Introduction
Malignant cancers are among the most prevalent
diseases, constituting a complex and dynamically evolving process.
The incidence of malignant tumors is continuously increasing each
year, and it is estimated that by 2050, the number of new cancer
cases per year will reach 35 million (1). Since the late 1990s, molecular
targeted therapy and bio-cellular immunotherapy have emerged as
prevailing therapeutic modalities in the oncological landscape
(2). To enhance the efficacy of
tumor treatment and improve patient prognoses, there is a continual
necessity to identify novel targets, thereby paving the way for the
identification of novel pharmaceutical agents and combinatorial
treatment strategies.
Collagen, the most abundant protein in mammals,
comprises a diverse family of 28 members, which are intricately
associated with the occurrence, progression and prognosis of
different cancer types, including breast, colorectal, gastric, lung
and cervical carcinomas (3–8). The findings of previous studies have
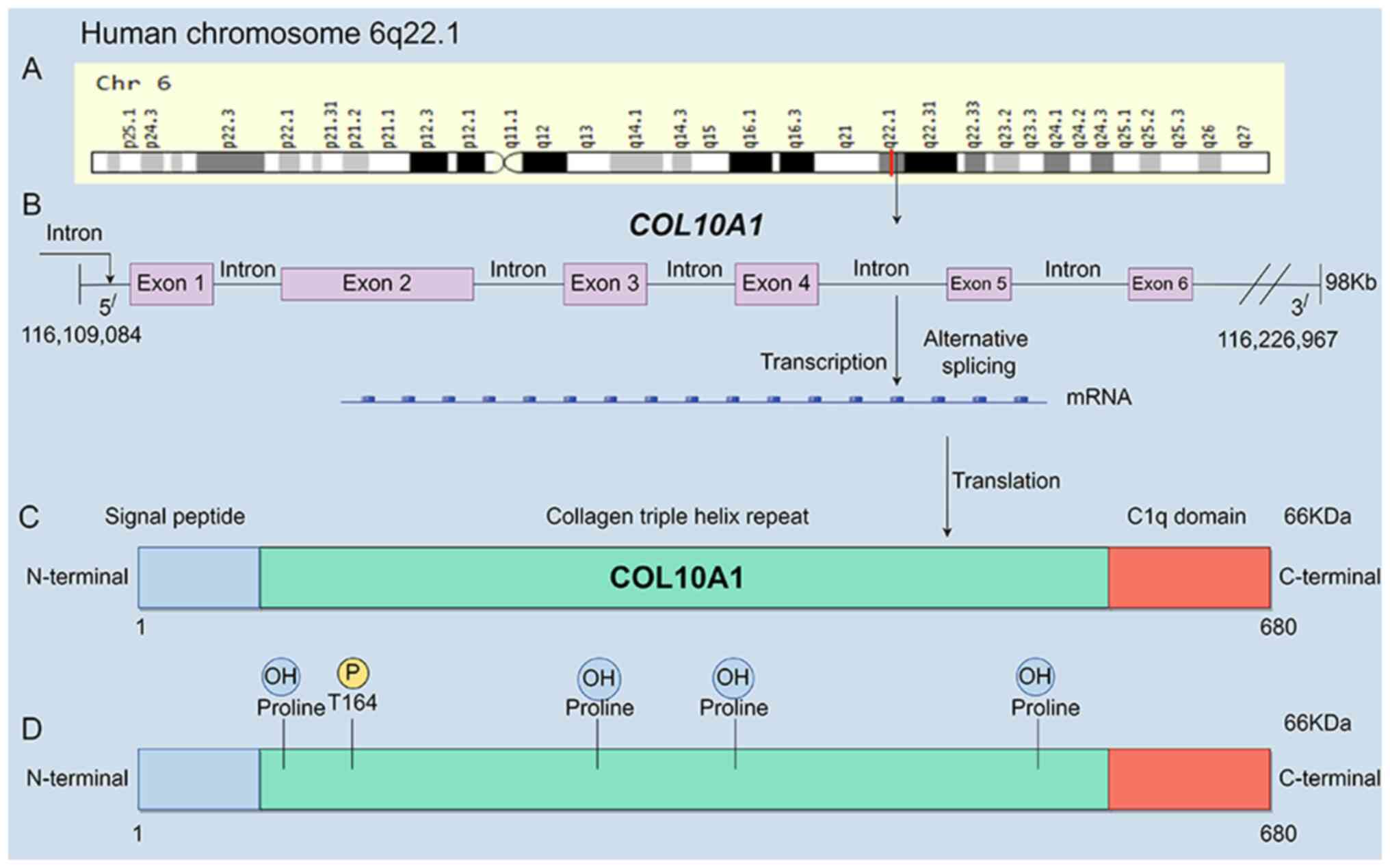
highlighted the significance of collagen type X α1 chain
(COL10A1), located on chromosome 6q22.1, as a gene
associated with disease progression. COL10A1, known as Xα collagen,
is a secretory, cartilage-specific short-chain collagen, serving as
a principal component of the interstitial matrix (9). As a secreted protein, it is
predominantly expressed in the cytoplasm and extracellular matrix
(ECM), and serves a role in the development of Schmid metaphyseal
chondrodysplasia (9). Upregulation
of COL10A1 expression has been observed in multiple human
malignancies, with its translated product primarily encompassing
three structural domains: Signal peptide, collagen triple helix
repeat and C1q domain (https://www.genecards.org/cgi-bin/carddisp.pl?gene=COL10A1&keywords=COL10A1)
(Fig. 1). Numerous studies have
focused on COL10A1, making it a focal point of evaluation. Research
indicates that the triple helical region of Xα collagen harbors
specific discoidin domain receptor tyrosine kinase 2 (DDR2) binding
sites capable of receptor activation, thereby facilitating
interactions with chondrocytes and other cells via the α2β1
integrin (10,11). As a ligand and coactivator, COL10A1
participates in a diverse range of cellular processes, including
the induction of epithelial-mesenchymal transition (EMT),
modulation of the ECM, regulation of the cell cycle and
proliferation (12–15). Upregulation of COL10A1 and
downstream signaling pathways influences cellular biology via
multiple mechanism, thereby promoting carcinogenesis. COL10A1 was
initially identified, alongside MMP13, cathelicidin antimicrobial
peptide and DNA damage-induced apoptosis suppressor, as one of the
genes encoding proteins that were more specifically secreted in
breast cancer based on differential gene expression analysis.
Subsequent research has also revealed high COL10A1 expression in
other human cancer types (16,17).
COL10A1 has been characterized as a bidirectional communication
regulator between tumor cells and cancer-associated fibroblasts
(CAFs). COL10A1, as one of the ECM-associated proteins, promotes
ECM deposition and remodeling in non-small cell lung cancer (NSCLC)
(18). Based on the evidence
obtained to date, high COL10A1 expression is associated with higher
histological grades, lower overall survival rates, dysregulated
apoptosis, tumor metastasis and recurrence in a range of malignant
tumors. However, the precise oncogenic signaling pathways mediated
by COL10A1 remain incompletely elucidated.
In humans, the upregulation of COL10A1 expression in
different types of malignant tumors results in an imbalance between
tumor cell proliferation and apoptosis, thereby contributing to
tumorigenesis (19). However, on
the basis of the considerable progress in research conducted to
date, it has been established that the primary mechanistic effect
of COL10A1 differs among different types of malignancies (Table I). The present comprehensive review
focuses on studies that have sought to elucidate the structure,
biological functions and role of COL10A1 in different types of
malignant tumors.
 | Table I.Expression and function of COL10A1 in
human malignant tumors. |
Table I.
Expression and function of COL10A1 in
human malignant tumors.
| First author/s,
year | Tumor type | COL10A1
expression | Function in
tumors | Mechanism | (Refs.) |
|---|
| Liang et al,
2020 | Lung cancer | Upregulation | Reconstructs the
ECM, and governs the proliferation and metastasis of LUAD
cells | COL10A1/DDR2/FAK
axis | (13) |
| Li et al,
2022 |
| Upregulation | Stimulates the
proliferation of LUSC cells and impedes apoptosis-induced oxidative
stress | Cancer-associated
fibroblasts undergo m6A methylation of COL10A1 mediated by
METTL3 | (35) |
| Guo et al,
2019 |
| Upregulation | Promotes cell
proliferation, and inhibits apoptosis and autophagy in NSCLC
cells | miR-384 | (60) |
| Li et al,
2018 | Gastric cancer | Upregulation | Facilitates EMT,
and promotes cell migration, invasion and metastasis | TGF-β1/SOX9
axis | (12) |
| Zhang et al,
2022 |
| Upregulation | Contributes to an
unfavorable prognosis in gastric cancer | Upregulation of
LEF1 and Wnt2 | (15) |
| Li et al,
2020 |
| Upregulation | Facilitates the
proliferation, migration and invasion of gastric cancer cells | miR-26a-5p | (59) |
| Aktas et al,
2022 |
| Upregulation | Contributes to the
progression of the tumor | Interactions
between the COL10A1 and SOX9 genes | (68) |
| Wen et al,
2022 | Pancreatic
cancer | Upregulation | Enhances the
proliferation and migration of PDAC cells, resulting in EMT and
accelerating the progression of pancreatic cancer | Activation of the
MEK/ERK signaling pathway via the COL10A1/DDR2 axis | (50) |
| Xu et al,
2023 |
| Upregulation | Promotes cell
proliferation, migration and invasion | Regulates
CD276 | (73) |
| Zhang et al,
2023 |
| Upregulation | Promotes the
progression of pancreatic cancer | Upregulation of
COL10A1/FAP/FN1, activating the PI3K/AKT signaling pathway | (76) |
| Liu et al,
2022 |
| Upregulation | Facilitates immune
infiltration in pancreatic cancer | TUG1/miR-144-3p
axis | (57) |
| Ma et al,
2020 | Breast cancer | Upregulation | Facilitates the
proliferation, migration and invasion of breast cancer cells | Upregulates the
expression of P4HB | (54) |
| Vishnubalaji et
al, 2019 |
| Upregulation | Associated with the
HR+/HER2+ breast cancer molecular
subtype | TGFβ and FAK signal
transduction | (107) |
| Cen et al,
2023 | Prostate
cancer | Upregulation | Exerts a
considerable impact on cancer proliferation, metastasis, immune
therapy, and resistance to radiotherapy and chemotherapy | Activates the
endoplasmic reticulum stress mechanism | (87) |
| He et al,
2022 | Colorectal
cancer | Upregulation | Promotes the
proliferation, migration and invasion of colorectal cancer | VSNL1/COL10A1
axis | (95) |
| Sun et al,
2022 | Cervical
cancer | Upregulation | Promotes cell
proliferation, migration and EMT in cervical cancer | Activates the
TGF-β/Smad signaling pathway | (27) |
| Karagoz et
al, 2016 | Esophageal squamous
cell carcinoma | Upregulation | Facilitates the
occurrence and development of esophageal squamous cell
carcinoma | Metabolic
dysfunction in arachidonic acid metabolism and steroid hormone
biosynthesis pathways | (100) |
| Xie et al,
2019 | Ovarian cancer | Upregulation | Stimulates the
proliferation, invasion and migration of oral cancer cells | miR-101-3p in
extracellular vesicles from human bone marrow mesenchymal stem
cells | (105) |
| Guo et al,
2021 | Nasopharyngeal
carcinoma | Upregulation | Promotes the
development of nasopharyngeal carcinoma cells | NF-κB signaling
pathway and extracellular matrix organization | (106) |
Mechanisms of action of COL10A1 in
tumors
Potential positive feedback regulation
of COL10A1 and TGF-β signaling
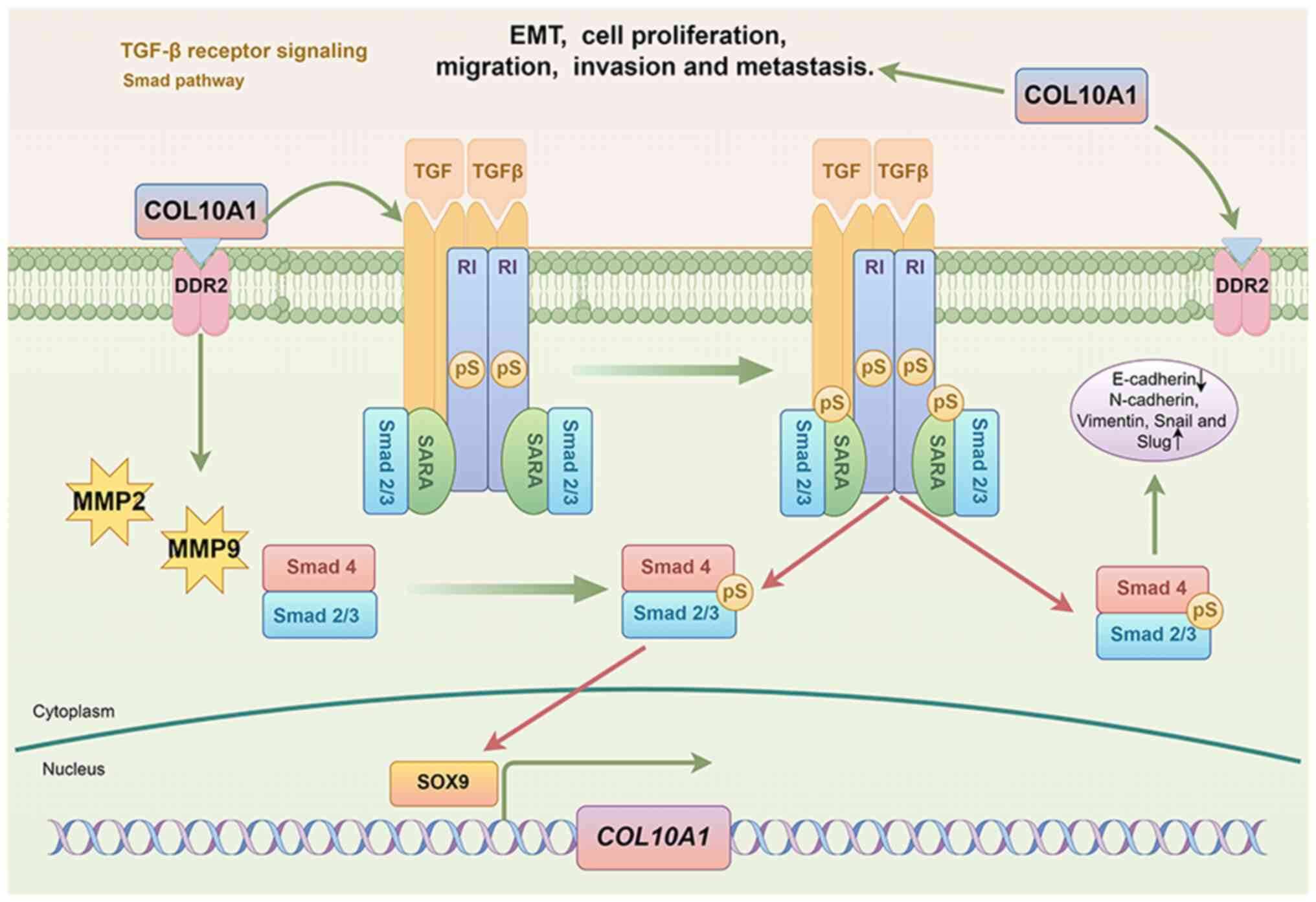
The findings of research conducted to date have
provided evidence to indicate that dysregulation of COL10A1 induces
EMT via the TGF-β1/SOX9 axis, thereby promoting invasion and
metastasis in gastric cancer (12).
The TGF-β/bone morphogenetic protein/SMAD signaling pathway, which
serves a pivotal role in cancer progression and metastasis, has
been the focus of a long history of research (20–22).
Upon activation by TGF-β1, the membrane receptor serine/threonine
kinase complex initiates a phosphorylation cascade of transcription
factors, including Smad1, Smad2 and Smad3. Having undergone
phosphorylation, these factors subsequently interact with Smad4,
forming complexes that undergo nuclear translocation (23). Bierie and Moses (24) observed that TGF-β signaling
modulates tumorigenesis, which is characterized by frequent
alterations in the associated signaling pathway during tumor
progression. SOX9, which functions as a transcription factor for
COL10A1, binds directly to the COL10A1 promoter, thus activating
its transcription and thereby enhancing COL10A1 expression. This
process mediates tumor EMT and promotes the malignant biological
behaviors of gastric cancer. Additionally, TGF-β1 stimulation has
been shown to increase the levels of SOX9, COL10A1 and
phosphorylated-Smad2 in MKN45 and SGC7901 gastric cancer cells.
TGF-β1 stimulation can activate Smad2 phosphorylation, leading to
changes in classical EMT markers (12) (Fig.
2). These findings provide further evidence to indicate that
COL10A1 may serve an essential role downstream of the TGF-β1/Smad2
signaling pathway (12). The
importance of the TGF-β signaling pathway in EMT has been
established in previous research. TGF-β signaling has been
implicated in coordinating immune suppression within the tumor
microenvironment and thereby contributing to cancer growth,
invasion, metastasis, recurrence and resistance to treatment
(25). Furthermore, TGF-β1
stimulation has been established to induce a reduction in
E-cadherin expression, and increases in vimentin, snail homolog 1
and snail homolog 2 expression, thereby promoting the migration and
invasion of tumor cells (26).
Conversely, the knockout of COL10A1 has been demonstrated to delay
TGF-β1-induced EMT (12).
Additionally, a study has indicated that by inactivating the
TGF-β/Smad signaling pathway, downregulation of COL10A1 can inhibit
the proliferation, migration and EMT of HeLa and C-33A cervical
cancer cells. Similarly, COL10A1 can enhance the expression of
TGF-β1 protein, modulate the phosphorylation of Smad2 and Smad3,
and activate the TGF-β/Smad signaling pathway (27). Collectively, the findings of these
studies tend to indicate that tumor cells induce EMT via a positive
feedback loop involving the TGF-β1/SMAD/SOX9/COL10A1 axis.
Activation of COL10A1 triggers the
focal adhesion kinase (FAK) signaling pathway
DDRs, including discoidin domain receptor tyrosine
kinase 1 and DDR2, constitute a unique class of receptor tyrosine
kinases activated by collagen at the cell-matrix interface. These
receptors are widely expressed in fetal and adult tissues, and both
experimental and clinical evidence indicates their dysregulation in
cancer (28). FAK, known for its
regulatory role in cellular survival, proliferation and migratory
processes, has been demonstrated to be upregulated in diverse
cancer types, including breast cancer (29). Disruption of DDR2 has been found to
alter focal adhesion orientation and subsequent ECM organization,
thereby modulating FAK and Yes1-associated transcriptional
regulator and WW domain-containing transcriptional regulator
1-mediated mesenchymal lineage cell signaling (30). It has been reported that DDR2 and
integrin β1 can interact with COL10A1 (11), and Chanez et al (31) found that the end-binding protein EB1
limits breast cancer cell invasive pseudopod formation and matrix
protein degradation via FAK. Another study has identified COL10A1
as a ligand for DDR2, with the interaction between COL10A1 and DDR2
promoting DDR2 phosphorylation, and thus, influencing downstream
FAK signaling, which contributes to the regulation of the malignant
progression of lung adenocarcinoma (LUAD) cells (13). Furthermore, integrins have been
established to induce EMT and activate non-canonical
FAK-independent signaling pathways, thereby preventing cancer cells
from undergoing integrin-mediated death, and thus, promoting cancer
cell metastasis (32). It is
hypothesized that COL10A1 induces EMT via its interaction with
DDR2, which, once activated, phosphorylates FAK, the expression
levels of which are thus maintained (Fig. 3A). As a consequence, expression of
the epithelial marker E-cadherin is downregulated, whereas
mesenchymal markers, such as N-cadherin and vimentin, are
upregulated, thereby promoting tumor cell migration and
proliferation. Furthermore, knockdown of COL10A1 in LUAD cells has
been demonstrated to result in a reduction in FAK expression,
whereas ectopic expression of COL10A1 leads to a marked increase in
FAK expression (13). These
findings indicate that COL10A1 contributes to malignant progression
by remodeling the ECM via the integrin/FAK signaling pathway.
 | Figure 3.COL10A1 activates signaling pathways
and regulates gene expression. (A) COL10A1 activates the FAK
signaling pathway. (B) COL10A1 regulates gene expression via
epitranscriptomic mechanisms. (C) COL10A1 regulates the
MAPK-related pathway. COL10A1 activates phosphorylated MEK and ERK
molecules by binding to DDR2 and subsequently activates a cascade
reaction. The figure was generated using Figdraw. CAFs,
cancer-associated fibroblasts; COL10A1, collagen type X α1 chain;
DDR2, discoidin domain receptor tyrosine kinase 2; EMT,
epithelial-mesenchymal transition; FAK, focal adhesion kinase; m6A,
N6-methyladenosine; me, methylation; METTL3, methyltransferase-like
3; p, phosphorylation; SAH, S-adenosylhomocysteine; SAM,
S-adenosylmethionine. |
COL10A1 regulates gene expression
through epitranscriptomic mechanisms
Cancer is a complex disease caused by genetic and
epitranscriptomic alterations in cell division control (33). Epitranscriptomics delineates the
realm of genetic modifications that have the capacity to influence
gene expression, whilst preserving the fundamental sequence of DNA
nucleotides (34). In lung squamous
cell carcinoma (LUSC), cancer-associated fibroblasts (CAFs) have
been shown to upregulate COL10A1 expression. This regulation occurs
through the enhancement of methyltransferase-like 3 (METTL3)
expression, which subsequently stabilizes COL10A1 expression within
CAFs via N6-methyladenosine (m6A) modification. This stabilization
facilitates the subsequent delivery of COL10A1 into LUSC cells,
thus promoting cell proliferation and suppressing oxidative
stress-induced apoptosis. Simultaneously, CAF-produced METTL3
promotes lung cancer cell proliferation by mediating the m6A
methylation of COL10A1 (35)
(Fig. 3B). This process contributes
to enhancing the levels of superoxide dismutase and glutathione
peroxidase, whilst reducing the rates of cell apoptosis and
reactive oxygen species production (35). m6A has been identified as the
predominant type of mRNA modification, catalyzed by the
methyltransferase complex. Within this complex, METTL3 assumes the
exclusive role of the catalytic subunit (36,37).
METTL3 has been extensively studied in the context of cancer,
including its role in regulating VGF nerve growth factor inducible
expression in LUAD cells, mediated by both transcriptional (via
histone modification) and post-transcriptional (via m6A
modification) mechanisms in a spatiotemporal manner (38). In this regard, Wang et al
(39) reported that elevated METTL3
expression promotes tumor angiogenesis and glycolysis in gastric
cancer, thereby enhancing tumor cell invasiveness. Additionally,
Guo et al (40) demonstrated
that a multi-omics model, specifically involving COL10A1, exhibited
superior prognostic accuracy for gastric cancer compared with
single-omics models. This model integrates data on mutations, copy
number variations, transcription, methylation and
clinicopathological changes, providing a more comprehensive
approach to prognosis prediction. In summary, the aforementioned
findings indicate that the functional activity of COL10A1 involves
epitranscriptomic mechanisms, via which it activates gene
transcription, thereby serving a pivotal role in cancer initiation
and progression by engaging CAFs within the tumor
microenvironment.
COL10A1 activates the MAPK signaling
pathway
Upregulated DDR2 expression has been detected in a
range of cancer cell types, in which it serves as a primary
regulator of EMT, promoting EMT processes in cancer types such as
hepatocellular carcinoma (41,42),
gastric cancer (43,44), breast cancer (45), and head and neck squamous cell
carcinoma (46). EMT is recognized
as a key regulatory process in tumor metastasis, endowing cells
with an invasive phenotype (47).
The MEK/ERK signaling pathway is a well-characterized MAPK
signaling pathway, and genetic alterations in this are among the
most common genetic alterations observed in human cancer (48). Cancer cells activate the MEK/ERK
signaling pathway to induce EMT, whereby ERK signal transduction
regulates key cellular processes such as proliferation, migration
and invasion (49). Research has
indicated that COL10A1 regulates PANC-1 and CFPAC-1 pancreatic
ductal adenocarcinoma (PDAC) cell proliferation and the MEK/ERK
signaling pathway by binding to DDR2, thereby promoting migration,
invasion and EMT (50). In PDAC
cells, COL10A1, through its binding to and phosphorylation of DDR2,
has been demonstrated to activate the MEK/ERK signaling pathway,
with the subsequent phosphorylation of MEK and ERK leading to
increases in the protein levels of EMT pathway markers (E-cadherin,
N-cadherin, vimentin and snail homolog 1), thereby promoting the
malignant biological behaviors of these cells, resulting in poor
patient prognosis. Furthermore, Wen et al (50) demonstrated that upregulation of
COL10A1 expression leads to a reduction in the expression levels of
the epithelial marker E-cadherin, and increases in the expression
levels of the mesenchymal markers N-cadherin and vimentin. However,
treatment with the ERK inhibitor SCH772984 was observed to reverse
EMT in PDAC cells in the group with COL10A1 overexpression. These
findings indicate an association between COL10A1-DDR2 expression
and the MEK/ERK signaling pathway (Fig.
3C).
COL10A1 targets prolyl 4-hydroxylase
subunit β (P4HB) to promote tumor cell migration and invasion
P4HB is a human chromosomal gene that encodes an
endoplasmic reticulum (ER) molecular chaperone protein with
oxidoreductase, co-chaperone and isomerase activities (51). P4HB has been demonstrated to serve
roles in carcinogenesis and cancer progression (52); for example, by acting as an
inhibitor of ferroptosis, thereby promoting tumor cell
proliferation (53). Ma et
al (54) found that EMT and the
β-catenin/Snail signaling pathway influence the regulatory role of
P4HB in the chemoresistance of hepatocellular carcinoma, and their
study has provided evidence indicating an important role for P4HB
in the progression of cancer. Furthermore, by positively regulating
P4HB expression, the upregulation of COL10A1 enhances the
proliferation and clonogenicity of MCF-7 and MDA-MB-231 breast
cancer cells, and promotes breast cancer cell migration and
invasion (55). Using rescue
experiments, it has been demonstrated that downregulating P4HB
suppresses the promotive effects of overexpression of COL10A1 on
the proliferation, migration and invasion of breast cancer cells
(55). In summary, by regulating
P4HB, COL10A1 can contribute to the control of tumor cell
phenotypes (Fig. 4). In breast
cancer cells, COL10A1 enhances cell proliferation, migration and
invasion by targeting P4HB. However, further in-depth research is
required to identify the signaling pathway associated with the
COL10A1/P4HB axis and its transcriptional regulation.
 | Figure 4.Role of COL10A1 in malignant tumors.
TUG1 inhibits miR-144-3p to enhance COL10A1 transcription, while
miR-384 and extracellular vesicle-contained miR-101-3p suppress
COL10A1 transcription. COL10A1, via the TGF-β1/SMAD, FAK, MAPK and
Wnt2 signaling pathways, and targeted action against P4HB, promotes
tumor cell proliferation. Additionally, COL10A1 promotes METTL3
expression, which upregulates the methylation of COL10A1 itself,
thereby further facilitating tumorigenesis. The figure was
generated using Figdraw. COL10A1, collagen type X α1 chain; DDR2,
discoidin domain receptor tyrosine kinase 2; EMT,
epithelial-mesenchymal transition; FAK, focal adhesion kinase; FAP,
fibroblast activation protein; FN1, fibronectin 1; LEF1, lymphoid
enhancer binding factor 1; METTL3, methyltransferase-like 3; miR,
microRNA; P4HB, prolyl 4-hydroxylase subunit β; TUG1, taurine
upregulated gene 1; VSNL1, visinin-like protein 1. |
Competing endogenous RNA (ceRNA)
network and microRNA (miRNA/miR) regulation of COL10A1
COL10A1 can undergo gene silencing by binding with
miRNAs, whereas long non-coding RNAs (lncRNAs) can modulate the
occurrence, invasion and metastasis of tumors by competitively
binding to miRNAs. The ceRNA network connects the functions of
protein-coding mRNAs with those of non-coding RNAs (such as miRNAs,
lncRNAs, pseudogene RNAs and circular RNAs), and dysregulation of
the ceRNA network is implicated in a range of human diseases,
including cancer (56). Using
bioinformatics analysis, Liu et al (57) found that lncRNA taurine upregulated
gene 1 (TUG1) participates in the miR-144-3p/COL10A1 axis,
promoting immune cell infiltration in pancreatic cancer. However,
this involvement has yet to be experimentally validated and has
only been assessed at the level of data analysis. Liu et al
(58) constructed a circular
RNA-miRNA-mRNA regulatory network, thereby providing a novel
perspective for elucidating the ceRNA regulatory mechanisms of
COL10A1 in stomach adenocarcinoma. Additionally, a study has
indicated that by targeting COL10A1, miR-26a-5p can enhance the
proliferation, migration and invasion of MKN-45 gastric cancer
cells (59). Furthermore,
miR-26a-5p has been found to inhibit the occurrence and development
of gastric cancer by reducing the expression levels of COL10A1, and
dual-luciferase assays have further confirmed the targeting
relationship between miR-26a-5p and COL10A1 (59). In addition, Guo et al
(60) found that miR-384 can
downregulate COL10A1 levels, thereby inhibiting NSCLC cell
proliferation, and promoting apoptosis and autophagy. Collectively,
these findings indicate that COL10A1 can be regulated by miRNAs,
thereby influencing the development of tumor cells in different
diseases (Fig. 4).
Function of COL10A1 in tumors
Lung cancer
As the most commonly diagnosed cancer, lung cancer
is a leading cause of cancer incidence and mortality in both men
and women (1). With the development
of numerous effective targeted therapies and immunotherapies,
immune checkpoint inhibitors have shown benefits in the treatment
of lung cancer. Currently, these inhibitors are used as a
first-line treatment for metastatic disease and consolidative
therapy for unresectable locally advanced disease
post-chemoradiotherapy, as well as adjuvant therapy for resectable
tumors following surgery and chemotherapy (61). In patients with LUAD, variations in
the expression levels of COL10A1 have been observed, with higher
levels of COL10A1 mRNA being associated with a shorter overall
survival, and upregulation of COL10A1 is positively associated with
white blood cell transendothelial migration, vascular smooth muscle
contraction and ECM-receptor interaction in LUAD (62). Wu et al (63) discerned notable distinctions in the
interplay between COL10A1 and different cellular pathways within
the context of NSCLC, including the inositol phosphate metabolism
pathway, focal adhesion signaling pathway, vascular smooth muscle
contraction signaling pathway, peroxisome proliferator-activated
receptor signaling pathway and calcium signaling pathway. A further
study has indicated that the diversity of interactions is
attributed to the regulation of LUAD cell proliferation and
migration by the COL10A1/DDR2/FAK axis, and elevated levels of
COL10A1 promote remodeling of the ECM, exhibiting a positive
association with lymph node metastasis (13). Research has also confirmed that
COL10A1, COL11A1 and secreted phosphoprotein 1, as core genes
originating from the embryonic mesoderm, serve pivotal roles in the
ECM-receptor interaction and cell adhesion signaling pathways,
potentially forming networks in lung and gastric cancer (64). Furthermore, miR-384 has been shown
to downregulate COL10A1 levels, thereby inhibiting NSCLC cell
proliferation, and promoting apoptosis and autophagy (60). CAFs, via METTL3-mediated mRNA
methylation modification, promote the secretion of COL10A1, the
upregulation of which promotes SW900 and LOU-NH91 LUSC cell
proliferation and inhibits apoptosis-induced oxidative stress
(35). As a soluble factor
associated with tumor-stroma crosstalk, COL10A1 may also serve as a
potential marker to distinguish patients with lung cancer from
heavy smokers (18). Upregulation
of COL10A1 expression has been established to be closely associated
with LUAD lymph node metastasis positivity, thereby serving as an
independent prognostic marker for poor outcomes in patients with
lung cancer.
Gastric cancer
Gastric cancer ranks fifth globally in terms of
incidence and fifth in terms of mortality among all cancer types
(1). Despite recent therapeutic
advances that have reduced the incidence and associated mortality,
the prognosis of gastric cancer remains poor, with a 5-year
survival rate of 20–30% (65).
Upregulation of COL10A1 expression has been identified as an
adverse prognostic indicator in gastric cancer, being associated
with tumor occurrence or pathological grading in gastrointestinal
cancer (66,67). COL10A1 has been found to regulate
gastric cancer progression via a number of mechanisms. First, the
TGF-β1/SOX9 axis promotes upregulation of COL10A1, thereby
contributing to invasive and metastatic tendencies in gastric
cancer via EMT (12,68). Second, the transcription factor
lymphoid enhancer-binding factor 1 (LEF1) upregulates COL10A1
expression, which is associated with reduced survival rates in
patients with gastric cancer exhibiting high COL10A1 levels
compared with those with lower levels. This upregulation
contributes to a poorer prognosis by driving the Wnt2 signaling
pathway (15). Third, by
attenuating the expression of COL10A1, miR-26a-5p has inhibitory
effects on gastric cancer cell proliferation, migration and
invasion (59). Fourth, COL10A1 may
influence tumor T staging and pathological staging by regulating
immune infiltration within the late-stage gastric cancer
microenvironment (69). The ECM
serves an undisputed role in tissue homeostasis, an imbalance of
which can lead to changes in mechanical and biochemical cues
influencing cancer initiation and progression (70). As a soluble ECM protein, increases
in the plasma levels of COL10A1 have been detected in patients with
gastric adenocarcinoma, and these elevated levels have been
demonstrated to be associated with cancer progression (71), which was validated by Li et
al (67), who used
comprehensive bioinformatics methods to identify a COL10A1 model
protein-protein interaction network involving ECM-receptor
interactions. In summary, upregulation of COL10A1 expression is
associated with clinical stage, and lymph node and distant
metastasis in gastric cancer, and thus, COL10A1 expression is an
independent prognostic factor for patients with gastric cancer.
Pancreatic cancer
Pancreatic cancer is among the most invasive,
high-mortality and poorly treatable types of tumors (72). COL10A1 is associated with the
malignant characteristics of pancreatic cancer. Increased COL10A1
expression in pancreatic cancer tissues has been established to
enhance the proliferation and migration of PDAC cells. The
COL10A1/DDR2 axis activates the MEK/ERK pathway, leading to EMT and
accelerating the progression of pancreatic cancer (50). Using bioinformatics analysis, Liu
et al (57) identified the
TUG1/miR-144-3p signaling pathway as the most likely upstream
non-coding RNA pathway for COL10A1, although further research is
needed, including experimental verification using luciferase
assays. Additionally, COL10A1 has been demonstrated to serve a key
role in regulating CD276 in Panc-1 pancreatic cancer cell
viability, migration, and invasion (73), with the knockdown of COL10A1
reducing CD276 expression, and upregulation of CD276 expression in
cells reversing the inhibition of proliferation and migration
induced by COL10A1 knockdown. In recent research, COL10A1, specific
to myofibroblast CAFs, has been observed to be elevated in
different solid tumor types and was associated with poor survival
(74). In related
gastroenteropancreatic neuroendocrine tumors, it has been
demonstrated that COL10A1 can also be used to differentiate between
primary and metastatic gastroenteropancreatic neuroendocrine
neoplasms with different antitumor phenotypes (75). Collectively, these findings
highlight the pivotal role of COL10A1 in the occurrence and
development of pancreatic cancer. Zhang et al (76) also reported that the upregulation of
characteristic genes (COL10A1/fibroblast-activation
protein/fibronectin 1) in Coronavirus Disease 2019 may promote the
progression of pancreatic cancer by activating the PI3K/AKT
signaling pathway. In conclusion, upregulation of COL10A1 appears
to be closely associated with increased proliferation and migratory
potential of pancreatic cancer cells.
Breast cancer
Breast cancer is the most common type of cancer in
women and the second leading cause of cancer-related deaths in
women worldwide (77). Although
treatment options for breast cancer have undergone improvement,
late-stage breast cancer and triple-negative breast cancer continue
to present challenges (78).
Although current immunotherapy and molecular targeted therapies
have contributed to a substantial improvement in patient survival,
challenges such as immune escape, drug resistance and poor
apoptosis still need to be addressed (79). As attention turns to the therapeutic
potential of targeting COL10A1 in breast cancer, it has been found
that knocking down this collagen protein can disrupt the
interaction between COL10A1 and P4HB, thereby inhibiting the
proliferation, migration and invasion of breast cancer cells
(54). A bioinformatics
investigation has revealed a favorable association between COL10A1
expression and estrogen receptor, progesterone receptor and HER-2
status, as well as lymph node status in breast cancer samples. In
tumor tissues, COL10A1 levels have been shown to be inversely
associated with age, Scarff-Bloom-Richardson grade, basal-like
status and triple-negative status, whereas these associations were
not observed in normal tissues (80). In addition, the downregulation of
COL14A1 has been found to be associated with invasive, basal-like
and Her-2/neu breast cancer subtypes (81). Brodsky et al (82) demonstrated that an increase in
stromal COL10A1 expression is associated with poor pathological
response in estrogen receptor-positive/HER2+ breast
tumors and low tumor-infiltrating lymphocyte levels. Furthermore,
COL10A1 has been found to be associated with immune cell
infiltration, being positively associated with γδT cells and M1
macrophages, and being negatively associated with CD8 T cells,
monocytes and follicular helper T cells (83,84).
In addition, the findings of a study have provided evidence to
indicate that COL10A1 is associated with markers of CAF subtypes,
with CAF+ cells being particularly enriched in the ECM
pathway (85). Changes in the
quantity and composition of the ECM are considered markers of tumor
development, and in this regard, protein imprinting analysis has
revealed an increase in the molecular levels of COL10A1 in
conditioned normal human dermal fibroblast cell lysates and
supernatants, potentially contributing to the diagnostic assessment
of suspicious breast nodules (86).
Furthermore, Zhang et al (80) conducted bioinformatics Gene Set
Enrichment Analysis to assess the significance of COL10A1 in breast
cancer prognosis, and observed enrichment of this protein in the
TGF-β signaling pathway, which may promote the migration and
invasion of tumor cells via this pathway. Consequently, targeting
of COL10A1 could emerge as a novel strategy in the clinical
treatment of breast cancer. Taken together, the evidence obtained
to date indicates that COL10A1 positively regulates the malignant
progression of breast cancer cells, and the development of
therapies targeting COL10A1 could provide novel strategies for the
treatment of invasive breast cancer.
Prostate cancer
Endoplasmic reticulum stress (ERS) has been
established to affect tumor growth, metastasis, immune therapy, and
resistance to radiotherapy and chemotherapy. COL10A1 has been found
to serve a key role in ERS in prostate cancer (87), where its high expression is
associated with poor patient prognosis. Analysis of the immune
relevance of COL10A1 in different cancer types has revealed an
association with tumor mutation burden, microsatellite instability
and immune cell infiltration. Additionally, knockdown of COL10A1
has been demonstrated to be associated with a substantial reduction
in the proliferation, migration and invasion of prostate cancer
cells (88). Further research has
indicated that COL10A1 may be involved in M2 macrophage
polarization in prostate cancer (89). Additionally, network analysis, based
on weighted gene co-expression network analysis modules, has been
used to predict the occurrence of bone metastasis in prostate
cancer, with the results indicating that the upregulation of
COL10A1 expression in prostate cancer is associated with disease
progression (90). However, given
the limited research on the involvement of COL10A1 in prostate
cancer, further clinical trials are required to validate these
findings.
Colorectal cancer
Colorectal cancer is the third most common type of
cancer and the fourth leading cause of cancer-related death
(91). COL10A1 expression is
frequently upregulated in most colorectal cancers, in which it is
considered to serve a carcinogenic role in progression, maintenance
and metastasis (92). For example,
colorectal cancer progression and EMT processes have been shown to
be associated with the aberrant expression of COL10A1 (93). As a consequence of EMT, cancer cells
acquire stronger migratory capacities, thereby facilitating a
dissociation from the primary tumor and the subsequent
establishment of distant metastatic foci. Research has indicated a
strong positive association between COL10A1 and the transcriptional
characteristics of CAFs and immune cell clusters (such as those of
B cells and macrophages), thereby providing evidence that COL10A1
transcription may mediate the interaction between tumor cells and
their stromal microenvironment (94). Furthermore, the levels of COL10A1
expression have been found to be associated with mismatch repair
defects and immune infiltration (95). He et al (95) reported that visinin-like protein 1
(VSNL1) could promote the proliferation, migration and invasion of
colorectal cancer cells by targeting COL10A1, whereas the
upregulation of COL10A1 could enhance the proliferation, migration
and invasion of colorectal cells, and reverse the effects of VSNL1
knockdown on SW480 and LoVo colorectal cancer cells.
2-cyanoacrylamido-4,5,6,7-tetrahydrobenzo[b]thiophene derivatives,
a novel class of compounds, have been found to downregulate COL10A1
expression and show promising anticancer activity in the treatment
of colon cancer (96). Accordingly,
although it has been established that COL10A1 serves a prominent
role in the occurrence and development of colorectal cancer,
research on targeting of COL10A1 for treatment remains limited.
Other types of malignant tumors
Research has revealed that COL10A1 expression is
upregulated in a range of different malignant tumor types, exerting
multiple pro-tumor effects on cell proliferation and invasion. In
cervical cancer, COL10A1 facilitates cell proliferation, migration
and the EMT process via modulation of the TGF-β/Smad signaling
pathway, and silencing of COL10A1 has been demonstrated to reduce
TGF-β1 protein levels and downregulate the phosphorylation of Smad2
and Smad3 (27). Additionally, as
an ECM-related protein, COL10A1 has been established to be
associated with immune cells, overall survival and bladder cancer
recurrence (97,98). For example, Wu et al
(99) demonstrated the efficacy of
a bladder cancer-associated COL10A1 genomic model in predicting
preoperative lymph node status, transurethral resection T stage and
lymphovascular invasion status. Upregulation of COL10A1 expression
is also associated with histological grading, tumor metastasis and
poor survival in esophageal squamous cell carcinoma, suggesting
that this protein may be a potential drug treatment target
(100–103). Similarly, Lapa et al
(104) revealed that COL10A1 could
serve as a drug target for laryngeal squamous cell carcinoma
(LSCC). By interacting with high-mobility group box DNA-binding
protein 1, COL10A1 modulates the cell cycle, and its abnormal
expression may regulate LSCC cell proliferation and survival
(14). The findings of a further
study have indicated that extracellular vesicles derived from human
bone marrow mesenchymal stem cells and carrying miR-101-3p
effectively restrain the proliferation, invasion and migration of
TCA8113 oral cancer cells, which is mediated via downregulation of
COL10A1 (105). Therefore, COL10A1
may function as a key regulatory factor in the development of oral
cancer. Furthermore, COL10A1 may participate in the occurrence of
nasopharyngeal carcinoma via the NF-κB signaling pathway and ECM
organization, and could thus serve as a molecular biomarker for the
early diagnosis of this cancer (106). However, research on the
involvement of COL10A1 in these aforementioned tumors is currently
limited, and thus, further clinical trials are required.
Analysis and future prospects
The present review provides a comprehensive overview
of the mechanisms and functions of COL10A1 in different cancer
types, including lung, gastric, pancreatic, breast, prostate and
colorectal cancer, and highlights the potential application of
COL10A1 as a promising biomarker for cancer diagnosis and in
therapeutic targeting. COL10A1 is implicated in tumor growth,
invasion, migration and EMT in multiple cancer types, involving the
TGF-β1/Smad, MEK/ERK and FAK signaling pathways, and P4HB protein
regulation. Additionally, by regulating COL10A1, miR-26a-5p and
miR-384 may serve important roles in influencing the development of
tumor cells in diseases. Preliminary findings suggest that COL10A1
could serve as a diagnostic biomarker or therapeutic target for
cancer, but further in vivo studies and clinical trials are
necessary to confirm these roles. Furthermore, on the basis of
METTL3-mediated m6A methylation, COL10A1 has been established to
serve pivotal roles in the epitranscriptomic mechanisms associated
with different malignancies, thereby providing novel therapeutic
avenues for cancer.
However, despite these promising therapeutic
applications, it should be emphasized that much of the data
reviewed in the present review is based on bioinformatics analyses,
highlighting the necessity for further experimental studies to
establish an optimal overview and minimize unnecessary batch
effects, whilst retaining biological signals. For example, the
specific interactions between COL10A1 and its transcription factor
LEF1, and the underlying regulatory mechanisms, as well as their
roles in oncogenesis, remain unclear. Furthermore, the
characteristics and involvement of the TUG1/miR-144-3p/COL10A1 axis
warrant further elucidation, and additional efforts are required to
develop specific inhibitors for COL10A1. Furthermore, given that
the pathological role served by COL10A1 in cancer development
appears to be dependent on the cell type and microenvironment, it
will be necessary develop novel COL10A1 subtype mouse models.
Nevertheless, COL10A1 remains a promising onco-promoter, with
considerable therapeutic potential in the diagnosis and treatment
of malignant tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82260604) and the National Natural
Science Foundation of Jiangxi Province (grant no.
20192BAB205053).
Availability of data and materials
Not applicable.
Authors' contributions
QY conceived the project and drafted the manuscript.
GZ, WZ, JW, XO and KY contributed to data analysis, manuscript
revision, discussions and language editing, and JZ revised the
manuscript. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
COL10A1
|
collagen type X α1 chain
|
|
TUG1
|
taurine upregulated gene 1
|
|
NSCLC
|
non-small cell lung cancer
|
|
FAK
|
focal adhesion kinase
|
|
EMT
|
epithelial-mesenchymal transition
|
|
LEF1
|
lymphoid enhancer binding factor 1
|
|
METTL3
|
methyltransferase-like 3
|
|
DDR2
|
discoidin domain receptor tyrosine
kinase 2
|
|
ECM
|
extracellular matrix
|
|
CAFs
|
cancer-associated fibroblasts
|
|
ERS
|
endoplasmic reticulum stress
|
|
LUAD
|
lung adenocarcinoma
|
|
VSNL1
|
visinin-like protein 1
|
|
LUSC
|
lung squamous cell carcinoma
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
LSCC
|
laryngeal squamous cell carcinoma
|
|
ER
|
endoplasmic reticulum
|
|
P4HB
|
prolyl 4-hydroxylase subunit β
|
|
ceRNA
|
competing endogenous RNA
|
|
miRNA
|
microRNA
|
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Min HY and Lee HY: Molecular targeted
therapy for anticancer treatment. Exp Mol Med. 54:1670–1694. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ricard-Blum S: The collagen family. Cold
Spring Harb Perspect Biol. 3:a0049782011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salimian N, Peymani M, Ghaedi K, Hashemi M
and Rahimi E: Collagen 1A1 (COL1A1) and Collagen11A1(COL11A1) as
diagnostic biomarkers in Breast, colorectal and gastric cancers.
Gene. 892:1478672024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu X, Cai J, Zuo Z and Li J: Collagen
facilitates the colorectal cancer stemness and metastasis through
an integrin/PI3K/AKT/Snail signaling pathway. Biomed Pharmacother.
114:1087082019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun H, Wang Y, Wang S, Xie Y, Sun K, Li S,
Cui W and Wang K: The involvement of collagen family genes in tumor
enlargement of gastric cancer. Sci Rep. 13:1002023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeltz C, Khalil M, Navab R and Tsao MS:
Collagen Type XI inhibits lung cancer-associated fibroblast
functions and restrains the integrin binding site availability on
collagen type I matrix. Int J Mol Sci. 23:117222022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Padežnik T, Oleksy A, Cokan A, Takač I and
Sobočan M: Changes in the extracellular matrix in endometrial and
cervical cancer: A systematic review. Int J Mol Sci. 24:54632023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Liang H, Liu W, Li X, Zhang W and
Shang X: A novel sequence variant in COL10A1 causing
spondylometaphyseal dysplasia accompanied with coxa valga: A case
report. Medicine (Baltimore). 98:e164852019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leitinger B and Kwan AP: The discoidin
domain receptor DDR2 is a receptor for type X collagen. Matrix
Biol. 25:355–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luckman SP, Rees E and Kwan AP: Partial
characterization of cell-type X collagen interactions. Biochem J.
372:485–493. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li T, Huang H, Shi G, Zhao L, Li T, Zhang
Z, Liu R, Hu Y, Liu H, Yu J and Li G: TGF-β1-SOX9 axis-inducible
COL10A1 promotes invasion and metastasis in gastric cancer via
epithelial-to-mesenchymal transition. Cell Death Dis. 9:8492018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang Y, Xia W, Zhang T, Chen B, Wang H,
Song X, Zhang Z, Xu L, Dong G and Jiang F: Upregulated collagen
COL10A1 remodels the extracellular matrix and promotes malignant
progression in lung adenocarcinoma. Front Oncol. 10:5735342020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu JY, Lan XL, Yan DM, Fang YY, Peng YX,
Liang FF, Jiang L, Huang SN, Mo M, Lin CX, et al: The clinical
significance of transcription factor WD repeat and HMG-box DNA
binding protein 1 in laryngeal squamous cell carcinoma and its
potential molecular mechanism. Pathol Res Pract. 230:1537512022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang M, Jin M, Gao Z, Yu W and Zhang W:
High COL10A1 expression potentially contributes to poor outcomes in
gastric cancer with the help of LEF1 and Wnt2. J Clin Lab Anal.
36:e246122022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang HJ, Yang MJ, Yang YH, Hou MF, Hsueh
EJ and Lin SR: MMP13 is potentially a new tumor marker for breast
cancer diagnosis. Oncol Rep. 22:1119–1127. 2009.PubMed/NCBI
|
|
17
|
Sun Y, Wang L, Jiang M, Huang J, Liu Z and
Wolfl S: Secreted phosphoprotein 1 upstream invasive network
construction and analysis of lung adenocarcinoma compared with
human normal adjacent tissues by integrative biocomputation. Cell
Biochem Biophys. 56:59–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andriani F, Landoni E, Mensah M,
Facchinetti F, Miceli R, Tagliabue E, Giussani M, Callari M, De
Cecco L, Colombo MP, et al: Diagnostic role of circulating
extracellular matrix-related proteins in non-small cell lung
cancer. BMC Cancer. 18:8992018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patra R, Das NC and Mukherjee S: Exploring
the differential expression and prognostic significance of the
COL11A1 gene in human colorectal carcinoma: An integrated
bioinformatics approach. Front Genet. 12:6083132021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung B, Staudacher JJ and Beauchamp D:
Transforming growth factor β superfamily signaling in development
of colorectal cancer. Gastroenterology. 152:36–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu R, Yin G, Tuo H, Guo Y, Zhu Y, Zhang
L, Yang W, Liu Q and Wang Y: METTL3-induced lncRNA GBAP1 promotes
hepatocellular carcinoma progression by activating BMP/SMAD
pathway. Biol Direct. 18:532023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iyengar PV, Marvin DL, Lama D, Tan TZ,
Suriyamurthy S, Xie F, van Dinther M, Mei H, Verma CS, Zhang L, et
al: TRAF4 inhibits bladder cancer progression by promoting BMP/SMAD
signaling. Mol Cancer Res. 20:1516–1531. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bierie B and Moses HL: TGF-beta and
cancer. Cytokine Growth Factor Rev. 17:29–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi X, Yang J, Deng S, Xu H, Wu D, Zeng Q,
Wang S, Hu T, Wu F and Zhou H: TGF-β signaling in the tumor
metabolic microenvironment and targeted therapies. J Hematol Oncol.
15:1352022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Yue GG, Yuen KK, Gao S, Leung PC,
Wong CK and Lau CB: Mechanistic insights into the anti-tumor and
anti-metastatic effects of Patrinia villosa aqueous extract in
colon cancer via modulation of TGF-β R1-smad2/3-E-cadherin and
FAK-RhoA-cofilin pathways. Phytomedicine. 117:1549002023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Ling J and Liu L: Collagen type X
alpha 1 promotes proliferation, invasion and epithelial-mesenchymal
transition of cervical cancer through activation of TGF-β/Smad
signaling. Physiol Int. May 18–2022.doi: 10.1556/2060.2022.00006
(Epub ahead of print). View Article : Google Scholar
|
|
28
|
Trono P, Ottavi F and Rosano L: Novel
insights into the role of Discoidin domain receptor 2 (DDR2) in
cancer progression: A new avenue of therapeutic intervention.
Matrix Biol. 125:31–39. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lv PC, Jiang AQ, Zhang WM and Zhu HL: FAK
inhibitors in cancer, a patent review. Expert Opin Ther Pat.
28:139–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pagani CA, Bancroft AC, Tower RJ,
Livingston N, Sun Y, Hong JY, Kent RN III, Strong AL, Nunez JH,
Medrano JMR, et al: Discoidin domain receptor 2 regulates aberrant
mesenchymal lineage cell fate and matrix organization. Sci Adv.
8:eabq61522022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chanez B, Ostacolo K, Badache A and
Thuault S: EB1 restricts breast cancer cell invadopodia formation
and matrix proteolysis via FAK. Cells. 10:3882021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li M, Wang Y, Li M, Wu X, Setrerrahmane S
and Xu H: Integrins as attractive targets for cancer therapeutics.
Acta Pharm Sin B. 11:2726–2737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Recillas-Targa F: Cancer epigenetics: An
overview. Arch Med Res. 53:732–740. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou S, Zeng H, Huang J, Lei L, Tong X, Li
S, Zhou Y, Guo H, Khan M, Luo L, et al: Epigenetic regulation of
melanogenesis. Ageing Res Rev. 69:1013492021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Li X, Deng M, Ye C, Peng Y and Lu Y:
Cancer-associated fibroblasts hinder lung squamous cell carcinoma
oxidative stress-induced apoptosis via METTL3 mediated m6A
methylation of COL10A1. Oxid Med Cell Longev. 2022:43208092022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng C, Huang W, Li Y and Weng H: Roles of
METTL3 in cancer: Mechanisms and therapeutic targeting. J Hematol
Oncol. 13:1172020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu Y, Song M, Hong Z, Chen W, Zhang Q,
Zhou J, Yang C, He Z, Yu J, Peng X, et al: The N6-methyladenosine
METTL3 regulates tumorigenesis and glycolysis by mediating m6A
methylation of the tumor suppressor LATS1 in breast cancer. J Exp
Clin Cancer Res. 42:102023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi K, Sa R, Dou L, Wu Y, Dong Z, Fu X and
Yu H: Correction: METTL3 exerts synergistic effects on m6A
methylation and histone modification to regulate the function of
VGF in lung adenocarcinoma. Clin Epigenetics. 16:22024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z,
Chen J, Jiang Z, Zhang Y, Xu G, Zhang J, et al: METTL3-mediated
m6A modification of HDGF mRNA promotes gastric cancer
progression and has prognostic significance. Gut. 69:1193–1205.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo S, Wang E, Wang B, Xue Y, Kuang Y and
Liu H: Comprehensive multiomics analyses establish the optimal
prognostic model for resectable gastric cancer: Prognosis
prediction for resectable GC. Ann Surg Oncol. 31:2078–2089. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cai Y, Lyu T, Li H, Liu C, Xie K, Xu L, Li
W, Liu H, Zhu J, Lyu Y, et al: LncRNA CEBPA-DT promotes liver
cancer metastasis through DDR2/β-catenin activation via interacting
with hnRNPC. J Exp Clin Cancer Res. 41:3352022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie B, Lin W, Ye J, Wang X, Zhang B, Xiong
S, Li H and Tan G: DDR2 facilitates hepatocellular carcinoma
invasion and metastasis via activating ERK signaling and
stabilizing SNAIL1. J Exp Clin Cancer Res. 34:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ren L, Ren Q, Wang J, He Y, Deng H, Wang X
and Liu C: miR-199a-3p promotes gastric cancer progression by
promoting its stemness potential via DDR2 mediation. Cell Signal.
106:1106362023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang YG, Xu L, Jia RR, Wu Q, Wang T, Wei
J, Ma JL, Shi M and Li ZS: DDR2 induces gastric cancer cell
activities via activating mTORC2 signaling and is associated with
clinicopathological characteristics of gastric cancer. Dig Dis Sci.
61:2272–2283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ren T, Zhang W, Liu X, Zhao H, Zhang J,
Zhang J, Li X, Zhang Y, Bu X, Shi M, et al: Discoidin domain
receptor 2 (DDR2) promotes breast cancer cell metastasis and the
mechanism implicates epithelial-mesenchymal transition programme
under hypoxia. J Pathol. 234:526–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu J, Lu W, Zhang S, Zhu C, Ren T, Zhu T,
Zhao H, Liu Y and Su J: Overexpression of DDR2 contributes to cell
invasion and migration in head and neck squamous cell carcinoma.
Cancer Biol Ther. 15:612–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ullah R, Yin Q, Snell AH and Wan L:
RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer
Biol. 85:123–154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng F, Wu J, Zhao S, Luo Q, Tang Q, Yang
L, Li L, Wu W and Hann SS: Baicalein increases the expression and
reciprocal interplay of RUNX3 and FOXO3a through crosstalk of AMPKα
and MEK/ERK1/2 signaling pathways in human non-small cell lung
cancer cells. J Exp Clin Cancer Res. 34:412015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wen Z, Sun J, Luo J, Fu Y, Qiu Y, Li Y, Xu
Y, Wu H and Zhang Q: COL10A1-DDR2 axis promotes the progression of
pancreatic cancer by regulating MEK/ERK signal transduction. Front
Oncol. 12:10493452022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang F, Yu Z, Liu X, Hu F, Liu X, Fu X,
Liu Y and Zou Z: A meta-analysis and bioinformatics analysis of
P4HB expression levels in the prognosis of cancer patients. Pathol
Res Pract. 245:1544742023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Feng D, Wang J, Li D, Wu R, Tuo Z, Yu Q,
Ye L, Miyamoto A, Yoo KH, Wang C, et al: Targeting Prolyl
4-hydroxylase subunit beta (P4HB) in cancer: New roads to travel.
Aging Dis. Nov 26–2023.
|
|
53
|
Feng D, Li L, Li D, Wu R, Zhu W, Wang J,
Ye L and Han P: Prolyl 4-hydroxylase subunit beta (P4HB) could
serve as a prognostic and radiosensitivity biomarker for prostate
cancer patients. Eur J Med Res. 28:2452023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma X, Wang J, Zhuang J, Ma X, Zheng N,
Song Y and Xia W: P4HB modulates epithelial-mesenchymal transition
and the β-catenin/Snail pathway influencing chemoresistance in
liver cancer cells. Oncol Lett. 20:257–265. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang W, Wu X and Zhou F: Collagen Type X
Alpha 1 (COL10A1) Contributes to cell proliferation, migration, and
invasion by targeting Prolyl 4-hydroxylase beta polypeptide (P4HB)
in breast cancer. Med Sci Monit. 27:e9289192021.PubMed/NCBI
|
|
56
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu Q, Zhao H, Guo Y, Zhang K, Shang F and
Liu T: Bioinformatics-based analysis: Noncoding RNA-Mediated
COL10A1 is associated with poor prognosis and immune cell
infiltration in pancreatic cancer. J Healthc Eng. 2022:79049822022.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu Q, Zhang W, Wu Z, Liu H, Hu H, Shi H,
Li S and Zhang X: Construction of a circular
RNA-microRNA-messengerRNA regulatory network in stomach
adenocarcinoma. J Cell Biochem. 121:1317–1331. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li HH, Wang JD, Wang W, Wang HF and Lv JQ:
Effect of miR-26a-5p on gastric cancer cell proliferation,
migration and invasion by targeting COL10A1. Eur Rev Med Pharmacol
Sci. 24:1186–1194. 2020.PubMed/NCBI
|
|
60
|
Guo Q, Zheng M, Xu Y, Wang N and Zhao W:
MiR-384 induces apoptosis and autophagy of non-small cell lung
cancer cells through the negative regulation of Collagen α-1(X)
chain gene. Biosci Rep. 39:2019. View Article : Google Scholar
|
|
61
|
Mamdani H, Matosevic S, Khalid AB, Durm G
and Jalal SI: Immunotherapy in lung cancer: Current landscape and
future directions. Front Immunol. 13:8236182022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu X, Li L, Xie X, Zhuang D and Hu C:
Integrated bioinformatics analysis of microarray data from the GEO
database to identify the candidate genes linked to poor prognosis
in lung adenocarcinoma. Technol Health Care. 31:579–592. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wu X, Zhang W, Hu Y and Yi X:
Bioinformatics approach reveals systematic mechanism underlying
lung adenocarcinoma. Tumori. 101:281–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shi Y, Chang D, Li W, Zhao F, Ren X and
Hou B: Identification of core genes and clinical outcomes in tumors
originated from endoderm (gastric cancer and lung carcinoma) via
bioinformatics analysis. Medicine (Baltimore). 100:e251542021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Díaz Del Arco C, Ortega Medina L, Estrada
Muñoz L, García Gómez de Las Heras S and Fernández Aceñero MJ: Is
there still a place for conventional histopathology in the age of
molecular medicine? Laurén classification, inflammatory
infiltration and other current topics in gastric cancer diagnosis
and prognosis. Histol Histopathol. 36:587–613. 2021.PubMed/NCBI
|
|
66
|
Cai Z, Wei Y, Chen S, Gong Y, Fu Y, Dai X,
Zhou Y, Yang H, Tang L and Liu H: Screening and identification of
key biomarkers in alimentary tract cancers: A bioinformatic
analysis. Cancer Biomark. 29:221–233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li Y, Luo Y, Tian Q, Lai Y, Xu L, Yun H,
Liang Y, Liao D, Gu R, Liu L, et al: Integrated bioinformatics
analysis for identifying the significant genes as poor prognostic
markers in gastric adenocarcinoma. J Oncol.
2022:90804602022.PubMed/NCBI
|
|
68
|
Aktas SH, Taskin-Tok T, Al-Khafaji K and
Akın-Balı DF: A detailed understanding of the COL10A1 and SOX9
genes interaction based on potentially damaging mutations in
gastric cancer using computational techniques. J Biomol Struct Dyn.
40:11533–11544. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shen N, Zhu S, Zhang Z and Yong X: High
Expression of COL10A1 is an independent predictive poor prognostic
biomarker and associated with immune infiltration in advanced
gastric cancer microenvironment. J Oncol. 2022:14633162022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Moreira AM, Ferreira RM, Carneiro P,
Figueiredo J, Osório H, Barbosa J, Preto J, Pinto-do-Ó P, Carneiro
F and Seruca R: Proteomic identification of a gastric tumor ECM
signature associated with cancer progression. Front Mol Biosci.
9:8185522022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Necula L, Matei L, Dragu D, Pitica I,
Neagu AI, Bleotu C, Dima S, Popescu I, Diaconu CC and
Chivu-Economescu M: High plasma levels of COL10A1 are associated
with advanced tumor stage in gastric cancer patients. World J
Gastroenterol. 26:3024–3033. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen L, Zhang X, Zhang Q, Zhang T, Xie J,
Wei W, Wang Y, Yu H and Zhou H: A necroptosis related prognostic
model of pancreatic cancer based on single cell sequencing analysis
and transcriptome analysis. Front Immunol. 13:10224202022.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu Q, Zheng J, Su Z, Chen B and Gu S:
COL10A1 promotes tumorigenesis by modulating CD276 in pancreatic
adenocarcinoma. BMC Gastroenterol. 23:3972023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Thorlacius-Ussing J, Jensen C, Nissen NI,
Cox TR, Kalluri R, Karsdal M and Willumsen N: The collagen
landscape in cancer: Profiling collagens in tumors and in
circulation reveals novel markers of cancer-associated fibroblast
subtypes. J Pathol. 262:22–36. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lou X, Gao H, Xu X, Ye Z, Zhang W, Wang F,
Chen J, Zhang Y, Chen X, Qin Y, et al: The Interplay of four main
pathways recomposes immune landscape in primary and metastatic
Gastroenteropancreatic neuroendocrine tumors. Front Oncol.
12:8084482022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang X, Chen B, Liu K, Ma Y, Liu Y, Zhou
H and Wei P: Infection with COVID-19 promotes the progression of
pancreatic cancer through the PI3K-AKT signaling pathway. Discov
Oncol. 14:2252023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Abdi E, Latifi-Navid S and Latifi-Navid H:
LncRNA polymorphisms and breast cancer risk. Pathol Res Pract.
229:1537292022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Won KA and Spruck C: Triple-negative
breast cancer therapy: Current and future perspectives (Review).
Int J Oncol. 57:1245–1261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Onkar SS, Carleton NM, Lucas PC, Bruno TC,
Lee AV, Vignali DAA and Oesterreich S: The great immune escape:
Understanding the divergent immune response in breast cancer
subtypes. Cancer Discov. 13:23–40. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang M, Chen H, Wang M, Bai F and Wu K:
Bioinformatics analysis of prognostic significance of COL10A1 in
breast cancer. Biosci Rep. 40:BSR201932862020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Malvia S, Chintamani C, Sarin R, Dubey US,
Saxena S and Bagadi SAR: Aberrant expression of COL14A1, CELRS3,
and CTHRC1 in breast cancer сells. Exp Oncol. 45:28–43. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brodsky AS, Xiong J, Yang D, Schorl C,
Fenton MA, Graves TA, Sikov WM, Resnick MB and Wang Y:
Identification of stromal ColXα1 and tumor-infiltrating lymphocytes
as putative predictive markers of neoadjuvant therapy in estrogen
receptor-positive/HER2-positive breast cancer. BMC Cancer.
16:2742016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhou W, Li Y, Gu D, Xu J, Wang R, Wang H
and Liu C: High expression COL10A1 promotes breast cancer
progression and predicts poor prognosis. Heliyon. 8:e110832022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bao S and He G: Identification of key
genes and key pathways in breast cancer based on machine learning.
Med Sci Monit. 28:e9355152022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang M, Feng R, Chen Z, Shi W, Li C, Liu
H, Wu K, Li D and Li X: Identification of cancer-associated
fibroblast subtype of triple-negative breast cancer. J Oncol.
2022:64526362022.PubMed/NCBI
|
|
86
|
Giussani M, Landoni E, Merlino G, Turdo F,
Veneroni S, Paolini B, Cappelletti V, Miceli R, Orlandi R, Triulzi
T and Tagliabue E: Extracellular matrix proteins as diagnostic
markers of breast carcinoma. J Cell Physiol. 233:6280–6290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cen S, Jiang D, Lv D, Xu R, Hou J, Yang Z,
Wu P, Xiong X and Gao X: Comprehensive analysis of the biological
functions of endoplasmic reticulum stress in prostate cancer. Front
Endocrinol (Lausanne). 14:10902772023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xu S, Liu D, Qin Z, Liang Z, Xie H, Yi B,
Wang K, Lin G, Liu R, Yang K, et al: Experimental validation and
pan-cancer analysis identified COL10A1 as a novel oncogene and
potential therapeutic target in prostate cancer. Aging (Albany NY).
15:15134–15160. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang C, Wang J, Chen S, Li K, Wan S and
Yang L: COL10A1 as a prognostic biomarker in association with
immune infiltration in prostate cancer. Curr Cancer Drug Targets.
24:340–353. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhao J, Wang R, Sun X, Huang K, Jin J, Lan
L, Jian Y, Xu Z, Wu H, Wang S and Wang J: An integrative
multi-omics analysis based on nomogram for predicting prostate
cancer bone metastasis incidence. Genet Res (Camb).
2022:82137232022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mármol I, Sánchez-de-Diego C, Pradilla
Dieste A, Cerrada E and Rodriguez Yoldi MJ: Colorectal carcinoma: A
general overview and future perspectives in colorectal cancer. Int
J Mol Sci. 18:1972017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Solé X, Crous-Bou M, Cordero D, Olivares
D, Guinó E, Sanz-Pamplona R, Rodriguez-Moranta F, Sanjuan X, de Oca
J, Salazar R and Moreno V: Discovery and validation of new
potential biomarkers for early detection of colon cancer. PLoS One.
9:e1067482014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Huang H, Li T, Ye G, Zhao L, Zhang Z, Mo
D, Wang Y, Zhang C, Deng H, Li G and Liu H: High expression of
COL10A1 is associated with poor prognosis in colorectal cancer.
Onco Targets Ther. 11:1571–1581. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kahlert UD, Shi W, Strecker M, Scherpinski
LA, Wartmann T, Dölling M, Perrakis A, Relja B, Mengoni M, Braun A
and Croner RS: COL10A1 allows stratification of invasiveness of
colon cancer and associates to extracellular matrix and immune cell
enrichment in the tumor parenchyma. Front Oncol. 12:10075142022.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
He C, Liu W, Xiong Y, Pan L, Luo L, Tu Y,
Song R and Chen W: VSNL1 promotes cell proliferation, migration,
and invasion in colorectal cancer by binding with COL10A1. Ann Clin
Lab Sci. 52:60–72. 2022.PubMed/NCBI
|
|
96
|
Sroor FM, Aboelenin MM, Mahrous KF,
Mahmoud K, Elwahy AHM and Abdelhamid IA: Novel
2-cyanoacrylamido-4,5,6,7-tetrahydrobenzo[b]thiophene derivatives
as potent anticancer agents. Arch Pharm (Weinheim).
353:e20000692020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhao H, Chen Z, Fang Y, Su M, Xu Y, Wang
Z, Gyamfi MA and Zhao J: Prediction of prognosis and recurrence of
bladder cancer by ECM-Related genes. J Immunol Res.
2022:17930052022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu Q, Diao R, Feng G, Mu X and Li A: Risk
score based on three mRNA expression predicts the survival of
bladder cancer. Oncotarget. 8:61583–61591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu SX, Huang J, Liu ZW, Chen HG, Guo P,
Cai QQ, Zheng JJ, Qin HD, Zheng ZS, Chen X, et al: A
Genomic-clinicopathologic Nomogram for the preoperative prediction
of lymph node metastasis in bladder cancer. EBioMedicine. 31:54–65.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Karagoz K, Lehman HL, Stairs DB, Sinha R
and Arga KY: Proteomic and metabolic signatures of esophageal
squamous cell carcinoma. Curr Cancer Drug Targets. Feb 2–2016.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li J, Wang X, Zheng K, Liu Y, Li J and
Wang S, Liu K, Song X, Li N, Xie S and Wang S: The clinical
significance of collagen family gene expression in esophageal
squamous cell carcinoma. PeerJ. 7:e77052019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li Y, Wang X, Shi L, Xu J and Sun B:
Predictions for high COL1A1 and COL10A1 expression resulting in a
poor prognosis in esophageal squamous cell carcinoma by
bioinformatics analyses. Transl Cancer Res. 9:85–94. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Song Y, Wang X, Wang F, Peng X, Li P, Liu
S and Zhang D: Identification of four genes and biological
characteristics of esophageal squamous cell carcinoma by integrated
bioinformatics analysis. Cancer Cell Int. 21:1232021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lapa RML, Barros-Filho MC, Marchi FA,
Domingues MAC, de Carvalho GB, Drigo SA, Kowalski LP and Rogatto
SR: Integrated miRNA and mRNA expression analysis uncovers drug
targets in laryngeal squamous cell carcinoma patients. Oral Oncol.
93:76–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Xie C, Du LY, Guo F, Li X and Cheng B:
Exosomes derived from microRNA-101-3p-overexpressing human bone
marrow mesenchymal stem cells suppress oral cancer cell
proliferation, invasion, and migration. Mol Cell Biochem.
458:11–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guo W, Zheng X, Hua L, Zheng X, Zhang Y,
Sun B, Tao Z and Gao J: Screening and bioinformatical analysis of
differentially expressed genes in nasopharyngeal carcinoma. J
Cancer. 12:1867–1883. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Vishnubalaji R, Shaath H, Elkord E and
Alajez NM: Long non-coding RNA (lncRNA) transcriptional landscape
in breast cancer identifies LINC01614 as non-favorable prognostic
biomarker regulated by TGFβ and focal adhesion kinase (FAK)
signaling. Cell Death Discov. 5:1092019. View Article : Google Scholar : PubMed/NCBI
|