Introduction
The Global Cancer Statistics for 2022 indicate that
nearly 20 million new cancer cases are expected in 2022, with 9.7
million cancer-related deaths anticipated in the same year. Cancer
remains a leading cause of mortality worldwide (1). Consequently, there is a pressing need
to identify novel biomarkers for the early diagnosis and prognosis
of cancer and to uncover potential molecular targets for therapy.
Research has demonstrated that miRNAs are closely linked to the
initiation and progression of cancer and serve as crucial
epigenetic regulators in cancer development (2).
MicroRNAs (miRNAs) are small endogenous non-coding
RNAs, typically ~22 nucleotides in length, that regulate
post-transcriptional gene expression in eukaryotes (3). MiRNAs are initially transcribed as
primary miRNAs by RNA polymerase II. These primary miRNAs possess a
localized hairpin structure and are cleaved by the Drosha-DGCR8
microprocessor complex subunit complex to form precursor miRNAs.
Subsequently, the precursor miRNAs are transported to the
cytoplasm, where they are processed by Dicer to generate mature
miRNAs. The mature miRNAs bind to Argonaute protein, forming
RNA-induced silencing complexes. These complexes generally bind to
the 3′-untranslated region (3′-UTR) of target mRNAs, leading to
miRNA degradation or the inhibition of protein translation
(4,5). As a result, miRNAs regulate various
important biological processes, including cell survival,
proliferation, autophagy and apoptosis. Dysregulation of miRNAs has
been implicated in the development of cancer (6).
Among the numerous miRNAs, miRNA-145-5p has gained
significant attention due to its altered expression across a wide
variety of cancers. Previous research indicated that miRNA-145-5p
levels are lower in prostate cancer cells compared to normal cells,
suggesting that its reduced expression may be linked to the
development of prostate cancer (7).
Furthermore, overexpression of miRNA-145-5p was shown to inhibit
epithelial-mesenchymal transition (EMT) and cell proliferation in
non-small cell lung cancer (NSCLC), while also enhancing the
sensitivity to pemetrexed in NSCLC (8). These results suggest that miRNA-145-5p
may be a valuable biomarker and therapeutic target.
The purpose of the present review was to highlight
the role of miRNA-145-5p in the progression of different cancers
and its effects on chemotherapy and radiotherapy in tumour cells.
The review also discusses the systemic delivery approaches for
miRNA-145-5p and evaluates its potential as a biomarker for
diagnosis, prognosis and treatment response.
Regulation of miRNA-145-5p in cancer
miRNA-145-5p is located on chromosome 5q33.1 and is
a member of the miRNA-145 family (9). It is typically transcribed as a
double-stranded primary transcript along with miRNA-143. The
epigenetic silencing or deletion of this region has been linked to
cancer initiation and progression (10). The abnormal expression of miRNAs in
cancer is often caused by the regulation of transcription factors
and epigenetic alterations, which disrupt the normally controlled
cellular RNA network. Several studies have shown that the
dysregulation of miRNA-145-5p in cancer is influenced by the
following factors:
i) Competing endogenous RNA (ceRNA). Long non-coding
RNAs (lncRNA) and circular RNA (circRNA) can act as ceRNAs for
miRNA target mRNAs. Both can function as upstream regulators of
miRNAs, binding to miRNAs and acting as sponges, which modulate
miRNA activity and reduce the suppressive effects of miRNAs on
target gene mRNAs. For instance, circRNA carboxypeptidase A4 and
lncRNA Hox transcript antisense intergenic RNA have been identified
as miRNA-145-5p sponges in cancer, decreasing miRNA-145-5p
expression (11,12).
ii) Transcription factors. Heat shock transcription
factor-1 (HSF-1) is a transcriptional regulator of miRNA-145-5p and
can enhance its inhibitory effect in peritoneal ovarian cancer
(13). The c-myb transcription
factor binds to the miRNA-145-5p promoter, activating its
transcription and increasing miRNA-145-5p expression in esophageal
cancer (14).
iii) DNA methylation. DNA methylation occurs at CpG
islands within the promoter region. In esophageal squamous cell
carcinoma, hypermethylation of the miRNA-145 promoter leads to
decreased expression of miRNA-145-5p. Furthermore, increased
methylation of the miRNA-145 promoter contributes to the silencing
of miRNA-145-5p expression, which facilitates brain metastasis in
lung cancer (15). Of note,
upstream regulators that enhance the methylation of the miRNA-145
promoter could themselves be downstream targets of miRNA-145
(16). Therefore, targeting these
upstream regulators to inhibit their expression and reduce
miRNA-145 promoter methylation may restore miRNA-145-5p expression
and help prevent disease progression (16).
iv) Other factors. Research has shown that hypoxia
triggers the upregulation of miRNA-145-5p expression in
glioblastoma, potentially through dependence on hypoxia-inducible
factor (HIF)-1 (17).
miRNA-145-5p in different types of
cancer
Currently, miRNA-145-5p is known as a tumour
suppressor in cholangiocarcinoma, cervical carcinoma,
retinoblastoma, renal cell carcinoma (RCC), oral squamous cell
carcinoma (OSCC) and osteosarcoma, as well as lung, breast,
gastric, pancreatic, ovarian, endometrial, bladder and prostate
cancer (18–31). However, in liver, esophageal and
colorectal cancer, miRNA-145-5p can act as both a tumour suppressor
and an oncogene (14,32–35).
Its complexity gives it potential as a therapeutic target.
miRNA-145-5p can regulate tumour cell proliferation, apoptosis,
metastasis, angiogenesis and other processes by targeting
downstream target genes (Fig. 1).
However, certain lncRNAs and circRNAs are upstream regulators of
miRNA-145-5p, which regulate miRNA-145-5p and downstream target
gene expression (Fig. 2). The roles
of miRNA-145-5p in different cancers are presented in Table I.
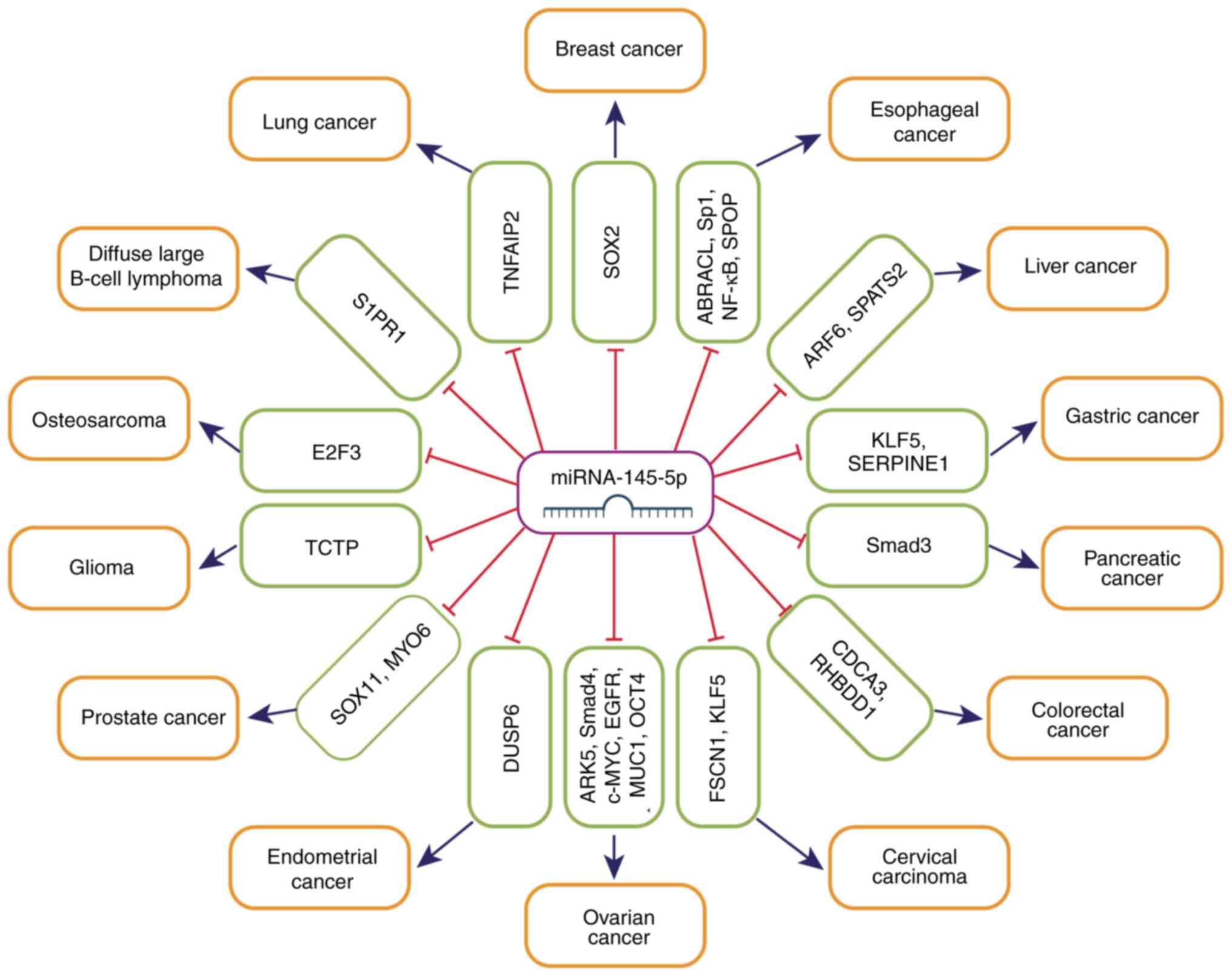 | Figure 1.Comprehensive illustration of the
interaction between miRNA-145-5p and its major target genes. miRNA,
microRNA. TNFAIP2, TNFα-induced protein 2; SOX2, sex determining
region Y-box2; ABRACL, ABPA C-terminal like; Sp1, specificity
protein 1; NF-κB, nuclear factor kappa-B; SPOP, speckled POZ
protein; ARF6, ADP-ribosylation factor 6; SPATS2,
spermatogenesis-associated serine-rich 2; KLF5, Krüppel-like factor
5; SERPINE1, serpin family E member 1; Smad3, smad family
member 3; CDCA3, cell division cycle-associated protein 3;
RHBDD1, rhomboid domain containing 1; FSCN1, fascin
actin-bundling protein 1; ARK5, AMPK-related protein kinase 5;
Smad4, smad family member 4; c-MYC, myelocytomatosis viral
oncogene homolog; EGFR, epidermal growth factor receptor;
MUC1, mucin 1; OCT4, octamer-binding transcription
factor 4; DUSP6, dual-specificity phosphatase 6; SOX11,
SRY-box transcription factor 11; MYO6, myosin VI; TCTP,
translationally controlled tumor protein; E2F3, E2F transcription
factor 3; S1PR1, sphingosine-1-phosphate receptor 1. |
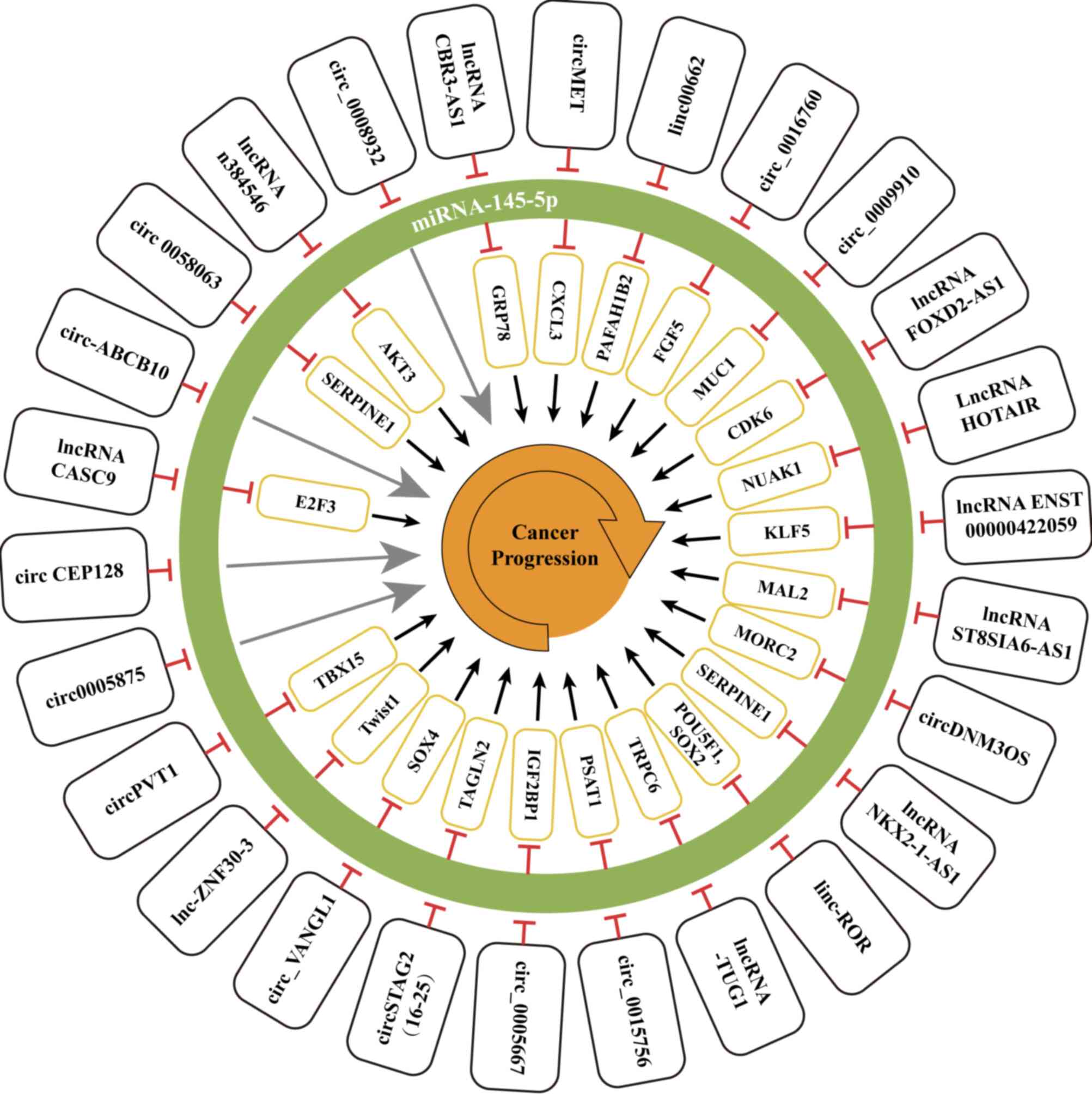 | Figure 2.Detailed overview of lncRNAs and
circRNAs as upstream regulators interacting with miRNA-145-5p in
malignant tumours. miRNA, microRNA; lncRNA, long non-coding RNA;
circRNA, circular RNA. circMET, circular RNA mesenchymal epithelial
transition factor receptor; CXCL3, C-X-C motif chemokine ligand 3;
linc00662, long intergenic RNA 00662; PAFAH1B2,
platelet-activating factor acetylhydrolase IB subunit beta;
circ_0016760, circRNA 0016760; FGF5, fibroblast growth factor-5;
MUC1, mucin 1; lncRNA FOXD2-AS1, lncRNA FOXD2 adjacent opposite
strand RNA 1; CDK6, cyclin-dependent kinase 6; lncRNA HOTAIR,
lncRNA Hox transcript antisense intergenic RNA; NUAK1, NUAK family
kinase 1; KLF5, Krüppel-like factor 5; ST8SIA6-AS1, ST8
α-N-acetyl-neuraminide α-2,8-sialyltransferase 6 antisense 1; MAL2,
Mal, T cell differentiation protein 2; circDNM3OS, circRNA DNM3
opposite strand/antisense RNA; MORC2, microrchidia family CW-type
zinc finger protein 2; lncRNA NKX2-1-AS1, lncRNA NK2 homeobox 1
antisense RNA 1; SERPINE1, serpin family E member 1; linc-ROR, long
intergenic non-protein coding RNA-regulator of reprogramming;
POU5F1, octamer-binding transcription factor; lncRNA-TUG1, lncRNA
taurine upregulated gene 1; TRPC6, transient receptor potential
cation channel subfamily C member 6; IGF2BP1, insulin-like growth
factor 2 mRNA-binding protein 1; circSTAG2(16–25), circRNA stromal
antigen 2(16–25); TAGLN2, transgelin-2; circ_VANGL1, circular RNA
VANGL1; SOX4, sex-determining region Y-related high-mobility group
box 4; lnc-ZNF30-3, lncRNA-zinc finger protein 30-3; Twist1,
Twist-related protein 1; circPVT1, circRNA plasmacytoma variant
translocation 1; TBX15, T-box transcription factor 15; circCEP128,
circRNA centrosomal protein 128; lncRNA CASC9, lncRNA cancer
susceptibility candidate 9; E2F3, E2F transcription factor 3;
circ-ABCB10, circRNA ATP binding cassette subfamily B member 10;
lncRNA CBR3-AS1, lncRNA carbonyl reductase 3 antisense RNA 1;
GRP78, glucose regulated protein 78 kD. |
 | Table I.Role of miRNA-145-5p in different
types of cancer. |
Table I.
Role of miRNA-145-5p in different
types of cancer.
| Type | Upstream
regulator | Target | Biological
function | Role | (Refs.) |
|---|
| Lung cancer | linc00662 | PAFAH1B2 | Inhibits cell
proliferation and colony formation | Tumour
suppressor | (18) |
|
|
Hsa_circ_0016760 | FGF5 | Inhibits cell
proliferation, migration and invasion | Tumour
suppressor | (36) |
|
| - | TNFAIP2 | Inhibits cell
migration and invasion | Tumour
suppressor | (37) |
|
| circMET | CXCL3 | Inhibits cell
proliferation, metastasis and immune evasion | Tumour
suppressor | (38) |
| Breast cancer | circ_0009910 | MUC1 | Inhibits cell
proliferation and migration | Tumour
suppressor | (19) |
|
| - | SOX2 | Inhibits breast
cancer progression | Tumour
suppressor | (39) |
| Esophageal
cancer | - | ABRACL | Inhibits cell
proliferation, migration and invasion | Tumour
suppressor | (40) |
|
| - | Sp1 and NF-κB | Inhibits cell
migration, invasion and EMT | Tumour
suppressor | (32) |
|
|
| SPOP | Promotes cell
proliferation, migration and immune evasion | Oncogene | (14) |
|
| lncRNA
FOXD2-AS1 | CDK6 | Inhibits cell
proliferation and invasion | Tumour
suppressor | (41) |
| Liver cancer | LncRNA HOTAIR | NUAK1 | Inhibits cell
migration, invasion and EMT | Tumour
suppressor | (12) |
|
| - | ARF6 | Inhibits cell
migration, invasion and metastasis | Tumour
suppressor | (42) |
|
| - | SPATS2 | Inhibits cell
proliferation and metastasis | Tumour
suppressor | (33) |
|
| LncRNA MEG3 | DAB2 | Promotes cell
metastasis and angiogenesis | Oncogene | (34) |
|
Cholangiocarcinoma | lncRNA
ST8SIA6-AS1 | MAL2 | Inhibits cell
growth and migration and promotes apoptosis | Tumour
suppressor | (20) |
|
| circDNM3OS | MORC2 | Inhibits cell
proliferation, migration, invasion and induces apoptosis | Tumour
suppressor | (43) |
| Gastric cancer | - | SERPINE1 | Inhibits cell
proliferation, migration and invasion | Tumour
suppressor | (9) |
|
| - | KLF5 | Promotes gastric
cancer differentiation | Tumour
suppressor | (21) |
|
| linc-ROR | POU5F1 and
SOX2 | Inhibits cell
growth, proliferation and migration | Tumour
suppressor | (44) |
| Pancreatic
cancer | - | Smad3 | Inhibits tumour
growth and promotes apoptosis | Tumour
suppressor | (22) |
| Colorectal
cancer | - | - | Inhibits
proliferation and invasion of colorectal cancer cells that have not
metastasized. Promotes proliferation and invasion of colorectal
cancer cells that develop metastasis | Tumour suppressor
and oncogene | (35) |
|
| lncRNA-TUG1 | TRPC6 | Inhibits cell
viability, proliferation and migration | Tumour
suppressor | (45) |
|
| - | CDCA3 | Inhibits cell
migration, invasion and EMT | Tumour
suppressor | (46) |
| Cervical
carcinoma | - | FSCN1 | Inhibits cell
migration, invasion and viability | Tumour
suppressor | (23) |
|
| - | KLF5 | Inhibits cell
proliferation, migration and invasion | Tumour
suppressor | (47) |
| Ovarian cancer | - | SMAD4 | Inhibits cell
proliferation and invasion | Tumour
suppressor | (24) |
|
| circ_0015756 | PSAT1 | Inhibits cell
growth, migration and invasion | Tumour
suppressor | (48) |
| Endometrial
cancer | - | DUSP6 | Inhibits cell
proliferation, migration and invasion, and promotes apoptosis | Tumour
suppressor | (25) |
| Bladder cancer | circ_VANGL1 | SOX4 | Inhibits cell
growth and promotes apoptosis | Tumour
suppressor | (26) |
|
| - | TAGLN2 | Inhibits cell
proliferation and migration | Tumour
suppressor | (49) |
| Prostate
cancer | lnc-ZNF30-3 | Twist1 | Inhibits cell
metastasis | Tumour
suppressor | (27) |
| Renal cell
carcinoma | CircPVT1 | TBX15 | Inhibits cell
growth and metastasis | Tumour
suppressor | (28) |
| Retinoblastoma | lncRNA CASC9 | E2F3 | Inhibits cell
proliferation, invasion and EMT | Tumour
suppressor | (29) |
| Oral squamous cell
carcinoma | circ-ABCB10 | - | Inhibits cell
viability, colony formation and migration | Tumour
suppressor | (30) |
| Osteosarcoma colony
formation | - | E2F3 | Inhibits cell
proliferation and suppressor | Tumour | (31) |
miRNA-145-5p as a tumour
suppressor
Lung cancer
Lung cancer is a common cancer, with ~2.5 million
new cases diagnosed worldwide in 2022 (1). Deletion of miRNA-145-5p was found to
lead to hyperactivation of HIF-2α, which in turn promotes
angiogenesis in lung cancer cells and is associated with poor
prognosis (50).
A study reported that miRNA-145-5p negatively
regulated TNFα-induced protein 2 levels through combining with its
3′UTR, thus reducing NSCLC cell viability and inhibiting their
migration and invasion (37). In
addition, circRNA mesenchymal epithelial transition factor receptor
(circMET) is associated with poor prognosis of NSCLC. CircMET
sponges miRNA-145-5p and promotes the expression of C-X-C motif
chemokine ligand 3, a downstream target gene of miRNA-145-5p, which
ultimately promotes NSCLC cell proliferation, metastasis and immune
evasion (38).
Breast cancer
Breast cancer is a frequent gynecological cancer,
with new breast cancer cases in females accounting for 11.6% of all
new cancers worldwide in 2022 (1).
It has been shown that miRNA-145-5p expression is decreased in both
breast cancer cells and tissues. miRNA-145-5p inhibits
paclitaxel-resistant breast cancer cell proliferation, migration
and invasion, while attenuating paclitaxel resistance by targeting
sex-determining region Y-box transcription factor 2 (SOX2)
(51).
In addition, circ_0009910 upregulates mucin 1 (MUC1)
expression and thus promotes breast cancer proliferation and
migration through binding to miRNA-145-5p, while overexpression of
miRNA-145-5p reverses this process (19). LncRNA ENST00000422059 also increases
Krüppel-like factor 5 (KLF5) protein expression via sponging of
miRNA-145-5p, promoting breast cancer cell proliferation and
inhibiting apoptosis (52).
Cholangiocarcinoma
Cholangiocarcinoma is a highly heterogeneous
malignant tumour of bile duct epithelial cells, prone to
metastasis, with an advanced 5-year survival rate of ~5% and a poor
prognosis (53). It was found that
lncRNA forkhead box D2 adjacent opposite strand RNA 1 [lncRNA
ST8SIA6-antisense 1 (AS1)] enhances cholangiocarcinoma cell
development and migration, specifically through combining with
miRNA-145-5p and upregulating Mal, T-cell differentiation protein 2
expression to promote cholangiocarcinoma progression (20). CircRNA hsa_circ_0005230 (circDNM3OS)
and MORC family CW-type zinc finger 2 (MORC2) were upregulated in
cholangiocarcinoma tissues, whereas miRNA-145-5p expression was
downregulated. CircDNM3OS can act as an miRNA-145-5p sponge to
upregulate MORC2 expression, induce cholangiocarcinoma glutamine
metabolism, promote cholangiocarcinoma cell proliferation,
migration and invasion, and reduce apoptosis, and overexpression of
miRNA-145-5p can reverse this process (43).
Gastric cancer
About 783,000 patients with gastric cancer died in
2018. Gastric cancer remains a great challenge that needs to be
urgently addressed globally (54).
miRNA-145-5p expression decreased within gastric cancer tissues and
cells. miRNA-145-5p negatively regulates serpin family E member 1
(SERPINE1) by combining with the 3′-UTR of SERPINE1 and inhibits
extracellular signal-regulated kinase-1/2 protein expression,
ultimately inhibiting gastric cancer cell proliferation, migration
and invasion (9). In addition,
miRNA-145-5p expression decreased within undifferentiated gastric
cancer compared with differentiated gastric cancer, suggesting that
miRNA-145-5p may be associated with the degree of malignancy of
gastric cancer. A further study found that miRNA-145-5p also
promotes gastric cancer differentiation and reduces gastric cancer
by targeting the KLF5-associated degree of malignancy (21). Teng et al (55) found that lncRNA NK2 homeobox-1-AS1
could bind to miRNA-145-5p, promote SERPINE1 expression and
activate the vascular endothelial growth factor receptor-2
signalling pathway to promote gastric cancer cell proliferation,
metastasis, invasion and angiogenesis.
Pancreatic cancer
Pancreatic cancer is an insidious malignancy and
most patients have developed metastases by the time it is detected
(56). It was found that
miRNA-145-5p expression decreased within pancreatic cancer tissues
relative to chronic pancreatitis or normal pancreatic tissues
(57). Ding et al (22) found that overexpression of
miRNA-145-5p in pancreatic cancer inhibited pancreatic cancer cell
proliferation and invasion, and promoted apoptosis, and the
regulatory mechanism was related to the inhibition of downstream
target gene Smad3 protein expression by miRNA-145-5p. These results
highlight that miRNA-145-5p may be utilized as a target in
pancreatic cancer.
Cervical carcinoma
Cervical carcinoma is a gynaecological malignancy
and according to global cancer statistics, there are 12.4 deaths
due to cervical cancer per 100,000 female individuals in certain
countries (58). Therefore, there
is a need to find new therapeutic strategies. miRNA-145-5p
expression decreased within cervical cancer tissues and cells, and
miRNA-145-5p overexpression suppressed their migration, invasion
and viability by targeting fascin 1, suggesting that miRNA-145-5p
is a promising anti-cervical cancer target (23).
Ovarian cancer
Approximately 207,252 deaths worldwide were
estimated to be related to ovarian cancer in 2020 (58). miRNA-145-5p expression significantly
decreases within ovarian cancer cells vs. healthy ovarian
epithelial cells, and miRNA-145-5p upregulation reduces their
proliferation while promoting their apoptosis; this result is
achieved by miRNA-145-5p by targeting the downstream target gene
AMP-activated protein kinase family member 5 (59). Smad4 protein is a signaling protein
whose dysfunction is closely associated with cancer (60). miRNA-145-5p prevents ovarian cancer
cell proliferation by inhibiting the expression of cell cycle
proteins E1, A2 and D1, and inhibits ovarian cancer cell migration
by targeting Smad4 (24).
Treatment of peritoneal metastatic ovarian cancer
cells using peritoneal heat infusion chemotherapy inhibited ovarian
cancer proliferation. The mechanism was associated with significant
upregulation miRNA-145-5p expression in ovarian cancer after
treatment, thereby inhibiting four downstream target genes, c-MYC,
EGFR, MUC1 and octamer-binding transcription factor 4 (OCT4); in
addition, HSF-1 acted as a transcriptional regulator in
miRNA-145-5p expression and inhibition of HSF-1 impaired the
inhibitory effect of miRNA-145-5p on ovarian cancer cells (13).
Endometrial carcinoma
Endometrial cancer is one of the most common
gynaecological malignancies in women, which affected ~66,200 female
individuals in the US in 2023 (61). It was shown that miRNA-145-5p
expression decreased within both cisplatin-resistant endometrial
cancer cells and tissues, and circ_0005667 sponged miRNA-145-5p but
increased miRNA-145-5p target gene insulin-like growth factor 2
mRNA-binding protein 1 (IGF2BP1) levels, which in turn enhanced the
resistance of endometrial cancer cells to cisplatin and promoted
cell proliferation, migration and invasion and inhibited apoptosis
(62). In addition, miRNA-145-5p
inhibits endometrial cancer cell proliferation, migration and
invasion, but promotes apoptosis, via targeting dual-specificity
phosphatase 6 and silencing its expression (25).
Bladder cancer
Bladder cancer is a highly heterogeneous disease
with ~200,000 deaths per year worldwide (58). It has been shown that circRNA
hsa_circ_0139697 [circSTAG2 (16–25)]
is highly expressed in bladder cancer cells, whereas miRNA-145-5p
is lowly expressed; high expression of circSTAG2 (16–25)
induces bladder cancer proliferation and invasion, and the specific
mechanism is related to circSTAG2 (16–25)
sponging miRNA-145-5p, which in turn upregulated transgelin-2
expression (63). In addition,
miRNA-145-5p can also be sponged by circRNA van Gogh-like 1, which
upregulated sex-determining region Y-related high-mobility group
box 4 (SOX4), promoted the viability of bladder cancer cells and
reduced bladder cancer sensitivity to adriamycin and apoptosis
(26).
Prostate cancer
Prostate cancer serves as a major factor inducing
male cancer-associated mortality worldwide, with new cases
accounting for 7.3% of all newly diagnosed cancers in 2020
(1). Neuroendocrine prostate cancer
is a specific subtype of prostate cancer that is highly malignant,
insensitive to endocrine therapy and associated with poor prognosis
and tumour progression (64). Low
expression of miRNA-145-5p predicts poor differentiation and poor
prognosis of prostate cancer; overexpression of miRNA-145-5p
suppresses proto-oncogene MYCN expression by targeting SOX11, and
then inhibit the neuroendocrine differentiation and cell
proliferation of prostate cancer, preventing it from
differentiating into prostate neuroendocrine carcinoma (65).
Myosin VI (MYO6) was found to be a protein involved
in cytoskeletal motility, which is associated with cancer
metastasis and invasion (66).
According to Armstrong et al (67), miRNA-145-5p expression decreased
within prostate cancer cell lines and MYO6 was the target of
miRNA-145-5p, while miRNA-145-5p negatively modulated MYO6
expression and downregulated the expression of waveform protein,
fibronectin 1 and actin α2, which in turn inhibited EMT and
significantly inhibited the proliferation and migration of prostate
cancer cells (67).
RCC
RCC is a cancer originating from the renal tubular
epithelium and its most common type is clear cell (cc)RCC (68). CircRNA plasmacytoma variant
translocation 1 can act as an miRNA-145-5p sponge and upregulates
T-box transcription factor 15 expression, which in turn promotes
ccRCC cell growth and metastasis (28). Circ0005875 expression was found to
be increased within both ccRCC tissues and cells, and it promoted
ccRCC cell proliferation, migration and invasion by sponging
miRNA-145-5p, and this may have increased the levels of a target
gene of miRNA-145-5p, zinc finger Ebox binding homeobox 2 (69).
Glioma
Glioma is the most frequent primary brain cancer,
and accurate and clean removal of the tumour is challenging due to
its invasiveness to the surrounding brain tissues (70). According to Zhang et al
(71), miRNA-145-5p expression
decreased within primary glioma stem cells, and its overexpression
suppressed transglial tumour stem cell proliferation while
promoting apoptosis, and the mechanism was related to the direct
targeting of translationally controlled tumour protein by
miRNA-145-5p, downregulating B-cell lymphoma-2 (Bcl-2) expression
and upregulating Bcl-2-associated X and cleaved caspase-3.
Overexpression of miRNA-145-5p promoted glioma cell sensitivity to
temozolomide, which in turn inhibited glioma cell proliferation,
the mechanism of which was that overexpression of miRNA-145-5p
inhibited the expression of ATP-binding cassette super-family G
member 2 (ABCG2) (73). ABCG2 is a
drug resistance protein and its decreased expression increases the
sensitivity of cancer cells to chemotherapeutic drugs (72); however, circCEP128 can target
miRNA-145-5p to reverse the inhibitiory effect of miRNA-145-5p on
glioma cells (73).
Retinoblastoma
Retinoblastoma is an intraocular malignant tumour
that develops in children, is frequently caused by genetic
mutations and is prone to metastasis and invasion (74). miRNA-145-5p expression decreased
within retinoblastoma relative to normal retinal tissue, while
lncRNA cancer susceptibility candidate 9 (CASC9) expression was
significantly increased. lncRNA CASC9 can bind to miRNA-145-5p and
then upregulate the expression of E2F transcription factor 3
(E2F3), the miRNA-145-5p target gene of upregulated N-cadherin and
Vimentin, and downregulated E-cadherin, and these changes enhanced
EMT, thus promoting retinoblastoma cell proliferation and invasion
and inhibiting their apoptosis (29).
OSCC
Owing to the prevalence of betel nut and tobacco,
OSCC has become a disease that makes it impossible to ignore, and
due to its rapid progression, more than half of the patients are
found to have metastases (75).
miRNA-145-5p expression decreased within OSCC tissues and cells,
and circ-ABCB10 was indicated to sponge miRNA-145-5p and inhibit
its expression, promoting OSCC cell proliferation and migration
(30). circ 0058063 expression was
significantly upregulated in OSCC cells. circ0058063 caused
SERPINE1 expression to be upregulated by binding to miRNA-145-5p,
which in turn promoted migration and proliferation, and facilitated
apoptosis of OSCC cells (76).
Thyroid cancer
Thyroid cancer is an endocrine system cancer and
papillary thyroid carcinoma (PTC) represents its major subtype,
accounting for ~85% of thyroid cancers (77). As discovered by Feng et al
(78), lncRNA n384546 expression
increased within PTC tissues and cells, which was related to tumour
size, lymph node metastasis and poor TNM staging of PTC. Further
studies suggested that lncRNA n384546 promoted PTC cell
proliferation, invasion and migration. Its mechanism was associated
with the lncRNA n384546 sponging and inhibiting the expression of
miRNA-145-5p, and upregulation of the expression of AKT
serine/threonine kinase 3, an oncogene that is a key protein in
several signalling pathways and promotes behaviours such as tumour
proliferation, metastasis and drug resistance (79).
Osteosarcoma
Osteosarcoma is a primary bone cancer in children
and adolescents, and most osteosarcomas infiltrate surrounding
tissues and metastasize; metastasis is a major challenge in the
treatment of osteosarcoma (80).
miRNA-145-5p could inhibit osteosarcoma proliferation via E2F3 and
by suppressing the expression of proteins such as cell cycle
protein D1, cyclin-dependent kinase 6 and cell cycle protein E
(31). Circ_0008932 sponges
miRNA-145-5p and inhibits its expression, promoting the migration
and invasion of osteosarcoma (81).
Of note, treatment of osteosarcoma Saos-2 cells with allicin
down-regulated lncRNA carbonyl reductase 3-AS1 expression,
upregulated miRNA-145-5p expression and inhibited target gene
glucose regulatory protein 78, causing increased expression of
light chain 3-II, Beclin1, CHOP, PERK, eIF2α and CD4+ T cells,
which activated endoplasmic reticulum stress, mitochondrial
autophagy and immune response, and promoted the apoptosis of
osteosarcoma cells (82).
Diffuse large B-cell lymphoma
(DLBCL)
DLBCL is the malignant tumour derived from mature
B-cells, with ~150,000 new cases each year (83). Gao and Ding (84) found that downregulation of
miRNA-145-5p was related to the survival of patients with DLBCL.
miRNA-145-5p can directly target sphingosine 1-phosphate receptor 1
(S1PR1), inhibiting the levels of AKT and STAT3 phosphorylation,
and thus inhibiting DLBCL cell proliferation, migration and
invasion. The miRNA-145-5p/S1PR1 axis brings new perspectives to
the treatment of DLBCL (84).
miRNA-145-5p acting as both an
oncogene and a tumour suppressor
Esophageal cancer
Esophageal cancer ranks seventh among cancers
worldwide; it has a high mortality with an estimated 544,000
esophageal cancer deaths in 2020 (85). Fan et al (40) found that miRNA-145-5p was negatively
related to ABRA C-terminal like (ABRACL). miRNA-145-5p inhibited
ABRACL expression, which in turn inhibited esophageal cancer cell
growth, invasion and migration (40). In addition, miRNA-145-5p targeted
nuclear factor κB and specificity protein 1 (Sp1) to inhibit
esophageal cancer cell EMT, migration and invasion (32).
Transcription activator C-myb promotes miRNA-145-5p
transcription and it subsequently targets speckled POZ protein to
upregulate programmed death receptor-ligand 1, while inhibiting
T-cell activity, promoting esophageal cancer cell growth and
invasion, and thereby inducing immune escape (14). miRNA-145-5p has dual roles in
esophageal cancer, possibly due to the different functions of
different downstream target genes.
Liver cancer
Hepatocellular carcinoma (HCC) is a cancer with a
high mortality rate and it is the most common histological type of
liver cancer; statistically, the number of liver cancer deaths
accounted for ~7.8% of global tumour deaths in 2022 (1). Wang et al (42) reported that miRNA-145-5p expression
decreased within HCC cells and tissues, which was related to poor
prognosis and metastatic features of patients with HCC.
miRNA-145-5p targeted ADP-ribosylation factor 6 and inhibited HCC
cell growth, invasion and migration (42). Furthermore,
spermatogenesis-associated serine-rich 2 (SPATS2) expression
increased within HCC, which is related to dismal survival and
prognosis. miRNA-145-5p directly targets SPATS2 and promotes HCC
cell apoptosis and cell cycle arrest in G1 phase (33).
Of note, lncRNA maternally expressed gene 3 could
inhibit M2 macrophage polarisation, and consequently HCC cell
proliferation, by sponging miRNA-145-5p and upregulating the
miRNA-145-5p downstream target gene disabled-2 to influence
metastasis and angiogenesis (34).
The reason for the dual role of miRNA-145-5p in HCC may be related
to the complex network of molecular interactions in cancer cells
and the function of targeting different downstream target
genes.
Colorectal cancer
Colorectal cancer is a frequent digestive tract
cancer. In 2020, epidemiological statistics showed that ~935,000
patients died due to colorectal cancer (58). miRNA-145-5p expression decreased
within colorectal cancer cells and tissues, and overexpression of
miRNA-145-5p inhibited colorectal cancer cell proliferation,
metastasis and EMT, which was associated with miRNA-145-5p
inhibiting the expression of a downstream target gene, cell
division cycle-associated protein 3 expression (46). Niu et al (86) discovered that miRNA-145-5p
downregulation was associated with poor prognosis in colorectal
cancer. miRNA-145-5p could target rhomboid domain containing 1 and
inhibit the EGFR signalling pathway, which in turn suppressed
colorectal cancer cell growth, migration and invasion, but induced
cell apoptosis. Cheng et al (35) found that overexpression of
miRNA-145-5p in non-metastatic colorectal cancer cells (SW480
cells) suppressed nodal cell growth and their invasive ability,
while miRNA-145-5p overexpression in advanced colorectal cancer
that had already metastasized (SW620 cells) promoted the
proliferation and invasive ability, suggesting the role of
miRNA-145-5p as the tumour suppressor of early-stage colorectal
cancers without metastasis and as an oncogene in advanced
colorectal cancers that have already metastasized (35). The potential mechanisms underlying
the dual role of miRNA-145-5p in colorectal cancer may be related
to different tumour microenvironments, tumour stages, cell types
and genetic backgrounds and this deserves further study.
miRNA-145-5p as a biomarker
Advances in sequencing technologies now allow for
the precise and quantitative detection of miRNA-145-5p,
underscoring its potential as a biomarker. Numerous studies have
confirmed that miRNA-145-5p holds promise as a biomarker for cancer
diagnosis, prognosis and prediction of treatment response,
providing valuable insights for cancer diagnosis and therapy. A
summary of these studies is provided in Table II.
 | Table II.miRNA-145-5p as a biomarker. |
Table II.
miRNA-145-5p as a biomarker.
| Type | Direction of
deregulated expression | Indication | (Refs.) |
|---|
| Colorectal
cancer | Down | Low expression is
associated with poor prognosis | (86) |
| Prostate
cancer | Down | Low expression is
associated with poor prognosis | (67) |
| Breast cancer | Down | Low expression is
associated with low survival in breast cancer patients | (87) |
|
| Down | Low expression is
associated with the achievement of complete pathological remission
in breast cancer treated with cisplatin chemotherapy | (88) |
| Chronic lymphocytic
leukemia | Down | Diagnosis of
chronic lymphocytic leukaemia | (89) |
| Glioblastoma | Down | Diagnosis of
glioblastoma; low expression implies poor prognosis | (90) |
| Ovarian cancer | Down | Low expression is
associated with poor prognosis and higher staging | (91) |
Biomarkers for diagnosis
It was found that miRNA-145-5p expression was
significantly reduced in glioblastoma compared to normal tissues,
with receiver operating characteristic analysis showing that
miRNA-145-5p could potentially be used as a diagnostic marker for
glioblastoma, yielding an area under the curve of 0.895 (90). Furthermore, a combination of three
miRNAs (miRNA-145-5p, miRNA-218-5p and miRNA-34a-5p) in urine was
capable of distinguishing precancerous cervical cancer from healthy
individuals as well as patients with cancer (92). By contrast, miRNA-145-5p expression
was significantly lower in chronic lymphocytic leukaemia, and its
expression levels could differentiate healthy individuals from
those with chronic lymphocytic leukaemia (89). In addition, miRNA-145-5p was able to
differentiate colorectal tumours from adjacent tissues (93). These findings suggest that
miRNA-145-5p has significant potential as a biomarker for cancer
diagnosis.
Biomarkers for prognosis
High expression of miRNA-145-5p has been associated
with improved patient outcomes in several cancers, including lung
cancer (18), prostate cancer
(67), breast cancer (87), ovarian cancer (91) and HCC (42). By contrast, low miRNA-145-5p
expression may serve as a biomarker for stem cell stemness in
breast cancer, which is associated with a worse prognosis in this
disease (87). In addition,
patients with colorectal cancer with low miRNA-145-5p expression
had a death risk 10 times higher than those with high miRNA-145-5p
expression levels (94). However,
in metastatic colorectal cancer, higher miRNA-145-5p expression was
linked to poorer prognosis, suggesting a dual role of miRNA-145-5p
in colorectal cancer (35). In
summary, miRNA-145-5p is an effective prognostic biomarker,
allowing for clinical predictions based on its expression levels in
specific cancers.
Biomarkers of therapeutic
response
Due to the heterogeneity of cancers, many of which
show resistance or no response to treatments, identifying
biomarkers of therapeutic response is critical. It was found that
miRNA-145-5p expression was significantly reduced in patients with
breast cancer who achieved complete pathological remission after
treatment with cisplatin or adriamycin compared to those who did
not respond to these treatments, suggesting that a decrease in
miRNA-145-5p levels after treatment may serve as an indicator of
treatment effectiveness (88).
Furthermore, a support vector machine model using four miRNAs,
including miRNA-145-5p, demonstrated an accuracy of 87.3% in
distinguishing responders from non-responders to treatment in
esophageal squamous cell carcinoma pathology (95). These findings suggest that
miRNA-145-5p could be a valuable biomarker for assessing
therapeutic response in certain cancers.
miRNA-145-5p in chemotherapy and
radiotherapy
Chemotherapy and radiotherapy are key treatments for
cancer. While patients may initially show good responses, cancer
cells often develop resistance over time, leading to decreased
effectiveness or relapse. Therefore, overcoming drug resistance
remains a central issue in cancer research. Recent studies have
indicated that miRNA-145-5p is involved in resistance to
chemotherapy and radiotherapy in cancer, suggesting that
miRNA-145-5p mimics or inhibitors could enhance the efficacy of
these treatments. This section explores the role of miRNA-145-5p in
platinum-based chemotherapy resistance and its relationship with
radiotherapy in cancer (Table
III).
 | Table III.Effect of miRNA-145-5p on
chemotherapy. |
Table III.
Effect of miRNA-145-5p on
chemotherapy.
|
| Cancer type | Role | Target | (Refs.) |
|---|
| Platinum | Endometrial
carcinoma | Increases cisplatin
sensitivity | IGF2BP1 | (62) |
|
| Ovarian cancer | Promotes cisplatin
sensitivity | - | (13) |
|
| Non-small cell lung
cancer | Reverses cisplatin
resistance | KLF4 | (95) |
|
| Colorectal
cancer | Promotes
oxaliplatin sensitivity | - | (96) |
| Pemetrexed | Non-small cell lung
cancer | Increases
pemetrexed sensitivity | Sp1 | (8) |
| Paclitaxel | Breast cancer | Promotes paclitaxel
sensitivity | SOX2 | (50) |
| Temozolomide | Glioma | Promotes
temozolomide sensitivity | - | (72) |
| 5-Fluorouracil | Gastric cancer | Promotes
5-fluorouracil sensitivity | - | (97) |
| Sorafenib | Hepatocellular
carcinoma | Promotes sorafenib
sensitivity | HDAC11 | (98) |
Platinum
Platinum-based chemotherapeutic agents, such as
cisplatin, oxaliplatin and carboplatin, are widely used in cancer
therapy. It has been shown that miRNA-145-5p expression is reduced
in both tissues and cells from cisplatin-resistant endometrial
cancer. miRNA-145-5p enhances the sensitivity of
cisplatin-resistant cells to cisplatin by targeting IGF2BP1, and
silencing miRNA-145-5p reverses this effect (62). In addition, increases in
miRNA-145-5p levels led to the downregulation of four downstream
target genes, c-MYC, EGFR, OCT4 and MUC1, which boosted the
sensitivity and anticancer effect of intraperitoneal hyperthermic
chemotherapy (cisplatin) in ovarian cancer (13). Similarly, in NSCLC, miRNA-145-5p
reversed cisplatin resistance in drug-resistant cells by targeting
KLF4 (97). On the other hand,
downregulation of miRNA-145-5p expression was associated with
increased resistance to oxaliplatin in colorectal cancer cells
(97). These findings suggest that
boosting miRNA-145-5p expression alongside platinum-based drugs
could offer a potential therapeutic approach for cancer
treatment.
Other chemotherapy drugs
miRNA-145-5p has been shown to enhance sensitivity
to various chemotherapeutic agents. For instance, it increased the
sensitivity of glioma to temozolomide (73). SOX2, a protein linked to cancer
growth and metastasis, was inhibited by miRNA-145-5p in breast
cancer cells, where it reduced resistance to paclitaxel in
drug-resistant cells by suppressing SOX2-driven tumour
proliferation, migration and invasion (51). In pemetrexed-resistant NSCLC,
miRNA-145-5p target Sp1 promoted E-cadherin expression, and reduced
snail family transcriptional repressor 1 and zinc finger E-box
binding homeobox 1 levels, ultimately reversing resistance to
pemetrexed and inhibiting EMT (8).
In addition, miRNA-145-5p is known to suppress histone deacetylase
11, enhancing sorafenib sensitivity in HCC (99).
Radiotherapy
Regarding radiotherapy, miRNA-145-5p expression was
significantly elevated in patients with colon cancer after 30
radiation treatments, indicating its potential tumour-suppressive
role in colon cancer radiotherapy (100). Furthermore, circ_0000392 can bind
to miRNA-145-5p to influence the regulator of kinase like
protein/MAPK pathway and decrease the sensitivity of cervical
cancer cells to radiotherapy (101), suggesting that miRNA-145-5p may
inhibit cervical cancer cell resistance to radiotherapy.
Systemic delivery strategies for
miRNA-145-5p
Delivering therapeutic miRNAs directly to tumours
remains a major challenge in miRNA-based cancer therapies due to
their vulnerability to enzymatic degradation. However, exosomal
vectors, viral vectors and nanoparticles represent promising
methods for effective miRNA delivery (102).
Exosomal vectors
Exosomes are extracellular vesicles, ranging in size
from 30 to 100 nm, surrounded by a lipid bilayer, and are crucial
for intercellular communication. A study by Ding et al
(22) demonstrated that exosomes
derived from human umbilical cord mesenchymal stromal cells can
deliver miRNA-145-5p into pancreatic ductal carcinoma, leading to a
significant increase in miRNA-145-5p expression. This upregulation
inhibited pancreatic ductal carcinoma cell proliferation and
invasion and promoted apoptosis, suggesting that exosomal delivery
of miRNA-145-5p using human umbilical cord mesenchymal stromal
cells could be a promising therapeutic approach for pancreatic
ductal carcinoma (22).
Viral vectors
Currently, viral vectors frequently used for
delivering miRNAs include adeno-associated viruses, lentiviruses
and adenoviruses (103). Sun et
al (104) developed a
lentiviral vector modified with liposome to create a
liposome-lentivirus hybrid, which efficiently delivered
miRNA-145-5p to liver cancer stem cells, resulting in its
overexpression. This overexpression inhibited the Wnt/β-catenin
signaling pathway, thereby suppressing self-renewal, migration and
invasion of liver cancer stem cells by targeting collagen type IV
alpha 3 chain (104). This
experiment showed that using liposome-lentiviral hybrid vectors to
deliver miRNA-145-5p is a viable method. However, the immunogenic
potential and risk of mutations with viral vectors have prompted
the search for safer and more effective alternatives.
Nanoparticle vectors
Nanoparticle drug delivery systems are composed of
nanoscale particles, typically ranging from 1 to 1,000 nm, made
from pharmaceutical materials and drugs. These systems have
advantages such as targeted delivery, controlled release, efficient
cellular uptake and enhanced drug stability (105).
A poly-l-lysine functionalized melanin nanoparticle
(MNP-PLL)/miRNA-145-5p system was developed (106), which successfully transfected
miRNA-145-5p into laryngeal squamous cell carcinoma cells, leading
to a significant increase in miRNA-145-5p expression. It was
further demonstrated that combining the MNP-PLL/miRNA-145-5p system
with photothermal therapy effectively inhibited the migration of
laryngeal squamous cell carcinoma cells, offering a novel strategy
for combining gene-targeted therapy with photothermal treatment in
cancer (106).
Dai et al (107) created a modular peptide probe
nanoparticle that efficiently delivered miRNA-145-5p into ovarian
cancer cells, increasing miRNA-145-5p levels and inducing apoptosis
in the cancer cells.
Ultrasound-targeted microbubble destruction (UTMD)
is a non-invasive method that enhances drug delivery to cells and
tissues, improving the efficiency of nanoparticle delivery systems
(108). Ren et al (109) applied UTMD to significantly boost
miRNA-145-5p delivery to breast cancer cells, increasing its
expression. This upregulation of miRNA-145-5p bound to and
inhibited actin gamma 1 expression, resulting in reduced growth,
migration and invasion of breast cancer cells (109).
Although current research indicates that systemic
delivery methods for miRNA-145-5p effectively target tumour cells
and enhance their expression, several challenges still exist with
these delivery systems. One major issue is the need to balance
maintaining efficient delivery with managing potential toxicity
from the vectors themselves or due to overdosing. For instance,
viral vectors, which integrate into the host genome, can trigger
immune responses, both innate and adaptive, resulting in cytotoxic
damage. In addition, cationic nanoparticles have been shown to
provoke immune reactions and excessive cationic components can
cause the nanocarriers to break down at the glomerular basement
membrane, leading to the clearance of miRNA by the kidneys
(110). Another concern is
off-target effects, as miRNAs can influence multiple genes
simultaneously, leading to unintended regulatory effects.
Furthermore, when viral vectors integrate into or near undesirable
genomic regions, they may disrupt gene expression and activate
proto-oncogenes, potentially contributing to the development of
other cancers (111). As such,
further research is necessary to address these issues and minimize
systemic adverse reactions.
Controversies
This study provides an overview of miRNA-145-5p's
role in various cancers, evaluating its potential as a biomarker,
its effect on radiotherapy and chemotherapy, and its systemic
delivery strategies, all of which may assist in cancer diagnosis
and treatment. However, as an miRNA, miRNA-145-5p is influenced by
environmental factors and it appears to have a dual role as both a
tumour suppressor and an oncogene in cancers such as esophageal,
colorectal, and liver cancer. This duality may be due to miRNAs
targeting distinct downstream genes, which can lead to conflicting
findings in research. Therefore, it is crucial to investigate the
relationship between miRNA-145-5p and its multiple downstream
target genes across different cancer types.
Furthermore, there are certain inconsistencies in
the reported expression levels of miRNA-145-5p. For instance,
variable results have been reported regarding its expression in
esophageal cancer cells and tissues (14,40),
as well as in plasma, serum and tissues of patients with breast
cancer (51,112,113). These discrepancies are not easily
explained by a single mechanism but may involve several factors.
First, specific cancers may selectively release certain miRNAs, and
miRNAs in circulating fluids could show higher levels in serum or
plasma, while their expression in tissues or cells may be reduced
(114). In addition, miRNAs may be
secreted into the extracellular space and incorporated into
extracellular vesicles, such as exosomes, to avoid degradation by
circulating fluids, leading to higher expression levels (115). Variations in the tumour
microenvironment could also contribute to differences in miRNA
expression in both cells and circulation (116). These conflicting results may stem
from differences in cancer cell lines, sample sizes, study
populations and cancer stages, and additional research is needed to
better understand these variations.
Conclusions
This review assessed the roles of miRNA-145-5p in
various cancer types. It was found that miRNA-145-5p generally acts
as a tumour suppressor in most cancers, although it demonstrates a
dual role in liver, esophageal and colorectal cancers.
Mechanistically, miRNA-145-5p can directly target its downstream
genes or be regulated by upstream factors, such as lncRNAs and
circRNAs, thereby influencing cancer development. In addition,
miRNA-145-5p has potential as a biomarker for cancer diagnosis,
prognosis and treatment response. It can also impact the
sensitivity to chemotherapy and radiotherapy, and the use of
miRNA-145-5p mimics or inhibitors could potentially overcome drug
resistance in certain cancers. Finally, systemic delivery methods
for miRNA-145-5p are promising for advancing effective miRNA-based
therapies targeting tumours. Together, these findings provide
comprehensive evidence that could inform future strategies for
cancer treatment and drug development.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZC performed the literature search, wrote major
parts of the manuscript, edited the manuscript and prepared the
figures and tables. YQ conceptualized the study and oversaw the
process. Data authentication is not applicable. Both authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang T, Hu Y, Yang N, Yu S and Pu X: The
microRNA-34 family and its functional role in lung cancer. Am J
Clin Oncol. 47:448–457. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morales-Martínez M and Vega MI: Role of
MicroRNA-7 (MiR-7) in cancer physiopathology. Int J Mol Sci.
23:90912022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saikia M, Paul S and Chakraborty S: Role
of microRNA in forming breast carcinoma. Life Sci. 259:1182562020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roufayel R and Kadry S. MicroRNAs: Crucial
regulators of stress. Microrna. 9:93–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwok ZH, Zhang B, Chew XH, Chan JJ, Teh V,
Yang H, Kappei D and Tay Y: Systematic analysis of intronic mirnas
reveals cooperativity within the multicomponent FTX locus to
promote colon cancer development. Cancer Res. 81:1308–1320. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun J, Deng L and Gong Y: MiR-145-5p
inhibits the invasion of prostate cancer and induces apoptosis by
inhibiting WIP1. J Oncol. 2021:44127052021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang WW, Wang BY, Chen SH, Chien PJ, Sheu
GT and Lin CH: miR-145-5p targets Sp1 in non-small cell lung cancer
cells and links to BMI1 induced pemetrexed resistance and
epithelial-mesenchymal transition. Int J Mol Sci. 23:153522022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bai HX, Qiu XM, Xu CH and Guo JQ:
MiRNA-145-5p inhibits gastric cancer progression via
the serpin family E member 1-extracellular signal-regulated
kinase-1/2 axis. World J Gastrointest Oncol. 16:2123–2140. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bellissimo T, Tito C, Ganci F, Sacconi A,
Masciarelli S, Di Martino G, Porta N, Cirenza M, Sorci M, De
Angelis L, et al: Argonaute 2 drives miR-145-5p-dependent gene
expression program in breast cancer cells. Cell Death Dis.
10:172019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Liu W, Huang T, Li J, Hu H, Xu X
and Fan Z: CircCPA4 induces ASCT2 expression to promote tumor
property of non-small cell lung cancer cells in a
miR-145-5p-dependent manner. Thorac Cancer. 15:764–777. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu DX, Jin Y, Wang BR, Jiao Y, Zhang CK,
Guo ZH, Hu SZ and Li N: LncRNA HOTAIR enhances
epithelial-to-mesenchymal transition to promote the migration and
invasion of liver cancer by regulating NUAK1 via epigenetic
inhibition miR-145-5p expression. J Cancer. 14:2329–2343. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Agostino S, Canu V, Donzelli S, Pulito
C, Sacconi A, Ganci F, Valenti F, Goeman F, Scalera S, Rollo F, et
al: HSF-1/miR-145-5p transcriptional axis enhances hyperthermic
intraperitoneal chemotherapy efficacy on peritoneal ovarian
carcinosis. Cell Death Dis. 14:5352023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Wang X, Li Y, Han J, Gao X, Li S
and Wang F: c-Myb facilitates immune escape of esophageal
adenocarcinoma cells through the miR-145-5p/SPOP/PD-L1 axis. Clin
Transl Med. 11:e4642021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donzelli S, Mori F, Bellissimo T, Sacconi
A, Casini B, Frixa T, Roscilli G, Aurisicchio L, Facciolo F,
Pompili A, et al: Epigenetic silencing of miR-145-5p contributes to
brain metastasis. Oncotarget. 6:35183–35201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu S, Zhang Y, Zhou G and Liu A:
Bidirectional negative feedback actions of DNMT3A and miR-145 in
regulating autophagy in cardiac fibroblasts and affecting
myocardial fibrosis. J Bioenerg Biomembr. 55:341–352. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agrawal R, Pandey P, Jha P, Dwivedi V,
Sarkar C and Kulshreshtha R: Hypoxic signature of microRNAs in
glioblastoma: Insights from small RNA deep sequencing. BMC
Genomics. 15:6862014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu ZY, Peng J, Shi ZZ, Chen XL, Cheng HZ,
Wang H, Wang Y, Wang GP, Jiang W and Peng H: Silencing linc00662
inhibits cell proliferation and colony formation of lung cancer
cells via regulating the miR-145-5p-PAFAH1B2 axis. Biochem Cell
Biol. 99:330–338. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abtin M, Nafisi N, Hosseinzadeh A,
Kadkhoda S, Omranipour R, Sahebi L, Razipour M, Ghafouri-Fard S and
Shakoori A: Inhibition of breast cancer cell growth and migration
through siRNA-mediated modulation of circ_0009910/miR-145-5p/MUC1
axis. Noncoding RNA Res. 9:367–375. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He J, Yan H, Wei S and Chen G: LncRNA
ST8SIA6-AS1 promotes cholangiocarcinoma progression by suppressing
the miR-145-5p/MAL2 axis. Onco Targets Ther. 14:3209–3223. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou T, Chen S and Mao X: miR-145-5p
affects the differentiation of gastric cancer by targeting KLF5
directly. J Cell Physiol. 234:7634–7644. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y,
Cui Q, Mei W and Li F: Exosomes derived from human umbilical cord
mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit
pancreatic ductal adenocarcinoma progression. Cancer Lett.
442:351–361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He S, Yu G, Peng K and Liu S:
MicroRNA-145-5p suppresses fascin to inhibit the invasion and
migration of cervical carcinoma cells. Mol Med Rep. 22:5282–5292.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Zhang X, Li W and Chen Y:
MicroRNA-145-5p regulates the proliferation of epithelial ovarian
cancer cells via targeting SMAD4. J Ovarian Res. 13:542020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Men Y, Zhang L and Ai H: MicroRNA-145-5p
over-expression suppresses proliferation, migration and invasion
and promotes apoptosis of human endometrial cancer cells by
targeting dual specific phosphatase 6. Nan Fang Yi Ke Da Xue Xue
Bao. 40:61–66. 2020.(In Chinese). PubMed/NCBI
|
|
26
|
Zhu J and Zhang F: Circular RNA VANGL1
knockdown suppressed viability, promoted apoptosis, and increased
doxorubicin sensitivity through targeting miR-145-5p to regulate
SOX4 in bladder cancer cells. Open Med (Wars). 16:1010–1021. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Hars M, Castro-Vega LJ, Rajabi F,
Tabatadze D, Romero M, Pinskaya M and Groisman I: Pro-tumorigenic
role of lnc-ZNF30-3 as a sponge counteracting miR-145-5p in
prostate cancer. Biol Direct. 18:382023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng Z, Chen Z, Zhong Q, Zhu D, Xie Y,
Shangguan W and Xie W: CircPVT1 promotes progression in clear cell
renal cell carcinoma by sponging miR-145-5p and regulating TBX15
expression. Cancer Sci. 112:1443–1456. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang T, Yang J, Gong F, Li L and Li A:
Long non-coding RNA CASC9 promotes the progression of
retinoblastoma via interacting with miR-145-5p. Cell Cycle.
19:2270–2280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen F, Li XH, Liu C, Zhang Y and Wang RJ:
Circ-ABCB10 accelerates the malignant progression of oral squamous
cell carcinoma by absorbing miRNA-145-5p. Eur Rev Med Pharmacol
Sci. 24:681–690. 2020.PubMed/NCBI
|
|
31
|
Li H, Pan R, Lu Q, Ren C, Sun J, Wu H, Wen
J and Chen H: MicroRNA-145-5p inhibits osteosarcoma cell
proliferation by targeting E2F transcription factor 3. Int J Mol
Med. 45:1317–1326. 2020.PubMed/NCBI
|
|
32
|
Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J and
Shi ZZ: miR-145-5p suppresses tumor cell migration, invasion and
epithelial to mesenchymal transition by regulating the Sp1/NF-κB
signaling pathway in esophageal squamous cell carcinoma. Int J Mol
Sci. 18:18332017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong G, Zhang S, Shen S, Sun L, Wang X,
Wang H, Wu J, Liu T, Wang C, Wang H, et al: SPATS2, negatively
regulated by miR-145-5p, promotes hepatocellular carcinoma
progression through regulating cell cycle. Cell Death Dis.
11:8372020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei Q, Liu G, Huang Z, Huang Y, Huang L,
Huang Z, Wu X, Wei H and Pu J: LncRNA MEG3 inhibits tumor
progression by modulating macrophage phenotypic polarization via
miR-145-5p/DAB2 axis in hepatocellular carcinoma. J Hepatocell
Carcinoma. 10:1019–1035. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng X, Shen T, Liu P, Fang S, Yang Z, Li
Y and Dong J: mir-145-5p is a suppressor of colorectal cancer at
early stage, while promotes colorectal cancer metastasis at late
stage through regulating AKT signaling evoked EMT-mediated anoikis.
BMC Cancer. 22:11512022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu Z, Wu Q, Zhang M, Tong J, Zhong B and
Yuan K: Hsa_circ_0016760 exacerbates the malignant development of
non-small cell lung cancer by sponging miR-145-5p/FGF5. Oncol Rep.
45:501–512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Song Y, Yu B and Yu Y: TNFAIP2
promotes non-small cell lung cancer cells and targeted by
miR-145-5p. DNA Cell Biol. 39:1256–1263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pei X, Chen SW, Long X, Zhu SQ, Qiu BQ,
Lin K, Lu F, Xu JJ, Zhang PF and Wu YB: circMET promotes NSCLC cell
proliferation, metastasis, and immune evasion by regulating the
miR-145-5p/CXCL3 axis. Aging (Albany NY). 12:13038–13058. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang W, Zhang X, Tan W, Gao J, Pan L, Ye
X, Chen L and Zheng W: miR-145-5p suppresses breast cancer
progression by inhibiting SOX2. J Surg Res. 236:278–287. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan S, Chen P and Li S: miR-145-5p
inhibits the proliferation, migration, and invasion of esophageal
carcinoma cells by targeting ABRACL. Biomed Res Int.
2021:66925442021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi W, Gao Z, Song J and Wang W: Silence
of FOXD2-AS1 inhibited the proliferation and invasion of esophagus
cells by regulating miR-145-5p/CDK6 axis. Histol Histopathol.
35:1013–1021. 2020.PubMed/NCBI
|
|
42
|
Wang S, Wang T and Gu P: microRNA-145-5p
inhibits migration, invasion, and metastasis in hepatocellular
carcinoma by inhibiting ARF6. Cancer Manag Res. 13:3473–3484. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Su Y, Yu T, Wang Y, Huang X and Wei X:
Circular RNA circDNM3OS functions as a miR-145-5p sponge to
accelerate cholangiocarcinoma growth and glutamine metabolism by
upregulating MORC2. Onco Targets Ther. 14:1117–1129. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu X, Ma X, Jia Y, Wu X and Yan Z:
Linc-ROR regulates POU5F1 and SOX2 b competitively binding
miR-145-5p to affect the proliferation and migration of gastric
cancer cells. Cell Transplant. 32:96368972311789022023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X, Bai X, Yan Z, Guo X and Zhang Y:
The lncRNA TUG1 promotes cell growth and migration in colorectal
cancer via the TUG1-miR-145-5p-TRPC6 pathway. Biochem Cell Biol.
99:249–260. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Q, Zhou L, Ye X, Tao M and Wu J:
miR-145-5p suppresses proliferation, metastasis and EMT of
colorectal cancer by targeting CDCA3. Pathol Res Pract.
216:1528722020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cao H, Pan G, Tang S, Zhong N, Liu H, Zhou
H, Peng Q and Zou Y: miR-145-5p regulates the proliferation,
migration and invasion in cervical carcinoma by targeting KLF5.
Onco Targets Ther. 13:2369–2376. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pan Y, Huang Q, Peng X, Yu S and Liu N:
Circ_0015756 promotes ovarian cancer progression via the
miR-145-5p/PSAT1 axis. Reprod Biol. 22:1007022022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang H, Jiang M, Liu Q, Han Z, Zhao Y and
Ji S: miR-145-5p inhibits the proliferation and migration of
bladder cancer cells by targeting TAGLN2. Oncol Lett. 16:6355–6360.
2018.PubMed/NCBI
|
|
50
|
Tsai YM, Wu KL, Chang YY, Chang WA, Huang
YC, Jian SF, Tsai PH, Lin YS, Chong IW, Hung JY and Hsu YL: Loss of
miR-145-5p causes ceruloplasmin interference with PHD-Iron axis and
HIF-2α stabilization in lung adenocarcinoma-mediated angiogenesis.
Int J Mol Sci. 21:50812020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guan X and Guan Y: miR-145-5p attenuates
paclitaxel resistance and suppresses the progression in
drug-resistant breast cancer cell lines. Neoplasma. 67:972–981.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhu Y, Ren J, Wu X, Zhang Y, Wang Y, Xu J,
Tan Q, Jiang Y and Li Y: lncRNA ENST00000422059 promotes cell
proliferation and inhibits cell apoptosis in breast cancer by
regulating the miR-145-5p/KLF5 axis. Acta Biochim Biophys Sin
(Shanghai). 55:1892–1901. 2023.PubMed/NCBI
|
|
53
|
Li Y, Yu J, Zhang Y, Peng C, Song Y and
Liu S: Advances in targeted therapy of cholangiocarcinoma. Ann Med.
56:23101962024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI
|
|
55
|
Teng F, Zhang JX, Chen Y, Shen XD, Su C,
Guo YJ, Wang PH, Shi CC, Lei M, Cao YO and Liu SQ: LncRNA
NKX2-1-AS1 promotes tumor progression and angiogenesis via
upregulation of SERPINE1 expression and activation of the VEGFR-2
signaling pathway in gastric cancer. Mol Oncol. 15:1234–1255. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Du Q, Zhang M, Gao A, He T and Guo M:
Epigenetic silencing ZSCAN23 promotes pancreatic cancer growth by
activating Wnt signaling. Cancer Biol Ther. 25:23029242024.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dobre M, Herlea V, Vlăduţ C, Ciocîrlan M,
Balaban VD, Constantinescu G, Diculescu M and Milanesi E:
Dysregulation of miRNAs targeting the IGF-1R pathway in pancreatic
ductal adenocarcinoma. Cells. 10:18562021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu L, Zhou WQ, Yuan LN, Li J and Pei ML:
Role of miR-145-5p targeting ARK5 in regulating the proliferation
and apoptosis of human epithelial ovarian cancer cells. Zhongguo Yi
Xue Ke Xue Yuan Xue Bao. 43:669–676. 2021.(In Chinese). PubMed/NCBI
|
|
60
|
Rohini M, Arumugam B, Vairamani M and
Selvamurugan N: Stimulation of ATF3 interaction with Smad4 via
TGF-β1 for matrix metalloproteinase 13 gene activation in human
breast cancer cells. Int J Biol Macromol. 134:954–961. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sun G, Tian J, Xiao Y and Zeng Y: Circular
RNA circ_0005667 promotes cisplatin resistance of endometrial
carcinoma cells by regulating IGF2BP1 through miR-145-5p.
Anticancer Drugs. 34:816–826. 2023.PubMed/NCBI
|
|
63
|
Du C, Waltzer WC, Wilusz JE, Spaliviero M,
Darras F and Romanov V: Circular STAG2 RNA modulates bladder cancer
progression via miR-145-5p/TAGLN2 and is considered as a biomarker
for recurrence. Cancers (Basel). 16:9782024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu C, Chen J, Cong Y, Chen K, Li H, He Q,
Chen L, Song Y and Xing Y: PROX1 drives neuroendocrine plasticity
and liver metastases in prostate cancer. Cancer Lett.
597:2170682024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ji S, Shi Y, Yang L, Zhang F, Li Y and Xu
F: miR-145-5p inhibits neuroendocrine differentiation and tumor
growth by regulating the SOX11/MYCN axis in prostate cancer. Front
Genet. 13:7906212022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang L, Yu R, Li C, Dang Y, Yi X and Wang
L: Circ_0026416 downregulation blocks the development of colorectal
cancer through depleting MYO6 expression by enriching miR-545-3p.
World J Surg Oncol. 19:2992021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Armstrong L, Willoughby CE and McKenna DJ:
The suppression of the epithelial to mesenchymal transition in
prostate cancer through the targeting of MYO6 using MiR-145-5p. Int
J Mol Sci. 25:43012024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tannir NM, Formiga MN, Penkov K, Kislov N,
Vasiliev A, Skare N, Hong W, Dai S, Tang L, Qureshi A, et al:
Bempegaldesleukin plus nivolumab versus sunitinib or cabozantinib
in previously untreated advanced clear cell renal cell carcinoma: A
phase III randomized study (PIVOT-09). J Clin Oncol. 42:2800–2811.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lv Q, Ma C, Li H, Tan X, Wang G, Zhang Y
and Wang P: Circular RNA microarray expression profile and
potential function of circ0005875 in clear cell renal cell
carcinoma. J Cancer. 11:7146–7156. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sun J, Cheng W, Guo S, Cai R, Liu G, Wu A
and Yin J: A ratiometric SERS strategy for the prediction of cancer
cell proportion and guidance of glioma surgical resection. Biosens
Bioelectron. 261:1164752024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang Q, Cheng Z, Shi L and Mao G:
miR-145-5p inhibits the proliferation of glioma stem cells by
targeting translationally controlled tumor protein. J Cancer.
13:1490–1500. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang Y, Ji N, Teng QX, Cai CY, Wang JQ, Wu
ZX, Lei ZN, Lusvarghi S, Ambudkar SV and Chen ZS: Sitravatinib, a
tyrosine kinase inhibitor, inhibits the transport function of ABCG2
and restores sensitivity to chemotherapy-resistant cancer cells in
vitro. Front Oncol. 10:7002020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hua L, Huang L, Zhang X, Feng H and Shen
B: Knockdown of circular RNA CEP128 suppresses proliferation and
improves cytotoxic efficacy of temozolomide in glioma cells by
regulating miR-145-5p. Neuroreport. 30:1231–1238. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gregersen PA, Jensen PS, Christensen R,
Lohmann D, Racher H, Gallie B and Urbak SF: Retinoblastoma caused
by an RB1 variant with unusually low penetrance in a Danish family.
Eur J Med Genet. 70:1049562024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Panchannavar GS and Angadi PV: Tumor
budding is a prognostic marker for overall survival and not for
lymph node metastasis in oral squamous cell carcinoma-systematic
review update and meta-analysis. J Oral Biol Craniofac Res.
14:362–369. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yu J, Lou Y, Hou M, Ma X and Wang L:
Circ_0058063 contributes to oral squamous cell carcinoma
development by sponging miR-145-5p and upregulating SERPINE1. J
Oral Pathol Med. 51:630–637. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Coca-Pelaz A, Shah JP, Hernandez-Prera JC,
Ghossein RA, Rodrigo JP, Hartl DM, Olsen KD, Shaha AR, Zafereo M,
Suarez C, et al: Papillary thyroid cancer-aggressive variants and
impact on management: A narrative review. Adv Ther. 37:3112–3128.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Feng J, Zhou Q, Yi H, Ma S, Li D, Xu Y,
Wang J and Yin S: A novel lncRNA n384546 promotes thyroid papillary
cancer progression and metastasis by acting as a competing
endogenous RNA of miR-145-5p to regulate AKT3. Cell Death Dis.
10:4332019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yang C and Hardy P: The multifunctional
nature of the MicroRNA/AKT3 regulatory axis in human cancers.
Cells. 12:25942023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liang H, Cui M, Tu J and Chen X:
Advancements in osteosarcoma management: Integrating immune
microenvironment insights with immunotherapeutic strategies. Front
Cell Dev Biol. 12:13943392024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cao C and Shu X: Suppression of
circ_0008932 inhibits tumor growth and metastasis in osteosarcoma
by targeting miR-145-5p. Exp Ther Med. 22:11062021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xie W, Ma F, Dou L, Chang W, Yuan D, Zhang
Z and Zhang Y: Allicin affects immunoreactivity of osteosarcoma
cells through lncRNA CBR3-AS1. Heliyon. 10:e319712024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
D'Alò F, Bellesi S, Maiolo E, Alma E,
Bellisario F, Malafronte R, Viscovo M, Campana F and Hohaus S:
Novel targets and advanced therapies in diffuse large B cell
lymphomas. Cancers (Basel). 16:22432024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gao Y and Ding X: miR-145-5p exerts
anti-tumor effects in diffuse large B-cell lymphoma by regulating
S1PR1/STAT3/AKT pathway. Leuk Lymphoma. 62:1884–1891. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Guo QQ, Ma SZ, Zhao Y, Beeraka NM, Gu H,
Zheng YF, Zhao RW, Li ST, Nikolenko VN, Bulygin KV, et al:
Association of definitive radiotherapy for esophageal cancer and
the incidence of secondary head and neck cancers: A SEER
population-based study. World J Oncol. 15:598–611. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Niu Y, Zhang J, Tong Y, Li J and Liu B:
miR-145-5p restrained cell growth, invasion, migration and
tumorigenesis via modulating RHBDD1 in colorectal cancer via the
EGFR-associated signaling pathway. Int J Biochem Cell Biol.
117:1056412019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rajarajan D, Kaur B, Penta D, Natesh J and
Meeran SM: miR-145-5p as a predictive biomarker for breast cancer
stemness by computational clinical investigation. Comput Biol Med.
135:1046012021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
García-García F, Salinas-Vera YM,
García-Vázquez R, Marchat LA, Rodríguez-Cuevas S, López-González
JS, Carlos-Reyes Á, Ramos-Payán R, Aguilar-Medina M,
Pérez-Plasencia C, et al: miR-145-5p is associated with
pathological complete response to neoadjuvant chemotherapy and
impairs cell proliferation by targeting TGFβR2 in breast cancer.
Oncol Rep. 41:3527–3534. 2019.PubMed/NCBI
|
|
89
|
Bagheri M, Khansarinejad B, Mosayebi G,
Moradabadi A and Mondanizadeh M: Diagnostic value of plasma miR-145
and miR-185 as targeting of the APRIL oncogene in the B-cell
chronic lymphocytic leukemia. Asian Pac J Cancer Prev. 22:111–117.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang Y, Ta WW, Sun PF, Meng YF and Zhao
CZ: Diagnostic and prognostic significance of serum miR-145-5p
expression in glioblastoma. Int J Clin Exp Pathol. 12:2536–2543.
2019.PubMed/NCBI
|
|
91
|
Hang W, Feng Y, Sang Z, Yang Y, Zhu Y,
Huang Q and Xi X: Downregulation of miR-145-5p in cancer cells and
their derived exosomes may contribute to the development of ovarian
cancer by targeting CT. Int J Mol Med. 43:256–266. 2019.PubMed/NCBI
|
|
92
|
Aftab M, Poojary SS, Seshan V, Kumar S,
Agarwal P, Tandon S, Zutshi V and Das BC: Urine miRNA signature as
a potential non-invasive diagnostic and prognostic biomarker in
cervical cancer. Sci Rep. 11:103232021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bahreini F, Saidijam M, Mousivand Z,
Najafi R and Afshar S: Assessment of lncRNA DANCR, miR-145-5p and
NRAS axis as biomarkers for the diagnosis of colorectal cancer. Mol
Biol Rep. 48:3541–3547. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bahreini F, Saidijam M, Afshar S,
Mousivand Z and Najafi R: The effect of miR-145-5p, DANCR and NRAS
expression levels on the survival rate of colorectal cancer
patients. Asian Pac J Cancer Prev. 22:4043–4049. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wen J, Luo K, Liu H, Liu S, Lin G, Hu Y,
Zhang X, Wang G, Chen Y, Chen Z, et al: MiRNA expression analysis
of pretreatment biopsies predicts the pathological response of
esophageal squamous cell carcinomas to neoadjuvant
chemoradiotherapy. Ann Surg. 263:942–948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tuo Z, Liang L and Zhou R: LINC00852 is
associated with poor prognosis in non-small cell lung cancer
patients and its inhibition suppresses cancer cell proliferation
and chemoresistance via the hsa-miR-145-5p/KLF4 axis. J Gene Med.
23:e33842021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Xie L, Cui G and Li T: Long Noncoding RNA
CBR3-AS1 promotes stem-like properties and oxaliplatin resistance
of colorectal cancer by sponging miR-145-5p. J Oncol.
2022:22602112022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou F, Ding W, Mao Q, Jiang X, Chen J,
Zhao X, Xu W, Huang J, Zhong L and Sun X: The regulation of
hsacirc_004413 promotes proliferation and drug resistance of
gastric cancer cells by acting as a competing endogenous RNA for
miR-145-5p. PeerJ. 10:e126292022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang W, Ding B, Lou W and Lin S: Promoter
hypomethylation and miR-145-5p downregulation- mediated HDAC11
overexpression promotes sorafenib resistance and metastasis of
hepatocellular carcinoma cells. Front Cell Dev Biol. 8:7242020.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Khalighfard S, Kalhori MR, Amiriani T,
Poorkhani A, Khori V, Esmati E, Lashkari M, Najafi A and Alizadeh
AM: A systematic approach introduced novel targets in rectal cancer
by considering miRNA/mRNA interactions in response to radiotherapy.
Cancer Biomark. 33:97–110. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tian J, Wang N, Wang C, Wu DP, Wang CH,
Ding XJ and Wang YK: Hsa_circ_0000392 affects the radiation
sensitivity of cervical cancer by targeting the
miR-145-5p/CRKL/MAPK pathway. Zhonghua Zhong Liu Za Zhi.
45:879–891. 2023.(In Chinese). PubMed/NCBI
|
|
102
|
Parayath NN, Gandham SK and Amiji MM:
Tumor-targeted miRNA nanomedicine for overcoming challenges in
immunity and therapeutic resistance. Nanomedicine (Lond).
17:1355–1373. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Luna C, Parker M, Challa P and Gonzalez P:
Long-term decrease of intraocular pressure in rats by viral
delivery of miR-146a. Transl Vis Sci Technol. 10:142021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sun F, Chen H, Dai X, Hou Y, Li J, Zhang
Y, Huang L, Guo B and Yang D: Liposome-lentivirus for miRNA therapy
with molecular mechanism study. J Nanobiotechnology. 22:3292024.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Xu J, Liu W, Fan F, Zhang B, Sun C and Hu
Y: Advances in nano-immunotherapy for hematological malignancies.
Exp Hematol Oncol. 13:572024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fan B, Yang X, Li X, Lv S, Zhang H, Sun J,
Li L, Wang L, Qu B, Peng X and Zhang R:
Photoacoustic-imaging-guided therapy of functionalized melanin
nanoparticles: Combination of photothermal ablation and gene
therapy against laryngeal squamous cell carcinoma. Nanoscale.
11:6285–6296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Dai J, Cheng Y, Wu J, Wang Q, Wang W, Yang
J, Zhao Z, Lou X, Xia F, Wang S and Tang BZ: Modular peptide probe
for Pre/Intra/Postoperative therapeutic to reduce recurrence in
ovarian cancer. ACS Nano. 14:14698–14714. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang X, Li F, Zhang J, Guo L, Shang M, Sun
X, Xiao S, Shi D, Meng D, Zhao Y, et al: A combination of
PD-L1-targeted IL-15 mRNA nanotherapy and ultrasound-targeted
microbubble destruction for tumor immunotherapy. J Control Release.
367:45–60. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ren L, Wang L, Yi X, Tan Y, Yi L, He J and
Li D: Ultrasound microbubble-stimulated miR-145-5p inhibits
malignant behaviors of breast cancer cells by targeting ACTG1.
Ultrasound Q. 40:136–143. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jiang T, Qiao Y, Ruan W, Zhang D, Yang Q,
Wang G, Chen Q, Zhu F, Yin J, Zou Y, et al: Cation-Free siRNA
micelles as effective drug delivery platform and potent RNAi
nanomedicines for glioblastoma therapy. Adv Mater. 33:e21047792021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Howe SJ, Mansour MR, Schwarzwaelder K,
Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K,
Chatters SJ, de Ridder D, et al: Insertional mutagenesis combined
with acquired somatic mutations causes leukemogenesis following
gene therapy of SCID-X1 patients. J Clin Invest. 118:3143–3150.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jusoh AR, Mohan SV, Ping TL, Din TADAAB,
Haron J, Romli RC, Jaafar H, Nafi SN, Salwani TI and Yahya MM:
Plasma circulating mirnas profiling for identification of potential
breast cancer early detection biomarkers. Asian Pac J Cancer Prev.
22:1375–1381. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
García-Magallanes N, Beltran-Ontiveros SA,
Leal-León EA, Luque-Ortega F, Romero-Quintana JG, Osuna-Ramirez I,
Barbosa-Jasso M and Arámbula-Meraz E: Underexpression of
circulating miR-145-5p and miR-133a-3p are associated with breast
cancer and immunohistochemical markers. J Cancer Res Ther.
16:1223–1228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Pigati L, Yaddanapudi SC, Iyengar R, Kim
DJ, Hearn SA, Danforth D, Hastings ML and Duelli DM: Selective
release of microRNA species from normal and malignant mammary
epithelial cells. PLoS One. 5:e135152010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hernandez BJ, Strain M, Suarez MF, Stamer
WD, Ashley-Koch A, Liu Y, Klingeborn M and Rickman CB: Small
extracellular vesicle-associated MiRNAs in polarized retinal
pigmented epithelium. Invest Ophthalmol Vis Sci. 65:572024.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Guo Y, Han Y, Zhang J, Zhou Y, Wei M and
Yu L: Identification and experimental validation of prognostic
miRNA signature and ferroptosis-related key genes in cervical
squamous cell carcinoma. Cancer Med. 13:e704152024. View Article : Google Scholar : PubMed/NCBI
|
















