Introduction
Despite preventive measures and advances in the
diagnosis and treatment of cancer, liver cancer is the third
leading cause of cancer-associated deaths worldwide, and
hepatocellular carcinoma (HCC), which is the most aggressive type
of primary liver cancer, accounts for nearly 90% of liver cancer
cases (1,2). In the USA in 2024, it is estimated
that 41,630 individuals will be diagnosed with cancer of the liver
and intrahepatic bile duct and 29,840 individuals will die of such
cancers (3).
Currently, the treatment options for HCC vary and
include surgery, chemotherapy, radiotherapy, targeted therapies
using multiple kinase inhibitors sorafenib, regorafenib,
lenvatinib, cabozantinib, ramucirumab and bevacizumab, and
treatment with checkpoint inhibitors such as ipilimumab, nivolumab,
pembrolizumab and atezolimimab (2,4). While
patients identified at the early stage of the disease can be cured
with surgery and local ablation, numerous patients are diagnosed at
a more advanced stage of the disease and consequently do not gain a
long-term benefit from such therapeutic interventions alone due to
primary and secondary resistance to other treatment options
(4–7). The heterogeneous nature of HCC
(intra-tumour and inter-tumour heterogeneity), presence of cancer
stem cells (CSCs), aberrant expression and mutation of heterologous
growth factor receptor signalling pathways,
epithelial-to-mesenchymal transition, and differences in the tumour
microenvironment have been highlighted as some of the factors
contributing to no response or a response of short duration to the
current therapeutic interventions (8–13). To
reduce mortality as a result of liver cancer, it is considered
essential to identify additional biomarkers, to determine novel
therapeutic targets and to develop more effective therapeutic
agents. These may be used in the early diagnosis of HCC, in
determining cancer prognosis and in predicting the response to
therapy. Furthermore, it is important to identify novel therapeutic
targets and to develop more effective therapeutic approaches. This
may be achieved by investigation of the therapeutic application of
drugs that have already been approved for other cancer types by
repurposing such drugs.
Since the early 1980s, increased expression,
amplification or mutation of human EGFR, which is the prototype of
the type I growth factor receptor family (also called HER) with
tyrosine kinase activity, have been reported in a wide range of
solid tumours and these have been associated with poor prognosis in
some patients (14,15). The HER family, in addition to EGFR,
contains three additional members: HER-2, HER-3 and HER-4. The
binding of ligand to the external domain of EGFR results in
homodimerization or heterodimerization with other members of the
HER family, phosphorylation of the tyrosine kinase domain of each
receptor and ultimately activation of downstream cell signalling
pathways, including the RAS/MAP, PI (3)K/AKT, phospholipase C (PLC)γ/PLC and
Janus kinase/STAT signalling pathways (14,15).
To date, several monoclonal antibodies (mAbs) and small molecules
tyrosine kinase inhibitors (TKIs) targeting one or more members of
the HER family have been approved for the treatment of patients
with a range of cancer types (14–17).
However, the clinical benefit gain may be short in some patients
and no HER inhibitor has been approved for the treatment of
patients with liver cancer at present (18). Furthermore, to the best of our
knowledge, there is currently no comprehensive study of the
relative expression of all members of the HER family and the
type-III deletion mutant EGFR (EGFRvIII), which is the most common
variant of EGFR containing deletion in the extracellular domain of
EGFR, in patients with HCC.
Therefore, to the best of our knowledge, the present
study is the first to examine the relative expression and
prognostic significance of all members of the HER family, EGFRvIII,
two putative liver CSC markers [CD44 and epithelial cell adhesion
molecule (EpCAM)] and the tumour proliferation biomarker Ki67 in
patients with HCC. Furthermore, the present study investigated the
effect of various agents targeting one or more members of the HER
family and CDK inhibitors compared with the Food and Drug
Administration (FDA)-approved sorafenib and regorafenib on the
proliferation of a panel of HCC cell lines.
Materials and methods
Patients
The present retrospective study included tumour
specimens from a total of 43 patients with HCC treated between 2010
and 2019 at the Royal Surrey Hospital (Guilford, UK). The median
age of the patient population was 70 years, ranging between 45 and
86 years. The ethical approval for the study was obtained from the
NHS Research Ethics Committee (IRAS Project ID 252931; UK). Tumour
samples from patients with insufficient tumour and no follow-up
information were excluded from the study. Data on the
clinicopathological features of each participant, including patient
age, sex, tumour grade, differentiation, and hepatitis B and C
status, were collected. All participant information was analysed
anonymously.
Immunohistochemistry and scoring
criteria
Immunohistochemical analysis of 43 paraffin-embedded
HCC samples was performed using the Ultra View DAB kit and the
Ventana Discovery Ultra autostainer (Roche Diagnostics, Ltd.) as
described previously (18,19). Serial sections of tumour specimens
were cut and stained with antibodies specific for various
biomarkers. The co-expression levels were also determined based on
whether the same tumour specimen was positive for two or more
biomarkers. The primary antibodies employed in this study were:
EGFRvIII (1:500; cat. no. NBP2-50599; Novus Biologicals, Ltd.;
Bio-Techne), wtEGFR (1:250; DAK-H1-WT; cat. no. M7298; Dako;
Agilent Technologies, Inc.), HER-2 (3B5; 1:200; cat. no. sc-33684;
Insight Biotechnology), HER-3 (1:50; cat. no. ab93739; Abcam),
HER-4 (1:100; cat. no. sc-53280; Insight Biotechnology), CD44
(1:40; cat. no. M7082; Agilent Technologies, Inc.), EpCAM (1:100;
cat. no. NB600-1182; Novus Biologicals, Ltd.; Bio-Techne) and Ki67
(1:100; cat. no. M7240; Agilent Technologies, Inc.). All samples
were scored independently by two observers including a consultant
histopathologist. If any discrepancy occurred between the
reviewers, the slides were re-examined. Briefly, the slides were
scored based on the following characteristics as described
previously (19,20): Intensity (negative, 0; weak, 1+;
moderately positive, 2+; and strongly positive, 3+), location
(membrane, cytoplasm or nucleus) and percentage of positively
stained tumour cells (>5, >10, >20 and >50%).
Flow cytometry and determination of
proliferation of liver cancer cells following treatment with
various targeted agents
The expression levels of HER family members,
including insulin-like growth factor 1 receptor (IGF IR; 4 µg/ml;
cat. no. MAB1120; Merck KGaA), cMET (1:1,000; cat. no. ab237711;
Abcam) and CD44 (10 µg/ml; cat. no. 555476; Becton, Dickinson and
Company), were determined in a panel of human liver cancer cell
lines (LCCLs) by flow cytometry as described previously (21). SNU-475 (ATCC CRL-2236), C3A (ATCC
CRL-3581), SNU-449 (ATCC CRL-2234), PLC/PRF/5 (ATCC CRL-8024),
SNU-387 (ATCC CRL-2237) and SNU-423 (ATCC CRL-2238) cells were
purchased from American Type Culture Collection, and HepG2 cells
were purchased from the UK Health Security Agency (cat. no.
85011430). The EGFR-overexpressing head neck cancer cell line
(HN5), HER-2-overexpressing ovarian cancer cell line (Skov3) and
CD44-overexpressing colorectal cancer cell line (Caco-2), used in
our previous study, were used as positive controls for these
biomarkers in the present study (22).
All cell lines were grown in an incubator set at 5%
CO2 in medium containing 10% FBS (Sigma-Aldrich; Merck
KGaA) enriched media with addition of antibiotics, including
penicillin (50 µg/ml), streptomycin (50 µg/ml) and neomycin (50
µg/ml) (Sigma-Aldrich; Merck KGaA). The HEP-G2, PLC/PRF/5, C3A
(HepG2/C3A, derivative of Hep G2), Caco-2, SKOV3 and HN5 cell lines
were grown in DMEM (Sigma-Aldrich; Merck KGaA). SNU 449, SNU 475,
SNU 387 and SNU 423 cells were all grown in RPMI-1640 Medium
(Sigma-Aldrich; Merck KGaA). Furthermore, 2 mM L-Glutamine
(Sigma-Aldrich; Merck KGaA) was added to the DMEM and RPMI-1640
medium.
The proliferation of a panel of human LCCLs
following treatment with various agents targeting different members
of the HER family, other growth factor receptors and downstream
cell signalling molecules was also determined using a
sulforhodamine B assay as described previously (21).
Statistical analysis
Statistical assessments were carried out using SPSS
software (SPSS statistics version 26; IBM Corp.). Pearson's
χ2 test and Fisher's exact test were used to determine
the association between clinicopathological features and
immunohistochemistry scores. Kaplan-Meier curves were used to
determine if there was a significant association between biomarker
expression and overall survival (OS; months). Cox regression
analysis was performed to determine whether the biomarkers were
significantly associated with OS and whether they were an
independent factor based on univariate and multivariate analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological characteristics of
patients with HCC
The clinicopathological features and their
associations with OS are presented in Table I. The mean OS time of all patients
was 60.03 months. Age was significantly associated with OS
(P=0.02). Patients >65 years old had an average OS time of 44.00
months, while those <65 years old had an OS time of 79.99
months. No association was observed between the other
characteristics and OS (Table
I).
 | Table I.Clinicopathological features of
patients with hepatocellular carcinoma. |
Table I.
Clinicopathological features of
patients with hepatocellular carcinoma.
|
Characteristics | No. of patients
(%) | Overall survival,
months (mean ± SE) | 95% CI | P-value |
|---|
| Age, years |
|
|
| 0.02 |
|
≥65 | 34 (79.10) | 44.00±13.64 | 17.26–70.74 |
|
|
<65 | 9 (20.90) | 79.99±6.13 | 63.98–88.04 |
|
| Sex |
|
|
| 0.63 |
|
Male | 31 (72.10) | 71.86±6.53 | 59.07–84.65 |
|
|
Female | 12 (27.90) | 66.49±14.61 | 37.86–95.12 |
|
| Tumour size,
mm |
|
|
| 0.79 |
|
≤20 | 2 (4.70) | 69.50±31.50 | 7.76–131.24 |
|
|
≤50 | 22 (51.10) | 63.97±8.52 | 47.26–80.68 |
|
|
>50 | 19 (44.20) | 79.33±8.50 | 62.68–95.98 |
|
| Focal status |
|
|
| 0.26 |
|
Unifocal | 33 (76.70) | 72.61±6.40 | 60.07–85.15 |
|
|
Multifocal | 10 (23.30) | 62.08±15.37 | 31.96–92.21 |
|
| Hepatitis B |
|
|
| 0.61 |
|
Positive | 3 (6.98) | 67.67±22.73 | 32.15–53.85 |
|
|
Negative | 40 (93.02) | 53.10±5.85 | 38.40–57.60 |
|
| Hepatitis C |
|
|
| 0.61 |
|
Positive | 1 (2.33) | 42.00±0.00 | 42.00–42.00 |
|
|
Negative | 42 (97.67) | 54.41±5.75 | 43.14–65.67 |
|
Expression levels of HER family
members in patients with HCC
To the best of our knowledge, the present study was
the first to determine the expression of all HER family members and
EGFRvIII in tumour specimens from patients with HCC. At the cut off
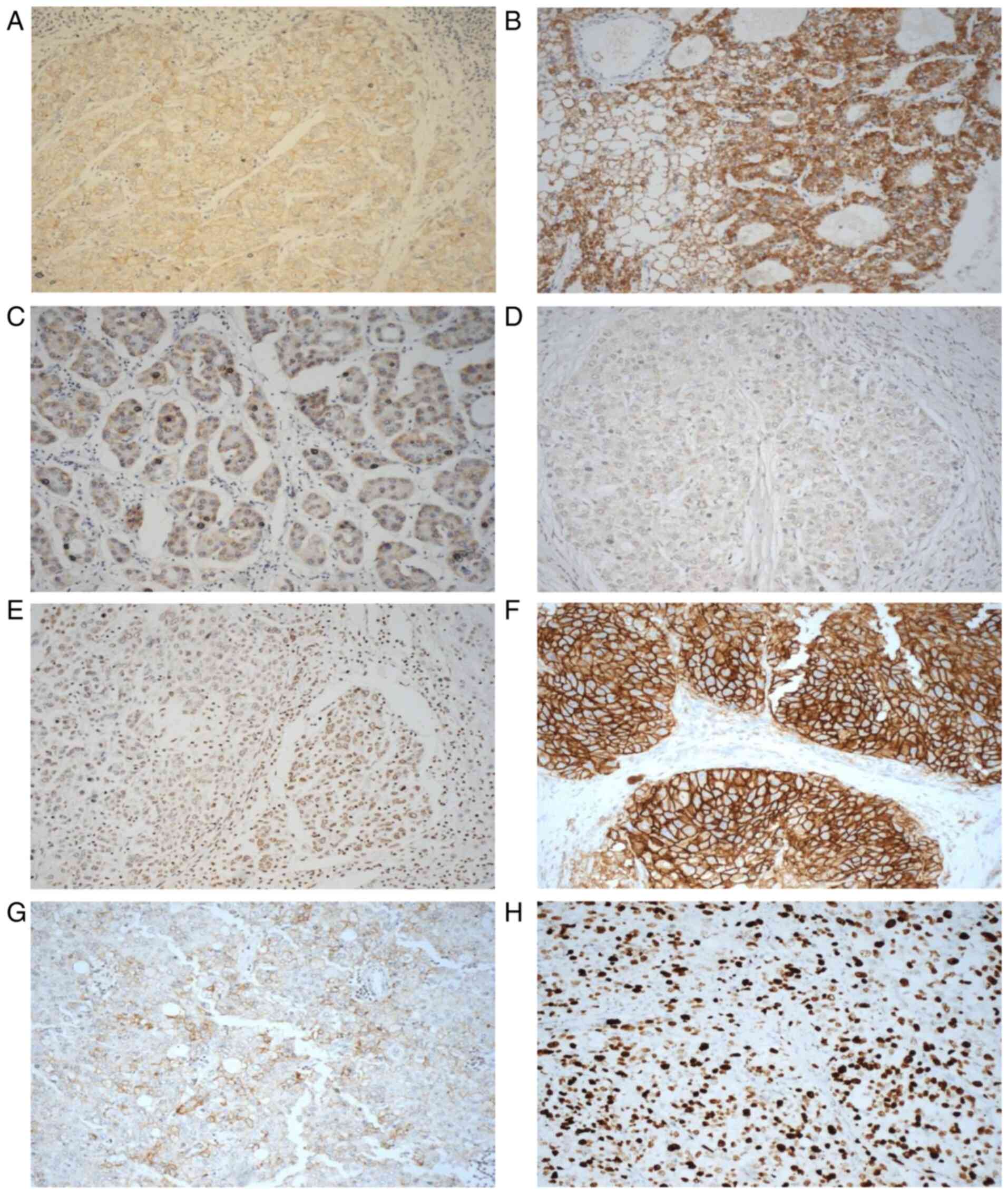
value of >5% of tumour cells with positive staining, 35% of the
cases were EGFR-positive. The cellular location of EGFR staining
was membranous and cytoplasmic in 33 and 12% of patients,
respectively. None of the staining was present at an intensity of
3+; however, 30 and 7% of the patients had an EGFR staining
intensity of 1+ and 2+, respectively (Table II). At the same cut off value, 58%
of patients were HER-2 positive, with the cellular location of
staining being membranous (14%) and cytoplasmic (58%), and with 12%
of patients having strong immunostaining intensity. While 19% of
the cases were HER-4 positive, none were HER-3 positive.
Furthermore, 26 and 9% of the patients were EGFRvIII-positive, when
the staining was scored at the cut off value of >5 and >10%,
respectively, of tumour cells with positive staining (Table II; Fig.
1). The results of immunostaining for these biomarkers at other
cut off values of >10, 20 and 50% are presented in Table II and images of positively stained
tumour cells are presented in Fig.
1.
 | Table II.Expression, cellular location and
intensity of the HER family members, CD44, EpCAM and Ki67 in
hepatocellular carcinoma. |
Table II.
Expression, cellular location and
intensity of the HER family members, CD44, EpCAM and Ki67 in
hepatocellular carcinoma.
|
| Number of positive
samples (%) |
|---|
|
|
|
|---|
| Variable | wtEGFR | HER-2 | HER-3 | HER-4 | EGFRvIII | CD44 | EpCAM | Ki67 |
|---|
| Positively stained
tumour cells (%) |
|
|
|
|
|
|
|
|
|
>5 | 15 (35) | 25 (58) | - | 8 (19) | 11 (26) | 17 (40) | 14 (33) | 29 (67) |
|
>10 | 11 (26) | 24 (56) | - | 3 (7) | 4 (9) | 13 (30) | 8 (19) | 21 (49) |
|
>20 | 7 (16) | 21 (49) | - | 3 (7) | 3 (7) | 10 (23) | 7 (16) | 14 (33) |
|
>50 | 4 (9) | 12 (28) | - | 1 (2) | 1 (2) | 3 (7) | 1 (2) | 7 (16) |
| Cellular
location |
|
|
|
|
|
|
|
|
|
Membranous | 14 (33) | 6 (14) | - | - | - | 11 (26) | 3 (7) | - |
|
Cytoplasmic | 5 (12) | 25 (58) | - | 7 (16) | 10 (23) | 6 (14) | 12 (28) | - |
|
Nuclear | - | - | - | 2 (5) | 1 (2) | - | 3 (7) | 29 (67) |
| Intensity |
|
|
|
|
|
|
|
|
| 1+ | 13 (30) | 17 (40) | - | 8 (19) | 11 (26) | 15 (35) | 10 (23) | - |
| 2+ | 3 (7) | 17 (40) | - | - | - | 2 (5) | 5 (12) | - |
| 3+ | - | 5 (12) | - | - | - | 1 (2) | - | - |
Expression levels of the CSC markers
CD44 and EpCAM, and Ki67 in patients with HCC
The presence of a subset of cancer cells with stem
cell properties has been associated with cancer recurrence and
resistance to therapeutic agents, and two putative biomarkers for
CSCs in HCC are CD44 and EpCAM (23). Therefore, the expression levels of
these two biomarkers, as well as the cell proliferation marker
Ki67, in tumour specimens were also determined. At a cut off value
of >5% of tumour cells with staining, 26 and 14% of the cases
exhibited membranous and cytoplasmic expression of CD44, with 5 and
35% of the cases having an immunostaining intensity of 2+ and 1+,
respectively (Table II; Fig. 1). At the same cut off value, 33% of
patients were EpCAM-positive, with the cellular location of
staining being membranous (7%), cytoplasmic (28%) and nuclear (7%)
(Table II; Fig. 1). Finally, at the same cut off
value, 67% of cases exhibited Ki67 staining in the nucleus
(Table II; Fig. 1).
However, at a higher cut off value of >10% of
tumour cells with positive staining, 30 and 19% of patients were
CD44-positive and EpCAM-positive, respectively (Table II). The cellular location of CD44
staining was membranous and cytoplasmic in 21 and 9% of patients,
respectively (Fig. 1). At the same
cut off value of >10, 19 and 49% of patients were EpCAM-positive
and Ki67-positive, respectively, with all staining for Ki67 being
nuclear (Table II). The results of
staining for these biomarkers at higher cut off values are
summarized in Table II.
Co-expression of the HER family
members, EGFRvIII, CD44, EpCAM and Ki67 in patients with HCC
Tumour heterogeneity is common in patients with HCC.
While the expression levels and prognostic significance of
individual markers in patients with HCC have been assessed
previously, to the best of our knowledge, there has been no study
investigating the co-expression of all these biomarkers and their
clinical significance (24). The
examination of co-expression of HER family members may reveal the
potential of cross-talk between different members of the HER family
in tumour progression and consequent poor response to therapy. The
identification of such cross-talk may lead to novel more effective
therapeutic interventions using a cocktail of mAbs, small molecule
TKIs and other therapeutic agents (25,26).
The results of co-expression analysis of two or more of the
biomarkers at cut off values of both >5 and >10% are
presented in Table SI. At the cut
off value of >5% of the tumour cells stained, 23% of the
patients exhibited co-expression of wtEGFR/HER-2. In 14, 14 and 2%
of the cases examined, EGFRvIII was co-expressed with wtEGFR, HER-2
and HER-4, respectively. However, the co-expression of all three
members of the HER family (EGFR/HER-2/HER-4) was found to be
uncommon and occurred in only 2% of the cases examined (Table SI). Next, the present study
examined the co-expression of one or more members of the HER family
with the CSC markers CD44 and EpCAM, and the tumour cell
proliferation marker Ki67, and the results are summarized in
Table SI. For example, at the cut
off value of >5% of tumour cells with positive immunostaining,
CD44 expression was accompanied by co-expression of wtEGFR (14%),
HER-2 (26%), HER-4 (9%), EGFRvIII (7%), EpCAM (14%), Ki67 (30%) and
Ki67/HER-2 (21%) (Table SI). At
the same cut off value of >5% of tumour cells with positive
staining, co-expression of EpCAM with EGFR, HER-2, HER-4 and Ki67
was present in 14, 23, 5 and 21% of the cases examined,
respectively. These results and those at a cut off value of >10%
of tumour cells with immunostaining are presented in Table SI.
Association of the expression of the
HER family members, CD44, EpCAM and Ki67 with clinicopathological
features and OS in patients with HCC
Following the determination of the expression
pattern of the HER family members, the present study investigated
the associations between the expression of these biomarkers and
clinicopathological features and their impact on OS using
Kaplan-Meier curves, the χ2 test and Fisher's exact
test. A significant association was observed between EGFRvIII
expression (>5% positive tumour cells) and focal status of the
tumour (P=0.04). However, there was no significant association
between any other biomarker and clinicopathological features (data
not shown). Next, the present study investigated the impact of
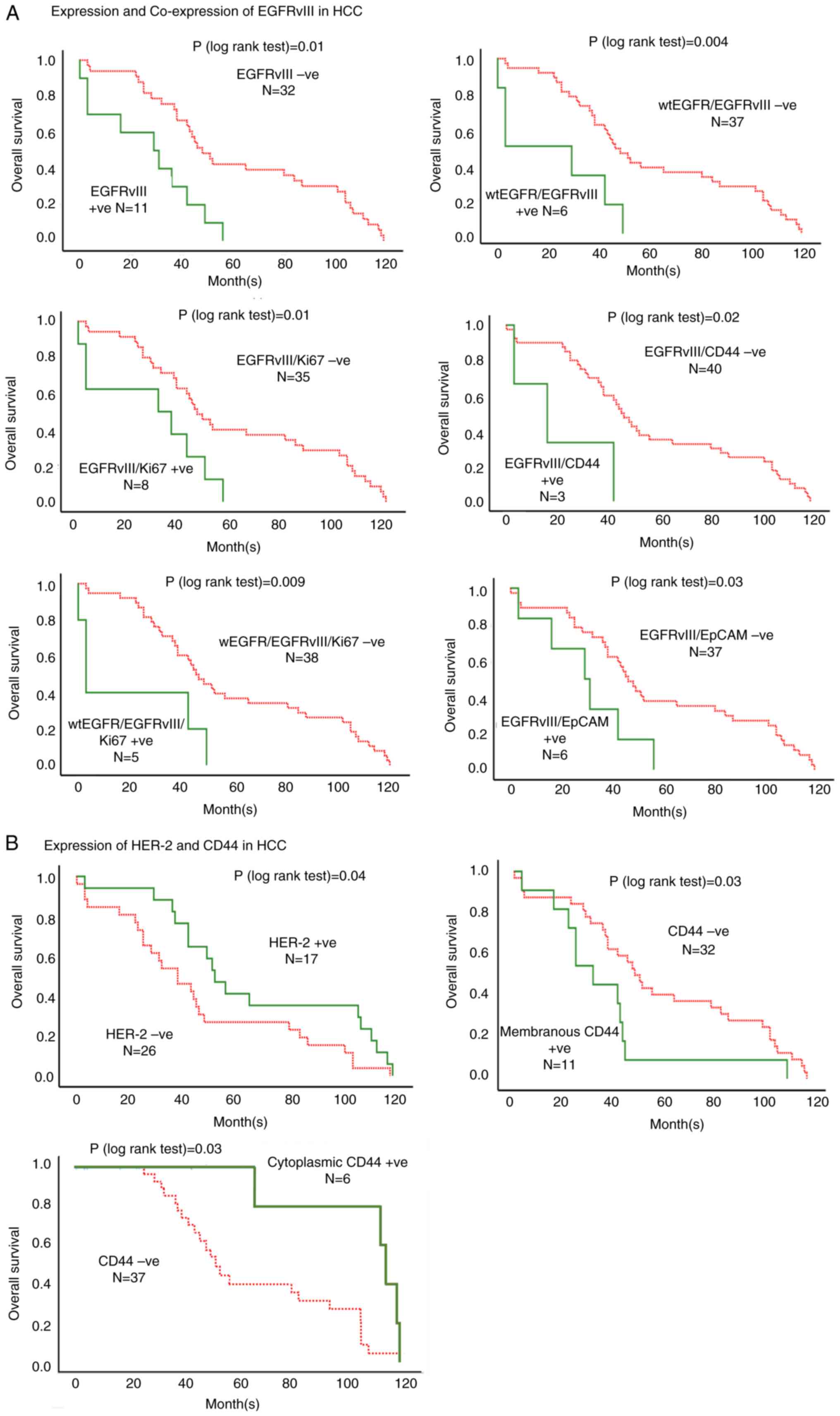
expression of EGFRvIII on the OS of patients with HCC (Table III). The expression of EGFRvIII
(at >5% of tumour cells stained) was associated with poor OS in
patients with HCC (Tables III and
SII; Fig. 2). In addition, patients whose
tumours expressed cytoplasmic EGFRvIII had a significantly worse OS
compared with those who were EGFRvIII-negative (63.66 vs. 28.70
months; P=0.002; Table SII). The
results of univariate and multivariate analyses further confirmed
the expression of EGFRvIII at >5 and 20% cut off values and
cytoplasmic expression of EGFRvIII to be independent biomarkers of
worse OS (Fig. 2; Table III).
 | Table III.Association between the expression
and co-expression of various biomarkers and overall survival in
univariate and multivariate analysis in patients with
hepatocellular carcinoma. |
Table III.
Association between the expression
and co-expression of various biomarkers and overall survival in
univariate and multivariate analysis in patients with
hepatocellular carcinoma.
|
| Overall
survival |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Expression of
biomarker | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| HER-2 1+
staining | 0.51 | 0.27–0.98 | 0.04 | 0.43 | 0.22–0.84 | 0.01 |
| EGFRvIII
>5% | 3.35 | 1.53–7.31 | 0.002 | 3.41 | 1.55–7.50 | 0.002 |
| EGFRvIII
>10% | 3.13 | 1.07–9.15 | 0.04 | - | - | NS |
| EGFRvIII
>20% | 7.22 | 1.94–26.92 | 0.003 | 7.66 | 2.00–29.36 | 0.03 |
| Membranous
CD44 | 2.12 | 1.04–4.36 | 0.04 | 2.15 | 1.02–4.53 | 0.04 |
| Cytoplasmic
CD44 | 0.30 | 0.11–0.81 | 0.02 | 0.20 | 0.07–0.59 | 0.003 |
| EGFRvIII +
wtEGFR | 3.57 | 1.42–8.96 | 0.01 | 3.36 | 1.33–8.52 | 0.01 |
| EGFRvIII +
CD44 | 4.03 | 1.17–13.91 | 0.03 | 3.97 | 1.15–13.67 | 0.03 |
| EGFRvIII+
EpCAM | 2.68 | 1.08–6.68 | 0.03 | - | - | NS |
| EGFRvIII +
Ki67 | 2.73 | 1.20–6.22 | 0.02 | 3.28 | 1.41–7.62 | 0.01 |
| EGFRvIII + wtEGFR +
Ki67 | 3.38 | 1.27–9.02 | 0.02 | 3.81 | 1.41–10.30 | 0.01 |
Of the HER family, patients with an HER-2
immunostaining intensity of 1+ had a significantly improved OS
compared with that of HER-2-negative patients (66.75 vs. 43.56
months; P=0.04; Fig. 2; Table III). The results of univariate and
multivariate analysis confirmed that the presence of HER-2 with an
immunostaining intensity of 1+ was associated with improved OS,
with a HR of 0.514 (P=0.04) and 0.43 (P=0.01) (Tables III and SII).
Of the two putative liver CSC biomarkers, the
cytoplasmic expression of CD44 was associated with significantly
improved OS (100.20 vs. 55.92 months; HR, 0.20; P=0.003; Fig. 2; Tables III and SII). By contrast, the membranous
expression of CD44 was associated with poor OS (37.09 vs. 55.92
months), with a HR of 2.12 and 2.15 in univariate and multivariate
analysis, respectively (P=0.04; Table
III). In addition, patients with nuclear EpCAM expression had a
significantly reduced OS (25.67 vs. 53.52 months; P=0.05; Table SII). Of these patients, only 1
patient (2%) exhibited cytoplasmic staining of EpCAM that was
present in >50% of tumour cells and was associated with
significantly reduced OS (6.00 vs. 53.52 months; P=0.002; Table SII). However, this requires
validation by examination of tumour specimens in a larger group of
patients.
Association between co-expression of
HER family members, EGFRvIII, CD44, EpCAM and Ki67 and OS in
patients with HCC
To date, to the best of our knowledge, there is no
comprehensive study of the co-expression and prognostic
significance of all members of the HER family and other biomarkers,
and their association with OS in patients with liver cancer. At a
cut off value of >5% of tumour cells with positive staining,
co-expression of EGFRvIII/wtEGFR was present in 14% of the cases
examined and associated with a poor OS (21.00 vs. 59.47 months;
P=0.004; Fig. 2; Table SII).
In addition, co-expression of EGFRvIII with CD44,
EpCAM or KI67 was associated with shorter OS, with a HR of 4.03
(P=0.03), 2.68 (P=0.03) and 2.73 (P=0.02), respectively (Fig. 2; Table
III). Furthermore, in the multivariate analysis, the
co-expression of EGFRvIII/CD44 and EGFRvIII/Ki67 was also
associated with a shorter OS (Table
III).
Impact of various targeted agents on
the proliferation of human LCCLs
Finally, the present study examined the expression
levels of HER family members, CD44, IGF IR and c-MET in a panel of
seven LCCLs established from patients at different stages of
disease. Overall, the majority of these cancer cell lines expressed
low levels of the HER family members, IGF IR and c-MET (Table IV; Fig. S1). By contrast, the expression
levels of the CSC marker CD44 were high in four out of the seven
LCCLs (Table IV; Fig. S1). Due to the heterogenous nature
of liver cancer, the present study subsequently investigated the
effect of 12 agents targeting one or more members of the HER
family, other growth factor receptors and cyclin-dependent kinases
compared with FDA-approved regorafenib and sorafenib on the
proliferation of LCCLs. Of the HER inhibitors targeting one or more
members of the HER family, the irreversible pan-HER family blocker
afatinib was the most effective drug by inhibiting the
proliferation of three out of seven human LCCLs (Table V). This was more evident when tumour
cells were cultured in a lower concentration of serum (2% FBS
rather than 10% FBS). In addition, while treatment with the
targeted agents crizotinib, NVP-AEW742 and LGK-974 inhibited the
proliferation of some LCCLs, they were less effective than the two
targeted drugs sorafenib and regorafenib, and doxorubicin, which is
approved for the treatment of patients with HCC (Table VI). Finally of the two CDK
inhibitors, dinaciclib (CDK1/2/5/9 inhibitor) was the most
effective targeted agent by inhibiting the proliferation of all
LCCLs, being more effective than the CDK4/6 inhibitor palbocicilib,
and sorafenib and regorafenib. Treatment with afatinib and
dinacicilib inhibited the proliferation of human LCCLs (Table VII).
 | Table IV.Cell surface expression of HER family
members, IGF IR, C-MET and CD44 in liver cancer cell lines
determined by flow cytometry. |
Table IV.
Cell surface expression of HER family
members, IGF IR, C-MET and CD44 in liver cancer cell lines
determined by flow cytometry.
|
| Mean fluorescence
intensity |
|---|
|
|
|
|---|
| Cell lines | Untreated
Sample | EGFR | HER-2 | HER-3 | HER-4 | IGF IR | c-MET | CD44 |
|---|
| HEPG-2 (HCC) | 6.6±1.0 | 14.9±0.1 | 11.8±1.5 | 10.4±0.1 | 7.3±0.2 | 52.7±2.5 | 10.1±0.9 | 17.3±0.6 |
| PLC/PRF/5
(hepatoma) | 5.9±0.2 | 30.6±0.3 | 18.1±0.7 | 17.9±3.5 | 10.1±0.4 | 26.9±0.4 | 12.3±0.3 | 15.7±2.9 |
| C3A (derivative of
HepG2) | 4.9±0.1 | 6.2±0.1 | 13.9±4.1 | 10.3±0.2 | 4.0±1.0 | 33.7±0.1 | 17.7±0.4 | 5.1±2.2 |
| SNU 449 (HCC;
hepatitis B positive) | 4.2±0.1 | 13.4±3.6 | 15.4±2.5 | 7.7±0.2 | 4.5±0.1 | 16.1±4.0 | 22.1±0.1 | 204.3±3.8 |
| SNU 475 (HCC;
hepatitis B positive) | 4.8±0.1 | 40.7±1.1 | 14.3±0.2 | 6.2±0.1 | 8.71±0.0 | 13.1±0.4 | 8.3±0.1 | 349.5±2.0 |
| SNU 387 (HCC;
hepatitis B positive) | 4.9±0.1 | 10.6±0.1 | 6.9±0.1 | 5.4±0.1 | 5.6±0.1 | 9.5±0.0 | 9.5±0.0 | 251.9±0.2 |
| SNU 423 (HCC;
hepatitis B positive) | 4.6±0.8 | 10.1±1.6 | 9.1±1.9 | 4.5±1.8 | 4.7±0.9 | 8.3±0.2 | 10.5±1.8 | 622.4±0.5 |
| Other cell
lines |
|
|
|
|
|
|
|
|
| HN5
(head and neck cancer) | 6.3 | 857.5±4.9 | n/a | n/a | n/a | n/a | n/a | n/a |
| Skov-3
(ovarian cancer) | 2.1 | n/a | 550.7±3.8 | n/a | n/a | n/a | n/a | n/a |
| Caco2
(colorectal cancer) | 4.6 | n/a | n/a | n/a | n/a | n/a | n/a | 126.1±7.0 |
 | Table V.Proliferation of human liver cancer
cell lines, when cultured in medium containing 2 vs. 10% FBS,
following treatment with various HER family-targeted agents. |
Table V.
Proliferation of human liver cancer
cell lines, when cultured in medium containing 2 vs. 10% FBS,
following treatment with various HER family-targeted agents.
|
| IC50
value (µM) |
|---|
|
|
|
|---|
|
| Gefitinib
(reversible selective EGFR TKI) | Lapatinib
(reversible dual EGFR/HER-2 TKI) | Sapitinib
(reversible pan EGFR/HER-2/HER-3 TKI) | Afatinib
(irreversible pan EGFR/HER-2/HER-4 TKI) |
|---|
|
|
|
|
|
|
|---|
| Cell line | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium |
|---|
| HEPG-2 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| PLC/PRF/5 | >10 | >10 | 2.43±1.20 | >10 | >10 | >10 | 0.09±0.44 | 3.54±2.57 |
| C3A | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| SNU 449 | 5.83±2.87 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| SNU 475 | >10 | >10 | >10 | >10 | >10 | >10 | 7.82±4.59 | >10 |
| SNU 387 | >10 | 0.12±0.42 | >10 | 0.90±0.70 | 6.37±2.54 | >10 | >10 | >10 |
| SNU 423 | 3.35±2.18 | >10 | >10 | >10 | 9.57±0.48 | >10 | 6.29±1.68 | >10 |
 | Table VI.Proliferation of human liver cancer
cell lines, when cultured in medium containing 2 vs. 10% FBS,
following treatment with small molecule targeted agents and
doxorubicin. |
Table VI.
Proliferation of human liver cancer
cell lines, when cultured in medium containing 2 vs. 10% FBS,
following treatment with small molecule targeted agents and
doxorubicin.
|
| IC50
value (µM) |
|---|
|
|
|
|---|
|
| Crizotinib
(c-MET/ALK TKI) | NVP-AEW 742 (IGF IR
TKI) | LGK-974 (Wnt
inhibitor) | Doxorubicin |
|---|
|
|
|
|
|
|
|---|
| Cell line | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium |
|---|
| HEPG-2 | >10 | 8.82±0.44 | >10 | 7.34±0.99 | >10 | >10 | 0.22±0.65 | 0.89±0.03 |
| PLC/PRF/5 | >10 | 7.99±0.94 | >10 | 8.45±0.75 | >10 | 9.95±2.69 | 1.20±2.77 | 0.01 |
| C3A | >10 | 7.89±0.55 | >10 | 8.27±0.63 | >10 | >10 | >10 | >10 |
| SNU 449 | >10 | >10 | 2.74±2.33 | >10 | >10 | >10 | 0.34±0.93 | 0.85±0.47 |
| SNU 475 | >10 | >10 | >10 | >10 | >10 | >10 | 0.03±1.74 | 0.02 |
| SNU 387 | >10 | 9.74±0.14 | 4.81±1.91 | >10 | >10 | 9.89±1.34 | 0.60±1.87 | 0.16±0.14 |
| SNU 423 | >10 | >10 | >10 | >10 | >10 | >10 | 0.01±0.84 | 0.01 |
 | Table VII.Proliferation of human liver cancer
cell lines, when cultured in medium containing 2 vs. 10% FBS,
following treatment with CDK inhibitors and Food and Drug
Administration-approved sorafenib and regorafenib. |
Table VII.
Proliferation of human liver cancer
cell lines, when cultured in medium containing 2 vs. 10% FBS,
following treatment with CDK inhibitors and Food and Drug
Administration-approved sorafenib and regorafenib.
|
| IC50
value (µM) |
|---|
|
|
|
|---|
|
| Palbociclib (CDK4/6
inhibitor) | Dinaciclib
(CDK1/2/5/9 inhibitor) | Sorafenib
(RAF1/BRAF/VEGFR1/2/4, PDGFR/KIT/FLT3/FGFR1/RET inhibitor) | Regorafenib
(VEGFR/VEGFR2/KDR/VEGFR3/FLT4/FGFR1/TEK/RAF1 inhibitor) |
|---|
|
|
|
|
|
|
|---|
| Cell line | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium | 2% FBS medium | 10% FBS medium |
|---|
| HEPG-2 | 9.29±1.10 | >10 | 5.09±3.75 | 3.21±2.12 | 8.10±1.88 | 7.39±2.05 | 8.26±1.57 | 6.22±1.45 |
| PLC/PRF/5 | 0.22±0.93 | 8.44±0.44 | 0.02±0.98 | 0.95±1.01 | 0.37±0.47 | 3.86±1.11 | 0.37±0.52 | 3.40±0.05 |
| C3A | >10 | 9.66±0.11 | 8.92±0.72 | 0.74±0.75 | 2.09±1.07 | 6.05±0.58 | 2.43±1.62 | 8.20±1.22 |
| SNU 449 | 9.50±2.42 | 8.66±2.11 | 0.18±0.72 | 8.62±1.20 | 2.60±0.99 | 8.32±0.47 | 2.08±0.88 | 8.96±1.02 |
| SNU 475 | 0.09±0.88 | 0.78±0.87 | 0.02±0.89 | 1.04±0.87 | 1.92±1.53 | 1.46±0.86 | 1.35±0.66 | 1.01±0.89 |
| SNU 387 | 4.09±2.17 | 8.84±1.35 | 0.01±0.67 | 0.97±0.95 | 2.10±2.15 | 1.10±0.24 | 0.72±0.78 | 3.01±1.12 |
| SNU 423 | 0.67±1.10 | 0.23±0.26 | 0.47±0.44 | 1.22±0.66 | 2.52±1.69 | 4.09±1.21 | 1.98±1.22 | 1.96±0.78 |
Discussion
HCC was the third most common cause of
cancer-related deaths worldwide in 2020 (1). Although there are more effective
therapeutic interventions for patients who are diagnosed at the
early stage of cancer, the great majority of patients with HCC are
diagnosed at the advanced stage of the disease and consequently
have a poorer response to current treatments with both traditional
and targeted cancer therapeutics (2,4,18). The
difference between tumours from different patients with HCC
(inter-tumour heterogeneity), and the presence of distinct tumour
cell populations within the same tumour specimen (intra-tumour
heterogeneity) and its microenvironment are some of the factors
contributing to the primary and acquired tumour cell resistance to
current therapies (2,4–5,7,9–10).
Therefore, in addition to preventative measures of avoiding the
known risk factors such as hepatitis B virus or hepatitis C virus
infections, to reduce the incidence and mortality of HCC, it is
considered important to identify additional biomarkers of
diagnostic, prognostic and predictive value in patients with HCC.
It is also important to identify more specific therapeutic targets
and to develop more effective therapeutic interventions.
EGFR and HER-2 are two of the most important
receptors for targeted therapy in patients with a wide range of
epithelial tumours, including colorectal, breast, lung, stomach,
thyroid, and head and neck cancer, in combination with chemotherapy
and radiotherapy (14–17). Despite the approval of several types
of HER-targeted drugs, including naked mAbs, antibody-drug
conjugates and small molecule TKIs targeting one or more members of
the HER family, no such drugs have currently been approved for the
treatment of patients with HCC (2,16–18).
In some studies, the cross-talk between different members of the
HER family and the cellular location of HER family members, such as
membranous or nuclear EGFR, have been associated with tumour
progression and poor response to treatment with HER inhibitors and
other therapeutics (24–33). While some studies have investigated
the expression of the individual members of the HER family in
patients with HCC, to the best of our knowledge, there has been no
comprehensive study of the co-expression, cellular location and
prognostic significance of all members of the HER family and
EGFRvIII, which is the most common deletion mutant type of EGFR at
its extracellular domain, in patients with HCC. Therefore, to the
best of our knowledge, the present study was the first to
investigate the relative expression, cellular location and
prognostic significance of all members of the HER family and
EGFRvIII, as well as the two putative CSC biomarkers CD44 and
EpCAM, and the cell proliferation biomarker Ki67 in patients with
HCC (10,34,35).
The present study investigated the expression levels and cellular
location of wtEGFR, which transmits the mitogenic action of seven
ligands belonging to the EGF family, including EGF, TGFα,
amphiregulin, heparin-binding EGF, betacellulin, epiregulin and
epigen, in patients with HCC. wtEGFR is recognized as the
signalling hub for mediating the mitogenic action of its ligands
via cross-talk with other growth factor signalling pathways. In
particular, EGFR and its ligands have been implicated in wound
repair and liver regeneration, and are also aberrantly expressed in
the development of HCC (3,4–6). In
the present study, at a cut off value of >5% of the tumour cells
with staining, 35% of the cases were wtEGFR-positive. The cellular
location of EGFR staining was membranous and cytoplasmic in 33 and
12% of cases, respectively, with 7% of the cases having an EGFR
intensity of 2+. None of the cases exhibited nuclear staining of
wtEGFR. Nuclear EGFR has been associated with poor prognosis and
resistance to therapy in other cancer types, and may be a potential
molecular target for anticancer strategies (36–38).
At the same cut off value, 58% of the cases were HER-2-positive,
and 14 and 40% of cases exhibited membranous expression of HER-2
and a staining intensity of 2+, respectively. Only HER-2 staining
at an intensity of 1+ was associated with improved prognosis, and
this warrants further investigation. While 19% of the cases were
HER-4-positive, none of the cases were HER-3-positive in the
present study.
Since the mid-1990s, membranous EGFR and HER-2 have
been important targets for therapy with various types of mAb-based
products (such as naked antibodies, antibody-drug conjugates,
bispecific antibodies or chimeric antigen receptor T cells) in a
wide range of epithelial tumours. However, the clinical benefit
obtained may be modest in some patients, with the expression level,
cellular location and mutational status of such receptors, and the
heterogenous expression of HER family members being some of the
contributing factors (14–17,39,40).
In the present study, wtEGFR expression was accompanied by
co-expression of HER-2 in 23% of the cases examined. It has been
shown recently that treatment of patients even with low HER-2
(immunohistochemistry score of 2+/in situ
hybridization-negative) metastatic breast cancer with the
anti-HER-2 antibody-drug conjugate fam-trastuzumab deruxtecan-mxki
resulted in improved OS in such patients, gaining FDA approval in
August 2022 (41,42). Therefore, the present results
support the need for investigation of the therapeutic potential of
various types of mAb-based drugs and small molecule TKIs targeting
the HER family members in patients with HCC whose tumours
co-express these receptors.
In addition to the examination of co-expression of
all members of the HER family, the present study investigated the
relative expression and prognostic significance of EGFRvIII, which
is the most common deletion mutant of EGFR containing deletions in
exons 2–7 of EGFR. EGFRvIII contains a tumour specific, truncated
extracellular domain incapable of ligand binding and is
constitutively active (14–16,19).
At the cut off value of >5% of the tumour cells with staining,
26% of the cases were EGFRvIII-positive. Notably, EGFRvIII
expression alone at all cut off values of >5, 10 and 20% of
cells with staining, and its co-expression with wtEGFR when the
staining was present in >5% of the tumour cells were associated
with poor prognosis, which was statistically significant in both
univariate and multivariate analysis, with the exception of
EGFRvIII staining at the cut off value of >10% of the tumour
cells, which was statistically significant only in univariate
analysis. EGFRvIII is currently an important target for therapeutic
interventions in patients with glioblastoma with various types of
mAb-based products, CAR-T cells, small molecule TKIs and EGFRvIII
cancer vaccines (43–50). However, the clinical benefits of
drugs targeting EGFRvIII have been modest. As cytoplasmic EGFRvIII
will not be accessible to mAb-based drugs, the cellular location of
EGFRvIII may also contribute to the poor response to therapy with
such drugs (44–50). In the present study, EGFRvIII
expression was mainly cytoplasmic, but its expression was
associated with poor OS in patients with HCC. Therefore, further
investigations of the co-expression, cellular location, prognostic
significance and predictive value of EGFRvIII and other members of
the HER family in a larger group of patients with HCC are
warranted. The therapeutic potential of drugs targeting one or more
members of the HER family when used in combination with other drugs
in patients with HCC should also be evaluated (51).
In the past few decades, the heterogeneous nature of
HCC has been associated not only with the presence of a diverse
population of bulky tumour cells, but also with the presence of
minor populations of CSCs in the tumour microenvironment. These are
capable of self-renewal and differentiation into different
populations of tumour cells, leading to tumour growth, metastasis,
tumour recurrence and resistance to therapies (52–55).
As a result, the present study examined the expression of two
putative CSC markers, CD44 and EpCAM, in patients with HCC
(56–60). At the cut off value of >5% of
tumour cells with staining, 40 and 33% of the cases were
CD44-positive and EpCAM-positive, respectively. The cellular
location of CD44 staining was membranous and cytoplasmic in 26 and
14%, respectively, of the cases examined. The membranous expression
of CD44 was significantly associated with poor OS in patients with
HCC in both univariate and multivariate analysis. By contrast, the
cytoplasmic expression of CD44 was associated with improved OS
(100.20 vs. 55.92 months; P=0.01). The results of a meta-analysis
involving 14 studies and 2,225 cases showed that CD44 expression
was associated with poor OS in patients with HCC (61). More recently, emodin, which is a
natural product found in the roots of a number of plants and an
anthraquinone derivate, has been shown to inhibit the proliferation
of CD44-positive tumour cells. Further research should investigate
whether the antitumour activity of emodin in HCC cells is
accompanied by increased toxicity (60,61).
While none of the patients in the present study exhibited nuclear
staining of CD44, 7% of the cases exhibited nuclear expression of
EpCAM, and the nuclear expression of EpCAM was associated with poor
prognosis (25.67 vs. 53.52 months). At a cut off value of >5% of
tumour cells with staining, not only the expression of EGFRvIII
alone but also its co-expression with the two HCC CSC markers CD44
and EpCAM, the cell proliferation marker Ki67, wtEGFR, and
wtEGFR/Ki67 were associated with poor OS in patients with HCC in
the univariate analysis. The present results provide further
support for the study of the expression pattern, prognostic
significance and targeting of EGFRvIII in patients with HCC.
Finally, due to the heterogenous nature of HCC
cells, the present study also investigated the relative expression
of all members of the HER family, two other growth factor receptors
(IGF IR and c-MET) and CD44 in a panel of human LCCLs. As increased
proliferation is a hallmark of human cancers, the sensitivity of
human LCCLs to treatment with agents targeting one or more members
of the HER family, IGF IR, c-MET and Wnt, and treatment with two
CDK inhibitors (palbocicilib and dinacicilib) was compared with the
sensitivity to treatment with the two FDA-approved drugs sorafenib
and regorafenib, and the cytotoxic drug doxorubicin. In general,
the expression of HER family members in LCCLs was lower than that
of EGFR and HER-2 in the positive control HN5 and SKOV3 cells, and
four out of seven LCCLs exhibited upregulation of CD44 expression.
As a result, of the HER inhibitors, the irreversible pan-HER family
blocker afatinib was the most effective drug by inhibiting the
proliferation of three out of seven human LCCLs, suggesting that
not all HCCs depend on HER signalling for their proliferation.
While treatment with crizotinib, NVP-AEW742 and LGK-974 inhibited
the proliferation of some LCCLs, they were less effective than the
two targeted drugs sorafenib and regorafenib, and doxorubicin,
which is approved for the treatment of patients with HCC. Finally
of the two CDK inhibitors, dinaciclib (CDK1/2/5/9) was the most
effective targeted agent by inhibiting the proliferation of all
LCCLs, being more effective than the CDK4/6 inhibitor palbocicilib
and the FDA-approved sorafenib and regorafenib. Therefore, it would
be interesting to investigate the therapeutic potential of drugs
targeting EGFRvIII, CDKs and other members of the HER family in
patients with HCC whose tumours are dependent on EGFRvIII and other
members of the HER family for cancer growth.
In summary, to the best of our knowledge, the
present study was the first to demonstrate that EGFRvIII expression
occurred in patients with HCC. While the expression of EGFRvIII was
mainly cytoplasmic, it was associated with poor survival when
expressed alone or in combination with wtEGFR or CSC biomarkers.
The present results highlighted the importance of EGFRvIII, which
is a tumour-specific and constitutively active form of EGFR, as a
biomarker of tumour progression. These results support targeting
EGFRvIII by repurposing drugs specific for EGFRvIII and other
members of the HER family when used in combination with CDK
inhibitors and other FDA-approved drugs in patients with HCC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Professor Albert J.
Wong (Department of Adult Neurosurgery, Stanford Cancer Institute,
Stanford University, Palo Alto, CA, USA) for providing
EGFRvIII-expressing cell lines as a model for the study of EGFRvIII
in human cancers. The original proposal on the study of HER family
in patients with HCC was designed in the memory of Helmout
Modjtahedi's Late cousin, Masoumeh Modjtahedi, who despite all
treatments passed away from liver cancer in 2018.
Funding
The present study was supported as part of a self-funded PhD
project at Kingston University London, UK.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HM conceived the original research idea for the
project. OS performed the experiments and conducted data analysis.
Both SAK and AMS co-supervised the project and helped with
technical training and data analysis. IB and OS performed the
scoring of immunohistochemistry staining. AD and SM were other
collaborators on this project on the clinical study of EGFR
expression in human cancers and made substantial contributions to
acquisition of data and/or analysis and interpretation of data. OS
wrote the manuscript. HM and SAK helped with final editing of the
manuscript and confirm the authenticity of all the raw data. All
authors have read and approved final version of the manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
NHS Research Ethics Committee (IRAS Project ID 252931; UK). Due to
the retrospective nature of the study, the requirement for patient
consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGFRvIII
|
type-III deletion mutant EGFR
|
|
HCC
|
hepatocellular carcinoma
|
|
CSC
|
cancer stem cell
|
|
TKIs
|
tyrosine kinase inhibitors
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Philips CA, Rajesh S, Nair DC, Ahamed R,
Abduljaleel JK and Augustine P: Hepatocellular Carcinoma in 2021:
An exhaustive update. Cureus. 13:e192742021.PubMed/NCBI
|
|
3
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lohitesh K, Chowdhury R and Mukherjee S:
Resistance a major hindrance to chemotherapy in hepatocellular
carcinoma: An insight. Cancer Cell Int. 18:442018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jing F, Li X, Jiang H, Sun J and Guo Q:
Combating drug resistance in hepatocellular carcinoma: No awareness
today, no action tomorrow. Biomed Pharmacother. 167:1155612023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lei YR, He XL, Li J and Mo CF: Drug
resistance in hepatocellular carcinoma: Theoretical basis and
therapeutic aspects. Front Biosci (Landmark Ed). 29:522024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Dang H and Wang XW: The
significance of intertumor and intratumor heterogeneity in liver
cancer. Exp Mol Med. 50:e4162018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khatib SA and Wang XW: Causes and
functional intricacies of inter- and intratumor heterogeneity of
primary liver cancers. Adv Cancer Res. 156:75–102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nio K, Yamashita T and Kaneko S: The
evolving concept of liver cancer stem cells. Mol Cancer. 16:42017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marin JJG, Macias RIR, Monte MJ, Romero
MR, Asensio M, Sanchez-Martin A, Cives-Losada C, Temprano AG,
Espinosa-Escudero R, Reviejo M, et al: Molecular Bases of Drug
Resistance in Hepatocellular Carcinoma. Cancers (Basel).
12:16632020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung A, Nasralla D and Quaglia A:
Understanding the immunoenvironment of primary liver cancer: A
Histopathology Perspective. J Hepatocell Carcinoma. 9:1149–1169.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ladd AD, Duarte S, Sahin I and Zarrinpar
A: Mechanisms of drug resistance in HCC. Hepatology. 79:926–940.
2024.PubMed/NCBI
|
|
14
|
Modjtahedi H and Dean C: The receptor for
EGF and its ligands-expression, prognostic value and target for
therapy in cancer (review). Int J Oncol. 4:277–296. 1994.PubMed/NCBI
|
|
15
|
Baselga J and Mendelsohn J: Receptor
blockade with monoclonal antibodies as anti-cancer therapy.
Pharmacol Ther. 64:127–154. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meric-Bernstam F, Johnson AM, Dumbrava
EEI, Raghav K, Balaji K, Bhatt M, Murthy RK, Rodon J and Piha-Paul
SA: Advances in HER2-Targeted Therapy: Novel agents and
opportunities beyond breast and gastric cancer. Clin Cancer Res.
25:2033–2041. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halder S, Basu S, Lall SP, Ganti AK, Batra
SK and Seshacharyulu P: Targeting the EGFR signaling pathway in
cancer therapy: What's new in 2023? Expert Opin Ther Targets.
27:305–324. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Selene II, Ozen M and Patel RA:
Hepatocellular Carcinoma: Advances in systemic therapy. Semin
Intervent Radiol. 41:56–62. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khelwatty SA, Puvanenthiran S, Essapen S,
Bagwan I, Seddon AM and Modjtahedi H: HER2 expression is predictive
of survival in cetuximab treated patients with RAS wild type
metastatic colorectal cancer. Cancers (Basel). 13:6382021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan T, Seddon A, Khelwatty S, Dalgleish
A, Bagwan I, Mudan S and Modjtahed H: The co-expression of HER
family members and CD109 is common in pancreatic cancer. Med Res
Arch. 11:1–35. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan T, Seddon AM, Dalgleish AG, Khelwatty
S, Ioannou N, Mudan S and Modjtahedi H: Synergistic activity of
agents targeting growth factor receptors, CDKs and downstream
signaling molecules in a panel of pancreatic cancer cell lines and
the identification of antagonistic combinations: implications for
future clinical trials in pancreatic cancer. Oncol Rep.
44:2581–2594. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mulliqi E, Khelwatty S, Morgan A, Ashkan K
and Modjtahedi H: Synergistic effects of neratinib in combination
with palbociclib or miransertib in brain cancer cells. World J
Oncol. 15:492–505. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu YC, Yeh CT and Lin KH: Cancer stem
cell functions in hepatocellular carcinoma and comprehensive
therapeutic strategies. Cells. 9:13312020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi JH, Guo WZ, Jin Y, Zhang HP, Pang C,
Li J, Line PD and Zhang SJ: Recognition of HER2 expression in
hepatocellular carcinoma and its significance in postoperative
tumor recurrence. Cancer Med. 8:1269–1278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin H, Shi Y, Lv Y, Yuan S, Ramirez CFA,
Lieftink C, Wang L, Wang S, Wang C, Dias MH, et al: EGFR activation
limits the response of liver cancer to lenvatinib. Nature.
595:730–734. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marshall G and Cao J: Mechanism-directed
combinational immunotherapies in liver cancer hold promise. Cell
Mol Immunol. 20:1395–1397. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steinway SN, Dang H, You H, Rountree CB
and Ding W: The EGFR/ErbB3 pathway acts as a compensatory survival
mechanism upon c-Met Inhibition in Human c-Met+ hepatocellular
carcinoma. PLoS One. 10:e01281592015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Zhang B and Sun Z: Spectrum of EGFR
aberrations and potential clinical implications: Insights from
integrative pan-cancer analysis. Cancer Commun (Lond). 40:43–59.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Puvanenthiran S, Essapen S, Haagsma B,
Bagwan I, Green M, Khelwatty SA, Seddon A and Modjtahedi H:
Co-expression and prognostic significance of the HER family
members, EGFRvIII, c-MET, CD44 in patients with ovarian cancer.
Oncotarget. 9:19662–19674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han W and Lo HW: Landscape of EGFR
signaling network in human cancers: Biology and therapeutic
response in relation to receptor subcellular locations. Cancer
Lett. 318:124–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li C, Iida M, Dunn EF, Ghia AJ and Wheeler
DL: Nuclear EGFR contributes to acquired resistance to cetuximab.
Oncogene. 28:3801–3813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tortora G, Gelardi T, Ciardiello F and
Bianco R: The rationale for the combination of selective EGFR
inhibitors with cytotoxic drugs and radiotherapy. Int J Biol
Markers. 22:47–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(Review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun X and Kaufman PD: Ki-67: More than a
proliferation marker. Chromosoma. 127:175–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Michalopoulos GK and Khan Z: Liver
regeneration, growth factors, and amphiregulin. Gastroenterology.
128:503–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Natarajan A, Wagner B and Sibilia M: The
EGF receptor is required for efficient liver regeneration. Proc
Natl Acad Sci USA. 104:17081–17086. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berasain C and Avila MA: The EGFR
signalling system in the liver: From hepatoprotection to
hepatocarcinogenesis. J Gastroenterol. 49:9–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lo HW and Hung MC: Nuclear EGFR signalling
network in cancers: linking EGFR pathway to cell cycle progression,
nitric oxide pathway and patient survival. Br J Cancer. 94:184–188.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brand TM, Iida M, Luthar N, Starr MM,
Huppert EJ and Wheeler DL: Nuclear EGFR as a molecular target in
cancer. Radiother Oncol. 108:370–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Modi S, Jacot W, Yamashita T, Sohn J,
Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al:
Trastuzumab deruxtecan in previously treated HER2-Low advanced
breast cancer. N Engl J Med. 387:9–20. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang C, Brezden-Masley C, Joy AA, Sehdev
S, Modi S, Simmons C and Henning JW: Targeting HER2-low in
metastatic breast cancer: An evolving treatment paradigm. Ther Adv
Med Oncol. 15:175883592311754402023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Platten M: EGFRvIII vaccine in
glioblastoma-InACT-IVe or not ReACTive enough? Neuro Oncol.
19:1425–1426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
An Z, Aksoy O, Zheng T, Fan QW and Weiss
WA: Epidermal growth factor receptor and EGFRvIII in glioblastoma:
Signaling pathways and targeted therapies. Oncogene. 37:1561–1575.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zebertavage L, Bambina S, Shugart J, Alice
A, Zens KD, Lauer P, Hanson B, Gough MJ, Crittenden MR and Bahjat
KS: A microbial-based cancer vaccine for induction of
EGFRvIII-specific CD8+ T cells and anti-tumor immunity. PLoS One.
14:e02091532019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Greenall SA, McKenzie M, Seminova E,
Dolezal O, Pearce L, Bentley J, Kuchibhotla M, Shengnan CC,
Mcdonald KL, Kornblum HI, et al: Most clinical anti-EGFR antibodies
do not neutralize both wtEGFR and EGFRvIII activation in glioma.
Neuro Oncol. 21:1016–1027. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rosenthal M, Curry R, Reardon DA,
Rasmussen E, Upreti VV, Damore MA, Henary HA, Hill JS and Cloughesy
T: Safety, tolerability, and pharmacokinetics of anti-EGFRvIII
antibody-drug conjugate AMG 595 in patients with recurrent
malignant glioma expressing EGFRvIII. Cancer Chemother Pharmacol.
84:327–336. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gedeon PC, Schaller TH, Chitneni SK, Choi
BD, Kuan CT, Suryadevara CM, Snyder DJ, Schmittling RJ, Szafranski
SE, Cui X, et al: A Rationally Designed Fully Human
EGFRvIII:CD3-Targeted Bispecific Antibody Redirects Human T Cells
to Treat Patient-derived Intracerebral Malignant Glioma. Clin
Cancer Res. 24:3611–3631. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iurlaro R, Waldhauer I, Planas-Rigol E,
Bonfill-Teixidor E, Arias A, Nicolini V, Freimoser-Grundschoberet
A, Cuartus I, Martinez-Moreno A, Martínez-Ricarte F, et al: A Novel
EGFRvIII T-Cell Bispecific Antibody for the Treatment of
Glioblastoma. Mol Cancer Ther. 21:1499–1509. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li F, Wu H, Du X, Sun Y, Rausseo BN,
Talukder A, Katailiha A, Elzohary L, Wang Y, Wang Z and Lizée G:
Epidermal growth factor receptor-targeted neoantigen peptide
vaccination for the treatment of non-small cell lung cancer and
glioblastoma. Vaccines (Basel). 11:14602023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chandramohan V, Bao X, Yu X, Parker S,
McDowall C, Yu YR, Healy P, Desjardins A, Gunn MD, Gromeier M, et
al: Improved efficacy against malignant brain tumors with
EGFRwt/EGFRvIII targeting immunotoxin and checkpoint inhibitor
combinations. J Immunother Cancer. 7:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li MM, Hi YT, Liang JK, Guan XY, Ma NF and
Liu M: Cancer stem cell-mediated therapeutic resistance in
hepatocellular carcinoma. Hepatoma Res. 8:362022. View Article : Google Scholar
|
|
53
|
Sukowati CHC: Heterogeneity of hepatic
cancer stem cells. Adv Exp Med Biol. 1139:59–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schulte LA, López-Gil JC, Sainz B Jr and
Hermann PC: The cancer stem cell in hepatocellular carcinoma.
Cancers (Basel). 12:6842020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jeng KS, Chang CF, Sheen IS, Jeng CJ and
Wang CH: Cellular and molecular biology of cancer stem cells of
hepatocellular carcinoma. Int J Mol Sci. 24:14172023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Endo K and Terada T: Protein expression of
CD44 (standard and variant isoforms) in hepatocellular carcinoma:
Relationships with tumor grade, clinicopathologic parameters, p53
expression, and patient survival. J Hepatol. 32:78–84. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Noh CK, Wang HJ, Kim CM, Kim J, Yoon SY,
Lee GH, Jo HJ, Yang MJ, Kim SS, Hwang JC, et al: EpCAM as a
predictive marker of tumor recurrence and survival in patients who
underwent surgical resection for hepatocellular carcinoma.
Anticancer Res. 38:4101–4109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou L and Zhu Y: The EpCAM overexpression
is associated with clinicopathological significance and prognosis
in hepatocellular carcinoma patients: A systematic review and
meta-analysis. Int J Surg. 56:274–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Luo Y and Tan Y: Prognostic value of CD44
expression in patients with hepatocellular carcinoma:
Meta-analysis. Cancer Cell Int. 16:472016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Akkol EK, Tatlı II, Karatoprak GŞ, Ağar
OT, Yücel Ç, Sobarzo-Sánchez E and Capasso R: Is emodin with
anticancer effects completely innocent? Two sides of the coin.
Cancers (Basel). 13:27332021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gao Y, Li Y, Zhu Y, Luo Q, Lu Y, Wen K, Du
B, Xi X and Li G: Emodin is a potential drug targeting
CD44-positive hepatocellular cancer. Curr Cancer Drug Targets.
24:510–518. 2024. View Article : Google Scholar : PubMed/NCBI
|