Introduction
Glioblastoma is the most common glioma-derived tumor
in the central nervous system, especially in the brain (1). The World Health Organization (WHO)
classifies central nervous system tumors according to histological,
molecular, and prognostic factors (2). Every year, 3–5/100,000 individuals are
diagnosed with a brain tumor of which glioblastoma multiforme (GBM)
represents the most common form (3). In addition, GBM constitutes the most
lethal form of primary brain tumor. According to the WHO, GBM is
the Grade IV stage (highest grade) of astrocytoma. The WHO GBM
grading was determined according to the core structure, mitotic
activity, vascularization, necrosis, proliferation rate, clinical
signs, and response to treatment (3,4). Due
to its highly heterogeneous structure, radical resection is not
possible, resulting in a shortened life expectancy in patients.
Despite intensive treatment and surgery in patients with GBM, the
overall life expectancy is 12–15 months (5). Rapid infiltrative growth of GBM cells
into peripheral structures causes the disease to progress more
aggressively (6).
Revealing the epidemiology, etiology, anatomy,
molecular structure and spreading of GBM will provide us with more
opportunities to create meaningful treatments and surgical
approaches. The aim of the present review is to reveal the
lateralization of GBM, the anatomical regions where it is
frequently located, the main molecular features in order to
associate these characteristics with tumor location and brain
anatomy.
Lateralization of the brain and functional
area
The right and left hemispheres, which are formed by
the symmetrical division of the brain into two halves through the
fissura longtidunalis, are connected to each other mainly by the
corpus callosum and the comissura anterior. Interhemispheric
connections occur between areas specialized for the same function
in the contralateral cortex (7).
However, morphological and physiological differences in the human
brain cause significant asymmetries between hemispheres. In most
individuals, the right hemisphere appears to be heavier than the
left, while the left hemisphere tends to have a denser structure,
which may signify that high-level control centers are located on
the left (8). This indicates that
one side is functionally dominant, and partially or completely
responsible for that function, and this tendency is defined as
lateralization. For example, in most individuals, speech function
is dominant on the left side, while visual and spatial functions
are specialized on the right side (9). From a different point of view, the
fact that the speech function is dominant on the left side causes
this hemisphere to be dominant in other verbal skills, which in
most individuals is the left side dominant (8).
Frontal lobe
The frontal lobe is the largest lobe of the brain in
terms of volume and contains numerous motor and cognitive control
centers (10). It is reported that
the systems located on the left side of the frontal lobe are
responsible for the cognitive preferences and behaviors given by
the existing memory, while the right side is mostly involved in the
behaviors directed by the external environment. This indicates that
the hemisphere plays a vital role in the healthy processing and
assembly of new information cognitively (11).
Parietal lobe
The parietal lobe includes cortical regions related
to sensory and language function at the subcortical level, and it
is also accepted as an intersection point of white matter pathways
related to motor, sensory, language, visuospatial and visual
function (12). There is general
agreement in the literature that visuospatially-oriented attention
is dependent on a network of frontal and parietal areas in the
right hemisphere. Visuspatially-oriented attention is also
considered to be related to some functions of the right parietal
lobe in the production of open-eye movements (13). It has been reported that the
parietal cortex tends to be more lateralized on the left side than
on the right side (14).
Extra-personal and personal spatial neglect can be serious and is
often observed in association with the right parietal lobe
(15).
Occipital lobe
The occipital lobe is the part of the brain that
processes visual data. It is associated with visual-spatial
processing, distance and depth perception, color identification,
object and face recognition, and memory formation (16). It has been reported that the brain
regions involved in visual word processing are lateralized to the
left hemisphere, which is considered logical considering that in
the majority of individuals, language-related cortical structures
are lateralized to the left hemisphere (17). Anatomically, what is called the
Yakovlevian torque (occipital bending) is the right hemisphere's
tendency to rotate slightly forward relative to the left, which can
cause the right frontal lobe to be larger and wider, and the left
occipital lobe to be wider and project to the right. This makes the
left Sylvian fissure longer and straighter, resulting in a larger
planum temporale (an extension of Wernicke's area) (18).
Temporal lobe
The temporal lobe is the part of the brain
responsible for various cognitive functions including memory,
senses, auditory, language processing, cognition, and semantics
(19). Some studies report that
while the temporal lobe has a larger area especially on the left
side, on the contrary, the cortex thickness is higher on the right
side (20,21). The presence of asymmetries in the
morphological development of the temporal lobes is considered an
important sign of lateralization. The most prominent asymmetry is
observed in the peri-Sylvian region and superior temporal sulcus
(21).
In general, when the whole brain is examined, it is
reported that the total surface area and volume of the left
hemisphere are higher, whereas the cortex thickness is higher on
the right side (20). Although such
lateralization is generally observed, it is also observed that
there are differences between the subunits in each lobe (22).
All these data suggest how asymmetries can be found
at different levels with different parameters such as regional
volume, cortical thickness, connections, cellular and molecular
organization, and surface area. The most clearly studied asymmetry
is the speech function and it is known to be lateralized to the
left side (8,23).
The right hemisphere is less exposed to external
influences in its development as it is responsible for the
functions necessary for survival. In addition, there is a general
dominance of the right hemisphere for all functions except
language, therefore the right hemisphere develops earlier (8).
Although genotype probably plays a role in the
development of structural asymmetries, lesions in any hemisphere
can trigger dominance of the unaffected side due to high
plasticity. On the other hand, environmental and/or physiological
factors can also cause asymmetries to occur, the best example of
which is the right-hand preference due to cultural pressures
(18,24).
Lateralization of the gliomas within the
brain
Considering the complementary functions, the human
brain usually has duality, and individuals are categorized as ‘left
or right-brained’ according to the dominant hemisphere (25). It is known that a number of
different functions, especially the preference for hand use, and
lateralization of speech, are located on one side of the cerebrum
(right or left) and it is accepted as the dominant hemisphere
(26). While the right hemisphere
is primarily associated with nonverbal abilities, the left one is
reported to be responsible for verbal memory and language functions
(27). It is suggested that
evaluating the localization of GBM can be accepted as an indicator
in determining the direction of spread (28). While magnetic resonance imaging
(MRI) can reveal the characteristic structure of the disease by
determining the volumetric information of the tumor and the
determination of the anatomical structures that are or may be
affected, these determinations are insufficient to reveal the
pathophysiology and prognosis (29).
Inskip et al (30), in their study conducted on 489
patients with glioma (354 high-grade, 135 low-grade), 197
meningiomas, and 96 acoustic neuromas, did not find a statistical
difference between the rates of incidence on the left and right
sides, although there was no significant difference between them,
in patients with low-grade glioma and meningioma. More
specifically, in this study, a more common distribution on the
right side compared with the left side was observed, although it
was not significant in patients with high-grade and acoustic
neuroma. These authors reported that aphasia and mental status
changes are more commonly observed in patients with glioma and
tumors affecting the left side of the brain. In a study by Jansma
and Rutten it was reported that 30 tumors from patients with
high-grade glioma were located on the left side and 26 were located
on the right side, while 37 patients with low-grade glioma had
tumors located on the left side and 16 had tumors located on the
right side (31).
In a study by Coluccia et al (32) performed on 235 patients, the
incidence of tumor spread was as follows: The frontal lobe (left,
29.8%; right, 43.0%), the temporal lobe (left, 42.1%; right,
43.8%), the occipital lobe (left, 15.8%; right, 9.9%), the parietal
lobe (left, 35.1%; right, 29.8), the basal ganglia (left, 4.4%;
right, 7.4%). These authors also observed that tumors located in
the right hemisphere were larger. In the same study, it was
reported that the patients with the tumor located on the right had
paralysis in the extremities, and the patients with the tumor
located on the left had more language problems (63.2% in the right
hemisphere and 10.0% in the left hemisphere). There was a decrease
in Karnofsky Performance Status after resection in patients with
left-sided tumors compared with patients with right-sided lesions.
While there was no difference in the overall survival (OS) of the
patients regardless of the side, it was reported that there was a
decrease in the progression-free survival (PFS) in left-sided
patients (7.4 months vs. 10.1 months). The authors stated that the
reason for this result was that total resection was performed with
less success on the left side.
Appropriate surgical resection is one of the
important points in achieving tumor control in GBM. The morbidity
risks and reduced quality of life are taken into account when
approaching tumors located in the vicinity of or directly within
important anatomical regions (33).
It has been observed that resection of the dominant hemisphere
carries great surgical risks. It has been reported that patients
with left temporal lobe glioma experience higher preoperative
neurocognitive impairment and demonstrate a more frequent and
severe decline in neurocognitive abilities after surgical resection
compared with patients with right temporal lobe glioma (34).
In a study involving 507 patients by Ellingson et
al (35), it was reported that
tumors were located to a greater extent on the left side. A
relationship between tumor lateralization and OS with an extended
survival time of up to 36 months in patients with left-sided tumors
compared with the survival time of 12 months observed in patients
with tumors affecting the right side, was also oberved in this
study. The authors also stated that the lesions were more inclined
to be located in the frontal lobe in young patients compared with
the elderly (35). In a study by
Larjavaara et al (36), on
331 patients with glioma, it was reported that tumors were mostly
located in the frontal lobe (40%), followed by the temporal lobe
(29%), parietal lobe (14%), and occipital lobe (3%), respectively.
The authors also stated that the tumor was located more frequently
on the right side than on the left (51% on the right and 40% on the
left).
One of the most comprehensive studies on
lateralization in recent years is the study by Kommers et al
(37), which was conducted on a
total of 1,596 patients with GBM from 13 centers. In this study,
the structure of the tumor, its volume, and the structures of the
affected brain regions were examined by automatic and manual
segmentation with MRI. In the automated segmentations, it was
identified that 785 (49.2%) tumors were located on the left side,
while 792 (49.6%) were on the right side. In manual segmentation,
794 (49.7%) patients had left-sided tumors, and 799 (50.1%) had
right-sided tumors. It was also observed that 19 patients (1.2%)
examined by automated segmentation and 3 patients (0.2%) examined
by manual segmentation, exhibited no laterality. The authors of
this study stated that automatic segmentation produced near-perfect
results. In the continuation of the study, cortical and subcortical
anatomical formations or anatomical regions in the affected
hemisphere were determined in detail.
Based on the study by Kommers et al (37), data obtained through automated
segmentation revealed that the tumors appeared to be most
frequently localized in the insular lobe, frontal lobe and temporal
lobe, respectively (37). According
to this study, these tumors affected a number of areas of the
frontal lobe and were frequently located in the precentral gyrus,
with a lesser percentage in the cingulate anterior gyrus and
frontal opercular cortex. In the parietal lobe, gliomas were mostly
localized in the posterior division of the cingulate gyrus,
posterior division of the marginal gyrus, and precuneus cortex. It
was observed that the areas involved in the temporal lobe were
several. Among these, tumors were often located in the superior
temporal gyrus, planum temporale, and central opercular cortex. The
incidence of the tumor was mainly observed to be located in the
occipital cortex and in the superior and inferior divisions of the
lateral occipital cortex. The incidence for each lobe was similar
for the cortex structure affected on the right and left sides.
Tractus and fascicles involved in subcortical structures appeared
to be the corticospinal tract, superior longitudinal I, II and III
fascicles, arcuate fascicle long segment, frontal strait tract, and
inferior frontal-occipital fascicle. Overall, the eclipse rate of
numerous of these structures was high on the right. It should be
noted that, except for the arcuate fascicle long segment, the
anterior segment was observed significantly higher on the right
side than on the left side (37).
Mickevicius et al (38) retrospectively studied 113 patients
with GBM, hypothesizing that the location of the white matter
structures with the tumor was associated with survival. The authors
found that OS times were reduced in patients with tumors located in
the right anterior thalamic radiation (ATR), right lower inferior
fronto-occipital fasciculus (IFOF), right and left cortico-spinal
tract (CST), and corpus callosum (CC). It was also revealed that
PFS times were decreased in patients with tumors located in the
CST, CC body, right ATR, posterior IFOF, and inferior longitudinal
fasciculus (ILF), and PFS times were increased in patients with
tumors located in the right genu of CC and anterior IFOF. The main
findings of the aforementioned studies are summarized in Table I.
 | Table I.Studies reporting the lateralization
of glioblastoma. |
Table I.
Studies reporting the lateralization
of glioblastoma.
|
|
|
| Lateralization |
|
|---|
|
|
|
|
|
|
|---|
| First author(s),
year | No. of
patients | Brain region | Left | Right | (Refs.) |
|---|
| Inskip et
al, 2003 | 354 HGG | Entire brain | Left |
| (30) |
|
| 135 LGG | Entire brain | Right |
|
|
| Ellingson et
al, 2013 | 507 |
| Left |
| (35) |
| Jansma and Rutten,
2017 | 56 | Entire brain | 53.6% | 46.4% | (31) |
|
| 53 | Entire brain | 69.8% | 30.2% |
|
| Coluccia et
al, 2018 | 235 | Frontal lobe | 29.8% | 43.0% | (32) |
|
|
| Temporal lobe | 42.1% | 43.8% |
|
|
|
| Parietal lobe | 35.1% | 29.8% |
|
|
|
| Occipital lobe | 15.8% | 9.9% |
|
|
|
| Basal ganglia | 4.4% | 7.4% |
|
| Larjavaara et
al, 2007 | 116 (GBM) | Entire brain | 44.5% | 55.5% | (36) |
|
|
| Frontal lobe |
|
|
|
|
|
| Temporal lobe |
|
|
|
|
|
| Parietal lobe |
|
|
|
|
|
| Occipital lobe |
|
|
|
| Kommers et
al, 2021 | 1,596 (Automated
segmentation) | Entire brain | 49.2% | 50.1% | (37) |
|
| 1,596 (Manual
segmentation) | Entire brain | 49.7% | 49.6% |
|
Anatomy of brain regions affected by
glioblastoma
CS
The CST is a complex system consisting of a series
of projection fibers that control spinal cord functions by the
brain, including the control of spinal reflexes and motor neuron
activity. CST axons (75–90% of the axons) form a crossing at the
level of the medulla oblongata and at the level of the midline,
called the pyramidal decussation. This signifies that the left side
of the brain controls the right side of the spinal cord and is
critical for motor functions (39,40).
Most CST axons originate from pyramidal neurons in layer V located
in the primary motor and sensory cortex (M1 and S1). A pioneer
study by Danks et al using diffusion tensor imaging (DTI)
revealed that the CST originates mainly from the M1 and S1, but
also receives input from the supplementary motor areas (SMA) and
the ventral and dorsal premotor cortices (PMC). Leaving the
neocortex, the CST reaches the brainstem by passing through the
posterior limb of the internal capsule and cerebral peduncles
before reaching the brainstem in the ventral position. Data
obtained through the use of DTI revealed that the CST content
originates from 37% M1, 32% S1, 25% SMA, and 7% PMC (41).
The control of complex motor functions such as the
selection of movement, making the final decision, initiating the
function, and monitoring the process is provided by the SMA. It is
known that this area has connections with the precentral gyrus,
prefrontal cortex, basal ganglia, limbic system, spinal cord,
contralateral SMA, superior parietal cortex, and inferior frontal
cortical areas, especially pars opercularis. When Salvati et
al (42) compared 127 patients
with GBM and SMA involvement (Group A) and patients with non-SMA
but M1 and CST involvement (Group B), it was reported that group A
patients had a higher volume, but there was no change in the OS and
PFS durations of the patients.
Superior longitudinal fasciculus
(SLF)
The primary function of the SLF is to provide
communication between the frontal and parietal lobes and partial
connections with the temporal lobe. It is accepted that there are
two different paths due to its proximity to the arcuate fascicle
that connects the posterior temporal lobe and the frontal lobe in
the peri-Slyvian region (43,44).
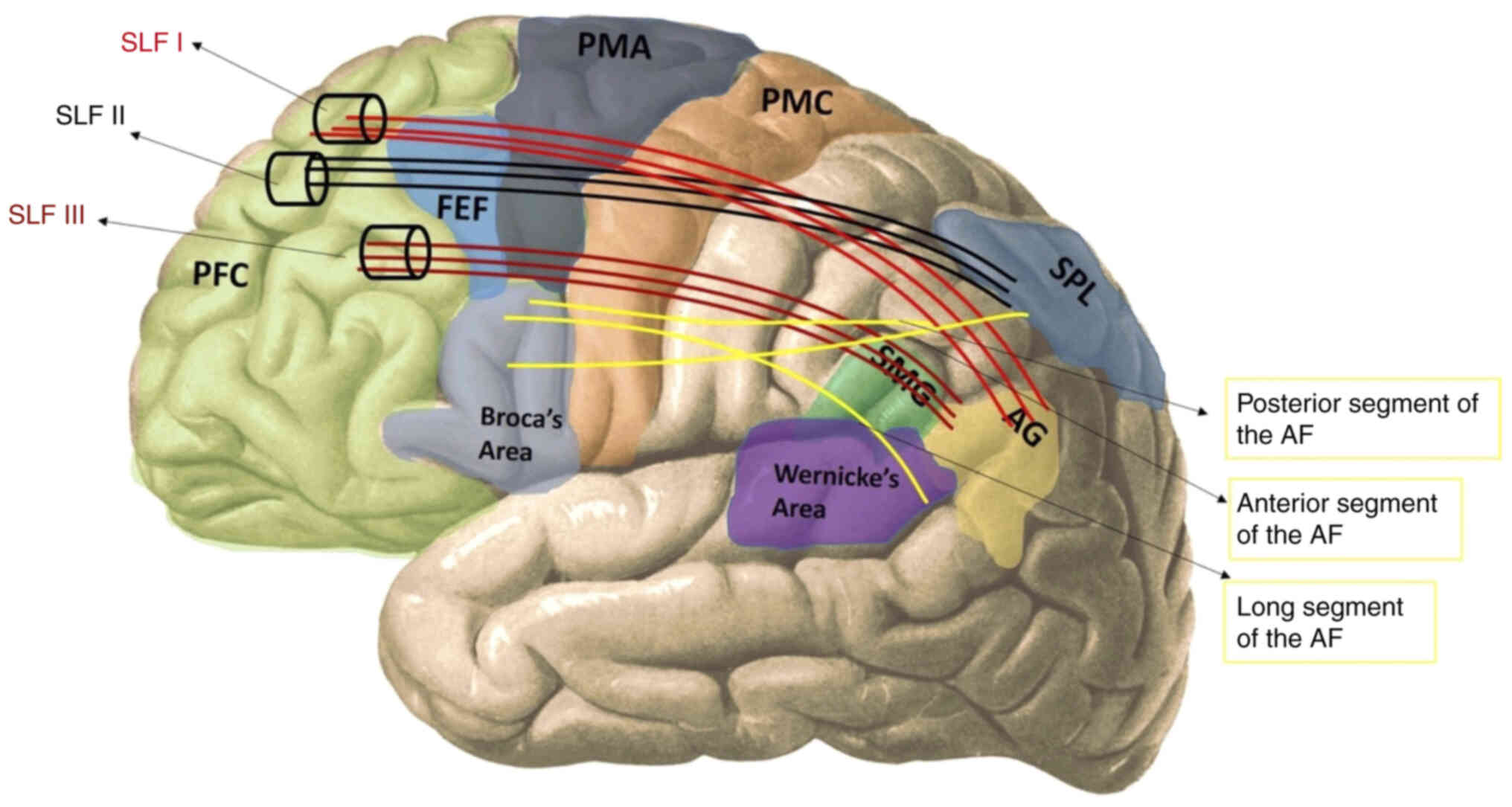
Makris et al (45) analyzed
the SLF by dividing it into four anatomical subsections. SLF I is
the dorsal part and provides the connection between the superior
parietal and superior frontal lobes. SLF II starts from the angular
gyrus, passes over the centrum semiovale on the insula, and ends in
the caudal-lateral prefrontal region. SLF III is the ventral part
of the pathway, and it travels between the anterior part of the
angular gyrus, and the supramarginal gyrus, and the ventral
premotor and prefrontal areas. The 4th subcomponent of the SLF,
identified as SLF IV in earlier studies on non-human primates,
corresponds to the arcuate fasciculus (AF). This segment connects
the posterior part of the superior temporal gyrus to the lateral
prefrontal cortex, running through the caudal extremity of the
Sylvian fissure. Nonetheless, the designation of the AF as the 4th
component of the SLF is not universally accepted (46,47).
In definitions by Catani and Thiebaut de Schotten,
each arcuate fascicle is divided into three parts (long, anterior
and posterior) connecting two regions of the Broca, Wernicke, or
Geschwind region (inferior parietal lobule) (46). The anterior segment of AF appears to
correspond to SLF III and the two terms are used interchangeably.
Therefore, although these two bundles are separate structures, some
of their subcomponents appear to overlap with each other (47).
Various studies have revealed that the SLF is
markedly closer to the cingulum, while it cannot be separated by
other SLF structures (SLF I–III and AF) (48,49);
in addition, according to Thiebaut de Schotten, the SLF is
symmetrical in both hemispheres (50). However, some studies in the
literature are not concordant indicating that SLF is greater on the
right side than on the left side (47,51),
while other studies have claimed that SLF II is dominant on the
left side through multiple components (49,52).
This anatomical feature may explain the lateralization of the
language in the left hemisphere (Fig.
1) (53).
Several studies have demonstrated the occurrence of
gliomas and glioblastoma in the SLF region. More specifically,
Davtian et al (54),
presented a case report of a 59-year-old woman with recurrent GBM
involving the left medial frontal and cingulate gyri with
impairment of the SLF region (54).
The authors described that the dissection of the tumor border in
contact with the SLF resulted in recurrent speech arrest
highlighting how the surgical resection of tumors in this region is
not recommended for large lesions (54). Previously, Nakajima et al
(55), observed visuospatial
dysfunction in patients with glioma affecting the right dorsal
SLF.
Similarly, Liu et al (56), observed cognitive deficits in
patients with glioma located in the right SLF temporal part.
Overall, all these data are concordant in
demonstrating the cognitive decline (in terms of visuospatial and
speech abilities) associated with glioma development in the SLF
region.
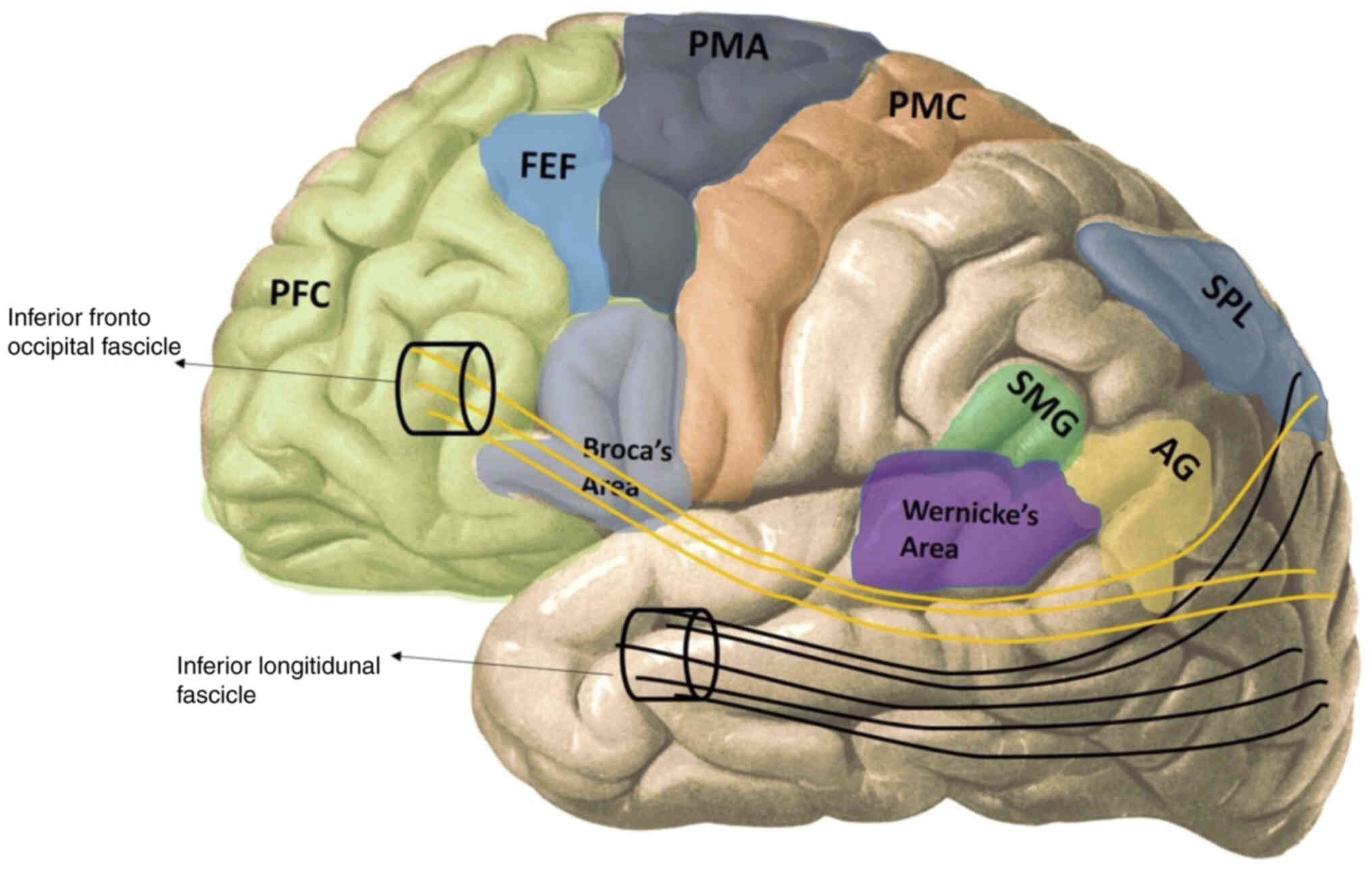
ILF
The ILF is the white matter structure that provides
the reciprocal connection between the temporal-parietal-occipital
lobes. It also functions in visual word recognition, connecting the
occipital cortex to the posterior occipitotemporal cortices
(57). Latini et al
(58) reported that the main
structure of the ILF is located more in the fusiform, lingual, and
dorsolateral-occipital regions of the occipital lobe and that these
are fixed components. It was revealed that there was no
lateralization for the subcomponents of the ILF, but that they were
located on the right in total volume (Fig. 2).
As regards the ILF, there are a few studies
describing the localization of GBM in this region. Specifically,
the postsurgical residual lesion volume located in the left ILF was
associated with lexical retrieval impairments supporting the data
already described in the aforemetioned section on SLF (59,60).
IFOF
The IFOF is the white matter structure that connects
the occipital cortex, temporo-basal areas, superior parietal
lobule, and precuneus to the frontal lobe. The region starting from
the periphery of the caudate nucleus and extending to the temporal
horn of the lateral ventricle is the sub-insular region, and the
IFOF is located on the ventral third of this region and the outer
side of the external capsule. This pathway is involved in mumerous
functions such as language, non-verbal semantic processing, object
identification, visual-spatial processing and planning, reading,
facial expression recognition, and memory (Fig. 2) (61). Vassal et al (62) examined the cortical terminations of
IFOF by tractography in 20 healthy individuals to determine
individual variations and asymmetry. According to their data, IFOF
terminations lateralize over the superior parietal lobule on the
right and the inferior frontal gyrus on the left. Altieri et
al (63) reported that of a
total of 23 patients with 38% GBM, 33% oligodendroglioma, and 29%
astrocytoma, 57% of tumors were located in the left hemisphere and
43% in the right henisphere. Moreover in this study, this pathway
was divided into three parts anatomically: A vertical that runs
along the frontal lobe, a horizontal segment that runs along the
frontal lobe, and a horizontal segment that runs from the limen
insulae. Caminis et al (64)
reported that 33 lesions of patients with tumors involving the
temporal lobe were located on the left side (97%), 14 were medial,
14 were lateral, and 6 were medial and lateral extensions. In
total, it was reported that the lesions were directed to the insula
in 22 cases.
Frontostriatal tract (FST)
The FST connects several cortical areas of the
frontal lobe, especially the prefrontal cortex, with the striatum
and the thalamus. It is responsible for a wide range of mental,
motor, limbic, and cognitive functions (65). In particular, the connections
between the caudate nucleus and the frontal cortex have a markedly
rigid and clear topographic organization in regionally specific
clusters. It is reported that these specific regions are located in
the subregions of the ventrolateral, dorsolateral, and
orbitofrontal cortex and that there is a similar organizational
scheme in both hemispheres (66).
From a different anatomical point of view, two
different association fiber structures originating from SMA and
connecting with different regions are mentioned in the literature.
One is the FST, which connects the pre-SMA to the anterior part of
the caudate nucleus, and the other is the fronto-aslant tract,
which connects the pre-SMA to the pars opercularis. The FST starts
from the caudate nucleus, passes through the lateral ventricle's
frontal horn, and terminates in the ipsilateral inferior frontal
gyrus (67). The frontal aslant
tract is a brain white matter pathway that connects the superior
frontal gyrus (SMA, pre-SMA) to the pars opercularis and pars
triangularis of the inferior frontal gyrus and the insula (68). Functionally, the left frontal aslant
tract is responsible for language and the right for executive
functions (69).
An in-depth analysis of the literature does not
reveal any studies on glioblastoma involvement in the FST. However,
Müller et al (70)
questioned the possibility of performing neurosurgery for the
removal of glioma affecting the caudate nucleus connected to the
supplementary motor area through the FST. In this case, the authors
discussed the functional role of both the caudate nucleus and the
FST that can be indicative of the limits of resection in the case
of supracomplete glioma resection (70).
A number of these pathways, which have a complex
structure, are still the subject of research. A summary of these
aforementioned pathways regarding GBM is included in Table II.
 | Table II.White matter brain pathways. |
Table II.
White matter brain pathways.
| Pathway | Connections |
|---|
| Corticospinal
tract | Primary motor and
sensory cortex to the spinal cord (lower motor neurons) |
| SLF
I | Superior parietal
lobe to the superior frontal lobe |
| SLF
II | Angular gyrus to
the caudal-lateral prefrontal region |
| SLF
III | Between the
anterior part of the angular gyrus, supramarginal gyrus, ventral
premotor and prefrontal areas |
| Inferior
longitudinal fasciculus | Between the
temporal, parietal and occipital lobes |
| Inferior
fronto-occipital fascicle | Occipital cortex,
temporo-basal areas, superior parietal lobule and precuneus to th
frontal lobe |
| Fronto-striatal
tract | Between the
prefrontal cortex, with the striatum and the thalamus |
Lateralization of molecular features in
glioblastoma
As aforementioned, the lateralization of glioma and
glioblastoma has profound anatomical implications that can limit
the surgical approaches available due to the impairment of
cognitive functions. In addition, it was widely demonstrated that
GBM lateralization also has effects on tumor molecular features
which influence the pathogenesis of the tumor and the clinical
outcomes (71). Despite extensive
research efforts, GBM remains a challenge due to its heterogeneity
and resistance to treatment, and such heterogeneity is also
reflected in the prevalence of molecular alterations observed in
the right and left side of the brain (72).
Genomic studies have demonstrated profound
differences existing between left and right brain tumors in terms
of molecular profiles. Notably, mutations in the isocitrate
dehydrogenase 1 (IDH1) gene are more prevalent in tumors located in
the frontal lobe, whereas tumors located in the temporal lobe
frequently exhibit epidermal growth factor receptor (EGFR)
amplification and phosphatase and tensin homolog (PTEN) loss
(73,74).
The lateralization of molecular features is not
limited only to gene mutations. Notably, DNA methylation, histone
modifications, and other epigenetic alterations have exhibited
lateralization patterns in GBM (75,76).
One of the most common epigenetic alterations observed in cancer,
including GBM, is the alteration of DNA methylation (75). The analysis of DNA methylation
patterns observed in GBM has revealed region-specific differences
in the methylome of GBM, with distinct CpG island methylator
phenotype (CIMP) subtypes associated with specific brain regions
(75,77). Moreover, differential histone
modifications have been observed in GBM located in different brain
regions suggesting a putative role of epigenetics in regional tumor
heterogeneity and specific subtypes such as the H3 G34 mutant type
(78).
In addition to the anatomical and biological
lateralization of GBM-associated molecular features, the
lateralization of both genetic and epigenetic alterations also has
important clinical and therapeutic implications. In this context,
the lateralization of molecular features in GBM can be useful to
improve the diagnosis and prognosis of this tumor. Indeed, the
integration of molecular information with clinical and
radio-imaging data could enhance the accuracy of tumor diagnosis
and guide personalized treatment strategies. For example, the
identification of specific molecular alterations associated with
different brain regions could aid in the preoperative prediction of
tumor location and facilitate surgical planning (79). In addition, the lateralization of
molecular alterations may provide useful prognostic information
correlating both side-specific genetic and epigenetic features with
the survival outcomes of patients and the route of metastatization
(80,81).
Some authors have also proposed to tailor the
treatment depending on the lateralization of the molecular features
in GBM (82,83). Due to the regional differences
observed for the genetic and epigenetic alterations in GBM,
targeted approaches could be designed to exploit specific
vulnerabilities associated with each brain region. For instance,
therapies targeting IDH1 mutations may be more effective in frontal
lobe tumors, while targeting EGFR amplification could be more
beneficial in temporal lobe tumors (84). In addition, the immune
microenvironment and tumor-stromal cell interactions may have a
role in the response to immunotherapies and other targeted
interventions, however, more in-depth investigations should be
performed to fully clarify these complex interactions.
Emerging evidence suggests that specific molecular
alterations may exhibit a predilection for GBM located in the right
side, left side, frontal lobe or other regions of the brain. This
section aims to explore the molecular alterations frequently
observed in different areas of the brain in patients with gliomas
and glioblastomas, shedding light on their potential clinical and
therapeutic implications (Fig. 3
and Table SI).
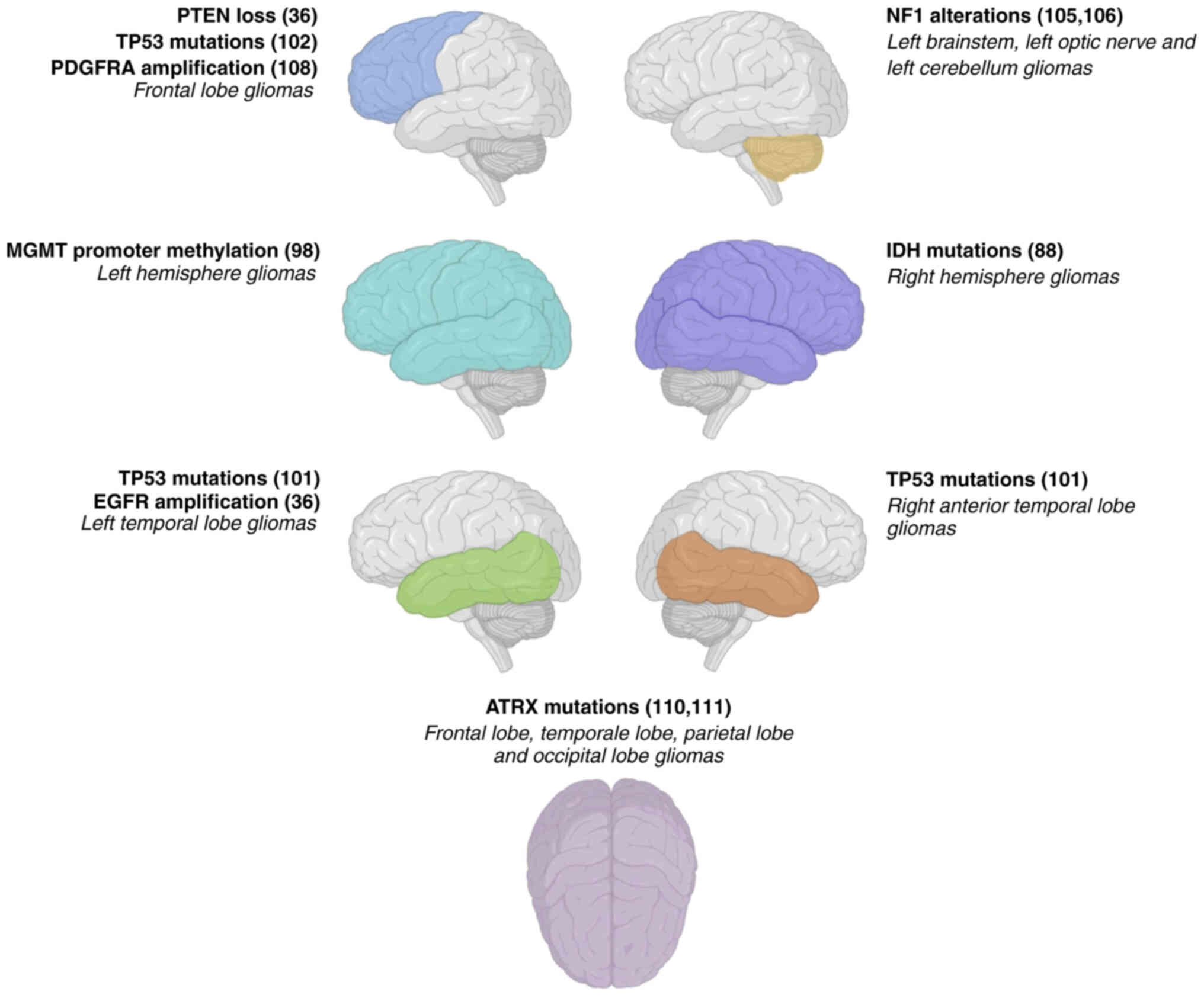
IDH1 mutations
IDH1 mutations represent one of the most observed
molecular alterations in both gliomas and glioblastoma (85,86). A
molecular and radio imaging study performed on patients with glioma
revealed a higher prevalence of IDH1 mutations in tumors mainly
affecting the right hemisphere compared with the left hemisphere
(87). However, the precise
mechanisms responsible for this lateralization pattern are not
widely understood, but can be associated with GBM regional
differences in IDH1 expression or genetic predispositions
associated with brain asymmetry (88). As a limitation of these findings,
the majority of the studies on the detection of IDH mutations do
not report the right or left lateralization of the mutation but
only the brain region affected.
EGFR amplification
Amplification of the EGFR gene is a common genetic
alteration observed in glioblastoma (89). Notably, a previous study indicated a
higher frequency of EGFR amplification in glioblastoma located on
the left temporal lobe of the brain (35). The reasons for this specific
lateralization are still under investigation, but could be
attributed to variations in the microenvironment or signaling
pathways specific to the left hemisphere (90). More importantly, the prevalence of
EGFR mutation in GBM may have important prognostic implications as
different targeted treatments for EGFR-positive tumors are
currently available (91).
PTEN loss
PTEN is a tumor suppressor gene frequently mutated
or deleted in glioblastoma. The loss-of-function of PTEN results in
the dysregulation of multiple associated signaling pathways
involved in cell proliferation, cell survival and apoptosis
(92,93). Contrary to what was observed for
IDH1 and EGFR, there are no studies describing the specific
distribution of PTEN alterations in the two brain hemispheres,
however, the study of Ellingson et al (35) revealed a higher incidence of PTEN
loss in glioblastomas affecting the frontal lobe. However, with
regard to this case, the underlying mechanisms for this
lateralization pattern require further investigation.
O6-methylguanine-DNA
methyltransferase (MGMT) promoter methylation
As regards the lateralization of epigenetics
alterations, the MGMT gene encodes a DNA repair protein that can be
silenced through the hypermethylation of its promoter (94). MGMT promoter methylation is
associated with increased sensitivity to alkylating chemotherapy,
such as temozolomide, one of the main drugs used for the treatment
of GBM (95,96). Studies have reported a higher
frequency of MGMT promoter methylation in left-sided glioblastomas
compared with the hypomethylation observed in tumors affecting the
right side (97) suggesting how in
the left hemisphere epigenetic alterations induced by stromal cells
or the microenvironment are more evident compared with the right
side (98).
TP53 mutations
A significant fraction of GBM harbor mutations
affecting the TP53 gene, which encodes the tumor suppressor protein
p53 and plays a key role in the regulation of DNA integrity
(99). A previous study reported a
higher prevalence of TP53 mutations in gliomas affecting the left
medial temporal lobe and the right anterior temporal lobe (100). Another study revealed that
p53-mutated glioblastomas were preferentially located in the
frontal lobe near the rostral extension of the lateral ventricles
(101). As for the other mutations
observed in GBM, the mechanisms responsible for this lateralization
remain unclear but it results in the alteration of DNA repair
capacity or regional variations in the response to mutagenic
factors (102).
Neurofibromin 1 (NF1) alterations
Alterations in the NF1 gene have been implicated in
the pathogenesis of gliomas (103). Notably, NF1 acts as a negative
regulator of the Ras signaling pathway thus playing a role in the
modulation of cell proliferation and cell survival. As described
for PTEN, NF1 alterations can affect GBM occurring in different
parts of the brain, however, there is a slight preference for the
left hemisphere as demonstrated by two independent studies where
NF1-mutated gliomas affected the left side of the brainstem, left
optic nerve and left cerebellum (104,105).
Platelet-derived growth factor
receptor-α (PDGFRA) amplification
Amplification of the PDGFRA gene is a recurrent
genetic alteration observed in glioblastoma (106). A previous study has indicated a
higher frequency of PDGFRA amplification in H3 G34 diffuse
hemispheric gliomas located on both the right and left frontal
lobes of the brain (107).
However, the specific mechanisms underlying this lateralization
pattern remain to be fully elucidated.
ATRX chromatin remodeler (ATRX)
mutations
ATRX is a gene involved in chromatin remodeling and
it is often mutated in astrocytoma and low-grade glioma (108). Previous studies demonstrated that
ATRX mutations can be found both in the right and left hemispheres
affecting different brain regions including the frontal lobe, the
temporal lobe, the parietal lobe, the occipital lobe, and in rare
cases also the brainstem, and the thalamus (108,110).
Overall, understanding the specific right- or
left-sided molecular alterations in glioma and glioblastoma has
fundamental diagnostic, prognostic, and clinical implications.
First of all, the molecular differences observed in right and left
tumors could aid the clinical diagnosis performed through imaging
techniques and identify potential hidden lesions. Secondly, the
precise characterization of the lateralization of molecular
alterations may contribute to the development of novel targeted
therapies. In particular, the presence of EGFR and TP53
alterations, along with other molecular features, may drive the
selection of the correct drug to be administered to the patients.
Finally, the definition of the lateralization of a tumor from an
anatomical and molecular point of view could improve the management
of this tumor.
Notably, different studies are concordant in
confirming a more common location of GBM within the frontal lobe
and insula with a consequent poor prognosis in terms of PFS times
when the tumor is located on the left side of the brain. It was
also reported that the white matter structures on the right side
are larger in volume, thus the tumors arising in this region are
usually of a larger volume. Based on the data obtained in the study
by Kommers et al it is demonstrated that tumors mainly
affect the SLF, ILF, IFOF, AF, FST, and CST regions (however, no
statistical comparison depending on the brain side was performed in
the study) (37). Considering the
anatomical features of these localizations and the areas they
connect, it is logical that the major involvements are observed in
the frontal-temporal-parietal-occipital lobes, respectively. This
supports the hypothesis that the tumor follows the white matter
structures during its spreading favored by both direct and indirect
mechanisms (for example, mediated by GBM-derived exosomes which
induce neurotoxicity in the surrounding structures) (111–113). Although tumor volumes are higher
on the right side, some sources state that the prognosis is worse
in left-sided patients. However, it should be noted here that there
is no difference in OS times, regardless of the localization of
tumors. The aforementioned pathways related to GBM and the
evaluation of the anatomy of the region for appropriate resection
are especially emphasized by surgeons. Some sources state that
establishing the location of tumors within brain regions could be
useful to determine the prognosis of the disease as well as the
surgical decisions (15). In the
literature it is reported that regular radiological imaging can be
used as a predictor in the evaluation of prognosis by detecting the
anatomical and volumetric position of the tumor, necrosis or extent
of edema, and pathophysiological changes in patients (16).
Overall, the anatomical data on GBM lateralization,
coupled with the growing findings on molecular alterations
affecting specific brain regions and epigenetics events involved in
GBM development and progression (114,115), may improve the clinical management
of patients with GBM and the positive effects on the outcomes of
patients. In addition, a deep understanding of both anatomical and
molecular lateralization is essential to propose novel effective
treatment in GBM, including photoacoustic nanoprobes (116,117).
Conclusions
By analyzing the studies reported in the literature,
it is not clear if a precise lateralization of brain tumors exists.
However, there is strong evidence supporting the anatomical
lateralization of both glioma and glioblastoma as well as the
molecular lateralization of some key molecular alterations which
influence the evolution of tumors and the prognosis of patients.
Recently, a broad meta-analysis has collected all the studies
reporting the anatomical lateralization of glioma and GBM revealing
no specific lateralization patterns in the right or left
hemispheres. However, a clear relationship between tumor location
in specific brain regions and glioma-associated symptoms were
established (118). As regards the
lateralization of glioma and GBM molecular features, more robust
investigations on this specific topic are needed to establish
lateralization patterns of mutations and epigenetics
alterations.
In conclusion, these data suggest that the specific
evaluation of glioma and GBM localization from both an anatomical
and molecular perspective can help clinicians in a number of areas.
It may help develop more specific drugs and treatment methods based
on different molecular changes in different brain regions. In
addition, predicting the location of the tumor and the possibility
of spread can provide the physician preliminary information
regarding the prognosis of the disease. Another important issue is
that technologies such as artificial intelligence can be used in
this field. Evaluating radiological images with artificial
intelligence, especially in the early stages, is a topic that can
be evaluated in terms of prognosis.
Supplementary Material
Supporting Data
Acknowledgements
MP acknowledges the PIA.CE.RI Program of the
University of Catania for its support in allowing young researchers
to promote and participate in research activities (PIA.CE.RI ID:
EBioCaSt).
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ATs and ATa conceptualized the study. MP, LF, PDM,
SG and NTC wrote the original draft of the manuscript. MP, LF, PDM,
DAS and ATa provided critical revisions. LF, SG and NTC prepared
the tables and figures, conducted the formal analysis, and
critically analyzed the literature. Data authentication is not
applicable. All authors contributed to the manuscript revision, as
well as read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this
article.
References
|
1
|
Bouget D, Eijgelaar RS, Pedersen A,
Kommers I, Ardon H, Barkhof F, Bello L, Berger MS, Nibali MC,
Furtner J, et al: Glioblastoma surgery imaging-reporting and data
system: Validation and performance of the automated segmentation
task. Cancers (Basel). 13:46742021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fontán-Lozano Á, Morcuende S, Davis-López
de Carrizosa MA, Benítez-Temiño B, Mejías R and Matarredona ER: To
Become or not to become tumorigenic: Subventricular zone versus
hippocampal neural stem cells. Front Oncol. 10:6022172020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu H, Chen X, Sun Y, Hu X, Zhang X, Wang
Y, Tang Q, Zhu Q, Song K, Chen H, et al: Comprehensive molecular
characterization of long-term glioblastoma survivors. Cancer Lett.
593:2169382024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taghizadehghalehjoughi A, Hacımüftüoglu A,
Cetin M, Ugur AB, Galatenau B, Mezhuev Y, Okkay U, Taspinar N,
Taspinar M, Uyanik A, et al: Effect of metformin/irinotecan-loaded
poly-lactic-co-glycolic acid nanoparticles on glioblastoma: In
vitro and in vivo studies. Nanomedicine. 13:1595–1606. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grochans S, Cybulska AM, Simińska D,
Korbecki J, Kojder K, Chlubek D and Baranowska-Bosiacka I:
Epidemiology of glioblastoma multiforme-literature review. Cancers
(Basel). 14:24122022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seker-Polat F, Pinarbasi Degirmenci N,
Solaroglu I and Bagci-Onder T: Tumor cell infiltration into the
brain in glioblastoma: From mechanisms to clinical perspectives.
Cancers (Basel). 14:4432022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pittella JEH: The uniqueness of the human
brain: A review. Dement Neuropsychol. 18:e202300782024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bisiacchi P and Cainelli E: Structural and
functional brain asymmetries in the early phases of life: A scoping
review. Brain Struct Funct. 227:479–496. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spaccavento S, Caliendo S, Galetta R,
Picciola E, Losavio E and Glueckauf R: Pragmatic communication
deficit and functional outcome in patients with right- and
left-brain damage: A pilot study. Brain Sci. 14:3872024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Catani M: The anatomy of the human frontal
lobe. Handb Clin Neurol. 163:95–122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldberg E, Podell K and Lovell M:
Lateralization of frontal lobe functions and cognitive novelty. J
Neuropsychiatry Clin Neurosci. 6:371–378. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Wang J, Zhang Y, Zheng D, Zhang J,
Rong M, Wu H, Wang Y, Zhou K and Jiang T: The neuroanatomical basis
for posterior superior parietal lobule control lateralization of
visuospatial attention. Front Neuroanat. 10:322016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dziedzic TA, Bala A and Marchel A:
Cortical and subcortical anatomy of the parietal lobe from the
neurosurgical perspective. Front Neurol. 12:7270552021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong SK and Xu Y: The impact of top-down
spatial attention on laterality and hemispheric asymmetry in the
human parietal cortex. J Vis. 16:22016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kondo M: The laterality of parietal
association areas: Hemispatial neglect, body images and body
schema. Brain Nerve. 70:1059–1066. 2018.(In Japanese). PubMed/NCBI
|
|
16
|
Rehman A and Al Khalili Y: Neuroanatomy,
Occipital Lobe. StatPearls Treasure Island, FL: StatPearls
Publishing; 2023, Available from:. https://www.ncbi.nlm.nih.gov/books/NBK544320/
|
|
17
|
Vonk JMJ, Borghesani V, Battistella G,
Younes K, DeLeon J, Welch A, Hubbard HI, Miller ZA, Miller BL and
Gorno-Tempini ML: Verbal semantics and the left dorsolateral
anterior temporal lobe: A longitudinal case of bilateral temporal
degeneration. Aphasiology. 34:865–885. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo F and Massoud TF: Structural
asymmetries in normal brain anatomy: A brief overview. Ann Anat.
241:1518942022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao F, Wu Z, Wang L, Lin W and Li G:
Longitudinally consistent registration and parcellation of cortical
surfaces using semi-supervised learning. Med Image Anal.
96:1031932024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong XZ, Mathias SR, Guadalupe T; ENIGMA
Laterality Working Group, ; Glahn DC, Franke B, Crivello F,
Tzourio-Mazoyer N, Fisher SE, Thompson PM, et al: Mapping cortical
brain asymmetry in 17,141 healthy individuals worldwide via the
ENIGMA Consortium. Proc Natl Acad Sci USA. 115:E5154–E5163. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu L, Wang Y, Fang H, Xiao X, Xiao T, Li
Y, Li C, Wu Q, Chu K, Xiao C and Ke X: Longitudinal study of brain
asymmetries in autism and developmental delays Aged 2–5 years.
Neuroscience. 432:137–149. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koelkebeck K, Miyata J, Kubota M, Kohl W,
Son S, Fukuyama H, Sawamoto N, Takahashi H and Murai T: The
contribution of cortical thickness and surface area to gray matter
asymmetries in the healthy human brain. Hum Brain Mapp.
35:6011–6022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esteves M, Ganz E, Sousa N and
Leite-Almeida H: Asymmetrical brain plasticity: Physiology and
pathology. Neuroscience. 454:3–14. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grant JH, Parker AJ, Hodgson JC, Hudson JM
and Bishop DVM: Testing the relationship between lateralization on
sequence-based motor tasks and language laterality using an online
battery. Laterality. 28:1–31. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Corballis MC: Evolution of cerebral
asymmetry. Prog Brain Res. 250:153–178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhai Z and Feng J: Left-right asymmetry
influenced the infarct volume and neurological dysfunction
following focal middle cerebral artery occlusion in rats. Brain
Behav. 8:e011662018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Edwards JD, Jacova C, Sepehry AA, Pratt B
and Benavente OR: A quantitative systematic review of
domain-specific cognitive impairment in lacunar stroke. Neurology.
80:315–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Luca C, Virtuoso A, Papa M, Certo F,
Barbagallo GMV and Altieri R: Regional development of Glioblastoma:
The anatomical conundrum of cancer biology and its surgical
implication. Cells. 11:13492022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nestler U, Lutz K, Pichlmeier U, Stummer
W, Franz K, Reulen HJ and Bink A; 5-ALA Glioma Study Group, :
Anatomic features of glioblastoma and their potential impact on
survival. Acta Neurochir (Wien). 157:179–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inskip PD, Tarone RE, Hatch EE, Wilcosky
TC, Selker RG, Fine HA, Black PM, Loeffler JS, Shapiro WR and Linet
MS: Laterality of brain tumors. Neuroepidemiology. 22:130–138.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jansma JM and Rutten G: P04.11 effect of
hemisphere and tumor grade on default mode deactivation in glioma
patients. Neuro Onco. 19:iii422017. View Article : Google Scholar
|
|
32
|
Coluccia D, Roth T, Marbacher S and
Fandino J: Impact of laterality on surgical outcome of glioblastoma
patients: A retrospective single-center study. World Neurosurg.
114:e121–e128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mazoyer B, Zago L, Jobard G, Crivello F,
Joliot M, Perchey G, Mellet E, Petit L and Tzourio-Mazoyer N:
Gaussian mixture modeling of hemispheric lateralization for
language in a large sample of healthy individuals balanced for
handedness. PLoS One. 9:e1011652014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noll KR, Ziu M, Weinberg JD and Wefel J:
Neurocognitive functioning in patients with glioma of the left and
right temporal lobes. J Neurooncol. 128:323–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ellingson BM, Lai A, Harris RJ, Selfridge
JM, Yong WH, Das K, Pope WB, Nghiemphu PL, Vinters HV, Liau LM, et
al: Probabilistic radiographic atlas of glioblastoma phenotypes.
AJNR Am J Neuroradiol. 34:533–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Larjavaara S, Mäntylä R, Salminen T,
Haapasalo H, Raitanen J, Jääskeläinen J and Auvinen A: Incidence of
gliomas by anatomic location. Neuro Oncol. 9:319–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kommers I, Bouget D, Pedersen A, Eijgelaar
RS, Ardon H, Barkhof F, Bello L, Berger MS, Conti Nibali M, Furtner
J, et al: Glioblastoma surgery imaging-reporting and data system:
Standardized reporting of tumor volume, location, and resectability
based on automated segmentations. Cancers (Basel). 13:28542021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mickevicius NJ, Carle AB, Bluemel T,
Santarriaga S, Schloemer F, Shumate D, Connelly J, Schmainda KM and
LaViolette PS: Location of brain tumor intersecting white matter
tracts predicts patient prognosis. J Neurooncol. 125:393–400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Filippopulos FM, Brem C, Seelos K,
Köglsperger T, Sonnenfeld S, Kellert L and Vollmar C: Uncrossed
corticospinal tract in health and genetic disorders: Review, case
report, and clinical implications. Eur J Neurol. 28:2804–2811.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Welniarz Q, Dusart I and Roze E: The
corticospinal tract: Evolution, development, and human disorders.
Devel Neurobio. 77:810–829. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Danks RA, Aglio LS, Gugino LD and Black
PM: Craniotomy under local anesthesia and monitored conscious
sedation for the resection of tumors involving eloquent cortex. J
Neurooncol. 49:131–139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Salvati M, Armocida D, Pesce A, Palmieri
M, Venditti E, D'Andrea G, Frati A and Santoro A: No prognostic
differences between GBM-patients presenting with postoperative
SMA-syndrome and GBM-patients involving cortico-spinal tract and
primary motor cortex. J Neurol Sci. 419:1171882020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Frye RE, Hasan K, Malmberg B, Desouza L,
Swank P, Smith K and Landry S: Superior longitudinal fasciculus and
cognitive dysfunction in adolescents born preterm and at term. Dev
Med Child Neurol. 52:760–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakajima R, Kinoshita M, Shinohara H and
Nakada M: The superior longitudinal fascicle: Reconsidering the
fronto-parietal neural network based on anatomy and function. Brain
Imaging Behav. 14:2817–2830. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Makris N, Kennedy DN, McInerney S,
Sorensen AG, Wang R, Caviness VS Jr and Pandya DN: Segmentation of
subcomponents within the superior longitudinal fascicle in humans:
A quantitative, in vivo, DT-MRI study. Cereb Cortex. 15:854–869.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Catani M and Thiebaut de Schotten M: Atlas
of Human Brain Connections. Oxford; University Press: 2012,
View Article : Google Scholar
|
|
47
|
Janelle F, Iorio-Morin C, D'Amour S and
Fortin D: Superior longitudinal fasciculus: A review of the
anatomical descriptions with functional correlates. Front Neurol.
13:7946182022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Martino J, De Witt Hamer PC, Berger MS,
Lawton MT, Arnold CM, de Lucas EM and Duffau H: Analysis of the
subcomponents and cortical terminations of the perisylvian superior
longitudinal fasciculus: A fiber dissection and DTI tractography
study. Brain Struct Funct. 218:105–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Pathak S, Stefaneanu L, Yeh FC, Li
S and Fernandez-Miranda JC: Subcomponents and connectivity of the
superior longitudinal fasciculus in the human brain. Brain Struct
Funct. 221:2075–2092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Thiebaut de Schotten M, Dell'Acqua F,
Forkel SJ, Simmons A, Vergani F, Murphy DG and Catani M: A
lateralized brain network for visuospatial attention. Nat Neurosci.
14:1245–1246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hecht EE, Gutman DA, Bradley BA, Preuss TM
and Stout D: Virtual dissection and comparative connectivity of the
superior longitudinal fasciculus in chimpanzees and humans.
Neuroimage. 108:124–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vernooij MW, Smits M, Wielopolski PA,
Houston GC, Krestin GP and van der Lugt A: Fiber density asymmetry
of the arcuate fasciculus in relation to functional hemispheric
language lateralization in both right- and lefthanded healthy
subjects: A combined fMRI and DTI study. Neuroimage. 35:1064–1076.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Netter FH: Atlas of Human Anatomy. 6th
edition. Elsevier; Philedelphia: 2008
|
|
54
|
Davtian M, Ulmer JL, Mueller WM, Gaggl W,
Mulane MP and Krouwer HG: The superior longitudinal fasciculus and
speech arrest. J Comput Assist Tomogr. 32:410–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nakajima R, Kinoshita M, Miyashita K,
Okita H, Genda R, Yahata T, Hayashi Y and Nakada M: Damage of the
right dorsal superior longitudinal fascicle by awake surgery for
glioma causes persistent visuospatial dysfunction. Sci Rep.
7:171582017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu D, Liu Y, Hu X, Hu G, Yang K, Xiao C,
Hu J, Li Z, Zou Y, Chen J, et al: Alterations of white matter
integrity associated with cognitive deficits in patients with
glioma. Brain Behav. 10:e016392020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tamai S, Kinoshita M, Nakajima R, Okita H
and Nakada M: Two different subcortical language networks
supporting distinct Japanese orthographies: Morphograms and
phonograms. Brain Struct Funct. 227:1145–1154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Latini F, Mårtensson J, Larsson EM,
Fredrikson M, Åhs F, Hjortberg M, Aldskogius H and Ryttlefors M:
Segmentation of the inferior longitudinal fasciculus in the human
brain: A white matter dissection and diffusion tensor tractography
study. Brain Res. 1675:102–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Duffau H: White matter tracts and diffuse
Lower-grade gliomas: The pivotal role of myelin plasticity in the
tumor pathogenesis, infiltration patterns, functional consequences
and therapeutic management. Front Oncol. 12:8555872022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Herbet G, Moritz-Gasser S, Boiseau M,
Duvaux S, Cochereau J and Duffau H: Converging evidence for a
cortico-subcortical network mediating lexical retrieval. Brain.
139:3007–3021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
DE Benedictis A, Marras CE, Petit L and
Sarubbo S: The inferior Fronto-occipital fascicle: A century of
controversies from anatomy theaters to operative neurosurgery. J
Neurosurg Sci. 65:605–615. 2021.PubMed/NCBI
|
|
62
|
Vassal F, Pommier B, Sontheimer A and
Lemaire JJ: Inter-individual variations and hemispheric asymmetries
in structural connectivity patterns of the inferior
Fronto-occipital fascicle: A diffusion tensor imaging tractography
study. Surg Radiol Anat. 40:129–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Altieri R, Melcarne A, Junemann C, Zeppa
P, Zenga F, Garbossa D, Certo F and Barbagallo G: Inferior
Fronto-occipital fascicle anatomy in brain tumor surgeries: From
anatomy lab to surgical theater. J Clin Neurosci. 68:290–294. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Camins À, Naval-Baudin P, Majós C,
Sierpowska J, Sanmillan JL, Cos M, Rodriguez-Fornells A and
Gabarrós A: Inferior Fronto-occipital fascicle displacement in
temporoinsular gliomas using diffusion tensor imaging. J
Neuroimaging. 32:638–646. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Heller C, Steinmann S, Levitt JJ, Makris
N, Antshel KM, Fremont W, Coman IL, Schweinberger SR, Weiß T, Bouix
S, et al: Abnormalities in white matter tracts in the
Fronto-striatal-thalamic circuit are associated with verbal
performance in 22q11.2DS. Schizophr Res. 224:141–150. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Levitt JJ, Zhang F, Vangel M, Nestor PG,
Rathi Y, Kubicki M, Shenton ME and O'Donnell LJ: The organization
of frontostriatal brain wiring in healthy subjects using a novel
diffusion imaging fiber cluster analysis. Cereb Cortex.
31:5308–5318. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kinoshita M, de Champfleur NM, Deverdun J,
Moritz-Gasser S, Herbet G and Duffau H: Role of Fronto-striatal
tract and frontal aslant tract in movement and speech: An axonal
mapping study. Brain Struct Funct. 220:3399–3412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
La Corte E, Eldahaby D, Greco E, Aquino D,
Bertolini G, Levi V, Ottenhausen M, Demichelis G, Romito LM, Acerbi
F, et al: The frontal aslant tract: A systematic review for
neurosurgical applications. Front Neurol. 12:6415862021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Landers MJF, Meesters SPL, van Zandvoort
M, de Baene W and Rutten GM: The frontal aslant tract and its role
in executive functions: A quantitative tractography study in glioma
patients. Brain Imaging Behav. 16:1026–1039. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Müller DMJ, Robe PAJT, Eijgelaar RS, Witte
MG, Visser M, de Munck JC, Broekman MLD, Seute T, Hendrikse J,
Noske DP, et al: Comparing Glioblastoma surgery decisions between
teams using brain maps of tumor locations, biopsies, and
resections. JCO Clin Cancer Inform. 3:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cancer Genome Atlas Research Network, .
Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA,
Rheinbay E, Miller CR, Vitucci M, et al: Comprehensive, integrative
genomic analysis of diffuse Lower-grade gliomas. N Engl J Med.
372:2481–2498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yabo YA, Niclou SP and Golebiewska A:
Cancer cell heterogeneity and plasticity: A paradigm shift in
glioblastoma. Neuro Oncol. 24:669–682. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Qi S, Yu L, Li H, Ou Y, Qiu X, Ding Y, Han
H and Zhang X: Isocitrate dehydrogenase mutation is associated with
tumor location and magnetic resonance imaging characteristics in
astrocytic neoplasms. Oncol Lett. 7:1895–1902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fathi Kazerooni A, Bakas S, Saligheh Rad H
and Davatzikos C: Imaging signatures of glioblastoma molecular
characteristics: A radiogenomics review. J Magn Reson Imaging.
52:54–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
J Dabrowski M and Wojtas B: Global DNA
methylation patterns in human gliomas and their interplay with
other epigenetic modifications. Int J Mol Sci. 20:34782019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Klughammer J, Kiesel B, Roetzer T,
Fortelny N, Nemc A, Nenning KH, Furtner J, Sheffield NC, Datlinger
P, Peter N, et al: The DNA methylation landscape of glioblastoma
disease progression shows extensive heterogeneity in time and
space. Nat Med. 24:1611–1624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
de Souza CF, Sabedot TS, Malta TM, Stetson
L, Morozova O, Sokolov A, Laird PW, Wiznerowicz M, Iavarone A,
Snyder J, et al: A Distinct DNA methylation shift in a subset of
Glioma CpG island methylator phenotypes during tumor recurrence.
Cell Rep. 23:637–651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lucas CG, Mueller S, Reddy A, Taylor JW,
Oberheim Bush NA, Clarke JL, Chang SM, Gupta N, Berger MS, Perry A,
et al: Diffuse hemispheric glioma, H3 G34-mutant: Genomic landscape
of a new tumor entity and prospects for targeted therapy. Neuro
Oncol. 23:1974–1976. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gatto L, Franceschi E, Tosoni A, Di Nunno
V, Tonon C, Lodi R, Agati R, Bartolini S and Brandes AA: Beyond
imaging and genetic signature in Glioblastoma: Radiogenomic
holistic approach in Neuro-oncology. Biomedicines. 10:32052022.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cui M, Gao X, Chi Y, Zhang M, Lin H, Chen
H, Sun C and Ma X: Molecular alterations and their correlation with
the survival of glioblastoma patients with corpus callosum
involvement. Front Neurosci. 15:7014262021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kannan S, Murugan AK, Balasubramanian S,
Munirajan AK and Alzahrani AS: Gliomas: Genetic alterations,
mechanisms of metastasis, recurrence, drug resistance, and recent
trends in molecular therapeutic options. Biochem Pharmacol.
201:1150902022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
El Atat O, Naser R, Abdelkhalek M, Habib
RA and El Sibai M: Molecular targeted therapy: A new avenue in
glioblastoma treatment. Oncol Lett. 25:462022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jiang H, Yu K, Cui Y, Ren X, Li M, Zhang
G, Yang C, Zhao X, Zhu Q and Lin S: Differential predictors and
clinical implications associated with long-term survivors in IDH
Wildtype and mutant Glioblastoma. Front Oncol. 11:6326632021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Senhaji N, Louati S, Chbani L, El Fatemi
H, Hammas N, Mikou K, Maaroufi M, Benzagmout M, Boujraf S, El
Bardai S, et al: EGFR amplification and IDH mutations in
glioblastoma patients of the northeast of morocco. Biomed Res Int.
2017:80458592017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Solomou G, Finch A, Asghar A and Bardella
C. Mutant IDH in Gliomas: Role in cancer and treatment options.
Cancers (Basel). 15:28832023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sharma N, Mallela AN, Shi DD, Tang LW,
Abou-Al-Shaar H, Gersey ZC, Zhang X, McBrayer SK and Abdullah KG:
Isocitrate dehydrogenase mutations in gliomas: A review of current
understanding and trials. Neurooncol Adv. 5:vdad0532023.PubMed/NCBI
|
|
87
|
Kudulaiti N, Zhang H, Qiu T, Lu J,
Aibaidula A, Zhang Z, Guan Y and Zhuang D: The Relationship between
IDH1 mutation status and metabolic imaging in nonenhancing
supratentorial diffuse gliomas: A 11C-MET PET study. Mol
Imaging. 18:15360121198940872019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kopal J, Kumar K, Shafighi K, Saltoun K,
Modenato C, Moreau CA, Huguet G, Jean-Louis M, Martin CO, Saci Z,
et al: Using rare genetic mutations to revisit structural brain
asymmetry. bioRxiv. Apr 18–2023.(Epub ahead of print). doi:
10.1101/2023.04.17.537199.
|
|
89
|
Xu H, Zong H, Ma C, Ming X, Shang M, Li K,
He X, Du H and Cao L: Epidermal growth factor receptor in
glioblastoma. Oncol Lett. 14:512–516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Oprita A, Baloi SC, Staicu GA, Alexandru
O, Tache DE, Danoiu S, Micu ES and Sevastre AS: Updated insights on
EGFR signaling pathways in glioma. Int J Mol Sci. 22:5872021.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ezzati S, Salib S, Balasubramaniam M and
Aboud O: Epidermal growth factor receptor inhibitors in
glioblastoma: Current status and future possibilities. Int J Mol
Sci. 25:23162024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Moghaddam M, Vivarelli S, Falzone L, Libra
M and Bonavida B: Cancer resistance via the downregulation of the
tumor suppressors RKIP and PTEN expressions: Therapeutic
implications. Explor Target Antitumor Ther. 4:170–207. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Dogan E, Yildirim Z, Akalin T, Ozgiray E,
Akinturk N, Aktan C, Solmaz AE, Biceroglu H, Caliskan KE, Ertan Y,
et al: Investigating the effects of PTEN mutations on cGAS-STING
pathway in glioblastoma tumours. J Neurooncol. 166:283–292. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Della Monica R, Cuomo M, Buonaiuto M,
Costabile D, Franca RA, Del Basso De Caro M, Catapano G, Chiariotti
L and Visconti R: MGMT and Whole-genome DNA methylation impacts on
diagnosis, prognosis and therapy of glioblastoma multiforme. Int J
Mol Sci. 23:71482022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Falzone L, Bordonaro R and Libra M:
SnapShot: Cancer chemotherapy. Cell. 186:18162023. View Article : Google Scholar
|
|
96
|
Alnahhas I, Alsawas M, Rayi A, Palmer JD,
Raval R, Ong S, Giglio P, Murad MH and Puduvalli V: Characterizing
benefit from temozolomide in MGMT promoter unmethylated and
methylated glioblastoma: A systematic review and meta-analysis.
Neurooncol Adv. 2:vdaa0822020.PubMed/NCBI
|
|
97
|
Ellingson BM, Cloughesy TF, Pope WB, Zaw
TM, Phillips H, Lalezari S, Nghiemphu PL, Ibrahim H, Naeini KM,
Harris RJ, et al: Anatomic localization of O6-methylguanine DNA
methyltransferase (MGMT) promoter methylated and unmethylated
tumors: A radiographic study in 358 de novo human glioblastomas.
Neuroimage. 59:908–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li P, Ensink E, Lang S, Marshall L,
Schilthuis M, Lamp J, Vega I and Labrie V: Hemispheric asymmetry in
the human brain and in Parkinson's disease is linked to divergent
epigenetic patterns in neurons. Genome Biol. 21:612020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang Y, Dube C, Gibert M Jr, Cruickshanks
N, Wang B, Coughlan M, Yang Y, Setiady I, Deveau C, Saoud K, et al:
The p53 pathway in glioblastoma. Cancers (Basel). 10:2972018.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang YY, Zhang T, Li SW, Qian TY, Fan X,
Peng XX, Ma J, Wang L and Jiang T: Mapping p53 mutations in
low-grade glioma: A voxel-based neuroimaging analysis. AJNR Am J
Neuroradiol. 36:70–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang T, Wang Y, Fan X, Ma J, Li S, Jiang
T and Wang L: Anatomical localization of p53 mutated tumors: A
radiographic study of human glioblastomas. J Neurol Sci. 346:94–98.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Marutani M, Tonoki H, Tada M, Takahashi M,
Kashiwazaki H, Hida Y, Hamada J, Asaka M and Moriuchi T:
Dominant-negative mutations of the tumor suppressor p53 relating to
early onset of glioblastoma multiforme. Cancer Res. 59:4765–4769.
1999.PubMed/NCBI
|
|
103
|
Scheer M, Leisz S, Sorge E, Storozhuk O,
Prell J, Ho I and Harder A: Neurofibromatosis type 1 gene
alterations define specific features of a subset of glioblastomas.
Int J Mol Sci. 23:3522021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Costa AA and Gutmann DH: Brain tumors in
neurofibromatosis type 1. Neurooncol Adv. 1:vdz0402019.PubMed/NCBI
|
|
105
|
Lobbous M, Bernstock JD, Coffee E,
Friedman GK, Metrock LK, Chagoya G, Elsayed G, Nakano I, Hackney
JR, Korf BR, et al: An update on neurofibromatosis type
1-associated gliomas. Cancers (Basel). 12:1142020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Carlotto BS, Trevisan P, Provenzi VO,
Soares FP, Rosa RFM, Varella-Garcia M and Zen PRG: PDGFRA, KIT, and
KDR gene amplification in glioblastoma: Heterogeneity and clinical
significance. Neuromolecular Med. 25:441–450. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hu W, Duan H, Zhong S, Zeng J and Mou Y:
High frequency of PDGFRA and MUC family gene mutations in diffuse
hemispheric glioma, H3 G34-mutant: A glimmer of hope? J Transl Med.
20:642022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Haase S, Garcia-Fabiani MB, Carney S,
Altshuler D, Núñez FJ, Méndez FM, Núñez F, Lowenstein PR and Castro
MG: Mutant ATRX: Uncovering a new therapeutic target for glioma.
Expert Opin Ther Targets. 22:599–613. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Meng L, Zhang R, Fa L, Zhang L, Wang L and
Shao G: ATRX status in patients with gliomas: Radiomics analysis.
Medicine (Baltimore). 101:e301892022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Silantyev AS, Falzone L, Libra M, Gurina
OI, Kardashova KS, Nikolouzakis TK, Nosyrev AE, Sutton CW, Mitsias
PD and Tsatsakis A: Current and future trends on diagnosis and
prognosis of glioblastoma: From molecular biology to proteomics.
Cells. 8:8632019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang S, Su X, Kemp GJ, Yang X, Wan X, Tan
Q, Yue Q and Gong Q: Two patterns of white matter connection in
multiple gliomas: Evidence from probabilistic fiber tracking. J
Clin Med. 11:36932022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Genc S, Pennisi M, Yeni Y, Yildirim S,
Gattuso G, Altinoz MA, Taghizadehghalehjoughi A, Bolat I, Tsatsakis
A, Hacımüftüoğlu A, et al: Potential neurotoxic effects of
Glioblastoma-derived exosomes in primary cultures of cerebellar
neurons via oxidant stress and glutathione depletion. Antioxidants
(Basel). 11:12252022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yeni Y, Taghizadehghalehjoughi A, Genc S,
Hacimüftüoglu A, Yıldırım S and Bolat I: Glioblastoma cell-derived
exosomes induce cell death and oxidative stress in primary cultures
of olfactory neurons. Role of redox stress. Mol Biol Rep.
50:3999–4009. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Stella M, Falzone L, Caponnetto A, Gattuso
G, Barbagallo C, Battaglia R, Mirabella F, Broggi G, Altieri R,
Certo F, et al: Serum extracellular Vesicle-derived circHIPK3 and
circSMARCA5 Are two novel diagnostic biomarkers for glioblastoma
multiforme. Pharmaceuticals (Basel). 14:6182021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Candido S, Lupo G, Pennisi M, Basile MS,
Anfuso CD, Petralia MC, Gattuso G, Vivarelli S, Spandidos DA, Libra
M, et al: The analysis of miRNA expression profiling datasets
reveals inverse microRNA patterns in glioblastoma and Alzheimer's
disease. Oncol Rep. 42:911–922. 2019.PubMed/NCBI
|
|
116
|
Zeng F, Fan Z, Li S, Li L, Sun T, Qiu Y,
Nie L and Huang G: Tumor microenvironment activated
photoacoustic-fluorescence bimodal nanoprobe for precise
Chemo-immunotherapy and immune response tracing of glioblastoma.
ACS Nano. 17:19753–19766. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Peng Y, Liu Y, Lu X, Wang S, Chen M, Huang
W, Wu Z, Lu G and Nie L: Ag-hybridized plasmonic Au-triangular
nanoplates: Highly sensitive photoacoustic/Raman evaluation and
improved antibacterial/photothermal combination therapy. J Mater
Chem B. 6:2813–2820. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Valenzuela-Fuenzalida JJ, Moyano-Valarezo
L, Silva-Bravo V, Milos-Brandenberg D, Orellana-Donoso M,
Nova-Baeza P, Suazo-Santibáñez A, Rodríguez-Luengo M,
Oyanedel-Amaro G, Sanchis-Gimeno J, et al: Association between the
anatomical location of glioblastoma and its evaluation with
clinical considerations: A systematic review and meta-analysis. J
Clin Med. 13:34602024. View Article : Google Scholar : PubMed/NCBI
|