Colorectal cancer (CRC) ranks as the third most
prevalent malignancy and the second leading cause of cancer-related
mortality worldwide (1). Previous
clinical studies have demonstrated that immunotherapy monotherapy
or in combination with chemotherapy confers a favorable survival
benefit for patients with CRC (2).
Previously, CRC immunotherapy focused primarily on αβ T cells,
which exert cytotoxicity by recognizing mutant antigens in tumor
cells through the major histocompatibility complex (MHC) (3). However, cancer cells typically exhibit
depletion of MHC molecules, which renders tumor cells immune to αβ
T-cell-mediated cell mortality (4).
Another T-cell type in humans, the γδ T cell, exhibits
MHC-unrestricted lytic activity against different tumor cells in
vitro, suggesting the possibility for application in cancer
treatment (5).
In humans, γδ T cells associated with CRC can be
generally categorized into three types according to the chains on
the T-cell receptor (TCR) surface: Vδ1, Vδ2 and Vδ3 T cells
(6). The thymus and mucosal
epithelial tissues contain the majority of Vδ1 T lymphocytes, which
release various cytokines, including tumor necrosis factor-alpha
(TNF-α) and interferon-gamma (IFN-γ), which have cytotoxic effects
on tumor cells and are crucial in the development of numerous
illnesses. Vδ2 T cells comprise 50–90% of all γδ T cells, mostly in
the peripheral circulation. The TCR of Vδ2 T cells primarily
utilizes Vγ9 and Vδ2, which may detect phosphorylated antigens for
activation and release perforin and granzymes, resulting in
cytotoxicity. Activated Vδ2 T cells can act as antigen-presenting
cells (6–8). The proportion of Vδ3 T cells among the
total γδ T cells is <1%, and Vδ3 T cells are predominantly
localized in the liver and intestine (9). These cells exhibit cytotoxicity
through the expression of genes encoding cytotoxic molecules such
as granzyme B, perforin, granulysin, and also possess NKG2D
receptors for tumor cell recognition and elimination (10). γδ T cells can be classified into
regulatory γδ, γδ T17, IFN-γ+ γδ, and other functional
types. The main impediment to the therapeutic application of these
cells lies in the immune evasion mechanisms employed by tumor cells
(11). Tumor cells can alter the
function of the host immune system and create a tumor
microenvironment (TME) conducive to tumor development, allowing
immune evasion (12). Additionally,
several studies have demonstrated a correlation between the gut
microbiota and γδ T cells (13),
with an imbalance in the gut microbiota potentially promoting the
progression of inflammation toward CRC (14). An understanding of γδ T-cell
characteristics and the mechanism of action involving the TME and
the gut microbiota with γδ T cells will facilitate the development
of novel anti-CRC therapeutics and establish a foundation for
clinical treatment combinations.
Currently, there is a paucity of research on Vδ1 T
cells in CRC, likely because of the heterogeneous nature of Vδ1
T-cell populations in this malignancy (21), which poses challenges for
investigation. The feasibility of categorizing Vδ1 T cells and
selectively acquiring distinct subsets of Vδ1 T cells for targeted
investigations may be explored in the future.
The reduced presence of Vδ2 T cells in patients with
colitis-induced cancers can potentially be attributed to impaired
recruitment of Vδ2 T cells from the peripheral circulation and
sustained inflammatory processes resulting in the depletion of Vδ2
T cells (22). A potential strategy
for treating tumors involves promoting the proliferation and
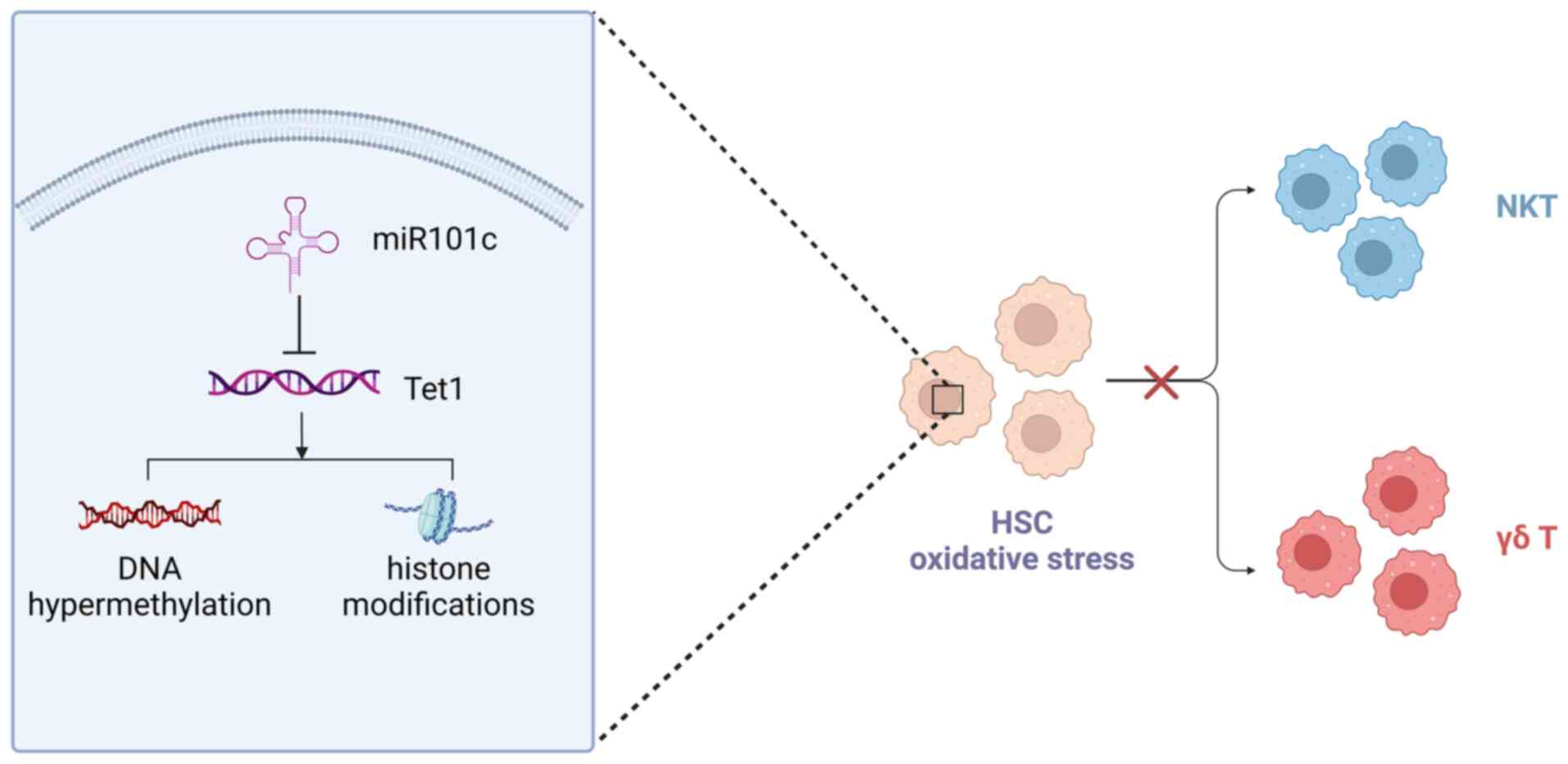
augmenting the functionality of Vδ2 T cells. Currently, research
efforts have focused predominantly on investigating the antitumor
potential of Vγ9Vδ2 T cells.
The recognition of tumor cells by Vγ9Vδ2 T
lymphocytes is predominantly MHC-unrestricted, with CRC cell lines
being recognized by ascites-derived Vγ9Vδ2 clones and regulated by
both TCR-dependent and TCR-independent signals (23,24).
It has been reported that Vγ9Vδ2 T cells recognize tumor cells
through the CDR3δ region of the γδ-TCR (25). In a subsequent study, Zhao et
al (26) engineered
CDR3δ-transplanted Vγ9Vδ2 T cells capable of producing antitumor
cytokines upon stimulation with tumor cell extracts. Furthermore,
this antitumor effect was attenuated by the administration of
anti-γδ-TCR monoclonal antibodies (26). Another study identified specific
sequence and structure patterns in CDR3δ, including rearrangement
within the J1 region, the presence of atypical T-cell receptor
genes, the positioning of hydrophobic amino acids in CDR3δ, the
distribution of CDR3δ lengths, and the number of N insertions.
These factors may impact the affinity between T-cell receptors and
antigens, consequently influencing T-cell activation and expansion
(27).
The activation of Vγ9Vδ2 T cells can be induced by
the overexpression of phospho-antigen (pAg) (18) and the interaction between NKG2D
receptors and ligands in CRC (28,29).
Once activated, Vγ9Vδ2 T cells can eliminate tumor cells through
various mechanisms, including the engagement of death
receptors/ligands with Fas ligands and tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) and the secretion
of perforins, cytokines (such as TNF-α), or granzymes (30). These pAgs are mainly pyrophosphates
produced in eukaryotes via the mevalonate pathway (31). Different phosphate antigens activate
Vγ9Vδ2 T cells through different mechanisms. For example,
bromo-hydro-pyrophosphate directly stimulates Vγ9Vδ2 T cells,
whereas amino-bisphosphonates, such as pamidronate and zoledronate,
indirectly activate Vγ9Vδ2 T cells by inhibiting the mevalonate
pathway, thereby increasing the intracellular accumulation of
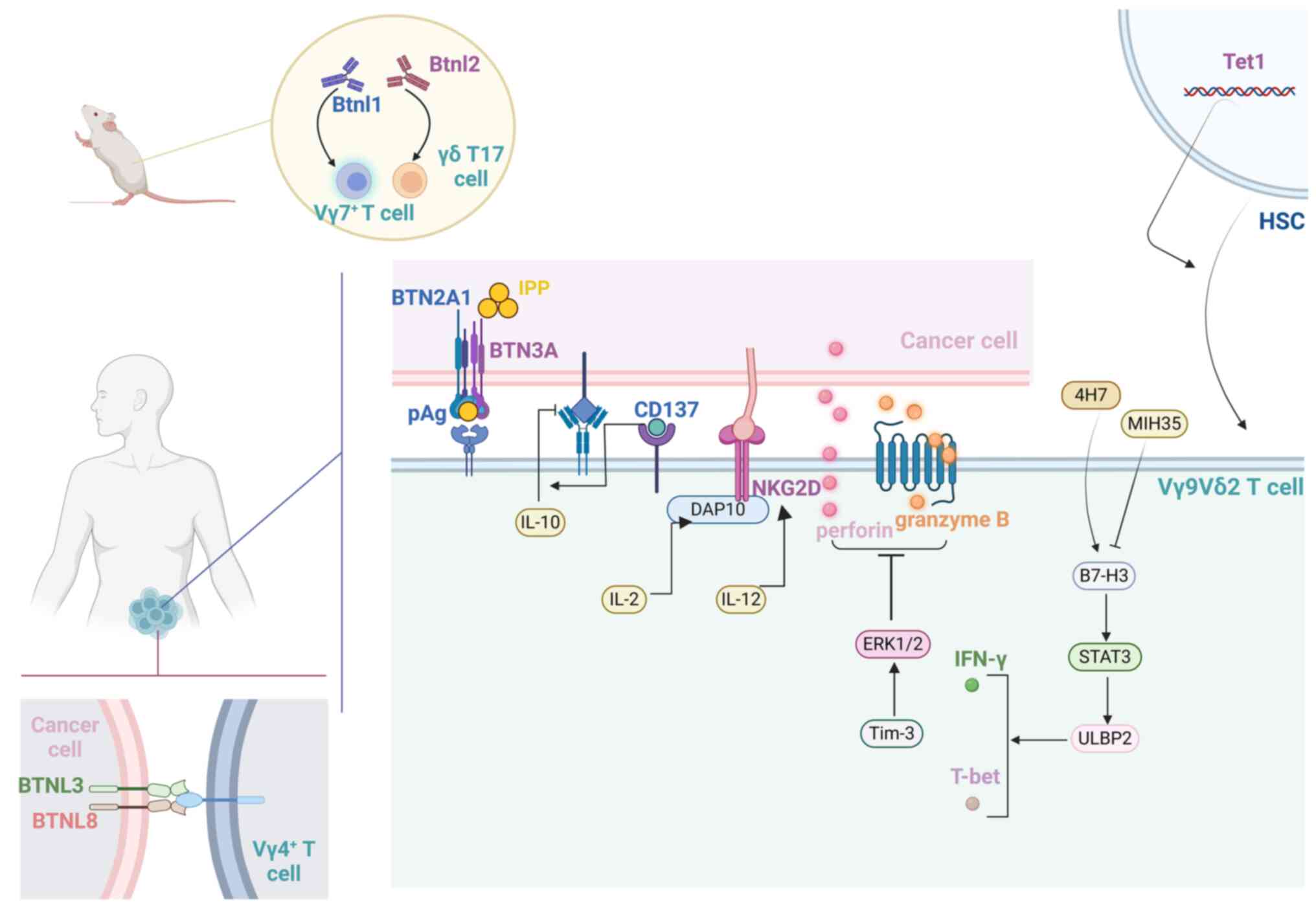
isopentenyl pyrophosphate (IPP) (32,33).
IPP accumulates in numerous types of cancer, and the resulting
disordered metabolic processes render cancer cells susceptible to
Vγ9Vδ2 T-cell-mediated mortality (6). Reportedly, interleukin-2 (IL-2)
stimulates the production of the adaptor molecule DAP10, increasing
the surface expression of NKG2D (34). Similarly, Smyth et al
(35) reported that the
cytotoxicity of IL-12-induced cells toward tumor cells is
contingent upon the interaction between NKG2D and its corresponding
ligand. Pei et al (36)
reported that CD137 co-stimulation can overcome the inhibitory
effect of endogenous IL-10 (hIL-10 and vIL-10) on the antitumor
activity of Vγ9Vδ2 T cells, thereby enhancing the efficacy of this
specific subset in tumor therapy. However, according to Zhang et
al (37), soluble NKG2DLs
impair the cytotoxicity of γδ T cells to tumor cells. Therefore,
increasing the expression of NKG2DLs within tumors or employing
targeted delivery of synthetic adhesives to tumors may be an
effective approach for enhancing the antitumor efficacy of γδ T
cells.
Evidence from three lines of investigation
demonstrated that chemotherapy enhances the susceptibility of
colonic cancer initiating stem cells (CICs) to Vγ9Vδ2 T-cell
toxicity. Pioneering work by Mattarollo et al (38) demonstrated that the combination of
Vγ9Vδ2 T cells and chemotherapeutic agents yields a high level of
cytotoxicity in cell lines derived from solid tumors.
IL-17-producing γδ T cells play a decisive role in immune responses
against cancer induced by chemotherapy in mice (39). Simultaneous or immediate in
vivo activation of Vγ9Vδ2 T cells or adoptive transfer of in
vitro-activated Vγ9Vδ2 T lymphocytes following treatment with
the chemotherapeutic drugs 5-fluorouracil (5-FU) and doxorubicin
(DXR) significantly increased antitumor activity (7).
Vγ9Vδ2 T-cell elimination post-chemotherapy in CICs
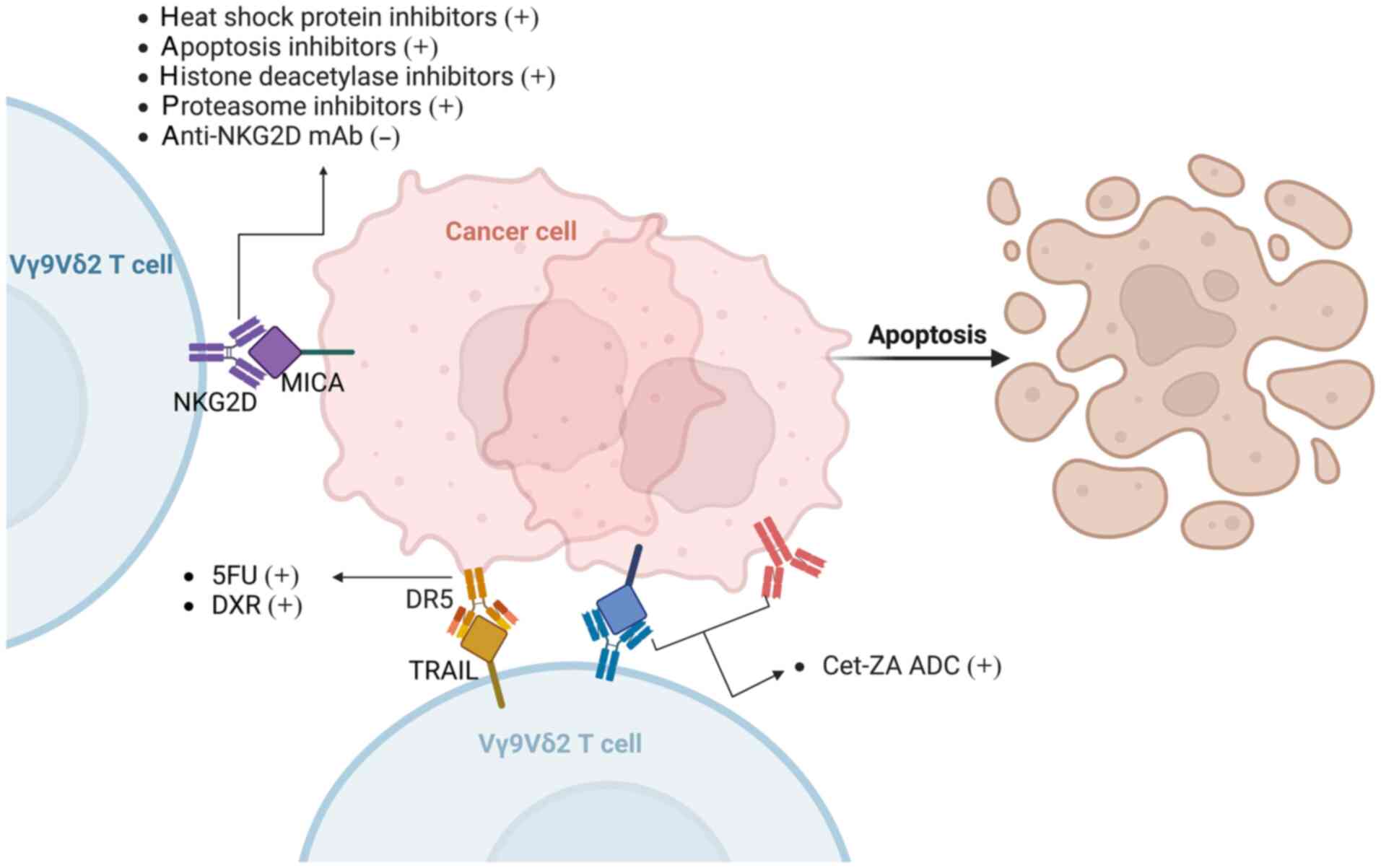
is mediated through the activation of NKG2D and TRAIL (7). 5-FU and DXR significantly increase the
expression of DR5 (TRAIL-R2) in colon cancer stem cells (CSCs).
Additionally, the anti-NKG2D mAb effectively suppresses the
cytotoxicity of Vγ9Vδ2 T cells against colon CSCs, whereas neither
anti-CD3 nor anti-TCR antibodies nor mevastatin (a
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor that
prevents endogenous pAg accumulation) demonstrate significant
inhibitory effects (34). The
expression of NKG2D ligands on tumor cells can be induced by
various drugs, including proteasomes, histone deacetylases, heat
shock proteins and apoptosis inhibitors (40–44),
thereby increasing the toxicity of Vγ9Vδ2 T cells and inhibiting
tumor development. In addition, Benelli et al (45) developed a Cet-ZA antibody-drug
conjugate (ADC) that targets CRC cells and enhances Vδ2 T-cell
cytotoxicity through the TCR pathway (Fig. 1). Although numerous studies are
underway, drug toxicity and targeting remain challenges.
γδ T17 cells represent a prominent source of IL-17
within the TME. Activated inflammatory dendritic cells (inf-DCs)
can induce γδ T17 cells to generate TNF-α, IL-8 and GM-CSF, while
immunosuppressive polymorphonuclear myeloid-derived suppressor
cells (PMN-MDSCs) accumulate in tumors. The regulatory axis of
inf-DC-γδT17-PMN-MDSCs in human CRC establishes a connection
between MDSC-mediated immunosuppression and tumor-induced
inflammation, highlighting the potential role of γδ T17 cells in
the progression of human CRC (46).
The percentage of tumor-infiltrating γδ T17 cells
positively correlates with the progression of TNM stage and other
clinicopathological characteristics, including tumor size, tumor
invasion, lymphatic and vascular invasion, lymph node metastasis
and the serum carcinoembryonic antigen level (46,47).
Furthermore, inf-DC, PMN-MDSC, IL-23 and IL-17 levels in tumor
tissue are significantly related to the proportion of
tumor-infiltrating γδ T17 cells (46,48).
Following acute intestinal injury,
IL-23R+RORγT+γδ T cells located in the
colonic lamina propria serve as pivotal sources of initial
protective IL-17 within the intestines, playing an indispensable
role in preserving and enhancing the integrity of the intestinal
mucosal epithelial barrier (49).
The dual role of γδ T17 cells in tumors poses a challenge for
developing immunotherapies targeting this specific cell subset.
Further comprehensive investigations are warranted to elucidate
their functional pathways within the TME and identify pivotal
breakthroughs.
TCR sequencing research has shown that γδ T cells
with antitumor characteristics include polyclonal Vγ7+
and Vγ1+ cells, and a minority of tumor-promoting cells
that produce IL-17 are Vγ4+ cells; most are clonally
expanded Vγ6Vδ1+ cells (54). Although Vγ6+ cells are
the predominant progenitor subset in tumors, Vγ4+ cells
appear to be able to compensate when Vγ6+ cells are
damaged, similar to Vγ7+ cells (mostly gut-specific γδ T
cells) and Vγ1+ cells (with broad tissue distribution);
elimination of Vγ1+ cells from the tumor is required
when performing antitumor functional analyses of Vγ7+
cells (54,55).
The utilization of synthetic immune checkpoint
inhibitors has emerged as a prominent area of research in the field
of CRC immunotherapy and has demonstrated remarkable efficacy,
especially in patients with microsatellite instability (MSI)-high
CRC (56). These agents target
immune checkpoints, such as the programmed cell death protein 1
(PD-1)/programmed cell death-Ligand 1 (PD-L1) pathway, which tumors
utilize to evade detection by the immune system. By obstructing
this interaction, these inhibitors can augment the immune response
against cancer cells (57). Despite
the potential for adverse effects, immune checkpoint inhibitors
have been shown to have a greater safety profile than chemotherapy
(58–60). Several studies on γδ T cells have
identified immune checkpoint genes, which are expected to be used
to screen drugs for the treatment of CRC.
Despite a significant reduction in the proportion of
γδ T cells in both peripheral blood mononuclear cells and tumor
areas among patients with colon cancer, there is an increase in the
proportion of B7-H3+γδ T lymphocytes. It is postulated
that B7-H3 functions as a negative immune checkpoint molecule,
modulating the activity and biological function of γδ T cells in
colon cancer. It has been revealed that blocking or reducing B7-H3
leads to enhanced proliferation, inhibition of apoptosis, and
upregulation of activation markers (CD25 and CD69) in Vδ2 T cells.
Conversely, the B7-H3 agonist 4H7 exerts the opposite effect. In
the presence of IL-2 and zoledronic acid, Vδ2 T cells treated with
MIH35 (a specific inhibitory antibody against B7-H3) or B7-H3 siRNA
presented increased cell viability, a reduced rate of apoptosis,
and increased expression of the signaling molecules CD25 and CD69
(63).
The inhibition of Vδ2 T cells by B7-H3 is mediated
mainly by the suppression of T-bet and a decrease in IFN-γ and
perforin/granzyme B expression, which involves STAT3 activation and
a reduction in ULBP2 expression (11,63).
Cryptotanshinone, an inhibitor of STAT3 phosphorylation, can
reverse the decrease in ULBP2 expression and attenuate the B7-H3
overexpression-induced elimination of colon cancer cells by Vδ2 T
cells (11). The B7-H3-mediated
STAT3/ULBP2 axis may be a potential target for enhancing the
efficiency of γδ T-cell-based colon cancer immunotherapy (11,63).
In mice, Btnl proteins are predominantly expressed
on the epithelial cells lining the intestinal villi (64). Previously, the expression of Btnl1
in intestinal villi in the early stage of life was shown to
selectively promote the maturation and proliferation of
Vγ7+ IELs in tissues (65), revealing its antitumor potential,
whereas the expression of Btnl2 in tumor cells specifically
recruits IL-17-producing γδ T cells that promote tumorigenesis
(66).
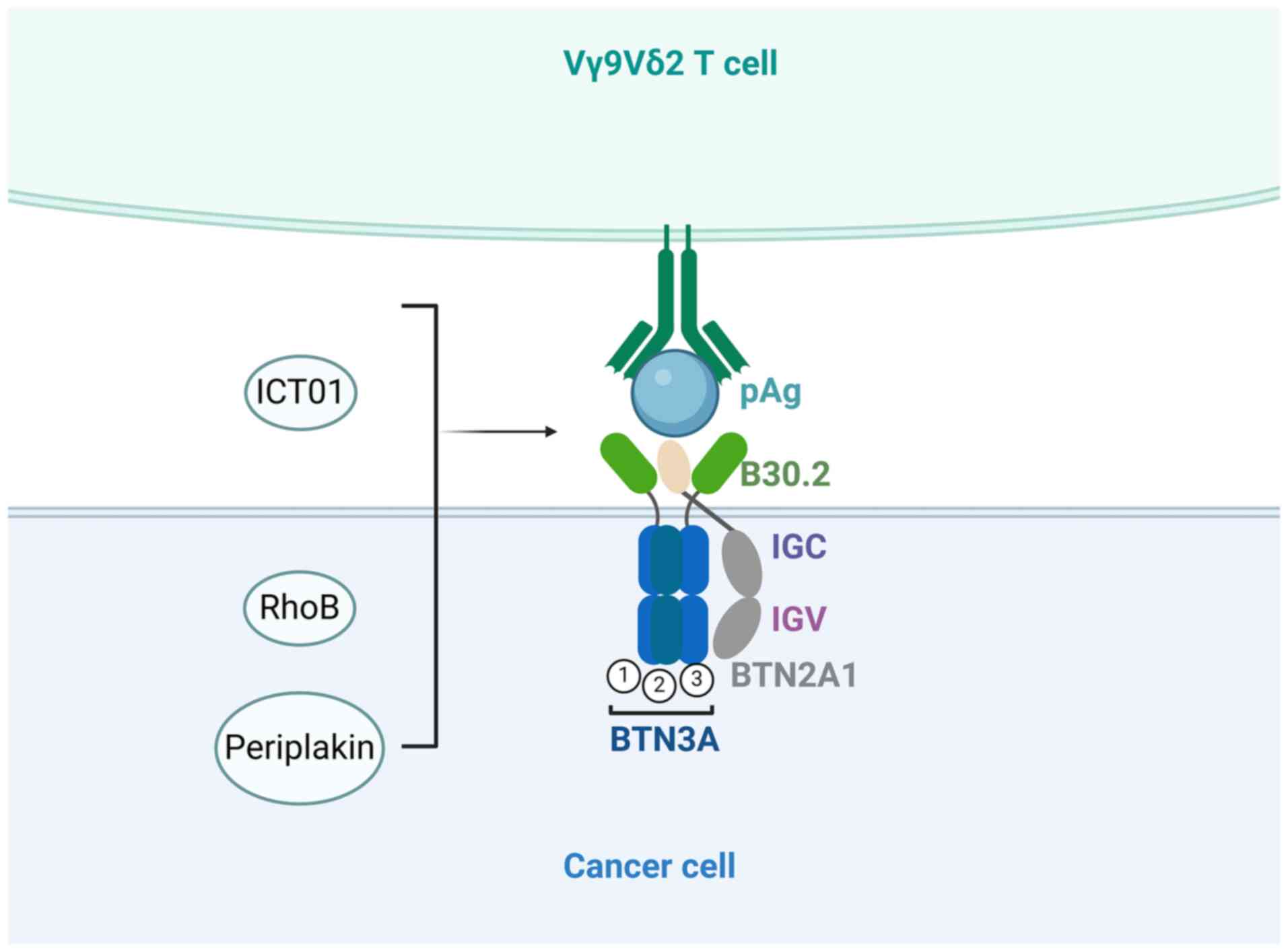
The Butinophil-3A (BTN3A, also known as CD277)
protein subfamily plays a crucial role in the antitumor process of
γδ T cells by serving as a pivotal mediator of pAg signal
transduction (67). The BTN3A
molecular subfamily is part of the B7 costimulatory molecular
family and includes the BTN3A1, BTN3A2 and BTN3A3 subtypes
(68). The three subtypes can
stimulate Vγ9Vδ2 T cells following treatment with the 20.1 agonist
mAb, activating the cells through mechanisms involving mobility
reduction (67) and BTN3A molecular
polymerization (69). However,
BTN3A1 cannot mediate the activation of Vγ9Vδ2 T cells without
BTN3A2 or BTN3A3; Cano et al (70) also demonstrated that BTN3A-mediated
cytotoxicity of Vγ9Vδ2 T cells toward cancer cells must involve
BTN2A1.
Human intestinal epithelial cells express BTNL3 and
BTNL8, and the concurrent expression of BTNL3+BTNL8
induces a selective TCR-dependent response in Vγ4+ cells
of the human colon (65). According
to the analysis by Blazquez et al (72), the homing and maintenance of BTNL3
and BTNL8 in the semi-activated state in human intestinal
Vγ4+ γδ T cells may be relevant to the pathogenesis of
intestinal autoimmune disorders, such as ulcerative colitis and
inflammatory bowel disease. Chronic intestinal inflammation can
promote the formation of colorectal tumors (73,74),
revealing the correlation between BTNL3 and BTNL8 and CRC.
Lebrero-Fernández et al (75) reported significantly lower levels of
BTNL3 and BTNL8 expression in colon cancer tissues than in adjacent
normal tissues, providing further support for this notion.
The expression of PD-1 can serve as a partial
indicator of γδ T-cell function and impact patient prognosis.
However, the upregulation of PD-1 alone is insufficient to fully
characterize the functional phenotype of γδ T cells in cancer,
necessitating comprehensive evaluation of other markers and
indicators (76). In academic
research, PD-1 is frequently investigated in conjunction with Tim-3
(77), whereas in clinical
practice, the combination of PD-1 and CTLA-4 antibodies has
demonstrated successful outcomes in the treatment of CRC (78). As a crucial negative regulator of
Vγ9Vδ2 T-cell activation, Tim-3 was found to downregulate the
expression of perforin and granzyme B in Vγ9Vδ2 T cells via an
ERK1/2 signaling pathway-dependent mechanism, thereby attenuating
the cytotoxicity of Vγ9Vδ2 T cells to colon cancer cells (79) (Fig.
4). The inhibitory receptors CTLA-4, LAG-3 and TIGIT have been
demonstrated to be present on the surface of T cells (80–82).
However, the specific mechanism underlying their interaction with
γδ T cells remains unclear, particularly in treating CRC. Further
investigations into the mechanisms underlying these immune
checkpoint genes and the development of diverse immune checkpoint
inhibitors for combination therapy may represent promising
approaches to enhance the current landscape of CRC treatment.
The CRC TME is a complex communication system
comprising cancer cells and various other cell types (including
endothelial cells, immune cells and cancer-associated fibroblasts).
This intricate communication relies on a dysregulated regulatory
network comprising chemokines, cytokines, growth factors and their
corresponding receptors. Consequently, this dynamic interaction
gives rise to an inflammatory TME that facilitates tumorigenesis
and progression (83).
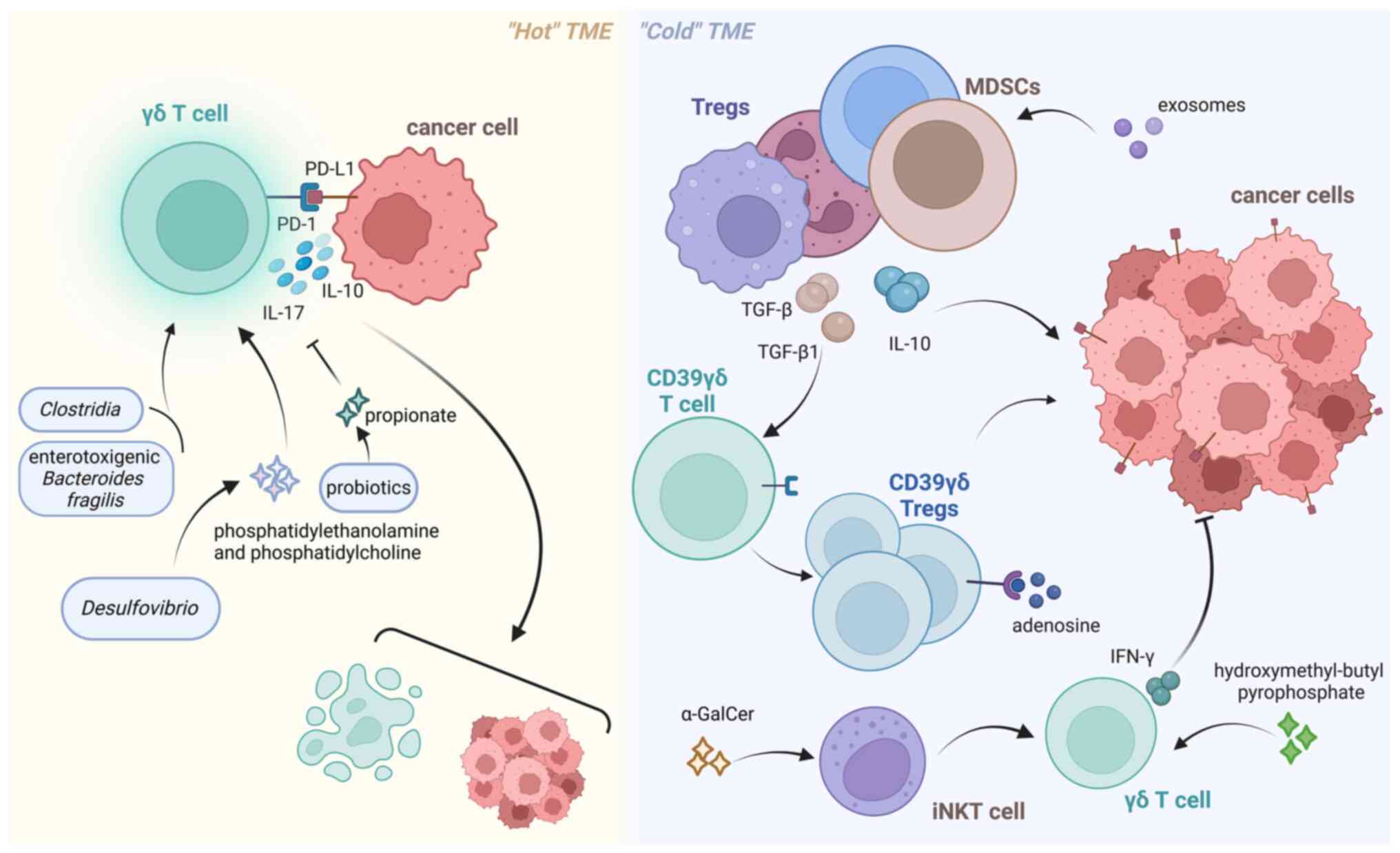
In the TME, CRC can be divided into ‘hot’ and ‘cold’
subtypes. Hot tumors are characterized by the presence of activated
immune cells that exhibit proinflammatory cytokine signaling, and
immune checkpoint inhibitors have shown promising efficacy in
inhibiting the growth of such tumors (84). By contrast, cold tumors typically
express receptors and ligands associated with immunosuppression and
are encompassed by populations of immunosuppressive cells,
including regulatory T cells (Tregs), MDSCs and tumor-associated
macrophages (85). T
Immunosuppressive cells can express IL-10 and TGF-β, thereby
impeding the infiltration and functionality of effector T cells,
including γδ T cells, while facilitating immune evasion (33,85,86).
Modification of the immune microenvironment is essential for
treating this type of tumor, including converting a cold tumor into
a hot tumor or enhancing effector cell function to achieve
effective immunotherapy (84).
Various studies have demonstrated the profound
impact of intestinal microbes on DNA damage, DNA methylation,
chromatin structure, and noncoding RNA expression in colon
epithelial cells (91).
Furthermore, alterations in certain genes and pathways induced by
intestinal microbes are closely associated with the CRC development
and influence the functionality of γδ T cells in this context.
According to previous reports, certain bacteria and their
metabolites, including Bacteroides fragilis, Lactobacillus
acidophilus, desulfurizing Vibrio and
Citrobacter, have been found to assist γδ T cells in
combating tumors. Conversely, specific gut bacteria, such as
Clostridia and enterotoxigenic Bacteroides fragilis,
may accelerate the development of CRC by activating γδ T cells that
promote tumor growth (13). Li
et al (92) reported that
phosphatidylethanolamine and phosphatidylcholine, metabolites of
Desulfovibrio, induced the proliferation of IL-17A-producing
γδ T cells, which aggravated intestinal injury. Some probiotics can
protect the normal intestinal mucosa in CRC by producing
short-chain fatty acids, such as acetate and propionate (93). Propionate can directly act on γδ T17
cells and inhibit IL-17 production in a histone
deacetylase-dependent manner (94),
whereas Akkermansia can reduce the number of IL-17-producing
γδ T cells in mice (95), thereby
improving intestinal inflammation. Hydroxymethyl-butyl
pyrophosphate produced by microorganisms can act as a pAg to
activate γδ T cells (96). A study
conducted by Roselli et al (97) demonstrated that the combination of
L. acidophilus and B. longum effectively impeded the
progression of colitis through the modulation of the γδ T-cell
population. The α-GalCer produced by Bacteroides fragilis,
Bacteroides vulgatus, Prevotella copri and other unidentified
bacteria can exert antitumor effects by indirectly inducing the
production of IFN-γ by γδ T cells through the activation of
invariant NKT cells (98) (Fig. 5). These studies suggested that the
gut microbiota actively participates in the antitumor process of γδ
T cells, assuming distinct roles. However, most of these studies
have focused primarily on the cellular level. Consequently, whether
clinical intervention targeting specific gut microbiota components
can effectively decelerate tumor progression remains uncertain. In
addition, several studies have demonstrated that the gut microbiota
can serve as a reliable biomarker for the non-invasive diagnosis of
CRC (99–101). However, the selection of
appropriate biomarkers and the development of highly sensitive
detection methods pose limitations for its clinical application,
and further research is needed to achieve breakthroughs.
In recent years, clinical studies on the application
of γδ T cells in CRC immunotherapy have focused primarily on their
role in MSI-high CRC and microsatellite-stable (MSS) CRC. MSI CRCs
can be categorized into MSI-H and MSI-L groups according to the
level of instability, with MSI-L and MSS often grouped together in
clinical studies. Given their greater mutation load and neoantigen
exposure, MSI-H tumors are more readily recognized and targeted by
the immune system, making conventional immune checkpoint inhibitors
more effective for treating MSI-H CRC than MSS CRC (56). However, MSS tumors account for the
majority of CRC cases, underscoring the pressing need for the
development of innovative immunotherapies targeting patients with
CRC with MSS tumors (102,103).
γδ T cells have emerged as pivotal players in the
immunotherapy landscape of CRC, exerting their antitumor effects
independently of MHC restrictions and exhibiting the ability to
recognize and respond to tumor cells that may have evaded
conventional αβ T-cell surveillance. With the discovery that Vδ2 T
cells can recognize pAgs and be activated by them to exert
antitumor activity, Vδ2 T cells have emerged as a prominent
research focus in recent years. Numerous researchers have dedicated
efforts to investigating pAgs and their associated activation
pathways, enhancing our understanding of Vδ2 T cells. Several
immune checkpoint genes have been identified during investigations
into the mechanism of action between Vδ2 T cells and tumor cells.
The combination of PD-1 and CTLA-4 inhibitors has demonstrated
efficacy in treating CRC. Moreover, ongoing research is exploring
additional immune checkpoint genes as promising therapeutic targets
for CRC in the future. The finding that the cytotoxic potential of
dysfunctional Vδ1 T cells in tumors can be reactivated in
vitro holds promise for the treatment of MSS CRC unresponsive
to immune checkpoint inhibitors. In addition, utilizing BiTE and
CAR modification technology, along with integrating multiple
approaches, significantly enhances CRC therapy efficacy. However,
given the intricate regulatory network of the TME, research on the
specific regulatory mechanism is lacking. In the future, combining
drugs that target diverse cellular components within the TME to
impede the progression of CRC may be possible. Recent studies have
shown that gut microbes and their metabolites also interact with γδ
T cells and tumors. However, the diversity of gut microbes and the
lack of methods for sample collection, storage and analysis pose
obstacles to related research. Therefore, the mode of interaction
between the gut microbiota and γδ T cells in CRC and the microbial
flora involved in this process remains unclear. In the future,
analyses of the gut microbial species involved in the antitumor
process of γδ T cells can be initiated to discover new methods for
treating or diagnosing CRC.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81972716).
Not applicable.
XH and XC conceived and designed the study. LP, YZ
and WW prepared and wrote the manuscript. YK and CW edited the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golshani G and Zhang Y: Advances in
immunotherapy for colorectal cancer: A review. Therap Adv
Gastroenterol. 13:17562848209175272020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morazan-Fernandez D, Mora J and
Molina-Mora JA: In Silico pipeline to identify Tumor-specific
antigens for cancer immunotherapy using exome sequencing data.
Phenomics. 3:130–137. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu X, Li T, Jiang R, Yang X, Guo H and
Yang R: Targeting MHC-I molecules for cancer: Function, mechanism,
and therapeutic prospects. Mol Cancer. 22:1942023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodrigues NV, Correia DV, Mensurado S,
Nobrega-Pereira S, deBarros A, Kyle-Cezar F, Tutt A, Hayday AC,
Norell H, Silva-Santos B and Dias S: Low-Density lipoprotein uptake
inhibits the activation and antitumor functions of human
Vgamma9Vdelta2 T cells. Cancer Immunol Res. 6:448–457. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki T, Hayman L, Kilbey A, Edwards J
and Coffelt SB: Gut γδ T cells as guardians, disruptors, and
instigators of cancer. Immunol Rev. 298:198–217. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Todaro M, Orlando V, Cicero G, Caccamo N,
Meraviglia S, Stassi G and Dieli F: Chemotherapy sensitizes colon
cancer initiating cells to Vγ9Vδ2 T Cell-mediated cytotoxicity.
PLoS One. 8:e651452013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo Presti E, Pizzolato G, Gulotta E,
Cocorullo G, Gulotta G, Dieli F and Meraviglia S: Current advances
in γδ T Cell-based tumor immunotherapy. Front Immunol. 8:14012017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arias-Badia M, Chang R and Fong L: γδ T
cells as critical Anti-tumor immune effectors. Nat Cancer.
5:1145–1157. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Vries NL, van de Haar J, Veninga V,
Chalabi M, Ijsselsteijn ME, van der Ploeg M, van den Bulk J, Ruano
D, van den Berg JG, Haanen JB, et al: γδ T cells are effectors of
immunotherapy in cancers with HLA class I defects. Nature.
613:743–750. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu H, Ma Y, Wang M, Shen J, Wu H, Li J,
Gao N, Gu Y, Zhang X, Zhang G, et al: B7-H3 confers resistance to
Vγ9Vδ2 T cell-mediated cytotoxicity in human colon cancer cells via
the STAT3/ULBP2 axis. Cancer Immunol Immunother. 70:1213–1226.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lo Presti E, Pizzolato G, Corsale AM,
Caccamo N, Sireci G, Dieli F and Meraviglia S: γδ T cells and tumor
microenvironment: From immunosurveillance to tumor evasion. Front
Immunol. 9:13952018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin P, Yan Y, Zhang Z, Dong Q, Yi J, Li Q,
Zhang A and Kong X: The γδ T cells dual function and crosstalk with
intestinal flora in treating colorectal cancer is a promising area
of study. Int Immunopharmacol. 123:1107332023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu LQ, Zhang L, Zhang J, Chang GL, Liu G,
Yu DD, Yu XM, Zhao MS and Ye B: Evodiamine inhibits high-fat
Diet-induced Colitis-associated cancer in mice through regulating
the gut microbiota. J Integr Med. 19:56–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rong L, Li K, Li R, Liu HM, Sun R and Liu
XY: Analysis of tumor-infiltrating gamma delta T cells in rectal
cancer. World J Gastroenterol. 22:3573–3580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma R, Yuan D, Guo Y, Yan R and Li K:
Immune effects of γδ T cells in colorectal cancer: A review. Front
Immunol. 11:16002020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu D, Wu P, Wu X, Ye J, Wang Z, Zhao S, Ni
C, Hu G, Xu J, Han Y, et al: Ex vivo expanded human circulating Vδ1
γδT cells exhibit favorable therapeutic potential for colon cancer.
Oncoimmunology. 4:e9927492015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mikulak J, Oriolo F, Bruni E, Roberto A,
Colombo FS, Villa A, Bosticardo M, Bortolomai I, Lo Presti E,
Meraviglia S, et al: NKp46-expressing human gut-resident
intraepithelial Vδ1 T cell subpopulation exhibits high antitumor
activity against colorectal cancer. JCI Insight. 4:e1258842019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bruni E, Cimino MM, Donadon M, Carriero R,
Terzoli S, Piazza R, Ravens S, Prinz I, Cazzetta V, Marzano P, et
al: Intrahepatic CD69+Vδ1 T cells re-circulate in the blood of
patients with metastatic colorectal cancer and limit tumor
progressionn. J Immunother Cancer. 10:e0045792022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Devaud C, Rousseau B, Netzer S, Pitard V,
Paroissin C, Khairallah C, Costet P, Moreau JF, Couillaud F,
Dechanet-Merville J and Capone M: Anti-metastatic potential of
human Vδ1(+) γδ T cells in an orthotopic mouse xenograft model of
colon carcinoma. Cancer Immunol Immunother. 62:1199–1210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bernard NJ: Expanding Vδ1 T cells. Nat
Immunol. 24:13962023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lo Presti E, Mocciaro F, Mitri RD, Corsale
AM, Di Simone M, Vieni S, Scibetta N, Unti E, Dieli F and
Meraviglia S: Analysis of colon-infiltrating γδ T cells in chronic
inflammatory bowel disease and in colitis-associated cancer. J
Leukoc Biol. 108:749–760. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bouet-Toussaint F, Cabillic F, Toutirais
O, Le Gallo M, Thomas de la Pintiere C, Daniel P, Genetet N,
Meunier B, Dupont-Bierre E, Boudjema K, et al: Vgamma9Vdelta2 T
cell-mediated recognition of human solid tumors. Potential for
immunotherapy of hepatocellular and colorectal carcinomas. Cancer
Immunol Immunother. 57:531–539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iovino F, Meraviglia S, Spina M, Orlando
V, Saladino V, Dieli F, Stassi G and Todaro M: Immunotherapy
targeting colon cancer stem cells. Immunotherapy. 3:97–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adams EJ, Strop P, Shin S, Chien YH and
Garcia KC: An autonomous CDR3delta is sufficient for recognition of
the nonclassical MHC class I molecules T10 and T22 by gammadelta T
cells. Nat Immunol. 9:777–784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H, Xi X, Cui L and He W:
CDR3δ-grafted γ9δ2T cells mediate effective antitumor reactivity.
Cell Mol Immunol. 9:147–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vyborova A, Janssen A, Gatti L, Karaiskaki
F, Yonika A, van Dooremalen S, Sanders J, Beringer DX, Straetemans
T, Sebestyen Z and Kuball J: γ9δ2 T-Cell expansion and phenotypic
profile are reflected in the CDR3δ repertoire of healthy adults.
Front Immunol. 13:9153662022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Silva-Santos B and Strid J: Working in ‘NK
Mode’: Natural Killer Group 2 Member D and natural cytotoxicity
receptors in Stress-surveillance by γδ T cells. Front Immunol.
9:8512018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong Y, Cao W, Xi X, Ma C, Cui L and He W:
The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces
cytotoxicity to tumor cells through both TCRgammadelta and NKG2D.
Blood. 114:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Todaro M, D'Asaro M, Caccamo N, Iovino F,
Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G,
Salerno A, et al: Efficient killing of human colon cancer stem
cells by gammadelta T lymphocytes. J Immunol. 182:7287–7296. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoeres T, Smetak M, Pretscher D and
Wilhelm M: Improving the efficiency of Vγ9Vδ2 T-Cell immunotherapy
in cancer. Front Immunol. 9:8002018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zocchi MR, Costa D, Vene R, Tosetti F,
Ferrari N, Minghelli S, Benelli R, Scabini S, Romairone E,
Catellani S, et al: Zoledronate can induce colorectal cancer
microenvironment expressing BTN3A1 to stimulate effector γδ T cells
with antitumor activity. Oncoimmunology. 6:e12780992017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JH and Lee HK: Function of γδ T cells
in tumor immunology and their application to cancer therapy. Exp
Mol Med. 53:318–327. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramutton T, Buccheri S, Dieli F, Todaro M,
Stassi G and Meraviglia S: γδ T cells as a potential tool in colon
cancer immunotherapy. Immunotherapy. 6:989–999. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smyth MJ, Swann J, Kelly JM, Cretney E,
Yokoyama WM, Diefenbach A, Sayers TJ and Hayakawa Y: NKG2D
recognition and perforin effector function mediate effective
cytokine immunotherapy of cancer. J Exp Med. 200:1325–1335. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pei Y, Xiang Z, Wen K, Tu CR, Wang X,
Zhang Y, Mu X, Liu Y and Tu W: CD137 costimulation enhances the
antitumor activity of Vγ9Vδ2-T cells in IL-10-Mediated
immunosuppressive tumor microenvironment. Front Immunol.
13:8721222022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang T, Wang J, Zhao A, Xia L, Jin H, Xia
S and Shi T: The way of interaction between Vγ9Vδ2 T cells and
tumor cells. Cytokine. 162:1561082023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mattarollo SR, Kenna T, Nieda M and Nicol
AJ: Chemotherapy and zoledronate sensitize solid tumour cells to
Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother.
56:1285–1297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma Y, Aymeric L, Locher C, Mattarollo SR,
Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F,
Casares N, et al: Contribution of IL-17-producing gamma delta T
cells to the efficacy of anticancer chemotherapy. J Exp Med.
208:491–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jinushi M, Vanneman M, Munshi NC, Tai YT,
Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR and
Dranoff G: MHC class I chain-related protein A antibodies and
shedding are associated with the progression of multiple myeloma.
Proc Natl Acad Sci USA. 105:1285–1290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vales-Gomez M, Chisholm SE, Cassady-Cain
RL, Roda-Navarro P and Reyburn HT: Selective induction of
expression of a ligand for the NKG2D receptor by proteasome
inhibitors. Cancer Res. 68:1546–1554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Diermayr S, Himmelreich H, Durovic B,
Mathys-Schneeberger A, Siegler U, Langenkamp U, Hofsteenge J,
Gratwohl A, Tichelli A, Paluszewska M, et al: NKG2D ligand
expression in AML increases in response to HDAC inhibitor valproic
acid and contributes to allorecognition by NK-cell lines with
single KIR-HLA class I specificities. Blood. 111:1428–1436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Armeanu S, Bitzer M, Lauer UM, Venturelli
S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, et
al: Natural killer Cell-mediated lysis of hepatoma cells via
specific induction of NKG2D ligands by the histone deacetylase
inhibitor sodium valproate. Cancer Res. 65:6321–6329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jones AB, Rocco A, Lamb LS, Friedman GK
and Hjelmeland AB: Regulation of NKG2D stress ligands and its
relevance in cancer progression. Cancers (Basel). 14:23392022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Benelli R, Costa D, Salvini L, Tardito S,
Tosetti F, Villa F, Zocchi MR and Poggi A: Targeting of colorectal
cancer organoids with zoledronic acid conjugated to the anti-EGFR
antibody cetuximab. J Immunother Cancer. 10:e0056602022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang
Z, Wang C, Zhang Z, Xia W, et al: gammadeltaT17 cells promote the
accumulation and expansion of myeloid-derived suppressor cells in
human colorectal cancer. Immunity. 40:785–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Corsale AM, Di Simone M, Lo Presti E,
Dieli F and Meraviglia S: γδ T cells and their clinical application
in colon cancer. Front Immunol. 14:10988472023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grivennikov SI, Wang K, Mucida D, Stewart
CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung
KE, et al: Adenoma-linked barrier defects and microbial products
drive IL-23/IL-17-mediated tumour growth. Nature. 491:254–258.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF,
Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan
TK, et al: Interleukin-23-Independent IL-17 production regulates
intestinal epithelial permeability. Immunity. 43:727–738. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reis BS, Darcy PW, Khan IZ, Moon CS,
Kornberg AE, Schneider VS, Alvarez Y, Eleso O, Zhu C, Schernthanner
M, et al: TCR-Vγδ usage distinguishes protumor from antitumor
intestinal γδ T cell subsets. Science. 377:276–284. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mu X, Xiang Z, Xu Y, He J, Lu J, Chen Y,
Wang X, Tu CR, Zhang Y, Zhang W, et al: Glucose metabolism controls
human γδ T-cell-mediated tumor immunosurveillance in diabetes. Cell
Mol Immunol. 19:944–956. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Agerholm R and Bekiaris V: Evolved to
protect, designed to destroy: IL-17-producing γδ T cells in
infection, inflammation, and cancer. Eur J Immunol. 51:2164–2177.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lopes N, McIntyre C, Martin S, Raverdeau
M, Sumaria N, Kohlgruber AC, Fiala GJ, Agudelo LZ, Dyck L, Kane H,
et al: Distinct metabolic programs established in the thymus
control effector functions of γδ T cell subsets in tumor
microenvironments. Nat Immunol. 22:179–192. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mensurado S and Silva-Santos B: Battle of
the γδ T cell subsets in the gut. Trends Cancer. 8:881–883. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Silva-Santos B, Mensurado S and Coffelt
SB: γδ T cells: Pleiotropic immune effectors with therapeutic
potential in cancer. Nat Rev Cancer. 19:392–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cai L, Chen A and Tang D: A new strategy
for immunotherapy of Microsatellite-stable (MSS)-type advanced
colorectal cancer: Multi-pathway combination therapy with
PD-1/PD-L1 inhibitors. Immunology. Mar 22–2024.doi:
10.1111/imm.13785 (Epub ahead of print). View Article : Google Scholar
|
|
57
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
58
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
Non-Small-Cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tie G, Messina KE, Yan J, Messina JA and
Messina LM: Hypercholesterolemia induces oxidant stress that
accelerates the ageing of hematopoietic stem cells. J Am Heart
Assoc. 3:e0002412014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tie G, Yan J, Khair L, Messina JA, Deng A,
Kang J, Fazzio T and Messina LM: Hypercholesterolemia increases
colorectal cancer incidence by reducing production of NKT and γδ T
cells from hematopoietic stem cells. Cancer Res. 77:2351–2362.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X,
Zhang G and Chen W: B7-H3 inhibits the IFN-γ-dependent cytotoxicity
of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology.
9:17489912020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bas A, Swamy M, Abeler-Dorner L, Williams
G, Pang DJ, Barbee SD and Hayday AC: Butyrophilin-like 1 encodes an
enterocyte protein that selectively regulates functional
interactions with T lymphocytes. Proc Natl Acad Sci USA.
108:4376–4381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Di Marco Barros R, Roberts NA, Dart RJ,
Vantourout P, Jandke A, Nussbaumer O, Deban L, Cipolat S, Hart R,
Iannitto ML, et al: Epithelia use Butyrophilin-like molecules to
shape organ-Specific γδ T cell compartments. Cell. 167:203–218.e17.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Du Y, Peng Q, Cheng D, Pan T, Sun W, Wang
H, Ma X, He R, Zhang H, Cui Z, et al: Cancer Cell-expressed BTNL2
facilitates tumour immune escape via engagement with
IL-17A-producing γδ T cells. Nat Commun. 13:2312022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Harly C, Guillaume Y, Nedellec S, Peigne
CM, Monkkonen H, Monkkonen J, Li J, Kuball J, Adams EJ, Netzer S,
et al: Key implication of CD277/butyrophilin-3 (BTN3A) in cellular
stress sensing by a major human γδ T-cell subset. Blood.
120:2269–2279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen S, Li Z, Huang W, Wang Y and Fan S:
Prognostic and therapeutic significance of BTN3A proteins in
tumors. J Cancer. 12:4505–4512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Palakodeti A, Sandstrom A, Sundaresan L,
Harly C, Nedellec S, Olive D, Scotet E, Bonneville M and Adams EJ:
The molecular basis for modulation of human Vγ9Vδ2 T cell responses
by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J Biol Chem.
287:32780–32790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cano CE, Pasero C, De Gassart A, Kerneur
C, Gabriac M, Fullana M, Granarolo E, Hoet R, Scotet E, Rafia C, et
al: BTN2A1, an immune checkpoint targeting Vγ9Vδ2 T cell
cytotoxicity against malignant cells. Cell Rep. 36:1093592021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
De Gassart A, Le KS, Brune P, Agaugue S,
Sims J, Goubard A, Castellano R, Joalland N, Scotet E, Collette Y,
et al: Development of ICT01, a first-in-class, anti-BTN3A antibody
for activating Vγ9Vδ2 T cell-mediated antitumor immune response.
Sci Transl Med. 13:eabj08352021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Blazquez JL, Benyamine A, Pasero C and
Olive D: New insights into the regulation of γδ T cells by BTN3A
and Other BTN/BTNL in tumor immunity. Front Immunol. 9:16012018.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Seiwert N, Adam J, Steinberg P, Wirtz S,
Schwerdtle T, Adams-Quack P, Hovelmeyer N, Kaina B, Foersch S and
Fahrer J: Chronic intestinal inflammation drives colorectal tumor
formation triggered by dietary heme iron in vivo. Arch Toxicol.
95:2507–2522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Santiago L, Castro M, Sanz-Pamplona R,
Garzon M, Ramirez-Labrada A, Tapia E, Moreno V, Layunta E,
Gil-Gomez G, Garrido M, et al: Extracellular granzyme A promotes
colorectal cancer development by enhancing gut inflammation. Cell
Rep. 32:1078472020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lebrero-Fernandez C, Wenzel UA, Akeus P,
Wang Y, Strid H, Simren M, Gustavsson B, Borjesson LG, Cardell SL,
Ohman L, et al: Altered expression of Butyrophilin (BTN) and
BTN-like (BTNL) genes in intestinal inflammation and colon cancer.
Immun Inflamm Dis. 4:191–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu J, Wu M, Yang Y, Wang Z, He S, Tian X
and Wang H: γδ T cells and the PD-1/PD-L1 axis: A love-hate
relationship in the tumor microenvironment. J Transl Med.
22:5532024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wu K, Feng J, Xiu Y, Li Z, Lin Z, Zhao H,
Zeng H, Xia W, Yu L and Xu B: Vδ2 T cell subsets, defined by PD-1
and TIM-3 expression, present varied cytokine responses in acute
myeloid leukemia patients. Int Immunopharmacol. 80:1061222020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pan T, Yang H, Wang WY, Rui YY, Deng ZJ,
Chen YC, Liu C and Hu H: Neoadjuvant immunotherapy with ipilimumab
plus nivolumab in mismatch repair Deficient/Microsatellite
Instability-High colorectal cancer: A preliminary report of case
series. Clin Colorectal Cancer. 23:104–110. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li X, Lu H, Gu Y, Zhang X, Zhang G, Shi T
and Chen W: Tim-3 suppresses the killing effect of Vγ9Vδ2 T cells
on colon cancer cells by reducing perforin and granzyme B
expression. Exp Cell Res. 386:1117192020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Guo C, Dai X, Du Y, Xiong X and Gui X:
Preclinical development of a novel CCR8/CTLA-4 bispecific antibody
for cancer treatment by disrupting CTLA-4 signaling on CD8 T cells
and specifically depleting tumor-resident Tregs. Cancer Immunol
Immunother. 73:2102024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Aggarwal V, Workman CJ and Vignali DAA:
LAG-3 as the third checkpoint inhibitor. Nat Immunol. 24:1415–1422.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Perales O, Jilaveanu L, Adeniran A, Su DG,
Hurwitz M, Braun DA, Kluger HM and Schoenfeld DA: TIGIT expression
in renal cell carcinoma infiltrating T cells is variable and
inversely correlated with PD-1 and LAG3. Cancer Immunol Immunother.
73:1922024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bhat AA, Nisar S, Singh M, Ashraf B,
Masoodi T, Prasad CP, Sharma A, Maacha S, Karedath T, Hashem S, et
al: Cytokine- and chemokine-induced inflammatory colorectal tumor
microenvironment: Emerging avenue for targeted therapy. Cancer
Commun (Lond). 42:689–715. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shu Y and Zheng S: The current status and
prospect of immunotherapy in colorectal cancer. Clin Transl Oncol.
26:39–51. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen DS and Mellman I: Elements of cancer
immunity and the Cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yi Y, He HW, Wang JX, Cai XY, Li YW, Zhou
J, Cheng YF, Jin JJ, Fan J and Qiu SJ: The functional impairment of
HCC-infiltrating γδ T cells, partially mediated by regulatory T
cells in a TGFβ- and IL-10-dependent manner. J Hepatol. 58:977–983.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hu G, Wu P, Cheng P, Zhang Z, Wang Z, Yu
X, Shao X, Wu D, Ye J, Zhang T, et al: Tumor-infiltrating CD39+γδ
Tregs are novel immunosuppressive T cells in human colorectal
cancer. Oncoimmunology. 6:e12773052017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhan Y, Zheng L, Liu J, Hu D, Wang J, Liu
K, Guo J, Zhang T and Kong D: PLA2G4A promotes Right-sided
colorectal cancer progression by inducing CD39+γδ Treg
polarization. JCI Insight. 6:e1480282021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen Z, Han F, Du Y, Shi H and Zhou W:
Hypoxic microenvironment in cancer: Molecular mechanisms and
therapeutic interventions. Signal Transduct Target Ther. 8:702023.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li L, Cao B, Liang X, Lu S, Luo H, Wang Z,
Wang S, Jiang J, Lang J and Zhu G: Microenvironmental oxygen
pressure orchestrates an anti- and pro-tumoral γδ T cell
equilibrium via tumor-derived exosomes. Oncogene. 38:2830–2843.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Allen J and Sears CL: Impact of the gut
microbiome on the genome and epigenome of colon epithelial cells:
Contributions to colorectal cancer development. Genome Med.
11:112019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li Y, Wang Y, Shi F, Zhang X, Zhang Y, Bi
K, Chen X, Li L and Diao H: Phospholipid metabolites of the gut
microbiota promote hypoxia-induced intestinal injury via
CD1d-dependent γδ T cells. Gut Microbes. 14:20969942022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Casanova MR, Azevedo-Silva J, Rodrigues LR
and Preto A: Colorectal cancer cells increase the production of
short chain fatty acids by propionibacterium freudenreichii
impacting on cancer cells survival. Front Nutr. 5:442018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Dupraz L, Magniez A, Rolhion N, Richard
ML, Da Costa G, Touch S, Mayeur C, Planchais J, Agus A, Danne C, et
al: Gut microbiota-derived short-chain fatty acids regulate IL-17
production by mouse and human intestinal γδ T cells. Cell Rep.
36:1093322021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cox LM, Maghzi AH, Liu S, Tankou SK, Dhang
FH, Willocq V, Song A, Wasen C, Tauhid S, Chu R, et al: Gut
microbiome in progressive multiple sclerosis. Ann Neurol.
89:1195–1211. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sandstrom A, Peigne CM, Leger A, Crooks
JE, Konczak F, Gesnel MC, Breathnach R, Bonneville M, Scotet E and
Adams EJ: The intracellular B30.2 domain of butyrophilin 3A1 binds
phosphoantigens to mediate activation of human Vγ9Vδ2 T cells.
Immunity. 40:490–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Roselli M, Finamore A, Nuccitelli S,
Carnevali P, Brigidi P, Vitali B, Nobili F, Rami R, Garaguso I and
Mengheri E: Prevention of TNBS-induced colitis by different
Lactobacillus and Bifidobacterium strains is associated with an
expansion of gammadeltaT and regulatory T cells of intestinal
intraepithelial lymphocytes. Inflamm Bowel Dis. 15:1526–1536. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ustjanzew A, Sencio V, Trottein F, Faber
J, Sandhoff R and Paret C: Interaction between bacteria and the
immune system for cancer immunotherapy: The α-GalCer alliance. Int
J Mol Sci. 23:58962022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Baxter NT, Ruffin MT, Rogers MAM and
Schloss PD: Microbiota-based model improves the sensitivity of
fecal immunochemical test for detecting colonic lesions. Genome
Medicine. 8:372016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liang Q, Chiu J, Chen Y, Huang Y,
Higashimori A, Fang J, Brim H, Ashktorab H, Ng SC, Ng SSM, et al:
Fecal bacteria act as novel biomarkers for noninvasive diagnosis of
colorectal cancer. Clin Cancer Res. 23:2061–2070. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yu J, Feng Q, Wong SH, Zhang D, Liang QY,
Qin Y, Tang L, Zhao H, Stenvang J, Li Y, et al: Metagenomic
analysis of faecal microbiome as a tool towards targeted
non-invasive biomarkers for colorectal cancer. Gut. 66:70–78. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ooki A, Shinozaki E and Yamaguchi K:
Immunotherapy in colorectal cancer: Current and future strategies.
J Anus Rectum Colon. 5:11–24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Heregger R, Huemer F, Steiner M,
Gonzalez-Martinez A, Greil R and Weiss L: Unraveling resistance to
immunotherapy in MSI-High colorectal cancer. Cancers (Basel).
15:50902023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Stary V, Pandey RV, List J, Kleissl L,
Deckert F, Kabiljo J, Laengle J, Gerakopoulos V, Oehler R, Watzke
L, et al: Dysfunctional tumor-infiltrating Vδ1 + T lymphocytes in
microsatellite-stable colorectal cancer. Nat Commun. 15:69492024.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wu Z, Lamao Q, Gu M, Jin X, Liu Y, Tian F,
Yu Y, Yuan P, Gao S, Fulford TS, et al: Unsynchronized butyrophilin
molecules dictate cancer cell evasion of Vγ9Vδ2 T-cell killing.
Cell Mol Immunol. 21:362–373. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Xu Y, Xiang Z, Alnaggar M, Kouakanou L, Li
J, He J, Yang J, Hu Y, Chen Y, Lin L, et al: Allogeneic Vγ9Vδ2
T-cell immunotherapy exhibits promising clinical safety and
prolongs the survival of patients with late-stage lung or liver
cancer. Cell Mol Immunol. 18:427–439. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yang XM, Lin XD, Shi W, Xie SX, Huang XN,
Yin SH, Jiang XB, Hammock BD, Xu ZP and Lu XL: Nanobody-based
bispecific T-cell engager (Nb-BiTE): A new platform for enhanced
T-cell immunotherapy. Signal Transduct Target Ther. 8:3282023.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Magee MS, Abraham TS, Baybutt TR,
Flickinger JC Jr, Ridge NA, Marszalowicz GP, Prajapati P,
Hersperger AR, Waldman SA and Snook AE: Human GUCY2C-targeted
chimeric antigen receptor (CAR)-expressing T cells eliminate
colorectal cancer metastases. Cancer Immunol Res. 6:509–516. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li M, Li S, Zhao R, Lv J, Zheng D, Qin L,
Li S, Wu Q, Long Y, Tang Z, et al: CD318 is a target of chimeric
antigen receptor T cells for the treatment of colorectal cancer.
Clin Exp Med. 23:2409–2419. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nicol AJ, Tokuyama H, Mattarollo SR, Hagi
T, Suzuki K, Yokokawa K and Nieda M: Clinical evaluation of
autologous gamma delta T cell-based immunotherapy for metastatic
solid tumours. Br J Cancer. 105:778–786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kamrani A, Nasiri H, Hassanzadeh A,
Ahmadian Heris J, Mohammadinasab R, Sadeghvand S, Sadeghi M,
Valedkarimi Z, Hosseinzadeh R, Shomali N, et al: New immunotherapy
approaches for colorectal cancer: Focusing on CAR-T cell, BiTE, and
oncolytic viruses. Cell Commun Signal. 22:562024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Van De Vyver AJ, Marrer-Berger E, Wang K,
Lehr T and Walz AC: Cytokine release syndrome by T-cell-Redirecting
therapies: Can we predict and modulate patient risk? Clin Cancer
Res. 27:6083–6094. 2021. View Article : Google Scholar : PubMed/NCBI
|