Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most prevalent type of cancer worldwide with 890,000 new
cases and 450,000 deaths reported in 2018 (1). Based on etiological factors, HNSCC is
classified into two types of disease: HPV-positive or HPV-negative.
HPV-positive HNSCC occurs mainly in oropharyngeal tissues, whereas
HPV-negative HNSCC is primarily found in the oral cavity and larynx
(2). The occurrence of HPV-negative
HNSCC is associated with the use of tobacco and excessive
consumption of alcohol, and is characterized by mutations in
diverse oncogenic driver genes. By contrast, HPV-positive HNSCC is
related to human papillomavirus (HPV) infection, whereby viral
proteins E6 and E7 cause oncogenic transformation of host cells
(3–6). Compared with HPV-negative HNSCC,
HPV-positive HNSCC expresses viral proteins and other neoantigens,
as well as mutagenesis caused by viral infection, which may be
advantageous to immunotherapy (2).
In addition, HPV-positive HNSCC has better clinical outcomes with
standard therapy in comparison to HPV-negative HNSCC (7). Reports have indicated that patients
with HPV-positive HNSCC have a higher 5-year survival rate (~80%)
than HPV-negative patients (~50%) (8); therefore, there is an urgent need for
new effective therapeutics to treat HPV-negative HNSCC (9,10).
Classical therapies for patients with HNSCC without
distant metastasis typically include surgical resection, radiation
therapy, chemotherapy or a combination of these regimens. The
specific therapeutic approach depends on various factors, such as
pre-existing clinical conditions, cancer location and the
Tumor-Node-Metastasis stage of the tumor (4,11). The
combination of these treatments could reduce the rate of recurrence
and distant metastasis for patients with metastatic disease
(12). However, chemotherapy
remains the primary option for patients with recurrent and distant
metastatic HNSCC (13). Cisplatin
is the most commonly used anticancer drug to treat advanced HNSCC;
however, while a number of newly diagnosed patients with advanced
HNSCC initially respond well to cisplatin-based chemotherapies,
most patients either have intrinsic resistance or will eventually
develop acquired resistance to cisplatin, which often leads to
death within 1 year (14).
Immunotherapy has been introduced to treat refractory HNSCC, but
its impact has been limited (14,15).
Therefore, it remains of the utmost importance to identify new
therapeutic alternatives to improve the efficacy of cisplatin-based
therapies, or to replace them, for the treatment of patients with
advanced HNSCC.
Epidermal growth factor receptor (EGFR), a member of
the ErbB kinase family, is reported to be upregulated in 90% of
HNSCC cases and cell lines, according to a study that included 24
patients with SCCHN and 10 cell lines, as opposed to seven healthy
control individuals (16) and
serves a crucial role in the pathogenesis and clinical course of
cancer (13,17,18).
EGFR controls the activation of several essential pathways, such as
PI3K/Akt/mTOR and RAS-RAF-MEK-ERK, which regulate cell
proliferation, survival and migration (19,20).
In 2006, the monoclonal EGFR antibody cetuximab was approved by the
Food and Drug Administration (FDA) to treat HNSCC in combination
with the standard therapy (21–23).
However, the use of cetuximab has been reported to result in very
limited improvement in survival rates for patients undergoing
cisplatin-based therapy (24). In
addition, small molecule kinase inhibitors, such as gefitinib and
erlotinib, while effective in targeted therapies for non-small cell
lung cancer, have not demonstrated any benefits for patients with
HNSCC (25,26). Increasing evidence has demonstrated
the importance of the ErbB family, which contains EGFR, HER2, HER3
and HER4, in the carcinogenesis of HNSCC and its response to
therapies (27). HER2 and HER3 form
heterodimers with EGFR and play a role in PI3K/Akt activation. In
addition, HER2 and HER3 have been shown to be associated with
resistance to EGFR and PI3K inhibitors in cancer (28,29).
These results indicated that targeting the ErbB family kinases
could more effectively suppress HNSCC compared with using EGFR
inhibitors alone (25,30). Notably, the FDA-approved ErbB family
inhibitor afatinib has shown positive results in HNSCC clinical
trials and is now listed in the National Comprehensive Cancer
Network (NCCN) guidelines as a third-line single agent for HNSCC
treatment (25,26,31–33).
Understanding the mechanisms behind resistance to afatinib and
exploring methods to avoid that resistance would be beneficial.
PI3K is one of the most important downstream
effectors of the EGFR/ErbB receptor family. The genes PIK3CA,
PIK3CB and PIK3CD encode three highly homologous catalytic isoforms
of class IA PI3K, p110α, p110β and p110δ, respectively. These
isoforms associate with any of five regulatory isoforms: p85α, its
splicing variants p55α and p50α, p85β and p55γ (34). The most important PI3K-p85 complex
is PI3Kα/p85α. The PI3K pathway has been reported to be the most
frequently mutated oncogenic pathway, detected in 30.5% of tumors,
according to whole-exome sequencing of the genes in the JAK/STAT,
MAPK and PI3K pathways in 151 HNSCC tumors (35). Previous studies have also shown that
PIK3CA, the gene that codes for PI3K p110α, is one of the most
frequently mutated genes in HNSCC (35–37),
and PIK3CA amplification and overexpression have also been
identified in HNSCC (38).
According to The Cancer Genome Atlas (TCGA), profiling of 279 HNSCC
tumors identified amplification or mutation of PIK3CA in 34% of
HPV-negative HNSCC tumors and 56% of HPV-positive tumors (39,40).
PI3K activation in turn activates Akt, which then phosphorylates
its substrates, such as TSC2, PRAS40, GSK3β and FOXO, to regulate
multiple cellular functions that consequently control cell
proliferation, survival and response to therapy. The tumor
suppressor gene PTEN encodes the PTEN protein that dephosphorylates
PIP3 to inhibit the PI3K pathway. Mutations that result in PTEN
loss or deceased PTEN expression have been frequently observed in
HPV-positive and negative HNSCC (41,42).
These alterations further result in PI3K/Akt activation (43). Therefore, PI3K is an attractive
target for the treatment of HNSCC (40,44,45).
Our previous study reported that co-targeting the
ErbB family and PI3K, through a combination of afatnib and
copanlisib, suppressed the growth of HPV-positive HNSCC (46). The present study further explored
whether this combination was also effective at suppressing the
growth of HPV-negative HNSCC. The present findings indicated that
the combination of afatnib and copanlisib may be a promising
treatment option for HPV-negative HNSCC.
Materials and methods
Cell culture
HNSCC cell lines, Cal27 and FaDu, were purchased
from American Type Culture Collection, authenticated by short
tandem repeat analysis and tested for Mycoplasma
contamination at the Translational Core Facility at Marlene and
Stewart Greenebaum Comprehensive Cancer Center, University of
Maryland (Baltimore, MD, USA). All cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum, 2 mM glutamine, and 100 U/ml penicillin and
streptomycin (all from Gibco; Thermo Fisher Scientific) at 37°C in
an incubator containing 5% CO2 for 24 h before each
experiment was performed.
Antibodies and inhibitors
The following antibodies were purchased from Cell
Signaling Technology, Inc.: Phosphorylated (P)-Akt-S473 (cat. no.
4058), P-Akt-T308 (cat. no. 9275), Akt (cat. no. 2938),
P-HER2-Y1248 (cat. no. 2247), HER2 (cat. no. 4290), P-HER3-Y1289
(cat. no. 2842), HER3 (cat. no. 12708), caspase-3 (cat. no. 9665),
cleaved-caspase-3 (cat. no. 9664) and β-actin (cat. no. 4967).
HRP-labeled anti-mouse (cat. no. W4028) and anti-rabbit (cat. no.
W4018) secondary antibodies were purchased from Promega
Corporation. Gefitinib, erlotinib and afatinib, as well as all PI3K
inhibitors (copanlisib, BKM120, GDC-0941, BYL179, AZD8186 and
CAL-101) were purchased from Selleck Chemicals. Copanlisib was
dissolved in 10% trifluoroacetic acid (TFA), while all other drugs
were dissolved in 100% DMSO. The final concentrations of TFA and
DMSO in cell culture media were 0.01% and 0.1%. For single
EGFR/ErbB inhibitor treatment, cells were treated with DMSO or
increasing concentrations of gefitinib (3 nM-30 µM), erlotinib (3
nM-30 µM) and afatinib (1 nM-10 µM) at 37°C for 96 h. For single
PI3K inhibitor treatment, cells were treated with TFA, DMSO or
increasing concentrations of copanlisib (0.01 nM-10 µM), GDC-0941
(1 nM-10 nM) and other PI3K inhibitors (3 nM-30 µM) at 37°C for 96
h. For the combination treatment, Cal27 cells were treated with the
combination of various concentrations of copanlisib (2.875–92 nM)
and afatinib (0.2875–9.2 nM), and FaDu cells were treated with the
combination of various concentrations of copanlisib (3.375–118 nM)
and afatinib (0.3125–11.6 nM) at 37°C for 96 h.
Cell lysis and western blot
analysis
Cell lysis and western blot analysis were performed
as previously described (46,47).
Briefly, cells grown on 100-mm dishes were rinsed twice with 1X
cold PBS, then lysed on ice for 30 min in 1 ml lysis buffer
(M-PER™ Mammalian Protein Extraction Reagent; cat. no.
78503; Pierce; Thermo Fisher Scientific, Inc.) with phosphatase
inhibitor and EDTA-free protease inhibitors (Roche Diagnostics).
After centrifugation at 13,000 × g for 20 min, lysates that
contained 30 µg protein were separated by SDS-PAGE on 4–12% gels,
and the proteins were transferred to Pure Nitrocellulose Membranes
(Bio-Rad Laboratories, Inc.), which were blocked in 5% nonfat milk
at room temperature for 1 h. The membranes were then incubated with
the indicated primary antibodies (1:1,000 dilutions) overnight at
4°C, and with HRP-conjugated secondary antibodies (1:4,000
dilutions) at room temperature for 1 h. Proteins were finally
visualized by using Clarity™ Western ECL Substrate (cat.
no. 1705060; Bio-Rad Laboratories, Inc.).
Analyzing apoptosis by Annexin
V/propidium iodide (PI) staining
Apoptosis analysis was performed using the FITC
Annexin V Apoptosis Detection Kit II (cat. no. 556570; BD
Biosciences) combined with PI (cat. no. P1304MP; Invitrogen; Thermo
Fisher Scientific, Inc.) staining as previously described (46,47).
Cells were plated into 6-well plates at a density of
4.5×105/well overnight, before being treated with
vehicle control, copanlisib (30 nM in Cal27 and 125 nM in FaDu),
afatinib (0.5 µM in Cal27 and 1 µM in FaDu), or a combination of
copanlisib and afatinib at 37°C for 48 h. Both floating and
adherent cells were collected and stained with Annexin V and PI,
according to the manufacturers' instructions. Samples and single
stained controls were subsequently analyzed using the BD FACSCanto
II flow cytometer (BD Biosciences). Data were analyzed using FCS
Express 7 (Research Edition; De Novo Software). Cells stained
solely with Annexin V (early apoptotic cells) or double stained
with Annexin V and PI (late apoptotic cells) were considered
apoptotic cells.
Cell viability assay
Cell viability was assessed using sulforhodamine B
(SRB) staining as described previously (48). Briefly, cells were plated in 96-well
plates at a density of 3,500 cells/well overnight before being
subjected to treatment with a serial concentration of the indicated
drugs or vehicle control for 96 h at 37°C. Following an overnight
fixation in methanol, the cells were stained with SRB for 1 h and
then dissolved with 10 mmol/l Tris base for 1 h while being shaken.
The aforementioned procedures were all carried out at room
temperature. Subsequently, the absorbance was read at 492 nm. The
half maximal effective concentration (EC50) values were
calculated using nonlinear regression analysis with variable slope
in Graph-Pad Prism v8.0 (Dotmatics). The effect of the inhibitors
on cell viability are represented as percentages compared with
vehicle control-treated cells. Each experiment was performed in
triplicate and repeated at least twice. The vehicle controls were
treated with the reagents that were used to dissolve the chemicals
(0.01% TFA for copanlisib and 0.1% DMSO for the other drugs). To
determine synergy of the drug combination, the combination index
(CI) values were determined according to the Chou-Talalay method
(49) using CalcuSyn software
(version 2.0; BIOSOFT).
Tumor xenograft formation in mice
In vivo experiments were conducted with the
approval of the Institutional Animal Care and Use Committee (IACUC)
of University of Maryland (IACUC #1021001) and within an
Association for Assessment and Accreditation of Laboratory Animal
Care International-accredited vivarium. Female 6-week-old nude mice
(n=45), purchased from Inotiv, Inc., were used for the animal
studies. All mice were housed under the following conditions:
Ambient temperature (21–22°C), regulated humidity (40–50%) ad
libitum access to food and water, 12-h light/dark cycle. The
animals were treated and handled in accordance with the guidelines
set by the IACUC of the University of Maryland. A total of 45 nude
mice received injection of 0.5×106 FaDu cells into the
right flank. The mice were split into four groups (n=7/group) 13
days after cell injection, so that the mean tumor volume was
similar (~150 mm3). Notably, 45 mice were injected but
only 28 mice entered the study; mice were excluded from entering
the study based on irregular shape of the tumors (difficult to
measure). One mouse per group was excluded from analysis due to
necrosis at termination of the study; mice were sacrificed as soon
as necrosis was observed. The day of sorting occurred on the first
day of dosing and was defined as Day 1. The four treatment groups
(n=6/group at termination) were: Vehicle control, copanlisib (6
mg/kg, intraperitoneal, 5 times/week, Monday to Friday), afatinib
(6 mg/kg, oral, 5 times/week, Monday to Friday), and a combination
of copanlisib and afatinib. Tumor volume was measured twice per
week using electronic calipers and the animals were weighed five
times per week. Tumor volume was calculated as (L ×
W2)/2, where W is the smaller dimension and L is tumor
length. At the end of the study (32 days), mice were euthanized by
CO2 asphyxiation (30% vol/min) followed by cervical
dislocation as noted in the IACUC-approved protocol, and the tumors
were excised, weighed and cut in half. Half of the tumors were
fixed in formalin (4%) at room temperature for 24 h and then
transferred to 70% ethanol (half), whereas the other half were
frozen at −80°C. Tumors were preserved by fixation and freezing for
future evaluation. All mice were euthanized 32 days after the start
of dosing. No animal who entered the study was found dead; however,
as aforementioned, one mouse from each group was removed from the
study and euthanized due to necrosis. The study was terminated
based on tumor volume (~2,000 mm3) as listed in the
IACUC-approved protocol as a humane endpoint. No analgesics were
administered during the study since flank tumor studies are
considered painless. Mice were briefly anesthetized with 2.5%
isoflurane during the subcutaneous injection of cells.
Statistical analysis
In vitro data were repeated at least three
times unless specifically described in figure legends. The data
were presented as the mean ± SD and animal data are shown as the
mean ± SEM. Statistical analysis was performed using GraphPad Prism
version 10.3.1 (Dotmatics). One-way ANOVA was used to statistically
analyze multiple groups, followed by Tukey's post hoc analysis.
P<0.05 was considered to indicate a statistically significantly
difference.
Results
HPV-negative HNSCC cell lines are
sensitive to afatinib
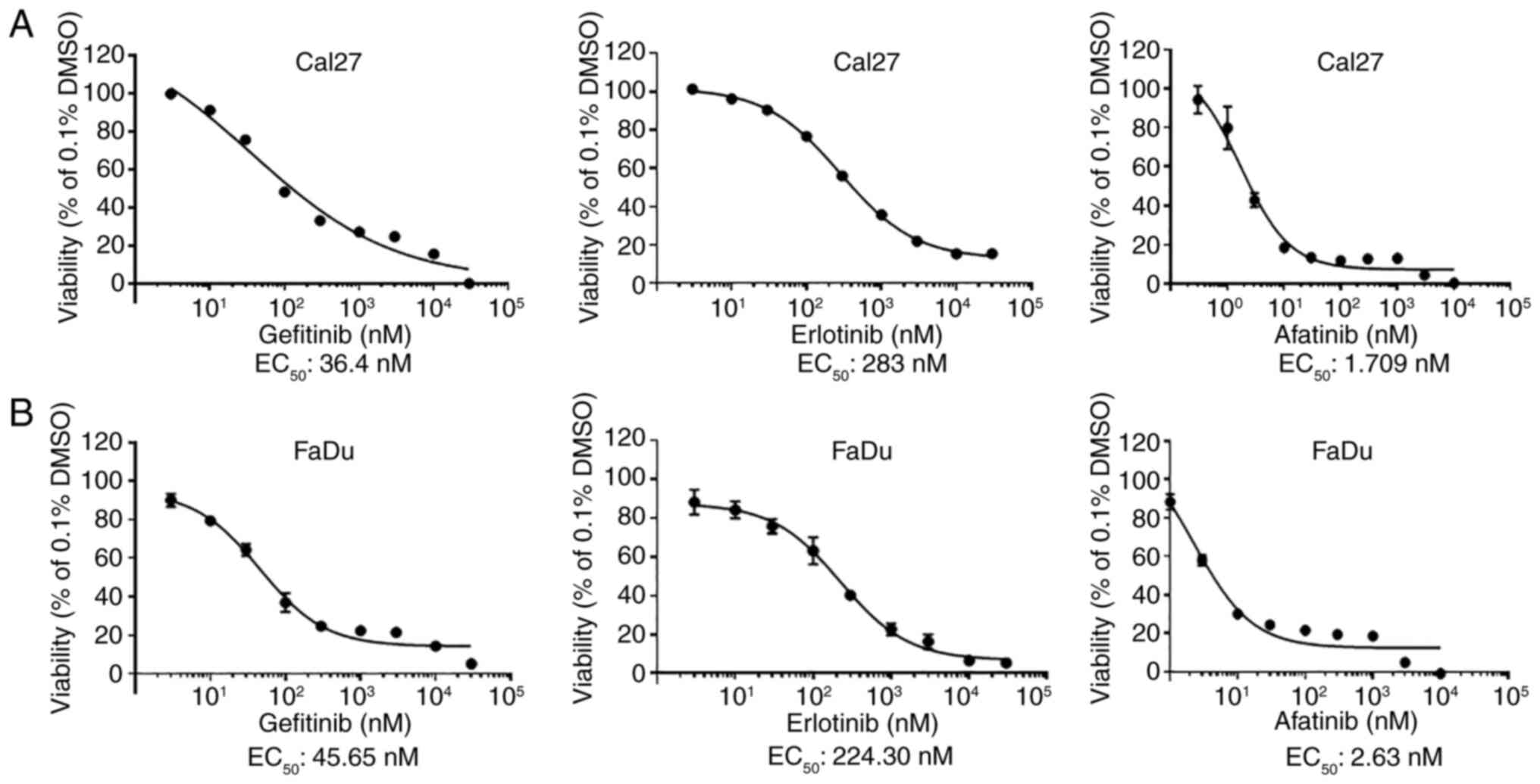
The EC50 values of EGFR/ErbB family
inhibitors, gefitinib, erlotinib and afatinib, were determined in
two HPV-negative HNSCC cell lines, Cal27 and FaDu. Both cell lines
were relatively resistant to erlotinib and sensitive to gefitinib
(Fig. 1A and B). However, they were
revealed to be very sensitive to afatinib, although Cal27 cells
were more sensitive to afatinib in comparison to FaDu cells. These
results suggested that afatinib was the more effective small
molecule inhibitor for the treatment of HPV-negative HNSCC in
comparison to other EGFR inhibitors.
Copanlisib is the most effective PI3K
inhibitor to suppress HPV-negative HNSCC viability
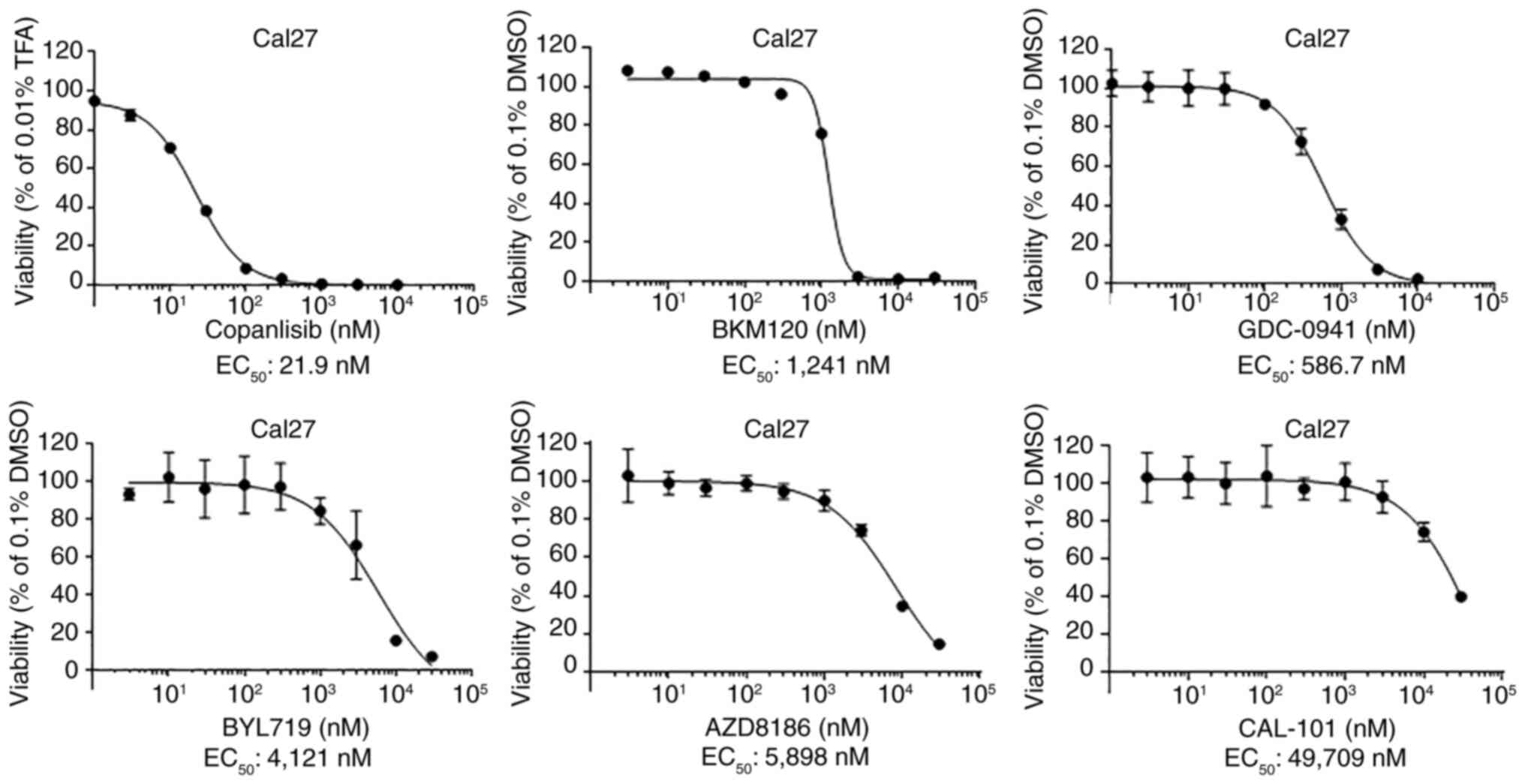
To identify the most effective PI3K inhibitor, the
EC50 values of six PI3K inhibitors were determined,
including three pan-PI3K inhibitors, copanlisib, BKM120 and
GDC-09410; the PI3Kα inhibitor Byl719; the PI3Kβ inhibitor AZD8186;
and the PI3Kδ inhibitor Cal-101 (Fig.
2). Cal27 cells were strongly resistant to the PI3Kδ inhibitor,
and relatively resistant to PI3Kα and PI3Kβ inhibitors. However,
they were much more sensitive to the pan-PI3K inhibitors
copanlisib, GDC-0941 and BKM120. Moreover, the EC50
value of copanlisib was much lower in comparison to those of BKM120
and GDC-0941. Similar results were found in FaDu cells (Fig. S1). These results suggested that
copanlisib may be the most effective small molecule PI3K inhibitor
to treat HPV-negative HNSCC.
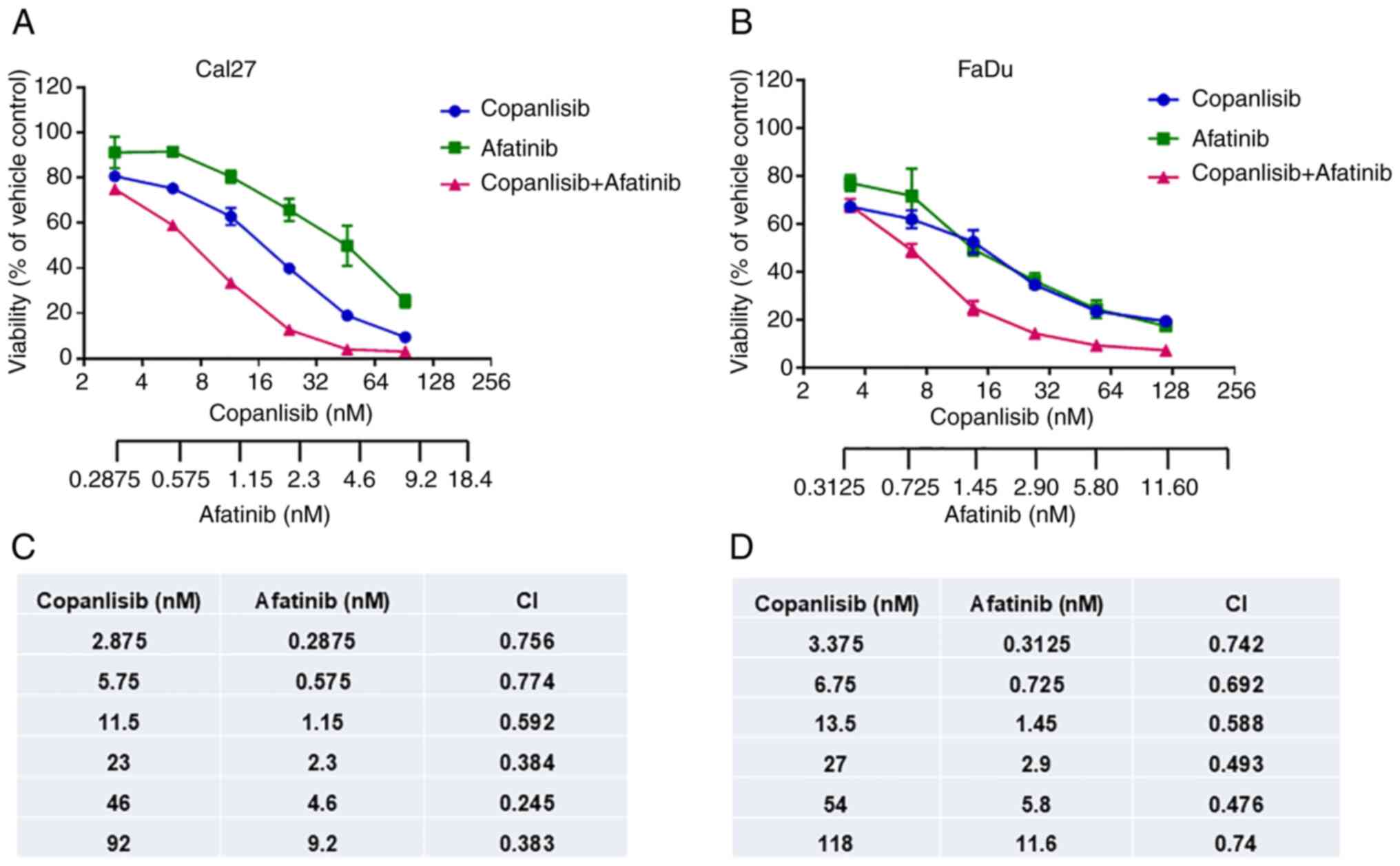
Synergistic inhibition of cell
viability using a combination of afatinib and copanlisib
The present study aimed to assess whether
simultaneous inhibition of ErbB family and PI3K pathways could more
effectively inhibit HPV-negative HNSCC cell viability. Based on the
aforementioned data, afatinib and copanlisib were selected for the
combination therapy. Similar to the results shown in Figs. 1 and 2, afatinib or copanlisib alone inhibited
cell viability; however, the combination caused enhanced inhibition
of viability in both Cal27 (Fig.
3A) and FaDu cells (Fig. 3B).
Furthermore, the related CI value for each combination was
calculated according to the Chou-Talalay method (49) using CalcuSyn software. The CI values
for all combinations were <1.0, which indicated a synergistic
effect in the combination of afatinib and copanlisib (Fig. 3C and D). These data indicated that
afatinib and copanlisib may synergistically inhibit HPV-negative
HNSCC cell viability.
Synergistic inhibition of xenograft
tumor growth via the combination of afatinib and copanlisib in
mice
The present study also evaluated the antitumor
activity of the afatinib and copanlisib combination in vivo
using a mouse xenograft model. FaDu cells were inoculated into the
mice, and when the tumors reached ~200 mm3, the mice
were randomly split into four groups, which were treated with a
vehicle control, copanlisib, afatinib or their combination. The
original aim was to treat the mice for more than 6–8 weeks;
however, the experiment was terminated on day 32 when tumor
necrosis was observed in the majority of the mice in the control
group. The average tumor volume in groups treated with either
copanlisib or afatinib was lower than that of the control group,
but there were no significant differences in tumor volume among
these three groups, which may be due to tumor necrosis-induced
decreases in tumor volumes in the control group (Fig. 4A). However, tumor volumes in the
combination treatment group were significantly lower than in the
single treatment groups, and they were more significantly lower
than those in the control group by the end of the study.
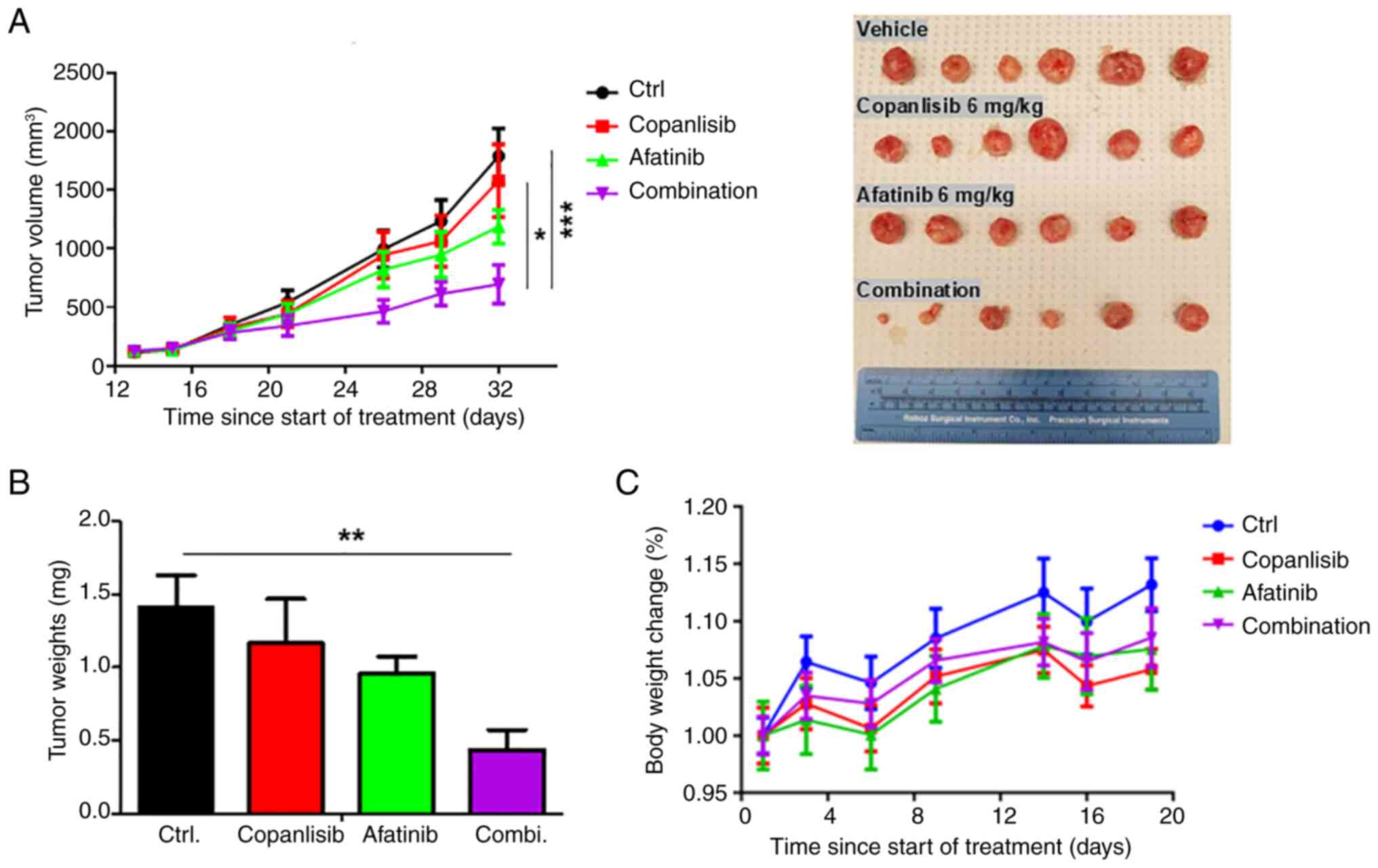 | Figure 4.Inhibition of head and neck squamous
cell carcinoma growth by a combination of afatinib and copanlisib
in vivo. FaDu cells were inoculated into mice. When tumors
reached ~150 mm3, mice were randomized to one of four
treatment groups (n=6/group): Vehicle control, copanlisib (6 mg/kg,
intraperitoneal, 5 times/week Monday to Friday), afatinib (6 mg/kg,
oral, 5 times/week, Monday to Friday), or a combination of
copanlisib and afatinib. The treatments were performed for 32 days.
(A) Xenograft tumor volumes and images, (B) final average weights
of tumors, and (C) average body weight changes of mice in each
group were compared by GraphPad Prism 10.3.1 software. After
one-way ANOVA, Tukey's post hoc test was performed for multiple
comparisons. ***P<0.001; **P<0.01; *P<0.05. |
Similarly, after the tumors were excised and weighed
at the end of the study, there was no significant difference in
tumor weight (mg) between the groups treated with either copanlisib
or afatinib (Fig. 4B). In addition,
there were no significant differences in tumor weight (mg) between
the control group and the single agent treatment groups due to
individual differences. However, tumor weight in the combination
treatment group was significantly lower compared with that in the
control agent treated group. In summary, while afatinib or
copanlisib alone had modest inhibitory effects on tumor growth,
they did not reach statistical significance, whereas the
combination of the two drugs significantly inhibited tumor
growth.
Notably, all doses of copanlisib and afatinib in
single and combination treatments were well-tolerated, as indicated
by no significant weight loss observed during the study (Fig. 4C). These results indicated the
feasibility of treatment with a combination of afatinib and
copanlisib to suppress HPV-negative HNSCC.
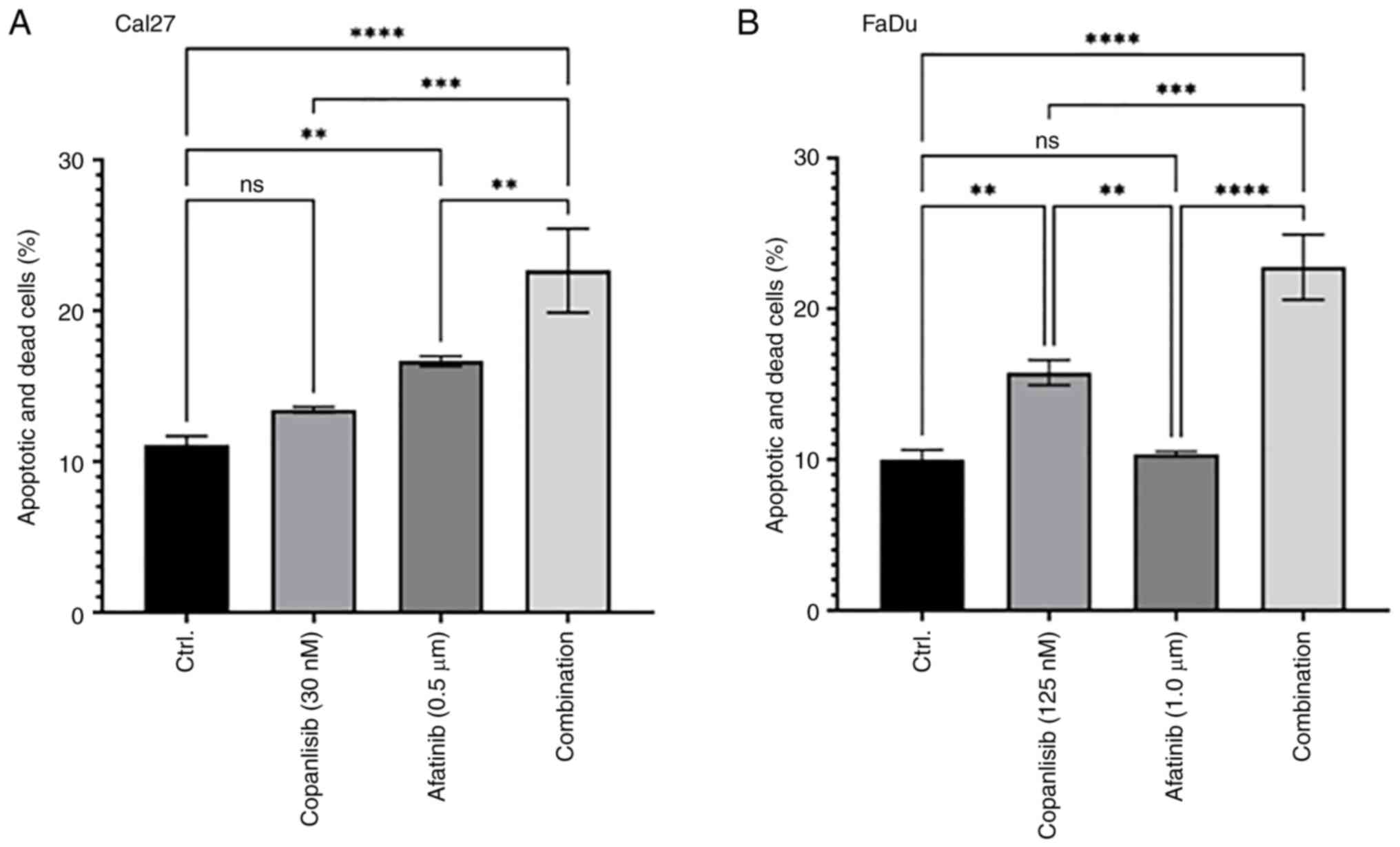
A combination of afatinib and
copanlisib induces apoptosis
The present study assessed whether a combination of
afatinib and copanlisib could induce more apoptosis compared with
either single treatment. Cal27 cells were treated with afatinib
(0.5 µM), copanlisib (30 nM) or their combination for 48 h before
an apoptosis assay was performed. Afatinib, but not copanlisib
alone, induced modest cell apoptosis, whereas their combination
significantly increased cell apoptosis (Figs. 5A and S2). Since FaDu cells exhibited higher
EC50 to afatinib and copanlisib in comparison to Cal27
cells (Figs. 1, 2 and S1),
higher concentrations of afatinib and copanlisib were chosen for
the treatments. Treatment with copanlisib (125 nM) significantly
enhanced apoptosis, whereas treatment with afatinib (1.0 µM) did
not significantly increase apoptosis. However, a combination of
copanlisib (125 nM) and afatinib (1.0 µM) led to significantly
increased apoptosis compared with either single treatment (Figs. 5B and S3). These data indicated that afatinib
and copanlisib may cooperate to induce apoptosis in HPV-negative
HNSCC.
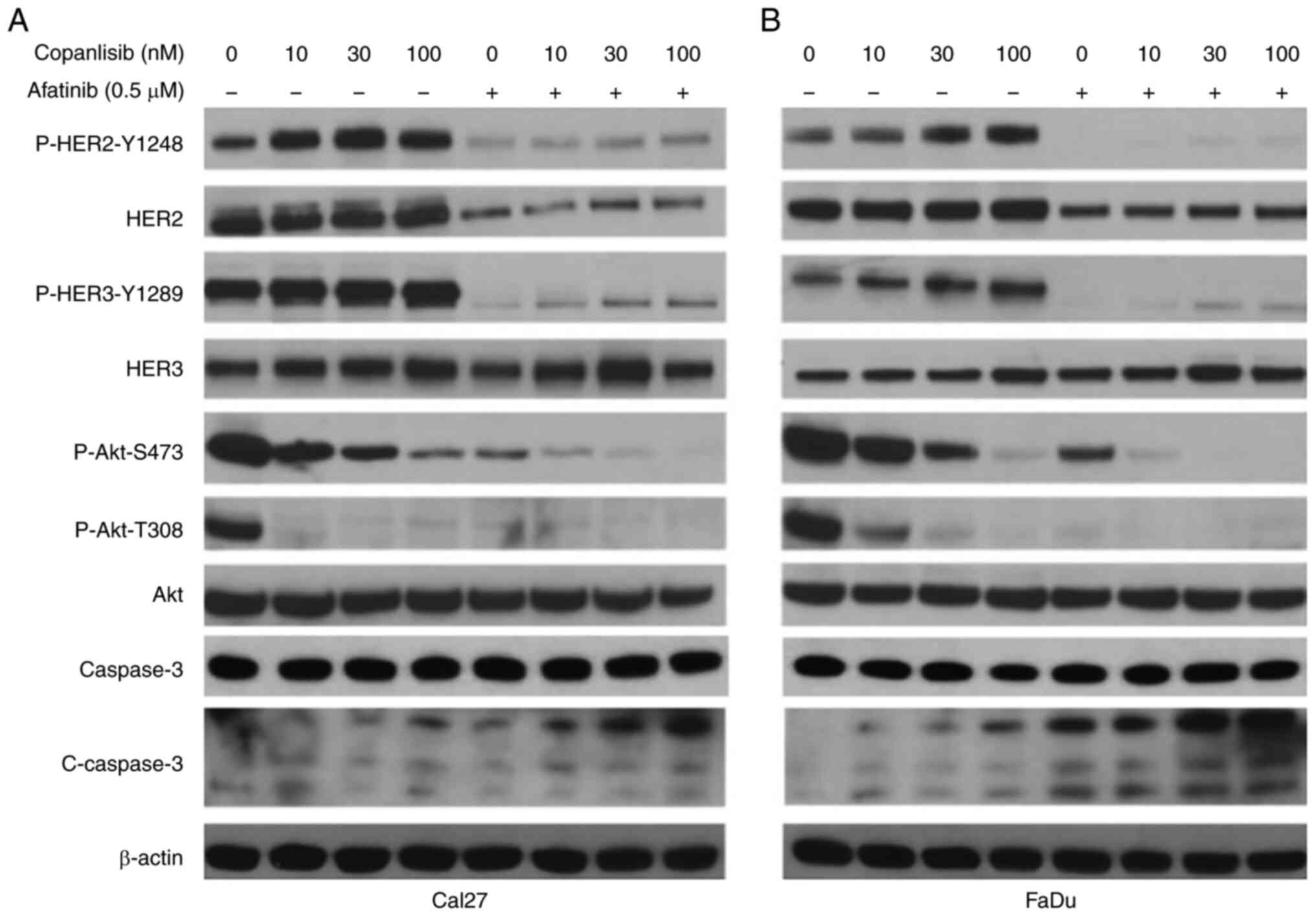
Combination of afatinib and copanlisib
completely inhibits ErbB and PI3K pathways, which results in
induction of caspase-3 cleavage
Previous studies have demonstrated that PI3K
inhibitors can induce HER2 and HER3 phosphorylation, thus
conferring resistance to PI3K inhibitors (50,51).
Our recent study showed that copanlisib induced an increase in
P-HER2 Y1248 and P-HER3 Y1289 in HPV-positive HNSCC cells, whereas
a combination of afatinib and copanlisib blocked the
phosphorylation of HER2 and HER3 (46). The present study further tested
whether copanlisib induced P-HER2 Y1248 and P-HER3 Y1289 in
HPV-negative Cal27 (Fig. 6A) and
FaDu cells (Fig. 6B). Similarly,
copanlisib markedly inhibited the phosphorylation of Akt and
induced phosphorylation of HER2 Y1248 and HER3 Y1289, whereas the
combination of copanlisib and afatinib completely blocked
phosphorylation of Akt, HER2 and HER3. In addition, increased
caspase-3 cleavage was induced by the combination compared with the
single treatments.
Discussion
Afatinib has shown positive results in HNSCC
clinical trials and is currently listed in the NCCN guidelines as a
third-line single agent for HNSCC treatment (25,26,31–33).
It is important to determine the mechanisms by which patients with
HNSCC develop resistance to afatinib in order to identify new
afatinib-based combination therapies for patients with advanced
HNSCC. The present study assessed the efficacy of co-inhibiting the
ErbB family and PI3K through a combination of afatinib and
copanlisib in the treatment of HPV-negative HNSCC. The results
showed that the combination of afatinib and copanlisib induced a
marked inhibition of cell viability and suppressed cell survival
in vitro in comparison to either treatment alone. Notably,
the combination also led to a significant inhibition of xenograft
tumor growth without affecting the body weight of the mice. These
results suggested that the combination of afatinib and copanlisib
may have clinical potential for the treatment of HPV-negative
HNSCC.
HNSCC is a heterogenous disease, and its gene
mutations/alterations are associated with EGFR inhibitor resistance
(5,40,52).
In a previous study by Cheng et al (53), the genomic and transcriptomic
changes for 15 HPV-negative and 11 HPV-positive HNSCC cell lines
were characterized and compared with data from 279 tumors from
TCGA. This study identified a number of genetic alterations,
including in TP53, PTEN, PIK3CA and FAT1, in HPV-negative and
HPV-positive HNSCC cells. The EGFR/ErbB and PI3K/Akt/mTOR pathways
are considered the most attractive pathways to target for treatment
of HNSCC due to overexpression or activating mutation of PIK3CA,
and loss of function mutations of PTEN (5,54–57).
It has been reported that constitutive activation of the
PI3K/Akt/mTOR pathway due to the alterations in PIK3CA, PTEN, Akt
or mTOR may be associated with resistance to EGFR inhibitors
(5). The present study used two
cell lines: Cal27 and FaDu cells. Both cell lines have TP53
mutations, whereas FaDu cells also have PIK3CA amplification, but
Cal27 cells do not (58,59). The present study showed that
treating both Cal27 and FaDu cells with afatinib alone blocked Akt
phosphorylation at Thr308, but only partially blocked the
phosphorylation of Akt at Ser473. Furthermore, it has been reported
that PI3K inhibition can lead to increased phosphorylation and
total levels of HER3, which confer resistance to PI3K inhibitors
(50,51,60–62).
The present data showed that copanlisib increased P-HER3 (Y1289),
which was counteracted by the addition of afatinib. Notably, the
combination of copanlisib and afatinib induced caspase-3 cleavage
in addition to the complete inhibition of ErbB and PI3K/Akt
pathways. These results provide a rationale for the co-inhibition
of ErbB and PI3K as a method to treat HNSCC with or without PIK3CA
amplification. However, it is important to test the efficacy of
this combination in more HNSCC cell lines with more genetic
alterations.
Our previous study reported that the combination of
afatinib and copanlisib effectively suppressed HPV-positive HNSCC.
The combination therapy blocked both ErbB and PI3K/Akt pathways,
which was accompanied by deceased E6 and E7, and the induction of
apoptosis, which indicated the increased efficacy of this
combination in HPV-positive HNSCC (46). Milewska et al (63) reported that cell lines from multiple
types of cancers, including HNSCC with PIK3CA mutations, are
sensitive to the combination of afatinib and copanlisib. As the
basal level of PI3K/Akt is also high in HPV-negative HNSCC and
serves essential roles in the regulation of growth, metastasis and
sensitivity to chemotherapy and targeted therapies (5,37,40,64),
it would be reasonable to predict that this combination would also
be beneficial in HPV-negative HNSCC with upregulated PI3K/Akt
signaling.
One of the challenges in treating patients with PI3K
pathway inhibitors is the associated toxicity from on-target and
off-target effects (65). The most
common side-effects observed with PI3K pathway inhibitors in
patients are hyperglycemia, rash, stomatitis, diarrhea, nausea and
fatigue (65,66). Due to the limitation of the
xenograft model, this information is lacking in this study.
Moreover, it is important to analyze the toxicity of the
combination of the two drugs to the liver and kidney of mice to
illustrate the feasibility of the combination; the lack of this
analysis is another limitation of the present study. Thus, to
accelerate its transition to treatment for patients with HNSCC, it
is important to further determine the drug dosage and
administration method of the combination in additional preclinical
models.
Cisplatin-based chemotherapy is the primary option
for the treatment of advanced HNSCC. It is interesting to compare
the efficacy and side effects of co-targeting ErbB and PI3K, or in
combination with cisplatin in preclinical models. We are currently
performing these experiments using HPV-negative HNSCC cell lines
with different PIK3CA and PTEN alterations.
Immunotherapy, which includes immune checkpoint
blockade that targets PD-L1/PD-1 using PD-L1 or PD-1 inhibitors,
has been an important advancement in the treatment of advanced
HNSCC. Afatinib modulates PD-L1 expression in multiple types of
cancer, including gastric cancer (67). In addition, it has been reported
that PI3K inhibitors, such as BKM120, can decrease the expression
of PD-L1 in HNSCC cells (68). It
would be interesting to determine the effects of afatinib,
copanlisib and their combination on the expression of PD-L1 in
HNSCC cells, in order to determine the impact of the combination of
afatinib and copanlisib on immune checkpoints.
In conclusion, the present results suggested that
co-targeting the ErbB family kinases and PI3K using a combination
of afatinib and copanlisib may have clinical potential for the
treatment of patients with HNSCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported, in part, by grants from the
National Cancer Institute and National Institute of Dental and
Craniofacial Research to HD (grant nos. R00CA149178, R01CA212094
and R56DE030423) and the Orakowa Foundation. This research was also
supported by funds through the National Cancer Institute-Cancer
Center Support Grant (grant no. P30CA134274) and the Maryland
Department of Health's Cigarette Restitution Fund Program (grant
no. CH-649-CRF).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XG contributed to the study conception and design,
performed the experiments and data analysis, wrote the original
draft, and reviewed and edited the manuscript. SA performed the
experiments and conducted data analysis. ZY, RGL and XF contributed
to data analysis. YT and RM were involved in data analysis, and
reviewing and editing the manuscript. KJC contributed to
conceptualization, data analysis, supervision, review, editing the
manuscript and funding acquisition. HD contributed to
conceptualization, data analysis, supervision, writing, review,
editing the manuscript and funding acquisition. XG, SA and HD
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
In vivo experiments were conducted with the
approval of the Institutional Animal Care and Use Committee of
University of Maryland.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Powell SF, Vu L, Spanos WC and Pyeon D:
The key differences between human papillomavirus-positive and
-negative head and neck cancers: Biological and clinical
implications. Cancers (Basel). 13:52062021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hashibe M, Boffetta P, Zaridze D, Shangina
O, Szeszenia-Dabrowska N, Mates D, Fabiánová E, Rudnai P and
Brennan P: Contribution of tobacco and alcohol to the high rates of
squamous cell carcinoma of the supraglottis and glottis in Central
Europe. Am J Epidemiol. 165:814–820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashibe M, Brennan P, Benhamou S,
Castellsague X, Chen C, Curado MP, Dal Maso LD, Daudt AW, Fabianova
E, Fernandez L, et al: Alcohol drinking in never users of tobacco,
cigarette smoking in never drinkers, and the risk of head and neck
cancer: Pooled analysis in the international head and neck cancer
epidemiology consortium. J Natl Cancer Inst. 99:777–789. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaryouh H, De Pauw I, Baysal H, Peeters M,
Vermorken JB, Lardon F and Wouters A: Recent insights in the
PI3K/Akt pathway as a promising therapeutic target in combination
with EGFR-targeting agents to treat head and neck squamous cell
carcinoma. Med Res Rev. 42:112–155. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fakhry C, Westra WH, Li S, Cmelak A, Ridge
JA, Pinto H, Forastiere A and Gillison ML: Improved survival of
patients with human papillomavirus-positive head and neck squamous
cell carcinoma in a prospective clinical trial. J Natl Cancer Inst.
100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin T, Li S, Henry LE, Liu S and Sartor
MA: Molecular tumor subtypes of HPV-positive head and neck cancers:
Biological characteristics and implications for clinical outcomes.
Cancers (Basel). 13:27212021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hennessey PT, Westra WH and Califano JA:
Human papillomavirus and head and neck squamous cell carcinoma:
Recent evidence and clinical implications. J Dent Res. 88:300–306.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferreira CC: The relation between human
papillomavirus (HPV) and oropharyngeal cancer: A review. PeerJ.
11:e155682023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tran NH, Sais D and Tran N: Advances in
human papillomavirus detection and molecular understanding in head
and neck cancers: Implications for clinical management. J Med
Virol. 96:e297462024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zumsteg ZS, Luu M, Yoshida EJ, Kim S,
Tighiouart M, David JM, Shiao SL, Mita AC, Scher KS, Sherman EJ, et
al: Combined high-intensity local treatment and systemic therapy in
metastatic head and neck squamous cell carcinoma: An analysis of
the national cancer data base. Cancer. 123:4583–4593. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen Y and Grandis JR: Emerging drugs for
head and neck cancer. Expert Opin Emerg Drugs. 20:313–329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YS, Johnson DE and Grandis JR: An
update: Emerging drugs to treat squamous cell carcinomas of the
head and neck. Expert Opin Emerg Drugs. 23:283–299. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perri F, Ionna F, Longo F, Della Vittoria
Scarpati G, De Angelis C, Ottaiano A, Botti G and Caponigro F:
Immune response against head and neck cancer: Biological mechanisms
and implication on therapy. Transl Oncol. 13:262–274. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grandis JR and Tweardy DJ: Elevated levels
of transforming growth factor alpha and epidermal growth factor
receptor messenger RNA are early markers of carcinogenesis in head
and neck cancer. Cancer Res. 53:3579–3584. 1993.PubMed/NCBI
|
|
17
|
Park BJ, Chiosea SI and Grandis JR:
Molecular changes in the multistage pathogenesis of head and neck
cancer. Cancer Biomark. 9:325–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharafinski ME, Ferris RL, Ferrone S and
Grandis JR: Epidermal growth factor receptor targeted therapy of
squamous cell carcinoma of the head and neck. Head Neck.
32:1412–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:522017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alorabi M, Shonka NA and Ganti AK: EGFR
monoclonal antibodies in locally advanced head and neck squamous
cell carcinoma: What is their current role? Crit Rev Oncol Hematol.
99:170–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blaszczak W, Barczak W, Wegner A,
Golusinski W and Suchorska WM: Clinical value of monoclonal
antibodies and tyrosine kinase inhibitors in the treatment of head
and neck squamous cell carcinoma. Med Oncol. 34:602017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mehra R, Cohen RB and Burtness BA: The
role of cetuximab for the treatment of squamous cell carcinoma of
the head and neck. Clin Adv Hematol Oncol. 6:742–750.
2008.PubMed/NCBI
|
|
24
|
Argiris A, Heron DE, Smith RP, Kim S,
Gibson MK, Lai SY, Branstetter BF, Posluszny DM, Wang L, Seethala
RR, et al: Induction docetaxel, cisplatin, and cetuximab followed
by concurrent radiotherapy, cisplatin, and cetuximab and
maintenance cetuximab in patients with locally advanced head and
neck cancer. J Clin Oncol. 28:5294–5300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Specenier P and Vermorken J: Afatinib in
squamous cell carcinoma of the head and neck. Expert Opin
Pharmacother. 17:1295–1301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Machiels JPH, Haddad RI, Fayette J,
Licitra LF, Tahara M, Vermorken JB, Clement PM, Gauler T, Cupissol
D, Grau JJ, et al: Afatinib versus methotrexate as second-line
treatment in patients with recurrent or metastatic squamous-cell
carcinoma of the head and neck progressing on or after
platinum-based therapy (LUX-Head & Neck 1): An open-label,
randomised phase 3 trial. Lancet Oncol. 16:583–594. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saddawi-Konefka R, Schokrpur S, Lui AJ and
Gutkind JS: HER2 and HER3 as therapeutic targets in head and neck
cancer. Cancer J. 28:339–345. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sacco AG and Worden FP: Molecularly
targeted therapy for the treatment of head and neck cancer: A
review of the ErbB family inhibitors. Onco Targets Ther.
9:1927–1943. 2016.PubMed/NCBI
|
|
29
|
Rysman B, Mouawad F, Gros A, Lansiaux A,
Chevalier D and Meignan S: Human epidermal growth factor receptor 3
in head and neck squamous cell carcinomas. Head Neck. 38 (Suppl
1):E2412–E2418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palumbo C, Benvenuto M, Focaccetti C,
Albonici L, Cifaldi L, Rufini A, Nardozi D, Angiolini V, Bei A,
Masuelli L and Bei R: Recent findings on the impact of ErbB
receptors status on prognosis and therapy of head and neck squamous
cell carcinoma. Front Med (Lausanne). 10:10660212023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kao HF, Liao BC, Huang YL, Huang HC, Chen
CN, Chen TC, Hong YJ, Chan CY, Chia JS and Hong RL: Afatinib and
pembrolizumab for recurrent or metastatic head and neck squamous
cell carcinoma (ALPHA Study): A phase II study with biomarker
analysis. Clin Cancer Res. 28:1560–1571. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Ahn MJ, Chan A, Wang CH, Kang JH,
Kim SB, Bello M, Arora RS, Zhang Q, He X, et al: Afatinib versus
methotrexate as second-line treatment in Asian patients with
recurrent or metastatic squamous cell carcinoma of the head and
neck progressing on or after platinum-based therapy (LUX-Head &
Neck 3): An open-label, randomised phase III trial. Ann Oncol.
30:1831–1839. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haddad R, Guigay J, Keilholz U, Clement
PM, Fayette J, de Souza Viana L, Rolland F, Cupissol D, Geoffrois
L, Kornek G, et al: Afatinib as second-line treatment in patients
with recurrent/metastatic squamous cell carcinoma of the head and
neck: Subgroup analyses of treatment adherence, safety and mode of
afatinib administration in the LUX-Head and Neck 1 trial. Oral
Oncol. 97:82–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mellor P, Furber LA, Nyarko JNK and
Anderson DH: Multiple roles for the p85α isoform in the regulation
and function of PI3K signalling and receptor trafficking. Biochem
J. 441:23–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lui VW, Hedberg ML, Li H, Vangara BS,
Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR, et al:
Frequent mutation of the PI3K pathway in head and neck cancer
defines predictive biomarkers. Cancer Discov. 3:761–769. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cochicho D, Esteves S, Rito M, Silva F,
Martins L, Montalvão P, Cunha M, Magalhães M, da Costa RMG and
Felix A: PIK3CA gene mutations in HNSCC: Systematic review and
correlations with HPV status and patient survival. Cancers (Basel).
14:12862022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jung K, Kang H and Mehra R: Targeting
phosphoinositide 3-kinase (PI3K) in head and neck squamous cell
carcinoma (HNSCC). Cancers Head Neck. 3:32018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
García-Escudero R, Segrelles C, Dueñas M,
Pombo M, Ballestín C, Alonso-Riaño M, Nenclares P,
Álvarez-Rodríguez R, Sánchez-Aniceto G, Ruíz-Alonso A, et al:
Overexpression of PIK3CA in head and neck squamous cell carcinoma
is associated with poor outcome and activation of the YAP pathway.
Oral Oncol. 79:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cancer Genome Atlas Network, .
Comprehensive genomic characterization of head and neck squamous
cell carcinomas. Nature. 517:576–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marquard FE and Jücker M: PI3K/AKT/mTOR
signaling as a molecular target in head and neck cancer. Biochem
Pharmacol. 172:1137292020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Poetsch M, Lorenz G and Kleist B:
Detection of new PTEN/MMAC1 mutations in head and neck squamous
cell carcinomas with loss of chromosome 10. Cancer Genet Cytogenet.
132:20–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sangale Z, Prass C, Carlson A, Tikishvili
E, Degrado J, Lanchbury J and Stone S: A robust immunohistochemical
assay for detecting PTEN expression in human tumors. Appl
Immunohistochem Mol Morphol. 19:173–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Squarize CH, Castilho RM, Abrahao AC,
Molinolo A, Lingen MW and Gutkind JS: PTEN deficiency contributes
to the development and progression of head and neck cancer.
Neoplasia. 15:461–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Psyrri A, Seiwert TY and Jimeno A:
Molecular pathways in head and neck cancer: EGFR, PI3K, and more.
Am Soc Clin Oncol Educ Book. 246–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pezzuto F, Buonaguro L, Caponigro F, Ionna
F, Starita N, Annunziata C, Buonaguro FM and Tornesello ML: Update
on head and neck cancer: Current knowledge on epidemiology, risk
factors, molecular features and novel therapies. Oncology.
89:125–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Z, Liao J, Schumaker L, Carter-Cooper
B, Lapidus RG, Fan X, Gaykalova DA, Mehra R, Cullen KJ and Dan H:
Simultaneously targeting ErbB family kinases and PI3K in
HPV-positive head and neck squamous cell carcinoma. Oral Oncol.
131:1059392022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang Z, Liao J, Carter-Cooper BA, Lapidus
RG, Cullen KJ and Dan H: Regulation of cisplatin-resistant head and
neck squamous cell carcinoma by the SRC/ETS-1 signaling pathway.
BMC Cancer. 19:4852019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Packer LM, Geng X, Bonazzi VF, Ju RJ,
Mahon CE, Cummings MC, Stephenson SA and Pollock PM: PI3K
inhibitors synergize with FGFR inhibitors to enhance antitumor
responses in FGFR2mutant endometrial cancers. Mol Cancer
Ther. 16:637–648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chakrabarty A, Sánchez V, Kuba MG,
Rinehart C and Arteaga CL: Feedback upregulation of HER3 (ErbB3)
expression and activity attenuates antitumor effect of PI3K
inhibitors. Proc Natl Acad Sci USA. 109:2718–2723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Meister KS, Godse NR, Khan NI, Hedberg ML,
Kemp C, Kulkarni S, Alvarado D, LaVallee T, Kim S, Grandis JR and
Duvvuri U: HER3 targeting potentiates growth suppressive effects of
the PI3K inhibitor BYL719 in pre-clinical models of head and neck
squamous cell carcinoma. Sci Rep. 9:91302019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Young NR, Liu J, Pierce C, Wei TF, Grushko
T, Olopade OI, Liu W, Shen C, Seiwert TY and Cohen EE: Molecular
phenotype predicts sensitivity of squamous cell carcinoma of the
head and neck to epidermal growth factor receptor inhibition. Mol
Oncol. 7:359–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheng H, Yang X, Si H, Saleh AD, Xiao W,
Coupar J, Gollin SM, Ferris RL, Issaeva N, Yarbrough WG, et al:
Genomic and transcriptomic characterization links cell lines with
aggressive head and neck cancers. Cell Rep. 25:1332–1345.e5. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zaryouh H, Van Loenhout J, Peeters M,
Vermorken JB, Lardon F and Wouters A: Co-targeting the EGFR and
PI3K/Akt pathway to overcome therapeutic resistance in head and
neck squamous cell carcinoma: What about autophagy? Cancers
(Basel). 14:61282022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zaryouh H, De Pauw I, Baysal H, Pauwels P,
Peeters M, Vermorken JB, Lardon F and Wouters A: The role of Akt in
acquired cetuximab resistant head and neck squamous cell carcinoma:
An in vitro study on a novel combination strategy. Front Oncol.
11:6979672021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mock A, Plath M, Moratin J, Tapken MJ,
Jäger D, Krauss J, Fröhling S, Hess J and Zaoui K: EGFR and PI3K
pathway activities might guide drug repurposing in HPV-negative
head and neck cancers. Front Oncol. 11:6789662021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Izumi H, Wang Z, Goto Y, Ando T, Wu X,
Zhang X, Li H, Johnson DE, Grandis JR and Gutkind JS:
Pathway-specific genome editing of PI3K/mTOR tumor suppressor genes
reveals that PTEN loss contributes to cetuximab resistance in head
and neck cancer. Mol Cancer Ther. 19:1562–1571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Martin D, Abba MC, Molinolo AA,
Vitale-Cross L, Wang Z, Zaida M, Delic NC, Samuels Y, Lyons JG and
Gutkind JS: The head and neck cancer cell oncogenome: A platform
for the development of precision molecular therapies. Oncotarget.
5:8906–8923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
van Harten AM, Poell JB, Buijze M, Brink
A, Wells SI, René Leemans C, Wolthuis RMF and Brakenhoff RH:
Characterization of a head and neck cancer-derived cell line panel
confirms the distinct TP53-proficient copy number-silent subclass.
Oral Oncol. 98:53–61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cook RS, Garrett JT, Sánchez V, Stanford
JC, Young C, Chakrabarty A, Rinehart C, Zhang Y, Wu Y, Greenberger
L, et al: ErbB3 ablation impairs PI3K/Akt-dependent mammary
tumorigenesis. Cancer Res. 71:3941–3951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mishra R, Patel H, Alanazi S, Yuan L and
Garrett JT: HER3 signaling and targeted therapy in cancer. Oncol
Rev. 12:3552018.PubMed/NCBI
|
|
62
|
Garrett JT, Sutton CR, Kurupi R, Bialucha
CU, Ettenberg SA, Collins SD, Sheng Q, Wallweber J, Defazio-Eli L
and Arteaga CL: Combination of antibody that inhibits
ligand-independent HER3 dimerization and a p110α inhibitor potently
blocks PI3K signaling and growth of HER2+ breast cancers. Cancer
Res. 73:6013–6023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Milewska M, Cremona M, Morgan C, O'Shea J,
Carr A, Vellanki SH, Hopkins AM, Toomey S, Madden SF, Hennessy BT
and Eustace AJ: Development of a personalized therapeutic strategy
for ERBB-gene-mutated cancers. Ther Adv Med Oncol.
10:17588340177460402018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Akbari Dilmaghani N, Safaroghli-Azar A,
Pourbagheri-Sigaroodi A and Bashash D: The PI3K/Akt/mTORC signaling
axis in head and neck squamous cell carcinoma: Possibilities for
therapeutic interventions either as single agents or in combination
with conventional therapies. IUBMB Life. 73:618–642. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nunnery SE and Mayer IA: Management of
toxicity to isoform α-specific PI3K inhibitors. Ann Oncol. 30
(Suppl 10):x21–x26. 2019. View Article : Google Scholar
|
|
66
|
Chia S, Gandhi S, Joy AA, Edwards S, Gorr
M, Hopkins S, Kondejewski J, Ayoub JP, Califaretti N, Rayson D and
Dent SF: Novel agents and associated toxicities of inhibitors of
the pi3k/Akt/mtor pathway for the treatment of breast cancer. Curr
Oncol. 22:33–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Suh KJ, Sung JH, Kim JW, Han SH, Lee HS,
Min A, Kang MH, Kim JE, Kim JW, Kim SH, et al: EGFR or HER2
inhibition modulates the tumor microenvironment by suppression of
PD-L1 and cytokines release. Oncotarget. 8:63901–63910. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fiedler M, Schulz D, Piendl G, Brockhoff
G, Eichberger J, Menevse AN, Beckhove P, Hautmann M, Reichert TE,
Ettl T and Bauer RJ: Buparlisib modulates PD-L1 expression in head
and neck squamous cell carcinoma cell lines. Exp Cell Res.
396:1122592020. View Article : Google Scholar : PubMed/NCBI
|