived
glioblastoma resection samples | Submerged culture
and co-culture | >2 months (>1
year of continuous culture without passaging) | 70 | Describing a novel
organoid culture system and studying the heterogenoous cell-cell
relationships | 2019 | (21) |
| Nasopharyngeal | Surgical
specimens | Submerged
culture | >6 months | 16 primary NPC, 23
recurrent NPC, 13 normal mucosa samples | Exploring the
pathogenesis and developing precision medicine | 2022 | (38) |
Methodologies of tumor organoids
Materials for tumor organoids are typically derived
from sources such as fine-needle aspirations, biopsies, resection
specimens, circulating tumor cells and PSCs (24,39). A
key factor for successful cultivation is obtaining an adequate
quantity of tumor cells. It is important to note that these diverse
techniques for obtaining organoids balance simplicity and ease of
use with precision.
Submerged culture
This method involves culturing the material within
gels of extracellular matrix (ECM), submerged beneath tissue
culture media (40). Submerged
culture is predominantly suited for tumor tissues such as those
from the digestive tract, glands and urogenital reproduction
system.
Induced stem cells
This category includes both iPSCs and embryonic stem
cells from human or murine sources. This approach involves using
customized tissue-specific differentiation protocols to generate
the corresponding organ type. However, it is important to note that
this process generally requires a significant duration, ranging
from weeks to months (41).
Air-liquid interface culture
In this technique, the top of the Transwell is
directly exposed to air, which enhances oxygen diffusion and
facilitates the growth of larger organoids (41). This method is extensively used in
respiratory system studies and is crucial when combined with
gene-editing technology for investigating tumor mechanisms or
signaling pathways (42).
Co-culture
This approach enriches the culture system by
incorporating a mix of cells, tissues and organs, fostering a more
complex and interactive environment (43).
Bioreactors
These devices are especially efficient in quickly
providing cells with the essential nutrients and growth factors;
they are primarily used for rapid organoid generation in the
nervous system over a brief period of 2–4 weeks (44).
Organoid-on-a-chip
Originating from the field of microfluidics, this
innovative device simulates human organ functional units ex
vivo. Organoid-on-a-chip enables precise control over the
physical and biochemical microenvironment, managing aspects such as
cytokine concentration gradients and nutrient supply, and modeling
interactions between tissues and multiple organs (45).
Common applications of tumor organoids
Patient-derived tumor organoids (PDTOs) reflect the
in vitro mutational modeling of all stages of malignancy,
and their construction is the same as that of the other tumor cells
such as iPSCs and embryonic stem cells. PDTOs are useful in
precision medicine as they are able to guide patient-specific
therapies. The organoids are celebrated for their ability to
include various cell types, accurately reflect the corresponding
native tissues and replicate definitive functionalities. A pivotal
feature of PDTOs is their capacity to display the genomic and
phenotypic heterogeneity within and between tumors. Employing
advanced sequencing analyses, such as whole-genome sequencing and
RNA sequencing, PDTOs maintain the unique somatic mutations of the
patient (18,19). The preservation of genetic profiles,
combined with rapid chemosensitivity testing post-diagnosis, is
crucial in precision medicine (46). Besides, PDTOs can mimic the tumor
under controlled laboratory conditions, provide results closer to
those of the clinic than cell lines and animals, and last but not
least, reduce the requirement for animal testing. Currently, there
is a lack of established guidelines for PDTOs between different
labotories, meaning that reproducibility cannot be ensured.
Meanwhile, the ethical considerations (17) of obtaining patient-derived tumor
materials also cannot be ignored.
In pancreatic cancer specifically, PDTOs demonstrate
a matching rate of up to 89%, showing therapeutic responses similar
to those of the patient they originate from. This similarity offers
a promising indicator for predicting patient responses to
treatments in clinical settings (14). PDTOs can accurately replicate
complex inter- and intra-tumoral heterogeneity. Notably, some
organoid lines exhibit biomarker overexpression, such as that of
human epidermal growth factor receptor 2 (HER2) in breast cancer
PDTOs, which consequently results in insensitivity to HER2-targeted
therapies (22). Likewise, lung
cancer PDTOs with epidermal growth factor receptor mutations may
exhibit resistance to erlotinib, while activin receptor-like kinase
1 mutations may show no response to crizotinib. Conversely, Erb-B2
receptor tyrosine kinase 2-mutated cancer organoids have shown
sensitivity to both erlotinib and gefitinib (36). These variations suggest that
predictive mutation biomarkers may sometimes be off-target or
influenced by sequencing biases.
Notably, insights from patient-derived glioblastoma
organoids have highlighted the cytotoxic effects of targeted
therapies such as everolimus (a mammalian target of rapamycin
inhibitor) and cobimetinib (a mitogen-activated protein kinase
inhibitor) (21). These findings
underscore the importance of clinicians being acutely aware of
potential adverse effects when administering these drugs to
patients. In summary, PDTOs offer a transformative approach to
oncology, blending tumor biology intricacies with personalized
medicine precision. The workflow of generation, characterization
and applications of tumor organoids is illustrated in Fig. 2.
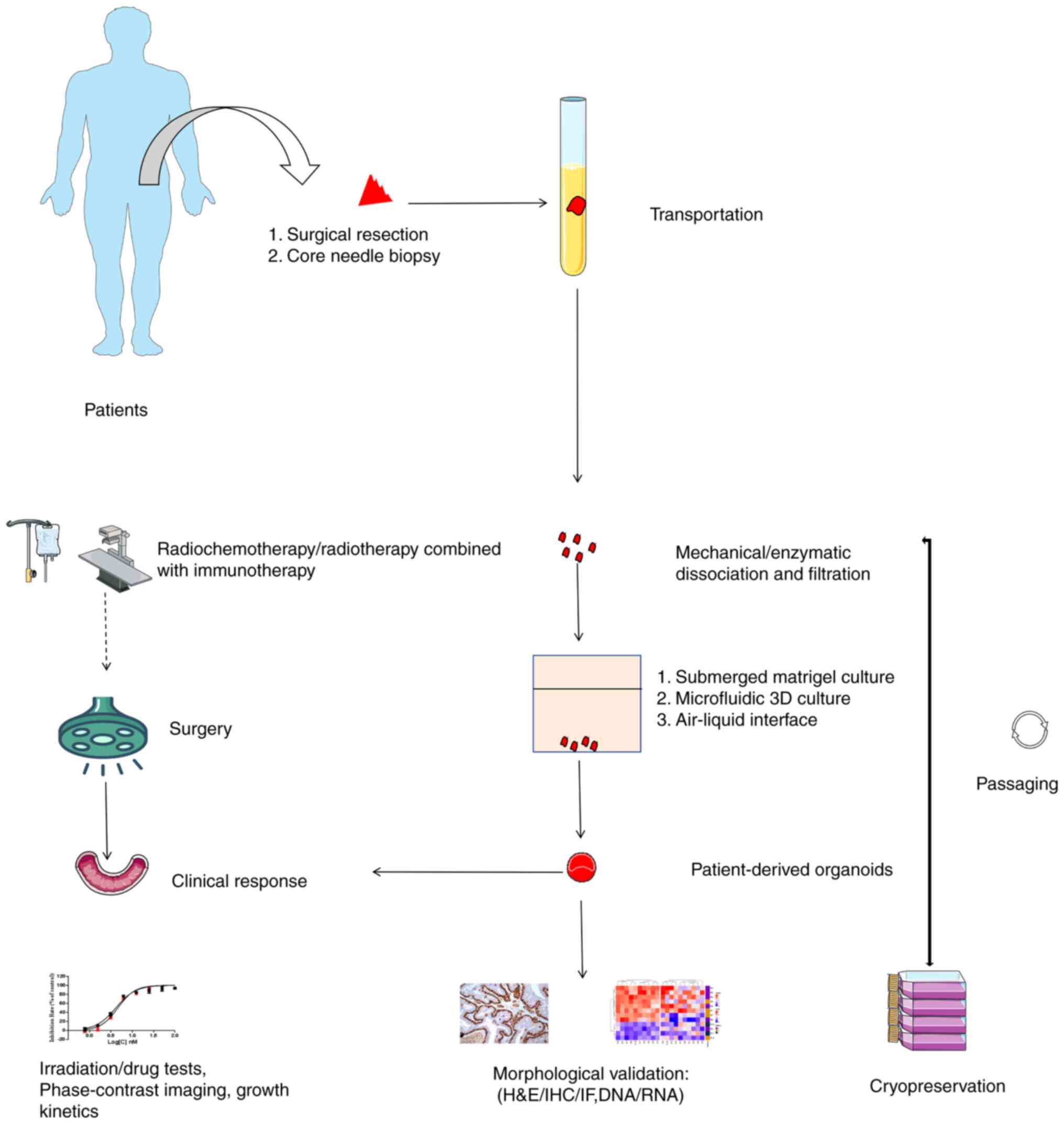 | Figure 2.Workflow of PDO generation,
characterization and applications. PDO generation begins with the
acquisition of tumor fragments via surgical resection or core
needle biopsy. These are then dissociated and filtered into a
single-cell suspension upon arrival at the laboratory. Cells are
seeded in conditional medium using methods such as submerged
Matrigel culture, microfluidic 3D culture or air-liquid interface.
Subsequently, PDOs are processed for cryopreservation to establish
a patient biobank and subjected to morphological assays, including
H&E/IHC/IF, DNA/RNA analysis, coupled with irradiation, drug
tests, phase-contrast imaging and growth kinetics. A special focus
is placed on neoadjuvant therapies such as radiochemotherapy and
radiotherapy combined with immunotherapy, aimed at reducing tumor
size, alleviating symptoms or improving surgical outcomes. Finally,
experimental results are applied to patients for precision
medicine. PDO, patient-derived organoid; H&E, hematoxylin and
eosin; IHC, immunohistochemistry; IF, immunofluorescence. |
GITOs
Animal models for predicting human gastrointestinal
responses to radiotherapy have previously been limited due to
disparities in gut microbiota, the microenvironment, dietary
structure, and anatomical variances between mice models carrying
carcinogenic genes and tumor lesions in patients, and advanced
stage tumors have also resulted in a shortened mouse lifespan
(47,48). Emerging GITOs have effectively
addressed the previous contradictions by achieving high fidelity
and precise matching of corresponding tumor structure in patients.
Yet, it is important to note that the cultural systems vary
significantly across different laboratories.
Comparison of the cultural systems of
GITOs
The [epidermal growth factor (EGF), Noggin and
R-Spondin 1] (ENR) culture system developed by Sato et al
(49) is recognized as the simplest
method for cultivating intestinal organoids, and is primarily used
in primary tissue cultures. This system triggers the expression of
the leucine-rich repeat-containing G protein-coupled receptor 5
(Lgr5) gene, which acts as a receptor for the Wnt activator
R-spondin 1; it also utilizes specific molecules such as Noggin,
EGF and Wnt to promote the growth of intestinal crypts (50). Notably, R-spondin 1, serving as a
Wnt modulator, can substitute for the role of Wnt3a, meaning that
organoid formation does not always require Wnt induction, and their
combined use does not lead to synergistic effects (51). Moreover, the composition of culture
systems varies among research teams. Some studies suggest that
adding IL-22 to a culture system lacking EGF can prevent
radiation-induced damage to ISCs by inducing Stat3 phosphorylation
in Lgr5+ ISCs. This method not only protects ISCs from
radiation damage, but also promotes the proliferation of remaining
stem cells, aiding in tissue repair (52).
In contrast to the traditional ENR culture system,
the 8C culture system [LDN193189, glycogen synthase kinase 3
inhibitor XV, pexmetinib, valproic acid, EPZ6438, EGF, R-Spondin 1
conditioned medium and basic fibroblast growth factor (bFGF)],
proposed by Qu et al (53),
exhibited a nearly 130-fold increase in expression of the stem cell
antigen-1 gene associated with organ regeneration after radiation.
Additionally, the repair genes clusterin, annexin A1 and
regenerating islet-derived β showed nearly 300-, 160- and 22-fold
increases, respectively. Consequently, this system significantly
accelerates the restoration of the crypt structure in the
intestinal epithelium following radiation injury (53). Furthermore, the Yap pathway plays a
crucial role in crypt regeneration post-ionizing radiation by
suppressing the Wnt signaling pathway while inducing the Egfr
signaling pathway, ultimately promoting the proliferation of
Lgr5+ ISCs (54).
Besides Lgr5+ stem cells, Farin et
al (55) discovered that a
culture medium containing Wnt11 could increase organoid
proliferation following radiation damage by activating the Wnt
signaling pathway. Therefore, Wnt11 holds promise as a potential
target for radiation injury repair. Consequently, growth factors
are not only crucial for the development of organoid culture
systems, but also influence the outcomes of radiotherapy. For
instance, R-spondin 1 can protect Lgr5+ stem cells in
the intestine from radiation-induced damage (56). Insulin-like growth factor 1 (IGF-1)
and FGF-1 both inhibit p53-dependent apoptosis and promote the
survival of ISCs after exposure to radiation (57).
GITOs for radiotherapy
Current research utilizing organoids in fields such
as biobanking, phenotype validation and drug screening is thriving.
However, research on radiotherapy using organoids is relatively
limited (34). The development of
tumor organoids provides a comprehensive understanding of clinical
heterogeneity in patients, offering a method for predicting patient
responsiveness to radiotherapy. The organoids developed by Ganesh
et al (58) not only align
with rectal cancer in terms of tissue pathology, but also maintain
consistent expression of intestinal epithelial cell differentiation
markers, aiding in the assessment of patient reaction to
radiotherapy. Yao et al (59) effectively used gastrointestinal
organoids for screening highly effective clinical radiochemotherapy
regimens, achieving an efficacy rate of up to 85% for tumor
patients who had experienced treatment failures or disease
progression. Given the significant benefits of drug screening in
organoids, this model is often combined with chemotherapy to
improve treatment effectiveness.
Additionally, research by Al Bitar et al
(60) demonstrated that using a
radiation sensitizer alone has minimal impact on tumor lesions.
However, when combined with low-dose radiation (2 Gy), just 10 µM
of the sensitizer belinostat is sufficient to induce damage in
colon cancer organoids. This model can also be used to investigate
pathways associated with radiation resistance, including the
PI3K/Akt/mTOR, MEK/ERK and Notch activation pathways, as well as
pathways linked to damage repair, such as the DNA-dependent protein
kinase, RAD51 homolog recombination and breast cancer type 2
susceptibility protein pathways (61). Considering the increased
susceptibility of rectal cancer to the cumulative effects of
radiation (23), improvements in
intestinal protectors and radiation techniques, such as
intensity-modulated radiotherapy and volumetric-modulated arc
therapy, can partially reduce damage to the surrounding pelvic
tissues. However, radiation proctitis remains a challenging issue,
and a definitive cure is still elusive.
In 2017, Schwartz et al (62) applied intestinal organoids to
decellularized matrices, forming a monolayer epithelial structure
reminiscent of the intestinal surface, which could be used to mend
sites affected by radiation-induced injury. Subsequently, Jee et
al (63) successfully
transplanted colon organoids onto the irradiated mucosa of mouse
rectums with damaged tissue, leading to the restoration of the
epithelial structure. This model can even indirectly highlight the
importance of the internal environment during radiotherapy. When
exposed to 4 Gy of radiation, in vitro intestinal organoids
experience a significant decrease in the number of Lgr5+
stem cells, making lesion repair challenging. However, at the same
radiation dose in vivo, reserve stem cells are activated,
resulting in a rapid increase in Lgr5+ cells and
expediting the repair of damaged tissue (57,61).
Table II provides a detailed
summary of tumor organoids that are presently being utilized in
studies, focused on radiotherapy dose (58–61,64–68).
 | Table II.Different tumor organoids contained
in reviews, especially applications in radiotherapy. |
Table II.
Different tumor organoids contained
in reviews, especially applications in radiotherapy.
| Tumor
organoids | RT dose, Gy | Applications | (Refs.) |
|---|
| RC | 0-8 | RC tumoroids
display varying sensitivity to ionizing radiation, which
corresponds to clinical radiotherapy responses | (58) |
| Locally advanced
RC | 8 | PDOs predict LARC
patient responses in the clinic and may represent a companion
diagnostic tool in RC treatment | (59) |
| Colorectal
cancer | Combining a low
dose of TQ (3 µM) with IR (2 Gy) | TQ sensitizes
cancer cells and stem/progenitor cells to radiation mainly through
the inhibition of cell survival, DNA repair and stemness in
addition to regulating major pathways implicated in this
process | (60) |
| Duodenum, ileum,
jejunum and colon | 0.003
(Gy/h)-30(Gy/h) | Cell competition,
through apical junctions and extracellular ligands, might
contribute to the dose-rate effect on Lgr5+ cell
replenishment | (61) |
| NPC | 0.2–30 | Study the
radioresistance of the hypoxic sub-volumes in recurrent
radioresistant NPC | (64) |
| Head and neck
squamous cell carcinoma | 0-10 | Recapitulating
genetic, histological and functional features for future therapy
screening | (65) |
| Esophageal
cancer | 5 | Reflecting clinical
response following neoadjuvant radiotherapy | (66) |
| Breast cancer | 20 | Co-culture
macrophages with irradiated mammary glands, as a model for studying
tumor-stromal interactions, infiltration of immune cells and
macrophage polarization within an irradiated microenvironment | (67) |
| Glioblastoma | 3 | Stem and non-stem
glioblastoma cell populations can be simultaneously cultured to
explore new facets of microenvironmental influences and cancer stem
cell biology, depending on receiving critical maintenance cues from
their microenvironment | (68) |
Radiotherapy with immunotherapy of
GITOs
Radiotherapy elicits a range of immune-related
responses, both locally and systemically. A key objective of our
research has been to induce antitumor immune effects that improve
the ability of the immune system to recognize and combat malignant
cells, ultimately aiming to control and eradicate tumors. The
immune system primarily achieves this by promoting the infiltration
of effector T cells, mesenchymal stem cells (MSCs) and macrophages
into the tumor microenvironment (TME). This process involves
upregulating transforming growth factor-β (TGF-β) to activate the
signal transducer and activator of transcription-3 (STAT3) pathway,
thereby influencing the post-radiotherapy immune-related biological
behaviors (62–63,69).
Radiotherapy generates free radicals and oxidative
stress through high-energy generation, causing cellular damage. It
has been observed that high doses of radiation (>10 Gy), can
lead to adverse effects, including vascular endothelial damage,
reduced blood supply and reduced oxygen-carrying capacity (70). This, in turn, reduces the
recruitment of effector T cells, reducing the immunogenicity of the
tumor and its abscopal effect (63). Consequently, single high-dose
radiotherapy does not seem conducive to triggering a sustained
systemic immune response.
MSCs, integral to the tissue microenvironment,
exhibit multifunctionality and immunoregulatory capabilities. The
cells can stimulate vascular regeneration, which is highly relevant
for repairing radiation-induced lesions. Cultivating MSCs alongside
digestive tract tumor organoids results in a more biologically
pertinent model (71). Radiotherapy
directly eradicates tumor cells, and immunotherapy aims to
strengthen the patient's antitumor abilities; therefore, obviously,
the combination of radiotherapy and MSCs can synergistically
leverage their respective strengths and enhance the therapeutic
outcome (72).
Gong et al (73) observed that MSC transplantation
promotes the Wnt/Notch signaling pathways, encouraging the
proliferation of ISCs and promoting the regeneration of the
epithelium in mice with irradiation-induced intestinal injuries
(74). Similarly, Moussa et
al (75) found that this model
overexpresses genes such as gremlin 1 DAN family BMP antagonist and
twisted gastrulation BMP signaling modulator 1, which by inhibiting
the BMP signal, co-operatively promote the proliferation of
Lgr5+ stem cells, contributing to lesion repair after
irradiation. This is particularly significant in mice subjected to
extracorporeal irradiation after allogeneic bone marrow
transplantation.
Moreover, the IL-22 dimer/Fc fusion protein F-652
has been shown to significantly protect intestinal Lgr5+
cells from radiation-induced damage in vivo. This treatment
also helps mitigate intestinal pathological damage and reduces
mortality associated with graft-vs.-host disease (76).
Other systems of tumor organoids in
radiotherapy
GITOs are widely employed in radiotherapy, for both
clinical treatment and fundamental research. Additionally, the
development of tumor organoids for other areas, such as the nervous
system, head and neck, and reproductive system, has been
increasing. However, developing cerebral cortex organoids involves
addressing complex challenges related to nutrient supply and neural
regulatory functions. Furthermore, the graded regulation and
feedback mechanisms of the reproductive system still require
further refinement. Research on head and neck tumor organoids is
currently quite comprehensive. Tumors originating in the head and
neck region are characterized by high invasiveness, a strong
potential for metastasis and a high recurrence rate (1). Historically, they were often treated
as a single type of solid tumor, which led to treatment failures.
Extensive two-dimensional cell experiments have deepened our
understanding of the heterogeneity of head and neck cancers
(62,63). Presently, the primary focus of
organoid models for head and neck cancers is on oropharyngeal and
nasopharyngeal cancers. The former exhibits notable differences in
terms of the outcomes of radiotherapy, while the culture systems of
the latter show variations in composition, concentration and
product batch numbers (64).
Ionizing radiation is widely employed to slow the
growth of glioma; however, its clinical efficacy is limited due to
brain edema and radiation-induced brain conditions, including loss
of consciousness (77). Liu et
al (12) identified long
non-coding RNAs (lncRNAs) as potential therapeutic targets through
glioma organoid screening. The knockout of these lncRNAs increased
tumor cell sensitivity to radiotherapy. Additionally, research
indicates that treatment regiments combining vemurafenib (Zelboraf)
and cobimetinib (Mekinist) can significantly improve the low
remission rates of postoperative radiotherapy and temozolomide
chemotherapy for patients, expediting clinical decision-making
(78–81). Driehuis et al (65) found that pharyngeal cancer organoids
exhibit particular sensitivity to radiotherapy in clinical
practice. There are reports of patients remaining recurrence-free
for up to 5 months after receiving 48 Gy of radiation treatment,
aligning with the results of complete local remission in organoids
following radiotherapy (65).
Patients with oropharyngeal cancer often experience reduced saliva
production and xerostomia, post-radiotherapy. Peng et al
(82) discovered that reducing the
secretion of senescence-associated secretory phenotype factors in
salivary gland organoids can delay the aging of salivary gland
stem/progenitor cells, providing a targeted solution for
radiotherapy-induced salivary secretion dysfunction. Seol et
al (83) analyzed the effects
of radiation doses (0–12 Gy) on cervical cancer organoids and found
that the organoids could serve as a suitable in vitro
platform for predicting radiation sensitivity. While most of the
aforementioned solid tumor lesions are often localized, lymph nodes
are distributed throughout the body and are frequently affected
during tumor metastasis. After breast cancer surgery, adjuvant
radiotherapy and lymph node dissection are often necessary, but
these procedures can cause substantial damage to lymphatic vessels,
resulting in complications such as blocked lymphatic drainage and
edema in the affected areas. Lenti et al (84) transplanted lymphatic organoids into
areas of the mouse where lymph nodes had been removed. These
transplants fully integrated into the endogenous lymphatic system,
restoring lymphatic drainage and alleviating edema problems.
Therefore, injecting lymphatic organoids into the lesion sites may
help restore lymphatic drainage.
In clinical practice, radiotherapy is the primary
treatment modality for nasopharyngeal cancer. However,
complications during treatment can further reduce the 5-year
survival rate of patients. Therefore, it is crucial to
comprehensively address the issue of radiotherapy dosage,
considering the patient's disease stage and physical condition.
Insufficient dosage may lead to suboptimal treatment effects, while
excessive dosage could exacerbate damage to the surrounding tissues
(85). Nasopharyngeal cancer
organoids exhibit an impressive 88% positive predictive value and a
perfect 100% negative predictive value, providing crucial insights
for accurately modeling the association between nasopharyngeal
cancer tissue and radiotherapy dosage. This promotes the
translation of basic research into clinical practice (86).
Nasopharyngeal cancer is categorized as a
lymphoepithelial tumor, with its growth and proliferation
intricately involving immune cells and the TME (9). Microfluidic technology enables the
simulation of this growth environment, gradually diffusing oxygen,
growth factors and nutrients, while modeling cell heterogeneity
resulting from changes in microenvironment concentration. Compared
with conventional 2D culture methods, microfluidic technology more
faithfully replicates the actual proliferation rate of in
vivo tumor cells (45). Current
research predominantly centers on oxygen supply. Hypoxia results in
a decrease in reactive oxygen species in irradiated cells, leading
to the induction of hypoxia-related transcription factors and
subsequent radiotherapy resistance (87). In clinical practice, a fractionated
irradiation approach can progressively oxygenate hypoxic tumor
cells, resulting in a gradual therapeutic effect. Moreover,
clinical cases of local recurrences in pleomorphic glioblastomas
and breast cancer, in addition to nasopharyngeal cancer, are often
linked to unresected hypoxic cancer cells. Rycaj and Tang (88) showed that increasing the radiation
dose to hypoxic organoids by 1.4 times could effectively eradicate
recurrent tumor lesions. Consequently, utilizing nasopharyngeal
cancer organoid models can be crucial for optimizing radiation
dosage and adjusting radiotherapy plans. Hill et al
(89) indicated that the
platelet-derived growth factor receptor-β/vascular endothelial
growth factor-2/STAT2 signaling pathway induces radiotherapy
resistance in ovarian cancer organoids by influencing the
oxygen-dependent glycolysis pathway. Additionally, in hypoxic
environments and culture systems, various cytokines, such as TGF-β,
the FGF family, EGF and hepatocyte growth factor, can induce or
increase the receptiveness of nasopharyngeal cancer organoids to
epithelial-mesenchymal transition induction signals. This aids in
studying the impact of radiotherapy on tumor metastasis (90).
Discussion
Tumor organoids have played a crucial role in
various aspects of radiotherapy-related research, including organ
development, injury repair, regeneration studies and
microenvironment homeostasis. The ability of organoids to maintain
stable genetic traits throughout long-term passages offers
significant advantages in constructing patient resource banks,
gaining substantial attention in cancer research and clinical
translation. However, the considerable size differences between
tumor organoids and in vivo tissue organs present
challenges. For example, the extensive digestive tract, which can
reach up to 16 to 23 feet in length, dwarfs the considerably
smaller tumor organoids (49).
Additionally, complex, multi-tiered organs, such as the lungs and
kidneys, pose challenges for effectively integrating organoids into
in vivo tumor tissues due to their limited maturity and
integrity. Additionally, the absence of neuroregulatory factors
further complicates the modeling of diseases with high neural
regulation, such as ulcerative colitis. These challenges highlight
several issues that need to be addressed (91).
Several factors, including specimen source, culture
components, immune cells, stromal cells and the microenvironment,
are crucial in the construction of tumor organoids. Compared with
the use of iPSCs and surgically resected tissues, core biopsies
contain a lower percentage of tumor cells, resulting in a
cultivation success rate of <20% (9). Furthermore, uncleared normal tissue
can compete with tumor growth, interfering with the intended
formation of organoids. Certain tumor tissues, such as esophageal
cancer tissues, are particularly susceptible to bacterial and/or
fungal contamination due to local narrowing and food obstruction
resulting from pathological conditions when attempting to culture
organoids from primary tissues (92). For rare malignancies such as
chordomas, although organoid biobanks have been successfully
established, research related to radiotherapy remains unexplored
(93).
Patient tissue specimens serve as sources of tumor
stem cells, with the ECM in the culture medium containing varying
types and concentrations of factors that influence organoid quality
and area parameters (94). While
Matrigel derived from Engelbreth-Holm-Swarm mouse sarcoma cells is
commonly used in constructing organoid culture systems, its
tumorigenic potential renders it unsuitable for clinical-grade
cultures. Consequently, studies such as that by Jee et al
(63) have explored the use of
fibronectin from connective tissues, showing their promise for
clinical regenerative therapy and the treatment of complex
conditions such as fistulas and refractory ulcers (95). Furthermore, cellular experiments
indicate that the ECM significantly affects tumor growth, invasion
and metastasis. Tumor cells that survive radiation exhibit
increased invasiveness, migrating to distant sites independently of
enzymatic conditions. Thus, tumor organoids provide a valuable
platform for studying tumor invasion and culture matrices,
effectively addressing a significant proportion (up to 90%) of
clinical concerns (96). In
addition to the ECM, various additives are essential for
constructing organoids, for freshly obtained specimens, the
ρ-kinase inhibitor, Y-27632, is necessary to reduce anoikis-related
apoptosis (22). Within tumor
organoid cultures, selective removal of Wnt3a is crucial to prevent
overgrowth. Therefore, the adjustment of cytokines within the
culture system is essential (30).
Although stem cells, the ECM and additive components form the
foundation of the organoid culture system, a significant gap exists
between this system and the growth environment of solid tumors.
Introducing immune cells through co-culturing is a preliminary step
in enriching the system. For instance, co-culturing
radiation-exposed breast cancer organoids with macrophages can
induce chemokine ligand 2 chemotaxis, increasing invasive
phenotypes and promoting cancer cell migration (67).
Upregulating cytokines in the culture system can
also trigger TGF-β signaling, leading to increased secretion of
IL-10 by macrophages and inhibiting immune responses. Co-culturing
tumor cells post-radiation with monocytes can also downregulate
cytochrome P450 family 1 subfamily A polypeptide 1, impacting tumor
cell transcription (97). These
findings underscore the significance of immune cell-organoid
interactions in more accurately replicating actual tumor
responses.
When utilizing tissue-cultured organoids, the
persistence of uncleared fibroblasts can lead to their activation
and dominance. These fibroblasts may then consume the cytokines
needed by the organoids and encase the entire structure, thereby
inhibiting organoid growth (98).
Additionally, immune cells act as essential intermediaries
connecting stromal cells and the microenvironment. Stromal cells
influence the migration, adhesion and activation of immune cells in
the context of radiotherapy. Microfluidic technology can integrate
all three components within a single system where fluid flow
velocity in the microenvironment can impact the immune
functionality of T cells. This offers a platform for deeper
exploration of radiotherapy and immunotherapy in tumor organoid
models (99).
Hypoxic damage to the vascular endothelium and poor
blood circulation also contribute to increase the resistance to
radiotherapy. Reduced oxygen supplementation can alter the TME,
thus affecting organoid responses to radiotherapy and
immunotherapy. For instance, head and neck tumors, which have a
rich blood supply, demonstrate significantly higher sensitivity to
radiotherapy compared with tumors in the extremities. Furthermore,
with prolonged culture periods, aging cells may appear within the
interior of the organoid and its proliferative boundaries,
indicating that oxygen gradients in the microenvironment and cell
proliferation can contribute to cellular aging. Despite the
millimeter-level precision achieved by stereotactic body
radiotherapy, which minimizes radiation doses to surrounding
structures, including bone marrow, cancer-related fatigue (CRF) is
inevitable. CRF is a subjective sense of fatigue commonly
experienced by radiotherapy patients, which cannot be replicated
using organoid models (100).
Challenges and future prospects
Radiotherapy is a cornerstone in the arsenal of
treatments against malignant tumors and is crucial for ~70% of
patients with cancer at various stages of their therapeutic journey
(101). Radiotherapy is
particularly vital in advanced stages or in cases of recurrent
malignancies, where it serves as a key strategy not only in
mitigating clinical symptoms, but in significantly prolonging
patient survival and enhancing quality of life. The field of
radiotherapy has seen considerable evolution in recent years,
marked by rapid technological advancements and continuous
refinement of its underlying physical principles. This evolution
has propelled tumor radiotherapy into an era of heightened
precision and sophisticated intelligence. The innovative
progression of radiotherapy techniques, along with
interdisciplinary synergy, heralds a transformative era in cancer
treatment.
The landscape of radiation oncology has undergone
significant transformation, evolving from conventional
two-dimensional radiotherapy, which focused on precise target
delineation and meticulous radiation delivery, to cutting-edge
integration of genomics- and radiomics-guided precision
radiotherapy. Today, we are witnessing a groundbreaking shift
towards integrating artificial intelligence and systems biology,
marking a new era of intelligent radiotherapy. This rapid
advancement is further underscored by the introduction of novel
equipment and technologies in the field. A prime example is the
incorporation of innovative flash technology (102) in medical linear accelerators. This
advanced technology enables the administration of ultra-high
radiation doses with unprecedented precision in beam flux control,
showing profoundly beneficial biological effects in clinical
settings. Such advancements not only redefine the potential of
radiotherapy but also emphasize its evolution towards a more
patient-centric approach.
Moreover, the integration of radiotherapy with
surgical procedures, chemotherapy, immunotherapy and targeted
antibody-drug conjugates (103)
represents a synergistic approach that significantly enhances
treatment effectiveness. The emergence of tumor organoids marks a
pivotal advancement in clinical applications. There have been
previous reviews of organoids. For example, Lv et al
(104) systematically reviewed the
construction of the TME, cell components and methods for creating
tumor organoids. Radiotherapy, especially in CRC, rectal cancer and
cervical cancer, just has an applicational role. The review by
Nagle and Coppes (9) aimed to
explore radiotherapy with organoids, and the shortcomings of
existing organoid models in radiotherapy and immunity in the field
of radiation biology research. The present review placed an
emphasis on transformation and the clinical applications in
radiotherapy of constructed diverse tumor organoids (not only for
CRC, but all included diseases), especially in immunotherapy after
irradiation.
By facilitating the acquisition of preclinical data
on radiotherapy (and/or other therapeutic modalities) in an ex
vivo setting, these organoids provide a forward-looking
framework for precise treatment strategy selection. This innovative
approach fosters a customized treatment landscape for diverse
cancer patients, optimizing therapeutic outcomes; it maximizes
patient benefits, strategically reduces healthcare costs, and
mitigates the incidence and severity of side effects, thereby
enhancing the overall treatment experience.
However, research exploring the intersection of
tumor organoids and radiotherapy remains relatively limited. Given
the inherent genomic instability of cancer cells, mutations may
occur during their treatment with radiotherapy. Therefore, future
research endeavors could focus on investigating the genomic
evolution within cancer organoid models before and after
radiotherapy.
In the coming years, investigations associated with
tumor organoids may primarily focus on elucidating the mechanisms
underlying cancer resistance in radiotherapy, refining screening
and staging methodologies, identifying prognostic and
treatment-associated biomarkers, optimizing therapeutic
interventions for distinct tumor subtypes and spearheading novel
therapeutic modalities.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JY, KW and YT conceptualized the study. KW was
responsible for investigation of the literature. YT provided
resources. DZ supervised the study and assisted in revising the
manuscript. JY wrote the original draft. JY and KW reviewed and
edited the manuscript. All authors have read and approved the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kreier F: Cancer will cost the world $25
trillion over next 30 years. Nature. Mar 7–2023.(Epub ahead of
print). doi: 10.1038/d41586-023-00634-9. View Article : Google Scholar
|
|
3
|
Hanahan D: Rethinking the war on cancer.
Lancet. 383:558–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JH and Wee CW: Treatment of Adult
Gliomas: A current update. Brain Neurorehabil. 15:e242022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SH, Hu W, Matulay JT, Silva MV,
Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, et
al: Tumor evolution and drug response in patient-derived organoid
models of bladder cancer. Cell. 173:515–528.e17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boustani J, Grapin M, Laurent PA, Apetoh L
and Mirjolet C: The 6th R of radiobiology: Reactivation of
anti-tumor immune response. Cancers (Basel). 11:8602019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barazzuol L, Coppes RP and van Luijk P:
Prevention and treatment of radiotherapy-induced side effects. Mol
Oncol. 14:1538–1554. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strong MJ, Baddoo M, Nanbo A, Xu M,
Puetter A and Lin Z: Comprehensive high-throughput RNA sequencing
analysis reveals contamination of multiple nasopharyngeal carcinoma
cell lines with HeLa cell genomes. J Virol. 88:10696–10704. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagle PW and Coppes RP: Current and future
perspectives of the use of organoids in radiobiology. Cells.
9:26492020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stewart-Ornstein J, Iwamoto Y, Miller MA,
Prytyskach MA, Ferretti S, Holzer P, Kallen J, Furet P, Jambhekar
A, Forrester WC, et al: p53 dynamics vary between tissues and are
linked with radiation sensitivity. Nat Commun. 12:8982021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hammond EM and Muschel RJ: Radiation and
ATM inhibition: The heart of the matter. J Clin Invest.
124:3289–3291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu SJ, Malatesta M, Lien BV, Saha P,
Thombare SS, Hong SJ, Pedraza L, Koontz M, Seo K, Horlbeck MA, et
al: CRISPRi-based radiation modifier screen identifies long
non-coding RNA therapeutic targets in glioma. Genome Biol.
21:832020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujii M, Shimokawa M, Date S, Takano A,
Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K, et
al: A colorectal tumor organoid library demonstrates progressive
loss of niche factor requirements during tumorigenesis. Cell Stem
Cell. 18:827–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boj SF, Hwang CI, Baker LA, Chio II, Engle
DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al:
Organoid models of human and mouse ductal pancreatic cancer. Cell.
160:324–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang Y, Zhou Y, Li Y, Han Y, Xu J, Niu W,
Li Z, Liu S, Feng H, Huang W, et al: A human forebrain organoid
model of fragile X syndrome exhibits altered neurogenesis and
highlights new treatment strategies. Nat Neurosci. 24:1377–1391.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao D, Vela I, Sboner A, Iaquinta PJ,
Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora
VK, et al: Organoid cultures derived from patients with advanced
prostate cancer. Cell. 159:176–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Boo J and Hendriksen C: Reduction
strategies in animal research: A review of scientific approaches at
the intra-experimental, supra-experimental and extra-experimental
levels. Altern Lab Anim. 33:369–377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lancaster MA and Knoblich JA:
Organogenesis in a dish: Modeling development and disease using
organoid technologies. Science. 345:12471252014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clevers H: Modeling development and
disease with organoids. Cell. 165:1586–1597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Method of the Year 2017, . Organoids. Nat
Methods. Jan 3–2018.(Epub ahead of print).
|
|
21
|
Jacob F, Salinas RD, Zhang DY, Nguyen PTT,
Schnoll JG, Wong SZH, Thokala R, Sheikh S, Saxena D, Prokop S, et
al: A patient-derived glioblastoma organoid model and biobank
recapitulates inter- and intra-tumoral heterogeneity. Cell.
180:188–204.e22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sachs N, de Ligt J, Kopper O, Gogola E,
Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H,
et al: A living biobank of breast cancer organoids captures disease
heterogeneity. Cell. 172:373–386.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grassi L, Alfonsi R, Francescangeli F,
Signore M, De Angelis ML, Addario A, Costantini M, Flex E, Ciolfi
A, Pizzi S, et al: Organoids as a new model for improving
regenerative medicine and cancer personalized therapy in renal
diseases. Cell Death Dis. 10:2012019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vlachogiannis G, Hedayat S, Vatsiou A,
Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford
I, Burke R, et al: Patient-derived organoids model treatment
response of metastatic gastrointestinal cancers. Science.
359:920–926. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hofbauer P, Jahnel SM, Papai N,
Giesshammer M, Deyett A, Schmidt C, Penc M, Tavernini K, Grdseloff
N, Meledeth C, et al: Cardioids reveal self-organizing principles
of human cardiogenesis. Cell. 184:3299–3317.e22. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheridan MA, Zhao X, Fernando RC, Gardner
L, Perez-Garcia V, Li Q, Marsh SGE, Hamilton R, Moffett A and Turco
MY: Characterization of primary models of human trophoblast.
Development. 148:dev1997492021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khan AO, Rodriguez-Romera A, Reyat JS,
Olijnik AA, Colombo M, Wang G, Wen WX, Sousos N, Murphy LC,
Grygielska B, et al: Human bone marrow organoids for disease
modeling, discovery, and validation of therapeutic targets in
hematologic malignancies. Cancer Discov. 13:364–385. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bouffi C, Wikenheiser-Brokamp KA,
Chaturvedi P, Sundaram N, Goddard GR, Wunderlich M, Brown NE, Staab
JF, Latanich R, Zachos NC, et al: In vivo development of immune
tissue in human intestinal organoids transplanted into humanized
mice. Nat Biotechnol. 41:824–831. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Broutier L, Mastrogiovanni G, Verstegen
MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R,
Sidorova O, Gaspersz MP, et al: Human primary liver cancer-derived
organoid cultures for disease modeling and drug screening. Nat Med.
23:1424–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sato T, Stange DE, Ferrante M, Vries RG,
Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J,
Siersema PD and Clevers H: Long-term expansion of epithelial
organoids from human colon, adenoma, adenocarcinoma, and Barrett's
epithelium. Gastroenterology. 141:1762–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren W, Lewandowski BC, Watson J, Aihara E,
Iwatsuki K, Bachmanov AA, Margolskee RF and Jiang P: Single Lgr5-
or Lgr6-expressing taste stem/progenitor cells generate taste bud
cells ex vivo. Proc Natl Acad Sci USA. 111:16401–16406. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kessler M, Hoffmann K, Brinkmann V, Thieck
O, Jackisch S, Toelle B, Berger H, Mollenkopf HJ, Mangler M,
Sehouli J, et al: The Notch and Wnt pathways regulate stemness and
differentiation in human fallopian tube organoids. Nat Commun.
6:89892015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turco MY, Gardner L, Hughes J,
Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE,
Brosens JJ, Critchley HO, et al: Long-term, hormone-responsive
organoid cultures of human endometrium in a chemically defined
medium. Nat Cell Biol. 19:568–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Francies HE, Secrier M, Perner J,
Miremadi A, Galeano-Dalmau N, Barendt WJ, Letchford L, Leyden GM,
Goffin EK, et al: Organoid cultures recapitulate esophageal
adenocarcinoma heterogeneity providing a model for clonality
studies and precision therapeutics. Nat Commun. 9:29832018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blau HM and Daley GQ: Stem Cells in the
Treatment of Disease. N Engl J Med. 380:1748–1760. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SY, Kim SM, Lim S, Lee JY, Choi SJ,
Yang SD, Yun MR, Kim CG, Gu SR, Park C, et al: Modeling clinical
responses to targeted therapies by patient-derived organoids of
advanced lung adenocarcinoma. Clin Cancer Res. 27:4397–4409. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saito Y, Muramatsu T, Kanai Y, Ojima H,
Sukeda A, Hiraoka N, Arai E, Sugiyama Y, Matsuzaki J, Uchida R, et
al: Establishment of patient-derived organoids and drug screening
for biliary tract carcinoma. Cell Rep. 27:1265–1276.e4. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang XW, Xia TL, Tang HC, Liu X, Han R,
Zou X, Zhao YT, Chen MY and Li G: Establishment of a
patient-derived organoid model and living biobank for
nasopharyngeal carcinoma. Ann Transl Med. 10:5262022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Xu H, Zhang L, Song L, Feng D, Peng
X, Wu M, Zou Y, Wang B, Zhan L, et al: Malignant ascites-derived
organoid (MADO) cultures for gastric cancer in vitro modelling and
drug screening. J Cancer Res Clin Oncol. 145:2637–2647. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuki K, Cheng N, Nakano M and Kuo CJ:
Organoid models of tumor immunology. Trends Immunol. 41:652–664.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lo YH, Karlsson K and Kuo CJ: Applications
of organoids for cancer biology and precision medicine. Nat Cancer.
1:761–773. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Joo H, Min S and Cho SW: Advanced lung
organoids for respiratory system and pulmonary disease modeling. J
Tissue Eng. 15:204173142412325022024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yuan J, Li X and Yu S: Cancer organoid
co-culture model system: Novel approach to guide precision
medicine. Front Immunol. 13:10613882023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Licata JP, Schwab KH, Har-El YE,
Gerstenhaber JA and Lelkes PI: Bioreactor technologies for enhanced
organoid culture. Int J Mol Sci. 24:114272023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SE, Georgescu A and Huh D:
Organoids-on-a-chip. Science. 364:960–965. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Weeber F, van de Wetering M, Hoogstraat M,
Dijkstra KK, Krijgsman O, Kuilman T, Gadellaa-van Hooijdonk CG, van
der Velden DL, Peeper DS, Cuppen EP, et al: Preserved genetic
diversity in organoids cultured from biopsies of human colorectal
cancer metastases. Proc Natl Acad Sci USA. 112:13308–13311. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
O'Rourke KP, Loizou E, Livshits G,
Schatoff EM, Baslan T, Manchado E, Simon J, Romesser PB, Leach B,
Han T, et al: Transplantation of engineered organoids enables rapid
generation of metastatic mouse models of colorectal cancer. Nat
Biotechnol. 35:577–582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Martin ML, Adileh M, Hsu KS, Hua G, Lee
SG, Li C, Fuller JD, Rotolo JA, Bodo S, Klingler S, et al:
Organoids reveal that inherent radiosensitivity of small and large
intestinal stem cells determines organ in review sensitivity.
Cancer Res. 80:1219–1227. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sato T, Vries RG, Snippert HJ, van de
Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters
PJ and Clevers H: Single Lgr5 stem cells build crypt-villus
structures in vitro without a mesenchymal niche. Nature.
459:262–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sato T and Clevers H: Growing
self-organizing mini-guts from a single intestinal stem cell:
Mechanism and applications. Science. 340:1190–1194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujimichi Y, Otsuka K, Tomita M and
Iwasaki T: An efficient intestinal organoid system of direct
sorting to evaluate stem cell competition in vitro. Sci Rep.
9:202972019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lindemans CA, Calafiore M, Mertelsmann AM,
O'Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM,
Lawrence G, et al: Interleukin-22 promotes
intestinal-stem-cell-mediated epithelial regeneration. Nature.
528:560–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qu M, Xiong L, Lyu Y, Zhang X, Shen J,
Guan J, Chai P, Lin Z, Nie B, Li C, et al: Establishment of
intestinal organoid cultures modeling injury-associated epithelial
regeneration. Cell Res. 31:259–271. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gregorieff A, Liu Y, Inanlou MR, Khomchuk
Y and Wrana JL: Yap-dependent reprogramming of Lgr5(+) stem cells
drives intestinal regeneration and cancer. Nature. 526:715–718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Farin HF, Van Es JH and Clevers H:
Redundant sources of wnt regulate intestinal stem cells and promote
formation of paneth cells. Gastroenterology. 143:1518–1529.e7.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bhanja P, Saha S, Kabarriti R, Liu L,
Roy-Chowdhury N, Roy-Chowdhury J, Sellers RS, Alfieri AA and Guha
C: Protective role of R-spondin1, an intestinal stem cell growth
factor, against radiation-induced gastrointestinal syndrome in
mice. PLoS One. 4:e80142009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Otsuka K, Hamada N, Magae J, Matsumoto H,
Hoshi Y and Iwasaki T: Ionizing radiation leads to the replacement
and de novo production of colonic Lgr5(+) stem cells. Radiat Res.
179:637–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ganesh K, Wu C, O'Rourke KP, Szeglin BC,
Zheng Y, Sauvé CG, Adileh M, Wasserman I, Marco MR, Kim AS, et al:
A rectal cancer organoid platform to study individual responses to
chemoradiation. Nat Med. 25:1607–1614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L,
Xia F, Fu G, Deng Y, Pan M, et al: Patient-Derived organoids
predict chemoradiation responses of locally advanced rectal cancer.
Cell Stem Cell. 26:17–26.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Al Bitar S, Ballout F, Monzer A, Kanso M,
Saheb N, Mukherji D, Faraj W, Tawil A, Doughan S, Hussein M, et al:
Thymoquinone radiosensitizes human colorectal cancer cells in 2D
and 3D culture models. Cancers (Basel). 14:13632022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Otsuka K, Suzuki K, Fujimichi Y, Tomita M
and Iwasaki T: Cellular responses and gene expression profiles of
colonic Lgr5+ stem cells after low-dose/low-dose-rate radiation
exposure. J Radiat Res. 59 (Suppl 2):ii18–ii22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Schwartz DM, Pehlivaner Kara MO, Goldstein
AM, Ott HC and Ekenseair AK: Spray delivery of intestinal organoids
to reconstitute epithelium on decellularized native extracellular
matrix. Tissue Eng Part C Methods. 23:565–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jee J, Park JH, Im JH, Kim MS, Park E, Lim
T, Choi WH, Kim JH, Kim WR, Ko JS, et al: Functional recovery by
colon organoid transplantation in a mouse model of radiation
proctitis. Biomaterials. 275:1209252021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lucky SS, Law M, Lui MH, Mong J, Shi J, Yu
S, Yoon DK, Djeng SK, Wang J, Lim CM and Tan MH: Patient-derived
nasopharyngeal cancer organoids for disease modeling and radiation
dose optimization. Front Oncol. 11:6222442021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Driehuis E, Kolders S, Spelier S,
Lõhmussaar K, Willems SM, Devriese LA, de Bree R, de Ruiter EJ,
Korving J, Begthel H, et al: Oral mucosal organoids as a potential
platform for personalized cancer therapy. Cancer Discov. 9:852–871.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Karakasheva TA, Kijima T, Shimonosono M,
Maekawa H, Sahu V, Gabre JT, Cruz-Acuña R, Giroux V, Sangwan V,
Whelan KA, et al: Generation and characterization of
patient-derived head and neck, oral, and esophageal cancer
organoids. Curr Protoc Stem Cell Biol. 53:e1092020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hacker BC, Gomez JD, Batista CAS and Rafat
M: Growth and characterization of irradiated organoids from mammary
glands. J Vis Exp. May 3–2019.(Epub ahead of print). doi:
10.3791/59293. View
Article : Google Scholar
|
|
68
|
Hubert CG, Rivera M, Spangler LC, Wu Q,
Mack SC, Prager BC, Couce M, McLendon RE, Sloan AE and Rich JN: A
three-dimensional organoid culture system derived from human
glioblastomas recapitulates the hypoxic gradients and cancer stem
cell heterogeneity of tumors found in vivo. Cancer Res.
76:2465–2477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lumniczky K, Candéias SM, Gaipl US and
Frey B: Editorial: Radiation and the immune system: Current
knowledge and future perspectives. Front Immunol. 8:19332018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Vanpouille-Box C, Alard A, Aryankalayil
MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN,
Formenti SC and Demaria S: DNA exonuclease Trex1 regulates
radiotherapy-induced tumour immunogenicity. Nat Commun.
8:156182017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Park HJ, Griffin RJ, Hui S, Levitt SH and
Song CW: Radiation-induced vascular damage in tumors: Implications
of vascular damage in ablative hypofractionated radiotherapy (SBRT
and SRS). Radiat Res. 77:311–327. 2012. View Article : Google Scholar
|
|
72
|
Zhang Z, Liu X, Chen D and Yu J:
Radiotherapy combined with immunotherapy: The dawn of cancer
treatment. Signal Transduct Target Ther. 7:2582022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gong W, Guo M, Han Z, Wang Y, Yang P, Xu
C, Wang Q, Du L, Li Q, Zhao H, et al: Mesenchymal stem cells
stimulate intestinal stem cells to repair radiation-induced
intestinal injury. Cell Death Dis. 7:e23872016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chiang CS, Fu SY, Wang SC, Yu CF, Chen FH,
Lin CM and Hong JH: Irradiation promotes an m2 macrophage phenotype
in tumor hypoxia. Front Oncol. 2:892012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Moussa L, Lapière A, Squiban C, Demarquay
C, Milliat F and Mathieu N: BMP antagonists secreted by mesenchymal
stromal cells improve colonic organoid formation: Application for
the treatment of radiation-induced injury. Cell Transplant.
29:9636897209296832020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gao B and Xiang X: Interleukin-22 from
bench to bedside: A promising drug for epithelial repair. Cell Mol
Immunol. 16:666–667. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Aiyappa-Maudsley R, Chalmers AJ and
Parsons JL: Factors affecting the radiation response in
glioblastoma. Neurooncol Adv. 4:vdac1562022.PubMed/NCBI
|
|
78
|
Kim YH, Han SH, Kim H, Lee SJ, Joo HW, Kim
MJ, Shim S, Kim K, Lee J, Jang WS, et al: Evaluation of the
radiation response and regenerative effects of mesenchymal stem
cell-conditioned medium in an intestinal organoid system.
Biotechnol Bioeng. 117:3639–3650. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Perumal V, Corica T, Dharmarajan AM, Sun
Z, Dhaliwal SS, Dass CR and Dass J: Circulating tumour cells (CTC),
head and neck cancer and radiotherapy; Future. Perspectives.
Cancers (Basel). 11:3672019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen CC, Li HW, Wang YL, Lee CC, Shen YC,
Hsieh CY, Lin HL, Chen XX, Cho DY, Hsieh CL, et al: Patient-derived
tumor organoids as a platform of precision treatment for malignant
brain tumors. Sci Rep. 12:163992022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Peng X, Wu Y, Brouwer U, van Vliet T, Wang
B, Demaria M, Barazzuol L and Coppes RP: Cellular senescence
contributes to radiation-induced hyposalivation by affecting the
stem/progenitor cell niche. Cell Death Dis. 11:8542020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Seol HS, Oh JH, Choi E, Kim S, Kim H and
Nam EJ: Preclinical investigation of patient-derived cervical
cancer organoids for precision medicine. J Gynecol Oncol.
34:e352023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lenti E, Bianchessi S, Proulx ST, Palano
MT, Genovese L, Raccosta L, Spinelli A, Drago D, Andolfo A, Alfano
M, et al: Therapeutic regeneration of lymphatic and immune cell
functions upon lympho-organoid transplantation. Stem Cell Reports.
12:1260–1268. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ding RB, Chen P, Rajendran BK, Lyu X, Wang
H, Bao J, Zeng J, Hao W, Sun H, Wong AH, et al: Molecular landscape
and subtype-specific therapeutic response of nasopharyngeal
carcinoma revealed by integrative pharmacogenomics. Nat Commun.
12:30462021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li W, Zhang XJ, Feng XY, Chen D, Luo JQ
and Zhu BJ: Three-dimensional culture and characterization of
patient-derived nasopharyngeal carcinoma organoids. Res Sq. Oct
13–2020.(Epub ahead of print). doi: 10.21203/rs.3.rs-90861/v1.
|
|
87
|
Yip YL, Lin WT, Deng W, Tsang CM and Tsao
SW: Establishment of nasopharyngeal carcinoma cell lines,
patient-derived xenografts, and immortalized nasopharyngeal
epithelial cell lines for nasopharyngeal carcinoma and epstein-barr
virus infection studies. Nasopharyngeal Carcinoma. Lee AWM, Lung ML
and Ng WT: Elsevier; Amsterdam: 2019, View Article : Google Scholar
|
|
88
|
Rycaj K and Tang DG: Cancer stem cells and
radioresistance. Int J Radiat Biol. 90:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hill SJ, Decker B, Roberts EA, Horowitz
NS, Muto MG, Worley MJ Jr, Feltmate CM, Nucci MR, Swisher EM,
Nguyen H, et al: Prediction of DNA repair inhibitor response in
short-term patient-derived ovarian cancer organoids. Cancer Discov.
8:1404–1421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lai X, Li Q, Wu F, Lin J, Chen J, Zheng H
and Guo L: Epithelial-mesenchymal transition and metabolic
switching in cancer: Lessons from somatic cell reprogramming. Front
Cell Dev Biol. 8:7602020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Populin L, Stebbing MJ and Furness JB:
Neuronal regulation of the gut immune system and neuromodulation
for treating inflammatory bowel disease. FASEB Bioadv. 3:953–966.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Takahashi T: Organoids for drug discovery
and personalized medicine. Annu Rev Pharmacol Toxicol. 59:447–462.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Foo MA, You M, Chan SL, Sethi G, Bonney
GK, Yong WP, Chow EK, Fong ELS, Wang L and Goh BC: Clinical
translation of patient-derived tumour organoids-bottlenecks and
strategies. Biomark Res. 10:102022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mollica PA, Booth-Creech EN, Reid JA,
Zamponi M, Sullivan SM, Palmer XL, Sachs PC and Bruno RD: 3D
bioprinted mammary organoids and tumoroids in human mammary derived
ECM hydrogels. Acta Biomater. 95:201–213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Conlon GA and Murray GI: Recent advances
in understanding the roles of matrix metalloproteinases in tumour
invasion and metastasis. J Pathol. 247:629–640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Long L, Yin M and Min W: 3D Co-culture
system of tumor-associated macrophages and ovarian cancer cells.
Bio Protoc. 8:e28152018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Schaue D, Xie MW, Ratikan JA and McBride
WH: Regulatory T cells in radiotherapeutic responses. Front Oncol.
2:902012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Pulze L, Congiu T, Brevini TAL, Grimaldi
A, Tettamanti G, D'Antona P, Baranzini N, Acquati F, Ferraro F and
de Eguileor M: MCF7 spheroid development: New insight about
spatio/temporal arrangements of TNTs, Amyloid Fibrils, Cell
Connections, and Cellular Bridges. Int J Mol Sci. 21:54002020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kroll KT, Mata MM, Homan KA, Micallef V,
Carpy A, Hiratsuka K, Morizane R, Moisan A, Gubler M, Walz AC, et
al: Immune-infiltrated kidney organoid-on-chip model for assessing
T cell bispecific antibodies. Proc Natl Acad Sci USA.
120:e23053221202023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fabi A, Bhargava R, Fatigoni S, Guglielmo
M, Horneber M, Roila F, Weis J, Jordan K and Ripamonti CI; ESMO
Guidelines Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Cancer-related fatigue: ESMO Clinical Practice Guidelines for
diagnosis and treatment. Ann Oncol. 31:713–723. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Baskar R, Yap SP, Chua KL and Itahana K:
The diverse and complex roles of radiation on cancer treatment:
Therapeutic target and genome maintenance. Am J Cancer Res.
2:372–382. 2012.PubMed/NCBI
|
|
102
|
Lin B, Gao F, Yang Y, Wu D, Zhang Y, Feng
G, Dai T and Du X: FLASH radiotherapy: History and future. Front
Oncol. 11:6444002021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hafeez U, Parakh S, Gan HK and Scott AM:
Antibody-drug conjugates for cancer therapy. Molecules.
25:47642020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lv J, Du X, Wang M, Su J, Wei Y and Xu C:
Construction of tumor organoids and their application to cancer
research and therapy. Theranostics. 14:1101–1125. 2021. View Article : Google Scholar : PubMed/NCBI
|