Introduction
In recent years, the incidence and mortality of
colon cancer have risen (1). Colon
cancer accounts for 10.0 and 9.4% of all cancer types, ranking
third and second, respectively, as the leading cause of
cancer-related mortality worldwide (2). By 2030, the burden of colon cancer is
expected to increase by 60% to >2.2 million new cases and 1.1
million mortalities (3). The
occurrence and development of colon cancer are closely related to
the tumor microenvironment, including inflammatory response and
heredity, and are associated with risk factors such as population
aging, dietary habits, obesity, lack of physical exercise and
smoking (4). Among colon cancer
cases, 20–30% are familial inherited due to genetic factors, and
~70% are affected by environmental factors (5). With the intervention of early
screening methods such as colonoscopy, the overall incidence of
colon cancer has shown a slow decreasing trend, but the age at
diagnosis tends to be younger (5).
In addition, the early symptoms of colon cancer are more insidious,
and the majority of patients are diagnosed in middle and advanced
stages (6).
At present, the combined treatment of surgery and
chemoradiotherapy is the standard modality for patients with
advanced rectal cancer (7).
Surgical resection of the lesion is the preferred treatment
modality (8). However, there is a
high incidence of local recurrence and distant metastasis after
surgical treatment alone (8).
Systematic radiotherapy (RT) and chemotherapy are essential to
relieve the symptoms and prolong the life of most patients,
particularly those with metastatic symptoms of cancer cells
(9). Current first-line
chemotherapy regimens for colon cancer include combination of
fluorouracil, leucovorin and oxaliplatin or fluorouracil,
irinotecan and bevacizumab (10).
However, the unavoidable side effects of chemotherapeutic drugs
(for example, diarrhea, nausea, vomiting, acute myocardial
infarction and cerebrovascular injury) greatly reduce the quality
of life of patients, and are serious and even potentially
life-threatening (11).
Preoperative RT can effectively eliminate subclinical lesions
around the tumor, reduce tumor volume, reduce tumor stage, and
increase the possibility of anus preservation for patients
(12). In addition, preoperative RT
can reduce local recurrence and increase patient survival
opportunities (13). However,
tolerance to radiation therapy for colon cancer as well as the side
effects of RT remain the biggest problems affecting clinicians
(13). Therefore, it is important
to screen and search for natural products with low toxicity and
high antitumor activity.
In recent years, the status of traditional Chinese
medicine has gradually increased in the study of radiosensitizers
(14). The antitumor effect of
isoegomaketone (IK) (PubChem Compound ID: 5318556) in the perilla
extract is particularly prominent (15). It has been shown that IK can promote
apoptosis and inhibit cell proliferation, and exert
radio-sensitizing effects on lung cancer cells by regulating the
expression of endoplasmic reticulum stress proteins (15). In addition, IK has biological
functions such as anti-inflammatory, antifungal, healing-promoting
activities and resistance to toxicity induced by immunotherapy
(16). However, the RT sensitizing
effect of IK on colon cancer and whether IK can alleviate the side
effects of RT on intestinal injury have not been reported to date.
Therefore, the present study intended to investigate the RT
sensitizing effect of IK in colon cancer and its effect on reducing
intestinal side effects and related potential mechanisms by using
the combination of RT and IK in in vitro and in vivo
experiments.
Materials and methods
Materials and reagents
HT-29 cells (cat. no. HTB-38) and CCD-18Co cells
(cat. no. CRL-1459) were purchased from the American Type Culture
Collection. IK was isolated from Perilla frutescens Britt by
the supercritical carbon dioxide (SC-CO2) method
(17). DMEM (cat. no. SH30285.02),
fetal bovine serum (FBS; cat. no. SH30088.03IR25-40), trypsin (cat.
no. SH30042.02) and penicillin-streptomycin (cat. no. SV30010) were
purchased from HyClone (Cytiva). Cell Counting Kit-8 (CCK-8) assay
kit (cat. no. PA137267) was purchased from Pierce (Thermo Fisher
Scientific, Inc.). Specific antibodies against HIF-1α (1:1,000;
cat. no. 36169), PI3K (1:1,000; cat. no. 4292), phosphorylated
(p)-PI3K (1:1,000; cat. no. 4228), AKT (cat. no. 9272), p-AKT
(1:500; cat. no. 9271), mTOR (1:500; cat. no. 2972), p-mTOR
(1:1,000; cat. no. 2971), BCL-2 (1:500; cat. no. 15071), BAX
(1:1,000; cat. no. 2772), LC3 I/II (1:1,000; cat. no. 4108S),
Beclin-1 (1:1,000; cat. no. 3738) and β-actin (1:1,000; cat. no.
4967) were purchased from Cell Signaling Technology, Inc. Specific
antibodies against γH2AX (1:1,000; cat. no. ab81299) were purchased
from Abcam. A total of 40 male BALB/c nude mice (age, 4 weeks-old;
weight, 18~22 g) were purchased from Beijing HFK Bioscience Co.
Ltd. X-ray irradiator (X-ray RAD 320; Precision X-ray Inc.). All
mice were housed in an SPF environment with free access to food or
water at a temperature of 21–25°C and humidity of 60–70%, ensuring
12 h of alternating light.
Cell culture and assays
HT-29 and CCD-18Co cells were cultured in DMEM
containing 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin,
and placed in an incubator at 37°C with 5% CO2 and
saturated humidity. After 48 h of incubation, cells were rinsed
twice with PBS, and 1 ml trypsin was added for digestion at 37°C
for 5–6 min. After complete digestion, 2 ml fresh DMEM was added to
terminate the reaction, and a cell suspension was prepared, which
was pipetted into a 10-ml Eppendorf tube and centrifuged at 1,000 ×
g for 3 min at room temperature. Next, fresh DMEM was added in a
1:3 ratio, and the mixture was transferred to a flask and incubated
in a 37°C in a 5% CO2 incubator. HT-29 and CCD-18Co
cells were incubated with different concentrations of IK (0, 50,
100 or 200 µg/ml) for 48 h at ≤65% cell confluence. At the same
time, all grouped cells were irradiated with different doses (0, 1,
2, 4, 6, 8, 12 or 16 Gy) of X-rays (X-ray RAD 320 irradiator;
Precision X-ray, Inc.). Cells from each group were seeded in
96-well plates at a concentration of 6×103 cells/well. A
total of 10 µl CCK-8 solution was added to each well and incubated
at 37°C for 4 h. Finally, the optical density (OD) 450 nm values
were determined by using a plate reader. Cell viability was
calculated by average cell viability of control wells. DNA damage
was detected by single cell gel electrophoresis at 0, 15, 30, 60
and 120 min after X-ray irradiation. Olive tail moment (OTM) was
analyzed with Comet assay software project (http://casplab.com/).
Animal experiments
BALB/c nude mice were fed normally for 7 days after
acquisition. A mouse tumor model was established by injecting
cancer cells into mice. Briefly, log-proliferating human colon
cancer HT-29 cells were washed with PBS and digested with trypsin
for 4–6 min, and the digestion was terminated by adding fresh DMEM.
After centrifugation, cell suspensions were obtained after two
resuspensions in serum-free DMEM. The cell concentration in the
mixture was adjusted to 2.5×107 cells/ml. A total of 40
healthy BALB/c-nu nude mice were inoculated subcutaneously with 150
µl (3.75×106 cells) cell suspension on the dorsal side
of the right axilla to obtain a HT-29 tumor-bearing nude mouse
model. All HT-29 tumor-bearing nude mice were randomly divided into
i) control group, ii) RT, iii) IK and iv) RT + IK groups (n=10).
Mice in both the RT and combination groups were treated with 8 Gy
doses of X-ray radiation once a day for 14 consecutive days, while
mice in the IK and combination groups were intraperitoneally
injected with 100 mg/kg IK daily for 14 consecutive days. Placebo
was administered during RT to the control group. Tumor size was
measured every other day and the survival of mice was counted. Mice
were euthanized when the tumor size exceeded 3,000 mm3.
All surviving mice were anesthetized 24 h after the last
administration. The anesthetic agent used was sodium pentobarbital,
prepared as a 3% sterile saline solution at a dose of 30 mg/kg body
weight. Sodium pentobarbital was administered via intraperitoneal
injection, and the animals' responses were continuously observed.
Once the animals were fully anesthetized, they were euthanized by
cervical dislocation.
The procedures for sampling tumor and intestinal
tissue were as follows: Using surgical instruments, the skin over
the tumor site of the mouse was carefully cut and exposed. Next,
the tumor was carefully separated from the surrounding tissues and
the intact tumor tissue was placed in a sterile container for
subsequent experimental analysis or preservation. For intestinal
tissues, upon disinfecting the skin with alcohol, the abdominal
skin and muscle layers were cut open to expose the abdominal
cavity. After locating the intestine, the desired intestinal
segment was excised. All operations were performed under sterile
conditions to ensure that the samples were not contaminated. The
obtained tumor and intestinal tissues were washed with normal
saline to remove residual blood, dried with absorbent paper and
stored at −80°C for later use. Intestinal tissues were fixed in a
4% paraformaldehyde solution, then embedded in paraffin and cut
into 5-µm thick sections. The sections were sequentially stained
with hematoxylin for 5 min and eosin for 2 min at room temperature.
Intestinal images were obtained using a light microscope.
Peripheral blood was collected from mice and analyzed for
peripheral blood leukocyte, neutrophil and monocyte numbers using a
LH-750 hematology analyzer (Beckman Coulter, Inc.).
The present animal studies complied with all
relevant national regulations and institutional policies, and were
approved [approval no. 2023 (30)]
by the Animal Experimental Ethics Committee of the First Affiliated
Hospital, Zhejiang University School of Medicine (Hangzhou,
China).
ELISA
The levels of malondialdehyde (MDA) (cat. no.
ab238537; Abcam), TNF-α (cat. no. ab181421; Abcam), NF-κB (cat. no.
ab279874; Abcam) and IL-1β (cat. no. BMS6002-2TEN; Invitrogen;
Thermo Fisher Scientific, Inc.), and the activities of glutathione
(GSH) (cat. no. EEL155; Invitrogen; Thermo Fisher Scientific, Inc.)
and catalase (CAT) (cat. no. KTB9040; Abbkine Scientific Co., Ltd.)
were measured by ELISA. Briefly, intestinal tissue frozen at −80°C
was mixed with 1 ml tissue lysis buffer, ground to homogenate in a
glass mill in an ice bath, lysed at 4°C for 30 min and centrifuged
at 3,000 × g for 20 min at 4°C. The levels of MDA, TNF-α, NF-κB and
IL-1β in tissue homogenate, and the activities of GSH and CAT were
measured using commercial ELISA kits.
Western blotting
Western blotting was used to analyze protein
expression levels in different cells and tissues. Briefly, tumor
and intestinal tissues frozen at −80°C were mixed with 1 ml tissue
NP-40 lysis buffer (cat. no. P0013F; Beyotime Institute of
Biotechnology), ground to homogenate in a glass mill in an ice
bath, lysed at 4°C for 30 min and centrifuged at 3,000 × g for 20
min at 4°C. A total of 10 µl HT-29 cell homogenates, tumor tissue
homogenates or intestinal tissue homogenates were isolated by 10%
SDS-PAGE and transferred to nitrocellulose membranes, which were
then blocked with 5% bovine serum albumin (cat. no. ST2249-5g;
Beyotime Institute of Biotechnology) for 1 h at room temperature
and washed with TBS-Tween 20 (TBST; 20 mM Tris buffer, 0.1% Tween
20, pH 7.4) for 5 min. Subsequently, the membranes were incubated
at 4°C with a primary antibody (1:500) overnight and then with a
horseradish peroxidase-labeled secondary antibody (1:1,000; cat.
no. 58802; Cell Signaling Technology, Inc.) for 1 h at room
temperature. After washing with TBST, the membrane was immersed in
an enhanced chemiluminescence solution (SuperSignal™ West Atto;
cat. no. A38556; Thermo Fisher Scientific, Inc.). The gray value of
the bands was analyzed by ImageJ software (version 1.8.0; National
Institutes of Health). The target protein expression level was
calculated as the target band gray value divided by the reference
material strip gray value.
Statistical analysis
Data were analyzed using SPSS 25.0 statistical
software (IBM Corp.), and the data were expressed as the mean ± SD.
Inter-group comparisons were performed using one-way ANOVA followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
IK enhances the sensitivity of HT29
cells to X-rays
To investigate whether IK can synergistically
promote the inhibitory effect of X-rays on the proliferation of
human colorectal cancer cells, the viability of HT-29 cells treated
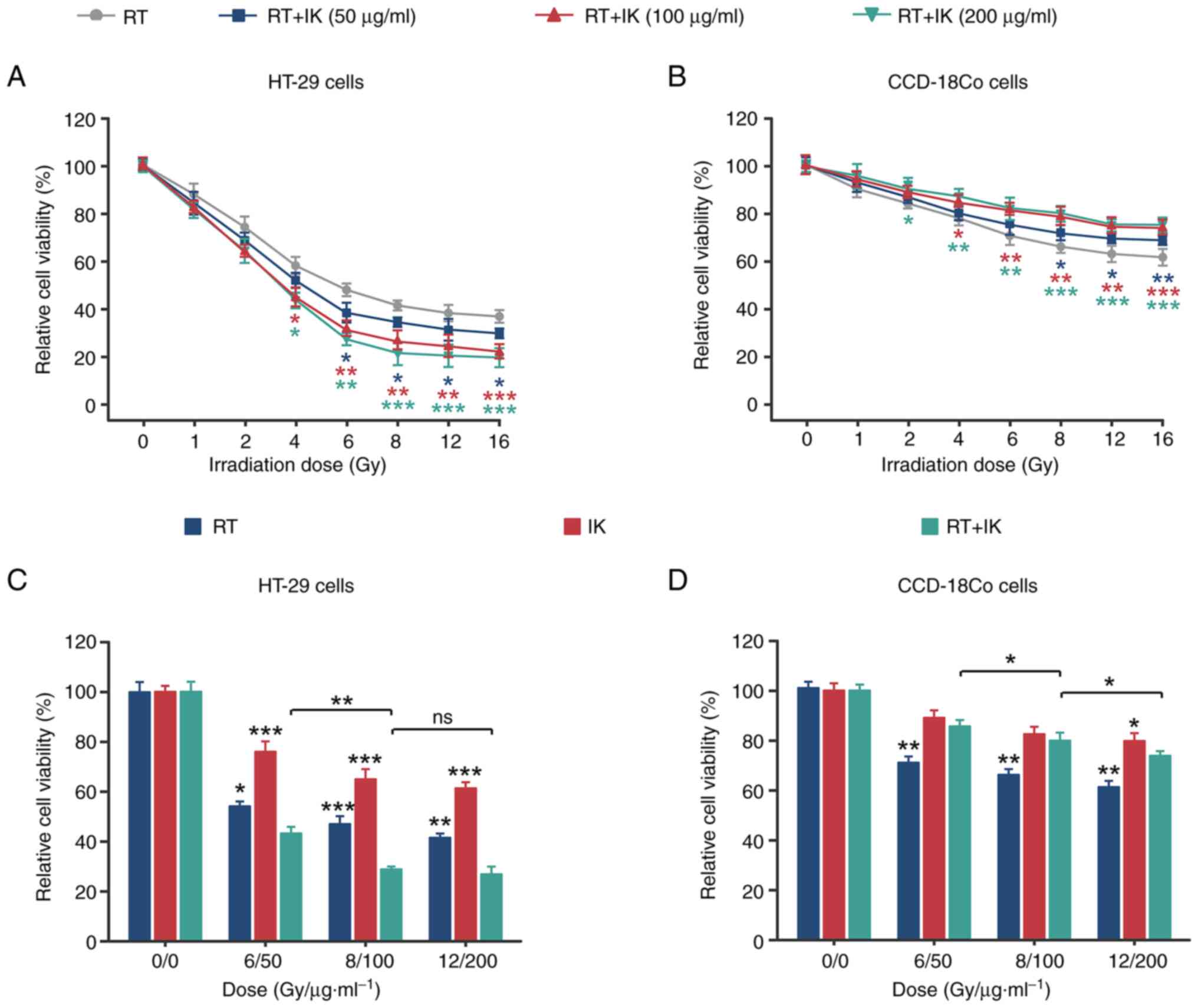
with RT combined with IK was examined by CCK-8 assay. As shown in
Fig. 1A, RT at different
irradiation doses inhibited the viability of HT-29 cells. In
addition, RT in combination with different concentrations of IK
further reduced HT-29 cell viability. Notably, for normal human
colon fibroblasts (CCD-18Co cells), IK effectively reversed the
RT-induced decrease in cell viability (Fig. 1B). The optimal dose ratio of RT in
combination with IK was further analyzed (Fig. 1C and D). At different doses of RT
and IK, the proliferation inhibition rate of HT-29 cells in the
combination group was significantly greater than that in the RT
alone or IK alone groups (both P<0.05). In addition, the
proliferation inhibition of CCD-18Co cells in the combination group
was significantly lower than that in the RT alone group (all
P<0.01). CCD-18Co cell viability was significantly decreased
(P<0.05) in the 12 Gy/200 µg/ml group compared with the RT/IK
dose of 8 Gy/100 µg/ml, but had no significant effect on HT-29 cell
viability (P>0.05). Therefore, 8 Gy/100 µg/ml was selected as
the optimal RT/IK dose ratio for subsequent studies. The
combination index (CI) values were further calculated (data not
shown), and they were observed to be <1 at each concentration,
suggesting that RT combined with IK had a synergistic inhibitory
effect on the proliferation of colon cancer cells.
IK enhances DNA damage in HT-29 cells
by inhibiting HIF-1α and the PI3K/AKT signaling pathway
After X-ray irradiation, the repair time of DNA
damage is usually 4–6 h. Therefore, single cell gel electrophoresis
was used in the present study to analyze the initial DNA damage in
HT-29 cells at 0, 15, 30, 60 and 120 min after irradiation. As
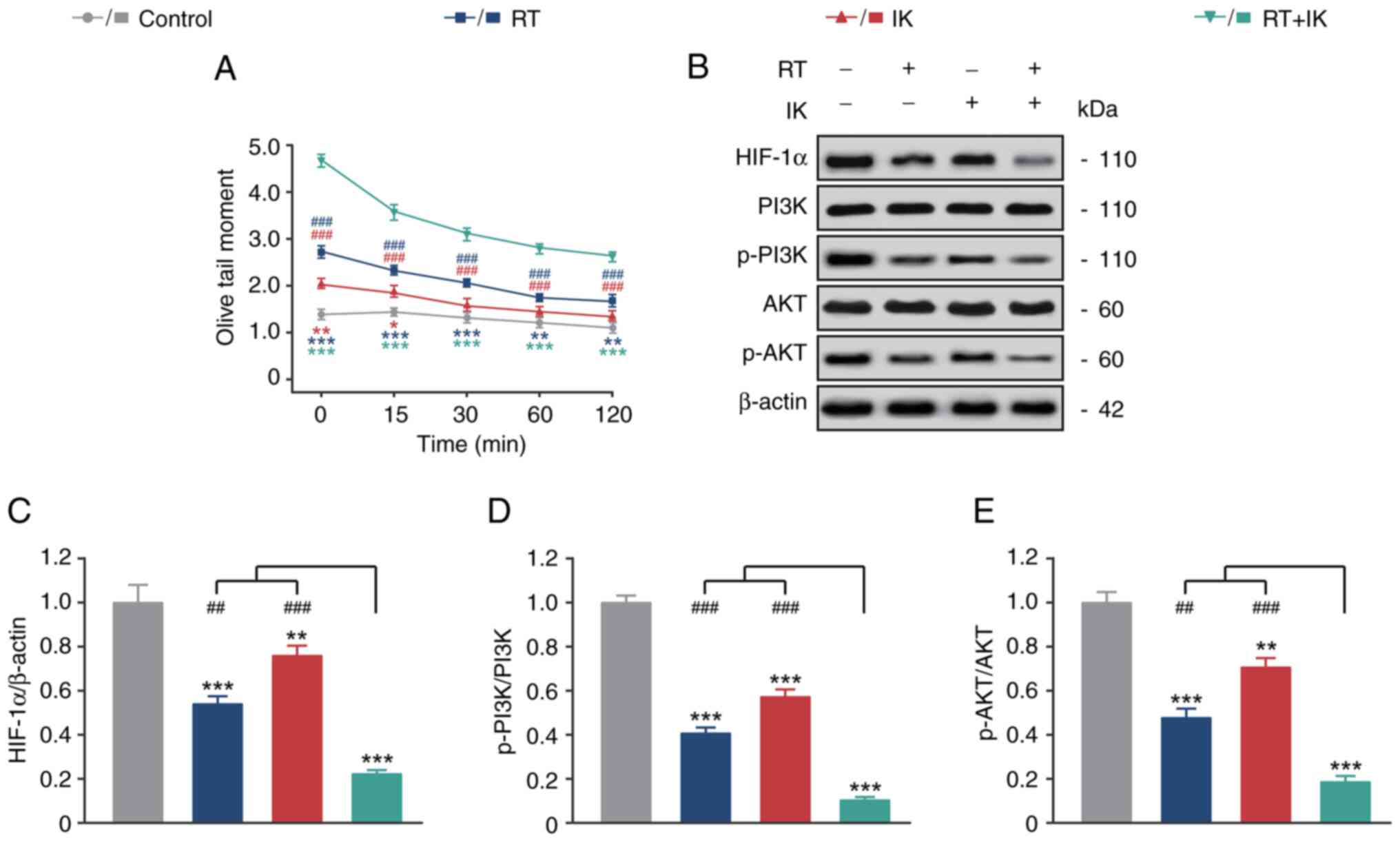
demonstrated in Fig. 2A, OTM in
control HT-29 cells remained low from 0 to 120 min. RT alone
significantly increased the OTM levels of HT-29 cells at 0, 15, 30,
60 and 120 min compared with the controls (P<0.01). Notably, IK
alone also slightly increased OTM levels in HT-29 cells at 0 and 15
min (both P<0.05). In addition, RT in combination with IK
increased the OTM levels of HT-29 cells to a greater extent and
significantly higher than either treatment alone (all
P<0.001).
The expression levels of HIF-1α and the P13K/AKT
signaling pathways in HT-29 cells were analyzed by western
blotting. As revealed in Fig. 2B-E,
RT or IK alone significantly decreased the expression levels of
HIF-1α, p-PI3K and p-AKT compared with controls (all P<0.01). In
addition, RT in combination with IK further reduced the expression
level of HIF-1α and the phosphorylation level of the PI3K/AKT
signaling pathway.
IK combined with RT promotes the
apoptosis and autophagy of HT-29 cells
The expression of apoptosis and autophagy-related
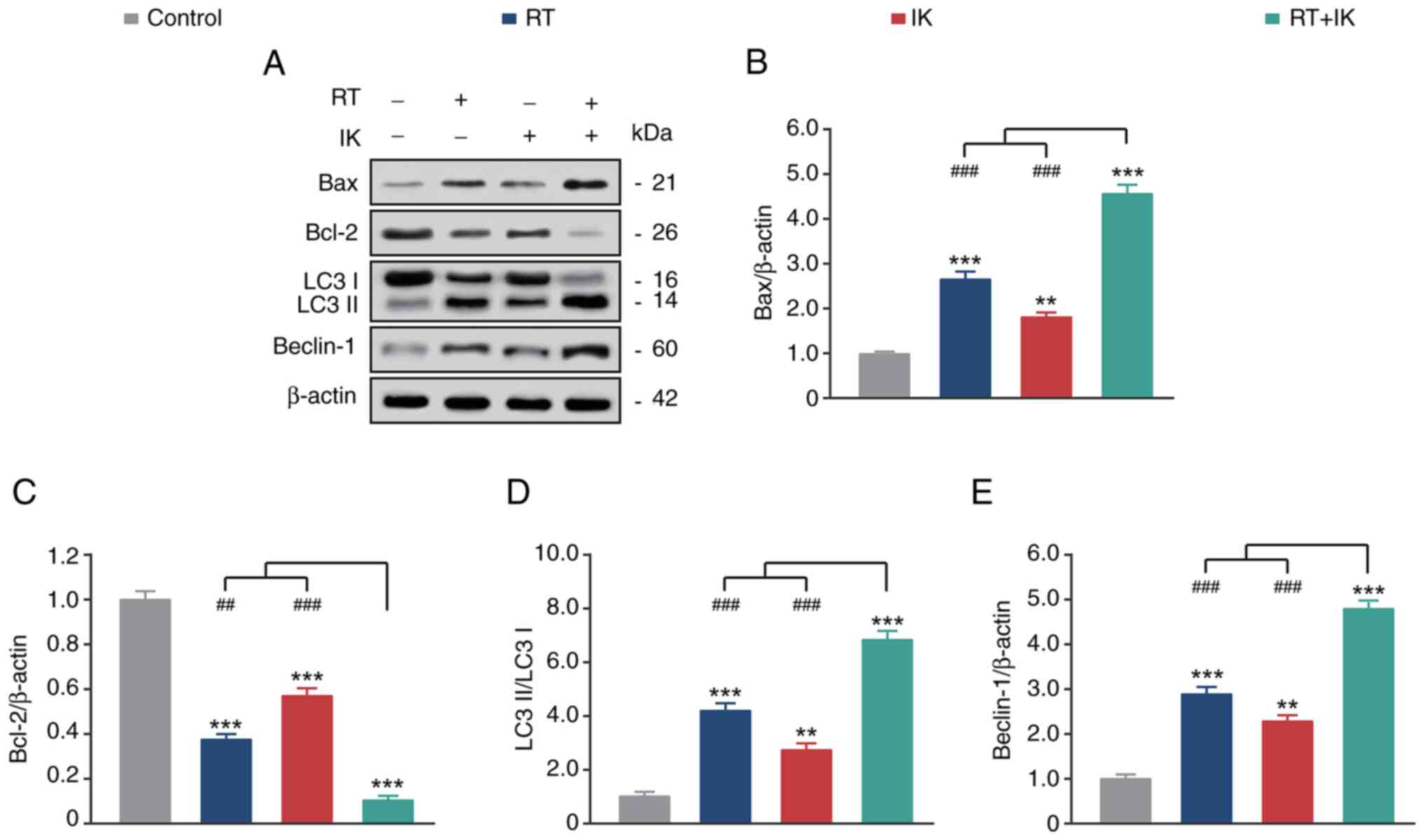
proteins in HT-29 cells was further analyzed by western blotting.
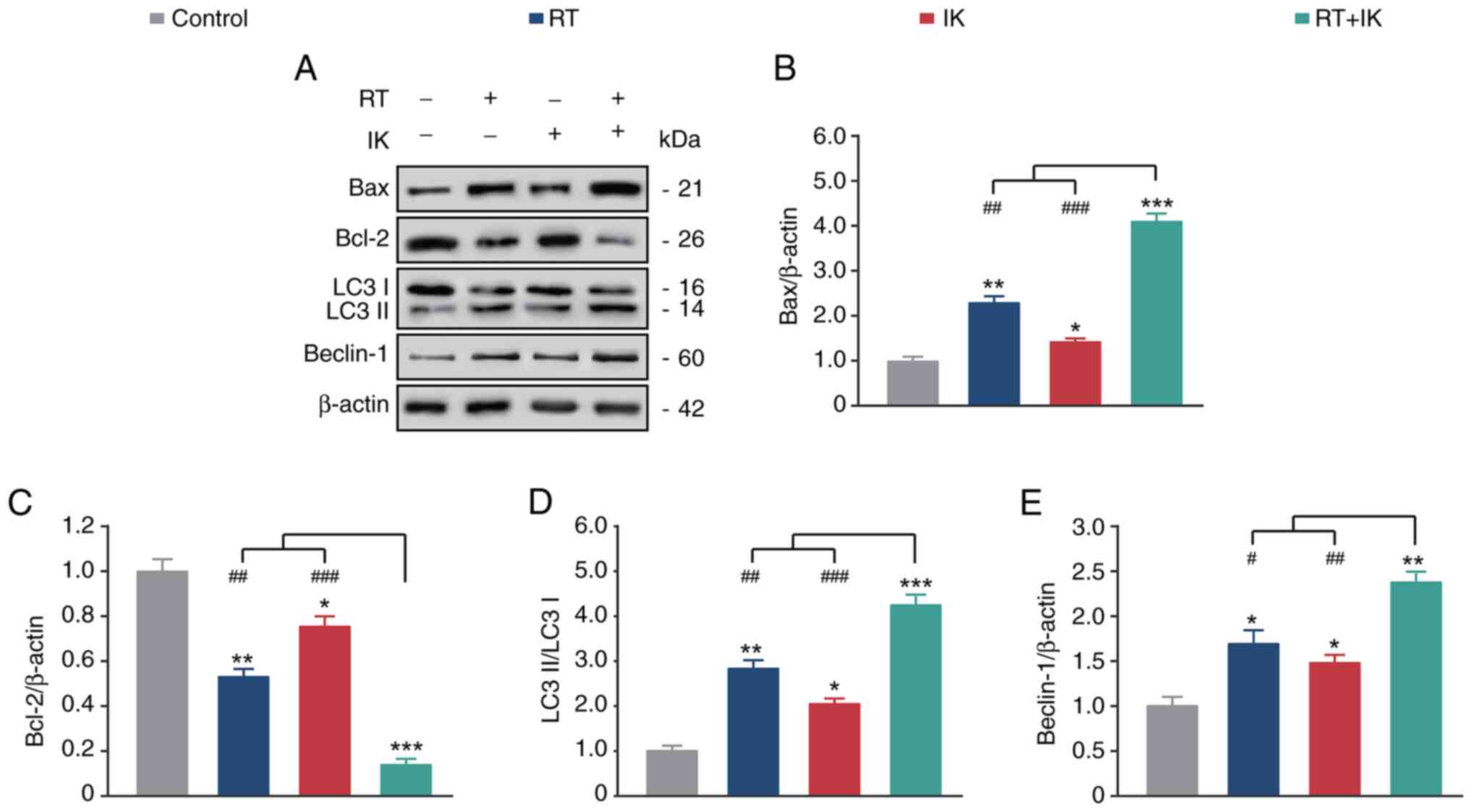
As demonstrated in Fig. 3A-C, both
RT or IK alone significantly increased the expression of the
proapoptotic factor BAX and decreased the expression of the
anti-apoptotic factor BCL-2 compared with the control group (all
P<0.05). As expected, RT in combination with IK increased BAX
but decreased BCL-2 expression, compared with controls (both
P<0.05), and the extent of the change was significantly higher
than either treatment alone. In addition, RT or IK alone promoted
the conversion of LC3 I to LC3 II and increased Beclin-1 expression
levels compared with controls (both P<0.05). Notably, the
combination treatment group further increased the changes in these
levels. These results suggest that RT combined with IK can
significantly promote apoptosis and autophagy in HT-29 cells.
Combination treatment with RT
effectively inhibits colon cancer growth in a xenograft model
Based on the efficacy of RT in combination with IK
in inhibiting HT-29 cell proliferation, the in vivo
antitumor potential of this combination therapy was further
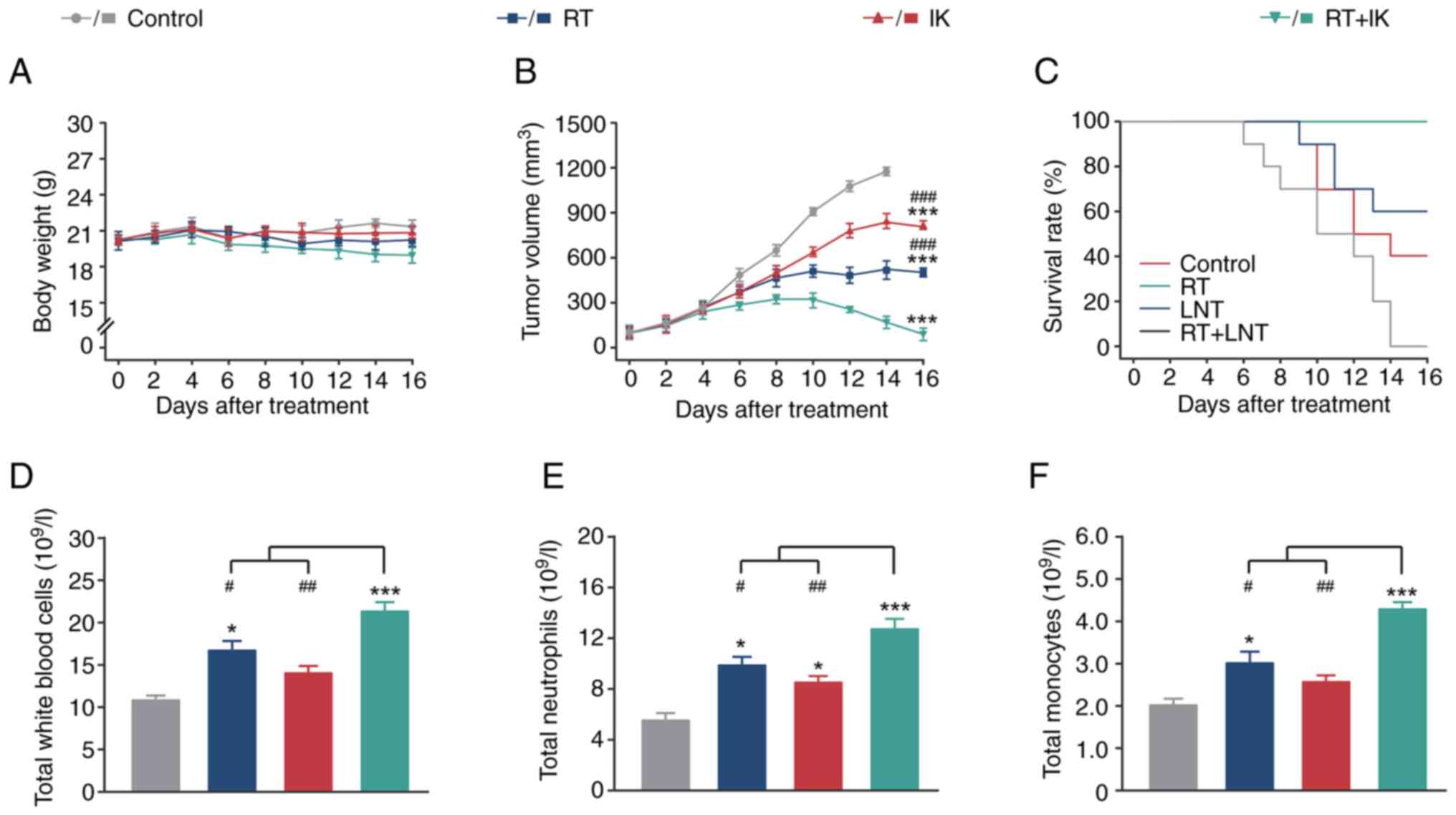
evaluated in HT-29-bearing mice. As shown in Fig. 4A and B, the tumor volume of control
mice continued to increase over the 14-day treatment period, but
there was no significant change in body weight. The maximum tumor
diameter and volume was 16.12 mm and 1,425 mm3,
respectively. Both RT and IK treatments significantly reduced tumor
size in mice compared with controls (both P<0.001). In addition,
the combination of RT and IK significantly reduced tumor size in
HT-29-bearing mice compared with controls (P<0.001), and to a
significantly greater extent than RT or IK alone. Notably, after 14
consecutive days of RT in combination with IK, the transplanted
tumors in HT-29-bearing mice were almost completely eliminated,
followed by no tumor recurrence. The survival rates of different
groups of mice during 14 days of continuous treatment were
statistically analyzed (Fig. 4C).
Xenograft mice in the control group commenced to succumb to disease
6 days after modeling and all succumbed within 14 days. Treatment
with RT or IK alone significantly improved mouse survival compared
with controls, but survival was only 40–60%. Of note, xenograft
mice in the combination treatment group did not die during 14
consecutive days of treatment (survival rate of 100%), indicating
that RT combined with IK treatment is effective in prolonging the
survival of HT-29-bearing mice.
Non-specific immunity plays an important role in the
treatment of solid tumors. Therefore, the levels of leukocytes,
neutrophils and monocytes in the peripheral blood of different
groups of HT-29-bearing mice were further analyzed. As revealed in
Fig. 4D-F, RT alone significantly
increased the levels of white blood cells, neutrophils and
monocytes in the peripheral blood of mice compared with controls
(all P<0.05). In addition, the levels of neutrophils in mice
treated with IK alone were significantly higher than those in the
control group (P<0.05), whereas the levels of white blood cells
and monocytes were not significantly different (both P>0.05). As
expected, the levels of white blood cells, neutrophils and
monocytes in peripheral blood were significantly enhanced in the
combination group, and were significantly higher than in the
monotherapy or control groups.
IK combined with RT enhances the
autophagy and apoptosis of tumor cells in a xenograft model
Western blotting was used to study the expression of
apoptosis and autophagy-related proteins in tumor tissues of
HT-29-bearing mice. As shown in Fig.
5A-C, RT or IK alone significantly upregulated the expression
of BAX and downregulated the expression of BCL-2 compared with the
control group (all P<0.01). Furthermore, RT in combination with
IK further significantly upregulated BAX expression and
downregulated BCL-2 expression compared with both treatment groups
alone (both P<0.01). On the other hand, both RT and IK alone
significantly upregulated LC3 II expression and downregulated LC3 I
expression compared with controls, indicating an increased
conversion of LC3 I to LC3 II (both P<0.01) (Fig. 5D). In addition, treatment with RT or
IK alone also significantly upregulated Beclin-1 expression (both
P<0.01) (Fig. 5E). The
combination of RT and IK further significantly enhanced the
conversion of LC3 I to LC3 II (both P<0.001), and significantly
upregulated Beclin-1 expression (both P<0.001) compared with the
two treatment groups alone. These results suggest that RT combined
with IK can promote tumor cell apoptosis by regulating the
expression of apoptosis-related proteins, and ultimately inhibit
the xenograft tumor growth.
Effect of IK on intestinal injury
induced by RT in mice
Radiation therapy damages normal tissue around the
lesion. Oxidative stress and inflammation are the main
manifestations of radiation side effects. Therefore, the present
study further investigated the protective effect of IK on normal
intestinal tissue after RT in mice. As demonstrated in Fig. 6A-C, after RT, MDA levels in normal
intestinal tissues of xenograft mice were significantly increased
(P<0.001), whereas the activities of GSH and CAT were
significantly decreased (both P<0.001), suggesting development
of oxidative stress. Notably, IK treatment significantly decreased
MDA levels in the intestinal tissue of irradiated mice (P<0.01),
and significantly increased the activities of GSH and CAT (both
P<0.01). On the other hand, RT treatment significantly increased
the levels of inflammatory factors, including TNF-α, NF-κB and
IL-1β, compared with controls (all P<0.001) (Fig. 6D-F). In addition, these changes in
inflammatory cytokine levels were significantly reversed by IK
treatment (all P<0.01). These results suggest that IK
significantly ameliorates radiation-induced oxidative stress and
inflammation.
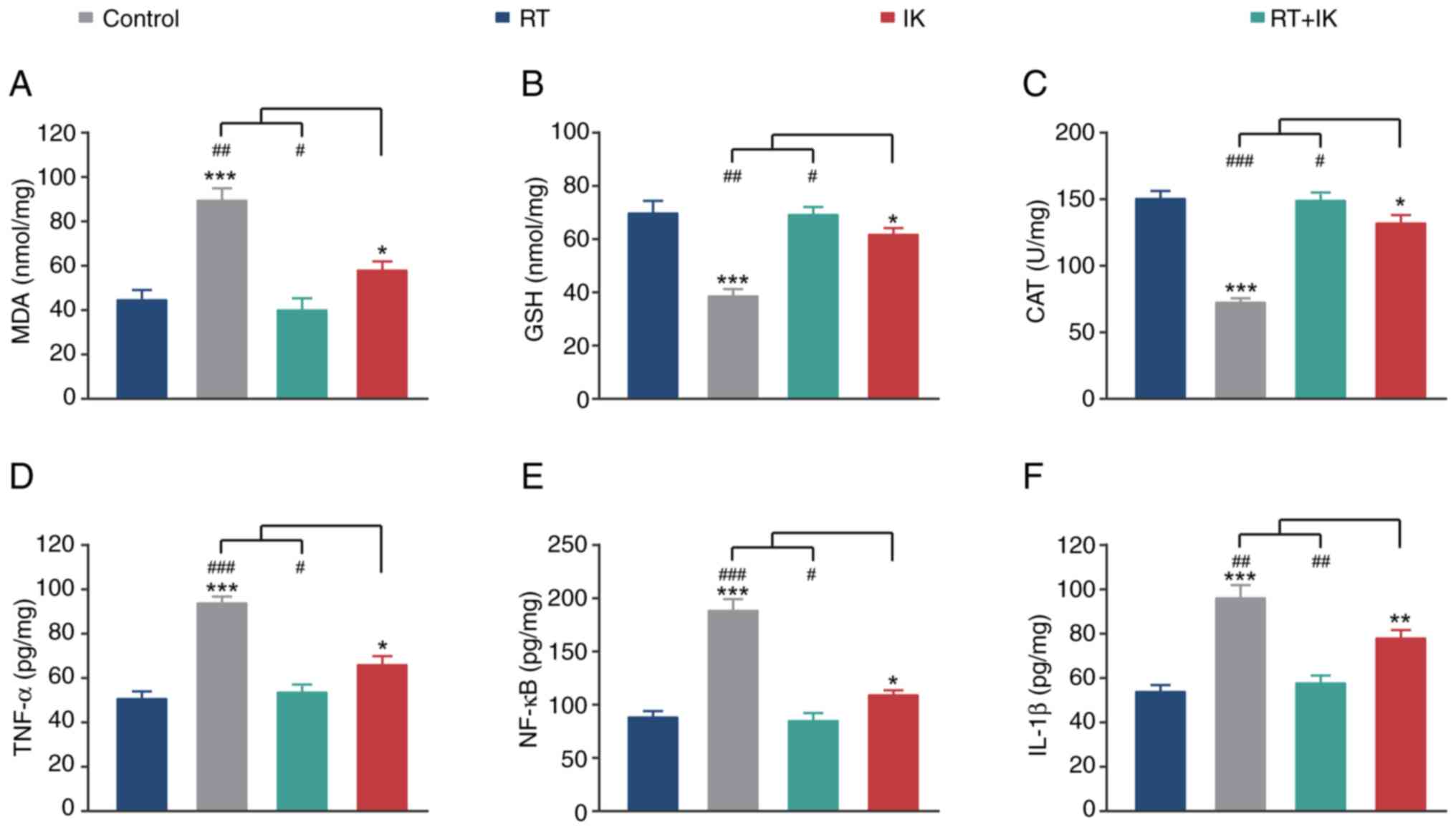 | Figure 6.Effect of RT combined with IK on
oxidative stress and inflammation in normal intestinal tissues of
HT-29-bearing mice. Quantitative analysis of (A) MDA, (B) GSH, (C)
CAT, (D) TNF-α, (E) NF-κB and (F) IL-1β of normal intestinal
tissues in HT-29-bearing mice treated with RT (8 Gy) combined with
IK (100 mg/kg). Data are presented as the mean ± SD (n=10).
*P<0.05, **P<0.01 and ***P<0.001 vs. the control group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the RT + IK group. RT, radiotherapy;
IK, isoegomaketone; MDA, malondialdehyde; GSH, glutathione; CAT,
catalase. |
IK attenuates radiation-induced
intestinal injury in rats by inhibiting the phosphorylation of
proteins in the PI3K/AKT/mTOR signaling pathway
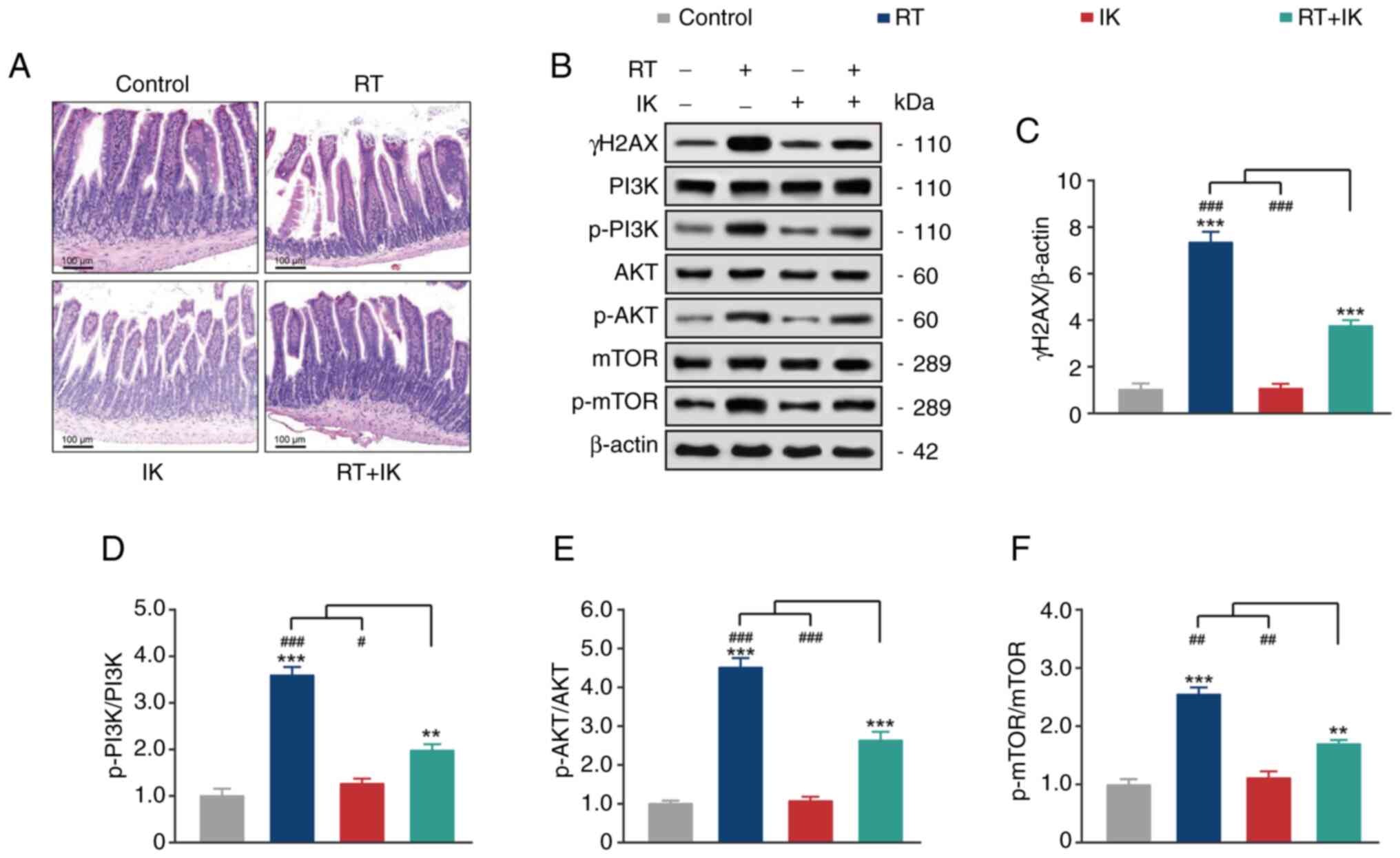
The results of H&E staining in normal intestinal
tissues of xenograft tumor models are shown in Fig. 7A. Intestinal microvilli were
structurally intact in the control and IK groups. By contrast, in
the RT group, the tip of some villi in intestinal mucosa was
necrotic, the height and width of villi were disorganized, and some
villi exhibited shortening and atrophy. In the IK group, only
partial villus shortening and structural integrity were observed in
intestinal microvilli. In the RT+IK group, histopathological
changes were relieved, and the submucosal, muscular and serosal
tissue structures were significantly improved.
The PI3K/AKT/mTOR signaling pathway plays an
important role in inflammation and oxidative stress. Activation of
the PI3K/AKT/mTOR signaling pathway promotes the expression of
numerous proinflammatory cytokines as well as the development of
oxidative stress. Thus, the expression of proteins of the
PI3K/AKT/mTOR signaling pathway in normal intestinal tissues of
tumor-bearing mice was further analyzed by western blotting. As
revealed in Fig. 7, RT treatment
significantly upregulated the expression level of γH2AX and the
phosphorylation levels of PI3K, AKT and mTOR compared with controls
(all P<0.001). In addition, the expression level of γH2AX and
the phosphorylation levels of PI3K, AKT and mTOR in the intestinal
tissue of mice treated with IK alone did not change significantly
compared with the control group (all P>0.05). As expected, IK
treatment significantly reversed the RT-induced phosphorylation of
PI3K/AKT/mTOR (all P<0.01). These results suggest that IK can
ameliorate radiation injury in the intestinal tissue of xenografted
mice by inhibiting the phosphorylation of the PI3K/AKT/mTOR
pathway.
Discussion
Numerous previous studies have focused on various
radiosensitive markers and sensitizers (18–20).
Radiosensitive markers include mutations in the tumor suppressor
gene P53, cell proliferation marker Ki-67 and high expression of
proliferating cell nuclear antigen (19). Radiosensitizers include
chemotherapeutic agents such as 5-fluorouracil and oxaliplatin, or
targeted therapeutic agents such as cetuximab (19). However, the reason of RT tolerance
of colorectal cancer cells remains unclear, and effective
radiosensitive markers and sensitizers need to be further explored.
In addition, radiation enteritis is one of the most common
potential complications of RT for abdominal tumors and a major
issue affecting patient quality of life and treatment outcome
(20). Therefore, it is necessary
to prevent or reduce radiation damage to the intestine. Perilla
frutescens is favored by researchers for its mild drug
properties and favorable antitumor effects. IK is the main
component of Perilla extract, which can promote enhanced
cleavage of BID, BAX translocation, release of cytochrome c,
translocation of apoptosis-inducing factors into the nucleus, and
enhancement of the activities of apoptosis-related enzymes such as
caspases-3, −8 and −9 (21). In
addition, IK can inhibit the phosphorylation of signal transducer
and activator of transcription 1 and the activation of NF-κB,
thereby blocking apoptosis escape and inhibiting cell proliferation
(21). In addition, it has been
identified that IK also attenuates the development of inflammation
and oxidative stress (22).
Therefore, the aim of the present study was to investigate whether
IK could act as a RT radiosensitizer for colon cancer and to
mitigate the side effects of RT on normal intestinal tissue.
Firstly, CCK-8 assay was used to investigate whether
IK could synergistically promote the inhibitory effect of RT on the
proliferation of human colorectal cancer cells. The results showed
that different concentrations of IK significantly enhanced the
viability of HT-29 cells, which was inhibited by RT. Remarkably, IK
significantly reduced the inhibitory effect of RT on the viability
of normal colonic histiocytes (CCD-18Co cells). Inhibition of HT-29
cell viability was maximal at RT and IK doses of 8 Gy and 100
µg/ml, respectively, whereas inhibition of CCD-18Co cell viability
was not evident. Therefore, 8 Gy/100 µg/ml was selected as the
optimal dose ratio for RT/IK in subsequent experiments.
Hypoxia is a ubiquitous phenomenon in the
microenvironment of solid tumors (23). The ability of tumor cells to resist
ionizing radiation exceeds that of cells with normal oxygen content
by 2-3-fold, mainly due to reduced damage to DNA proliferation by
oxygen free radicals (24). Hypoxia
in solid tumors is the main cause of RT tolerance, and overcoming
radiation resistance due to hypoxia is an important approach to
improve the therapeutic effect of tumors (25). HIF-1 is a transcription factor for
hypoxic cells and consists of two subunits, α and β, in which
HIF-1α is oxygen-regulated (26).
High expression of HIF-1α predicts adverse outcomes such as poor RT
effect, local recurrence and reduced overall survival time
(26). In addition, the PI3K/AKT
pathway is one of the major pathways regulating HIF-1α protein
expression and is also closely associated with RT resistance
(27). Freudlsperger et al
(27) found that PI3K/AKT-mediated
DNA damage repair may be an important mechanism of radiation
resistance, and inhibition of the PI3K/AKT pathway could increase
radiation-induced apoptosis and attenuate intrinsic radiation
resistance of cells to exert a role in radiosensitization. Miyasaka
et al (28) found that
regulation of the protein expression of HIF-1α downstream of the
PI3K/AKT pathway similarly enhanced the radiosensitivity of
endometrial cancer cells. Blockade of the PI3K/AKT pathway could
inhibit the expression of HIF-1α and improve the hypoxic status of
tumor cells, thereby increasing the sensitivity of endometrial
cancer cells to radiation, which is consistent with the results of
the present study (28). The
current findings revealed that IK could enhance the DNA damage
induced by RT in HT-29 cells by reducing the expression level of
HIF-1α and the phosphorylation level of proteins in the PI3K/AKT
signaling pathway. In addition, apoptosis, also known as programmed
cell death, is an important process of cell life-sustaining
activities (29), and is considered
key to effective anticancer treatment options (29).
The BCL-2 family plays an important role in
apoptosis, with the pro-apoptotic gene BAX and the anti-apoptotic
gene BCL-2 leading to programmed cell death by activating
downstream caspase proteins (30).
Autophagy is a critical cellular process that typically protects
cells and organisms from stressors such as nutritional deficiencies
(29). In addition to their roles
in normal physiological processes, they also play important roles
in pathological processes such as cancer (31). Autophagy has been found to inhibit
tumor growth, and deletion of autophagy genes leads to
tumorigenesis (32). Beclin-1 was
originally identified as a tumor suppressor gene, and is one of the
key molecules between apoptosis and autophagy, and can suppress
tumors by promoting autophagy (33). When the autophagic process begins,
LC3 I is converted to LC3 II; thus, the levels of autophagy can be
reflected by detecting LC3 I and LC3 II levels (34). The present results demonstrated that
RT combined with IK significantly upregulated the expression of BAX
and downregulated the expression of BCL-2. In addition, the
combination of RT and IK further enhanced the conversion of LC3 I
to LC3 II, and upregulated Beclin-1 expression to a greater extent
than that observed in the treatment alone and control groups. These
results confirmed that the combination of RT and IK significantly
promoted apoptosis and autophagy in HT-29 cells.
Based on the antitumor effect of RT in combination
with IK in vitro, HT-29-bearing mice were further generated,
and the antitumor potential of RT in combination with IK in
vivo was evaluated in the present study. Combination therapy
for 14 consecutive days significantly reduced tumor size in
xenograft mice to an extent of almost complete elimination. In
addition, combination therapy significantly improved the survival
of mice with xenografts, with no mice dying during the 14-day
treatment period, and the survival rate being 100%. Mice in the
combination treatment group had significantly reduced tumor size
and significantly improved survival compared with controls and
either monotherapy groups. Furthermore, the levels of non-specific
immune factors in the peripheral blood of tumor-bearing mice were
analyzed. As expected, peripheral blood leukocyte, neutrophil and
monocyte levels were significantly enhanced in the combination
group compared with the monotherapies and control groups. In
addition, the levels of apoptosis- and autophagy-related proteins
in tumor tissues were evaluated by western blotting. The results
showed that the combination of RT and IK significantly upregulated
and downregulated the expression of pro- and anti-apoptotic
factors, respectively, and promoted the upregulation of
autophagy-related protein levels. These results suggest that the
combination of RT and IK can significantly increase the
non-specific immunity of HT-29 bearing mice, promote the apoptosis
and autophagy of tumor tissue cells, and further inhibit the growth
of tumor xenografts.
Radiation therapy is widely used for the treatment
of various abdominal malignancies (35). However, radiation damages
surrounding normal tissues, leading to epithelial barrier
dysfunction and mucosal inflammation, delayed mucosal atrophy and
intestinal wall fibrosis, thus reducing the quality of life of
patients with cancer and causing enterotoxicity or chronic
intestinal dysfunction in long-term cancer survivors, leading to
acute gastrointestinal syndromes such as abdominal pain, anorexia
and bleeding (35). The
pathogenesis of radiation-induced intestinal injury is
multifaceted, with inflammatory cytokines potentially involved in
ionizing radiation-induced cell injury (36). It has been observed that TNF-α,
IL-1β and NF-κB are markedly elevated in patients after RT
(36). At the same time, the
PI3K/Akt/mTOR pathway is involved in various cellular processes,
including cell proliferation, differentiation, survival,
metabolism, cellular immunity, inflammation and intestinal
epithelial apoptosis (37). There
is considerable evidence that the PI3K pathway modulates
inflammatory responses and is strongly associated with
chemokine-mediated recruitment of immune cells to sites of
inflammation (38). Inhibition of
the PI3K/Akt/mTOR signaling pathway has been reported to be a
potential target for inflammation-related diseases (39). Furthermore, the PI3K/Akt/mTOR
signaling pathway is equally closely associated with the
development of oxidative stress (40). Consistent with the aforementioned
studies, RT combined with IK treatment reduced the development of
intestinal tissue inflammation and oxidative stress in the present
study. The underlying mechanism may be through downregulation of
phosphorylation levels in the PI3K/Akt/mTOR signaling pathway,
thereby reducing radiation-induced intestinal injury. In addition,
paradoxical effects were observed in tumor and normal intestinal
tissues. Notably, the underlying molecular mechanisms are not
contradictory. Specifically, the combination of RT and IK
significantly decreased PI3K/AKT phosphorylation compared with the
RT alone group in both HT-29 cells and normal intestinal tissues.
In HT-29 cells, decreased phosphorylation of PI3K/AKT further
improved DNA damage in HT-29 cells. On the other hand, decreased
PI3K/AKT phosphorylation is a potential molecular mechanism
underlying decreased inflammation and oxidative stress in normal
intestinal tissues. Moreover, the specific mechanism by which IK
enhances the efficacy of RT remains elusive and needs to be
elucidated in subsequent in-depth studies. In addition, the
toxicological effects of RT combined with IK therapy are future
research topics. In the future study, the IK neutralizers,
inhibitors, or even similar competitors, will be applied in an
attempt to reverse the radiosensitivities of IK to colorectal
cancer and to explore how does the IK sensitize the tumor cells to
radiation.
In summary, IK synergistically increased the
antitumor effect of RT in vitro and attenuated radiation
damage to normal cells. In addition, IK could inhibit RT-induced
DNA damage repair through the HIF-1α/PI3K/AKT signaling pathway,
and further promote apoptosis and autophagy in HT-29 cells.
Furthermore, the combination of RT and IK could effectively exert
antitumor effects in HT-29 bearing mice, inhibit the growth of
xenografts and improve the survival rate of mice. RT and IK
combination therapy has potential therapeutic value in improving
radiation-induced inflammation and oxidative stress, and reducing
intestinal inflammation and oxidative stress in irradiated mice. IK
may be a promising RT adjuvant to improve therapeutic effect and
safety by enhancing radiosensitivity to tumor tissue and reducing
intestinal complications after RT.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Competitive Science and
Technology Research Project of Quzhou (grant no. 2023K122).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
SFX and HYW analyzed the data and drafted the
manuscript. XWH and LLY contributed to the study design and
critical revision. All authors read and approved the final version
of the manuscript. SFX and XWH confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Research related to animal use complies with all
relevant national regulations, institutional policies, and was
approved [approval no. 2023 (30)]
by the animal Experimental Ethics Committee of the First Affiliated
Hospital, Zhejiang University School of Medicine (Hangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morgan E, Arnold M, Gini A, Lorenzoni V,
Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N and Bray F:
Global burden of colorectal cancer in 2020 and 2040: Incidence and
mortality estimates from GLOBOCAN. Gut. 72:338–344. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sung H, Siegel RL, Rosenberg PS and Jemal
A: Emerging cancer trends among young adults in the USA: Analysis
of a population-based cancer registry. Lancet Public Health.
4:e137–e147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Araghi M, Soerjomataram I, Bardot A,
Ferlay J, Cabasag CJ, Morrison DS, De P, Tervonen H, Walsh PM,
Bucher O, et al: Changes in colorectal cancer incidence in seven
high-income countries: A population-based study. Lancet
Gastroenterol Hepatol. 4:511–518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buccafusca G, Proserpio I, Tralongo AC,
Rametta Giuliano S and Tralongo P: Early colorectal cancer:
Diagnosis, treatment and survivorship care. Crit Rev Oncol Hematol.
136:20–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dariya B, Aliya S, Merchant N, Alam A and
Nagaraju GP: Colorectal cancer biology, diagnosis, and therapeutic
approaches. Crit Rev Oncog. 25:71–94. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kusano M, Aoyama T, Okabayashi K, Hirata
K, Tsuji Y, Nakamori S, Asahara T, Ohashi Y, Yoshikawa T, Sakamoto
J, et al: A randomized phase III study of hepatic arterial infusion
chemotherapy with 5-fluorouracil and subsequent systemic
chemotherapy versus systemic chemotherapy alone for colorectal
cancer patients with curatively resected liver metastases (Japanese
foundation for multidisciplinary treatment of cancer 32). J Cancer
Res Ther. 14 (Suppl):S761–S766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zielińska A, Włodarczyk M, Makaro A,
Sałaga M and Fichna J: Management of pain in colorectal cancer
patients. Crit Rev Oncol Hematol. 157:1031222021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Li X, Peng R, Wang Y and Wang J:
Stereotactic ablative radiotherapy for colorectal cancer liver
metastasis. Semin Cancer Biol. 71:21–32. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu J, Li N, Tang Y, Wang X, Tang Y, Wang
SL, Song YW, Liu YP, Li YX and Jin J: Outcomes after
hypofractionated stereotactic radiotherapy for colorectal cancer
oligometastases. J Surg Oncol. 119:532–538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Qiu H, Li C, Cai P and Qi F: The
positive role of traditional Chinese medicine as an adjunctive
therapy for cancer. Biosci Trends. 15:283–298. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho BO, Jin CH, Park YD, Ryu HW, Byun MW,
Seo KI and Jeong IY: Isoegomaketone induces apoptosis through
caspase-dependent and caspase-independent pathways in human DLD1
cells. Biosci Biotechnol Biochem. 75:1306–1311. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang R, Zhang Q, Feng C, Zhang J, Qin Y
and Meng L: Advances in the pharmacological activities and effects
of perilla ketone and isoegomaketone. Evid Based Complement
Alternat Med. 2022:88097922022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin CH, Park HC, So Y, Nam B, Han SN and
Kim JB: Comparison of the anti-inflammatory activities of
supercritical carbon dioxide versus ethanol extracts from leaves of
perilla frutescens Britt. radiation mutant. Molecules. 22:3112017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chi HC, Tsai CY, Tsai MM and Lin KH:
Impact of DNA and RNA methylation on radiobiology and cancer
progression. Int J Mol Sci. 19:5552018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lapierre A, Gourgou S, Brengues M, Quéro
L, Deutsch É, Milliat F, Riou O and Azria D: Tumour and normal
tissue radiosensitivity. Cancer Radiother. 26:96–103. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwak SY, Jang WI, Lee SB, Kim MJ, Park S,
Cho SS, Kim H, Lee SJ, Shim S and Jang H: Centella asiatica-derived
endothelial paracrine restores epithelial barrier dysfunction in
radiation-induced enteritis. Cells. 11:25442022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Wang R, Qin Y and Feng C:
Defining the potential targets for biological activity of
isoegomaketone based on network pharmacology and molecular docking
methods. Life (Basel). 12:21152022.PubMed/NCBI
|
|
22
|
Jin CH, So Y, Nam B, Han SN and Kim JB:
Isoegomaketone alleviates the development of collagen
antibody-induced arthritis in male balb/c mice. Molecules.
22:12092017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jing X, Yang F, Shao C, Wei K, Xie M, Shen
H and Shu Y: Role of hypoxia in cancer therapy by regulating the
tumor microenvironment. Mol Cancer. 18:1572019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan CS, Deng ZW, Qin D, Mu YZ, Chen XG
and Liu Y: Hypoxia-modulatory nanomaterials to relieve tumor
hypoxic microenvironment and enhance immunotherapy: Where do we
stand? Acta Biomater. 125:1–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brown JM: Tumor hypoxia in cancer therapy.
Methods Enzymol. 435:297–321. 2007.PubMed/NCBI
|
|
26
|
Kilic M, Kasperczyk H, Fulda S and Debatin
KM: Role of hypoxia inducible factor-1 alpha in modulation of
apoptosis resistance. Oncogene. 26:2027–2038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Freudlsperger C, Horn D, Weißfuß S,
Weichert W, Weber KJ, Saure D, Sharma S, Dyckhoff G, Grabe N,
Plinkert P, et al: Phosphorylation of AKT(Ser473) serves as an
independent prognostic marker for radiosensitivity in advanced head
and neck squamous cell carcinoma. Int J Cancer. 136:2775–2785.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyasaka A, Oda K, Ikeda Y, Sone K, Fukuda
T, Inaba K, Makii C, Enomoto A, Hosoya N, Tanikawa M, et al:
PI3K/mTOR pathway inhibition overcomes radioresistance via
suppression of the HIF1-α/VEGF pathway in endometrial cancer.
Gynecol Oncol. 138:174–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimmelman AC and White E: Autophagy and
tumor metabolism. Cell Metab. 25:1037–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding GB, Sun J, Wu G, Li B, Yang P, Li Z
and Nie G: Robust anticancer efficacy of a biologically synthesized
tumor acidity-responsive and autophagy-inducing functional beclin
1. ACS Appl Mater Interfaces. 10:5227–5239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao Y, Luo Y, Zou J, Ouyang J, Cai Z, Zeng
X, Ling H and Zeng T: Autophagy and its role in gastric cancer.
Clin Chim Acta. 489:10–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu L, Sun C, Su Q, Wang Y, Li J, Guo Z,
Chen L and Zhang H: Radiation-induced lung injury: Latest molecular
developments, therapeutic approaches, and clinical guidance. Clin
Exp Med. 19:417–426. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barker CA, Kim SK, Budhu S, Matsoukas K,
Daniyan AF and D'Angelo SP: Cytokine release syndrome after
radiation therapy: Case report and review of the literature. J
Immunother Cancer. 6:12018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ran Z, Zhang Y, Wen X and Ma J: Curcumin
inhibits high glucose-induced inflammatory injury in human retinal
pigment epithelial cells through the ROS-PI3K/AKT/mTOR signaling
pathway. Mol Med Rep. 19:1024–1031. 2019.PubMed/NCBI
|
|
38
|
Hawkins PT and Stephens LR: PI3K
signalling in inflammation. Biochim Biophys Acta. 1851:882–897.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang JT, Xie WQ, Liu FQ, Bi Y, Zhu XJ,
Wang QE and Zheng YF: NADH protect against radiation enteritis by
enhancing autophagy and inhibiting inflammation through PI3K/AKT
pathway. Am J Transl Res. 10:1713–1721. 2018.PubMed/NCBI
|
|
40
|
Huang CY, Deng JS, Huang WC, Jiang WP and
Huang GJ: Attenuation of lipopolysaccharide-induced acute lung
injury by hispolon in mice, through regulating the
TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing
oxidative stress-mediated er stress-induced apoptosis and
autophagy. Nutrients. 12:17422020. View Article : Google Scholar : PubMed/NCBI
|