The high incidence of gynaecological malignancies
threatens women's health and longevity. Depending on their origin,
gynaecological malignancies are often categorized individually, and
they present different risk factors, symptoms, growth predictions
and treatments (1). As the leading
cause of cancer death in women, breast cancer (BCa) remains a
global public health problem (2).
Cervical cancer is second after BCa and causes >300,000 deaths
per year (3). The low 5-year
survival rate (4–6) of ovarian cancer (OC) is because most
patients are at the terminal stage when they are diagnosed, and
some exhibit chemoresistance (7).
In addition to the ovary and cervix, the endometrium may also be
affected by malignant tumours. The development of endometrial
cancer is mainly influenced by metabolic imbalance and genetic
susceptibility (8,9). The main treatment options for
gynaecological malignancies are surgery, chemotherapy and
radiotherapy. Chemotherapy aims to induce apoptosis in tumour
cells, selectively eliminating cancer cells without harming normal
cells, but some patients develop escape from apoptosis and
chemotherapy resistance. Regulatory cell death (RCD) can be
modulated by pharmacological or genetic interventions and is
controlled by specific signalling pathways. Exploring nonapoptotic
RCD processes may provide another strategy for breaking through the
antiapoptotic characteristics of tumours to inhibit tumour
growth.
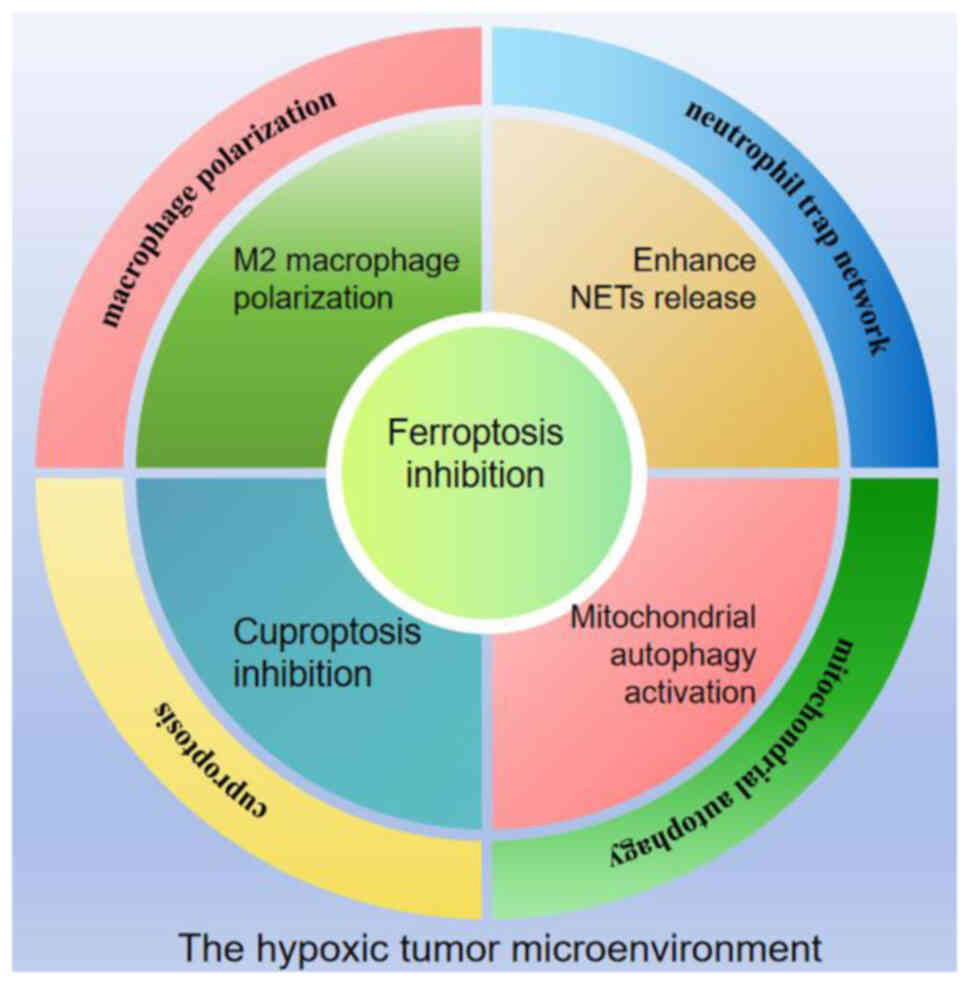
The vigorous metabolism and rapid proliferation of
tumour cells cause a hypoxic tumour microenvironment (TME). Cancer
cells undergo changes in signalling pathways and molecular
expression to adapt to the hypoxic environment and escape immune
surveillance, and hypoxia affects tumour biological behaviour and
treatment effects (17). Hypoxia
inducible factor (HIF) is the main regulator of the hypoxic
microenvironment (18). It is
activated under hypoxic conditions, and it promotes tumour
angiogenesis, regulates metabolic reprogramming, and increases
chemoradiotherapy resistance (19).
Hypoxia directly affects the expression of ferroptosis-related
molecules, upregulates the expression of iron oxidase and
stearoyl-CoA desaturase 1 (SCD1), downregulates iron
autophagy-related protein nuclear receptor coactivator 4 (NCOA4),
limits intracellular Fe2+, and inhibits ferroptosis
(20). Moreover, HIF and nuclear
factor erythroid 2-related factor 2 (Nrf2) are involved in
regulating and coordinating the antioxidant mechanisms of
ferroptosis and iron homeostasis (21). HIF-1 increases the transcription of
SLC7A11 and HO-1, inhibiting ferroptosis (22). Hypoxia increases Nrf2 activity,
increases HO-1 expression, and inhibits ferroptosis (23,24).
Inflammatory molecules released by cancer cells (such as IL-8,
CXCL1 and CTSC) and cells in the TME, such as cancer-associated
fibroblasts, can induce neutrophil extracellular trap (NET)
formation, and cancer cells in a hypoxic environment may have an
increased ability to induce NETs (25). Hypoxia-activated HIF-1α promotes M2
macrophage polarization by increasing the expression of VEGF, Arg1
and other M2-related genes (26).
Hypoxia also affects mitochondrial behaviour, inducing the
production of mitochondrial ROS (mtROS) through mitochondrial
complex I dysfunction and the activation of the mitochondrial
Na+/Ca2+ exchanger NCLX (27). The increase in mtROS induces
mitochondrial autophagy, which is related to the mTOR/AKT/HIF1α
signalling axis (28), further
improving the oxygen tolerance of cancer cells (29). Ferredoxin 1 (FDX1) and protein
acylation are key regulators of cuproptosis. Tumour hypoxia
significantly downregulates the expression of FDX1 in cells,
thereby significantly inhibiting cuproptosis (30). Tumour cells are highly adaptable in
hypoxic microenvironments; have heightened resistance to adverse
factors such as ferroptosis and cuproptosis; and promote
proliferation, oxygen resistance and invasion by inducing
macrophages to polarize to M2 (protumour type), increasing NET
release, and activating mitochondrial autophagy (Fig. 1).
To date, the intrinsic network between ferroptosis
and other tumour-related mechanisms has not been fully
characterized. Therefore, the latest research progress on the
crosstalk between ferroptosis and macrophage polarization, NETs,
mitochondrial autophagy and cuproptosis is reviewed. The role of
ferroptosis in female cancers has gradually emerged in recent
years. In the present review, research advances in the field of
ferroptosis in gynaecological malignancies and the implications for
gynaecological cancer therapy are discussed.
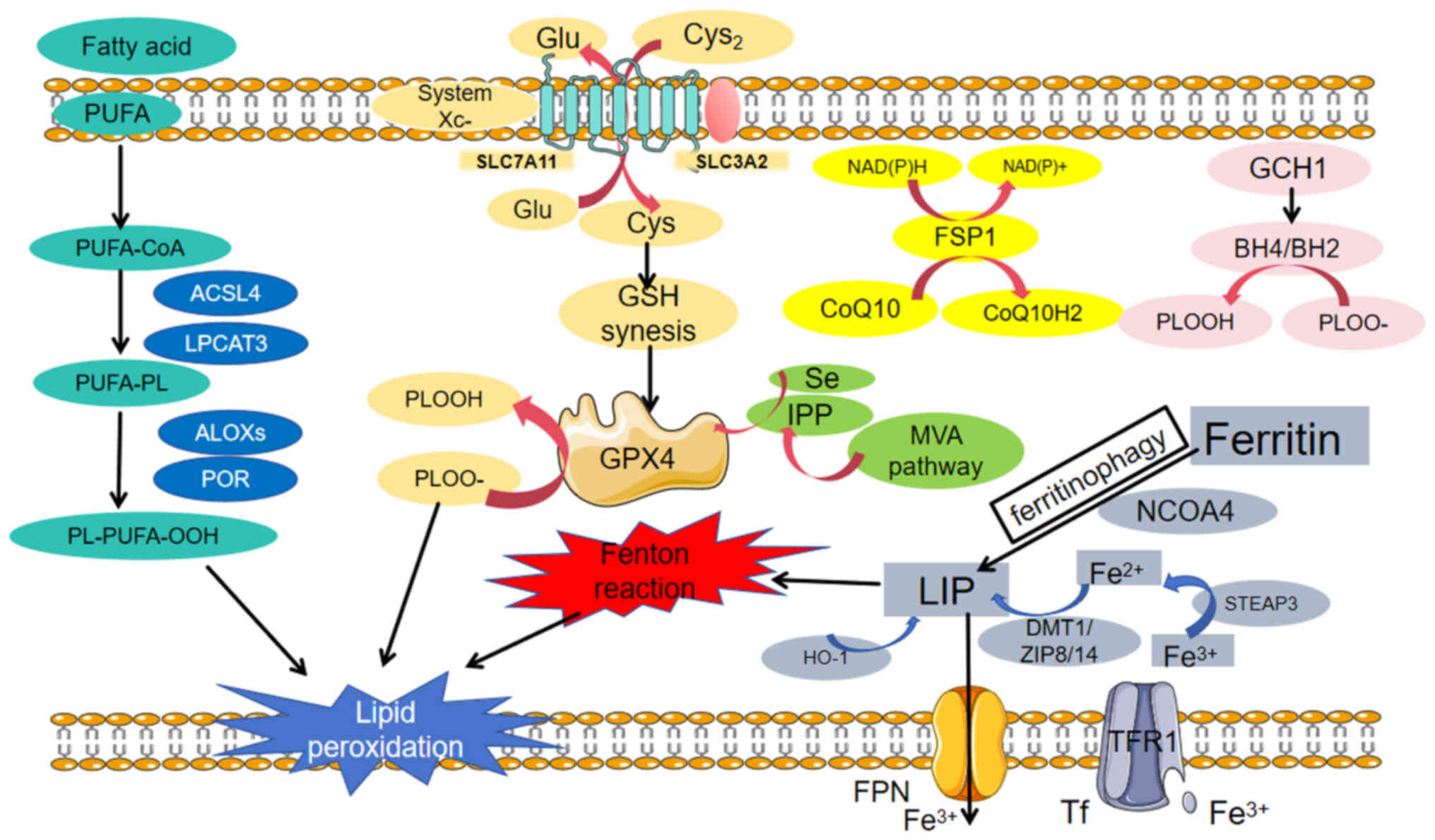
Ferroptosis is a special type of regulated cell
death characterized by intracellular iron overload, with excessive
accumulation of lipid peroxides on the cell membrane damaging
membrane integrity. It occurs when the ferroptosis defence
mechanism is out of balance (Fig.
2). Thus, lipid peroxidation, iron metabolism and the
anti-ferroptosis system constitute the cornerstones of ferroptosis;
conversely, ferroptosis can be induced or inhibited by genetic or
pharmacological intervention in these three aspects.
Polyunsaturated fatty acids (PUFAs), which are
produced from food, acetyl-CoA carboxylase (ACC), or lipid
phagocytes, are indispensable substrates for lipid peroxidation
under iron overload conditions. They bind with specific membrane
phospholipids (PLs) to form PUFA-PLs (12). Acyl-CoA synthetase long-chain family
member 4 (ACSL4) and lysolipid phosphatidylcholine acyltransferase
3 (LPCAT3) are crucial drivers of ferroptosis (31,32).
ACSL4 attaches long-chain PUFAs to CoA, catalysing the conversion
of free PUFAs to acyl-CoA derivatives (PUFA-CoAs) (33). The latter are further incorporated
into membrane phospholipids by LPCAT3 and possibly other enzymes,
such as AGPAT3, increasing the amount of long-chain PUFAs in
cellular lipids and membranes. Lipid peroxides interact with
Fe2+ to produce peroxide radicals, which extract
hydrogen from adjacent acyl chains in the lipid membrane,
propagating the lipid peroxidation process. This process is
mediated by lipoxygenases (ALOXs) or cytochrome P450
oxidoreductases (34). The products
of lipid peroxidation include initial lipid hydroperoxides (LOOHs)
and subsequent reactive aldehydes such as malondialdehyde (MDA).
The accumulation of lipid peroxides leads to membrane damage and
instability, eventually resulting in cell death.
Iron metabolism is a static and dynamic regulatory
process involving the absorption, storage, utilization and
excretion of iron and the participation of numerous proteins and
molecules. Iron absorption occurs mainly in the duodenum and upper
jejunum, and dietary iron is absorbed in the form of
Fe2+. Non-haem iron in food is mainly in the form of
insoluble Fe3+, which binds to transferrin (TF) in serum
and is subsequently recognized by the TF receptor (TFRC) on cell
membranes (35). TF carrying
Fe3+ binds to TFR1 to form a complex that is
internalized into the endosome. In endosomes, prostate epithelial
antigen 3 reduces Fe3+ to Fe2+, which is then
transported by ZIP8/14 (zinc transporter 8/14) or divalent metal
transporter 1 (DMT1), thus promoting the formation of labile iron
pools (LIP) (36). In addition to
extracellular iron transport, LIP expansion can be facilitated by
haem degradation and ferritinophagy mediated by NCOA4 (37), which can be excreted from cells via
the lysosome DMT1 (38). The iron
in a cell's LIP can be used in the mitochondria, sequenced in
ferritin, or excreted from the cell via ferritransporter (FPN).
Iron plays a key role in the induction and propagation of lipid
peroxidation, possibly by increasing the activity of ALOX or EGLN
prolyl hydroxylase and promoting free radical production, which
leads to lipid peroxidation and cell membrane damage, causing an
imbalance of enzyme-regulated lipid peroxidation and oxygen
homeostasis. In iron-overloaded cells, iron is released from these
compartments and increases the intracytoplasmic iron concentration
(39), thus promoting the Fenton
reaction and enhancing cell ROS production.
The glutathione peroxidase 4 (GPX4)-glutathione
(GSH) axis represents a pivotal pathway in countering ferroptosis.
GPX4 effectively combats lipid peroxidation by utilizing GSH as a
reducing agent. The synthesis of GSH relies on cystine, whose
uptake is mediated by the cystine reverse transport system Xc-.
This system comprises solute carrier family 7 member 11 (SLC7A11)
and solute carrier family 7 member 11 (SLC3A2). The proteins
encoded by the SLC7A11 and SLC3A2 genes form a transmembrane
transporter that is responsible for importing cystine into cells,
where it is then used to synthesize GSH. With GSH as a cofactor,
GPX4 reduces LOOHs to lipid alcohols, protecting cells from the
threat of ferroptosis. Additionally, nicotinamide adenine
dinucleotide phosphate (NADPH) plays a vital role in the GPX4-GSH
axis (11). As an electron donor,
NADPH participates in the recycling of GSH, enabling it to
continuously exert its antioxidant effects. Any disturbance to the
GPX4-GSH axis may trigger excessive generation of ROS, leading to
the occurrence of lipid peroxidation. These disturbances include
the degradation of GPX4 through autophagy or the
ubiquitin-proteasome system (40);
the inhibition of system Xc- by drugs such as erastin and
sorafenib; the direct inhibition of GPX4 by drugs such as
RAS-synthetic lethal 3 (RSL3) and the nitro-isoxazole-containing
compound (ML210); or defects in GSH, cysteine, or NADPH (41). Previously, scientists discovered a
ferroptosis inhibition mechanism that is independent of the GPX4
antioxidant pathway (42). The
FSP1-CoQ10-NAD(P)H system, which acts in parallel and
independently, synergizes with GPX4 and glutathione to inhibit
phospholipid peroxidation and ferroptosis. As a powerful
ferroptosis inhibitor, FSP1 (ferroptosis suppressor protein 1)
(43) exerts antioxidant effects
via coenzyme Q10 (CoQ10) with NADPH to regenerate reduced CoQ10
(CoQ10H2). In the mitochondrial lipid protection system,
dihydrolactate dehydrogenase (DHODH), an enzyme located on the
outer surface of the inner mitochondrial membrane, is essential. It
oxidizes dihydrolactate (DHO) to lactate (OA) in the inner
mitochondrial membrane and simultaneously reduces CoQ to CoQH2
(44,45). Therefore, DHODH/CoQ can protect
mitochondria from oxidative damage (46). Furthermore, GTP cyclohydrolase 1
plays a significant role in this antioxidant system. It produces
tetrahydrobiopterin (BH4), which can capture lipid-derived peroxyl
radicals and reduce oxidized lipids. Simultaneously, BH4
contributes to the production of CoQ10H2, further enhancing the
resistance of cells to oxidative stress and ferroptosis (47). In summary, the combined
dysregulation of iron metabolism and the redox system leads to the
accumulation of LOOHs in cells, ultimately triggering ferroptosis.
The GPX4-GSH axis and other related mechanisms play crucial roles
in this life-and-death struggle, jointly maintaining cellular
homeostasis and survival.
Ferroptosis is a regulated mode of cell death that
can be induced or inhibited by targeting iron metabolism, lipid
metabolism and the antioxidant system (GSH/GPX4 axis, CoQ/FSP1)
(11,48–50).
Erastin induces cell death in an iron-dependent manner. It targets
voltage-dependent anion channels (VDACs) and binds to VDAC2,
inducing lipid ROS production and mitochondrial dysfunction
(48). RSL5 (49), which binds to VDAC3, acts similarly
on VDACs. Additionally, diisothiocyanatostilbene-2′,2-disulfonic
acid (DIDS) (50) can block VDACs
and inhibit DNA damage repair, thereby inducing ferroptosis.
Temozolomide (TMZ) can disrupt intracellular iron levels and iron
homeostasis by enhancing DMT1 (51), thereby inducing ferroptosis
(52). MMRi62, a small-molecule
quinolinol, induces the degradation of the ferritin heavy chain,
disrupting intracellular iron homeostasis and leading to the
accumulation of iron ions within cells and an increase in ROS,
which ultimately induces ferroptosis (53). Ferroptosis is closely related to
lipid metabolism, and disrupting lipid metabolism can also regulate
ferroptosis. Sorafenib (54) is an
antitumour drug, and the expression level of ACSL4 is associated
with cellular sensitivity to sorafenib. The addition of sorafenib
directly affects the metabolic pathway of lipid ROS generation in
cells, leading to oxidative stress and DNA damage, which ultimately
induces ferroptosis. The small-molecule compound tert-butyl
hydroperoxide (55) can directly
affect lipid ROS levels, causing abnormalities in the mitochondrial
membrane potential and inducing ferroptosis. By targeting the
GSH-GPX4 axis, multiple steps can be regulated. RSL3, ML162,
diphenyleneiodonium (DPI) and ferroptosis-inducing 56 (FIN56) can
induce ferroptosis by promoting the degradation of GPX4 (56,57).
In addition to blocking VDAC, erastin depletes GSH to further
weaken the antioxidant capacity of cells, simultaneously inducing
GPX4 degradation, exacerbating lipid peroxidation, and inducing
ferroptosis (58). By targeting the
FSP1/CoQ-related pathway, NDP4928 binds and inhibits FSP1,
enhancing the GSH-induced suppression of ferroptosis (59). FIN56 inhibits farnesyl diphosphate
farnesyltransferase (SQS), reduces cholesterol synthesis, depletes
CoQ, degrades GPX4, causes mitochondrial dysfunction, and promotes
the induction of ferroptosis. Different compounds target different
steps of the GSH-GPX4 axis, including promoting GPX4 degradation,
depleting GSH, and inhibiting FSP1 and SQS, to induce ferroptosis
through multiple mechanisms. The emergence of ferroptosis
inhibitors has also advanced research on the regulation of
ferroptosis. For example, cyclopyrrolone (11), deferoxamine, deferiprone and
deferasirox (60) inhibit
ferroptosis by chelating iron ions, whereas ferrostatin-1 (Fer-1)
inhibits lipid peroxidation induced by aromatic amines.
microRNA-522 inhibits ferroptosis by targeting arachidonate ALOX15
(61). β-ME helps to produce
cystine, increases GPX4 expression, and inhibits ferroptosis by
reacting with cystine to form mixed disulphide bonds (62). 2-Cyano-3,12-dioxooleana-1,9
(11)-dien-28-oic acid inhibits the
function of heat shock protein (HSP) 90, thereby inhibiting the
degradation of GPX4 and protecting cells from ferroptosis (58). These findings provide new strategies
and ideas for cancer treatment, and they offer important clues for
elucidating the regulatory network of ferroptosis and developing
new anticancer strategies.
Mitochondrial autophagy refers to the selective
removal of damaged or incomplete mitochondria through autophagy,
which serves as a ‘scavenger’ for maintaining mitochondrial network
homeostasis and functional integrity. Mitochondrial autophagy is a
promising biomarker and potential therapeutic target (104) because its abnormal activity is
associated with the growth and metastasis of cancers, particularly
OC (105). In response to a
certain level of oxidative stress, mitochondria can temporarily
protect cells by promoting mitochondrial fusion (106), mitigating oxidative stress,
inhibiting ferroptosis and maintaining their own stability
(107). However, when damage
exceeds the threshold for mitochondrial fusion, mitochondrial
autophagy is activated (108),
which helps maintain mitochondrial stability by reducing the
accumulation of ROS, preserving iron homeostasis, activating
cellular antioxidant systems, and enhancing cellular resistance to
oxidative stress (109).
Nevertheless, excessive activation of mitochondrial autophagy can
have negative consequences. Sustained activation of mitochondrial
autophagy can lead to the release of metal ions such as iron from
mitochondria into the cytoplasm, providing an unstable iron source.
Iron reacts with H2O2 in the subsequent
Fenton reaction, generating large amounts of hydroxyl radicals
(·OH), which are highly reactive oxidants that can initiate lipid
peroxidation, damage cell membrane structures, impair functions,
and promote ferroptosis (110).
During tumour development, mitochondrial autophagy plays a dual
role. On the one hand, it can eliminate dysfunctional mitochondria,
alleviate oxidative stress, and prevent carcinogenesis (109). On the other hand, under adverse
conditions (such as nutrient deprivation and hypoxia),
mitochondrial autophagy can promote tumour cell survival and
protect cells from apoptosis or necrosis. Therefore, mitochondrial
autophagy is a crucial factor in controlling cancer cell
quality.
The concentration of copper is closely related to
cellular activities such as cell proliferation and angiogenesis, as
well as metabolic processes such as glycolysis and lipid
transformation (111,112). Rapid cancer cell division and
immune infiltration are inseparable from copper levels, and
increased copper concentrations can be observed in various
malignant tumours, including breast, gynaecological, lung,
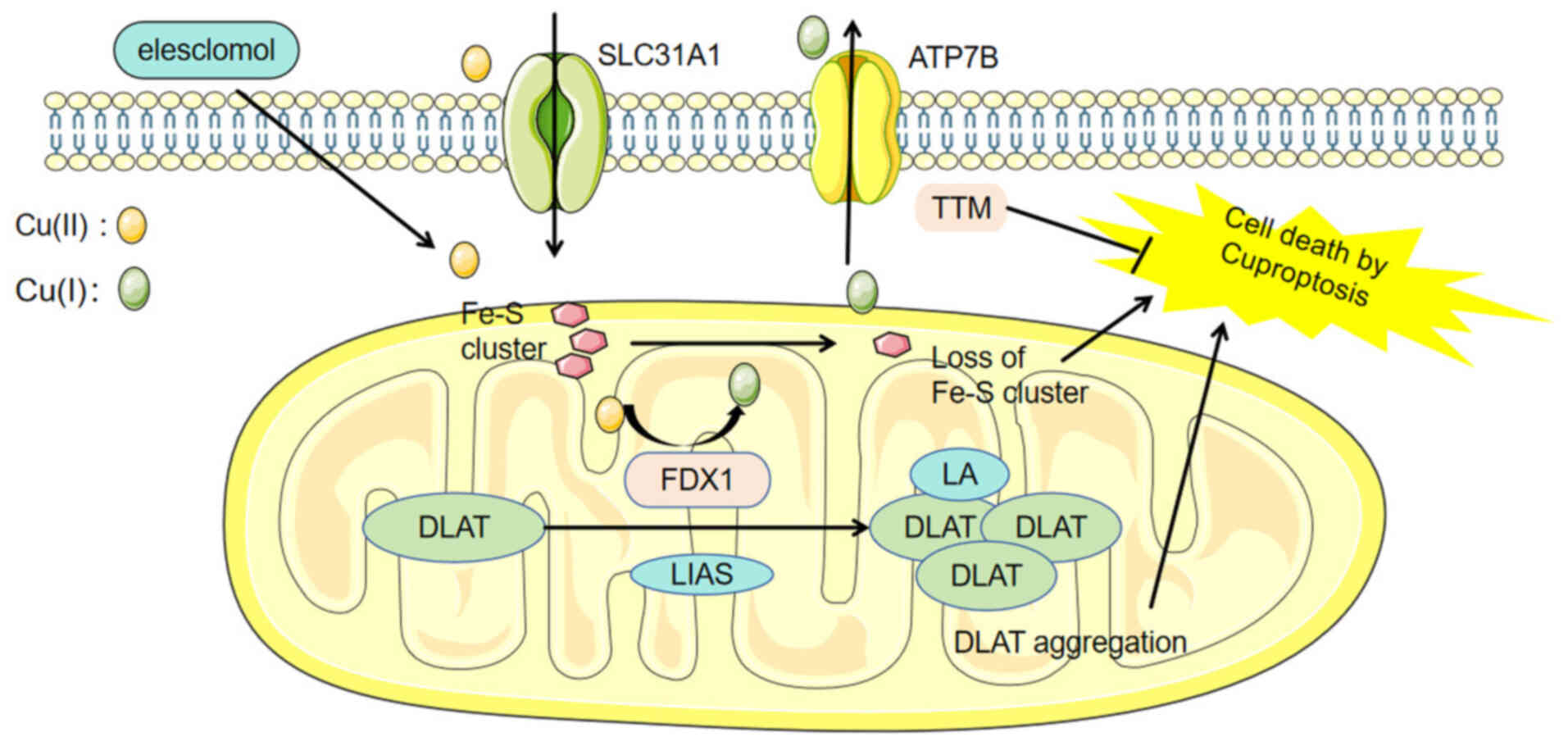
pancreatic, and gastric cancer (113–115). In 2022, Peter Tsvetkov first
proposed the concept of ‘cuproptosis’, a type of regulated cell
death that differs from apoptosis and follows ferroptosis. The
process of cuproptosis involves the accumulation of
copper-dependent fatty acid-acylated proteins and the reduction of
Fe-S cluster proteins (116)
(Fig. 4). Copper ions in the
extracellular environment can be transported into cells by binding
to copper ionophores such as elesclomol. The upstream regulator of
protein acylation, FDX1/lipoyl synthase, is responsible for
reducing Cu(II) to Cu(I) (117),
which subsequently binds to lipoylated proteins within the
tricarboxylic acid cycle (TCA) cycle in mitochondria, such as
dihydrolipoamide S-acetyltransferase (118,119). During this process, lipoylated
proteins accumulate, leading to increased ROS generation.
Additionally, the stability of Fe-S clusters is disruptive, and the
resulting protein toxicity stress serves as a trigger for cell
death. This process can be reversed by copper chelators such as
tetrathiomolybdate (120).
Ferroptosis and cuproptosis involve similar regulatory processes,
such as alterations in metal valence states, metabolism of
macronutrients, and energy conversion, all of which affect cancer
signalling pathways (121). There
are also interactions between ferroptosis and cuproptosis. First,
there are overlapping molecular components between these two modes
of cell death. In a study investigating the pathogenesis of
osteoarthritis, He et al (122) reported that 63 ferroptosis-related
genes were closely related to 11 cuproptosis-related genes, and
among these, they identified 40 novel characteristic genes
associated primarily with inflammation, extracellular stimuli and
autophagy. Luo et al (123)
reported six ferroptosis genes (including TRIB3, PML and CD44) to
be related to cuproptosis and were negatively correlated with
survival rates. Second, copper can increase the ubiquitination and
aggregation of GPX4, promoting its degradation and initiating
ferroptosis. Copper chelators can specifically inhibit ferroptosis
but have no effect on other forms of cell death, such as necrosis
or apoptosis (124). Third, both
forms of cell death can be triggered by the same stimuli, such as
P53 activation and excessive ROS production (125,126). Finally, the progression of a
single disease can involve both forms of cell death. In clear-cell
renal cell carcinoma (ccRCC), the downregulation of FDX1, a key
factor in cuproptosis, has been linked to tumorigenesis (123). The overexpression of Kruppel-like
factor 2 downregulates GPX4 (127), increasing the sensitivity of ccRCC
to ferroptosis and thereby hindering its growth and invasion
(128).
Ferroptosis has dual effects on tumours. Resistance
to ferroptosis is the nature of tumors. Inhibiting ferroptosis can
promote cancer progression, whereas inducing ferroptosis has
promising applications in tumour treatment. Ferroptosis can
increase the sensitivity of tumour cells to traditional antitumour
therapies such as chemotherapy and radiotherapy, providing a new
strategy for targeting cancer cells that are difficult to eliminate
with traditional treatments.
BCa. BCa is the most common malignant tumour in
women, and non-surgical treatments for this disease include
chemotherapy, human epidermal growth factor receptor 2-targeted
therapy and endocrine therapy (129). Ferroptosis is involved in the drug
resistance (130) and prognosis
(131) of BCa. Zou et al
(132) reported that VDAC3-derived
crRNA binds to HSPB1 and inhibits its ubiquitination degradation,
reducing the accumulation of ROS and LIP and thereby inhibiting
ferroptosis in BCa cells with low levels of HER2, which in turn
mediates trastuzumab deruxtecan resistance. Breast tissue has
vigorous fatty acid metabolism, the expression of IL-6 and leptin
is significantly increased in cancer-associated adipocytes, and
these two substances are key factors in promoting tumour growth
(133–135). Studies have shown that disrupting
lipid metabolism reprogramming in BCa cells through ferroptosis can
inhibit tumour activity and prevent tumour metastasis (136). Ferroptosis interferes with fatty
acid metabolism by causing the oxidation of PUFAs to increase the
production of PLOO−. This process damages the metabolism
of cancer cells and thus promotes cancer cell death. Bobińskir
et al (137) proposed that
inducing ferroptosis causes an unbalanced fatty acid ratio in
cancer cells, limiting the consumption and biosynthesis of
BCa-related fatty acids and consequently leading to fatty acid
deficiency and inhibiting tumour progression. In addition to
interfering with fat metabolism, ferroptosis can reverse resistance
to endocrine therapy in BCa. Tamoxifen (TAM) is a long-term
endocrine drug for patients with oestrogen receptor
(ER)+ BCa (138).
Unfortunately, TAM cannot escape the development of chemoresistance
(139). shown It has been reported
that ferroptosis and the non-canonical NF-κB pathway activated by
RelB are involved in BCa-TAM resistance: Activated RelB inhibits
TAM-induced ferroptosis by upregulating GPX4, thereby promoting TAM
resistance (140). By contrast,
sustained inhibition of RelB transcriptional activation
re-sensitizes TAM-resistant cells by increasing ferroptosis. The
development of drugs related to RelB inhibitors is expected to
promote the reversal of BCa resistance. The overexpression of
DNAJC12, a member of the HSP family (HSP40), is a negative
predictor of the response to neoadjuvant concurrent
chemoradiotherapy (141). It has
been revealed that overexpression of DNAJC12 inhibits ferroptosis
and apoptosis through the HSP70-AKT signalling axis, thereby
promoting BC resistance to chemotherapy and azithromycin. Studies
have also shown that AKT or HSP70 inhibitors can reverse this
process by repairing broken caspase3 and reducing GPX4 and SLC7A11
levels, providing new treatments for BCa chemotherapy resistance
(142). TNBC, characterized by the
absence of ER, progesterone receptor (PR) and HER2 (143), is the subtype of BCa that is the
most difficult to treat (144).
TNBC is rich in iron and lipids, making the induction of
ferroptosis a viable therapeutic strategy (31,145).
Yu et al (146) reported
that TFRC is highly expressed in ER+ tissues and that
reduced ER expression can increase TFRC expression, suggesting that
ER plays a regulatory role in TFRC expression. Timmerman et
al (147) reported that TNBC
cells rely on glutamine and that reducing intracellular glutamine
or inhibiting system Xc-can increase ROS in TNBC, inhibiting tumour
progression.
The incidence rate of cervical cancer among young
women has been increasing, which is a cause of serious concern. The
annual mortality rate of cervical cancer exceeds 300,000, making it
one of the cancers with the highest mortality rate among women
worldwide, along with BCa, colorectal and lung cancer (3). Research has revealed the involvement
of ferroptosis in the transformation of normal cervical cells into
squamous intraepithelial lesion (SIL), the progression of SIL, and
its transformation into cervical squamous cell carcinoma (148). Cervical cancer cells can inhibit
ferroptosis through circular RNAs (149,150), hypoxia (151) and the proliferation of M1
macrophages (152), enabling them
to survive and proliferate under ferroptotic stress (153). Inducing ferroptosis by targeting
the characteristics of cervical cancer that resist ferroptosis
provides a new prospective therapeutic approach. Oleanolic acid
(OA) is a natural anticancer agent (154). It has been identified that OA
targets and promotes ACSL4-mediated ferroptosis, which promotes the
biosynthesis of PUFA-PLs and increases lipid peroxidation, thereby
inhibiting the proliferation of cervical cancer cells (155). Dihydroartemisinin (DHA) is the
main active metabolite of artemisinin and its derivatives and has a
variety of low-toxicity anticancer properties. DHA can induce
NCOA4-mediated ferritin autophagy, thereby leading to an increase
in the intracellular LIP, aggravating the Fenton reaction to
produce excessive ROS, and consequently enhancing ferroptosis in
cervical cancer. The combination of DHA and doxorubicin has a
highly synergistic elimination effect on cervical cancer cells,
which is also related to ferroptosis (156). In addition to chemotherapy,
ferroptosis induction combined with radiotherapy has unexpected
effects. Radiotherapy can not only activate NRF2-mediated GPX4
transcription but also inhibit lysosome-mediated GPX4 degradation,
thereby inducing cancer cell tolerance to ferroptosis and
radioresistance. Tubastatin A, a histone deacetylase 6 inhibitor,
significantly promotes radiotherapy-induced lipid peroxidation and
tumour suppression by inhibiting GPX4 enzyme activity, overcoming
the ferroptosis resistance and radioresistance of cancer cells
(157). Various ferroptosis
inducers, such as sorafenib and sulfaquinoxaline, can act as
radiosensitizers by inhibiting the activity of SLC7A11 and GPX4.
Combining radiotherapy with ferroptosis inducers is expected to
overcome radiotherapy resistance in patients with cervical cancer
(158).
In total, ~70% of patients are already in the
advanced stage at the time of their first diagnosis of OC (159). Multiple mechanisms, including
glycolysis, fatty acid synthesis and angiogenesis mimicry,
collectively contribute to the development of OC (160–162). Platinum drugs combined with
paclitaxel are the traditional first-line treatment options for OC,
but chemotherapy resistance and high recurrence rates often occur
during treatment (163,164). Studies have shown that both
cisplatin and paclitaxel can act on the GPX4-GSH axis. The former
forms a complex with glutathione (165), and the latter downregulates the
expression of system Xc-. Both can reduce GSH levels, increase
oxidative stress and lipid peroxidation, and effectively induce
ferroptosis (166). The
characteristics of platinum-resistant cancer cells may confer
therapeutic benefits. Wang et al (167) reported that overexpression of the
Wnt receptor frzzled-7 (FZD7) activates the oncogenic factor TP63,
upregulates the glutathione metabolic pathway, increases GPX
expression, and protects cancer cells from chemotherapy-induced
peroxidative damage. After treatment with GPX4 inhibitors,
FZD7+ platinum-resistant OC cells become more sensitive
to platinum drugs, filling the therapeutic gap in treating
platinum-resistant cancers (168).
Curcumin sensitizes cisplatin-resistant OC cells to
cisplatin-induced apoptosis. However, its low bioavailability
limits its application. The development of the curcumin derivative
NLO1 has greatly improved the antitumour effects of curcumin.
Ferroptosis is involved in this process (169). NLO1 can downregulate HCAR1/MCT1
expression, activate the AMPK-SREBP1 pathway, downregulate GPX4
expression, induce ferroptosis in the Anglne and HO8910PM OC cell
lines, and inhibit OC proliferation. Erastin induces lipid ROS
production and mitochondrial dysfunction, consumes GSH, weakens the
antioxidant capacity of cells, and triggers ferroptosis. It has a
synergistic effect with cisplatin in inducing ferroptosis to
inhibit the growth of OC cells in vitro and in vivo,
thereby increasing the cytotoxic effect of cisplatin while reducing
side effects (170). In addition,
it has been revealed that combined treatment with cisplatin and
natural antitumour compounds isolated from the roots of
Lithospermum officinale can increase the levels of the
ferroptosis-related molecular markers ROS, LPO and Fe2+,
downregulate GPX4, induce ferroptosis, and synergistically reduce
the viability of cisplatin-resistant OC cells (171). PARP is an important target for
cancer treatment and is involved in DNA repair, methylation,
transcriptional regulation and transcriptional metabolism (172,173). The pharmacological inhibition or
genetic deletion of PARP promotes ferroptosis by inhibiting
SLC7A11-mediated GSH biosynthesis in a p53-dependent manner.
Olaparib is the most classic and effective PARP inhibitor (174,175). It is used in combination with a
ferroptosis inducer (FIN) to sensitize BRCA-mutated OC cells to
PARP inhibitors (176). Arsenic
trioxide is used in combination with olaparib to activate the AMPKα
pathway, inhibit SCD1 expression, promote lipid peroxidation, and
ultimately induce ferroptosis, increasing the effect of olaparib on
platinum-resistant OC (177).
Targeted therapy against ferroptosis is expected to open new
avenues for the treatment of platinum-resistant OC.
Endometrial cancer (EC) is a type of cancer that
arises from the malignant transformation of endometrial epithelial
cells (178). Metabolic
reprogramming is involved in the development of EC. Studies have
shown that EC is dependent on glucose and glutamine and
overexpresses SLC7A11 (179,180), which not only affects cellular
antioxidant defence (181) but
also influences the induction of ferroptosis in tumour cells. EC
cells rely on glycolysis-lipogenesis metabolism (182). A high glycolytic rate inhibits the
TCA cycle, reducing the production of NADH and ROS. Conversely,
inhibiting glycolysis in ECs promotes the TCA cycle and oxidative
phosphorylation, thereby inducing ferroptosis and inhibiting tumour
progression (183). Glucose
oxidase nanoparticles have been utilized to target tumour cells
(184), where they catalyse
glucose decomposition and increase H2O2
concentrations, thereby reducing glucose levels and increasing ROS
in tumours (185). The glutamine
dependency of EC can be exploited by upregulating the glutamine
transporter ASCT2 in ECs, which in turn reduces intracellular
glutamine levels and inhibits EC cell proliferation (179). Given that SLC7A11 is a critical
player in ferroptosis, therapies targeting SLC7A11 can also be
applied to EC. The use of ferroptosis inducers (such as
sulfasalazine, erastin and RSL3) to treat megestrol acetate
(MPA)-resistant EC downregulates SLC7A11 and GPX4, significantly
reducing the survival rate of MPA-resistant EC-1 cells (185). In addition, juglone (186) activates ferroptosis in EC cells by
upregulating heme oxygenase 1, resulting in the release of free
iron from haem in ECs, the production of lipid peroxides, and the
reduction of MDA. These results suggest a new therapeutic approach
for the treatment of EC.
The mechanism of ferroptosis involves iron
homeostasis, lipid metabolism and antioxidant systems. Ferroptosis
is a programmed cell death process that can be regulated by drugs
or genetic means, either inducers or inhibitors. In the TME, tumour
cells resist ferroptosis by activating hypoxia-related induction
factors to reduce intracellular iron storage or by increasing
antioxidant signals (187). In the
tumour immune microenvironment, inducing M2 macrophage polarization
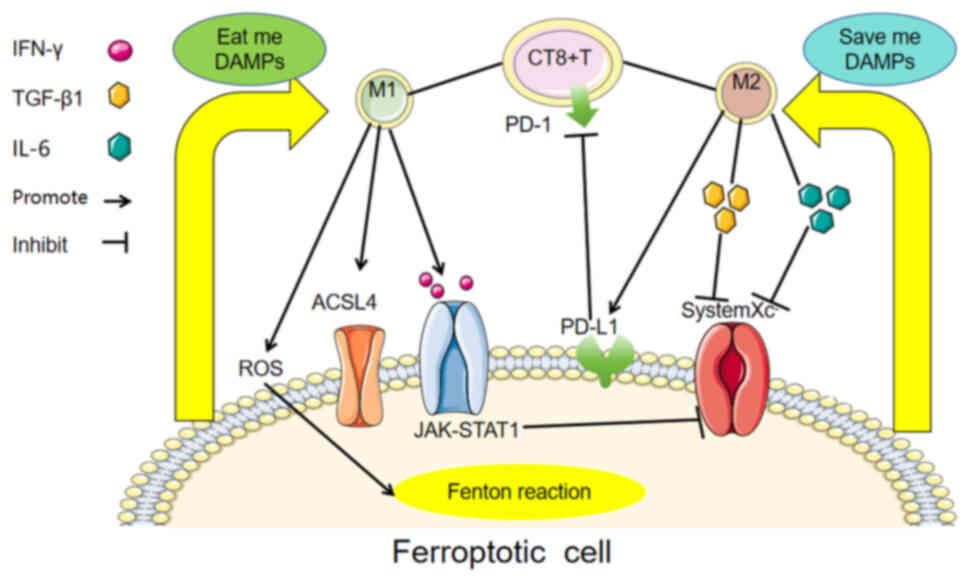
is beneficial for tumour survival and progression. Ferroptotic
cells can release DAMPs to activate the immune system, induce
macrophage polarization, and initiate signals related to protection
or resistance to ferroptosis. The depletion of M2 tumour-associated
macrophages in the TME and M1 repolarization can activate
ferroptosis and prevent tumour progression and metastasis (70,72).
The polarization state and function of macrophages in the TME are
significantly influenced by iron metabolism. Future research needs
to further explore the specific mechanisms of iron metabolism in
macrophage polarization and tumour immunotherapy and discover how
to balance the anticancer and cancer-promoting effects of
ferroptosis in macrophages and direct iron regulation in
macrophages towards the inhibition of cancer progression to develop
more effective therapeutic strategies. Inflammatory factors
secreted by tumours can induce the formation of NETs, which protect
tumour cells from cytotoxic immunity and impair tumour clearance
(188). In BCa (92), they can act as chemokines to mediate
the distant metastasis of tumours and can also promote tumour
progression by mediating ferroptosis resistance (103). Interestingly, in non-tumour cells,
such as alveolar epithelial cells (189) and intestinal endothelial cells
(190), NETs can cause disease
phenotypes by inducing ferroptosis. The mechanism by which NETs
resist ferroptosis in tumour tissues but induce ferroptosis in
non-tumour tissues has not yet been elucidated. Abnormalities in
mitochondrial structure and function lead to abnormal levels and
distributions of metal ions (191). Mitochondrial autophagy contributes
to mitochondrial quality control. The resulting autophagic
mitochondria cannot only isolate abnormal mitochondria but also
serve as new iron storage space to prevent the generation of ROS by
the Fenton reaction from inducing further cell death (110). However, excessive mitochondrial
autophagy is a sufficient source of iron for ferroptosis.
Cuproptosis is another form of metal ion-dependent cell death that
was discovered after ferroptosis and is closely related to multiple
signalling pathways and tumour-related biological behaviours
(192). The ferroptosis inducers
sorafenib and erastin can increase cuproptosis in primary liver
cancer, upregulate protein fatty acylation by blocking
mitochondrial matrix-associated protease-mediated degradation of
the FDX1 protein, and reduce the synthesis of the intracellular
copper chelator GSH by inhibiting cystine import (193). Cuproptosis and ferroptosis can be
induced by the same stimuli and share interacting molecules and
genes, and cuproptosis can promote ferroptosis (194). These findings indicate that
ferroptosis is not an independent pathway involved in disease.
Ferroptosis combines mechanisms involved in tumour occurrence and
development, such as cellular immunity-macrophage polarization,
organelle defence-mitochondrial autophagy, the cell
clearance-neutrophil capture network, and the interactions of other
metal ions with copper. Among these mechanisms, whether ferroptosis
is the main trunk or a side branch, has great application prospects
in oncology research. Examples of inducers and inhibitors of
ferroptosis are included in Table
I.
At present, the drug resistance of gynaecological
tumours is a worldwide problem that urgently needs to be solved.
Ferroptosis has been found to be involved in tumorigenesis, the
destruction of the immune microenvironment, tumour proliferation
and metastasis, and the treatment of malignant gynaecological
tumours. The current therapeutic strategies for inducing
ferroptosis in tumour cells include targeting anti-iron oxidation
pathways and ferroptosis metabolic pathways. The former weakens the
antioxidant capacity of cancer cells mainly by inhibiting the
GSH-GPX4 axis and inducing tumour cell death. The latter induces
ferroptosis in cancer cells by regulating ferroptosis metabolic
systems such as iron metabolism and lipid metabolism. Research on
related drugs in female patients is also in full swing. Ferroptosis
can supplement the therapeutic mechanisms of existing drugs, such
as curcumin derivatives, or it can be combined with existing drugs,
such as tamoxifen combined with RELB inhibitors, erastin combined
with platinum, and FIN combined with olaparib, to promote the
antitumour effect, reverse chemotherapy resistance or reduce
adverse drug reactions. In addition, ferroptosis combined with
radiotherapy is expected to reverse the radiotherapy resistance of
tumour cells. Ferroptosis has the potential to overcome
difficulties in the traditional treatment of gynaecological
malignancies, inhibit tumour cell proliferation and metastasis, and
resolve tumour resistance. However, the existing research on
ferroptosis remains experimental, and further research is needed to
enable clinical translation.
Not applicable.
The present study was supported by the Natural Science
Foundation of China (82303246), the Natural Science Foundation of
Hunan Province (2023JJ41066), Health Research Project of Hunan
Health Commission in 2024 (W20243173) and the Project of Hunan
Provincial Health Commission (202205034020).
Not applicable.
PTW and YKL structured the ideas for the document
and drafted the outline. PTW, JLC and HL were responsible for the
writing of the original manuscript and the creation of figures and
table. HYL and JZ reviewed and revised the manuscript. All authors
read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global cancer in women: Burden and trends. Cancer
Epidemiol Biomarkers Prev. 26:444–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abu Samaan TM, Samec M, Liskova A, Kubatka
P and Büsselberg D: Paclitaxel's mechanistic and clinical effects
on breast cancer. Biomolecules. 9:7892019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vu M, Yu J, Awolude OA and Chuang L:
Cervical cancer worldwide. Curr Probl Cancer. 42:457–465. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ledermann JA: Front-line therapy of
advanced ovarian cancer: New approaches. Ann Oncol. 28
(Suppl_8):viii46–viii50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fillon M: Opportunistic salpingectomy may
reduce ovarian cancer risk. CA Cancer J Clin. 72:97–99. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mirza MR, Coleman RL, González-Martín A,
Moore KN, Colombo N, Ray-Coquard I and Pignata S: The forefront of
ovarian cancer therapy: Update on PARP inhibitors. Ann Oncol.
31:1148–1159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalampokas E, Giannis G, Kalampokas T,
Papathanasiou AA, Mitsopoulou D, Tsironi E, Triantafyllidou O,
Gurumurthy M, Parkin DE, Cairns M and Vlahos NF: Current approaches
to the management of patients with endometrial cancer. Cancers
(Basel). 14:45002022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brooks RA, Fleming GF, Lastra RR, Lee NK,
Moroney JW, Son CH, Tatebe K and Veneris JL: Current
recommendations and recent progress in endometrial cancer. CA
Cancer J Clin. 69:258–279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolma S, Lessnick SL, Hahn WC and
Stockwell BR: Identification of genotype-selective antitumor agents
using synthetic lethal chemical screening in engineered human tumor
cells. Cancer Cell. 3:285–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conrad M and Pratt DA: The chemical basis
of ferroptosis. Nat Chem Biol. 15:1137–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi J, Zhu J, Wu J, Thompson CB and Jiang
X: Oncogenic activation of PI3K-AKT-mTOR signaling suppresses
ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA.
117:31189–31197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedmann Angeli JP, Krysko DV and Conrad
M: Ferroptosis at the crossroads of cancer-acquired drug resistance
and immune evasion. Nat Rev Cancer. 19:405–414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao X, Liu K, Shen S, Meng L and Chen S:
Ferroptosis, a new form of cell death: Mechanisms, biology and role
in gynecological malignant tumor. Am J Cancer Res. 13:2751–2762.
2023.PubMed/NCBI
|
|
17
|
Chen Z, Han F, Du Y, Shi H and Zhou W:
Hypoxic microenvironment in cancer: Molecular mechanisms and
therapeutic interventions. Signal Transduct Target Ther. 8:702023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Q, You L, Nepovimova E, Heger Z, Wu W,
Kuca K and Adam V: Hypoxia-inducible factors: Master regulators of
hypoxic tumor immune escape. J Hematol Oncol. 15:772022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semenza GL: Pharmacologic targeting of
Hypoxia-inducible factors. Annu Rev Pharmacol Toxicol. 59:379–403.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuhrmann DC, Mondorf A, Beifuß J, Jung M
and Brüne B: Hypoxia inhibits ferritinophagy, increases
mitochondrial ferritin, and protects from ferroptosis. Redox Biol.
36:1016702020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuhrmann DC and Brüne B: A graphical
journey through iron metabolism, microRNAs, and hypoxia in
ferroptosis. Redox Biol. 54:1023652022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng X, Wang S, Sun Z, Dong H, Yu H, Huang
M and Gao X: Ferroptosis enhanced diabetic renal tubular injury via
HIF-1α/HO-1 pathway in db/db mice. Front Endocrinol (Lausanne).
12:6263902021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan S, Wei C, Liu G, Zhang L, Li J, Li L,
Cai S and Fang L: Sorafenib attenuates liver fibrosis by triggering
hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell
Prolif. 55:e131582022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu XJ, Lv YF, Cui WZ, Li Y, Liu Y, Xue YT
and Dong F: Icariin inhibits hypoxia/reoxygenation-induced
ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1
signaling pathway. FEBS Open Bio. 11:2966–2976. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adrover JM, McDowell SAC, He XY, Quail DF
and Egeblad M: NETworking with cancer: The bidirectional interplay
between cancer and neutrophil extracellular traps. Cancer Cell.
41:505–526. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge W and Wu W: Influencing Factors and
significance of Tumor-associated Macrophage polarization in tumor
microenvironment. Zhongguo Fei Ai Za Zhi. 26:228–237. 2023.(In
Chinese). PubMed/NCBI
|
|
27
|
Hernansanz-Agustín P, Choya-Foces C,
Carregal-Romero S, Ramos E, Oliva T, Villa-Piña T, Moreno L,
Izquierdo-Álvarez A, Cabrera-García JD, Cortés A, et al: Na+
controls hypoxic signalling by the mitochondrial respiratory chain.
Nature. 586:287–291. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung J, Zhang Y, Celiku O, Zhang W, Song
H, Williams BJ, Giles AJ, Rich JN, Abounader R, Gilbert MR and Park
DM: Mitochondrial NIX promotes tumor survival in the hypoxic niche
of glioblastoma. Cancer Res. 79:5218–5232, 20193. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuo CL, Ponneri Babuharisankar A, Lin YC,
Lien HW, Lo YK, Chou HY, Tangeda V, Cheng LC, Cheng AN and Lee AY:
Mitochondrial oxidative stress in the tumor microenvironment and
cancer immunoescape: Foe or friend? J Biomed Sci. 29:742022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao C, Wang X, Li S, Zhang Z, Li J, Deng
Q, Chen X, Yang X and Li Z: A cuproptosis-based nanomedicine
suppresses triple negative breast cancers by regulating tumor
microenvironment and eliminating cancer stem cells. Biomaterials.
313:1227632025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dixon SJ, Winter GE, Musavi LS, Lee ED,
Snijder B, Rebsamen M, Superti-Furga G and Stockwell BR: Human
haploid cell genetics reveals roles for lipid metabolism genes in
nonapoptotic cell death. ACS Chem Biol. 10:1604–1609. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan H, Li X, Zhang X, Kang R and Tang D:
Identification of ACSL4 as a biomarker and contributor of
ferroptosis. Biochem Biophys Res Commun. 478:1338–1343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou Y, Li H, Graham ET, Deik AA, Eaton JK,
Wang W, Sandoval-Gomez G, Clish CB, Doench JG and Schreiber SL:
Cytochrome P450 oxidoreductase contributes to phospholipid
peroxidation in ferroptosis. Nat Chem Biol. 16:302–309. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koleini N, Shapiro JS, Geier J and
Ardehali H: Ironing out mechanisms of iron homeostasis and
disorders of iron deficiency. J Clin Invest. 131:e1486712021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luck AN and Mason AB: Transferrin-mediated
cellular iron delivery. Curr Top Membr. 69:3–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mancias JD, Wang X, Gygi SP, Harper JW and
Kimmelman AC: Quantitative proteomics identifies NCOA4 as the cargo
receptor mediating ferritinophagy. Nature. 509:105–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yambire KF, Rostosky C, Watanabe T,
Pacheu-Grau D, Torres-Odio S, Sanchez-Guerrero A, Senderovich O,
Meyron-Holtz EG, Milosevic I, Frahm J, et al: Impaired lysosomal
acidification triggers iron deficiency and inflammation in vivo.
Elife. 8:e510312019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang DD: Ironing out the details of
ferroptosis. Nat Cell Biol. 26:1386–1393. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen X, Yu C, Kang R, Kroemer G and Tang
D: Cellular degradation systems in ferroptosis. Cell Death Differ.
28:1135–1148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu J, Kang R and Tang D: Signaling
pathways and defense mechanisms of ferroptosis. FEBS J.
289:7038–7050. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ and Zoncu R: The
CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garcia-Bermudez J and Birsoy K: A
mitochondrial gatekeeper that helps cells escape death by
ferroptosis. Nature. 593:514–515. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao L, Zhou X, Xie F and Zhang L, Yan H,
Huang J, Zhang C, Zhou F, Chen J and Zhang L: Ferroptosis in cancer
and cancer immunotherapy. Cancer Commun (Lond). 42:88–116. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP Cyclohydrolase
1/Tetrahydrobiopterin Counteract Ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yagoda N, von Rechenberg M, Zaganjor E,
Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM,
Boniface JJ, et al: RAS-RAF-MEK-dependent oxidative cell death
involving voltage-dependent anion channels. Nature. 447:864–868.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang WS and Stockwell BR: Synthetic lethal
screening identifies compounds activating iron-dependent,
nonapoptotic cell death in oncogenic-RAS-harboring cancer cells.
Chem Biol. 15:234–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tomaskova Z, Gaburjakova J, Brezova A and
Gaburjakova M: Inhibition of anion channels derived from
mitochondrial membranes of the rat heart by stilbene
disulfonate-DIDS. J Bioenerg Biomembr. 39:301–311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xue X, Ramakrishnan SK, Weisz K, Triner D,
Xie L, Attili D, Pant A, Győrffy B, Zhan M, Carter-Su C, et al:
Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling
to promote colorectal tumorigenesis. Cell Metab. 24:447–461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Song Q, Peng S, Sun Z, Heng X and Zhu X:
Temozolomide drives ferroptosis via a DMT1-Dependent pathway in
glioblastoma cells. Yonsei Med J. 62:843–849. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li J, Lama R, Galster SL, Inigo JR, Wu J,
Chandra D, Chemler SR and Wang X: Small-Molecule MMRi62 induces
ferroptosis and inhibits metastasis in pancreatic cancer via
degradation of ferritin heavy chain and mutant p53. Mol Cancer
Ther. 21:535–545. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng J, Lu PZ, Zhu GZ, Hooi SC, Wu Y,
Huang XW, Dai HQ, Chen PH, Li ZJ, Su WJ, et al: ACSL4 is a
predictive biomarker of sorafenib sensitivity in hepatocellular
carcinoma. Acta Pharmacol Sin. 42:160–170. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wenz C, Faust D, Linz B, Turmann C,
Nikolova T, Bertin J, Gough P, Wipf P, Schröder AS, Krautwald S and
Dietrich C: t-BuOOH induces ferroptosis in human and murine cell
lines. Arch Toxicol. 92:759–775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF and Clish CB: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M,
Shan B, Pan H and Yuan J: Chaperone-mediated autophagy is involved
in the execution of ferroptosis. Proc Natl Acad Sci USA.
116:2996–3005. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yoshioka H, Kawamura T, Muroi M, Kondoh Y,
Honda K, Kawatani M, Aono H, Waldmann H, Watanabe N and Osada H:
Identification of a small molecule that enhances ferroptosis via
inhibition of ferroptosis suppressor protein 1 (FSP1). ACS Chem
Biol. 17:483–491. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao Y, Yin W, Yang Z, Sun J, Chang J,
Huang L, Xue L, Zhang X, Zhi H, Chen S, et al:
Nanotechnology-enabled M2 macrophage polarization and ferroptosis
inhibition for targeted inflammatory bowel disease treatment. J
Control Release. 367:339–353. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang H, Deng T, Liu R, Ning T, Yang H,
Liu D, Zhang Q, Lin D, Ge S, Bai M, et al: CAF secreted miR-522
suppresses ferroptosis and promotes acquired chemo-resistance in
gastric cancer. Mol Cancer. 19:432020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ishii T, Bannai S and Sugita Y: Mechanism
of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro.
Role of the mixed disulfide of 2-mercaptoethanol and cysteine. J
Biol Chem. 256:12387–12392. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Murdoch C, Giannoudis A and Lewis CE:
Mechanisms regulating the recruitment of macrophages into hypoxic
areas of tumors and other ischemic tissues. Blood. 104:2224–2234.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Boutilier AJ and Elsawa SF: Macrophage
polarization states in the tumor microenvironment. Int J Mol Sci.
22:69952021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone
S, Xu X, Zhang H, Santin AD, Lou G and Min W: Tumor-associated
macrophages drive spheroid formation during early transcoelomic
metastasis of ovarian cancer. J Clin Invest. 126:4157–4173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Recalcati S, Locati M, Gammella E,
Invernizzi P and Cairo G: Iron levels in polarized macrophages:
Regulation of immunity and autoimmunity. Autoimmun Rev. 11:883–889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gu Z, Liu T, Liu C, Yang Y, Tang J, Song
H, Wang Y, Yang Y and Yu C: Ferroptosis-strengthened metabolic and
inflammatory regulation of Tumor-associated macrophages provokes
potent tumoricidal activities. Nano Lett. 21:6471–6479. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hao X, Zheng Z, Liu H, Zhang Y, Kang J,
Kong X, Rong D, Sun G, Sun G, Liu L, et al: Inhibition of APOC1
promotes the transformation of M2 into M1 macrophages via the
ferroptosis pathway and enhances anti-PD1 immunotherapy in
hepatocellular carcinoma based on single-cell RNA sequencing. Redox
Biol. 56:1024632022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li LG, Peng XC, Yu TT, Xu HZ, Han N, Yang
XX, Li QR, Hu J, Liu B, Yang ZY, et al: Dihydroartemisinin remodels
macrophage into an M1 phenotype via ferroptosis-mediated DNA
damage. Front Pharmacol. 13:9498352022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Recalcati S, Locati M, Marini A,
Santambrogio P, Zaninotto F, De Pizzol M, Zammataro L, Girelli D
and Cairo G: Differential regulation of iron homeostasis during
human macrophage polarized activation. Eur J Immunol. 40:824–835.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhao YY, Lian JX, Lan Z, Zou KL, Wang WM
and Yu GT: Ferroptosis promotes anti-tumor immune response by
inducing immunogenic exposure in HNSCC. Oral Dis. 29:933–941. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen W, Zuo F, Zhang K, Xia T, Lei W,
Zhang Z, Bao L and You Y: Exosomal MIF derived from nasopharyngeal
carcinoma promotes metastasis by repressing ferroptosis of
macrophages. Front Cell Dev Biol. 9:7911872021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jakubczyk K, Dec K, Kałduńska J, Kawczuga
D, Kochman J and Janda K: Reactive oxygen species-sources,
functions, oxidative damage. Pol Merkur Lekarski. 48:124–127.
2020.PubMed/NCBI
|
|
75
|
Wang W, Green M, Choi JE, Gijón M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al: CD8+ T
cells regulate tumour ferroptosis during cancer immunotherapy.
Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Haschka D, Hoffmann A and Weiss G: Iron in
immune cell function and host defense. Semin Cell Dev Biol.
115:27–36. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wen ZF, Liu H, Gao R, Zhou M, Ma J, Zhang
Y, Zhao J, Chen Y, Zhang T, Huang F, et al: Tumor cell-released
autophagosomes (TRAPs) promote immunosuppression through induction
of M2-like macrophages with increased expression of PD-L1. J
Immunother Cancer. 6:1512018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Farhood B, Najafi M and Mortezaee K: CD8+
cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell
Physiol. 234:8509–8521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yang L, Guo J, Yu N, Liu Y, Song H, Niu J
and Gu Y: Tocilizumab mimotope alleviates kidney injury and
fibrosis by inhibiting IL-6 signaling and ferroptosis in UUO model.
Life Sci. 261:1184872020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Carmona-Cuenca I, Roncero C, Sancho P,
Caja L, Fausto N, Fernández M and Fabregat I: Upregulation of the
NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its
pro-apoptotic activity. J Hepatol. 49:965–976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang L, Xing R, Li C, Liu Y, Sun L, Liu X
and Wang Y: Active immunization with Tocilizumab mimotopes induces
specific immune responses. BMC Biotechnol. 15:462015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen P, Wang D, Xiao T, Gu W, Yang H, Yang
M and Wang H: ACSL4 promotes ferroptosis and M1 macrophage
polarization to regulate the tumorigenesis of nasopharyngeal
carcinoma. Int Immunopharmacol. 122:1106292023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Puylaert P, Roth L, Van Praet M, Pintelon
I, Dumitrascu C, van Nuijs A, Klejborowska G, Guns PJ, Berghe TV,
Augustyns K, et al: Effect of erythrophagocytosis-induced
ferroptosis during angiogenesis in atherosclerotic plaques.
Angiogenesis. 26:505–522. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tang R, Xu J, Zhang B, Liu J, Liang C, Hua
J, Meng Q, Yu X and Shi S: Ferroptosis, necroptosis, and pyroptosis
in anticancer immunity. J Hematol Oncol. 13:1102020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wen Q, Liu J, Kang R, Zhou B and Tang D:
The release and activity of HMGB1 in ferroptosis. Biochem Biophys
Res Commun. 510:278–283. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dai E, Han L, Liu J, Xie Y, Kroemer G,
Klionsky DJ, Zeh HJ, Kang R, Wang J and Tang D: Autophagy-dependent
ferroptosis drives tumor-associated macrophage polarization via
release and uptake of oncogenic KRAS protein. Autophagy.
16:2069–2083. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Takei H, Araki A, Watanabe H, Ichinose A
and Sendo F: Rapid killing of human neutrophils by the potent
activator phorbol 12-myristate 13-acetate (PMA) accompanied by
changes different from typical apoptosis or necrosis. J Leukoc
Biol. 59:229–240. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fuchs TA, Abed U, Goosmann C, Hurwitz R,
Schulze I, Wahn V, Weinrauch Y, Brinkmann V and Zychlinsky A: Novel
cell death program leads to neutrophil extracellular traps. J Cell
Biol. 176:231–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Papayannopoulos V and Zychlinsky A: NETs:
A new strategy for using old weapons. Trends Immunol. 30:513–521.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Monti M, De Rosa V, Iommelli F, Carriero
MV, Terlizzi C, Camerlingo R, Di Minno G and Del Vecchio S:
Neutrophil extracellular traps as an adhesion substrate for
different tumor cells expressing RGD-Binding integrins. Int J Mol
Sci. 19:23502018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pieterse E, Rother N, Garsen M, Hofstra
JM, Satchell SC, Hoffmann M, Loeven MA, Knaapen HK, van der Heijden
OWH, Berden JHM, et al: Neutrophil extracellular traps drive
Endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc
Biol. 37:1371–1379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xiao Y, Cong M, Li J, He D, Wu Q, Tian P,
Wang Y, Yang S, Liang C, Liang Y, et al: Cathepsin C promotes
breast cancer lung metastasis by modulating neutrophil infiltration
and neutrophil extracellular trap formation. Cancer Cell.
39:423–437.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xia X, Zhang Z, Zhu C, Ni B, Wang S, Yang
S, Yu F, Zhao E, Li Q and Zhao G: Neutrophil extracellular traps
promote metastasis in gastric cancer patients with postoperative
abdominal infectious complications. Nat Commun. 13:10172022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Awasthi D and Sarode A: Neutrophils at the
crossroads: Unraveling the multifaceted role in the tumor
microenvironment. Int J Mol Sci. 25:29292024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li C, Chen T, Liu J, Wang Y, Zhang C, Guo
L, Shi D, Zhang T, Wang X and Li J: FGF19-induced inflammatory CAF
promoted neutrophil extracellular trap formation in the liver
metastasis of colorectal cancer. Adv Sci (Weinh). 10:e23026132023.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yang L, Liu Q, Zhang X, Liu X, Zhou B,
Chen J, Huang D, Li J, Li H, Chen F, et al: DNA of neutrophil
extracellular traps promotes cancer metastasis via CCDC25. Nature.
583:133–138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lee W, Ko SY, Mohamed MS, Kenny HA,
Lengyel E and Naora H: Neutrophils facilitate ovarian cancer
premetastatic niche formation in the omentum. J Exp Med.
216:176–194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Aldabbous L, Abdul-Salam V, McKinnon T,
Duluc L, Pepke-Zaba J, Southwood M, Ainscough AJ, Hadinnapola C,
Wilkins MR, Toshner M and Wojciak-Stothard B: Neutrophil
extracellular traps promote angiogenesis: Evidence from vascular
pathology in pulmonary hypertension. Arterioscler Thromb Vasc Biol.
36:2078–2087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang H, Wu D, Wang Y, Guo K, Spencer CB,
Ortoga L, Qu M, Shi Y, Shao Y, Wang Z, et al: METTL3-mediated
N6-methyladenosine exacerbates ferroptosis via
m6A-IGF2BP2-dependent mitochondrial metabolic reprogramming in
sepsis-induced acute lung injury. Clin Transl Med. 13:e13892023.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang Y and Ertl HC: Starved and
Asphyxiated: How Can CD8(+) T cells within a tumor microenvironment
prevent tumor progression. Front Immunol. 7:322016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen X, Song M, Zhang B and Zhang Y:
Reactive oxygen species regulate T cell immune response in the
tumor microenvironment. Oxid Med Cell Longev. 2016:15809672016.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Demers M, Krause DS, Schatzberg D,
Martinod K, Voorhees JR, Fuchs TA, Scadden DT and Wagner DD:
Cancers predispose neutrophils to release extracellular DNA traps
that contribute to cancer-associated thrombosis. Proc Natl Acad Sci
USA. 109:13076–13081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yao L, Sheng X, Dong X, Zhou W, Li Y, Ma
X, Song Y, Dai H and Du Y: Neutrophil extracellular traps mediate
TLR9/Merlin axis to resist ferroptosis and promote triple negative
breast cancer progression. Apoptosis. 28:1484–1495. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang W: The mitophagy receptor FUN14
domain-containing 1 (FUNDC1): A promising biomarker and potential
therapeutic target of human diseases. Genes Dis. 8:640–654. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Al-Faze R, Ahmed HA, El-Atawy MA, Zagloul
H, Alshammari EM, Jaremko M, Emwas AH, Nabil GM and Hanna DH:
Mitochondrial dysfunction route as a possible biomarker and therapy
target for human cancer. Biomed J. March 5–2024.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sugioka R, Shimizu S and Tsujimoto Y:
Fzo1, a protein involved in mitochondrial fusion, inhibits
apoptosis. J Biol Chem. 279:52726–52734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen QM: Nrf2 for protection against
oxidant generation and mitochondrial damage in cardiac injury. Free
Radic Biol Med. 179:133–143. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tian C, Liu Y, Li Z, Zhu P and Zhao M:
Mitochondria related cell death modalities and disease. Front Cell
Dev Biol. 10:8323562022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li Y, Wang X, Huang Z, Zhou Y, Xia J, Hu
W, Wang X, Du J, Tong X and Wang Y: CISD3 inhibition drives
cystine-deprivation induced ferroptosis. Cell Death Dis.
12:8392021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yu F, Zhang Q, Liu H, Liu J, Yang S, Luo
X, Liu W, Zheng H, Liu Q, Cui Y, et al: Dynamic O-GlcNAcylation
coordinates ferritinophagy and mitophagy to activate ferroptosis.
Cell Discov. 8:402022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yu Z, Cao W, Ren Y, Zhang Q and Liu J:
ATPase copper transporter A, negatively regulated by miR-148a-3p,
contributes to cisplatin resistance in breast cancer cells. Clin
Transl Med. 10:57–73. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Finney L, Mandava S, Ursos L, Zhang W,
Rodi D, Vogt S, Legnini D, Maser J, Ikpatt F and Olopade OI: X-ray
fluorescence microscopy reveals large-scale relocalization and
extracellular translocation of cellular copper during angiogenesis.
Proc Natl Acad Sci USA. 104:2247–2252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lopez J, Ramchandani D and Vahdat L:
Copper depletion as a therapeutic strategy in cancer. Met Ions Life
Sci. 303–330. 2019.PubMed/NCBI
|
|
114
|
Shanbhag VC, Gudekar N, Jasmer K,
Papageorgiou C, Singh K and Petris MJ: Copper metabolism as a
unique vulnerability in cancer. Biochim Biophys Acta Mol Cell Res.
1868:1188932021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lossow K, Schwarz M and Kipp AP: Are trace
element concentrations suitable biomarkers for the diagnosis of
cancer? Redox Biol. 42:1019002021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Target Ther. 7:3782022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Tsvetkov P, Detappe A, Cai K, Keys HR,
Brune Z, Ying W, Thiru P, Reidy M, Kugener G, Rossen J, et al:
Mitochondrial metabolism promotes adaptation to proteotoxic stress.
Nat Chem Biol. 15:681–689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Mayr JA, Feichtinger RG, Tort F, Ribes A
and Sperl W: Lipoic acid biosynthesis defects. J Inherit Metab Dis.
37:553–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Solmonson A and DeBerardinis RJ: Lipoic
acid metabolism and mitochondrial redox regulation. J Biol Chem.
293:7522–7530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Brewer GJ, Askari F, Lorincz MT, Carlson
M, Schilsky M, Kluin KJ, Hedera P, Moretti P, Fink JK, Tankanow R,
et al: Treatment of Wilson disease with ammonium
tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and
trientine in a double-blind study of treatment of the neurologic
presentation of Wilson disease. Arch Neurol. 63:521–527. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chen T, Liang L, Wang Y, Li X and Yang C:
Ferroptosis and cuproptposis in kidney diseases: Dysfunction of
cell metabolism. Apoptosis. 29:289–302. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
He B, Liao Y, Tian M, Tang C, Tang Q, Ma
F, Zhou W, Leng Y and Zhong D: Identification and verification of a
novel signature that combines cuproptosis-related genes with
ferroptosis-related genes in osteoarthritis using bioinformatics
analysis and experimental validation. Arthritis Res Ther.
26:1002024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Luo G, Wang L, Zheng Z, Gao B and Lei C:
Cuproptosis-related ferroptosis genes for predicting prognosis in
kidney renal clear cell carcinoma. Eur J Med Res. 28:1762023.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Xue Q, Yan D, Chen X, Li X, Kang R,
Klionsky DJ, Kroemer G, Chen X, Tang D and Liu J: Copper-dependent
autophagic degradation of GPX4 drives ferroptosis. Autophagy.
19:1982–1996. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Xiong C, Ling H, Hao Q and Zhou X:
Cuproptosis: A53-regulated metabolic cell death? Cell Death Differ.
30:876–884. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Shao L, Zhu L, Su R, Yang C, Gao X, Xu Y,
Wang H, Guo C and Li H: Baicalin enhances the chemotherapy
sensitivity of oxaliplatin-resistant gastric cancer cells by
activating p53-mediated ferroptosis. Sci Rep. 14:107452024.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zou Y, Palte MJ, Deik AA, Li H, Eaton JK,
Wang W, Tseng YY, Deasy R, Kost-Alimova M, Dančík V, et al: A
GPX4-dependent cancer cell state underlies the clear-cell
morphology and confers sensitivity to ferroptosis. Nat Commun.
10:16172019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lu Y, Qin H, Jiang B, Lu W, Hao J, Cao W,
Du L, Chen W, Zhao X, Guo H, et al: KLF2 inhibits cancer cell
migration and invasion by regulating ferroptosis through GPX4 in
clear cell renal cell carcinoma. Cancer Lett. 522:1–13. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Gradishar WJ, Moran MS, Abraham J, Aft R,
Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et
al: Breast cancer, version 3.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:691–722. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zou Y, Zheng S, Xie X, Ye F, Hu X, Tian Z,
Yan SM, Yang L, Kong Y, Tang Y, et al: N6-methyladenosine regulated
FGFR4 attenuates ferroptotic cell death in recalcitrant
HER2-positive breast cancer. Nat Commun. 13:26722022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wu S, Pan R, Lu J, Wu X, Xie J, Tang H and
Li X: Development and verification of a prognostic
Ferroptosis-related gene model in triple-negative breast cancer.
Front Oncol. 12:8969272022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zou Y, Yang A, Chen B, Deng X, Xie J, Dai
D, Zhang J, Tang H, Wu T, Zhou Z, et al: crVDAC3 alleviates
ferroptosis by impeding HSPB1 ubiquitination and confers
trastuzumab deruxtecan resistance in HER2-low breast cancer. Drug
Resist Updat. 77:1011262024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Giordano C, Chemi F, Panza S, Barone I,
Bonofiglio D, Lanzino M, Cordella A, Campana A, Hashim A, Rizza P,
et al: Leptin as a mediator of tumor-stromal interactions promotes
breast cancer stem cell activity. Oncotarget. 7:1262–1275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Balaban S, Shearer RF, Lee LS, van
Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC,
Fazakerley DJ, Grewal T, et al: Adipocyte lipolysis links obesity
to breast cancer growth: Adipocyte-derived fatty acids drive breast
cancer cell proliferation and migration. Cancer Metab. 5:12017.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
He JY, Wei XH, Li SJ, Liu Y, Hu HL, Li ZZ,
Kuang XH, Wang L, Shi X, Yuan ST and Sun L: Adipocyte-derived IL-6
and leptin promote breast Cancer metastasis via upregulation of
Lysyl Hydroxylase-2 expression. Cell Commun Signal. 16:1002018.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Koundouros N and Poulogiannis G:
Reprogramming of fatty acid metabolism in cancer. Br J Cancer.
122:4–22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Bobiński R, Dutka M, Pizon M, Waksmańska W
and Pielesz A: Ferroptosis, Acyl starvation, and breast cancer. Mol
Pharmacol. 103:132–144. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Pondé NF, Zardavas D and Piccart M:
Progress in adjuvant systemic therapy for breast cancer. Nat Rev
Clin Oncol. 16:27–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Bi M, Zhang Z, Jiang YZ, Xue P, Wang H,
Lai Z, Fu X, De Angelis C, Gong Y, Gao Z, et al: Enhancer
reprogramming driven by high-order assemblies of transcription
factors promotes phenotypic plasticity and breast cancer endocrine
resistance. Nat Cell Biol. 22:701–715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xu Z, Wang X, Sun W, Xu F, Kou H, Hu W,
Zhang Y, Jiang Q, Tang J and Xu Y: RelB-activated GPX4 inhibits
ferroptosis and confers tamoxifen resistance in breast cancer.
Redox Biol. 68:1029522023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
He HL, Lee YE, Chen HP, Hsing CH, Chang
IW, Shiue YL, Lee SW, Hsu CT, Lin LC, Wu TF and Li CF:
Overexpression of DNAJC12 predicts poor response to neoadjuvant
concurrent chemoradiotherapy in patients with rectal cancer. Exp
Mol Pathol. 98:338–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Shen M, Cao S, Long X, Xiao L, Yang L,
Zhang P, Li L, Chen F, Lei T, Gao H, et al: DNAJC12 causes breast
cancer chemotherapy resistance by repressing doxorubicin-induced
ferroptosis and apoptosis via activation of AKT. Redox Biol.
70:1030352024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Denkert C, Liedtke C, Tutt A and von
Minckwitz G: Molecular alterations in triple-negative breast
cancer-the road to new treatment strategies. Lancet. 389:2430–2442.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Xiao Y, Ma D, Zhao S, Suo C, Shi J, Xue
MZ, Ruan M, Wang H, Zhao J, Li Q, et al: Multi-Omics Profiling
reveals distinct microenvironment characterization and suggests
immune escape mechanisms of Triple-negative breast cancer. Clin
Cancer Res. 25:5002–5014. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yu H, Yang C, Jian L, Guo S, Chen R, Li K,
Qu F, Tao K, Fu Y, Luo F and Liu S: Sulfasalazine-induced
ferroptosis in breast cancer cells is reduced by the inhibitory
effect of estrogen receptor on the transferrin receptor. Oncol Rep.
42:826–838. 2019.PubMed/NCBI
|
|
147
|
Timmerman LA, Holton T, Yuneva M, Louie
RJ, Padró M, Daemen A, Hu M, Chan DA, Ethier SP, van ‘t Veer LJ, et
al: Glutamine sensitivity analysis identifies the xCT antiporter as
a common triple-negative breast tumor therapeutic target. Cancer
Cell. 24:450–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wang Z, Dong J, Tian W, Qiao S and Wang H:
Role of TRPV1 ion channel in cervical squamous cell carcinoma
genesis. Front Mol Biosci. 9:9802622022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Liu Y, Li L, Yang Z, Wen D and Hu Z:
Circular RNA circACAP2 suppresses ferroptosis of cervical cancer
during malignant progression by miR-193a-5p/GPX4. J Oncol.
2022:52288742022.PubMed/NCBI
|
|
150
|
Wu P, Li C, Ye DM, Yu K, Li Y, Tang H, Xu
G, Yi S and Zhang Z: Circular RNA circEPSTI1 accelerates cervical
cancer progression via miR-375/409-3P/515-5p-SLC7A11 axis. Aging
(Albany NY). 13:4663–4673. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Xiong J, Nie M, Fu C, Chai X, Zhang Y, He
L and Sun S: Hypoxia enhances HIF1α transcription activity by
Upregulating KDM4A and mediating H3K9me3, thus inducing ferroptosis
resistance in cervical cancer cells. Stem Cells Int.
2022:16088062022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wu Y, Fang B, Yang XQ, Wang L, Chen D,
Krasnykh V, Carter BZ, Morris JS and Shureiqi I: Therapeutic
molecular targeting of 15-lipoxygenase-1 in colon cancer. Mol Ther.
16:886–892. 2008. View Article : Google Scholar
|
|
153
|
Abdurahman A, Li Y, Jia SZ, Xu XW, Lin SJ,
Ouyang P, Jun He Z, Zhang ZH, Liu Q, Xu Y and Song GL: Knockdown of
the SELENOK gene induces ferroptosis in cervical cancer cells.
Metallomics. 15:mfad0192023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ray Chaudhuri A and Nussenzweig A: The
multifaceted roles of PARP1 in DNA repair and chromatin
remodelling. Nat Rev Mol Cell Biol. 18:610–621. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Sánchez-Quesada C, López-Biedma A and
Gaforio JJ: Oleanolic acid, a compound present in grapes and
olives, protects against genotoxicity in human mammary epithelial
cells. Molecules. 20:13670–13688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Shi H, Xiong L, Yan G, Du S, Liu J and Shi
Y: Susceptibility of cervical cancer to dihydroartemisinin-induced
ferritinophagy-dependent ferroptosis. Front Mol Biosci.
10:11560622023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Liu S, Zhang HL, Li J, Ye ZP, Du T, Li LC,
Guo YQ, Yang D, Li ZL, Cao JH, et al: Tubastatin A potently
inhibits GPX4 activity to potentiate cancer radiotherapy through
boosting ferroptosis. Redox Biol. 62:1026772023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhang J, Ouyang F, Gao A, Zeng T, Li M, Li
H, Zhou W, Gao Q, Tang X, Zhang Q, et al: ESM1 enhances fatty acid
synthesis and vascular mimicry in ovarian cancer by utilizing the
PKM2-dependent Warburg effect within the hypoxic tumor
microenvironment. Mol Cancer. 23:942024. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Fan Z, Ye M, Liu D, Zhou W, Zeng T, He S
and Li Y: Lactate drives the ESM1-SCD1 axis to inhibit the
antitumor CD8+ T-cell response by activating the Wnt/β-catenin
pathway in ovarian cancer cells and inducing cisplatin resistance.
Int Immunopharmacol. 137:1124612024. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Li YK, Gao AB, Zeng T, Liu D, Zhang QF,
Ran XM, Tang ZZ, Li Y, Liu J, Zhang T, et al: ANGPTL4 accelerates
ovarian serous cystadenocarcinoma carcinogenesis and angiogenesis
in the tumor microenvironment by activating the JAK2/STAT3 pathway
and interacting with ESM1. J Transl Med. 22:462024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Christie EL and Bowtell DDL: Acquired
chemotherapy resistance in ovarian cancer. Ann Oncol. 28
(Suppl_8):viii13–viii5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ruan D, Wen J, Fang F, Lei Y, Zhao Z and
Miao Y: Ferroptosis in epithelial ovarian cancer: A burgeoning
target with extraordinary therapeutic potential. Cell Death Discov.
9:4342023. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Fu R, Zhao B, Chen M, Fu X, Zhang Q, Cui
Y, Fu X, Li R, Zhong G and Zhou X: Moving beyond cisplatin
resistance: mechanisms, challenges, and prospects for overcoming
recurrence in clinical cancer therapy. Med Oncol. 41:92023.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lv C, Qu H, Zhu W, Xu K, Xu A, Jia B, Qing
Y, Li H, Wei HJ and Zhao HY: Low-dose paclitaxel inhibits tumor
cell growth by regulating glutaminolysis in colorectal carcinoma
cells. Front Pharmacol. 8:2442017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wang Y, Zhao G, Condello S, Huang H,
Cardenas H, Tanner EJ, Wei J, Ji Y, Li J, Tan Y, et al: Frizzled-7
identifies platinum-tolerant ovarian cancer cells susceptible to
ferroptosis. Cancer Res. 81:384–399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Shi M, Zhang MJ, Yu Y, Ou R, Wang Y, Li H
and Ge RS: Curcumin derivative NL01 induces ferroptosis in ovarian
cancer cells via HCAR1/MCT1 signaling. Cell Signal. 109:1107912023.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Cheng Q, Bao L, Li M, Chang K and Yi X:
Erastin synergizes with cisplatin via ferroptosis to inhibit
ovarian cancer growth in vitro and in vivo. J Obstet Gynaecol Res.
47:2481–2491. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ni M, Zhou J, Zhu Z, Xu Q, Yin Z, Wang Y,
Zheng Z and Zhao H: Shikonin and cisplatin synergistically overcome
cisplatin resistance of ovarian cancer by inducing ferroptosis via
upregulation of HMOX1 to promote Fe2+ accumulation. Phytomedicine.
112:1547012023. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Krishnakumar R and Kraus WL: The PARP side
of the nucleus: Molecular actions, physiological outcomes, and
clinical targets. Mol Cell. 39:8–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Gibson BA and Kraus WL: New insights into
the molecular and cellular functions of poly(ADP-ribose) and PARPs.
Nat Rev Mol Cell Biol. 13:411–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Audeh MW, Carmichael J, Penson RT,
Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN,
Oaknin A, Loman N, et al: Oral poly(ADP-ribose) polymerase
inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and
recurrent ovarian cancer: A proof-of-concept trial. Lancet.
376:245–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Alsop K, Fereday S, Meldrum C, deFazio A,
Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, et
al: BRCA mutation frequency and patterns of treatment response in
BRCA mutation-positive women with ovarian cancer: A report from the
Australian Ovarian Cancer Study Group. J Clin Oncol. 30:2654–2663.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Hong T, Lei G, Chen X, Li H, Zhang X, Wu
N, Zhao Y, Zhang Y and Wang J: PARP inhibition promotes ferroptosis
via repressing SLC7A11 and synergizes with ferroptosis inducers in
BRCA-proficient ovarian cancer. Redox Biol. 42:1019282021.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Tang S, Shen Y, Wei X, Shen Z, Lu W and Xu
J: Olaparib synergizes with arsenic trioxide by promoting apoptosis
and ferroptosis in platinum-resistant ovarian cancer. Cell Death
Dis. 13:8262022. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Fang X, Zhang T and Chen Z: Solute carrier
family 7 member 11 (SLC7A11) is a potential prognostic biomarker in
uterine corpus endometrial carcinoma. Int J Gen Med. 16:481–497.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Marshall AD, van Geldermalsen M, Otte NJ,
Lum T, Vellozzi M, Thoeng A, Pang A, Nagarajah R, Zhang B, Wang Q,
et al: ASCT2 regulates glutamine uptake and cell growth in
endometrial carcinoma. Oncogenesis. 6:e3672017. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Liu X, Olszewski K, Zhang Y, Lim EW, Shi
J, Zhang X, Zhang J, Lee H, Koppula P, Lei G, et al: Cystine
transporter regulation of pentose phosphate pathway dependency and
disulfide stress exposes a targetable metabolic vulnerability in
cancer. Nat Cell Biol. 22:476–486. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Byrne FL, Poon IK, Modesitt SC, Tomsig JL,
Chow JD, Healy ME, Baker WD, Atkins KA, Lancaster JM, Marchion DC,
et al: Metabolic vulnerabilities in endometrial cancer. Cancer Res.
74:5832–5845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Han X, Ren C, Yang T, Qiao P, Wang L,
Jiang A, Meng Y, Liu Z, Du Y and Yu Z: Negative regulation of
AMPKα1 by PIM2 promotes aerobic glycolysis and tumorigenesis in
endometrial cancer. Oncogene. 38:6537–6549. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Cheng H, Jiang XY, Zheng RR, Zuo SJ, Zhao
LP, Fan GL, Xie BR, Yu XY, Li SY and Zhang XZ: A biomimetic cascade
nanoreactor for tumor targeted starvation therapy-amplified
chemotherapy. Biomaterials. 195:75–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Yu S, Chen Z, Zeng X, Chen X and Gu Z:
Advances in nanomedicine for cancer starvation therapy.
Theranostics. 9:8026–8047. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Murakami H, Hayashi M, Terada S and
Ohmichi M: Medroxyprogesterone acetate-resistant endometrial cancer
cells are susceptible to ferroptosis inducers. Life Sci.
325:1217532023. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Zhang YY, Ni ZJ, Elam E, Zhang F, Thakur
K, Wang S, Zhang JG and Wei ZJ: Juglone, a novel activator of
ferroptosis, induces cell death in endometrial carcinoma Ishikawa
cells. Food Funct. 12:4947–4959. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Hua Y, Yang S, Zhang Y, Li J, Wang M,
Yeerkenbieke P, Liao Q and Liu Q: Modulating ferroptosis
sensitivity: Environmental and cellular targets within the tumor
microenvironment. J Exp Clin Cancer Res. 43:192024. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Hu Y, Wang H and Liu Y: NETosis: Sculpting
tumor metastasis and immunotherapy. Immunol Rev. 321:263–279. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zhang H, Liu J, Zhou Y, Qu M, Wang Y, Guo
K, Shen R, Sun Z, Cata JP, Yang S, et al: Neutrophil extracellular
traps mediate m6A modification and regulates sepsis-associated
acute lung injury by activating ferroptosis in alveolar epithelial
cells. Int J Biol Sci. 18:3337–3357. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Chu C, Wang X, Yang C, Chen F, Shi L, Xu
W, Wang K, Liu B, Wang C, Sun D and Ding W: Neutrophil
extracellular traps drive intestinal microvascular endothelial
ferroptosis by impairing Fundc1-dependent mitophagy. Redox Biol.
67:1029062023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Medlock AE, Hixon JC, Bhuiyan T and Cobine
PA: Prime real estate: Metals, cofactors and MICOS. Front Cell Dev
Biol. 10:8923252022. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Xie J, Yang Y, Gao Y and He J:
Cuproptosis: Mechanisms and links with cancers. Mol Cancer.
22:462023. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Wang W, Lu K, Jiang X, Wei Q, Zhu L, Wang
X, Jin H and Feng L: Ferroptosis inducers enhanced cuproptosis
induced by copper ionophores in primary liver cancer. J Exp Clin
Cancer Res. 42:1422023. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Li Y, Du Y, Zhou Y, Chen Q, Luo Z, Ren Y,
Chen X and Chen G: Iron and copper: Critical executioners of
ferroptosis, cuproptosis and other forms of cell death. Cell Commun
Signal. 21:3272023. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Liu T, Liu W, Zhang M, Yu W, Gao F, Li C,
Wang SB, Feng J and Zhang XZ: Ferrous-supply-regeneration
nanoengineering for cancer-cell-specific ferroptosis in combination
with imaging-guided photodynamic therapy. ACS Nano. 12:12181–12192.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31:e19041972019. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Yang J, Jia Z, Zhang J, Pan X, Wei Y, Ma
S, Yang N, Liu Z and Shen Q: Metabolic intervention nanoparticles
for triple-negative breast cancer therapy via overcoming
FSP1-mediated ferroptosis resistance. Adv Healthc Mater.
11:e21027992022. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Radadiya PS, Thornton MM, Puri RV,
Yerrathota S, Dinh-Phan J, Magenheimer B, Subramaniam D, Tran PV,
Zhu H, Bolisetty S, et al: Ciclopirox olamine induces
ferritinophagy and reduces cyst burden in polycystic kidney
disease. JCI Insight. 6:e1412992021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Yao X, Zhang Y, Hao J, Duan HQ, Zhao CX,
Sun C, Li B, Fan BY, Wang X, Li WX, et al: Deferoxamine promotes
recovery of traumatic spinal cord injury by inhibiting ferroptosis.
Neural Regen Res. 14:532–541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Zheng H, Jiang J, Xu S, Liu W, Xie Q, Cai
X, Zhang J, Liu S and Li R: Nanoparticle-induced ferroptosis:
Detection methods, mechanisms and applications. Nanoscale.
13:2266–2285. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Brown CW, Amante JJ, Chhoy P, Elaimy AL,
Liu H, Zhu LJ, Baer CE, Dixon SJ and Mercurio AM: Prominin2 drives
ferroptosis resistance by stimulating iron export. Dev Cell.
51:575–586.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Wu H and Liu A: Long non-coding RNA NEAT1
regulates ferroptosis sensitivity in non-small-cell lung cancer. J
Int Med Res. 49:3000605219961832021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Alim I, Caulfield JT, Chen Y, Swarup V,
Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie
EJ, et al: Selenium drives a transcriptional adaptive program to
block ferroptosis and treat stroke. Cell. 177:1262–1279.e25. 2019.
View Article : Google Scholar : PubMed/NCBI
|