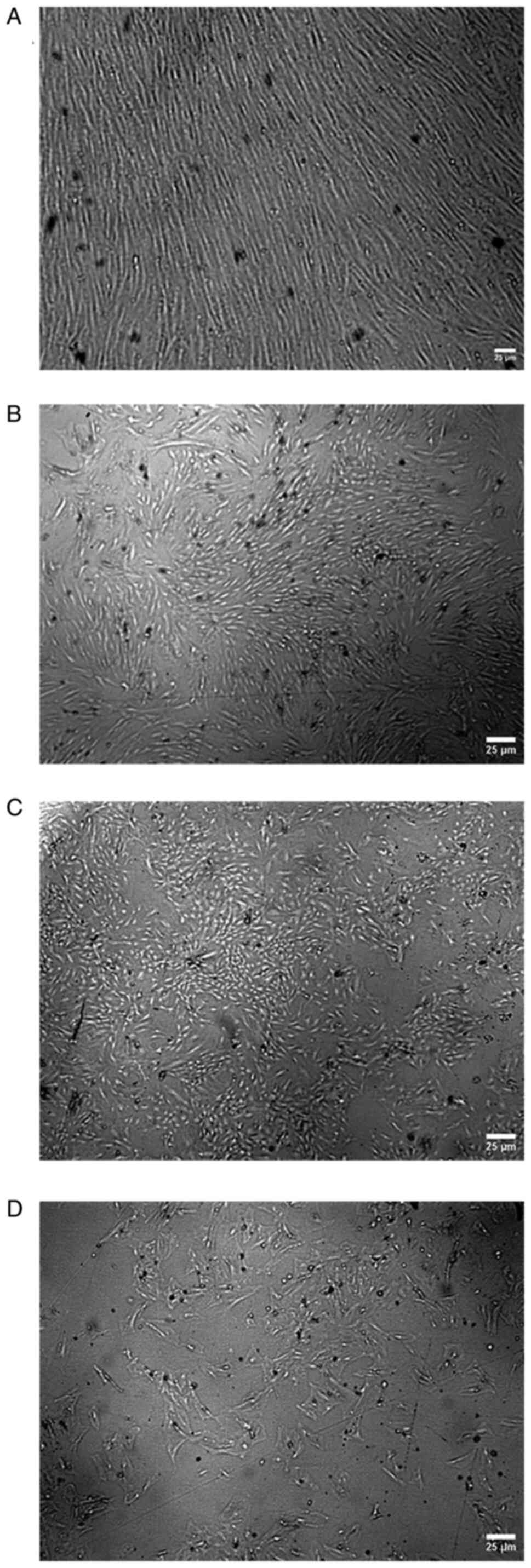
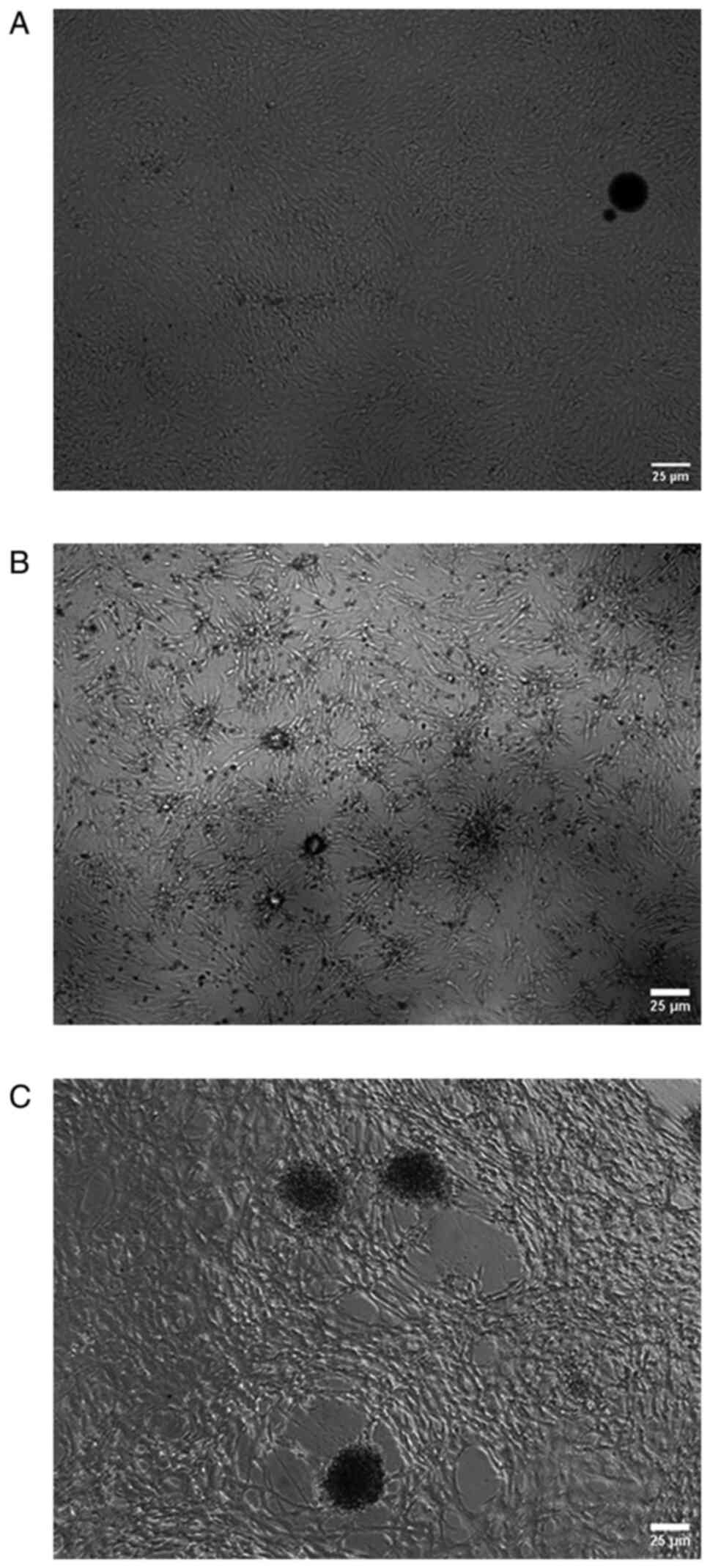
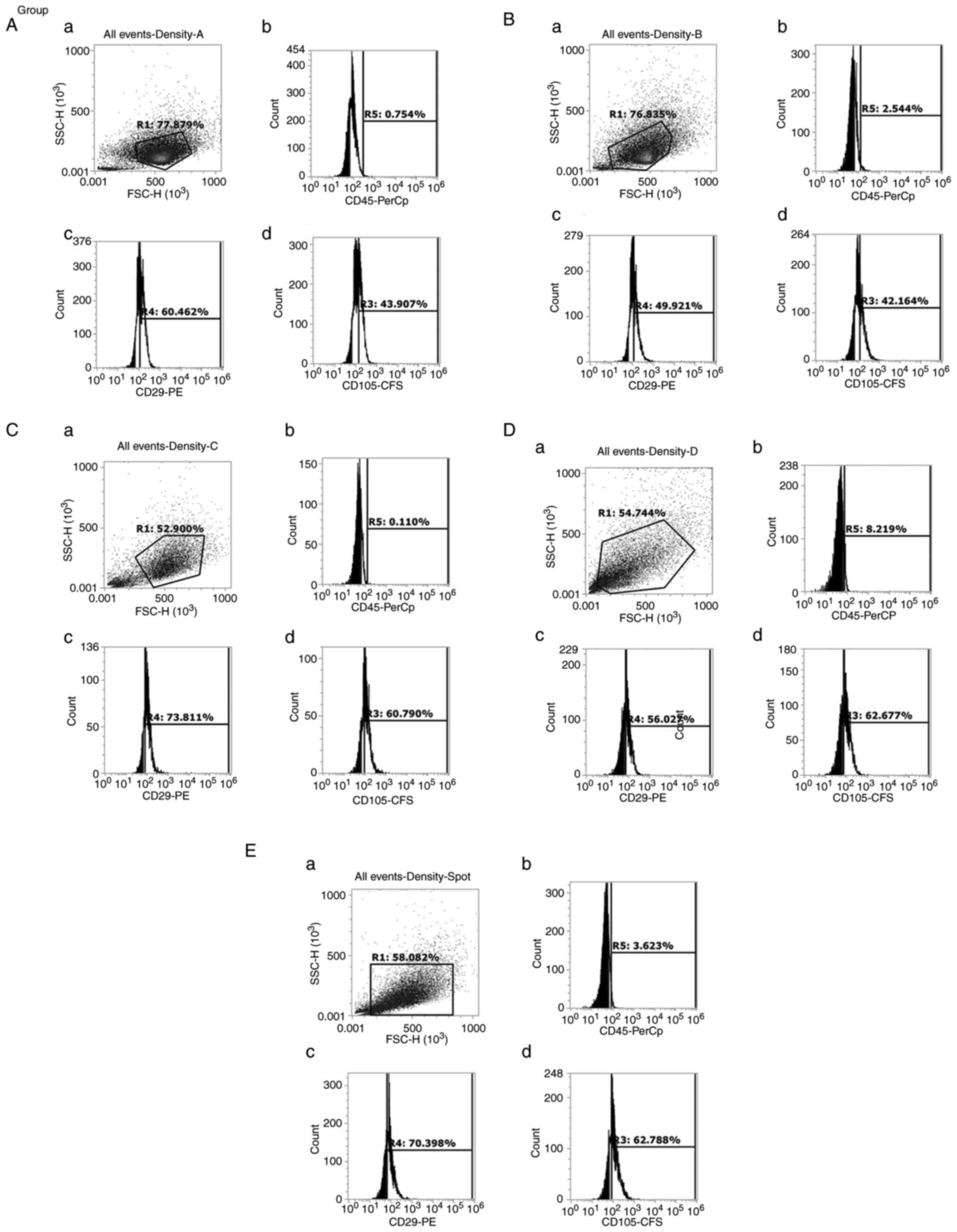
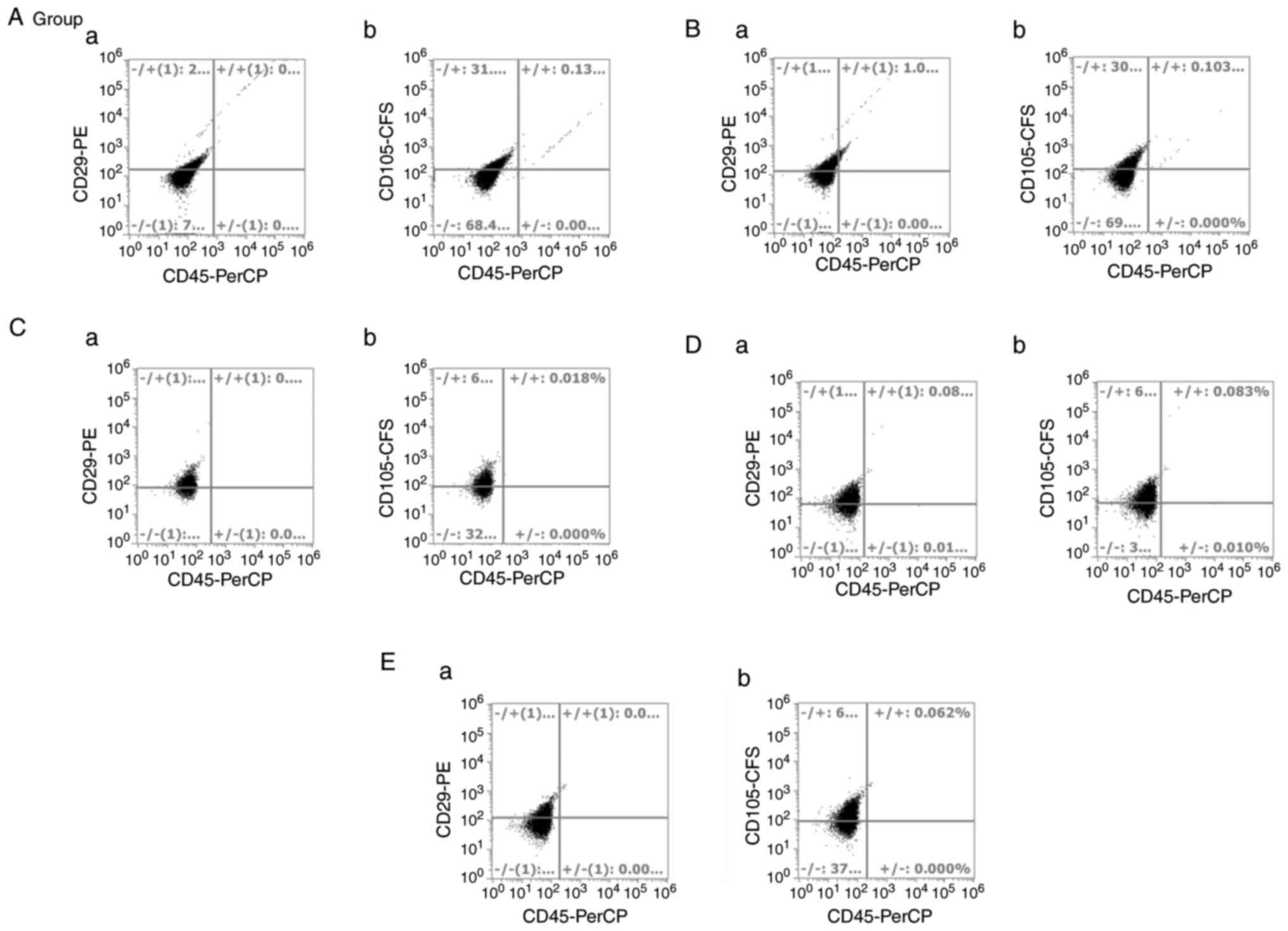
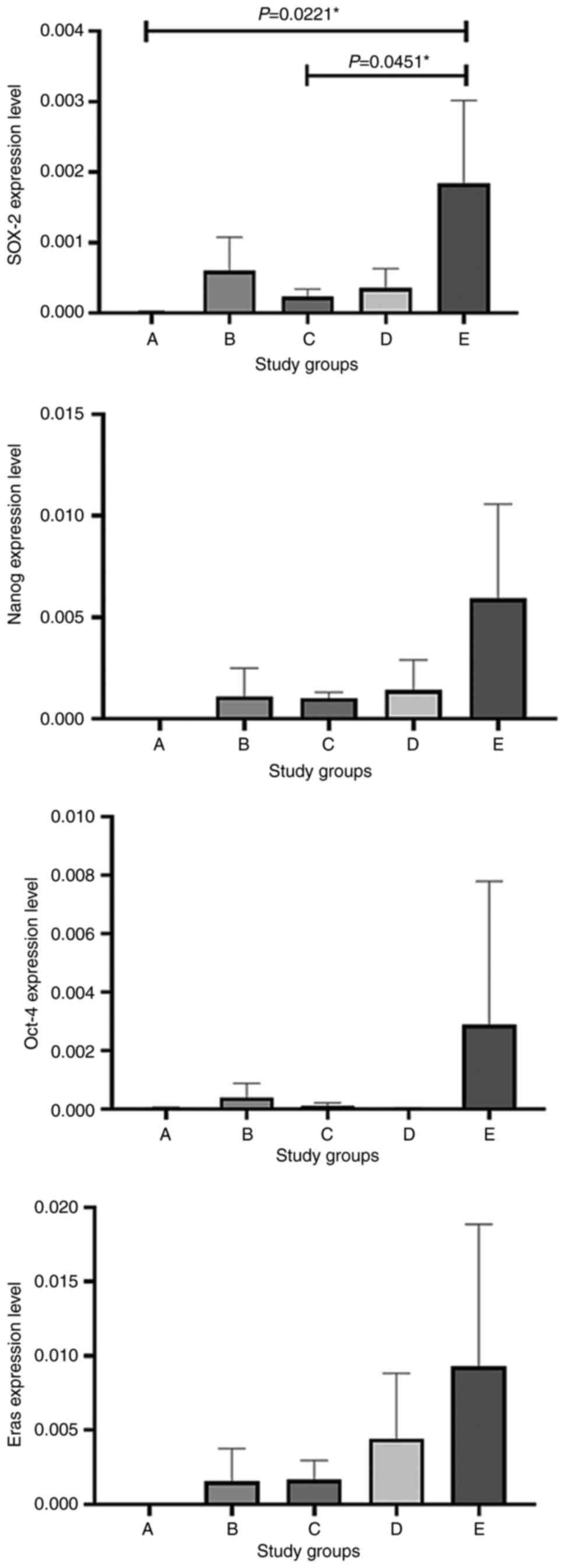
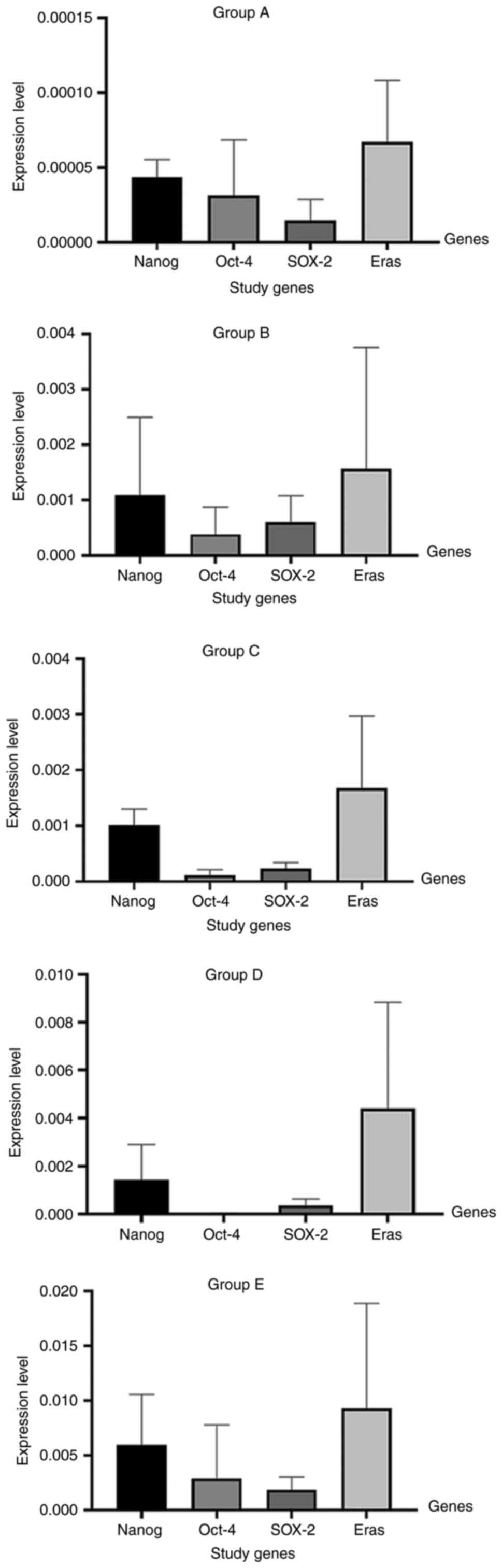
|
1
|
Zakrzewski W, Dobrzyński M, Szymonowicz M
and Rybak Z: Stem cells: Past, present, and future. Stem Cell Res
Ther. 10(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pavlović M and Radotić K: Essential
characteristics of stem cells: Self-renewal, and plasticity. Animal
and plant stem cells: Concepts. Propagation and Engineering. 17–21.
2017.
|
|
3
|
Shah AA and Khan FA: Types and
classification of stem cells. Advances in application of stem
cells: From Bench to Clinics: 25-49, 2021.
|
|
4
|
Barky AR, Ali EMM and Mohamed TM: Stem
cells, classifications and their clinical applications. Am J
Pharmacol Ther. 1:001–007. 2017.
|
|
5
|
Khan FA, Almohazey D, Alomari M and
Almofty SA: Isolation, culture, and functional characterization of
human embryonic stem cells: Current trends and challenges. Stem
Cells Int. 2018(1429351)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Volarevic V, Markovic BS, Gazdic M,
Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako
M and Stojkovic M: Ethical and safety issues of stem cell-based
therapy. Int J Med Sci. 15:36–45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim A, Lee KG, Kwon Y, Lee KI, Yang HM,
Habib O, Kim J, Kim ST, Kim SJ, Kim JS and Hwang DY: Off-the-Shelf,
immune-compatible human embryonic stem cells generated via
CRISPR-mediated genome editing. Stem Cell Rev Rep. 17:1053–1067.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Doğan A: Embryonic stem cells in
development and regenerative medicine. Adv Exp Med Biol. 1079:1–15.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Margiana R, Markov A, Zekiy AO, Hamza MU,
Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar
HH, et al: Clinical application of mesenchymal stem cell in
regenerative medicine: A narrative review. Stem Cell Res Ther.
13(366)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rehman A, Nigam A, Laino L, Russo D,
Todisco C, Esposito G, Svolacchia F, Giuzio F, Desiderio V and
Ferraro G: Mesenchymal stem cells in soft tissue regenerative
medicine: A comprehensive review. Medicina (Kaunas).
59(1449)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kobolak J, Dinnyes A, Memic A,
Khademhosseini A and Mobasheri A: Mesenchymal stem cells:
Identification, phenotypic characterization, biological properties
and potential for regenerative medicine through biomaterial
micro-engineering of their niche. Methods. 99:62–68.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Z, Zhang C, Weiner LP, Zhang Y and
Zhong JF: Molecular characterization of heterogeneous mesenchymal
stem cells with single-cell transcriptomes. Biotechnol Adv.
31:312–317. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Meurer SK, Neß M, Weiskirchen S, Kim P,
Tag CG, Kauffmann M, Huber M and Weiskirchen R: Isolation of mature
(Peritoneum-Derived) mast cells and immature (Bone Marrow-Derived)
mast cell precursors from mice. PLoS One.
11(e0158104)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fafián-Labora J, Fernández-Pernas P,
Fuentes I, De Toro J, Oreiro N, Sangiao-Alvarellos S, Mateos J and
Arufe MC: Influence of age on rat bone-marrow mesenchymal stem
cells potential. Sci Rep. 5(16765)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jung HG, Ahn EK, Lee JH, Kim YH, Leem SH,
Heo J and Kim H: Effects of harvesting sites and ages on adipose
tissue-derived stem cells in rat. Tissue Engineering and
Regenerative Medicine. 11:137–142. 2014.
|
|
18
|
Siennicka K, Zołocińska A, Dębski T and
Pojda Z: Comparison of the donor age-dependent and in vitro
culture-dependent mesenchymal stem cell aging in rat model. Stem
Cells Int. 2021(6665358)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bhat S, Viswanathan P, Chandanala S,
Prasanna SJ and Seetharam RN: Expansion and characterization of
bone marrow derived human mesenchymal stromal cells in serum-free
conditions. Sci Rep. 11(3403)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Haasters F, Prall WC, Anz D, Bourquin C,
Pautke C, Endres S, Mutschler W, Docheva D and Schieker M:
Morphological and immunocytochemical characteristics indicate the
yield of early progenitors and represent a quality control for
human mesenchymal stem cell culturing. J Anat. 214:759–767.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoon DS, Kim YH, Jung HS, Paik S and Lee
JW: Importance of Sox2 in maintenance of cell proliferation and
multipotency of mesenchymal stem cells in low-density culture. Cell
Prolif. 44:428–440. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Heo JS, Choi Y, Kim HS and Kim HO:
Comparison of molecular profiles of human mesenchymal stem cells
derived from bone marrow, umbilical cord blood, placenta and
adipose tissue. Int J Mol Med. 37:115–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Labedz-Maslowska A, Kamycka E,
Bobis-Wozowicz S, Madeja Z and Zuba-Surma EK: Identification of new
rat bone marrow-derived population of very small stem cell with
Oct-4A and Nanog expression by flow cytometric platforms. Stem
Cells Int. 2016(5069857)2016.PubMed/NCBI View Article : Google Scholar
|