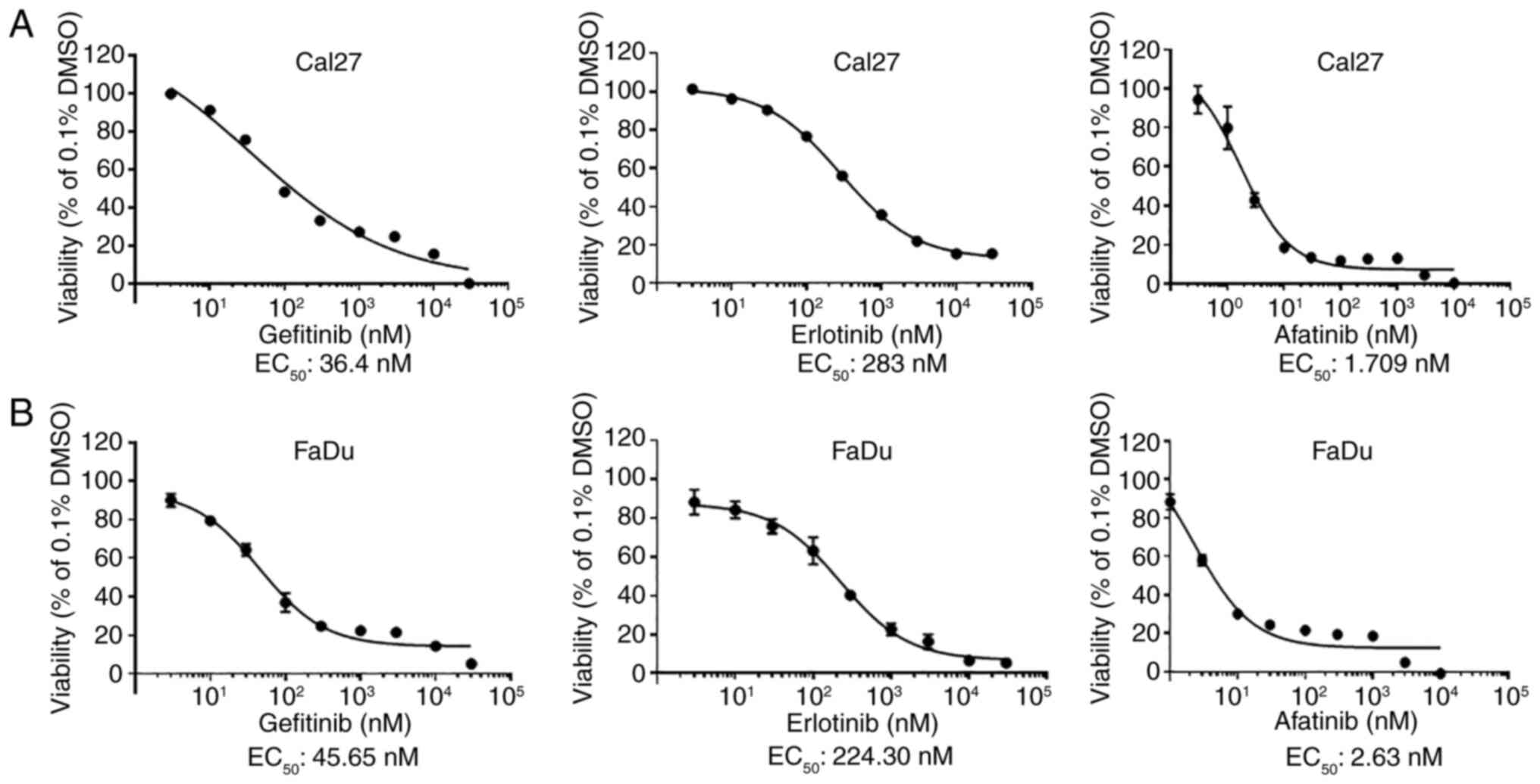
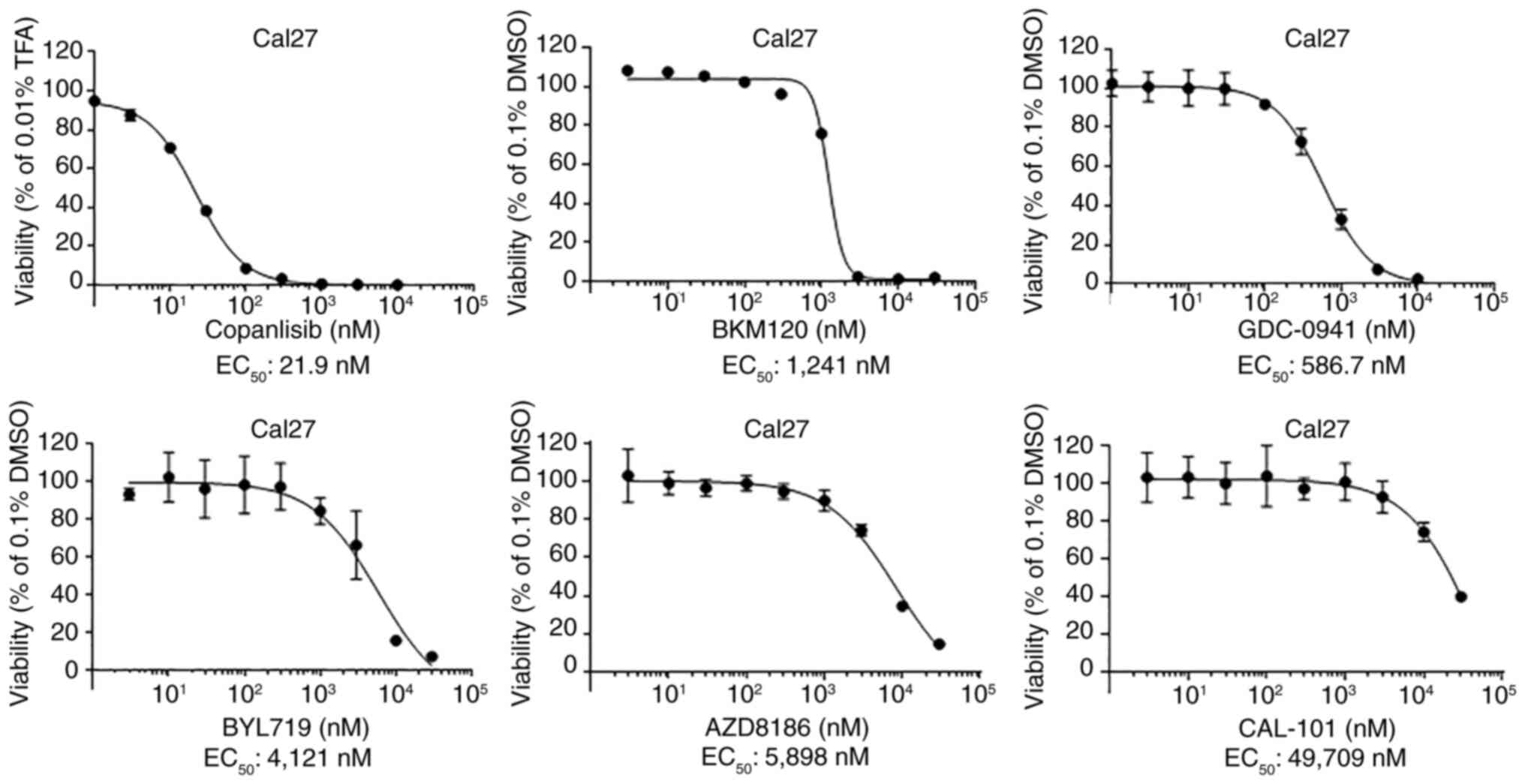
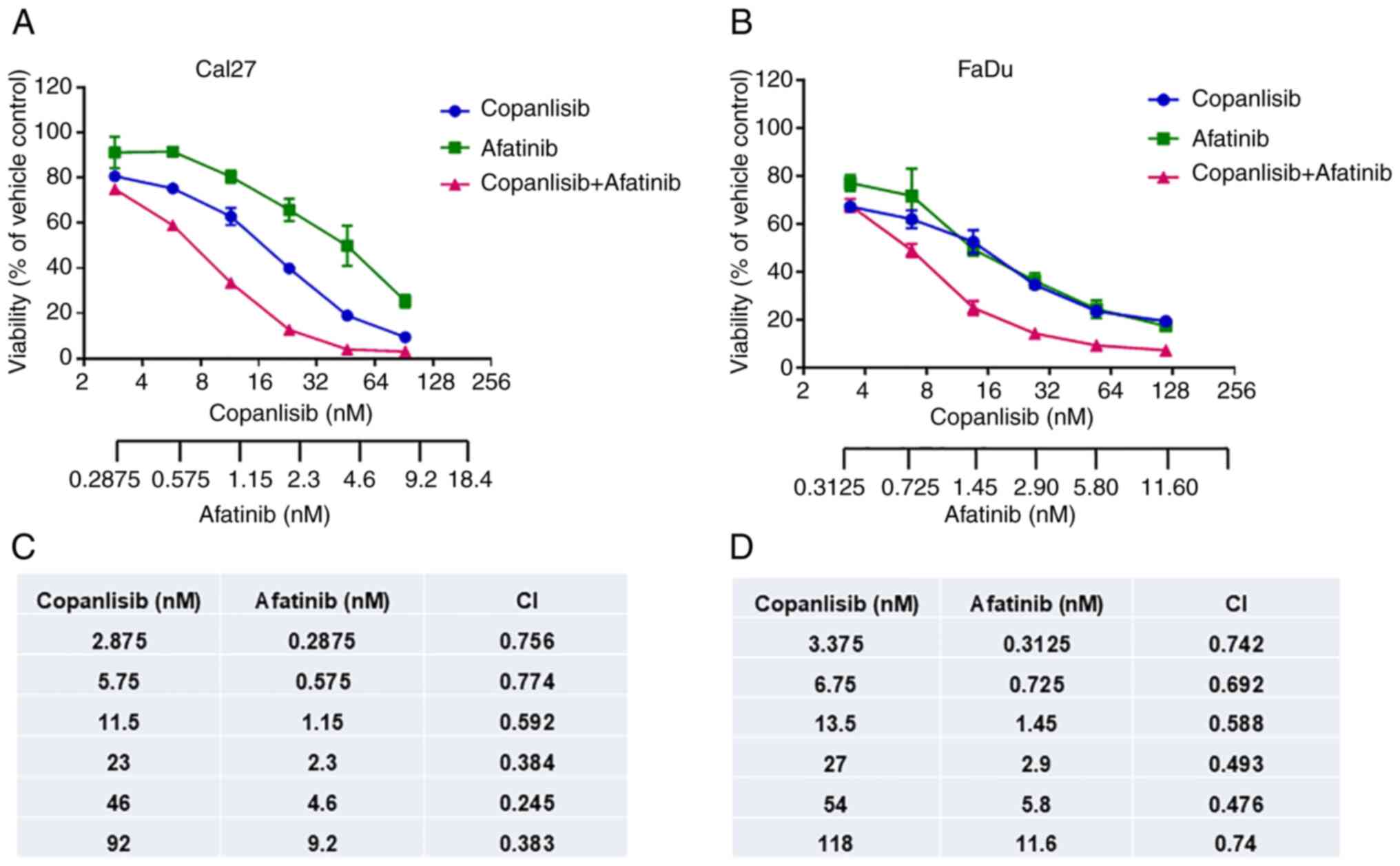
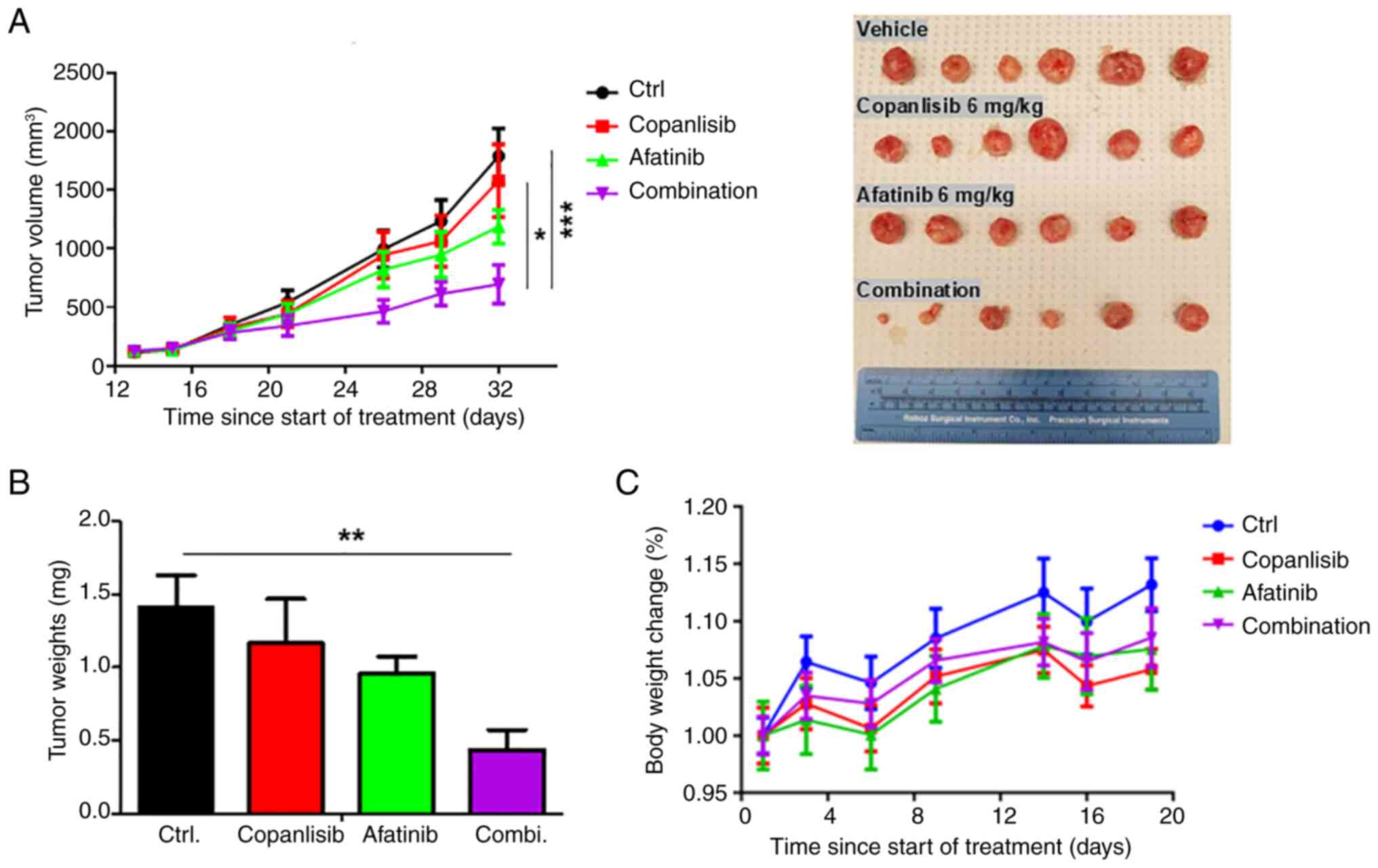
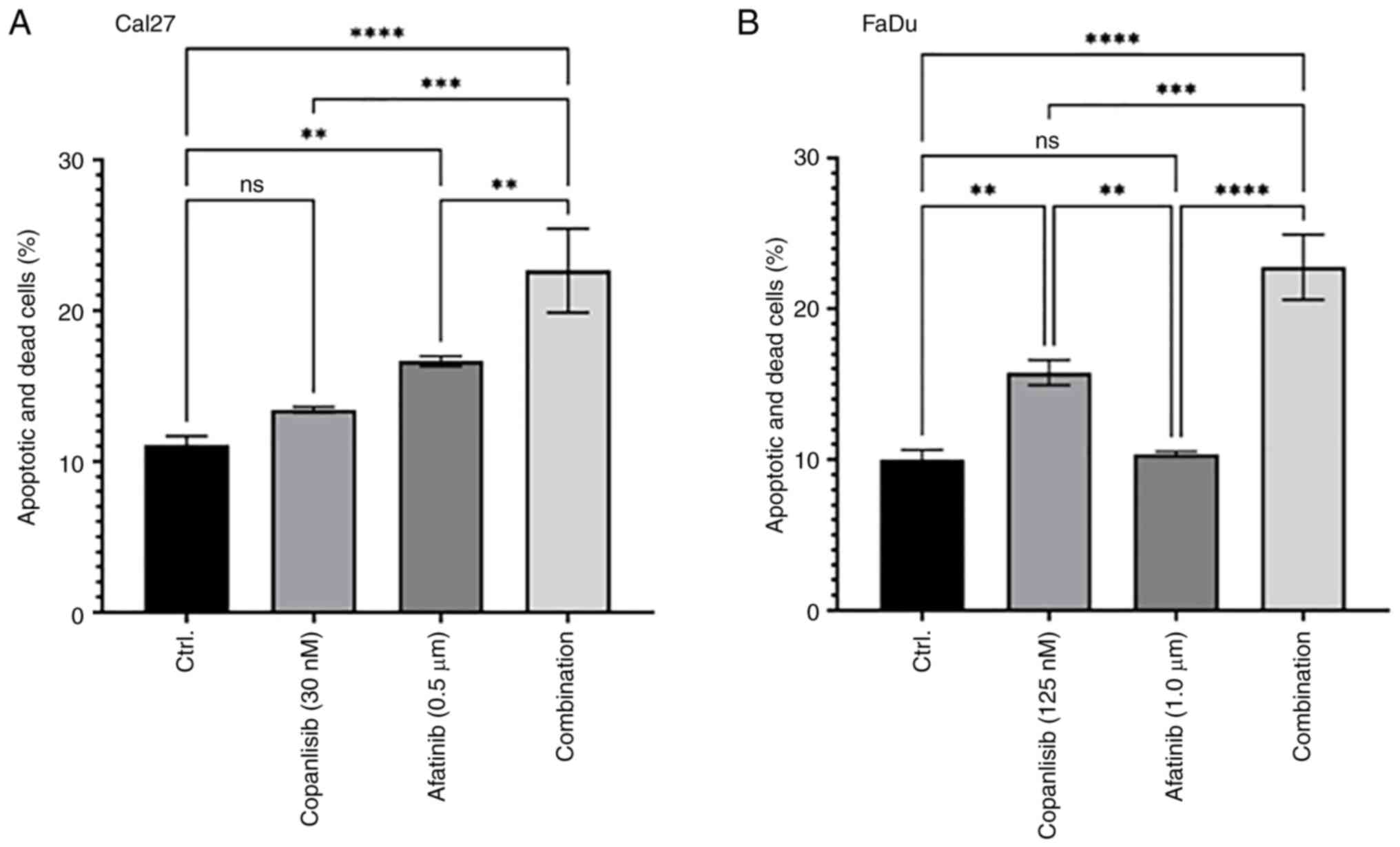
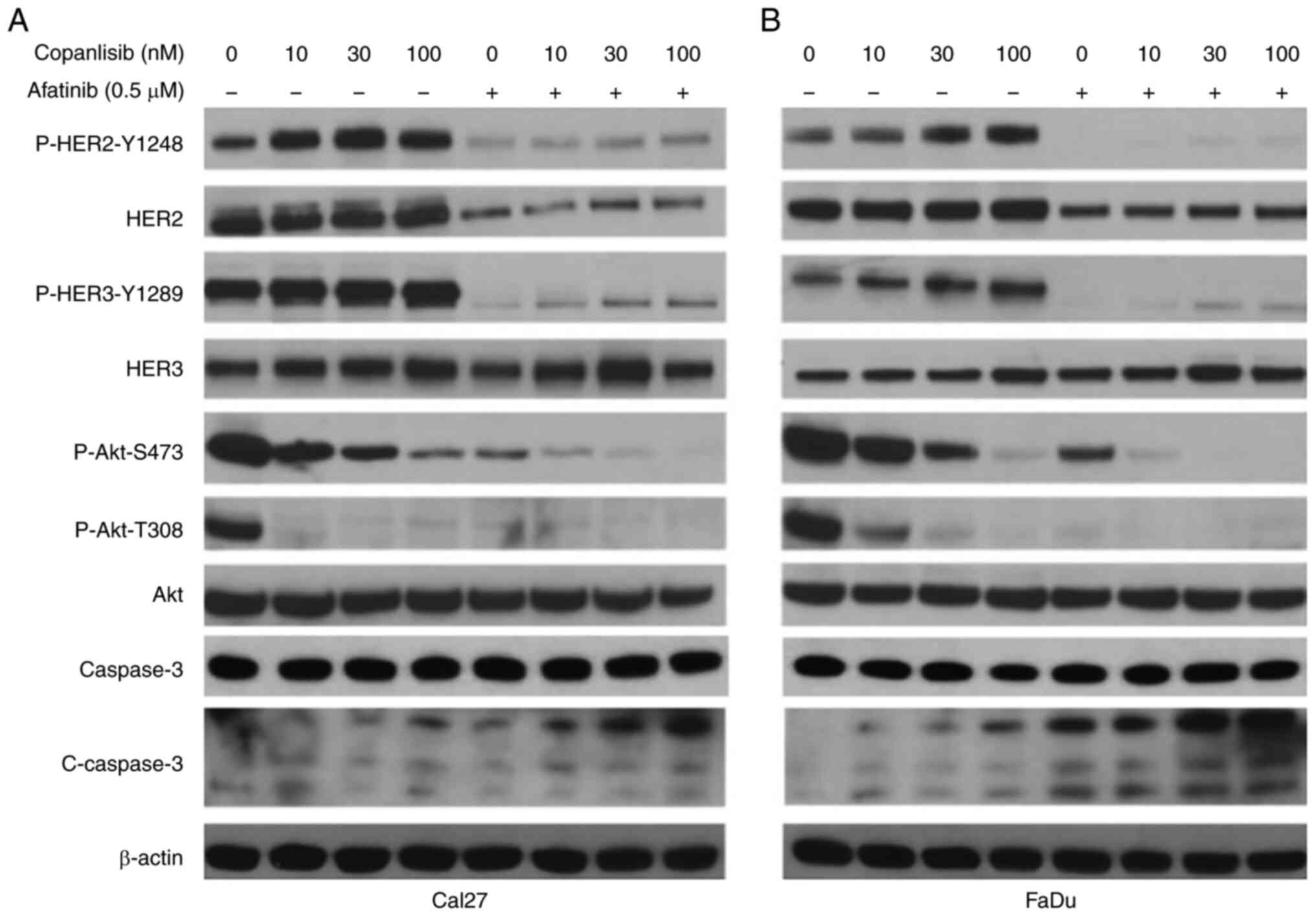
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Powell SF, Vu L, Spanos WC and Pyeon D:
The key differences between human papillomavirus-positive and
-negative head and neck cancers: Biological and clinical
implications. Cancers (Basel). 13:52062021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hashibe M, Boffetta P, Zaridze D, Shangina
O, Szeszenia-Dabrowska N, Mates D, Fabiánová E, Rudnai P and
Brennan P: Contribution of tobacco and alcohol to the high rates of
squamous cell carcinoma of the supraglottis and glottis in Central
Europe. Am J Epidemiol. 165:814–820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashibe M, Brennan P, Benhamou S,
Castellsague X, Chen C, Curado MP, Dal Maso LD, Daudt AW, Fabianova
E, Fernandez L, et al: Alcohol drinking in never users of tobacco,
cigarette smoking in never drinkers, and the risk of head and neck
cancer: Pooled analysis in the international head and neck cancer
epidemiology consortium. J Natl Cancer Inst. 99:777–789. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaryouh H, De Pauw I, Baysal H, Peeters M,
Vermorken JB, Lardon F and Wouters A: Recent insights in the
PI3K/Akt pathway as a promising therapeutic target in combination
with EGFR-targeting agents to treat head and neck squamous cell
carcinoma. Med Res Rev. 42:112–155. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fakhry C, Westra WH, Li S, Cmelak A, Ridge
JA, Pinto H, Forastiere A and Gillison ML: Improved survival of
patients with human papillomavirus-positive head and neck squamous
cell carcinoma in a prospective clinical trial. J Natl Cancer Inst.
100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin T, Li S, Henry LE, Liu S and Sartor
MA: Molecular tumor subtypes of HPV-positive head and neck cancers:
Biological characteristics and implications for clinical outcomes.
Cancers (Basel). 13:27212021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hennessey PT, Westra WH and Califano JA:
Human papillomavirus and head and neck squamous cell carcinoma:
Recent evidence and clinical implications. J Dent Res. 88:300–306.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferreira CC: The relation between human
papillomavirus (HPV) and oropharyngeal cancer: A review. PeerJ.
11:e155682023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tran NH, Sais D and Tran N: Advances in
human papillomavirus detection and molecular understanding in head
and neck cancers: Implications for clinical management. J Med
Virol. 96:e297462024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zumsteg ZS, Luu M, Yoshida EJ, Kim S,
Tighiouart M, David JM, Shiao SL, Mita AC, Scher KS, Sherman EJ, et
al: Combined high-intensity local treatment and systemic therapy in
metastatic head and neck squamous cell carcinoma: An analysis of
the national cancer data base. Cancer. 123:4583–4593. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen Y and Grandis JR: Emerging drugs for
head and neck cancer. Expert Opin Emerg Drugs. 20:313–329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YS, Johnson DE and Grandis JR: An
update: Emerging drugs to treat squamous cell carcinomas of the
head and neck. Expert Opin Emerg Drugs. 23:283–299. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perri F, Ionna F, Longo F, Della Vittoria
Scarpati G, De Angelis C, Ottaiano A, Botti G and Caponigro F:
Immune response against head and neck cancer: Biological mechanisms
and implication on therapy. Transl Oncol. 13:262–274. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grandis JR and Tweardy DJ: Elevated levels
of transforming growth factor alpha and epidermal growth factor
receptor messenger RNA are early markers of carcinogenesis in head
and neck cancer. Cancer Res. 53:3579–3584. 1993.PubMed/NCBI
|
|
17
|
Park BJ, Chiosea SI and Grandis JR:
Molecular changes in the multistage pathogenesis of head and neck
cancer. Cancer Biomark. 9:325–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharafinski ME, Ferris RL, Ferrone S and
Grandis JR: Epidermal growth factor receptor targeted therapy of
squamous cell carcinoma of the head and neck. Head Neck.
32:1412–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:522017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alorabi M, Shonka NA and Ganti AK: EGFR
monoclonal antibodies in locally advanced head and neck squamous
cell carcinoma: What is their current role? Crit Rev Oncol Hematol.
99:170–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blaszczak W, Barczak W, Wegner A,
Golusinski W and Suchorska WM: Clinical value of monoclonal
antibodies and tyrosine kinase inhibitors in the treatment of head
and neck squamous cell carcinoma. Med Oncol. 34:602017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mehra R, Cohen RB and Burtness BA: The
role of cetuximab for the treatment of squamous cell carcinoma of
the head and neck. Clin Adv Hematol Oncol. 6:742–750.
2008.PubMed/NCBI
|
|
24
|
Argiris A, Heron DE, Smith RP, Kim S,
Gibson MK, Lai SY, Branstetter BF, Posluszny DM, Wang L, Seethala
RR, et al: Induction docetaxel, cisplatin, and cetuximab followed
by concurrent radiotherapy, cisplatin, and cetuximab and
maintenance cetuximab in patients with locally advanced head and
neck cancer. J Clin Oncol. 28:5294–5300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Specenier P and Vermorken J: Afatinib in
squamous cell carcinoma of the head and neck. Expert Opin
Pharmacother. 17:1295–1301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Machiels JPH, Haddad RI, Fayette J,
Licitra LF, Tahara M, Vermorken JB, Clement PM, Gauler T, Cupissol
D, Grau JJ, et al: Afatinib versus methotrexate as second-line
treatment in patients with recurrent or metastatic squamous-cell
carcinoma of the head and neck progressing on or after
platinum-based therapy (LUX-Head & Neck 1): An open-label,
randomised phase 3 trial. Lancet Oncol. 16:583–594. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saddawi-Konefka R, Schokrpur S, Lui AJ and
Gutkind JS: HER2 and HER3 as therapeutic targets in head and neck
cancer. Cancer J. 28:339–345. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sacco AG and Worden FP: Molecularly
targeted therapy for the treatment of head and neck cancer: A
review of the ErbB family inhibitors. Onco Targets Ther.
9:1927–1943. 2016.PubMed/NCBI
|
|
29
|
Rysman B, Mouawad F, Gros A, Lansiaux A,
Chevalier D and Meignan S: Human epidermal growth factor receptor 3
in head and neck squamous cell carcinomas. Head Neck. 38 (Suppl
1):E2412–E2418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palumbo C, Benvenuto M, Focaccetti C,
Albonici L, Cifaldi L, Rufini A, Nardozi D, Angiolini V, Bei A,
Masuelli L and Bei R: Recent findings on the impact of ErbB
receptors status on prognosis and therapy of head and neck squamous
cell carcinoma. Front Med (Lausanne). 10:10660212023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kao HF, Liao BC, Huang YL, Huang HC, Chen
CN, Chen TC, Hong YJ, Chan CY, Chia JS and Hong RL: Afatinib and
pembrolizumab for recurrent or metastatic head and neck squamous
cell carcinoma (ALPHA Study): A phase II study with biomarker
analysis. Clin Cancer Res. 28:1560–1571. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Ahn MJ, Chan A, Wang CH, Kang JH,
Kim SB, Bello M, Arora RS, Zhang Q, He X, et al: Afatinib versus
methotrexate as second-line treatment in Asian patients with
recurrent or metastatic squamous cell carcinoma of the head and
neck progressing on or after platinum-based therapy (LUX-Head &
Neck 3): An open-label, randomised phase III trial. Ann Oncol.
30:1831–1839. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haddad R, Guigay J, Keilholz U, Clement
PM, Fayette J, de Souza Viana L, Rolland F, Cupissol D, Geoffrois
L, Kornek G, et al: Afatinib as second-line treatment in patients
with recurrent/metastatic squamous cell carcinoma of the head and
neck: Subgroup analyses of treatment adherence, safety and mode of
afatinib administration in the LUX-Head and Neck 1 trial. Oral
Oncol. 97:82–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mellor P, Furber LA, Nyarko JNK and
Anderson DH: Multiple roles for the p85α isoform in the regulation
and function of PI3K signalling and receptor trafficking. Biochem
J. 441:23–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lui VW, Hedberg ML, Li H, Vangara BS,
Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR, et al:
Frequent mutation of the PI3K pathway in head and neck cancer
defines predictive biomarkers. Cancer Discov. 3:761–769. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cochicho D, Esteves S, Rito M, Silva F,
Martins L, Montalvão P, Cunha M, Magalhães M, da Costa RMG and
Felix A: PIK3CA gene mutations in HNSCC: Systematic review and
correlations with HPV status and patient survival. Cancers (Basel).
14:12862022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jung K, Kang H and Mehra R: Targeting
phosphoinositide 3-kinase (PI3K) in head and neck squamous cell
carcinoma (HNSCC). Cancers Head Neck. 3:32018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
García-Escudero R, Segrelles C, Dueñas M,
Pombo M, Ballestín C, Alonso-Riaño M, Nenclares P,
Álvarez-Rodríguez R, Sánchez-Aniceto G, Ruíz-Alonso A, et al:
Overexpression of PIK3CA in head and neck squamous cell carcinoma
is associated with poor outcome and activation of the YAP pathway.
Oral Oncol. 79:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cancer Genome Atlas Network, .
Comprehensive genomic characterization of head and neck squamous
cell carcinomas. Nature. 517:576–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marquard FE and Jücker M: PI3K/AKT/mTOR
signaling as a molecular target in head and neck cancer. Biochem
Pharmacol. 172:1137292020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Poetsch M, Lorenz G and Kleist B:
Detection of new PTEN/MMAC1 mutations in head and neck squamous
cell carcinomas with loss of chromosome 10. Cancer Genet Cytogenet.
132:20–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sangale Z, Prass C, Carlson A, Tikishvili
E, Degrado J, Lanchbury J and Stone S: A robust immunohistochemical
assay for detecting PTEN expression in human tumors. Appl
Immunohistochem Mol Morphol. 19:173–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Squarize CH, Castilho RM, Abrahao AC,
Molinolo A, Lingen MW and Gutkind JS: PTEN deficiency contributes
to the development and progression of head and neck cancer.
Neoplasia. 15:461–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Psyrri A, Seiwert TY and Jimeno A:
Molecular pathways in head and neck cancer: EGFR, PI3K, and more.
Am Soc Clin Oncol Educ Book. 246–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pezzuto F, Buonaguro L, Caponigro F, Ionna
F, Starita N, Annunziata C, Buonaguro FM and Tornesello ML: Update
on head and neck cancer: Current knowledge on epidemiology, risk
factors, molecular features and novel therapies. Oncology.
89:125–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Z, Liao J, Schumaker L, Carter-Cooper
B, Lapidus RG, Fan X, Gaykalova DA, Mehra R, Cullen KJ and Dan H:
Simultaneously targeting ErbB family kinases and PI3K in
HPV-positive head and neck squamous cell carcinoma. Oral Oncol.
131:1059392022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang Z, Liao J, Carter-Cooper BA, Lapidus
RG, Cullen KJ and Dan H: Regulation of cisplatin-resistant head and
neck squamous cell carcinoma by the SRC/ETS-1 signaling pathway.
BMC Cancer. 19:4852019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Packer LM, Geng X, Bonazzi VF, Ju RJ,
Mahon CE, Cummings MC, Stephenson SA and Pollock PM: PI3K
inhibitors synergize with FGFR inhibitors to enhance antitumor
responses in FGFR2mutant endometrial cancers. Mol Cancer
Ther. 16:637–648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chakrabarty A, Sánchez V, Kuba MG,
Rinehart C and Arteaga CL: Feedback upregulation of HER3 (ErbB3)
expression and activity attenuates antitumor effect of PI3K
inhibitors. Proc Natl Acad Sci USA. 109:2718–2723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Meister KS, Godse NR, Khan NI, Hedberg ML,
Kemp C, Kulkarni S, Alvarado D, LaVallee T, Kim S, Grandis JR and
Duvvuri U: HER3 targeting potentiates growth suppressive effects of
the PI3K inhibitor BYL719 in pre-clinical models of head and neck
squamous cell carcinoma. Sci Rep. 9:91302019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Young NR, Liu J, Pierce C, Wei TF, Grushko
T, Olopade OI, Liu W, Shen C, Seiwert TY and Cohen EE: Molecular
phenotype predicts sensitivity of squamous cell carcinoma of the
head and neck to epidermal growth factor receptor inhibition. Mol
Oncol. 7:359–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheng H, Yang X, Si H, Saleh AD, Xiao W,
Coupar J, Gollin SM, Ferris RL, Issaeva N, Yarbrough WG, et al:
Genomic and transcriptomic characterization links cell lines with
aggressive head and neck cancers. Cell Rep. 25:1332–1345.e5. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zaryouh H, Van Loenhout J, Peeters M,
Vermorken JB, Lardon F and Wouters A: Co-targeting the EGFR and
PI3K/Akt pathway to overcome therapeutic resistance in head and
neck squamous cell carcinoma: What about autophagy? Cancers
(Basel). 14:61282022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zaryouh H, De Pauw I, Baysal H, Pauwels P,
Peeters M, Vermorken JB, Lardon F and Wouters A: The role of Akt in
acquired cetuximab resistant head and neck squamous cell carcinoma:
An in vitro study on a novel combination strategy. Front Oncol.
11:6979672021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mock A, Plath M, Moratin J, Tapken MJ,
Jäger D, Krauss J, Fröhling S, Hess J and Zaoui K: EGFR and PI3K
pathway activities might guide drug repurposing in HPV-negative
head and neck cancers. Front Oncol. 11:6789662021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Izumi H, Wang Z, Goto Y, Ando T, Wu X,
Zhang X, Li H, Johnson DE, Grandis JR and Gutkind JS:
Pathway-specific genome editing of PI3K/mTOR tumor suppressor genes
reveals that PTEN loss contributes to cetuximab resistance in head
and neck cancer. Mol Cancer Ther. 19:1562–1571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Martin D, Abba MC, Molinolo AA,
Vitale-Cross L, Wang Z, Zaida M, Delic NC, Samuels Y, Lyons JG and
Gutkind JS: The head and neck cancer cell oncogenome: A platform
for the development of precision molecular therapies. Oncotarget.
5:8906–8923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
van Harten AM, Poell JB, Buijze M, Brink
A, Wells SI, René Leemans C, Wolthuis RMF and Brakenhoff RH:
Characterization of a head and neck cancer-derived cell line panel
confirms the distinct TP53-proficient copy number-silent subclass.
Oral Oncol. 98:53–61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cook RS, Garrett JT, Sánchez V, Stanford
JC, Young C, Chakrabarty A, Rinehart C, Zhang Y, Wu Y, Greenberger
L, et al: ErbB3 ablation impairs PI3K/Akt-dependent mammary
tumorigenesis. Cancer Res. 71:3941–3951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mishra R, Patel H, Alanazi S, Yuan L and
Garrett JT: HER3 signaling and targeted therapy in cancer. Oncol
Rev. 12:3552018.PubMed/NCBI
|
|
62
|
Garrett JT, Sutton CR, Kurupi R, Bialucha
CU, Ettenberg SA, Collins SD, Sheng Q, Wallweber J, Defazio-Eli L
and Arteaga CL: Combination of antibody that inhibits
ligand-independent HER3 dimerization and a p110α inhibitor potently
blocks PI3K signaling and growth of HER2+ breast cancers. Cancer
Res. 73:6013–6023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Milewska M, Cremona M, Morgan C, O'Shea J,
Carr A, Vellanki SH, Hopkins AM, Toomey S, Madden SF, Hennessy BT
and Eustace AJ: Development of a personalized therapeutic strategy
for ERBB-gene-mutated cancers. Ther Adv Med Oncol.
10:17588340177460402018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Akbari Dilmaghani N, Safaroghli-Azar A,
Pourbagheri-Sigaroodi A and Bashash D: The PI3K/Akt/mTORC signaling
axis in head and neck squamous cell carcinoma: Possibilities for
therapeutic interventions either as single agents or in combination
with conventional therapies. IUBMB Life. 73:618–642. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nunnery SE and Mayer IA: Management of
toxicity to isoform α-specific PI3K inhibitors. Ann Oncol. 30
(Suppl 10):x21–x26. 2019. View Article : Google Scholar
|
|
66
|
Chia S, Gandhi S, Joy AA, Edwards S, Gorr
M, Hopkins S, Kondejewski J, Ayoub JP, Califaretti N, Rayson D and
Dent SF: Novel agents and associated toxicities of inhibitors of
the pi3k/Akt/mtor pathway for the treatment of breast cancer. Curr
Oncol. 22:33–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Suh KJ, Sung JH, Kim JW, Han SH, Lee HS,
Min A, Kang MH, Kim JE, Kim JW, Kim SH, et al: EGFR or HER2
inhibition modulates the tumor microenvironment by suppression of
PD-L1 and cytokines release. Oncotarget. 8:63901–63910. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fiedler M, Schulz D, Piendl G, Brockhoff
G, Eichberger J, Menevse AN, Beckhove P, Hautmann M, Reichert TE,
Ettl T and Bauer RJ: Buparlisib modulates PD-L1 expression in head
and neck squamous cell carcinoma cell lines. Exp Cell Res.
396:1122592020. View Article : Google Scholar : PubMed/NCBI
|