Among all malignancies, prostate cancer (PCa) is the
primary cancer in men and the second highest contributor to
mortality (1,2). Metastatic PCa, which is often deemed
incurable, has a 5-year survival rate of only 25% in contrast to
the 99% survival rate of localized PCa (3,4).
Studies have indicated that 80–90% of individuals diagnosed with
advanced PCa will ultimately experience the development of bone
metastases (5–8). Advanced PCa is usually treated with
drugs or surgical castration (9,10).
Most castration-resistant PCa (CRPC) is ultimately incurable, and
CRPC is often accompanied by the risk of bone metastasis (11,12).
Patients with bone metastases are prone to skeleton-related events,
including unbearable bone pain, which reduces the quality of life
of patients and increases the chance of death (13,14).
The axial bone is the most common site of PCa metastases,
especially in the pelvis or spine (15).
The identification and management of bone metastases
in patients with PCa pose difficulties. In the past few years, the
introduction of positron emission tomography/computed tomography
(PET/CT) has transformed the way patients with PCa-induced bone
metastasis are diagnosed and treated. Scientists created
PET/CT-targeted prostate specific membrane antigen (PSMA) (16), which can be used for molecular
imaging-based diagnosis. This technique has been widely used in the
diagnosis of PCa-related bone metastases (17–20).
Furthermore, conducting a bone tissue biopsy has remained the most
precise technique for detecting bone metastases at present.
However, the limited clinical application of these methods is
attributed to invasive surgical procedures, a lack of specificity,
high expenses and the potential hazards of radiation exposure
(21). Therefore, in-depth
exploration of the molecular mechanisms of PCa-induced bone
metastasis is needed to identify valuable diagnostic and
therapeutic biomarkers.
The high tendency of bone metastasis of PCa is
related to numerous factors, and the complete molecular process
that underlies the formation of bone metastases remains unclear. A
study has indicated that the factors influencing the tendency of
PCa to metastasize to bones mainly include the tumor
microenvironment, interactions between cancer cells and bone matrix
cells, the expression of specific proteins such as bone
morphogenetic protein (BMP) and receptor activator of NF-κB ligand
(RANKL), and the activation of signaling pathways that promote the
activities of osteoblasts and osteoclasts (22). Previous studies have confirmed that
numerous RNAs (protein-coding genes and non-coding RNAs) in the
primary tumor tissue and the microenvironment serve a role in the
chemotactic movement, colonization and destruction of bone tissue
by cancer cells during PCa-induced bone metastasis (23–28).
Bone metastasis occurs when tumor cells disrupt the physiological
remodeling process through the same molecular mechanisms as those
of bone precursors. This process involves molecular communication
among bone cells, osteoblasts and osteoclasts. Tumor cells use bone
tissue to absorb and release certain factors, thereby establishing
a vicious cycle that promotes the establishing process (27). Exosomes, soluble substances and
ligands that bind to the membrane affect the microenvironment of
the bone marrow (22). Upon
establishing certain conditions, a specialized environment is
created that supports the survival of PCa cells, thereby
facilitating the advancement of lesions toward a dominant state
(22).
The present review summarizes the mechanism of
action of protein-coding genes and non-coding RNAs in PCa-induced
bone metastasis, discusses the value of applying the identified
targets in the diagnosis and treatment of the disease, and provides
novel ideas for the diagnosis and treatment of PCa-induced bone
metastasis.
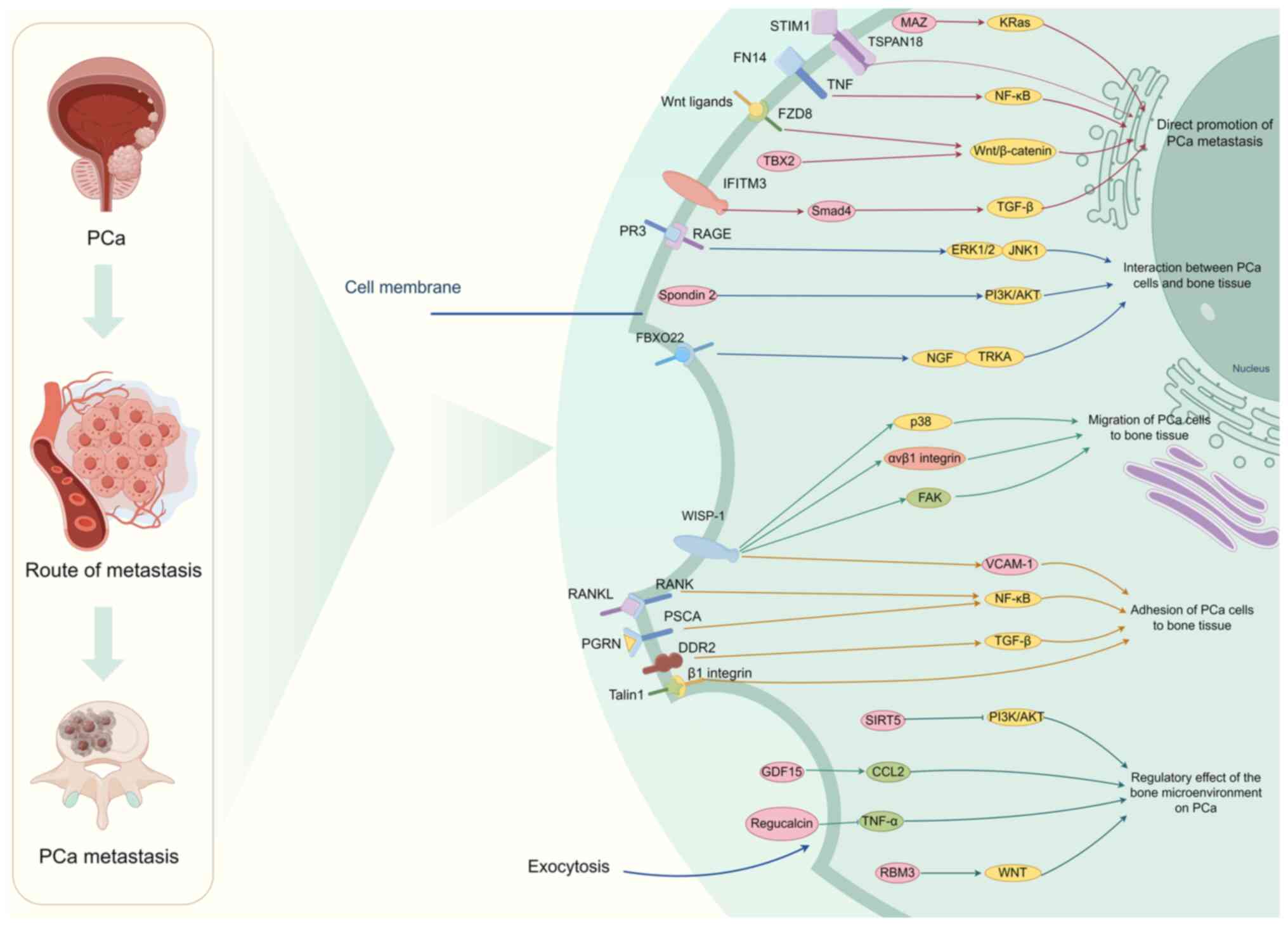
Similar to other tumors, protein-coding genes serve
an important role in regulating bone metastasis in PCa (22). By thoroughly examining the
literature, it has been revealed that the bone metastasis of PCa is
primarily influenced by the direct promotion of the spread to the
bones of PCa, the interaction between PCa and bone tissue, the
adhesion of PCa to bone tissue and the regulation of PCa by the
bone microenvironment (22,29–31)
(Table I). Through the
aforementioned processes, most mRNAs participate in bone metastases
and bone destruction after metastases of PCa (23,24,28,32–34).
In addition, some mRNAs also have an inhibitory effect (35–38)
(Fig. 1). Therefore, focusing on
these molecules could potentially provide therapeutic benefits for
PCa-induced bone metastasis.
Numerous studies have validated the contribution of
protein-coding genes in facilitating the spread of PCa to the bones
(23,24,29).
Tetraspanin-18 (TSPAN18) (23),
interferon induced transmembrane protein 3 (IFITM3) (24), fibroblast growth factor-inducible 14
(Fn14) (28), Frizzled 8 (FZD8)
(32), T-Box protein 2 (TBX2)
(33) and myc associated zinc
finger protein (MAZ) (34) promote
the migration and invasion of PCa cells by participating in
Wnt/β-catenin, Ca (2+), TGF-β, NF-κB, kRas and other signaling
pathways, and finally achieve the purpose of promoting PCa-induced
bone metastasis.
TBX2, a member of the T-box family of transcription
factors, has a critical function in embryonic development by acting
as a negative regulator of the cell cycle inhibitor p21 (39). In human PCa specimens and human PCa
xenograft mouse models, TBX2 expression was increased in bone
metastases. In vitro, inhibiting the natural expression of
TBX2 in PCa cell models could decrease the proliferation, formation
of colonies and invasion of tumor cells. Inhibiting natural TBX2 in
mouse xenografts of human PCa decreased the invasion of tumor cells
and the spread of cancer to bones and soft tissues (33). In addition, blocking the endogenous
TBX2 of PCa cells was found to reduce bone colonization in an
intratibial mouse model (33). The
study of the mechanism revealed that TBX2 is a downstream target of
WNT3A, suggesting that the use of WNT3A antagonists could
potentially serve as a novel medication for managing metastatic and
skeletal issues in patients with PCa (33). Li et al (32) reported that FZD8 from the FZD family
was highly expressed in bone metastatic PCa cell lines and tissues.
Clinical tumor progression and bone metastases were positively
associated with the elevated expression of FZD8. Furthermore, the
excessive expression of FZD8 was observed to enhance the movement,
infiltration and stem-like characteristics of PCa cells in
laboratory settings through the activation of conventional
Wnt/β-catenin signaling. Conversely, the inhibition of FZD8
resulted in the suppression of these characteristics. The
suppression of FZD8 markedly reduced the occurrence of bone
metastases in vivo in patients with PCa. Collectively, these
observations revealed a novel process of bone spread in PCa and
suggested that TBX2 and FZD8 could serve as promising targets for
the treatment of PCa-induced bone metastasis.
According to the latest research, ~85% of patients
with PCa experience bone metastasis in the advanced stages
(44); however, the underlying
biological mechanism for this specific preference remains mostly
unexplained. There is an interaction mechanism between PCa and bone
tissue through some protein-coding genes. Kolonin et al
(30) revealed that the connection
between receptor for advanced glycation end products (RAGE) and
protease 3 (PR3) facilitated the migration of PCa cells to the bone
marrow. In vitro, PR3 interacted with RAGE on the surface of
PCa cells, leading to the stimulation of tumor cell movement
through the activation and phosphorylation of a non-proteolytic
signaling pathway involving ERK1/2 and JNK1. Mouse tumor
experiments validated that the abnormal expression of RAGE on human
PCa cells was enough to facilitate the migration to the bone marrow
temporarily. The results illustrated the role of RAGE-PR3
interactions in facilitating bone metastases during the progression
of PCa in human cells, highlighting their potential impact on
prognosis and therapeutic approaches. Spondin 2 is a specific
diagnostic marker for PCa. Research has verified the function of
spondin 2 in stimulating the development of bone-forming cells in
PCa cells (45). Spondin 2 enhances
the production of Osterix and RUNX family transcription factor 2
(RUNX2) in bone cells, and it facilitates bone creation via the
PI3K/AKT/mTOR pathway during the advancement of prostate tumors
(45). In addition, Zhang et
al (46) deliberated the
specific regulatory mechanism and biological role of E3 ubiquitin
ligase F-box protein 22 (FBXO22) in the metastasis of PCa. FBXO22
was upregulated in PCa tissues compared with paracancerous tissues
and metastatic bone tissues compared with bone tissues without bone
metastases. Downregulation of FBXO22 decreased bone metastases and
M2-type polarization of macrophages in mice. The activity of PCa
cells and osteoblasts was detected by co-culturing them with
macrophages. Knockdown of FBXO22 inhibited tumor activity, while
restoring the osteoblast capacity of promoting bone formation and
remodeling. The findings indicated that FBXO22 enhanced the
proliferation activity of PCa cells and caused osteogenic damage by
inducing polarization of M2 macrophages.
Collagen binding can activate discoidin domain
receptor 2 (DDR2), which belongs to the receptor tyrosine kinase
family (51). Yan et al
(52) explored the role and
mechanism of DDR2 in bone spread of PCa. The study found that
enhanced DDR2 expression led to increased motility and
aggressiveness of PCa cells, while knockdown of DDR2 by specific
short hairpin RNAs (shRNAs) led to inhibition. Knockdown of DDR2 in
PCa cells resulted in reduced proliferation, differentiation and
function of osteoblasts. Animal models of bone metastases using
intraosseous injection showed that DDR2 promoted osteolytic
metastasis in vivo. According to molecular evidence, DDR2
serves a role in the activation of osteoclasts and the breakdown of
bone vis TGF-β, thus facilitating the attachment of PCa cells to
type I collagen (52). Adaptor
proteins called talins control adhesion signals by connecting
integrins to the cytoskeleton. Talins serve a crucial role in
activating integrins by directly binding to them (53). Jin et al (54) revealed that the activation of β1
integrin in metastatic PCa cells leads to an increase in PCa
metastases to both lymph nodes and bone. Their research has shown
that the phosphorylation of talin1 by CDK5, resulting in the
activation of β1 integrin, can enhance the metastatic capability of
PCa cells.
Wnt-1-induced secreted protein 1 (WISP-1), a member
of the cysteine rich angiogenic inducer 61, connective tissue
growth factor, Nov family of matricellular proteins, has a crucial
function in the development of bones (55). Tai et al (56) reported that introducing WISP-1 shRNA
into osteoblasts reduced vascular adhesion molecule-1 (VCAM-1)
expression and PCa migration. The study revealed that WISP-1,
derived from osteoblasts, suppressed microRNA (miRNA/miR)-126
expression by utilizing αvβ1 integrin, focal adhesion kinase and
p38 signaling pathways, which facilitated the movement of human PCa
cells and enhanced the expression of VCAM-1 (56). Chang et al (57) revealed that WISP-1 controlled the
process of bone mineralization by stimulating the production of
BMP2, BMP4 and osteopontin in osteoblasts. WISP-1, derived from
osteoblasts, increased the VCAM-1 expression in PCa cells, leading
to the promotion of cancer cell adhesion to osteoblasts. Therefore,
WISP-1 may be a novel molecular therapeutic target for PCa-induced
bone metastasis.
Metastasis of PCa to the bone can result in the
induction of epigenome reprogramming and dry remodeling of cancer
cells, consequently enhancing the adaptability of cancer cells to
the bone microenvironment and potentially causing secondary tumor
metastases (22). The interaction
between PCa cells and the bone microenvironment establishes a
harmful cycle that controls the bone microenvironment, enhances
bone abnormalities and stimulates the growth of bone tumors
(22). However, the molecular
mechanism by which PCa regulates the bone microenvironment is
complex. Prior research has validated that certain protein-coding
gene molecules contribute to the control of the bone
microenvironment, facilitating the proliferation of PCa within the
skeletal system (58).
Some mRNAs are involved in the negative regulation
of PCa progression in the bone microenvironment (35–37).
Zhang et al (35) revealed
that RNAs binding motif 3 (RBM3) disrupted CD44 alternative
splicing, thereby influencing the stem-like characteristics of PCa.
Methyltransferase 3 increased the methylation of N6-methyladenosine
on catenin β1 mRNA, as induced by RBM3 (35). Furthermore, the expression of
regucalcin impeded the migration and invasion of human PCa cells
in vitro. It also resulted in reduced levels of Ras, Akt,
MAPK, ribosomal S6 kinase 2, mTOR, caveolin-1 and integrin β1,
which are crucial proteins for metastasis (36). Yamaguchi et al (37) reported that the excessive expression
of regucalcin could hinder the generation of TNF-α. TNF-α may be an
important mediator in the interaction between tumor cells and bone
cells. The excessive expression of regucalcin had the ability to
impede the movement, infiltration and spread of human PCa cells to
the bones. The findings of this research offer a novel approach for
the treatment of PCa-induced bone metastasis by focusing on
regucalcin. Sirtuin 5 (SIRT5) is an NAD(+)-dependent deacylase that
is considered a key regulator of multiple cancer types such as
gastric cancer, colorectal cancer and clear cell renal cell
carcinoma (38). The suppression of
the PI3K/AKT/NK-κB pathway by SIRT5 has been observed to decrease
when PCa metastasizes from the bone to other tissues (59). Furthermore, Siddiqui et al
(60) investigated the role of
growth differentiation factor-15 (GDF15) in the bone metastasis of
advanced-stage PCa. Using preclinical mouse models and in
vitro coculture systems, the study demonstrated that
PCa-secreted GDF15 not only promoted bone metastases but also
induced structural changes in the bone microenvironment.
Mechanistically, GDF15 enhances osteoblast function and drives
osteoclastogenesis by upregulating CCL2 and RANKL, thereby
facilitating PCa growth in the bone. These findings underscore the
critical influence of GDF15 on the bone microenvironment and its
contribution to PCa bone metastasis.
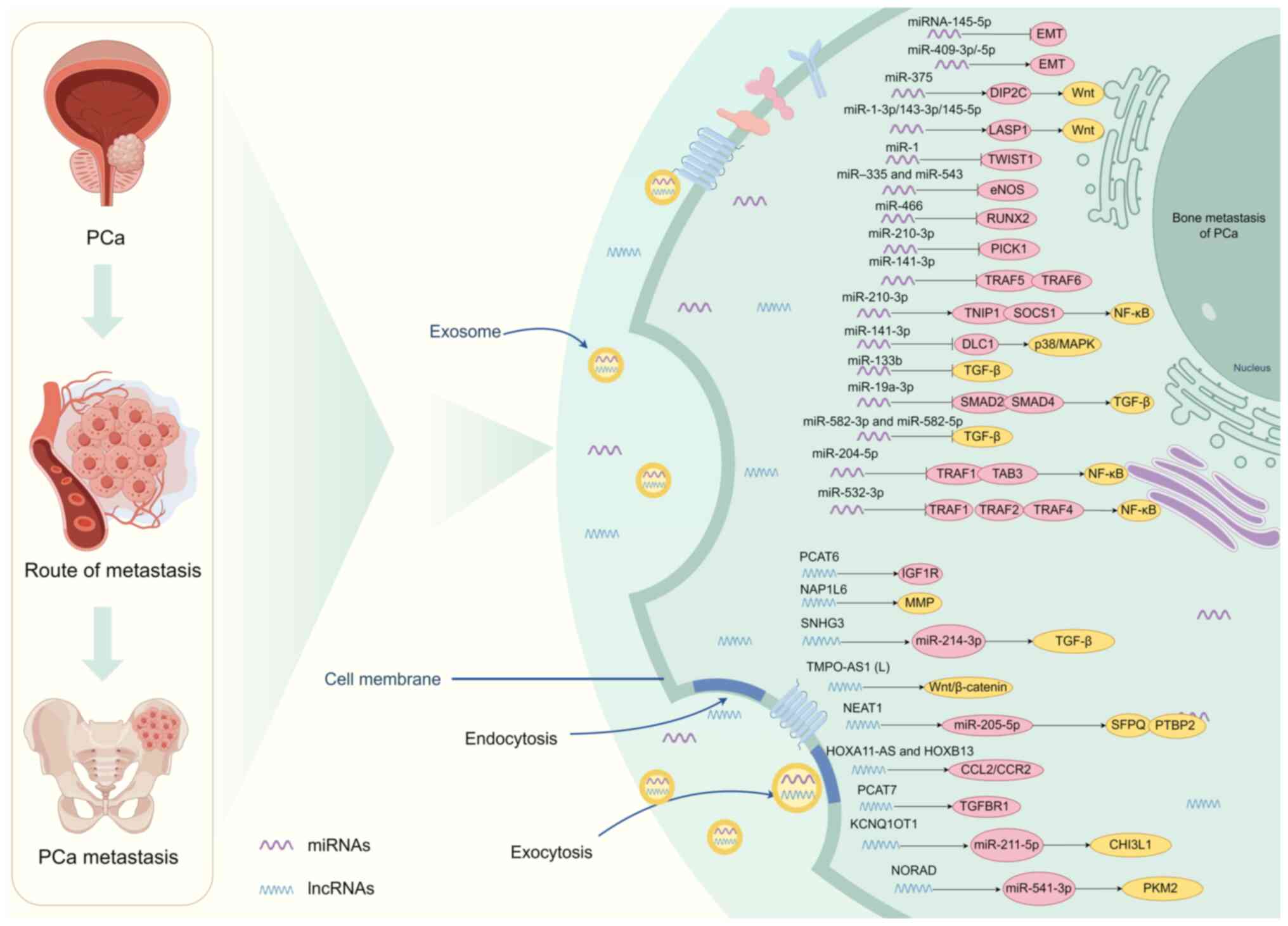
Approximately 98% of the human genome is considered
to be transcribed into non-coding RNAs, which serve a crucial role
in regulating gene expression at the epigenetic, transcriptional
and post-transcriptional stages (61). The significance of non-coding RNAs
in cancer is slowly being comprehended and has emerged as a crucial
field of research focus. According to their size, they are mainly
divided into miRNAs, long non-coding RNAs (lncRNAs) and circular
RNAs. miRNAs, which are small RNA molecules that are 19–25
nucleotides long, control gene expression by blocking the
translation of mRNA (62).
Approximately 30% of the protein-coding genes within the human
genome undergo regulation through the inherent expression of
miRNAs. Therefore, the significance of miRNAs lies in their role in
regulating cellular pathways linked to the development and
advancement of PCa-induced bone metastasis (25) (Fig.
2). lncRNAs, which are longer than 200 nucleotides, serve a
role in numerous biological processes such as enlisting
transcriptional regulators, interacting with chromatin, controlling
proteins and regulating epigenetics (63). They can also prevent targeted mRNA
degradation by absorbing miRNAs as sponges in PCa (64) (Fig.
2). Exosomes consist of a range of diminutive compounds, such
as non-coding RNAs, that are considered to have a strong
association with the spread of PCa to the bones (65). In summary, non-coding RNAs have
emerged as a subject of investigation, with multiple studies now
indicating their involvement in PCa-induced bone metastasis
(26,66–68)
(Tables II and III).
Over the past decade, research has revealed that
miRNAs serve a crucial role in the regulation of tumor progression
and metastases. Research has additionally verified that miRNAs also
control the progression of PCa-induced bone metastasis (66). Exosomes derived from PCa have been
demonstrated to enhance events associated with metastasis,
including the differentiation and proliferation of osteoblasts and
osteoclasts. Dysregulated miRNA expression in PCa can potentially
cause irregular bone restructuring and facilitate tumor growth
(66). Research has verified that
miR-375 (67),
miR-1-3p/143-3p/145-5p (69),
miR-409-3p/-5p (70), miR-210-3p
(71), miR-141-3p (72), miR-532-3p (73) and additional factors contribute to
the advancement of PCa-induced bone metastasis through epigenetic
regulatory mechanisms (Table II).
These miRNAs can promote or inhibit PCa-induced bone metastasis by
acting on downstream target genes and finally regulating the
signaling pathway of PCa-induced bone metastasis (Fig. 2).
The mechanism of action of the aforementioned miRNAs
was studied. miR-375 directly targets disco interacting protein 2
homolog C and upregulates the Wnt signaling pathway, thus promoting
the differentiation of human mesenchymal stem cells into
osteoblasts. Furthermore, miR-375 facilitated the in vitro
proliferation, invasion and migration of PCa cells, while also
promoting tumor advancement and osteogenic metastasis in
vivo (67). Experimental
evidence has revealed that miR-1-3p/143-3p/145-5p enhanced the
in vitro proliferation and migration of PCa. Interaction
with β-catenin enabled LIM and SH3 protein 1 to serve as a shared
target for these three miRNAs, potentially triggering the
activation of the Wnt signaling pathway (69). Josson et al (70) confirmed the increased expression of
miR-409-3p/-5p in bone metastatic PCa cell lines and PCa tissues of
men with a high Gleason score. Elevated levels of miR-409-3p
expression were associated with poor progression-free survival in
patients with PCa. The research indicated that miR-409-3p/-5p
serves a role in the biology of PCa by facilitating the growth of
tumors, promoting EMT and causing bone metastases. PCa-induced bone
metastasis is a well-known consequence of the constant activation
of TGF-β signaling (70). Protein
interacting with protein kinase C α (PRKCA) 1 (PICK1), the protein
that interacts with PRKCA1, serves a crucial role in inhibiting the
TGF-β pathway (70). Dai et
al (71) confirmed that the
excessive expression of miR-210-3p resulted in the reduction of
PICK1 in PCa tissues that had spread to the bones. In vitro,
exosomes facilitated the transfer of miR-141-3p to osteoblasts,
subsequently enhancing osteoblast activity. Additionally, the
protein level of target gene DLC1 Rho GTPase activating protein is
suppressed by miR-141-3p, which serves a crucial role in the
activation of the p38/MAPK pathway (71). Controlling the microenvironment of
bone metastases is crucial for the development of bone metastases
and osteogenic damage in PCa (72).
miR-210-3p is a widely studied cancer-causing miRNA
that is linked to different aspects of cancer development,
advancement and spread (74,75). A
study revealed that miR-210-3p expression was higher in PCa tissues
with bone metastases compared with those without bone metastases.
In patients with PCa, there was a positive association between high
expression levels of miR-210-3p and serum prostate-specific antigen
(PSA) levels, Gleason grade and the presence of bone metastases.
In vitro, enhancement of the EMT, invasion and migration of
PCa cells was observed with the upregulation of miR-210-3p, whereas
the inhibition of invasion and migration of PCa cells was observed
with the silencing of miR-210-3p. The findings additionally
indicated that miR-210-3p sustained continuous activation of NF-κB
signaling in PCa cells (74). This
is achieved by targeting TNFAIP3 interacting protein 1 and
suppressor of cytokine signaling 1, which are negative regulators
of NF-κB signaling, resulting in EMT, invasion, migration and bone
metastases (74). Furthermore,
miR-532-3p is a miRNA that serves a role in numerous aspects of
cancer onset and spread (76).
Overexpression of miR-532-3p inhibits activation of NF-κB signaling
by simultaneously targeting tumor necrosis factor
receptor-associated factor 1 (TRAF1), TRAF2 and TRAF4, thereby
enhancing the invasion, migration and bone metastases of PCa cells
(73). The results hold
significance in diagnosing and treating bone metastases in the
prostate, and miRNAs appear to be a promising focus for therapeutic
intervention in PCa-induced bone metastasis.
In addition, some miRNAs have been found to serve an
inhibitory role in regulating bone metastases of PCa. It has been
confirmed that miR-145-5p (77),
miR-1 (78), miR-335, miR-543
(79), miR-466 (80), miR-141-3p (81), miR-133b (82), miR-582-3p, miR-582-5p (83) and miR-204-5p (84) inhibited PCa-induced bone metastasis
as epigenetic regulatory mechanisms (Fig. 2). The specific regulatory mechanism
has also been deeply studied.
Research has revealed that miR-145-5p has the
ability to suppress the growth, movement and infiltration of
PCa-induced bone metastasis. In PC3 cells, miR-145-5p suppressed
the expression of basic fibroblast growth factor, insulin-like
growth factor and TGF-β. The expression of E-cadherin, an
epithelial marker, was enhanced by miR-145-5p, while the expression
of MMP-2 and MMP-9 was reduced. miRNA-145-5p mediated EMT and
induced apoptosis. miR-145-5p negatively regulated EMT, inhibited
PCa-induced bone metastasis, and promoted apoptosis of PCa-induced
bone metastasis cells (77).
Conversely, Guo et al (69)
identified its association with the regulation of LASP1, suggesting
a context-dependent role that may facilitate bone metastasis in
certain scenarios. These findings underscore the complex
interactions of microRNA-145-5p in prostate cancer, highlighting
its potential as a target for both diagnostic and therapeutic
strategies in managing bone metastatic disease. miR-466 plays a
significant role in inhibiting bone metastasis of PCa by directly
regulating the osteogenic transcription factor RUNX2 (80). Research indicates that miR-466
targets RUNX2, leading to a downregulation of its expression, which
subsequently impairs osteoblastic activity and tumor growth in the
bone microenvironment. This regulatory mechanism contributes to the
suppression of metastatic spread by altering key pathways
associated with bone remodeling and cancer cell interactions with
bone tissue (80). Therefore,
miR-466 represents a potential therapeutic target for preventing
bone metastasis in prostate cancer, providing insights into novel
treatment approaches.
Multiple cancer types, including breast cancer,
osteosarcoma and gastric cancer, have been found to involve the
participation of miR-204-5p in their development and spread, where
miR-204-5p functions as a tumor suppressor by targeting specific
oncogenes (86–88). Wa et al (84) found that miR-204-5p expression was
decreased in PCa tissues and serum samples with bone metastases
compared with those without bone metastases, which was related to
the late clinicopathological features of patients with PCa and the
poor survival rate without bone metastases. Furthermore, under
laboratory conditions, the increase in miR-204-5p expression
hindered the movement and infiltration of PCa cells. Notably, the
enhancement of miR-204-5p expression suppressed the spread of PCa
cells to the bones when tested in vivo. The findings
additionally indicated that miR-204-5p hindered the infiltration,
movement and bone spread of PCa cells by targeting TRAF1, TGF-β
activated kinase 1 (MAP3K7) binding protein 3 and MAP3K3,
deactivating the NF-κB pathway. The research revealed a novel
functional role of miR-204-5p in the spread of PCa to the bones and
highlighted the possible significance of miR-204-5p as a serum
biomarker for diagnosing PCa-induced bone metastasis. Furthermore,
miR-141-3p has been extensively studied as a miRNA in cancer, and
the decrease in miR-141-3p levels has been extensively documented
to serve a role in the advancement and spread of various types of
human cancer, including colorectal cancer and renal cell carcinoma
(89,90). Huang et al (81) found that miR-141-3p expression was
decreased in bone metastatic PCa tissues compared with non-bone
metastatic PCa tissues. There was a positive association between
the reduced expression of miR-141-3p and the serum PSA level,
Gleason grade, and bone metastases status in patients with PCa.
Furthermore, overexpression of miR-141-3p suppressed the process of
EMT, as well as the invasion and migration of PCa cells in
experimental settings. An in vivo reduction of bone
metastases in PC-3 cells was observed with the upregulation of
miR-141-3p. By directly targeting TRAF5 and TRAF6, miR-141-3p
hindered the activation of NF-κB signaling, consequently
suppressing the invasion, migration and bone metastases of PCa
cells.
lncRNAs serve as crucial controllers of gene
expression and contribute to the initiation and progression of
tumors (91). Mounting evidence
suggests that lncRNAs serve a role in the progression of
PCa-induced bone metastasis. Due to its special growth environment
and biomechanical properties, bone has become the preferred growth
site of PCa (92–98). Identifying novel molecular entities
that could potentially function as an initial indicator of the
metastasis procedure may provide an opportunity to establish
innovative and improved therapies and diagnostics. Research has
established that PCAT6 (26),
NAP1L6 (68), TMPO-AS1 (L)
(92), SNHG3 (93), HOXA11-AS (94), PCAT7 (95), kcnq10t1 (96), NORAD (97), NEAT1 (98) and other similar lncRNAs contribute
to the spread of PCa to the bones (Table III). These lncRNAs regulate the
signaling pathway of PCa-induced bone metastasis by acting on
downstream target genes, thus promoting PCa-induced bone metastasis
(Fig. 2).
Dynamic interactions among metastatic cancer cells,
the cellular components of the bone marrow microenvironment
(osteoblasts, osteoclasts and osteocytes) and bone stroma regulate
the process of bone metastases (112). In the past few years, there has
been a focus on the identification of EVs and their various
functions in the regulation of PCa metastasis. Multiple research
studies have demonstrated that interactions facilitated by EVs
between cancer cells and the microenvironment of the bone
effectively enhanced pathological bone metabolism at sites where
metastasis occurs (113,114). miRNAs transmitted through EVs in
the serum of patients were identified as a marker for PCa-induced
bone metastasis (115). Wang et
al (115) identified four
prospective EV-delivered miRNAs, including miR-181a-5p, using miRNA
deep sequencing and a miRNA microarray, and their expression in the
bone metastases group was significantly higher than that in the
non-bone metastases group (P<0.05). The diagnostic association
between candidate miR-181a-5p and bone metastases was evaluated by
logistic regression analysis, and the results showed that
miR-181a-5p was associated with bone metastases of PCa. miR-181a-5p
expressed by EVs is expected to be a diagnostic marker for bone
metastases of PCa. Additionally, it has been identified that PCa
exosomes are key mediators in regulating bone homeostasis, leading
to osteoclastic lesions, and thus, promoting bone tumor growth
(116). miR-92a-1-5p downregulates
type I collagen expression by directly targeting COL1A1, which
leads to the promotion of osteoclast differentiation and the
inhibition of osteoblast generation (116). Wang et al (117) reported that EVs derived from
tumors, which contained miR-378a-3p, had an impact on the spread of
PCa to the bones. These EVs triggered the progression of bone
destruction by activating the dual specificity tyrosine
phosphorylation regulated kinase 1A/nuclear factor of activated T
cells 1/angiopoietin like 2 pathway in bone marrow macrophages.
Therefore, miR-378a-3p holds promise as a potential indicator for
the spread of PCa. Treating PCa metastasis could potentially
involve strategies such as decreasing the secretion of EVs
containing miR-378a-3p or impeding the incorporation of miR-378a-3p
into EVs. In addition, Zeng et al (118) first revealed that miR-18a-5p was
upregulated within the bone microenvironment of individuals with
bone metastases caused by PCa. Additional research revealed that
miR-18a-5p was transmitted to osteoblasts through exosomes derived
from PCa cells. Subsequently, it specifically affected the
Hist1h2bc gene, leading to an increase in Ctnnb1 expression in the
Wnt/β-catenin signaling pathway. Therefore, miR-18a-5p derived from
exosomes may serve as a diagnostic indicator for bone metastases of
PCa (118).
More than half of the patients with advanced PCa
will develop bone metastases, and current treatment options are
only aimed at the control of clinical symptoms, rather than a
fundamental cure (119).
Currently, the primary focus of clinical diagnosis and treatment is
the development of novel targeted medications and the mitigation of
bone-related occurrences, considering the difficulties encountered
in managing PCa-induced bone metastasis (Table IV) (120). These mechanism studies indicate
that PCa cells and bone tissue-related molecules are expected to be
novel targets for anti-PCa-induced bone metastasis (121–123). Research has indicated that
denosumab has the ability to decrease bone frailty and postpone the
advancement of bone metastases in the prostate (124). Promising outcomes have also been
observed in clinical trials for bone metastatic PCa when examining
tyrosine kinase inhibitors such as cabozantinib (NCT01522443)
(125) and dasatinib (NCT00918385)
(126). Furthermore, atlacetam, a
blocker of endothelin-A receptor, has been identified to decrease
PSA levels and the occurrence of bone pain in individuals with
metastasis (127). Therefore,
targeted therapy of bone metastases of PCa has great potential.
In the past few years, choosing targeted therapy
according to the tumor metastasis mechanism has emerged as a novel
option in the realm of tumor treatment. This approach not only
offers effective tumor eradication but also minimally affects
normal cells (128). Genetic
testing of patients with PCa with bone metastases is critical to
find appropriate targeted therapies based on the specific molecular
characteristics of each patient (129). The successful response to
anti-programmed cell death protein 1 (PD-1) antibody therapy in
certain patients with PCa is largely attributed to underlying
characteristics such as defective mismatch repair mechanisms and
the presence of microsatellite instability (130). However, a study confirmed that the
CDK12 mutant cell line also showed a good response against PD-1
treatment (131). Individuals
diagnosed with mCRPC exhibit resistance to androgen receptor (AR)
inhibitors and androgen synthesis inhibitors, rendering them
suitable candidates for targeted therapy involving poly ADP-ribose
polymerase (PARP) inhibitors (132). Studies have shown that the PARP
inhibitors used are olaparib or lucaparib, which are particularly
effective in PCa cases associated with BRCA1/2 mutations and
homologous recombination deficiencies (132,133). PARP inhibitor has shown
effectiveness in treating cancer cells with homologous
recombination defects that can be affected by alterations in base
excision repair pathways (134).
Because of its specific expression in PCa, PSMA is
not only an important diagnostic tool for bone metastases of PCa,
but also a therapeutic target for patients with metastatic PCa
(135,136). Numerous studies have focused on
PSMA antibody-drug conjugates and PSMA-targeted chimeric antigen
receptor therapy as potential treatments for CRPC (137,138). Lutetium-177-PSMA has emerged as a
valuable treatment for mCRPC. Recent studies have demonstrated its
ability to selectively target PSMA expressed on mCRPC cells,
delivering targeted radiotherapy that effectively reduces the tumor
burden (139,140). In clinical trials,
Lutetium-177-PSMA has shown promising efficacy, leading to
improvements in overall survival and quality of life of patients
with mCRPC (141,142). This targeted approach not only
enhances treatment outcomes but also minimizes damage to
surrounding healthy tissue, marking an advancement in mCRPC
therapy. PSMA BiTE, a builder of bispecific antibodies targeting
CD3 and PSMA, redirects and activates T cells towards
PSMA-expressing cells. This treatment has also been utilized for
other malignancies, such as kidney cancer and melanoma, showing
promising outcomes in these tumors as well as in PCa (143). Clinical trials are currently
evaluating three additional participants in bispecific T cell
therapy, namely glypican-1, disintegrin and metalloproteinase 17,
and prostatic six transmembrane epithelial antigen 1 (144,145). Blocking the interactions between
MDM2 and p53 to activate tumor suppressors is a promising target
for mCRPC due to the role of TP53 inactivation in second-generation
anti-androgen resistance and neuroendocrine differentiation
(146). Idasanutlin is currently
undergoing several clinical trials. It targets cell lines altered
by p53, which, in the case of PCa, along with alterations in RB
transcriptional corepressor 1, leads to castration-resistant,
aggressive tumors with a poor prognosis (147). Future investigations should
examine the effectiveness of idasanutlin in various hematological
malignancies, analyzing the tumor response, biomarkers for
treatment efficacy and potential resistance mechanisms. This
research is vital for informing upcoming clinical trials and
optimizing therapeutic strategies for patients with hematological
malignancies and PCa.
Due to the significance of AKT in the PI3K pathway,
the effectiveness of PI3K inhibitors in treating PCa-induced bone
metastasis has been limited, resulting in only a partial response
observed in the initial clinical trial (148). ATP-competitive inhibitors such as
ipatasertib or capivasertib (NCT04404140 and NCT04087174), as well
as allosteric inhibitors such as perifosine or MK-2206 (NCT00060437
and NCT00058214), are among the AKT inhibitors currently being
tested in clinical trials. In a phase 2 trial, ipatasertib showed
some degree of prolongation of the progression-free interval
(NCT03072238) (149). An ongoing
clinical trial is currently investigating the use of docetaxel and
capivasertib in combination (NCT05348577). Radiology confirmed that
the combination of ipavasertib and abiraterone improved
progression-free survival (149).
However, several studies are needed before it can be incorporated
into clinical practice as a treatment option for PCa.
Targeted therapies have shown promise in the
treatment of mCRPC, particularly in reducing bone metastases. A
pivotal study by Fizazi et al (150) compared denosumab with zoledronic
acid for managing bone metastases in men with CRPC. The findings
revealed that denosumab delayed skeletal-related events,
highlighting its effectiveness in preventing complications
associated with bone metastases. Furthermore, recent trials, such
as the one led by Shenderov et al (151), which explored the combination of
nivolumab and ipilimumab with enzalutamide in patients expressing
AR-variant 7, have demonstrated moderate activity. These results
underscore the capability of targeted therapies to modulate the
immune response and directly affect cancer progression in the bone
microenvironment.
Further advancements have been made with
combination therapies, demonstrating the feasibility and clinical
potential of integrating immune checkpoint inhibitors such as
pembrolizumab with traditional AR inhibitors. Graff et al
(152) reported that the
combination not only improved treatment outcomes but also presented
a manageable safety profile in patients previously progressing on
enzalutamide alone. Additionally, the work of McNeel et al
(153) on T-cell activation using
MVI-816 alongside pembrolizumab provided evidence for the role of
targeted immunotherapy in enhancing antitumor responses. Overall,
these clinical studies collectively reflect the effectiveness and
safety of targeted therapies in managing bone metastases in mCRPC,
paving the way for innovative treatment strategies that could
ultimately improve the survival and quality of life of
patients.
Furthermore, AR inhibitors remain a cornerstone in
the treatment of mCRPC, particularly in cases involving bone
metastases (154). Clinical
studies, such as the results from the SPARTAN and PROSPER trials,
have demonstrated that AR inhibitors, including apalutamide and
enzalutamide, improve overall survival and delay disease
progression in patients with mCRPC (155). These agents block the AR signaling
pathway, which is crucial for the growth and survival of PCa cells,
even in castration-resistant settings (156). Furthermore, investigations have
shown that combining AR inhibitors with other therapies, such as
radiopharmaceuticals and immunotherapy, may enhance treatment
efficacy, further bolstering their role in managing mCRPC with bone
metastases (152,157). As a result, AR inhibitors continue
to be integral to both first-line and subsequent treatment
strategies, promoting improved outcomes and extending survival of
patients with this aggressive disease.
Most patients with advanced PCa inevitably face the
development of bone metastases. Conventional treatments for
patients with PCa-induced bone metastasis have limited efficacy
(158). Gaining knowledge
regarding the molecular process of PCa-induced bone metastasis is
beneficial for the advancement of novel targeted approaches for the
diagnosis and treatment of this disease. Research has indicated
that numerous protein-coding genes (TSPAN18, IFITM3, Fn14, FZD8,
TBX2 and MAZ) and non-coding RNAs [miR-375, miR-1-3p/143-3p/145-5p,
miR-409-3p/-5p, miR-210-3p, miR-141-3p, miR-532-3p, PCAT6, NAP1L6,
TMPO-AS1(L), SNHG3, HOXA11-AS, PCAT7, kcnq10t1, NORAD and NEAT1]
serve crucial roles in controlling the advancement of PCa-induced
bone metastasis (23,24,26,28,32–34,67–73,92–98).
These molecules control various substances and ultimately
contribute to the progression of tumors through well-known tumor
pathways, such as the Wnt/β-catenin (32), TGF-β (24), NF-κB (28) and kRas (34) pathways.
Research has indicated that protein-coding genes
serve roles in the physiological mechanism of PCa-induced bone
metastasis. Among them, TSPAN18 (23), IFITM3 (24), FN14 (28), FZD8 (32), TBX2 (33) and MAZ (34) directly promote PCa-induced bone
metastasis through related molecular pathways. PCa-derived RAGE
(30), spondin 2 (45) and FBXO22 (46) are involved in the interaction with
bone tissue to promote the differentiation and osteogenic injury of
PCa cells. In addition, the adhesion of PCa metastasis to bone is
accomplished by RANKL (47), PSCA
(50), DDR2 (51), β1 integrin (54) and WISP-1 (56) molecules. PCa cells are regulated by
the bone microenvironment after colonizing bone tissue, and the
molecules involved in this mechanism mainly include GDF15 (60), RBM3 (35), regucalcin (37) and SIRT5 (38).
Exosomes derived from PCa have been demonstrated to
enhance metastasis-associated processes, including the
differentiation and proliferation of osteoblasts and osteoclasts
(66). Exosomes contain a large
number of miRNAs, and abnormal expression of miRNAs leads to
abnormal bone remodeling in PCa. Studies have confirmed that
miR-375 (67),
miR-1-3p/143-3p/145-5p (69),
miR-409-3p/-5p (70), miR-210-3p
(71), miR-141-3p (74), miR-532-3p (73) and others promote PCa-induced bone
metastasis as epigenetic regulatory mechanisms. In addition,
studies have found that lncRNAs are involved in the bone metastases
process of PCa. Studies have confirmed that PCAT6 (26), NAP1L6 (68), TMPO-AS1 (L) (92), SNHG3 (93), HOXA11-AS (94), PCAT7 (95), KCNQ1OT1 (96), NORAD (97) and NEAT1 (98) exert crucial roles in the bone
metastases of PCa.
Currently, the utilization of PSMA PET/CT
technology for the detection of PCa has been progressively
implemented and has shown promising therapeutic outcomes.
Furthermore, PSMA PET/CT offers enhanced sensitivity and
specificity in detecting bone metastases in patients with PCa,
enabling improved staging, personalized treatment planning and
improved monitoring of disease progression (104). In the face of the challenges in
the treatment of PCa-induced bone metastasis, the development of
novel targeted drugs and the reduction of bone-related events are
currently the focus of clinical diagnosis and treatment (120). A study has indicated that PCa
cells and bone tissue-related molecules have the potential to be
novel targets for the prevention of PCa-related bone metastasis
(159). Additionally, clinical
trials have confirmed that dinomumab has the ability to decrease
bone brittleness and delay the advancement of bone metastases in
patients with PCa (124,160). Promising outcomes for patients
with bone-metastatic PCa have also been reported in a clinical
trial that examined tyrosine kinase inhibitors, such as
cabozantinib and dasatinib (127).
Therefore, targeted therapy of PCa-related bone metastases has
great potential. More basic research is needed to confirm that the
identified targets have specific effects on PCa-related bone
metastases. The phenomenon of the same target acting on PCa-related
bone metastases through different signaling pathways also exists
(42,43,60,61),
which poses a challenge for targeted therapy. Additionally, the
effectiveness and potential adverse reactions of utilizing these
targets for the treatment of PCa-induced bone metastasis require
validation in additional clinical trials.
Furthermore, the development of resistance to AR
inhibitors in metastatic PCa poses a challenge in treatment
efficacy. Mechanisms such as AR mutations, alternative splicing and
activation of bypass signaling pathways contribute to this
resistance, rendering standard therapies less effective (161). Additionally, the tumor
microenvironment in the bone can facilitate adaptive changes that
promote survival and proliferation of cancer cells despite AR
inhibition (44). To overcome these
hurdles, novel therapeutic strategies could involve combination
therapies targeting not only the AR but also the accompanying
signaling pathways and the unique bone microenvironment. These
innovative approaches may enhance treatment responses and improve
patient outcomes.
In the context of PCa bone metastasis treatment,
stem cells offer innovative therapeutic avenues beyond bone
differentiation. For instance, targeting the stem cell niche
microenvironment could enhance therapeutic strategies, as shown by
a study indicating that manipulating this niche can potentially
reverse aging-associated changes and improve regenerative outcomes
(162). Additionally, the
investigation of CD33+ leukemic stem cells highlights
the potential for targeted therapies in PCa. Their modulation may
provide insights into overcoming resistance mechanisms that hinder
current treatment modalities (163). Additionally, insights from cardiac
c-Kit+ progenitor cells have elucidated that stem cell
signaling pathways, such as the PI3K and MAPK pathways, serve
critical roles in cellular regeneration and may be leveraged to
develop novel treatments for PCa (164). Thus, harnessing stem cell
properties and their microenvironment can pave the way for
innovative approaches in combating PCa bone metastasis.
In summary, both coding and non-coding RNAs have
effects on the advancement of PCa-related bone metastases, with
dual effects of both promotion and inhibition. The identification
of specific pathway targets will have an impact on the management
of bone metastases in patients with PCa. Furthermore, PSMA, PARP
and PD-1 have been employed for the detection and management of
bone metastases in patients with PCa. Major changes are expected to
occur in the diagnosis and treatment of this disease, as the
molecular mechanisms of bone metastases in patients with PCa are
gradually understood.
Not applicable.
The present study was supported by the Project Category:
National Natural Science Fund (project no. 22JR11RA178).
Not applicable.
XG and SL were responsible for the conception and
execution of this article, and both authors were responsible for
the production of pictures and language polishing. Both authors
contributed to the manuscript and agreed to contribute to this
edition. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simmons JK, Hildreth BE III, Supsavhad W,
Elshafae SM, Hassan BB, Dirksen WP, Toribio RE and Rosol TJ: Animal
models of bone metastasis. Vet Pathol. 52:827–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang SH, Kao YH, Muller CJF, Joubert E
and Chuu CP: Aspalathin-rich green Aspalathus linearis extract
suppresses migration and invasion of human castration-resistant
prostate cancer cells via inhibition of YAP signaling.
Phytomedicine. 69:1532102020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gebrael G, Fortuna GG, Sayegh N, Swami U
and Agarwal N: Advances in the treatment of metastatic prostate
cancer. Trends Cancer. 9:840–854. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skotheim RI, Bogaard M, Carm KT, Axcrona U
and Axcrona K: Prostate cancer: Molecular aspects, consequences,
and opportunities of the multifocal nature. Biochim Biophys Acta
Rev Cancer. 1879:1890802024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu Z, Zou H, Wang H, Li Q and Yu D:
Identification of key gene signatures associated with bone
metastasis in castration-resistant prostate cancer using
co-expression analysis. Front Oncol. 10:5715242020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu L, Li S, Zhuo Y, Chen J, Qin X and Guo
G: Anticancer effect of triterpenes from Ganoderma lucidum in human
prostate cancer cells. Oncol Lett. 14:7467–7472. 2017.PubMed/NCBI
|
|
9
|
Morale MG, Tamura RE and Rubio IGS:
Metformin and cancer hallmarks: Molecular mechanisms in thyroid,
prostate and head and neck cancer models. Biomolecules. 12:3572022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chi JT, Lin PH, Tolstikov V, Oyekunle T,
Chen EY, Bussberg V, Greenwood B, Sarangarajan R, Narain NR,
Kiebish MA and Freedland SJ: Metabolomic effects of androgen
deprivation therapy treatment for prostate cancer. Cancer Med.
9:3691–3702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salji M, Hendry J, Patel A, Ahmad I, Nixon
C and Leung HY: Peri-prostatic fat volume measurement as a
predictive tool for castration resistance in advanced prostate
cancer. Eur Urol Focus. 4:858–866. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Jin M, Park SJ, Seo SY and Jeong
KW: SETD1A promotes proliferation of castration-resistant prostate
cancer cells via FOXM1 transcription. Cancers (Basel). 12:17362020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boopathi E, Birbe R, Shoyele SA, Den RB
and Thangavel C: Bone health management in the continuum of
prostate cancer disease. Cancers (Basel). 14:43052022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Talreja DB: Importance of antiresorptive
therapies for patients with bone metastases from solid tumors.
Cancer Manag Res. 4:287–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clézardin P, Coleman R, Puppo M, Ottewell
P, Bonnelye E, Paycha F, Confavreux CB and Holen I: Bone
metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev.
101:797–855. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sgouros G, Bodei L, McDevitt MR and Nedrow
JR: Radiopharmaceutical therapy in cancer: Clinical advances and
challenges. Nat Rev Drug Discov. 19:589–608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lawal IO, Ndlovu H, Kgatle M, Mokoala KMG
and Sathekge MM: Prognostic value of PSMA PET/CT in prostate
cancer. Semin Nucl Med. 54:46–59. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Houshmand S, Lawhn-Heath C and Behr S:
PSMA PET imaging in the diagnosis and management of prostate
cancer. Abdom Radiol (NY). 48:3610–3623. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pyka T, Okamoto S, Dahlbender M, Tauber R,
Retz M, Heck M, Tamaki N, Schwaiger M, Maurer T and Eiber M:
Comparison of bone scintigraphy and (68)Ga-PSMA PET for skeletal
staging in prostate cancer. Eur J Nucl Med Mol Imaging.
43:2114–2121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harmon SA, Bergvall E, Mena E, Shih JH,
Adler S, McKinney Y, Mehralivand S, Citrin DE, Couvillon A, Madan
RA, et al: A prospective comparison of 18F-sodium
fluoride PET/CT and PSMA-Targeted 18F-DCFBC PET/CT in
metastatic prostate cancer. J Nucl Med. 59:1665–1671. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luna A, Vilanova JC and Alcalá Mata L:
Total body MRI in early detection of bone metastasis and its
indication in comparison to bone scan and other imaging techniques.
Arch Esp Urol. 68:371–390. 2015.(In Spanish). PubMed/NCBI
|
|
22
|
Kang J, La Manna F, Bonollo F, Sampson N,
Alberts IL, Mingels C, Afshar-Oromieh A, Thalmann GN and
Karkampouna S: Tumor microenvironment mechanisms and bone
metastatic disease progression of prostate cancer. Cancer Lett.
530:156–169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Q, Chen X, Yao K, Zhang Y, He H,
Huang H, Chen H, Peng S, Huang M, Cheng L, et al: TSPAN18
facilitates bone metastasis of prostate cancer by protecting STIM1
from TRIM32-mediated ubiquitination. J Exp Clin Cancer Res.
42:1952023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Chen L, Fan Y, Hong Y, Yang X, Li
Y, Lu J, Lv J, Pan X, Qu F, et al: IFITM3 promotes bone metastasis
of prostate cancer cells by mediating activation of the TGF-β
signaling pathway. Cell Death Dis. 10:5172019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abramovic I, Ulamec M, Katusic Bojanac A,
Bulic-Jakus F, Jezek D and Sincic N: miRNA in prostate cancer:
Challenges toward translation. Epigenomics. 12:543–558. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z,
Du H, Ren D, Dai Y and Peng X: m6A modification of
lncRNA PCAT6 promotes bone metastasis in prostate cancer through
IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med.
11:e4262021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li FX, Liu JJ, Xu F, Lin X, Zhong JY, Wu F
and Yuan LQ: Role of tumor-derived exosomes in bone metastasis.
Oncol Lett. 18:3935–3945. 2019.PubMed/NCBI
|
|
28
|
Yin J, Liu YN, Tillman H, Barrett B,
Hewitt S, Ylaya K, Fang L, Lake R, Corey E, Morrissey C, et al:
AR-regulated TWEAK-FN14 pathway promotes prostate cancer bone
metastasis. Cancer Res. 74:4306–4317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang H, Weng H and Chen J: m6A
modification in coding and non-coding RNAs: Roles and therapeutic
implications in cancer. Cancer Cell. 37:270–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kolonin MG, Sergeeva A, Staquicini DI,
Smith TL, Tarleton CA, Molldrem JJ, Sidman RL, Marchiò S,
Pasqualini R and Arap W: Interaction between tumor cell surface
receptor RAGE and proteinase 3 mediates prostate cancer metastasis
to bone. Cancer Res. 77:3144–3150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park M, Cho YJ, Kim B, Ko YJ, Jang Y, Moon
YH, Hyun H and Lim W: RANKL immunisation inhibits prostate cancer
metastasis by modulating EMT through a RANKL-dependent pathway. Sci
Rep. 11:121862021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Q, Ye L, Zhang X, Wang M, Lin C, Huang
S, Guo W, Lai Y, Du H, Li J, et al: FZD8, a target of p53, promotes
bone metastasis in prostate cancer by activating canonical
Wnt/β-catenin signaling. Cancer Lett. 402:166–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nandana S, Tripathi M, Duan P, Chu CY,
Mishra R, Liu C, Jin R, Yamashita H, Zayzafoon M, Bhowmick NA, et
al: Bone metastasis of prostate cancer can be therapeutically
targeted at the TBX2-WNT signaling axis. Cancer Res. 77:1331–1344.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Q, Lang C, Wu Z, Dai Y, He S, Guo W,
Huang S, Du H, Ren D and Peng X: MAZ promotes prostate cancer bone
metastasis through transcriptionally activating the KRas-dependent
RalGEFs pathway. J Exp Clin Cancer Res. 38:3912019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Lv C, Niu Y, Li C, Li X, Shang Y,
Zhang Y, Zhang Y, Zhang Y and Zeng Y: RBM3 suppresses stemness
remodeling of prostate cancer in bone microenvironment by
modulating N6-methyladenosine on CTNNB1 mRNA. Cell Death Dis.
14:912023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaguchi M, Murata T and Ramos JW:
Extracellular regucalcin suppresses the growth, migration,
invasion, and adhesion of metastatic human prostate cancer cells.
Oncology. 100:399–412. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamaguchi M, Murata T and Ramos JW:
Overexpression of regucalcin blocks the migration, invasion, and
bone metastatic activity of human prostate cancer cells: Crosstalk
between cancer cells and bone cells. Prostate. 82:1025–1039. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lagunas-Rangel FA: Role of SIRT5 in
cancer. Friend or Foe? Biochimie. 209:131–141. 2023.PubMed/NCBI
|
|
39
|
Wang X, Li Z and Sun Y: T-box
transcription factor 2 mediates antitumor immune response in
cutaneous squamous cell carcinoma by regulating the expression of
programmed death ligand 1. Skin Res Technol. 29:e132542023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Trivedi T, Pagnotti GM, Guise TA and
Mohammad KS: The role of TGF-β in bone metastases. Biomolecules.
11:16432021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gerratana L, Davis AA, Polano M, Zhang Q,
Shah AN, Lin C, Basile D, Toffoli G, Wehbe F, Puglisi F, et al:
Understanding the organ tropism of metastatic breast cancer through
the combination of liquid biopsy tools. Eur J Cancer. 143:147–157.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yonezawa I, Waki M, Tamura Y, Onoda R,
Narushima M, Ishizuka T and Tajima S: Gemcitabine-based regimen for
primary ovarian angiosarcoma with MYC amplification. Curr Oncol.
21:e782–e789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stopsack KH, Nandakumar S, Wibmer AG,
Haywood S, Weg ES, Barnett ES, Kim CJ, Carbone EA, Vasselman SE,
Nguyen B, et al: Oncogenic genomic alterations, clinical
phenotypes, and outcomes in metastatic castration-sensitive
prostate cancer. Clin Cancer Res. 26:3230–3238. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Archer Goode E, Wang N and Munkley J:
Prostate cancer bone metastases biology and clinical management
(Review). Oncol Lett. 25:1632023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang H, Zhang M, Lu W and Yuan C: Prostate
cancer cell-derived spondin 2 boosts osteogenic factor levels in
osteoblasts via the PI3K/AKT/mTOR pathway. Oncol Rep. 49:232023.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Y, Li W, Guo S, Wu Z, Zhang L, Liu
Y, Li X, Guo X, Cao J, Yang C and Wang Z: FBXO22 mediates the
NGF/TRKA signaling pathway in bone metastases in prostate cancer.
Am J Pathol. 193:1248–1266. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ziaee S and Chung LWK: Induction of
integrin α2 in a highly bone metastatic human prostate cancer cell
line: Roles of RANKL and AR under three-dimensional suspension
culture. Mol Cancer. 13:2082014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ye XC, Choueiri M, Tu SM and Lin SH:
Biology and clinical management of prostate cancer bone metastasis.
Front Biosci. 12:3273–3286. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nayerpour Dizaj T, Doustmihan A,
Sadeghzadeh Oskouei B, Akbari M, Jaymand M, Mazloomi M and
Jahanban-Esfahlan R: Significance of PSCA as a novel prognostic
marker and therapeutic target for cancer. Cancer Cell Int.
24:1352024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao Z, Li E, Luo L, Zhao S, Liu L, Wang
J, Kang R and Luo J: A PSCA/PGRN-NF-κB-integrin-α4 axis promotes
prostate cancer cell adhesion to bone marrow endothelium and
enhances metastatic potential. Mol Cancer Res. 18:501–513. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Azemikhah M, Ashtiani HA, Aghaei M and
Rastegar H: Evaluation of discoidin domain receptor-2 (DDR2)
expression level in normal, benign, and malignant human prostate
tissues. Res Pharm Sci. 10:356–363. 2015.PubMed/NCBI
|
|
52
|
Yan Z, Jin S, Wei Z, Huilian H, Zhanhai Y,
Yue T, Juan L, Jing L, Libo Y and Xu L: Discoidin domain receptor 2
facilitates prostate cancer bone metastasis via regulating
parathyroid hormone-related protein. Biochim Biophys Acta.
1842:1350–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Calderwood DA, Campbell ID and Critchley
DR: Talins and kindlins: Partners in integrin-mediated adhesion.
Nat Rev Mol Cell Biol. 14:503–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jin JK, Tien PC, Cheng CJ, Song JH, Huang
C, Lin SH and Gallick GE: Talin1 phosphorylation activates β1
integrins: A novel mechanism to promote prostate cancer bone
metastasis. Oncogene. 34:1811–1821. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Holbourn KP, Acharya KR and Perbal B: The
CCN family of proteins: Structure-function relationships. Trends
Biochem Sci. 33:461–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tai HC, Chang AC, Yu HJ, Huang CY, Tsai
YC, Lai YW, Sun HL, Tang CH and Wang SW: Osteoblast-derived
WNT-induced secreted protein 1 increases VCAM-1 expression and
enhances prostate cancer metastasis by down-regulating miR-126.
Oncotarget. 5:7589–7598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chang AC, Chen PC, Lin YF, Su CM, Liu JF,
Lin TH, Chuang SM and Tang CH: Osteoblast-secreted WISP-1 promotes
adherence of prostate cancer cells to bone via the VCAM-1/integrin
α4β1 system. Cancer Lett. 426:47–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yao L and Zhang X: Interaction between
prostate cancer stem cells and bone microenvironment regulates
prostate cancer bone metastasis and treatment resistance. J Cancer.
13:2757–2767. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Choi SY, Jeon JM, Na AY, Kwon OK, Bang IH,
Ha YS, Bae EJ, Park BH, Lee EH, Kwon TG, et al: SIRT5 directly
inhibits the PI3K/AKT pathway in prostate cancer cell lines. Cancer
Genomics Proteomics. 19:50–59. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Siddiqui JA, Seshacharyulu P, Muniyan S,
Pothuraju R, Khan P, Vengoji R, Chaudhary S, Maurya SK, Lele SM,
Jain M, et al: GDF15 promotes prostate cancer bone metastasis and
colonization through osteoblastic CCL2 and RANKL activation. Bone
Res. 10:62022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Toden S, Zumwalt TJ and Goel A: Non-coding
RNAs and potential therapeutic targeting in cancer. Biochim Biophys
Acta Rev Cancer. 1875:1884912021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Loganathan T and Doss CGP: Non-coding RNAs
in human health and disease: Potential function as biomarkers and
therapeutic targets. Funct Integr Genomics. 23:332023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ma L, Bajic VB and Zhang Z: On the
classification of long non-coding RNAs. RNA Biol. 10:925–933. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang J, Yue BL, Huang YZ, Lan XY, Liu WJ
and Chen H: Exosomal RNAs: Novel potential biomarkers for
diseases-a review. Int J Mol Sci. 23:24612022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Prigol AN, Rode MP, da Luz Efe F, Saleh NA
and Creczynski-Pasa TB: The bone microenvironment soil in prostate
cancer metastasis: An miRNA approach. Cancers (Basel). 15:40272023.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu Y, Yang C, Chen S, Liu W, Liang J, He
S and Hui J: Cancer-derived exosomal miR-375 targets DIP2C and
promotes osteoblastic metastasis and prostate cancer progression by
regulating the Wnt signaling pathway. Cancer Gene Ther. 30:437–449.
2023.PubMed/NCBI
|
|
68
|
Zheng Y, Qi F, Li L, Yu B, Cheng Y, Ge M,
Qin C and Li X: LncNAP1L6 activates MMP pathway by stabilizing the
m6A-modified NAP1L2 to promote malignant progression in prostate
cancer. Cancer Gene Ther. 30:209–218. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Guo H, Zhao J, Li X, Sun F, Qin Y, Yang X,
Xiong X, Yin Q, Wang X, Gao L, et al: Identification of miR-1-3p,
miR-143-3p and miR-145-5p association with bone metastasis of
Gleason 3+4 prostate cancer and involvement of LASP1 regulation.
Mol Cell Probes. 68:1019012023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dai Y, Ren D, Yang Q, Cui Y, Guo W, Lai Y,
Du H, Lin C, Li J, Song L and Peng X: The TGF-β signalling negative
regulator PICK1 represses prostate cancer metastasis to bone. Br J
Cancer. 117:685–694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ye Y, Li SL, Ma YY, Diao YJ, Yang L, Su
MQ, Li Z, Ji Y, Wang J, Lei L, et al: Exosomal miR-141-3p regulates
osteoblast activity to promote the osteoblastic metastasis of
prostate cancer. Oncotarget. 8:94834–94849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wa Q, Zou C, Lin Z, Huang S, Peng X, Yang
C, Ren D, Xu D, Guo Y, Liao Z, et al: Ectopic expression of
miR-532-3p suppresses bone metastasis of prostate cancer cells via
inactivating NF-κB signaling. Mol Ther Oncolytics. 17:267–277.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ren D, Yang Q, Dai Y, Guo W, Du H, Song L
and Peng X: Oncogenic miR-210-3p promotes prostate cancer cell EMT
and bone metastasis via NF-κB signaling pathway. Mol Cancer.
16:1172017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen Q, Zhang H, Zhang J, Shen L, Yang J,
Wang Y, Ma J and Zhuan B: miR-210-3p promotes lung cancer
development and progression by modulating USF1 and PCGF3. Onco
Targets Ther. 14:3687–3700. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tuo X, Zhou Y, Yang X, Ma S, Liu D, Zhang
X, Hou H, Wang R, Li X and Zhao L: miR-532-3p suppresses
proliferation and invasion of ovarian cancer cells via
GPNMB/HIF-1α/HK2 axis. Pathol Res Pract. 237:1540322022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Luo B, Yuan Y, Zhu Y, Liang S, Dong R, Hou
J, Li P, Xing Y, Lu Z, Lo R and Kuang GM: microRNA-145-5p inhibits
prostate cancer bone metastatic by modulating the
epithelial-mesenchymal transition. Front Oncol. 12:9887942022.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chang YS, Chen WY, Yin JJ,
Sheppard-Tillman H, Huang J and Liu YN: EGF receptor promotes
prostate cancer bone metastasis by downregulating miR-1 and
activating TWIST1. Cancer Res. 75:3077–3086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fu Q, Liu X, Liu Y, Yang J, Lv G and Dong
S: MicroRNA-335 and −543 suppress bone metastasis in prostate
cancer via targeting endothelial nitric oxide synthase. Int J Mol
Med. 36:1417–1425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Colden M, Dar AA, Saini S, Dahiya PV,
Shahryari V, Yamamura S, Tanaka Y, Stein G, Dahiya R and Majid S:
MicroRNA-466 inhibits tumor growth and bone metastasis in prostate
cancer by direct regulation of osteogenic transcription factor
RUNX2. Cell Death Dis. 8:e25722017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Huang S, Wa Q, Pan J, Peng X, Ren D, Huang
Y, Chen X and Tang Y: Downregulation of miR-141-3p promotes bone
metastasis via activating NF-κB signaling in prostate cancer. J Exp
Clin Cancer Res. 36:1732017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang S, Wa Q, Pan J, Peng X, Ren D, Li Q,
Dai Y, Yang Q, Huang Y, Zhang X, et al: Transcriptional
downregulation of miR-133b by REST promotes prostate cancer
metastasis to bone via activating TGF-β signaling. Cell Death Dis.
9:7792018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Huang S, Zou C, Tang Y, Wa Q, Peng X, Chen
X, Yang C, Ren D, Huang Y, Liao Z, et al: miR-582-3p and miR-582-5p
suppress prostate cancer metastasis to bone by repressing TGF-β
signaling. Mol Ther Nucleic Acids. 16:91–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wa Q, Huang S, Pan J, Tang Y, He S, Fu X,
Peng X, Chen X, Yang C, Ren D, et al: miR-204-5p represses bone
metastasis via inactivating NF-κB signaling in prostate cancer. Mol
Ther Nucleic Acids. 18:567–579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wa Q, Li L, Lin H, Peng X, Ren D, Huang Y,
He P and Huang S: Downregulation of miR-19a-3p promotes invasion,
migration and bone metastasis via activating TGF-β signaling in
prostate cancer. Oncol Rep. 39:81–90. 2018.PubMed/NCBI
|
|
86
|
Qu L, Li Z and Liu P: mir-204-5p Acts as a
tumor suppressor by targeting DNM2 in osteosarcoma cells. J Healthc
Eng. 2022:89445882022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sun R, Wei T, Ding D, Zhang J, Chen S, He
HH, Wang L and Huang H: CYCLIN K down-regulation induces androgen
receptor gene intronic polyadenylation, variant expression and PARP
inhibitor vulnerability in castration-resistant prostate cancer.
Proc Natl Acad Sci USA. 119:e22055091192022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yang S, Chen B, Zhang B, Li C, Qiu Y, Yang
H and Huang Z: miR-204-5p promotes apoptosis and inhibits migration
of gastric cancer cells by targeting HER-2. Mol Med Rep.
22:2645–2654. 2020.PubMed/NCBI
|
|
89
|
Peng L, Li P and Peng Z: miR-141-3p
enhanced radiosensitivity of CRC cells. Comb Chem High Throughput
Screen. 27:118–126. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu Y, Fu W, Yin F, Xia L, Zhang Y, Wang
B, Li T, Zhang T, Cheng L, Wei Y and Gao B: miR-141-3p suppresses
development of clear cell renal cell carcinoma by regulating NEK6.
Anticancer Drugs. 33:e125–e133. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ferraz RS, Cavalcante JVF, Magalhães L,
Ribeiro-Dos-Santos  and Dalmolin RJS: Revealing metastatic
castration-resistant prostate cancer master regulator through
lncRNAs-centered regulatory network. Cancer Med. 12:19279–19290.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang M, Yin C, Wu Z, Wang X, Lin Q, Jiang
X, Du H, Lang C, Peng X and Dai Y: The long transcript of lncRNA
TMPO-AS1 promotes bone metastases of prostate cancer by regulating
the CSNK2A1/DDX3X complex in Wnt/β-catenin signaling. Cell Death
Discov. 9:2872023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xi X, Hu Z, Wu Q, Hu K, Cao Z, Zhou J,
Liao J, Zhang Z, Hu Y, Zhong X and Bao Y: High expression of small
nucleolar RNA host gene 3 predicts poor prognosis and promotes bone
metastasis in prostate cancer by activating transforming growth
factor-beta signaling. Bioengineered. 13:1895–1907. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Misawa A, Kondo Y, Takei H and Takizawa T:
Long noncoding RNA HOXA11-AS and transcription factor HOXB13
modulate the expression of bone metastasis-related genes in
prostate cancer. Genes (Basel). 12:1822021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lang C, Dai Y, Wu Z, Yang Q, He S, Zhang
X, Guo W, Lai Y, Du H, Wang H, et al: SMAD3/SP1 complex-mediated
constitutive active loop between lncRNA PCAT7 and TGF-β signaling
promotes prostate cancer bone metastasis. Mol Oncol. 14:808–828.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hao H, Chen H, Xie L, Liu H and Wang D:
LncRNA KCNQ1OT1 promotes proliferation, invasion and metastasis of
prostate cancer by regulating miR-211-5p/CHI3L1 pathway. Onco
Targets Ther. 14:1659–1671. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hu CY, Chen J, Qin XH, You P, Ma J, Zhang
J, Zhang H and Xu JD: Long non-coding RNA NORAD promotes the
prostate cancer cell extracellular vesicle release via
microRNA-541-3p-regulated PKM2 to induce bone metastasis of
prostate cancer. J Exp Clin Cancer Res. 40:982021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mo C, Huang B, Zhuang J, Jiang S, Guo S
and Mao X: LncRNA nuclear-enriched abundant transcript 1 shuttled
by prostate cancer cells-secreted exosomes initiates osteoblastic
phenotypes in the bone metastatic microenvironment via
miR-205-5p/runt-related transcription factor 2/splicing factor
proline- and glutamine-rich/polypyrimidine tract-binding protein 2
axis. Clin Transl Med. 11:e4932021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ma Q, Qi X, Lin X, Li L, Chen L and Hu W:
LncRNA SNHG3 promotes cell proliferation and invasion through the
miR-384/hepatoma-derived growth factor axis in breast cancer. Hum
Cell. 33:232–242. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zheng S, Jiang F, Ge D, Tang J, Chen H,
Yang J, Yao Y, Yan J, Qiu J, Yin Z, et al: LncRNA
SNHG3/miRNA-151a-3p/RAB22A axis regulates invasion and migration of
osteosarcoma. Biomed Pharmacother. 112:1086952019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xuan Y and Wang Y: Long non-coding RNA
SNHG3 promotes progression of gastric cancer by regulating
neighboring MED18 gene methylation. Cell Death Dis. 10:6942019.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fendler WP, Eiber M, Beheshti M, Bomanji
J, Calais J, Ceci F, Cho SY, Fanti S, Giesel FL, Goffin K, et al:
PSMA PET/CT: Joint EANM procedure guideline/SNMMI procedure
standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol
Imaging. 50:1466–1486. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Eiber M, Herrmann K, Calais J, Hadaschik
B, Giesel FL, Hartenbach M, Hope T, Reiter R, Maurer T, Weber WA
and Fendler WP: Prostate cancer molecular imaging standardized
evaluation (PROMISE): Proposed miTNM classification for the
interpretation of PSMA-ligand PET/CT. J Nucl Med. 59:469–478. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Regula N, Kostaras V, Johansson S, Trampal
C, Lindström E, Lubberink M, Iyer V, Velikyan I and Sörensen J:
Comparison of 68Ga-PSMA PET/CT with fluoride PET/CT for
detection of bone metastatic disease in prostate cancer. Eur J
Hybrid Imaging. 6:52022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brenner AI, Koshy J, Morey J, Lin C and
DiPoce J: The bone scan. Semin Nucl Med. 42:11–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Even-Sapir E, Metser U, Mishani E,
Lievshitz G, Lerman H and Leibovitch I: The detection of bone
metastases in patients with high-risk prostate cancer: 99mTc-MDP
Planar bone scintigraphy, single- and multi-field-of-view SPECT,
18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 47:287–297.
2006.PubMed/NCBI
|
|
107
|
Calais J, Ceci F, Eiber M, Hope TA, Hofman
MS, Rischpler C, Bach-Gansmo T, Nanni C, Savir-Baruch B, Elashoff
D, et al: 18F-fluciclovine PET-CT and
68Ga-PSMA-11 PET-CT in patients with early biochemical
recurrence after prostatectomy: A prospective, single-centre,
single-arm, comparative imaging trial. Lancet Oncol. 20:1286–1294.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
van Boxtel W, Lütje S, van Engen-van
Grunsven ICH, Verhaegh GW, Schalken JA, Jonker MA, Nagarajah J,
Gotthardt M and van Herpen CML: 68Ga-PSMA-HBED-CC PET/CT
imaging for adenoid cystic carcinoma and salivary duct carcinoma: A
phase 2 imaging study. Theranostics. 10:2273–2283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sonni I, Felker ER, Lenis AT, Sisk AE,
Bahri S, Allen-Auerbach M, Armstrong WR, Suvannarerg V, Tubtawee T,
Grogan T, et al: Head-to-head comparison of 68Ga-PSMA-11
PET/CT and mpMRI with a histopathology gold standard in the
detection, intraprostatic localization, and determination of local
extension of primary prostate cancer: Results from a prospective
single-center imaging trial. J Nucl Med. 63:847–854. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhou J, Gou Z, Wu R, Yuan Y, Yu G and Zhao
Y: Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and
bone scintigraphy in the diagnosis of bone metastases in patients
with prostate cancer: A systematic review and meta-analysis.
Skeletal Radiol. 48:1915–1924. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhou J, Wu R, Wang W, Zhao Y and Liu X:
68Ga-PSMA PET/CT for the detection of bone metastases in
prostate cancer: A systematic review of the published literature.
Clin Physiol Funct Imaging. Oct 29–2017.(Epub ahead of print).
|
|
112
|
Coleman RE, Croucher PI, Padhani AR,
Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R and
Costa L: Bone metastases. Nat Rev Dis Primers. 6:832020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Urabe F, Kosaka N, Yamamoto Y, Ito K,
Otsuka K, Soekmadji C, Egawa S, Kimura T and Ochiya T: Metastatic
prostate cancer-derived extracellular vesicles facilitate
osteoclastogenesis by transferring the CDCP1 protein. J Extracell
Vesicles. 12:e123122023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hu C, Chen Q, Wu T, Du X, Dong Y, Peng Z,
Xue W, Sunkara V, Cho YK and Dong L: The role of extracellular
vesicles in the treatment of prostate cancer. Small.
20:e23110712024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang Y, Fang YX, Dong B, Du X, Wang J,
Wang X, Gao WQ and Xue W: Discovery of extracellular vesicles
derived miR-181a-5p in patient's serum as an indicator for
bone-metastatic prostate cancer. Theranostics. 11:878–892. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yu L, Sui B, Fan W, Lei L, Zhou L, Yang L,
Diao Y, Zhang Y, Li Z, Liu J and Hao X: Exosomes derived from
osteogenic tumor activate osteoclast differentiation and
concurrently inhibit osteogenesis by transferring COL1A1-targeting
miRNA-92a-1-5p. J Extracell Vesicles. 10:e120562021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang J, Du X, Wang X, Xiao H, Jing N, Xue
W, Dong B, Gao WQ and Fang YX: Tumor-derived miR-378a-3p-containing
extracellular vesicles promote osteolysis by activating the
Dyrk1a/Nfatc1/Angptl2 axis for bone metastasis. Cancer Lett.
526:76–90. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zeng F, Zhao C, Wang R, Ren L, Qiu H, Zou
Z, Ding H, Sun Z, Li J and Dong S: Antagonizing exosomal miR-18a-5p
derived from prostate cancer cells ameliorates metastasis-induced
osteoblastic lesions by targeting Hist1h2bc and activating
Wnt/β-catenin pathway. Genes Dis. 10:1626–1640. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Baldessari C, Pipitone S, Molinaro E,
Cerma K, Fanelli M, Nasso C, Oltrecolli M, Pirola M, D'Agostino E,
Pugliese G, et al: Bone metastases and health in prostate cancer:
From pathophysiology to clinical implications. Cancers (Basel).
15:15182023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Schwartz GG: Prostate cancer, serum
parathyroid hormone, and the progression of skeletal metastases.
Cancer Epidemiol Biomarkers Prev. 17:478–483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yuan S, Hoggard NK, Kantake N, Hildreth BE
III and Rosol TJ: Effects of dickkopf-1 (DKK-1) on prostate cancer
growth and bone metastasis. Cells. 12:26952023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang B, Li Y, Wu Q, Xie L, Barwick B, Fu
C, Li X, Wu D, Xia S, Chen J, et al: Acetylation of KLF5 maintains
EMT and tumorigenicity to cause chemoresistant bone metastasis in
prostate cancer. Nat Commun. 12:17142021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gomez-Veiga F, Ponce-Reixa J,
Martinez-Breijo S, Planas J and Morote J: Advances in prevention
and treatment of bone metastases in prostate cancer. Role of
RANK/RANKL inhibition. Actas Urol Esp. 37:292–304. 2013.(In
English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Mizuta K, Oshiro H, Katsuki R, Tsuha Y,
Aoki Y, Tome Y and Nishida K: Denosumab administration for bone
metastases from solid tumors: A retrospective cross-sectional
study. BMC Cancer. 23:9992023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Agarwal N, McGregor B, Maughan BL, Dorff
TB, Kelly W, Fang B, McKay RR, Singh P, Pagliaro L, Dreicer R, et
al: Cabozantinib in combination with atezolizumab in patients with
metastatic castration-resistant prostate cancer: Results from an
expansion cohort of a multicentre, open-label, phase 1b trial
(COSMIC-021). Lancet Oncol. 23:899–909. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yu EY, Wilding G, Posadas E, Gross M,
Culine S, Massard C, Morris MJ, Hudes G, Calabrò F, Cheng S, et al:
Phase II study of dasatinib in patients with metastatic
castration-resistant prostate cancer. Clin Cancer Res.
15:7421–7428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Qiao L, Liang Y, Li N, Hu X, Luo D, Gu J,
Lu Y and Zheng Q: Endothelin-A receptor antagonists in prostate
cancer treatment-a meta-analysis. Int J Clin Exp Med. 8:3465–3473.
2015.PubMed/NCBI
|
|
128
|
Lee YT, Tan YJ and Oon CE: Molecular
targeted therapy: Treating cancer with specificity. Eur J
Pharmacol. 834:188–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Reichert ZR, Urrutia J and Alumkal JJ:
Microsatellite Instability as an emerging biomarker for checkpoint
inhibitor response in advanced prostate cancer. JAMA Oncology.
5:478–479. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mitsogiannis I, Tzelves L, Dellis A, Issa
H, Papatsoris A and Moussa M: Prostate cancer immunotherapy. Expert
Opin Biol Ther. 22:577–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wu YM, Cieślik M, Lonigro RJ, Vats P,
Reimers MA, Cao X, Ning Y, Wang L, Kunju LP, de Sarkar N, et al:
Inactivation of CDK12 delineates a distinct immunogenic class of
advanced prostate cancer. Cell. 173:1770–1782.e14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Bochum S, Berger S and Martens UM:
Olaparib. Recent Results Cancer Res. 211:217–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Risdon EN, Chau CH, Price DK, Sartor O and
Figg WD: PARP inhibitors and prostate cancer: To infinity and
beyond BRCA. Oncologist. 26:e115–e129. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chen A: PARP inhibitors: Its role in
treatment of cancer. Chin J Cancer. 30:463–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Stamatakos PV, Fragkoulis C, Leventi A,
Gklinos K, Kontolatis N, Papatsoris A and Dellis A: PSMA-based
therapeutics for prostate cancer. Expert Opin Pharmacother.
25:1405–1419. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Combes AD, Palma CA, Calopedos R, Wen L,
Woo H, Fulham M and Leslie S: PSMA PET-CT in the diagnosis and
staging of prostate cancer. Diagnostics (Basel). 12:25942022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Sun M, Niaz MO, Nelson A, Skafida M and
Niaz MJ: Review of 177Lu-PSMA-617 in patients with metastatic
castration-resistant prostate cancer. Cureus.
12:e89212020.PubMed/NCBI
|
|
138
|
Narayan V, Barber-Rotenberg JS, Jung IY,
Lacey SF, Rech AJ, Davis MM, Hwang WT, Lal P, Carpenter EL, Maude
SL, et al: PSMA-targeting TGFβ-insensitive armored CAR T cells in
metastatic castration-resistant prostate cancer: A phase 1 trial.
Nat Med. 28:724–734. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Almuradova E, Seyyar M, Arak H, Tamer F,
Kefeli U, Koca S, Sen E, Telli TA, Karatas F, Gokmen I, et al: The
real-world outcomes of Lutetium-177 PSMA-617 radioligand therapy in
metastatic castration-resistant prostate cancer: Turkish oncology
group multicenter study. Int J Cancer. 154:692–700. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Maharaj M, Heslop L, Govender T, Korowlay
N, Singh A, Choudhary P and Sathekge M: The outcome and safety of
re-challenge Lutetium-177 PSMA (177Lu-PSMA) therapy with
low-dose docetaxel as a radiosensitizer-a promising combination in
metastatic castrate-resistant prostate cancer (mCRPC): A case
report. Nucl Med Mol Imaging. 55:136–140. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Flegar L, Thoduka SG, Librizzi D, Luster
M, Zacharis A, Heers H, Eisenmenger N, Ahmadzadehfar H, Eiber M,
Weber W, et al: Adoption of Lutetium-177 PSMA
radioligand therapy for metastatic castration resistant prostate
cancer: A total population analysis in Germany from 2016 to 2020.
Eur J Nucl Med Mol Imaging. 50:2188–2195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Khreish F, Ghazal Z, Marlowe RJ, Rosar F,
Sabet A, Maus S, Linxweiler J, Bartholomä M and Ezziddin S: 177
Lu-PSMA-617 radioligand therapy of metastatic castration-resistant
prostate cancer: Initial 254-patient results from a prospective
registry (REALITY study). Eur J Nucl Med Mol Imaging. 49:1075–1085.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zarrabi KK, Narayan V, Mille PJ, Zibelman
MR, Miron B, Bashir B and Kelly WK: Bispecific PSMA antibodies and
CAR-T in metastatic castration-resistant prostate cancer. Ther Adv
Urol. 15:175628722311822192023. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lund ME, Howard CB, Thurecht KJ, Campbell
DH, Mahler SM and Walsh BJ: A bispecific T cell engager targeting
Glypican-1 redirects T cell cytolytic activity to kill prostate
cancer cells. BMC Cancer. 20:12142020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yamamoto K, Trad A, Baumgart A, Hüske L,
Lorenzen I, Chalaris A, Grötzinger J, Dechow T, Scheller J and
Rose-John S: A novel bispecific single-chain antibody for ADAM17
and CD3 induces T-cell-mediated lysis of prostate cancer cells.
Biochem J. 445:135–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Nyquist MD, Corella A, Coleman I, De
Sarkar N, Kaipainen A, Ha G, Gulati R, Ang L, Chatterjee P, Lucas
J, et al: Combined TP53 and RB1 loss promotes prostate cancer
resistance to a spectrum of therapeutics and confers vulnerability
to replication stress. Cell Rep. 31:1076692020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Patel SA: Managing the unmanageable:
Evidence-driven approaches to real-world patient prototypes of
TP53-mutant myelodysplastic neoplasms and acute myeloid leukemia.
Leukemia. Sep 30–2024.(Epub ahead of print). View Article : Google Scholar
|
|
148
|
Lee YC, Lee YL and Li CY: BRCA genes and
related cancers: A meta-analysis from epidemiological cohort
studies. Medicina (Kaunas). 57:9052021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Sweeney C, Bracarda S, Sternberg CN, Chi
KN, Olmos D, Sandhu S, Massard C, Matsubara N, Alekseev B, Parnis
F, et al: Ipatasertib plus abiraterone and prednisolone in
metastatic castration-resistant prostate cancer (IPATential150): A
multicentre, randomised, double-blind, phase 3 trial. Lancet.
398:131–142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Fizazi K, Carducci M, Smith M, Damião R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Shenderov E, Boudadi K, Fu W, Wang H,
Sullivan R, Jordan A, Dowling D, Harb R, Schonhoft J, Jendrisak A,
et al: Nivolumab plus ipilimumab, with or without enzalutamide, in
AR-V7-expressing metastatic castration-resistant prostate cancer: A
phase-2 nonrandomized clinical trial. Prostate. 81:326–338. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Graff JN, Beer TM, Alumkal JJ, Slottke RE,
Redmond WL, Thomas GV, Thompson RF, Wood MA, Koguchi Y, Chen Y, et
al: A phase II single-arm study of pembrolizumab with enzalutamide
in men with metastatic castration-resistant prostate cancer
progressing on enzalutamide alone. J Immunother Cancer.
8:e0006422020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
McNeel DG, Eickhoff JC, Wargowski E,
Johnson LE, Kyriakopoulos CE, Emamekhoo H, Lang JM, Brennan MJ and
Liu G: Phase 2 trial of T-cell activation using MVI-816 and
pembrolizumab in patients with metastatic, castration-resistant
prostate cancer (mCRPC). J Immunother Cancer. 10:e0041982022.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Xia QD, Zhang SH, Zeng N, Lu YC, Qin BL
and Wang SG: Novel androgen receptor inhibitors for metastatic
hormone-sensitive prostate cancer: Current application and future
perspectives. Biomed Pharmacother. 168:1158062023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Shah H and Vaishampayan U: Therapy of
advanced prostate cancer: Targeting the androgen receptor axis in
earlier lines of treatment. Target Oncol. 13:679–689. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhang Y, Ming A, Wang J, Chen W and Fang
Z: PROTACs targeting androgen receptor signaling: Potential
therapeutic agents for castration-resistant prostate cancer.
Pharmacol Res. 205:1072342024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Petrylak DP, Vaishampayan UN, Patel KR,
Higano CS, Albany C, Dawson NA, Mehlhaff BA, Quinn DI, Nordquist
LT, Wagner VJ, et al: A randomized phase IIa study of quantified
bone scan response in patients with metastatic castration-resistant
prostate cancer (mCRPC) treated with radium-223 dichloride alone or
in combination with abiraterone acetate/prednisone or enzalutamide.
ESMO Open. 6:1000822021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Manna F, Karkampouna S, Zoni E, De Menna
M, Hensel J, Thalmann GN and Kruithof-de Julio M: Metastases in
prostate cancer. Cold Spring Harb Perspect Med. 9:a0336882019.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang X: Interactions between cancer cells
and bone microenvironment promote bone metastasis in prostate
cancer. Cancer Commun (Lond). 39:762019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Lu J, Hu D, Zhang Y, Ma C, Shen L and
Shuai B: Current comprehensive understanding of denosumab (the
RANKL neutralizing antibody) in the treatment of bone metastasis of
malignant tumors, including pharmacological mechanism and clinical
trials. Front Oncol. 13:11338282023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Shiota M, Akamatsu S, Tsukahara S,
Nagakawa S, Matsumoto T and Eto M: Androgen receptor mutations for
precision medicine in prostate cancer. Endocr Relat Cancer.
29:R143–R155. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Farahzadi R, Valipour B, Montazersaheb S
and Fathi E: Targeting the stem cell niche micro-environment as
therapeutic strategies in aging. Front Cell Dev Biol.
11:11621362023. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Fathi E, Farahzadi R, Sheervalilou R,
Sanaat Z and Vietor I: A general view of CD33+ leukemic
stem cells and CAR-T cells as interesting targets in acute
myeloblatsic leukemia therapy. Blood Res. 55:10–16. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Fathi E, Valipour B, Vietor I and
Farahzadi R: An overview of the myocardial regeneration potential
of cardiac c-Kit+ progenitor cells via PI3K and MAPK
signaling pathways. Future Cardiol. 16:199–209. 2020. View Article : Google Scholar : PubMed/NCBI
|