Introduction
Colorectal cancer (CRC) is the third most common
malignant tumor globally in terms of incidence and mortality, with
>1.85 million diagnoses and 850,000 deaths annually (1). Despite significant progress in
surveillance and the development of personalized therapeutic
strategies, the 5-year survival rate for patients with CRC remains
<20%. Identifying and screening more effective therapeutic
agents is therefore of paramount importance in the clinical
management of CRC (2).
MicroRNAs (miRNAs or miRs), a class of short
non-coding RNAs comprising 20–22 nucleotides, play a pivotal role
in gene regulation by targeting mRNAs for translational repression
or degradation (3). The
dysregulation of miRNAs has been implicated in numerous
pathological processes associated with human cancers, including the
regulation of cell proliferation and apoptosis (4,5).
Several miRNAs have been identified as key players in the
pathogenesis of CRC (6,7). For instance, Zhou et al
(8) demonstrated that miR-483
fostered CRC cell proliferation in vivo, while Lin et
al (9) reported that miR-202,
which was under-expressed in CRC tissues, suppressed CRC cell
proliferation and invasion by targeting the ubiquitin-like with PHD
and RING finger domain 1 (UHRF1) (9). Intriguingly, miR-25 had been
recognized as an oncogenic miRNA across a spectrum of malignancies,
including those of the lung, stomach and liver (10–12).
Although miR-25 is known to be overexpressed in CRC and associated
with tumor growth (13), the
downstream mechanisms by which it influences the pathological
processes of CRC remain to be fully elucidated.
F-box and WD repeat containing domain 7 (FBXW7) is
one of the best-studied multiprotein ubiquitin E3 ligases. Due to
its involvement in the ubiquitination and degradation of numerous
oncoproteins, including c-Myc, Cyclin E, Notch and numerous others,
FBXW7 has been demonstrated in numerous studies to act as a tumor
suppressor (14–16). Low FBXW7 expression has been linked
to subpar clinical outcomes for patients with CRC, and FBXW7 mRNA
expression is considerably downregulated in tumor tissues in CRC
(17). Additionally, FBXW7 reduced
colon cancer cell migration and proliferation as a result of
abnormal enolase 1 expression and activity (18). Despite these findings, the
regulatory mechanisms governing FBXW7 in CRC remain to be fully
elucidated. Prior studies had suggested that a subset of miRNAs,
including miR-223, miR-25, miR-92 and miR-27b, may exert their
antitumor effects by directly binding to the 3′ untranslated region
(3′UTR) of FBXW7, thereby inhibiting its activity and downstream
targets across various cancer types (19–22).
Consequently, further investigation was warranted to improve
understanding of the role and underlying processes of Fbxw7 in the
pathogenesis of CRC.
Utilizing microarray data obtained from the Gene
Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/), a comprehensive
screening of differentially expressed miRNAs was conducted in the
present study. Among these, miR-25-3p stood out as the most
significantly upregulated miRNA and was thus selected for in-depth
analysis. To elucidate the functional contributions of miR-25-3p in
CRC cells and to unravel the molecular mechanisms at play, a series
of functional assays were meticulously performed. The current
investigation has uncovered evidence suggesting that miR-25-3p may
be instrumental in driving the oncogenic processes associated with
the development of CRC.
Materials and methods
Cell culture and tissue samples
Human normal colorectal mucosa NCM460 cell line (cat
no. SNL-519; SUNNCELL Co., Ltd.), four colon cancer cell lines,
namely HCT116 (cat no. CCL-247EMT), SW480 (cat no. CCL-228), SW620
(cat no. CCL-227) and Caco-2 (cat no. HTB-37), and 293T (cat no.
CRL-3216) cells were purchased from American Type Culture
Collection. These cell lines were cultured in high-glucose DMEM
(Thermo Fisher Scientific, Inc.) containing 10% FBS (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(Sigma-Aldrich; Merck KGaA) in a 37°C incubator with 5%
CO2.
Between January 2020 and July 2021, 50 CRC tissues
and associated normal tissues were obtained from the Department of
Colorectal Surgery, Zhejiang Provincial People's Hospital
(Hangzhou, China). Before surgery, neither radiation nor
chemotherapy had been administered to any of the patients. The
adjacent normal tissues that were >5 cm from the tumor's margin
were received. The present study was approved (approval no.
2020059) by the ethical committee of the Zhejiang Provincial
People's Hospital (Hangzhou, China). Every participant signed a
statement of informed consent. Clinicopathological characteristics
of patients with CRC (including sex and age distribution) are
presented in Table I.
 | Table I.Associations of miR-25-3p expression
with the clinicopathological characteristics of colorectal
cancer. |
Table I.
Associations of miR-25-3p expression
with the clinicopathological characteristics of colorectal
cancer.
|
|
| Expression level of
miR-25-3p |
|
|---|
| Clinicopathological
characteristics | All cases
(n=50) |
|
|
|---|
| High (31) | Low (19) | P-value |
|---|
| Sex |
|
|
| 0.771 |
|
Male | 25 | 16 | 9 |
|
|
Female | 25 | 15 | 10 |
|
| Age, years |
|
|
| 0.320 |
|
≥60 | 37 | 21 | 16 |
|
|
<60 | 13 | 10 | 3 |
|
| Location |
|
|
| 0.812 |
|
Proximal colon | 30 | 19 | 11 |
|
| Distal
colon and rectum | 20 | 12 | 8 |
|
| Tumor size, cm |
|
|
| 0.029 |
| ≥5 | 33 | 24 | 9 |
|
|
<5 | 17 | 7 | 10 |
|
| cTNM stage |
|
|
| 0.006 |
| I +
II | 15 | 5 | 10 |
|
| III +
IV | 35 | 26 | 9 |
|
| Distant
metastasis |
|
|
| 0.011 |
|
Absent | 28 | 13 | 15 |
|
|
Present | 22 | 18 | 4 |
|
| Histological
grade |
|
|
| 0.285 |
| Well
and moderate | 19 | 10 | 9 |
|
|
Poorly | 31 | 21 | 10 |
|
| Lymph node
metastasis |
|
|
| 0.057 |
|
Present | 27 | 20 | 7 |
|
|
Absent | 23 | 11 | 12 |
|
MicroRNA expression profile data from
GEO
The microRNA data (accession nos. GSE183437 and
GSE156719) were downloaded from GEO databases in NCBI (23). Microarray data of GSE183437 was on
account of GPL16791 Platform Illumina HiSeq 2500 (Homo
sapiens), which included 5 CRC tissues and 5 non-cancerous
tumor-adjacent tissues, while GSE156719 was on account of GPL20712
Platform Agilent-070156 human miRNA [miRNA version], which included
3 pairs of colorectal tumor tissues and normal tissues.
Differentially expressed miRNAs (DEmiRNAs) were identified through
GEO2R online platform (https://www.ncbi.nlm.nih.gov/geo/geo2r/), which is a
widely used tool that can be used to analyze data from any GEO
series and significance analysis of microarray (SAM), to determine
the differential expression of miRNAs among groups. miRNAs were
considered to be differentially expressed according to the
P<0.05 threshold from the limma analysis and median false
discovery rate <0.05 from SAM. miRs with the top 46 differences
were selected for heat mapping using GeneSpring GX statistical
software (version 7.3; Agilent Technologies, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA of the cultured cells and the tissues
was extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.), and total RNA was reverse-transcribed to cDNA
using the iScript advanced cDNA Synthesis Kit (Bio-Rad
Laboratories, Inc.) according to the manufacturer's protocol.
RT-qPCR was performed using SYBR®PrimeScript™ RT-PCR kit
(Takara Bio, Inc.) on the Applied Biosystems Quantstudio6 flex
(Applied Biosystems; Thermo Fisher Scientific, Inc.). For detection
of microRNAs, a universal reverse primer that is complementary to a
sequence within the RT stem-loop primer is
5′-GCAGGGTCCGAGGTATTC-3′. The specific forward primers for
microRNAs were as follows: miR-25 forward,
5′-CATTGCACTTGTCTCGGT-3′; miR-18a forward,
5′-ACTGCCCTAAGTGCTCCT-3′; miR-92a forward,
5′-AATTATTGCACTTGTCCC-3′; miR-203 forward,
5′-GTGAAATGTTTAGGACCA-3′; miR-143 forward,
5′-TGAGATGAAGCACTGTAG-3′; miR-375 forward,
5′-TTTGTTCGTTCGGCTCGC-3′; miR-29a forward,
5′-TAGCACCATCTGAAATCG-3′; miR-137 forward,
5′-TTATTGCTTAAGAATACG-3′. The other qPCR primers used in the
present study were as follows: FBWX7 forward,
5′-CACTCAAAGTGTGGAATGCAGAGAC-3′ and reverse,
5′-GCATCTCGAGAACCGCTAACAA-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; and GAPDH forward,
5′-TCAACGACCCCTTCATTGACC-3′ and reverse,
5′-CTTCCCGTTGATGACAAGCTTC-3′. The thermocycling conditions were as
follows: 95°C for 5 min, followed by 40 cycles of 95°C for 10 sec
and 60°C for 30 sec. The fold changes were calculated using the
2−∆∆Cq method (24).
Transfection
When HCT116 and Caco-2 cells (5×105
cells/well) in six-well plates reached ~80% confluence, miR-25-3p
mimics (20 nM), mimics negative control (mimics NC, 20 nM),
miR-25-3p inhibitor (20 nM), inhibitor NC (20 nM), small
interfering (si) FBXW7 (50 nM) and si-Scramble (50 nM) were
transfected into cells at 37°C for 24 h using
Lipofectamine®2000 according to manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). siRNA, miR mimics and
miR inhibitors were provided by GenScript (Nanjing) Co., Ltd. The
sequences are as follows: miR-25-3p mimics,
5′-CAUUGCACUUGUCUCGGUCUGA-3′; mimics NC,
5′-CUGUAACUGCUCUGCUGUCGUA-3′; miR-25-3p inhibitor,
5′-UCAGACCGAGACAAGUGCAAUG-3′; inhibitor NC,
5′-UAGAUAGUGACGAGAAACCCCG-3′; siFBXW7, 5′-CAAUUGUGUAGACGAUAUACU-3′;
si-Scramble, 5′-UAUAUUCUCAUGAACAGGAUG-3′.
The FBXW7 gene was amplified by PCR utilizing human
cDNA extracted from adjacent normal tissues. The amplified
fragments were cloned into the pcDNA3.1(+) plasmid (Shanghai
GenePharma Co., Ltd.), named pcDNA3.1-FBXW7. Transfection was
conducted using Lipofectamine®2000 (Invitrogen; Thermo
Fisher Scientific, Inc.).
RT-qPCR analysis and western blotting were used to
detect the successful knockdown or upregulation of miR-25-3p and
FBXW7 expression, respectively.
Cellular proliferation assay
The proliferation of HCT116 and Caco-2 cells was
measured using a Cell Counting Kit-8 (CCK-8) kit (Dojindo Molecular
Technologies, Inc.). After 24, 48 and 72 h post-transfection, CCK-8
reagent (10 µl/well) was added to HCT116 and Caco-2
(1×106 cells/well), and then incubated at 37°C with 5%
CO2 for another 3 h. Subsequently, cell proliferation
was analyzed at 450 nm, using a microplate reader (Multiskan
SkyHigh; Thermo Fisher Scientific, Inc.).
Apoptosis assay
HCT116 and Caco-2 cells (5×105 cells)
were seeded in 6-well plates overnight at 37°C. Apoptotic rates
were determined using the Annexin V-FITC Apoptosis Detection Kit
(cat. no. ab14082; Abcam) 48 h post-transfection. Cells were
digested with 0.25% trypsin (Millipore Sigma; Merck KGaA),
centrifuged at 300 × g for 5 min at 4°C, resuspended in 20 µl
binding buffer, and incubated with 5 µl Annexin V-FITC and 1 µl
Propidium Iodide (PI) in a dark room at room temperature for 20
min. The stained cells were then analyzed using BD FACSCalibur Flow
Cytometer System (BD Biosciences). Data analysis was performed
using BD FACSuite™ software (Version 6.0; BD Biosciences). The
results demonstrated healthy viable cells in the lower left
quadrant on the scatter plot as (FITC−/PI−).
The lower right quadrant (Q3) represented the early-stage apoptotic
cells as (FITC+/PI−). The upper right
quadrant (Q2) represented late-stage apoptotic cells as
(FITC+/PI+). The calculation was made as
follows: Apoptotic rate=percentage of early-stage apoptotic cells
(Q3) + percentage of late-stage apoptotic cells (Q2).
Caspase 3 activity assay
Caspase-3 activity assay was performed in HCT116 and
Caco-2 cells using a Caspase-3 colorimetric assay kit (cat no.
556485; BD Biosciences) according to the manufacturer's
protocol.
Luciferase reporter gene assay
Using the online bioinformatics tools TargetScan 7.0
(http://www.targetscan.org/vert_70/)
and miRanda (v3.3a; http://www.microrna.org), the binding sites between
miR-25-3p and FBXW7 were predicted. The FBXW7 3′-UTR partial
sequence, which contains the binding site or mutated binding site,
was generated and inserted into a dual-luciferase pGL3 plasmid
(Promega Corporation) by GenScript (Nanjing) Co., Ltd. Using
Lipofectamine®2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), a total of 200 ng of either wild-type
(wt)-FBXW7-3′UTR-pGL3 or mutant (mut)-FBXW7-3′UTR-pGL3 reporter
plasmids were co-transfected with 40 nM miR-25-3p mimics, mimics
NC, miR-25-3p inhibitor, and inhibitor NC into 293T cells.
Luciferase assays were performed 48 h post transfection using the
Dual-luciferase® Reporter Assay System (Promega
Corporation). The luciferase activity was normalized to
Renilla luciferase activity.
Western blot analysis
Total protein was extracted from cells using the
RIPA lysis buffer 48 h after transfection (Beyotime Institute of
Biotechnology). A BCA protein assay reagent kit was used to measure
the protein concentration. Proteins (25 µg/lane) were separated by
SDS-PAGE using 8% gels and then electroblotted on PVDF membranes.
The membranes were blocked by soaking in 5% skimmed milk for 1 h at
4°C overnight. Specific primary antibodies were used to stain the
membranes at 4°C overnight, followed by the corresponding secondary
antibody at room temperature for 1 h. The enhanced
chemiluminescence (ECL) detection system (Pierce; Thermo Fisher
Scientific, Inc.) was used to identify protein bands, and ImageJ
software (version 1.46; National Institutes of Health) was used to
quantify them. The specific antibodies used in the present study
were as follows: FBXW7 (1:200; cat. no. ab105752; Abcam), c-Myc
(Y69; 1:1,000; cat no. ab32072; Abcam), Notch 2 (1:1,000; cat. no.
ab118824; Abcam), YAP (1:1,000; cat. no. ab52771; Abcam), cyclin E
(1:1,000; cat. no. ab33911; Abcam), β-actin (1:1,000; cat no.
ab8277; Abcam) and horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:5,000; cat no. ab6721;
Abcam).
Statistical analysis
SPSS 21.0 software (IBM Corp.) was used to conduct
the statistical analysis. The data are presented as the mean ±
standard deviation. One-way ANOVA was used to analyze comparisons
between various groups, and Tukey's post hoc test was used to
confirm the results. Using the unpaired Student's t-test, two
groups were compared. The correlation between miR-25-3p and
clinicopathological features of patients with CRC was analyzed
using the chi-square test and Fisher's exact test. The correlation
coefficient between miR-25-3p and FBXW7 was calculated using
Spearman's rank correlation coefficient. The Kaplan-Meier method
was used to create the survival curves, and the log-rank test was
used to compare them statistically. The threshold for statistical
significance was set at P<0.05.
Results
miR-25-3p is upregulated in CRC
tissues and associated with worse survival of patients with
CRC
To delve into the function of miRNAs in the context
of CRC, an analysis of DEmiRNAs from two GEO datasets was conducted
(GSE183437 and GSE156719) using the GEO2R online platform
(https://www.ncbi.nlm.nih.gov/geo/geo2r/). The present
analysis revealed a total of 46 dysregulated miRNAs in CRC tissues,
with 20 miRNAs exhibiting increased expression and 26 showing
decreased expression relative to the adjacent normal tissues
(Fig. 1A). Subsequently, further
validation of the five most significantly upregulated miRNAs
(miR-18a, miR-92a, miR-25, miR-203 and miR-143) and the three most
notably downregulated miRNAs (miR-375, miR-29a and miR-137) was
performed across the two GEO datasets. The significantly DEmiRNAs,
which were consistent with previous results (25–31),
were presented in Fig. 1B,
indicating the reliability of the screening results obtained by the
microarray analysis. Of these miRNAs, the expression of miR-25-3p
was found to be the most significantly increased in the CRC tissue.
miR-25-3p has previously been linked to tumorigenesis of CRC
(32). By contrast, little is known
about the roles of miR-25-3p for CRC. It was therefore decided to
focus on miR-25-3p for further molecular analyses, to clarify the
previously unknown role of miR-25-3p in CRC.
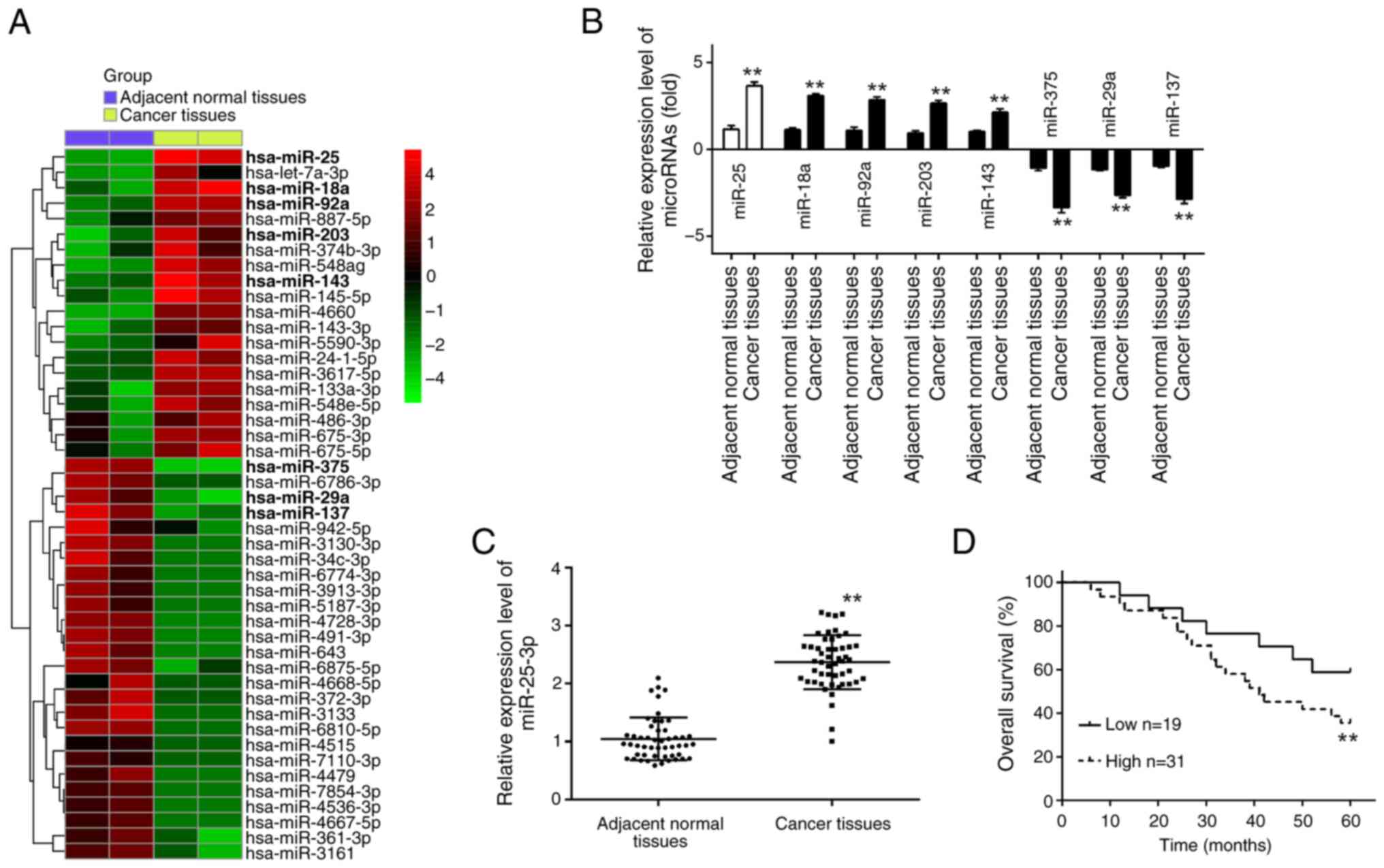 | Figure 1.miR-25-3p is upregulated in CRC
tissues. (A) Differentially expressed miRNAs were analyzed between
CRC cancer tissue and the adjacent normal tissue. Data were
retrieved from the Gene Expression Omnibus datasets, with the
accession numbers GSE183437 and GSE156719. The color code in the
heat map is linear and the expression levels of miRNAs that were
upregulated are shown in green to red, whereas the miRNAs that were
downregulated are shown from red to green. (B) The expression
levels of miR-18a, miR-92a, miR-25-3p, miR-203, miR-143, miR-375,
miR-29a and miR-137 were detected in CRC tissues and adjacent
normal tissues by RT-qPCR (n=3). Data represent the mean ± SD of
three independent experiments. (C) The expression levels of
miR-25-3p, were detected in CRC tissues and adjacent normal tissues
by RT-qPCR (n=50). Data represent the mean ± SD of three
independent experiments. (D) Kaplan-Meier survival curves of
patients with CRC according to the expression of miR-25-3p.
**P<0.01. miR or miRNA, microRNA; CRC, colorectal cancer;
RT-qPCR, reverse transcription-quantitative PCR. |
In order to investigate the relevance of miR-25-3p
in CRC, 50 pairs of CRC tissues and adjacent normal tissues were
utilized for the validation of its aberrant expression. RT-qPCR
analysis confirmed a significant upregulation of miR-25-3p in the
CRC tissues as opposed to the adjacent normal tissues. This
observation was in alignment with the differential expression
patterns observed in the GEO datasets, revealing a consistently
differential expression with the GEO datasets (Fig. 1C). Furthermore, the relationship
between miR-25-3p expression and clinicopathological features was
also analyzed. The samples were divided into high and low miRNA
expression groups according to the median expression value as the
cutoff point. It was found that high expression of miR-25-3p was
closely associated with tumor size, distant metastasis and
tumor-node-metastasis stage (Table
I). Furthermore, the association between miR-25-3p expression
and overall survival rate in CRC was investigated, and the overall
survival rate of patients with CRC with high miR-25-3p expression
was significantly shorter compared with low miR-25-3p expression
(Fig. 1D). Therefore, the findings
revealed that miR-25-3p might act as an oncogene in the development
of CRC.
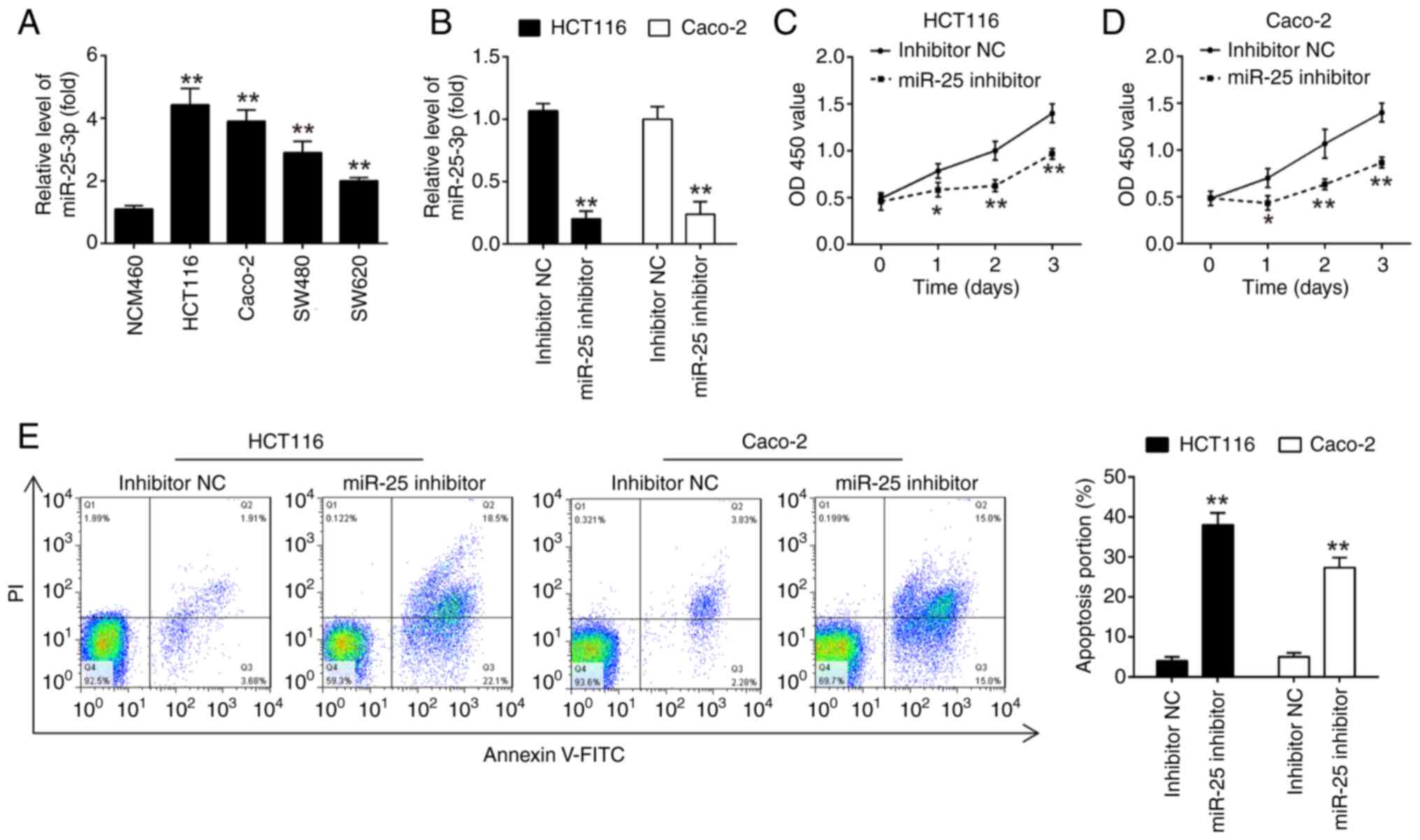
Knockdown of miR-25-3p inhibits CRC
cell proliferation and promotes cell apoptosis
Taken a step further, to validate whether the
altered expression of miR-25-3p was also present in CRC cell lines,
the expression of miR-25-3p was detected in HCT116, SW480, SW620
and Caco-2 cell lines and human normal colorectal mucosa NCM460
cell line was used as a control. It was found that miR-25-3p
expression was significantly upregulated in CRC cells compared with
NCM460 cells, which exhibited the same trend as the results in CRC
tissues (Fig. 2A).
To determine the effect of miR-25-3p on CRC cell
proliferation, HCT116 and Caco-2 cells were transfected with
miR-25-3p inhibitor. Post-transfection with the miR-25-3p
inhibitor, a significant reduction in miR-25-3p levels was observed
in both HCT116 and Caco-2 cells, as depicted in Fig. 2B. Moreover, the suppression of
miR-25-3p led to a significant decrease in cell proliferation and a
concurrent increase in the rate of apoptosis in HCT116 and Caco-2
cells when compared with the NC group (Fig. 2C-E). Collectively, these results
revealed that knockdown of miR-25-3p suppressed CRC cell
proliferation and promoted cell apoptosis.
FBXW7 is a direct of miR-25-3p in
CRC
To further explore the possible mechanisms of
miR-25-3p in CRC cells, potential target genes of miR-25-3p were
searched using TargetScan and miRanda databases. FBXW7 was chosen
as a target gene of miR-25-3p in the present study (Fig. 3A). RT-qPCR assay confirmed the
successful overexpression or knockdown of miR-25-3p after
transfection of miR-25-3p mimics or inhibitor, respectively, as
demonstrated in Fig. 3B. As it has
been previously reported, FBXW7 is a tumor suppressor with a role
in various types of human cancers, including CRC (33–35).
Additionally, a luciferase reporter assay was conducted to confirm
the interaction between miR-25-3p and the 3′UTR of FBXW7. As
illustrated in Fig. 3C, the
overexpression of miR-25-3p led to a significant decrease in
luciferase activity when the wt-FBXW7 3′UTR was present, whereas
the activity of the mut-FBXW7 3′UTR remained unaltered in 293T
cells. Conversely, the knockdown of miR-25-3p resulted in an
increase in luciferase activity for the wt-FBXW7 3′UTR. Moreover,
the overexpression of miR-25-3p in HCT116 and Caco-2 cells led to a
significant reduction in both mRNA and protein levels of FBXW7
(Fig. 3D and E), whereas the
knockdown of miR-25-3p yielded the opposite effect in mRNA level as
shown in Fig. 3D. These results
collectively suggested a direct and functional interaction between
miR-25-3p and FBXW7. Next, the relationship between miR-25-3p and
FBXW7 in CRC tissues was investigated. First, the level of FBXW7
mRNA in four CRC cell lines and 50 patients with CRC was measured
by RT-qPCR. The results of RT-qPCR showed that FBXW7 was
significantly decreased in the CRC cells and tissues compared with
NCM460 cells and the adjacent normal tissues, respectively
(Fig. 3F and G). Furthermore, as
revealed in Fig. 3H, there was an
inverse correlation between miR-25-3p and FBXW7 expression levels
in the 50 patients with CRC (r=−0.7989; P<0.01). These data
demonstrated that FBXW7 may be a functional target of miR-25-3p in
CRC.
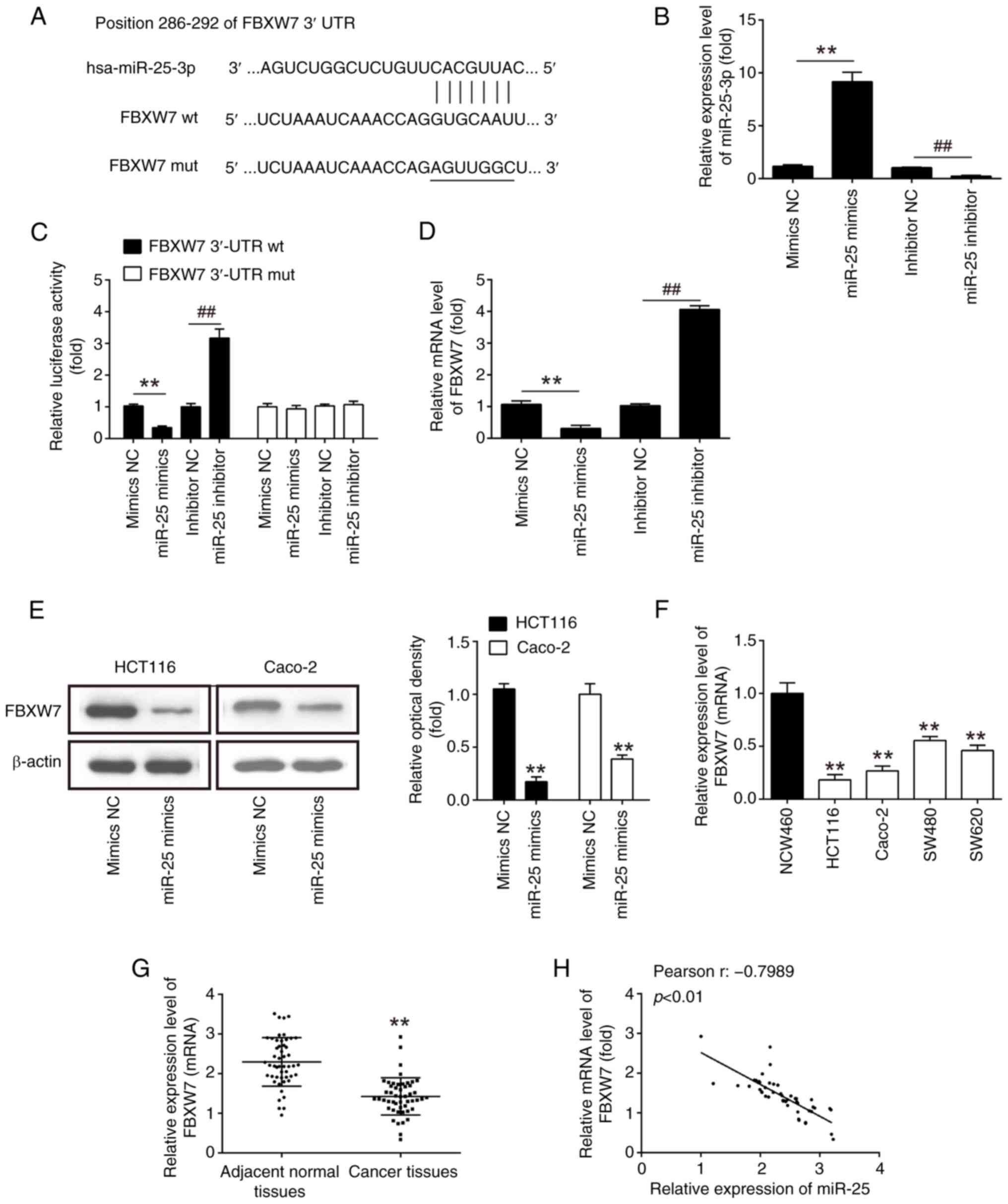 | Figure 3.FBXW7 is a direct target of
miR-25-3p. (A) Putative binding sites of miR-25-3p and FBXW7. (B)
Transfection efficiency of miR-25-3p mimics and inhibitor was
assessed by RT-qPCR analysis. (C) The relative luciferase activity
of FBXW7 wt or mut 3′UTR in 293T cells following transfection with
miR-25-3p mimics, mimics NC, miR-25-3p inhibitor or inhibitor NC,
as indicated (n=3). Data are presented as the mean ± SD of three
independent experiments. **P<0.01 vs. mimics NC;
##P<0.01 vs. inhibitor NC. (D and E) The mRNA and
protein expression of FBXW7 following transfection with miR-25-3p
mimics, mimics NC, miR-25-3p inhibitor, or inhibitor NC in HCT116
and Caco-2 cells were measured by RT-qPCR and western blot analysis
(n=3). **P<0.01 vs. mimics NC; ##P<0.01 vs.
inhibitor NC. (F) Expression of FBXW7 was examined in four CRC cell
lines and NCM460 cells, used as a control, by RT-qPCR analysis
(n=3). Data are presented as the mean ± SD of three independent
experiments. **P<0.01 vs. NCM460. (G) Expression of FBXW7 was
measured by RT-qPCR analysis in CRC tissues and adjacent normal
tissues (n=50). **P<0.01 vs. adjacent normal tissues. (H)
Pearson correlation analysis revealed a negative correlation
between the expression of FBXW7 and miR-25-3p (r = −0.7989;
P<0.01). FBXW7, F-box and WD repeat containing domain 7; miR,
microRNA; RT-qPCR, reverse transcription-quantitative PCR; wt,
wild-type; mut, mutant; UTR, untranslated region; NC, negative
control; CRC, colorectal cancer. |
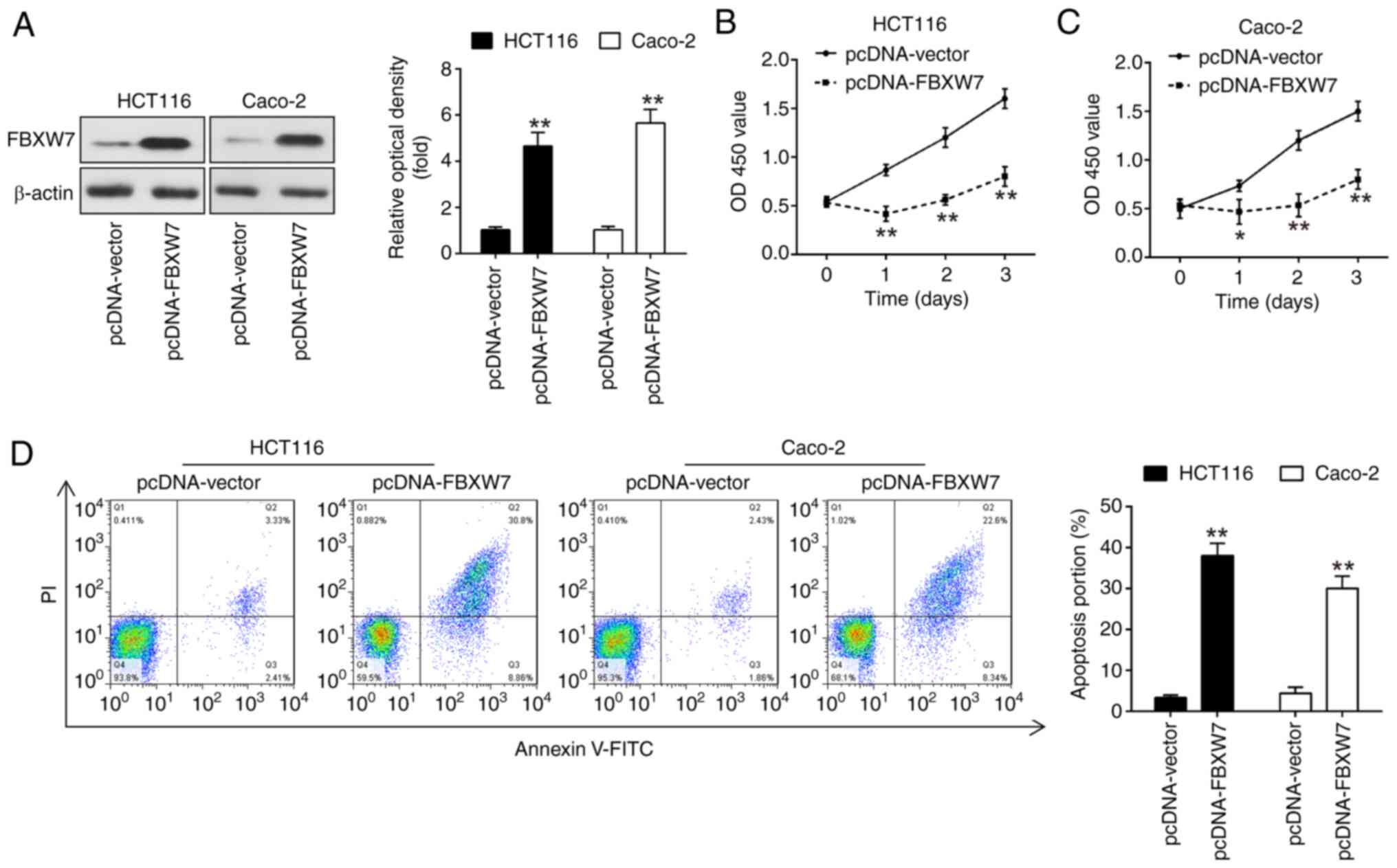
Overexpression of FBXW7 inhibits CRC
cell proliferation and promotes cell apoptosis
To elucidate the functional role of FBXW7 in CRC,
FBXW7 was overexpressed in CRC cell lines and the impact on cell
proliferation and apoptosis was subsequently assessed. For this
purpose, plasmids named pcDNA-FBXW7 were constructed and introduced
into HCT116 and Caco-2 cells. Western blot analysis confirmed the
successful overexpression of FBXW7 following the introduction of
the pcDNA-FBXW7 plasmids, as depicted in Fig. 4A. The overexpression of FBXW7 led to
a significant reduction of cell proliferation in both HCT116 and
Caco-2 cells, as determined by cell proliferation assays, compared
with the control group (Fig. 4B and
C). Moreover, the apoptotic rate in cells overexpressing FBXW7
was significantly higher than that observed in the control group
(Fig. 4D), underscoring the
potential of FBXW7 as a tumor suppressor in CRC. Overall, the
present findings revealed that FBXW7 overexpression inhibited CRC
cell proliferation and enhanced cell apoptosis.
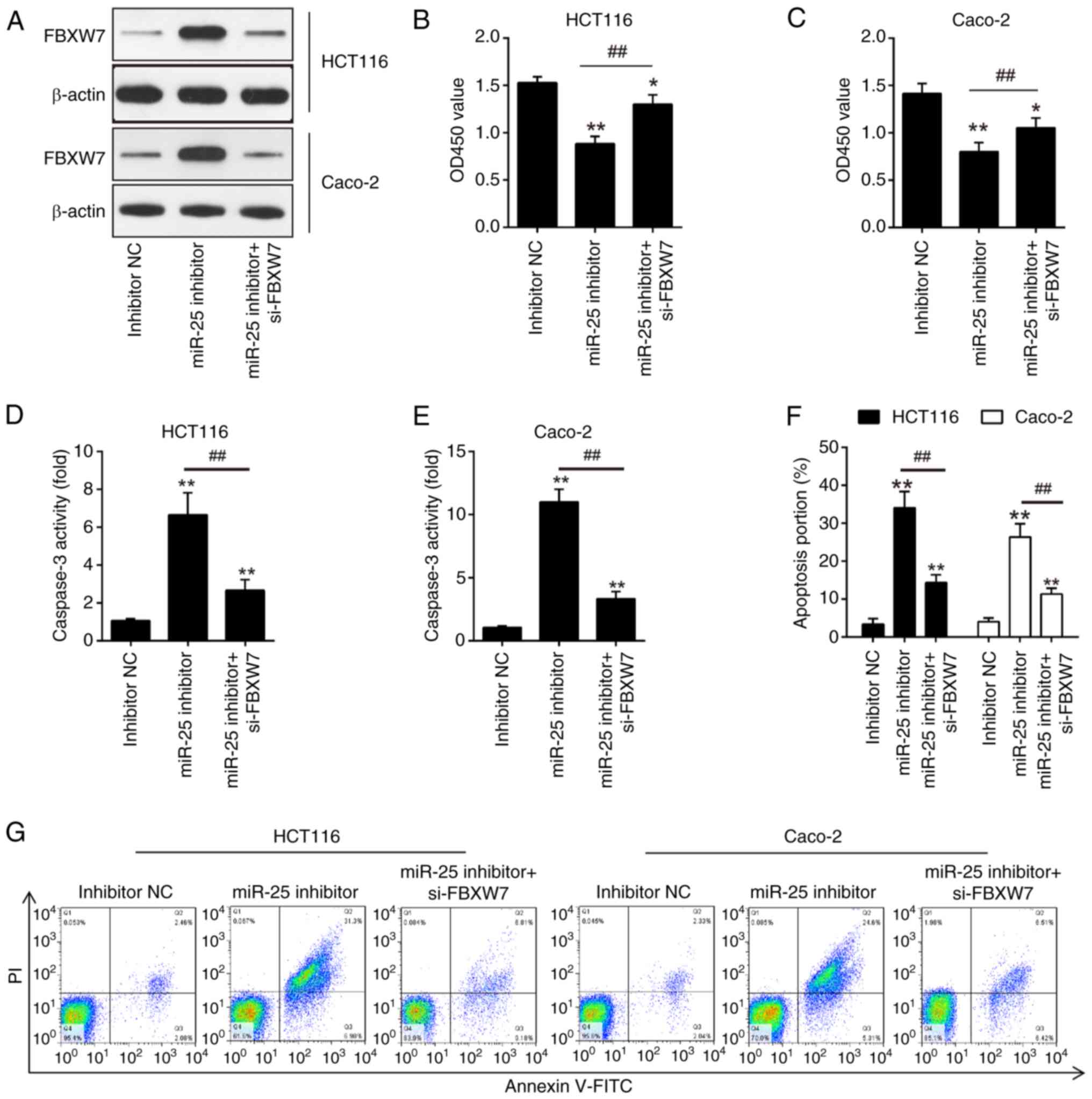
Knockdown of miR-25-3p exerts
antitumor effects by regulating FBXW7
It was anticipated that miR-25-3p targets FBXW7 in
colon cancer cells to have antitumor effects in light of the
aforementioned results showing that overexpression of FBXW7
decreased CRC cell proliferation and enhanced cell apoptosis.
HCT116 and Caco-2 cells received simultaneous transfections of
si-FBXW7 and miR-25-3p inhibitor. Western blot assay results
identified that FBXW7 was successfully knocked down by the si-FBXW7
(Fig. 5A). Then, apoptosis and
proliferation of CRC cells were investigated. The findings
demonstrated that miR-25-3p inhibitor-transfected HCT116 and Caco-2
cells showed reduced cell proliferation when compared with
inhibitor NC-transfected cells; however, these effects were
partially diminished by si-FBXW7 (Fig.
5B and C). In the caspase 3 activity assay, HCT116 and Caco-2
cells treated with the miR-25-3p inhibitor exhibited increased
caspase 3 activity compared with cells treated with the NC
inhibitor. However, the silencing of FBXW7 with si-FBXW7 negated
the enhanced caspase 3 activity observed with miR-25-3p inhibition
(Fig. 5D and E). A similar pattern
of apoptotic effects was observed in HCT116 and Caco-2 cells
following transfection with the miR-25-3p inhibitor, which were
counteracted by the concurrent knockdown of FBXW7 using si-FBXW7
(Fig. 5F and G). Collectively, the
present results indicated that the pro-apoptotic effects of
miR-25-3p inhibition on CRC cells are partially mitigated by the
suppression of FBXW7.
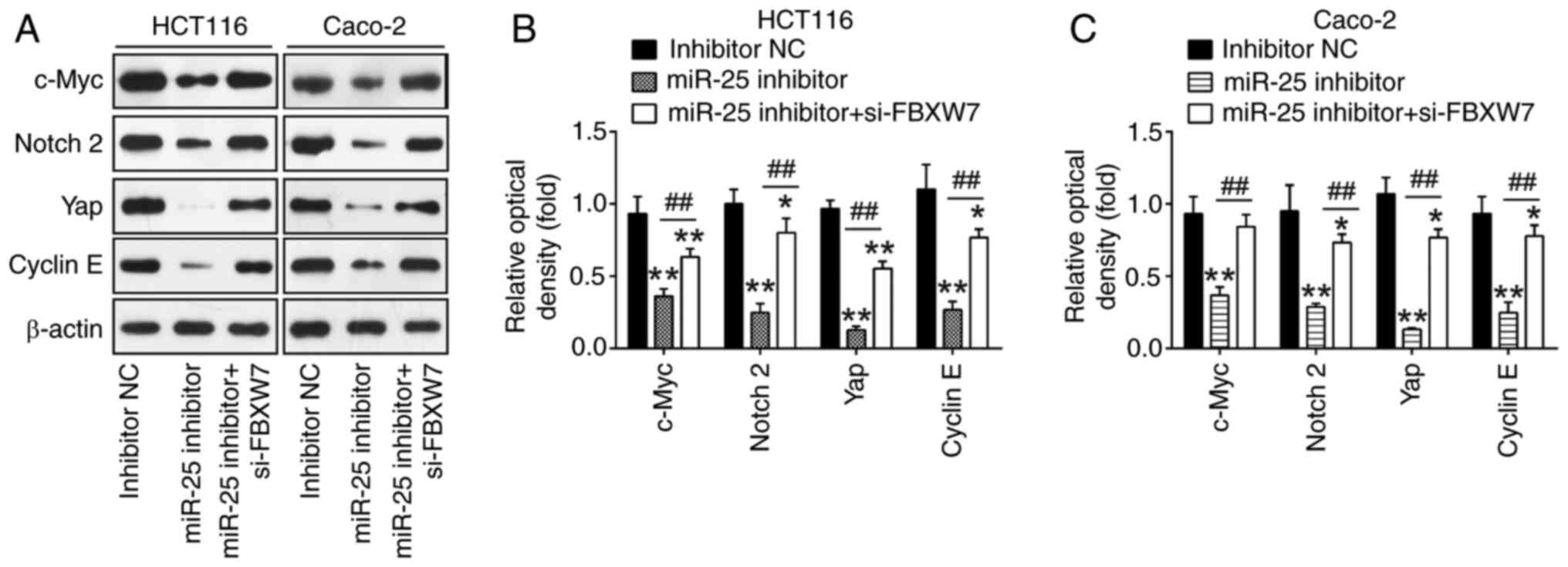
Knockdown of miR-25-3p influences
FBXW7-mediated degradation of the oncogenes
It has been revealed that FBXW7 is responsible for
the binding to and the degradation of several oncogenes, including
cyclin E, Yap, Notch and c-Myc, and thus inhibits tumor cell
proliferation and invasion, to exert its tumor-suppressive effects
(36–39). The FBXW7-related oncogenic proteins
were detected: cyclin E, Yap, Notch and c-Myc. Compared with the
inhibitor NC group, it was discovered that miR-25-3p knockdown
significantly reduced the expression of cyclin E, Yap, Notch and
c-Myc. However, these inhibitory effects were reversed by si-FBXW7
in HCT116 and Caco-2 cells (Fig.
6A-C). All of these findings pointed to the possibility that
miR-25-3p functions as an oncogene in CRC cells by suppressing
FBXW7 and subsequently indirectly promoting these oncoproteins.
Discussion
In the present study, it was found that miR-25-3p
expression was upregulated in CRC tissues and cell lines, and its
expression was strongly associated with the overall survival of
patients with CRC. In vitro, miR-25-3p downregulation
significantly reduced cell proliferation and increased cell
apoptosis. The oncogenic effects of miR-25-3p may also be partially
mediated by the inhibition of FBXW7 on the oncoproteins, as FBXW7
was discovered to be a target of miR-25-3p. These results indicated
that miR-25-3p targeting may be a possible treatment strategy for
patients with CRC.
Ectopic expression of miRNAs in CRC tissues plays
significant roles in the development of CRC, according to mounting
evidence (40–42). By targeting FBXL2 and triggering the
β-catenin signaling pathway, for instance, Pan et al
(43) revealed that miR-346-5p may
enhance CRC growth both in vivo and in vitro. By
targeting UHRF1, Lin et al (44) demonstrated that miR-202
overexpression prevented the growth and invasion of CRC. Due to
their high tissue specificity and part in carcinogenesis, miRNAs
can be employed for the diagnosis and prognosis monitoring of
patients with CRC, making them unique biomarkers for detecting
cancer and forecasting patient outcomes. For instance, it has been
shown that miR-21-5p, miR-92a-3p and their cluster have great
potential for early CRC screening (45). Consequently, identifying more miRNAs
that exhibit aberrant expression in CRC and tackling their
underlying biological pathways could prove beneficial in devising
treatment approaches and identifying CRC. miR-25-3p was found to be
one of the most increased miRNAs in CRC tissues after the
differentially expressed miRNAs were screened using the GSE183437
and GSE156719 microarray data that were retrieved from GEO and used
in the present study. miR-25-3p, which has previously been
identified, is important as an oncogene in several human
malignancies, including CRC (46).
It was identified that miR-25-3p was increased in CRC tissues and
that miR-25-3p levels beyond a certain threshold can serve as a
molecular predictor for poorer prognosis of patients with CRC.
miR-25-3p was discovered to function as an oncogene
in earlier investigations. For instance, Zhang et al
(47) reported that miR-25-3p
targeted PTEN to encourage the migration and invasion of esophageal
cancer cells and to suppress apoptosis via the PI3K/AKT pathway.
Through targeting EGR2, Yang et al (48) demonstrated that miR-25 boosted
gastric cancer cell proliferation and prevented their apoptosis. It
has been noted that miR-25-3p acts as an oncogenic miRNA in
osteosarcoma by targeting Merlin (49). Additionally, it was shown that
miR-25-3p, which functions as a tumor suppressor, was downregulated
in tongue squamous cell carcinoma (50). These results firmly establish the
dual roles of miR-25-3p as an antitumor and carcinogenic molecule
in a variety of human malignancies. miR-25-3p expression was
previously found to increase the proliferation and migration of
human colon cancer cells while inhibiting apoptosis in CRC
(32). In particular, miR-25-3p is
a possible prognostic sign for patients with CRC (51). The present study showed that
miR-25-3p knockdown accelerated apoptosis and decreased HCT116 and
Caco-2 cell proliferation, indicating that miR-25-3p may function
as an oncogene in CRC.
FBXW7, often referred to as hCDC4, serve as the SCF
E3 ubiquitin ligase's substrate recognition subunit (52). One of the most frequently
dysregulated ubiquitin-proteasome system proteins in human cancer,
FBXW7 is a crucial tumor suppressor (53,54).
It has been reported that certain oncoproteins including cyclin E,
c-Myc, Mcl-1, mTOR, Jun and Notch are degraded by the proteasome
under the supervision of FBXW7 (55,56).
Abnormal expression of these targets has been detected in several
human malignancies, including CRC (22). Previous studies have revealed that
the expression of FBXW7 plays a crucial part in the development of
CRC (57,58). For instance, Wei et al
(59) discovered that FBXW7
loss-of-function increased FASN-mediated lipogenesis and encouraged
the growth of CRC. The present study revealed that FBXW7 is a
target of miR-25-3p, and miR-25-3p knockdown in CRC cells inhibited
tumor cell proliferation and enhanced cell death by targeting
FBXW7. It was also confirmed that tumor suppressor actions of FBXW7
are achieved by regulating the proteolytic activity of oncogenic
substrates such as cyclin E, Yap, c-Myc and Notch2. In the future,
more investigations are needed to fully understand how FBXW7 and
its downstream pathways contribute to the development of CRC.
However, there are still some limitations to the
present study. Firstly, though not altered as markedly as
miR-25-3p, several miRNAs have also been screened; the authors'
ongoing experiments will mainly focus on the function and the
mechanism of these miRNAs in CRC. Secondly, other genes may be
targeted by miR-25-3p; FBXW7 is not unique as a target gene of
miR-25-3p. More importantly, the FBXW7 gene will be knocked out in
CRC cells to investigate the function of miR-25-3p, and further
verify the targeting relationship between miR-25-3p and FBXW7 in
animal models. Moreover, it was found that the level of miR-25-3p
varies among different CRC cell lines, which may be related to the
invasive ability of different tumor cells. In future research, this
correlation will be demonstrated to gain a more comprehensive
understanding of the important role of miR-25-3p in CRC.
In conclusion, the present study demonstrated that
the high level of miR-25-3p was associated with poor prognosis in
CRC, suggesting that miR-25-3p might be used as a potential
prognostic indicator in clinical CRC. Downregulation of miR-25-3p
suppressed the proliferation and promoted apoptosis of CRC cells by
targeting FBXW7. These findings demonstrated the carcinogenic
function of miR-25-3p and the underlying molecular mechanism behind
it, which may open the door to the development of more effective
and practical CRC treatments.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Public Welfare
Research Program of Zhejiang Provincial Science and Technology
Department (grant no. LTGY23H160033).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YC, BC, ST and HY performed all the experiments and
collected the data. HY and ST conceived and designed the study. YC
and HY wrote the main manuscript and analyzed the data. HY and ST
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2020059) by the ethical committee of the Zhejiang Provincial
People's Hospital (Hangzhou, China). Every participant signed a
statement of informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Biller LH and Schrag Dg: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Liu W, Zhao L, Güngör C, Xu Y, Song
X, Wang D, Zhou Z, Zhou Y, Li C, et al: Nomograms predicting
overall survival and cancer-specific survival for synchronous
colorectal liver-limited metastasis. J Cancer. 11:6213–6225. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Yin W and Chen P: MicroRNA-374a-5p
promotes metastasis of colorectal cancer by targeting GRB7.
Panminerva Med. 63:555–557. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu QD, Zhou QQ, Dong L, Huang Z, Wu F and
Deng X: MiR-199a-5p inhibits the growth and metastasis of
colorectal cancer cells by targeting ROCK1. Technol Cancer Res
Treat. 17:15330346187755092018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang N, Hu X, Du Y and Du J: The role of
miRNAs in colorectal cancer progression and chemoradiotherapy.
Biomed Pharmacother. 134:1110992021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santos DAR, Gaiteiro C, Santos M, Santos
L, Dinis-Ribeiro M and Lima L: MicroRNA biomarkers as promising
tools for early colorectal cancer screening-a comprehensive review.
Int J Mol Sci. 24:110232023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou W, Yang W, Duan L, Wang X, Lv P, Hu
Z, Zhao Y, Wu Z, Zhang Y and Hong L: MicroRNA-483 functions as an
oncogene in colorectal cancer. Ann Clin Lab Sci. 51:30–37.
2021.PubMed/NCBI
|
|
9
|
Lin Y, Chen Z, Lin S, Zheng Y, Liu Y, Gao
J and Chen S: MiR-202 inhibits the proliferation and invasion of
colorectal cancer by targeting UHRF1. Acta Biochim Biophys Sin
(Shanghai). 51:598–606. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu T, Chen W, Kong D, Li X, Lu H, Liu S,
Wang J, Du L, Kong Q, Huang X and Lu Z: miR-25 targets the
modulator of apoptosis 1 gene in lung cancer. Carcinogenesis.
36:925–935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li BS, Zuo QF, Zhao YL, Xiao B, Zhuang Y,
Mao XH, Wu C, Yang SM, Zeng H, Zou QM and Guo G: MicroRNA-25
promotes gastric cancer migration, invasion and proliferation by
directly targeting transducer of ERBB2, 1 and correlates with poor
survival. Oncogene. 34:2556–2565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su ZX, Zhao J, Rong ZH, Geng WM, Wu YG and
Qin CK: Upregulation of microRNA-25 associates with prognosis in
hepatocellular carcinoma. Diagn Pathol. 9:472014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Zhang Z and Gao Q: Transfer of
microRNA-25 by colorectal cancer cell-derived extracellular
vesicles facilitates colorectal cancer development and metastasis.
Mol Ther Nucleic Acids. 23:552–564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mo X, Shen X, Mo X, Yu F, Tan W, Deng Z,
He J, Luo Z, Chen Z and Yang J: CEMIP promotes small cell lung
cancer proliferation by activation of glutamine metabolism via
FBXW7/c-Myc-dependent axis. Biochem Pharmacol. 209:1154462023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis RJ, Welcker M and Clurman BE: Tumor
suppression by the Fbw7 ubiquitin ligase: Mechanisms and
opportunities. Cancer Cell. 26:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye Z, Zhuo Q, Hu Q, Xu X, Liu M, Zhang Z,
Xu W, Liu W, Fan G, Qin Y, et al: FBW7-NRA41-SCD1 axis
synchronously regulates apoptosis and ferroptosis in pancreatic
cancer cells. Redox Biol. 38:1018072021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iwatsuki M, Mimori K, Ishii H, Yokobori T,
Takatsuno Y, Sato T, Toh H, Onoyama I, Nakayama KI, Baba H and Mori
M: Loss of FBXW7, a cell cycle regulating gene, in colorectal
cancer: Clinical significance. Int J Cancer. 126:1828–1837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan P, Wang Y, Zhao S, Liu C, Wang Y, Wen
M, Mao JH, Wei G and Zhang P: FBXW7 negatively regulates ENO1
expression and function in colorectal cancer. Lab Invest.
95:995–1004. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grafals-Ruiz N, Sánchez-Álvarez AO,
Santana-Rivera Y, Lozada-Delgado EL, Rabelo-Fernandez RJ,
Rios-Vicil CI, Valiyeva F and Vivas-Mejia PE: MicroRNA-92b targets
tumor suppressor gene FBXW7 in glioblastoma. Front Oncol.
13:12496492023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun XF, Sun JP, Hou HT, Li K, Liu X and Ge
QX: MicroRNA-27b exerts an oncogenic function by targeting Fbxw7 in
human hepatocellular carcinoma. Tumour Biol. 37:15325–15332. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Mezayen H, Yamamura K, Yusa T, Nakao Y,
Uemura N, Kitamura F, Itoyama R, Yamao T, Higashi T, Hayashi H, et
al: MicroRNA-25 exerts an oncogenic function by regulating the
ubiquitin ligase Fbxw7 in hepatocellular carcinoma. Ann Surg Oncol.
28:7973–7982. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Ma T, Duan J, Liu X and Liu L:
MicroRNA-223-induced inhibition of the FBXW7 gene affects the
proliferation and apoptosis of colorectal cancer cells via the
Notch and Akt/mTOR pathways. Mol Med Rep. 23:1542021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hozaka Y, Kita Y, Yasudome R, Tanaka T,
Wada M, Idichi T, Tanabe K, Asai S, Moriya S, Toda H, et al:
RNA-sequencing based microRNA expression signature of colorectal
cancer: The impact of oncogenic targets regulated by miR-490-3p.
Int J Mol Sci. 22:98762021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang G, Wu X, Li S, Xu X, Zhu H and Chen
X: The long noncoding RNA CASC2 functions as a competing endogenous
RNA by sponging miR-18a in colorectal cancer. Sci Rep. 6:265242016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada NO and Senda T: Circulating
microRNA-92a-3p in colorectal cancer: A review. Med Mol Morphol.
54:193–202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hur K, Toiyama Y, Okugawa Y, Ide S, Imaoka
H, Boland CR and Goel A: Circulating microRNA-203 predicts
prognosis and metastasis in human colorectal cancer. Gut.
66:654–665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sahami-Fard MH, Kheirandish S and Sheikhha
MH: Expression levels of miR-143-3p and −424-5p in colorectal
cancer and their clinical significance. Cancer Biomark. 24:291–297.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu X, Chen X, Xu M, Liu X, Pan B, Qin J,
Xu T, Zeng K, Pan Y, He B, et al: miR-375-3p suppresses
tumorigenesis and partially reverses chemoresistance by targeting
YAP1 and SP1 in colorectal cancer cells. Aging (Albany NY).
11:7357–7385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mo WY and Cao SQ: MiR-29a-3p: A potential
biomarker and therapeutic target in colorectal cancer. Clin Transl
Oncol. 25:563–577. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding X, Zhang J, Feng Z, Tang Q and Zhou
X: MiR-137-3p inhibits colorectal cancer cell migration by
regulating a KDM1A-dependent epithelial-mesenchymal transition. Dig
Dis Sci. 66:2272–2282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li D, Zhang T, Lai J, Zhang J, Wang T,
Ling Y, He S and Hu Z: MicroRNA-25/ATXN3 interaction regulates
human colon cancer cell growth and migration. Mol Med Rep.
19:4213–4221. 2019.PubMed/NCBI
|
|
33
|
Pan Y, Liu J, Gao Y, Guo Y, Wang C, Liang
Z, Wu M, Qian Y, Li Y, Shen J, et al: FBXW7 loss of function
promotes esophageal squamous cell carcinoma progression via
elevating MAP4 and ERK phosphorylation. J Exp Clin Cancer Res.
42:752023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li N, Lorenzi F, Kalakouti E, Normatova M,
Babaei-Jadidi R, Tomlinson I and Nateri AS: FBXW7-mutated
colorectal cancer cells exhibit aberrant expression of
phosphorylated-p53 at Serine-15. Oncotarget. 6:9240–9256. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen S, Leng P, Guo J and Zhou H: FBXW7 in
breast cancer: Mechanism of action and therapeutic potential. J Exp
Clin Cancer Res. 42:2262023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Ouyang L, Zheng Z, Xiang D, Ti A, Li
L, Dan Y, Yu C and Li W: E3 ubiquitin ligase FBW7α inhibits
cholangiocarcinoma cell proliferation by downregulating c-Myc and
cyclin E. Oncol Rep. 37:1627–1636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kanatsu-Shinohara M, Onoyama I, Nakayama
KI and Shinohara T: Skp1-Cullin-F-box (SCF)-type ubiquitin ligase
FBXW7 negatively regulates spermatogonial stem cell self-renewal.
Proc Natl Acad Sci USA. 111:8826–8831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C,
Yao Y and Liu Q: Fbxw7 is an independent prognostic marker and
induces apoptosis and growth arrest by regulating YAP abundance in
hepatocellular carcinoma. Mol Cancer. 13:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aydin IT, Melamed RD, Adams SJ,
Castillo-Martin M, Demir A, Bryk D, Brunner G, Cordon-Cardo C,
Osman I, Rabadan R and Celebi JT: FBXW7 mutations in melanoma and a
new therapeutic paradigm. J Natl Cancer Inst. 106:dju1072014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu K, Dou R, Yang C, Di Z, Shi D, Zhang
C, Song J, Fang Y, Huang S, Xiang Z, et al: Exosome-transmitted
miR-29a induces colorectal cancer metastasis by destroying the
vascular endothelial barrier. Carcinogenesis. 44:356–367. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang XJ, Zhang D, Yang YT, Li XY, Li HN,
Zhang XP, Long JY, Lu YQ, Liu L, Yang G, et al: Suppression of
microRNA-222-3p ameliorates ulcerative colitis and
colitis-associated colorectal cancer to protect against oxidative
stress via targeting BRG1 to activate Nrf2/HO-1 signaling pathway.
Front Immunol. 14:10898092023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mo JS, Lamichhane S, Yun KJ and Chae SC:
MicroRNA 452 regulates SHC1 expression in human colorectal cancer
and colitis. Genes Genomics. 45:1295–1304. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pan S, Wu W, Ren F, Li L, Li Y, Li W, Wang
A, Liu D and Dong Y: MiR-346-5p promotes colorectal cancer cell
proliferation in vitro and in vivo by targeting FBXL2 and
activating the β-catenin signaling pathway. Life Sci.
244:1173002020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin Y, Chen Z, Lin S, Zheng Y, Liu Y, Gao
J and Chen S: MiR-202 inhibits the proliferation and invasion of
colorectal cancer by targeting UHRF1. Acta Biochim Biophys Sin
(Shanghai). 51:1305–1306. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen B, Xia Z, Deng YN, Yang Y, Zhang P,
Zhu H, Xu N and Liang S: Emerging microRNA biomarkers for
colorectal cancer diagnosis and prognosis. Open Biol. 9:1802122019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J,
Zhou K, Liu X, Ren X, Wang F, et al: Cancer-derived exosomal
miR-25-3p promotes pre-metastatic niche formation by inducing
vascular permeability and angiogenesis. Nat Commun. 9:53952018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang L, Tong Z, Sun Z, Zhu G, Shen E and
Huang Y: MiR-25-3p targets PTEN to regulate the migration,
invasion, and apoptosis of esophageal cancer cells via the PI3K/AKT
pathway. Biosci Rep. 40:BSR202019012020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang L, Li L, Chang P, Wei M, Chen J, Zhu
C and Jia J: miR-25 regulates gastric cancer cell growth and
apoptosis by targeting EGR2. Front Genet. 12:6901962021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rao HC, Wu ZK, Wei SD, Jiang Y, Guo QX,
Wang JW, Chen CX and Yang HY: MiR-25-3p serves as an oncogenic
MicroRNA by downregulating the expression of merlin in
osteosarcoma. Cancer Manag Res. 12:8989–9001. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu JY, Yang LL, Ma C, Huang YL, Zhu GX and
Chen QL: MiR-25-3p attenuates the proliferation of tongue squamous
cell carcinoma cell line Tca8113. Asian Pac J Trop Med. 6:743–747.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li X, Yang C, Wang X, Zhang J, Zhang R and
Liu R: The expression of miR-25 is increased in colorectal cancer
and is associated with patient prognosis. Med Oncol. 31:7812014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yeh CH, Bellon M and Nicot C: FBXW7: A
critical tumor suppressor of human cancers. Mol Cancer. 17:1152018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tan Y, Sangfelt O and Spruck C: The
Fbxw7/hCdc4 tumor suppressor in human cancer. Cancer Lett.
271:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Akhoondi S, Sun D, von der Lehr N,
Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D,
Marth C, et al: FBXW7/hCDC4 is a general tumor suppressor in human
cancer. Cancer Res. 67:9006–9012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Romano M, De Francesco F, Pirozzi G,
Gringeri E, Boetto R, Di Domenico M, Zavan B, Ferraro GA and Cillo
U: Expression of cancer stem cell biomarkers as a tool for a
correct therapeutic approach to hepatocellular carcinoma.
Oncoscience. 2:443–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kawashita Y, Morine Y, Ikemoto T, Saito Y,
Iwahashi S, Yamada S, Higashijima J, Imura S, Ogawa H, Yagi T and
Shimada M: Loss of Fbxw7 expression is a predictor of recurrence in
colorectal liver metastasis. J Hepatobiliary Pancreat Sci.
24:576–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chang CC, Lin HH, Lin JK, Lin CC, Lan YT,
Wang HS, Yang SH, Chen WS, Lin TC, Jiang JK and Chang SC: FBXW7
mutation analysis and its correlation with clinicopathological
features and prognosis in colorectal cancer patients. Int J Biol
Markers. 30:e88–e95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wei W, Qin B, Wen W, Zhang B, Luo H, Wang
Y, Xu H, Xie X, Liu S, Jiang X, et al: FBXW7β loss-of-function
enhances FASN-mediated lipogenesis and promotes colorectal cancer
growth. Signal Transduct Target Ther. 8:1872023. View Article : Google Scholar : PubMed/NCBI
|