Compounds with various pharmacological properties
can be found in abundance in nature (1). The unique perspective on nature is
attributable to the availability of anticarcinogenic medicines with
minimal harmfulness and the ability to inhibit a large range of
tumors (2). In addition, natural
products are less costly and more specific than synthetic
medications (3,4). As a result, finding unique
phytonutrients, and their therapeutic impact, and releasing them
into the market may be regarded as a novel approach to cancer
treatment. A number of effective anticancer medicines are either
obtained from botanical sources or are molecular modifications of
natural compounds (5,6).
Polyphenols found in fruits and vegetables have been
shown to attenuate the progression of cancer (4,7-9).
Polyphenols such as curcumin, genistein, resveratrol, apigenin,
fisetin, luteolin etc., have demonstrated anti-neoplastic activity
in a variety of malignancies such as leukemia, breast, cervical,
skin, colon etc. by targeting different hallmarks of cancer
(10-13).
In particular, flavonoids are the most common type of plant
secondary metabolites with beneficial health effects, such as
antioxidant, anti-inflammatory, anti-allergic and anti-viral
properties (8,14). They are ubiquitous polyphenolic
phytochemicals, which have been extensively investigated in recent
years for their cytotoxic and anticancer properties (6,7).
Antioxidant activity, antitumor action, cell cycle halting, the
stimulation of self-programmed cell death, the manipulation of
signaling pathways, the suppression of cancer cell movement and the
increasing efficiency of chemotherapeutics are among the anticancer
properties of these agents (11,13,14).
Experiments reported recently have revealed the efficacy of
flavonoids in cancer treatment. The treatment with natural
antitumor substances inhibits the growth of cancer cells with
minimal toxicity (1,2,13,15,16).
Since these critical molecules operate through numerous
physiological pathways and affect a wide variety of communication
networks (6,7), there has been a surge of interest in
flavonoids. Flavonoids are predicted to be consumed between 50 and
800 mg per day in the diet (8).
The present review discusses the anticancer properties of chrysin,
a flavonoid, in various types of cancer as stand-alone candidate
and in combination with chemotherapeutics.
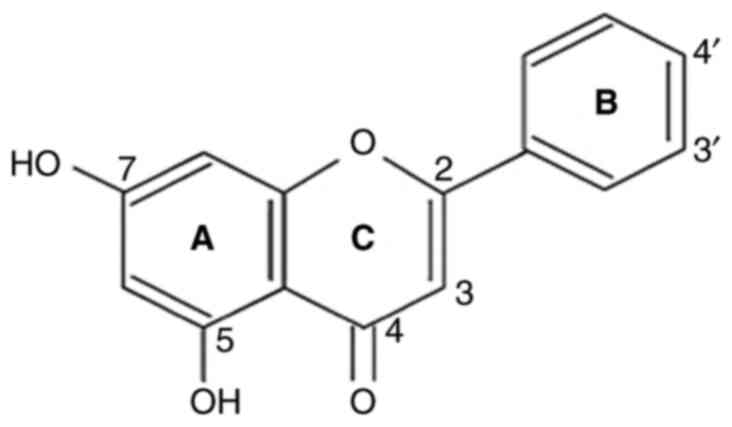
Chrysin (5,7-dihydroxyflavone) found in honey and
passion flower is a cost-effective and potent anticancer agent.
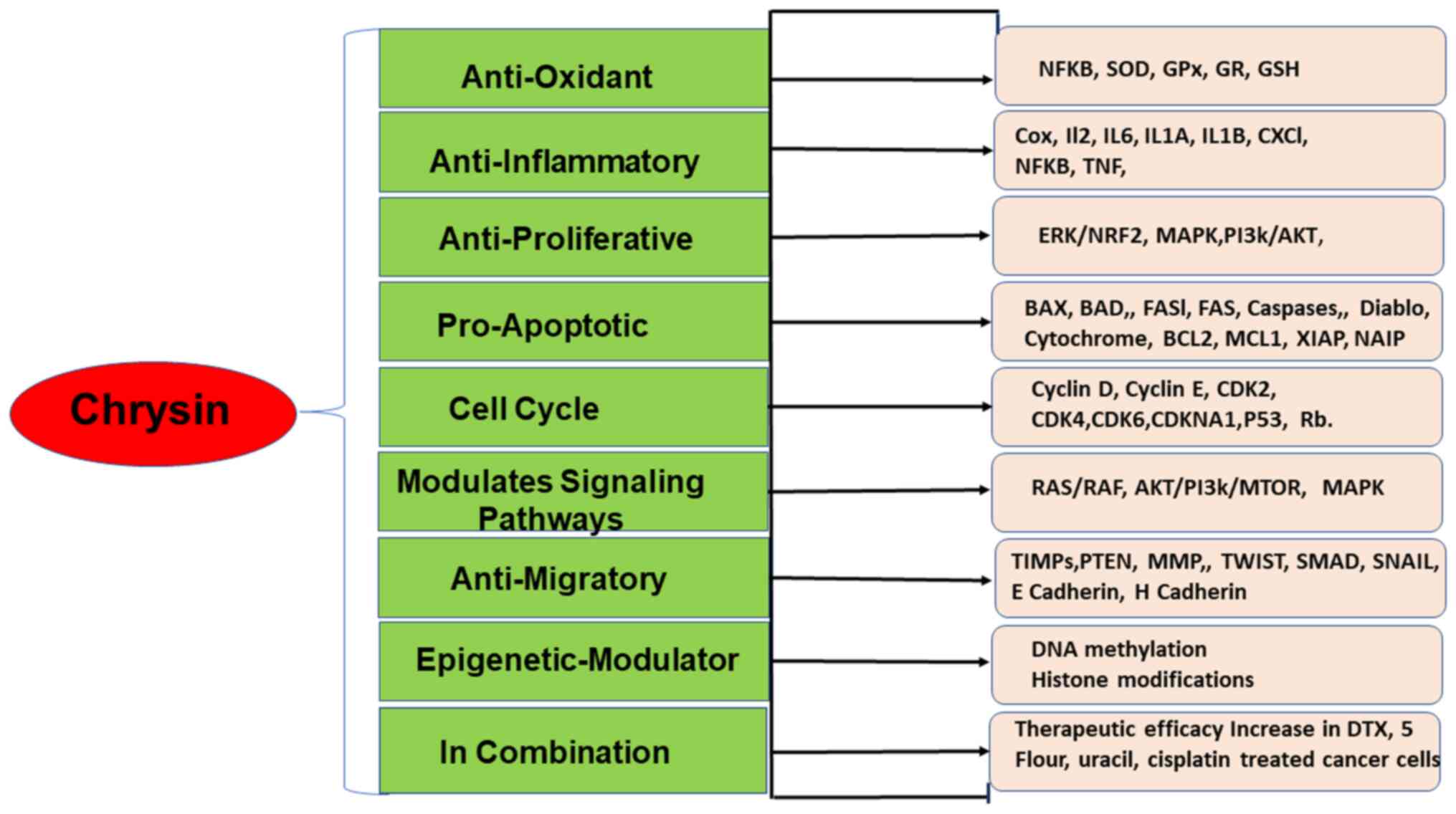
Chrysin possesses numerous biological properties, such as
anti-inflammatory, anti-viral, antioxidant and anti-diabetic
properties (3,4) (Fig.
1). The most appealing characteristic of chrysin is its
anticancer potential, which has been demonstrated through
investigations on a wide range of cancer cell lines and animal
tumor models. Thus, knowledge of the molecular processes triggered
by chrysin in tumor cells may lead to the development of novel
cancer treatment approaches with fewer side effects (10). The present review focuses on the
anticancer effects of chrysin on various types of cancer and the
molecular mechanisms involved.
Chrysin (5,7-dihydroxy flavone) is a flavone and has
a natural 15-carbon structure (Fig.
2). Chrysin is ubiquitously found in passion flower, honey and
propolis (3,17,18).
Chrysin produced from propolis and honey offers boundless curiosity
to researchers (7). Chrysin has
two benzene rings (A and B) and one oxygen-containing heterocyclic
ring in its structure. It has 2-3 double-bound carbons, with a
carbonyl group on the fourth carbon, but no 3-carbon hydroxyl
group. Chrysin is classified as a flavone based on its structural
categorization. It has -OH groups on the fifth and seventh carbon
atoms. Unlike other flavonoids, chrysin has no oxygenation in
ring-B. Diverse ring-A oxygenation is primarily responsible for
numerous chrysin derivatives, such as wogonin (18,19).
The biological actions of chrysin are linked to a lack of
oxygenation in the B and C rings, which is associated with
anti-inflammatory and antitoxic properties. Flavones show different
levels of oxidative potential which is reliant on their difference
in structure. The antioxidant property of chrysin is dependent on
carbonyl group on C4 and double bond between C2 and C3. At lower
doses, flavones are beneficial; however, they can be toxic at
higher doses and the recommended dose for chrysin is <3 g/day
(18,20).
Chrysin has an extremely low bioavailability in
humans due to rapid quick metabolism, removal and restricted
assimilation. The bioavailability of chrysin when taken orally has
been estimated to be between 0.003 to 0.02% (21). In intestinal and hepatic cells,
chrysin undergoes metabolism primarily by conjugation processes
(glucuronidation and sulfation) and less by oxidation (22). The highest concentrations of
chrysin sulfate and glucuronide have been found in the bile in
studies on chrysin metabolism in mice (23). As a result, excretion through feces
is the main recommended method for the elimination of chrysin and
its metabolites (22,23). Chrysin sulfonate and glucuronide
have been found in the urine and plasma at low concentrations
(22). The poor bioavailability of
chrysin has been best addressed by encapsulating it in
nanoparticles (24). One of the
optimal methods with which to obtain a therapeutic drug delivery
platform to treat recurrent oral ulcers and increase chrysin
bioavailability is to entrap the drug in niosomal oromuco-adhesive
films (25). The release kinetics
and cytotoxicity of chrysin are also regulated by encasing it in
poly (d, l-lactic-coglycolic acid) poly (ethylene glycol)
(PLGA-PEG) nanoparticles (26).
Notably, chrysin has been proven to be safe and effective in
various studies where volunteers have taken oral doses ranging from
300 to 625 mg without experiencing any documented effect (10,27).
Chrysin has been observed to be beneficial in
various metabolic disorders, such as diabetes, cardiac disease,
neurodegenerative diseases and above all, cancer (28). In both human and animal cancer
models, it has been shown that tumors release cytokines,
immunological mediators, classical neurotransmitters, pituitary and
hypothalamus hormones, melatonin and glucocorticoids. The body and
brain activities can be impacted by catecholamines, serotonin,
melatonin, neuropeptides and other neurotransmitters generated from
tumors. Cancers can take over the immunological and neuroendocrine
systems, resetting the body's equilibrium to favor their growth at
the expense of the host (29).
Chrysin has been shown to exert neuroprotective effects via a
variety of mechanisms, such as gamma-aminobutyric acid mimetic
properties, monoamine oxidase inhibition, antioxidant,
anti-inflammatory and anti-apoptotic activities (30).
Elevated glucose levels have been shown to cause
self-programmed death in glomerular specialized cells, and this
process can be minimized following treatment with chrysin (18). The main mechanism involved in the
effects of chrysin is the decrease in the splitting of DNA and the
repair of ratio of Bax and Bcl-2, as well as the inhibition of
cytochrome c and apoptotic protease activating factor 1 in
glomerular cells subjected to a higher concentration of glucose
(18,31). Chrysin therapy has been shown to
reduce NF-κB p65 unit, TNF-α, IL-1, IL-6 and caspase-3 levels in
the cerebral cortex and hippocampus, resulting in the antidiabetic
protection of cognitive decline (32). The use of chrysin also results in
reduced blood sugar and insulin signaling molecules and glucose
tolerance inhibition (33).
Another study revealed that treatment with chrysin between 20-80
mg/kg/day decreased the levels of low-density lipoprotein
cholesterol, triglycerides and cholesterol, and at the same time
increased the levels of high-density lipoprotein cholesterol,
glutathione S-transferase, superoxide dismutase and catalase
(34). Chrysin is a potent
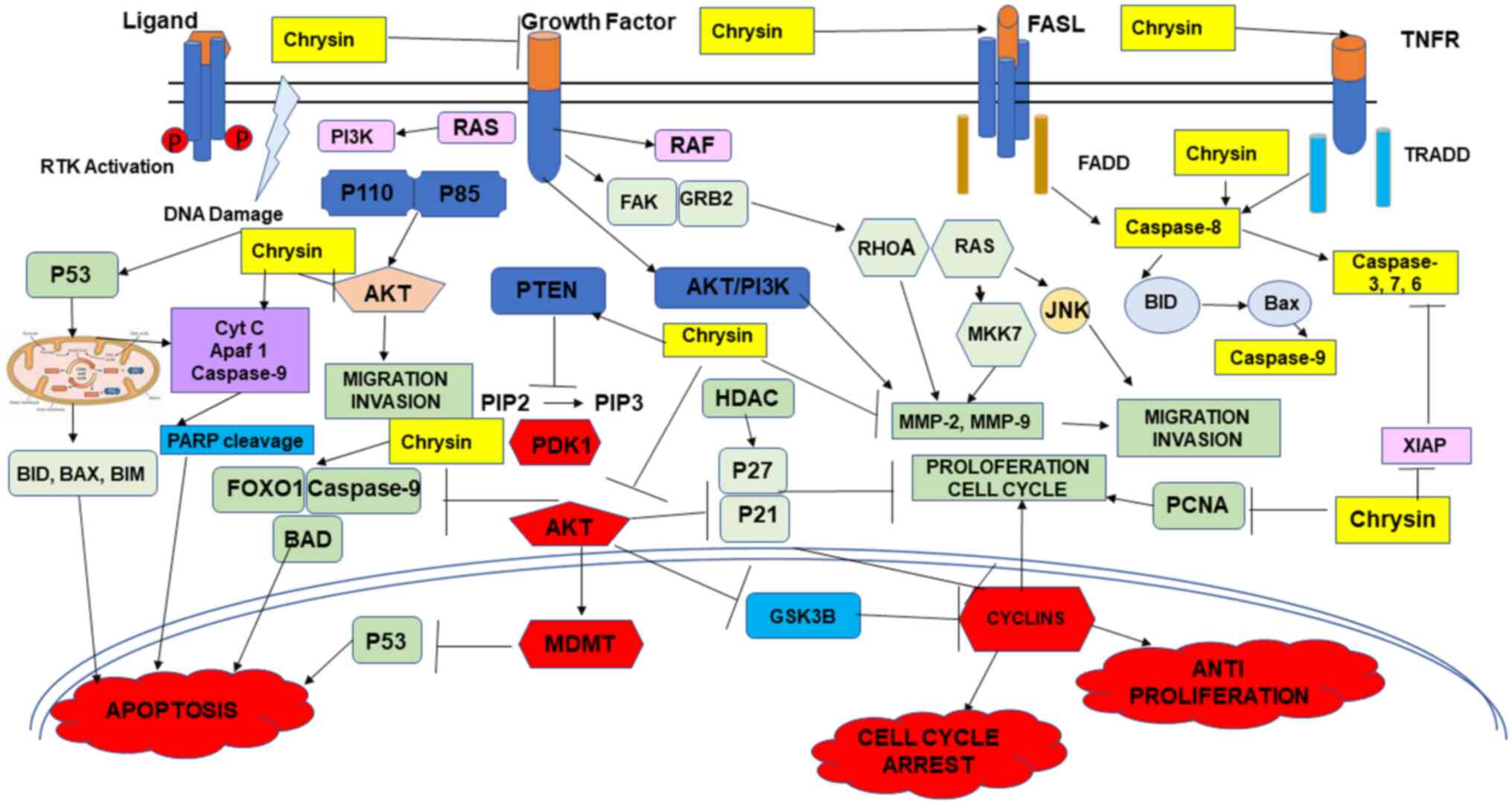
antioxidant and this has been reported by various studies. In rats,
chrysin has been shown to reduce lipid peroxidation, increase
antioxidant enzyme levels, decrease the expression of p53 and
intrinsic apoptosis-related proteins, including Bax, Noxa,
cytochrome c and caspase-3, increase the activity of Bcl-2,
inactivate the MAPK/JNK pathway and suppress the NF-κB pathways,
and at the same time upregulate the expression of PTEN, and
activate the VEGF/AKT pathway (Fig.
3) (18,35). Chrysin inhibits cytochrome P450
2E1, alcohol dehydrogenase and xanthine oxidase at various dosages
(20 and 40 mg/kg body weight) and protects Wistar rats against
oxidative stress. It also reduces serum aspartate aminotransferase,
alanine aminotransferase and glutamate aminotransferase levels
(36).
To identify published research, searches were
conducted using databases, such as Medline, PubMed, Scopus, Science
Direct and Google Scholar. Of note, two authors conducted a
literature search for this purpose using the terms chrysin,
anticancer, cancer therapy, chemotherapeutics and their
combinations. In addition, an investigation of the references of
the article was performed to search for any further research. The
search approach was used to filter the article titles and
abstracts.
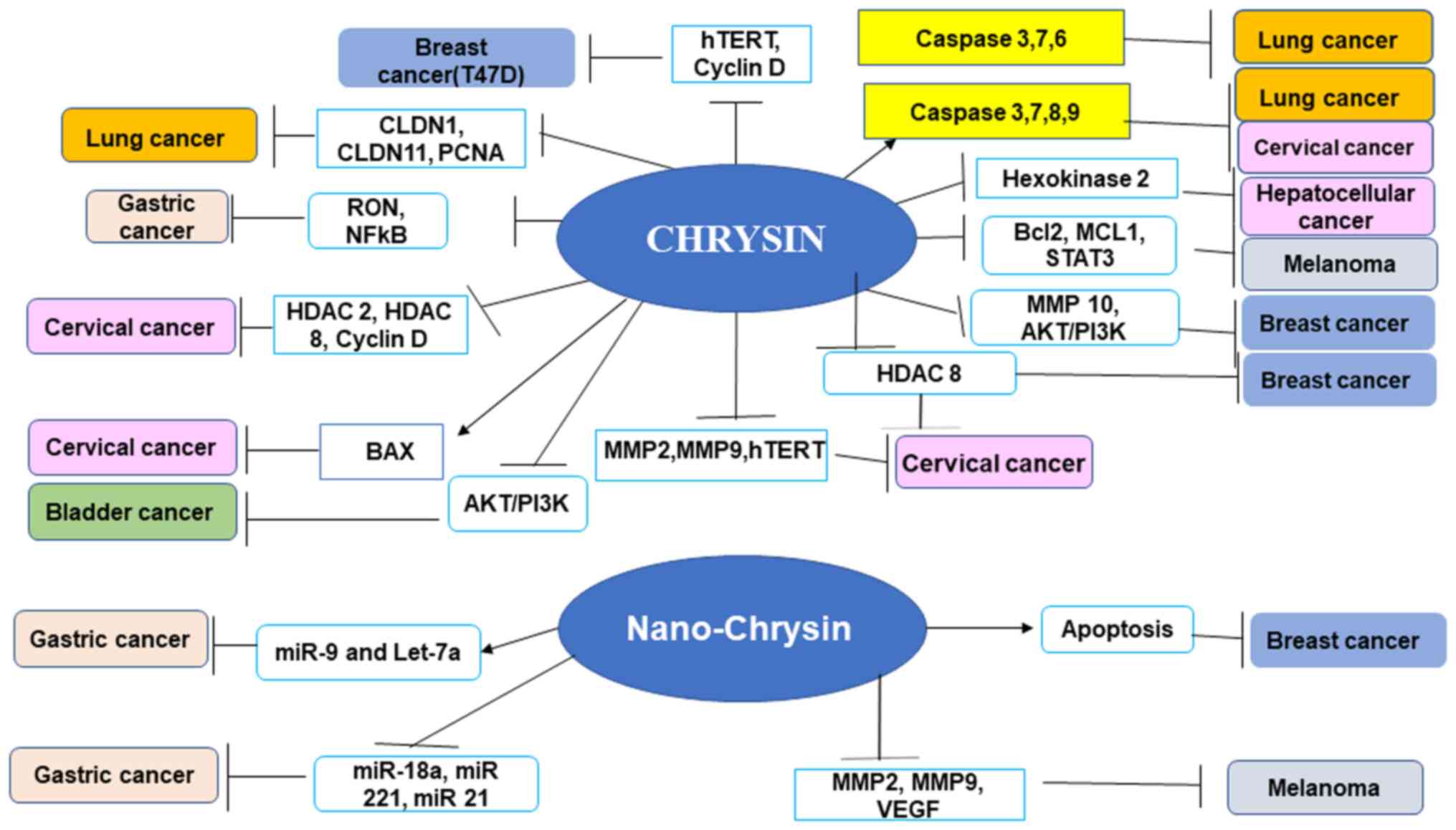
Chrysin demonstrates anticarcinogenic action in a
number of leukemias and most solid cancers. Chrysin has been shown
to have beneficial effects against numerous types of cancer,
including hepatic, breast, lung, cervical and prostate carcinomas
(10,37) (Fig.
3). Chrysin reduces cancer growth by selectively modulating
various cell signaling pathways associated with inflammation,
cancer cell survival, proliferation, angiogenesis, invasion and
metastasis. A number of studies have reported the anticancer
properties of chrysin in cell lines, as well as animal models,
demonstrating different pathways (10,37-39)
(Fig. 3). A previous study
demonstrated that the chrysin treatment in ovarian cancer led to
the augmented generation of reactive oxygen species, a decrease in
MMP and an increase in cytoplasmic Ca2+, together with
the initiation of cell demise (40). Chrysin has been shown to promote
the apoptosis of lymphocytic leukemia cells, via the mitochondrial
pathway (41). It has been found
that chrysin has no cytotoxic effect on normal cells, such as
fibroblasts (42). Additionally,
chrysin has depicted notable anticancer effects in combination with
chemotherapeutic drugs by overcoming resistance (43) (Fig.
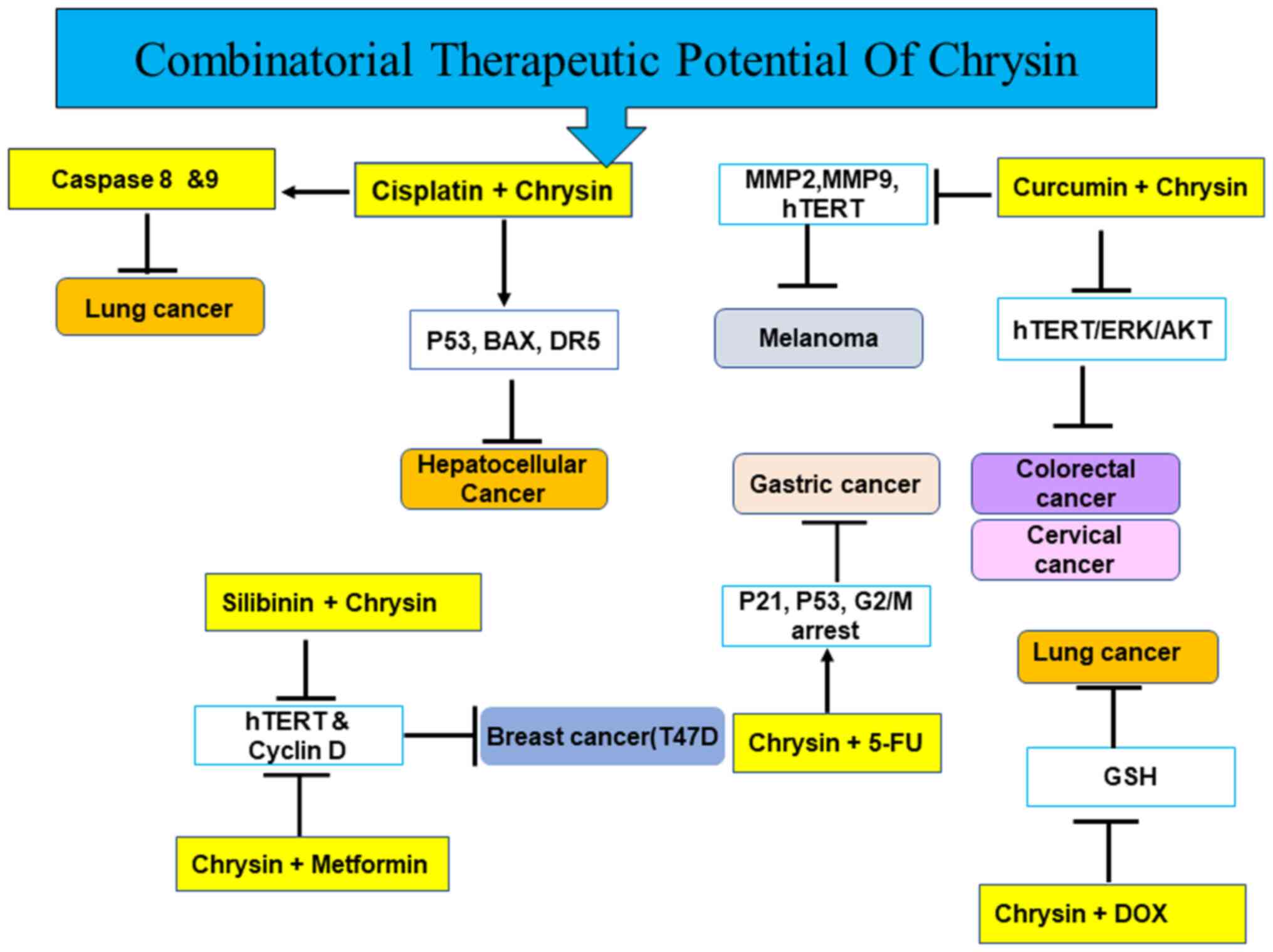
4). The present review discusses the anticancer effects of
chrysin in various types of cancer alone and in combination
(Figs. 1, and Fig. 3, Fig.
4, Fig. 5) with
chemotherapeutic agents.
The term ‘cervical cancer’ refers to a malignant
tumor that arises from cells in the cervix uteri (44,45).
It is a malignancy which frequently affects women and is considered
to be one of the chief reasons of cancer-related mortality
worldwide (46-48).
According to the WHO 2021 report, it is the fourth most frequent
type of cancer among women globally, both in regards of prevalence
and mortality rates, with an estimated 6 lakhs new cancer cases and
3.5 lakh deaths (49). The primary
cause of cervical cancer is manifestation of human papillomavirus
(HPV), and virtually all cervical cancers include one or more HPV
genotypes (50,51). There are no antiviral therapies
available for HPV infection; however, prophylactic vaccination is
an excellent primary preventive technique for cervical cancer
(52,53). Gardasil 9 has been approved for
nine types of HPV types (16 and 18 being most prevalent) and also,
these vaccinations provide minimal benefit to those who are
previously infested with the virus, and also are unavailable to
general populations in developing countries where cervical cancer
is prevalent maximum (52-54).
There are various chemotherapeutics available for cervical cancer
treatment; however, they all have severe side-effects; hence, the
search for selective cancer treatment strategies with which to
reduce the cervical cancer morbidity and motility continues
(3,11).
Various studies have reported the anticancer effects
of chrysin on different types of cervical cancer cells (Table I). Chrysin incudes the death of
cervical cancer cells via the modulation of various pathways, such
as MAPK, AKT/mTOR and genes responsible for tumor growth (Fig. 3) (4). Chrysin also induces apoptosis via
PI3K pathway in HeLa cells (55)
Chrysin likewise downregulates proliferating cell nuclear antigen
(PCNA) expression in cervical carcinoma cells (10). Chrysin at a dose of 30 µM has also
been shown to promote nuclear factor kappa-light-chain-enhancer of
activated B-cells in HeLa cells (56,57).
Additionally chrysin reactivates various TSGs and genes related to
apoptosis and migration by decreasing the methylation percentage of
these genes, and decreasing the expression of various epigenetic
enzymes, such as DNA methyl transferase, histone acetyl
transferase, histone deacetyl transferase and histone methyl
transferase at the biochemical and transcript level, leading to the
modulation of H3 and H4 histone modification marks and decreased
DNA methylation following the treatment of Hela cells with chrysin
(37). Chrysin encourages
TRAIL-induced apoptosis by sensitizing HeLa cells to chrysin
(58). Chrysin functions as a dual
inhibitor of methylation at the DNA and histone level (59). Chrysin decreases the expression of
TWIST 1 and NF-κB and thus suppresses epithelial-mesenchymal
transition (EMT) in HeLa cells (60).
In addition to the anticancer effects of chrysin
used alone, the use of chrysin in combination with other
chemotherapeutics and natural compounds has depicted additional
effects. It has been shown that chrysin and capsaicin promote early
senescence and programmed cell death via mitochondrial dysfunction
and an increase in p53 levels (61). Chrysin and cisplatin have also been
shown to exert synergistic effects on induction of apoptosis and
the inhibition of migration (62)
(Table II).
Previous research has demonstrated that chrysin
suppresses the proliferation in the MCF-7 breast cancer model in a
dose and time-dependent manner with IC50 values of 19.5 and 9.2 M
for 48 and 72 h, respectively (34,70).
This anti-proliferative action is credited to its capability to
induce the apoptosis of these cancer cell lines. Chrysin
significantly inhibits histone deacetylase (HDAC)8 activity with an
IC50 of 40.2 µM that significantly decreased the progression of
breast cancer (MDA-MB-231). It was previously shown that the oral
administration of 90 mg/kg/day of chrysin for 6 weeks evidently
decreased tumor size in the MDA-MB-231 xenograft model. Chrysin
administration led to the upregulation of CDKN1 at the transcript
and protein level (71). Chrysin
decreased the viability of 4T1 breast cancer cells by suppressing
hypoxia-induced phosphorylation of STAT3 and suppressed
hypoxia-induced VEGF gene expression. A similar effect was observed
in animal models implanted with 4T1 cells (42,72).
Chrysin also obstructs the migratory capacity of metastatic human
triple-negative breast cancer cells by modifying MMP10, EMT
transition, and PI3K/AKT pathway (68) (Table
I). Another study demonstrated that chrysin-loaded PGLA/PEG
nanoparticles modulated TIMPS and MMP2 and 9, and PI3K expression
in a mouse 4T1 breast tumor model (73).
In addition to its stand-alone anticancer
properties, chrysin has been shown to enhance the efficiency of
chemotherapeutic drugs. Nano technology has further increased the
efficiency of drugs (74). Chrysin
and metformin in combination have been found to exert
anti-proliferative effects against T47D breast cancer cells.
Chrysin used alone and as an adjuvant with metformin has been found
to downregulate cyclin D and hTERT expression in the breast cancer
cell line (69). Similar results
were obtained with the combination of chrysin and silibinin in T47D
breast cancer (75). Another study
demonstrated that chrysin ruthenium complex promoted the apoptosis
of MCF-7 cancer cells via the modulation of Bcl-2, p53 and Bax
(76). It has been shown that
nano-chrysin inhibits the proliferation of SKOV-3 and MCF-7 cells
in a concentration-dependent manner. Nano-chrysin had a much lower
IC50 value than pure chrysin and triggers the apoptosis of cancer
cells (77). Polymeric micelles
have also been created to administer chrysin and methotrexate to
breast cancer cells during chemotherapy (78). It has been found that PLGA-PEG
loaded with chrysin increases the solubility of the drug and
decreases the disputatious effects of chrysin. Chrysin proficiently
collects in the T47D cancer cells and augments the cytotoxicity of
chrysin on breast cancer cells (79). The combination of chrysin and
silibinin decreases the expression hTERT and cyclin D1 in T47D
breast cancer cells (75)
(Fig. 5). Curcumin and chrysin
have been shown to exert significant cooperative cytotoxicity, halt
the cell cycle at the G2/M stage and promote apoptosis through the
upregulation of expression of miR-321 and miR-502c in comparison to
the drugs used alone (80)
(Table II).
Chrysin (5,7-dihydroxyflavone) is a naturally
occurring flavonoid found in several therapeutic plants (4,28).
The anti-proliferative abilities of chrysin against human lung
cancer cells have been established by a number of research groups
(28,81) (Table
III). Treatment with chrysin has been shown to lead to the
activation of AMPKA and the suppression of AKT, the induction of
apoptosis and the growth inhibition of A549 lung cancer cells
(81).
CLDN1 and CLDN11 expression have been found to be
higher in human lung squamous cell carcinoma. Treatment with
chrysin treatment reduces both the mRNA and protein expression of
these claudin genes (85)
(Fig. 3). Chrysin exerts cytotoxic
effects on and promotes the death of human lung cancer and lymphoma
cells from mice at doses ranging from 25 to 75 g/ml, with no overt
damage to normal cells. Chrysin induces G1/S phase arrest at
specific doses. Treatment with chrysin treatment (1.3 mg/kg body
weight) significantly decreases tumor volume, resulting in a 52.6%
increase in mouse survival (86).
Chrysin restores the cellular equilibrium of cells
subjected to benzopyrene by downregulating the expression of
elevated proteins, such as PCNA, NF-κB and COX-2 (7,42).
Chrysin promotes the apoptosis of lung adenocarcinoma cells via the
modulation of caspases, Bax and Bcl-2(87). Chrysin, together with doxorubicin,
promote the activation of AMPK to induce A549 programmed cell death
which is attributed to AKT inhibition (81). It has also been shown that chrysin
can prevent the constitutive activation of STAT3, leading to the
decreased expression of myeloid cell leukemia-1 (Mcl-1); this
action motivates the deactivating TRAIL resistance of A549 human
lung cancer cells by chrysin (58). A previous study demonstrated that
quercetin and chrysin together decreased the levels of
pro-inflammatory molecules, such as IL-6, -1 and -10, and the
levels of TNF via the NF-κB pathway. In addition, chrysin and
quercetin downregulated the expression of Myd88 and Toll-like
receptor 4, as well as MMP9(88).
Chrysin has been shown to increase the efficacy of docetaxel in
non-small cell lung cancer by inducing cytotoxicity, suppressing
cell proliferation and promoting apoptosis (89) (Fig.
5). A previous study demonstrated that in A549 lung cancer
cells, curcumin- and chrysin-loaded nanoparticles led to the
downregulation of hTERT and MMPs (90). Another study demonstrated that the
combination of chrysin and doxorubicin decreased the IC50 value of
four cell lines, namely H460, A549, H157 and H1975(91). Chrysin at concentrations between 5
and 30 µM and doxorubicin at concentrations between 0.025 and 3.0
µM in combination functioned synergistically in lung cancer cells
to induce cell death and reduce the toxicity of doxorubicin
(91) (Table II). However, despite the numerous
biological properties of chrysin, its limited bioavailability is
the primary barrier to its use in pharmaceuticals. Chrysin-loaded
nanoparticles have depicted improved therapeutic activity in animal
models and may serve as a useful formulation for pharmacological
intervention (89).
Skin cancer is a common disease that affects
numerous individuals worldwide. The increase in the number of skin
cancer cases over the past few decades may be due to multiple
factors. These could include individual and collective habits, as
well as changes in the climate, particularly in the ozone layer.
The most common types of skin cancer encountered by physicians are
melanomas and non-melanomas. More specifically, there are two forms
of non-melanoma cancer: Squamous cell carcinoma and basal cell
carcinoma (92). The prognosis of
patients with this type of cancer is typically very poor (93). Of note, 80% of skin malignancies
that are not melanomas are caused by basal cell carcinoma. UV
radiation exposure is the main factor that may result in basal cell
carcinoma. Aggressive melanomas account for 60% of skin
cancer-related mortality, despite representing only 1% of all cases
of skin cancer cases (94).
Chrysin has been shown to inhibit squamous cell carcinoma via the
modulation of Rb and by decreasing the expression of CDK2 and
CDK4(95).
Melanoma is an extremely resistant and aggressive
skin tumor that accounts for >2-3% of all cancer occurrences.
Melanoma incidence has risen dramatically in recent decades and is
responsible for more than 75% of all skin cancer fatalities due to
its aggressiveness (96,97). Melanoma can be treated with surgery
in its early stages; however, treatment is impossible after it
metastasizes to other areas (97).
As melanin synthesis occurs inside the specialized membrane-bound
organelles known as melanosomes, it is a highly regulated process
in normal melanocytes. Under these circumstances, the synthesis of
melanin serves as a defense against attacks from the environment
and UVR-induced malignancies. However, melanin pigment appears to
have a function in the malignant transformation of melanocytes
despite its protective effect against UVR (98). This process can become dysregulated
in melanoma cells when melanogenesis intermediates seep outside of
melanosomes, influencing the behavior of the cells or the
environment around them (99).
Thus, unchecked melanogenesis plays a role, perhaps a crucial one,
in the development of melanotic melanoma and, in conjunction with
melanin pigment, it can reduce the effects of chemo- and
radiotherapy. The protective properties of melanin pigment under
normal circumstances and its destructive properties under
pathological ones represent the yin and yang of melanogenesis
(98).
Bioactive compounds, including chrysin derived from
plants, are able to induce apoptosis, as well as hinder migration;
they have been evaluated as possible medications in melanoma
treatment (100,101). In vitro, it has been shown
that chrysin selectively exhibits toxicity and induces the
self-programed death of human uveal melanoma cells (M17 and SP6.5)
without having any effect on normal cells (101). In vitro and in
vivo, chrysin has been shown to exert profound toxicity against
melanoma cells (Table III) by
encouraging self-programed death along with halting the cell cycle
at the G2/M or G1/S phases. In animal models, 2 and 3 weeks of
treatment with chrysin was found to decrease the tumor volume by
>55 and >65%, respectively (96,101) O note, chrysin promotes the
toxicity of natural killer cells, T-cells and macrophages, towards
cancer cells) (96). MMPs, such as
MMP2 and MMP9 play a prominent role in cancer cell migration via
the degradation of the extracellular matrix (102). Chrysin inhibits the migration of
melanoma cells and HeLa cells via the downregulation of MMP2, and
the upregulation of E-cadherin and the downregulation of cadherin
(37,103). The AKT/PI3K and NF-κB pathways
play a role in cancer cells and chrysin is associated with the
inhiation of the PI3K/Akt and NF-κB pathways in melanoma. Chrysin
decreases melanoma cell migration via the downregulation of the
EMT-related proteins, E-cadherin, Snail and Smad; WNT/β-catenin
target proteins, such as MMP2, MMP9, and VEGF have also been found
to be downregulated by chrysin in melanoma cells (104) (Table III and Fig. 3). To overcome drug resistance in
melanoma cells, nano-chrysin and nano-curcumin previous research
used for the treatment of C57B16 mice bearing B16F10 melanoma
tumors. It was observed that the combination of the two in an
encapsulated form decreased the migration of these highly
metastatic cancer cells via the downregulation of MMPs, tissue
inhibitor of metalloproteases and hTERT gene expression, more so in
the mouse B16F10 melanoma tumor model (3,105)
(Table II).
Bladder cancer is another main type of cancer found
in progressive countries. Its incidence rate is 6 lakh and >2
lakh deaths yearly according to the GLOBOCAN report in
2020(106). As all conventional
therapies are toxic and bladder cancer has exhibited resistance to
chemotherapeutics, research is being conducted to discover and
develop new strategies (107).
Plant-derived bioactive compounds are being used for the treatment
and prevention of different types of cancer, including bladder
cancer (4,9,107).
Notably, bladder cell cancer development is aided by the activation
of AKT/ERK/PI3K and STAT (108-110).
Chrysin has been shown to modulate the AKT pathway in bladder
fibrosis (111) (Fig. 3). Chrysin is a phytochemical found
in honey and bee propolis and it causes increase in ROS leading to
upregulation of caspases, downregulation of Bcl2, Bcl-xL and
inhibition of STAT3 and Mcl-1(112). Chrysin promotes the apoptosis of
bladder cancer cells (T-24 and 5637) via the upregulation of
caspase-9 and -3, and the downregulation of Bcl-xL, Mcl-1 and Bcl-2
and the intrinsic pathway (112)
(Table IV).
The mutation of TP53 is the main reason for the poor
survival rates of patients with bladder cell cancer. Chrysin
inhibits the propagation of bladder cancer cells having mutated and
wild-type TP53. Chrysin increases in reactive oxygen species, halts
the cell cycle, and promotes the downregulation of SRC, PLK1 and
HOXB3 in cells having mutated. Chrysin also promotes DNA
hypermethylation in grade 2 cells, and downregulates mTOR and c-MYC
in grade 3 cells. It has been proven that chrysin activity is
related to the TP53 status (113,114). The combined use of chrysin with
other chemotherapeutics exerts a synergistic effect. A previous
study demonstrates that the combination of TRAIL and chrysin led to
a decrease in the resistance of bladder cells to treatment and
increased cell death (115).
The effects of chrysin in hepatocellular carcinoma
have been reported by various studies (Table IV). Among the different factors
that affect cancer cell metabolism, namely the change from
oxidative phosphorylation to aerobic glycolysis, hexokonase2 is a
key protein (116). Chrysin
revokes the initial development of hepatic cancer and encourages
programmed cell death in N-nitroso-diethylamine-created early
neoplastic lumps in rats (117).
In a previous study, Chrysin decreased expression of HK-2 in
mitochondria, and the interaction between HK-2 and VDAC 2 was
disrupted, which resulted in a marked increase in membrane
permeability and the release of pro-apoptotic enzymes, such as
cytochrome c and the release of Bax in hepatocellular
carcinoma cells and animal models (118). As previously demonstrated, by
decreasing the expression of specified hexokinase2, chrysin
decreased glucose absorption and lactate generation in
hepatocellular carcinoma cells and apoptosis was induced by chrysin
due to the translocation of Bax from the cytoplasm to the
mitochondria. Furthermore, in hepatocellular carcinoma xenograft
models, Chrysin resulted in a promising decrease in tumor
development via hexokinase-2 downregulation (118). Chrysin exerted its effect on
propagation and programmed cell death in diethyl
nitrosamine-induced early hepatocarcinogenesis in male Wistar rats.
COX-2, NF-κB p65 and BcL-xL downregulation was observed, and Bax,
p53 and caspase-3 exhibited an amplified expression (117).
The emergence of chemoresistance has been linked to
the constitutive activation of the Nrf2-mediated signaling pathway.
Gao et al (119) examined
whether Nrf2 expression was connected to drug resistance in
BEL-7402 (BEL-7402/ADM) cells that were resistant to doxorubicin,
and whether chrysin could reverse drug resistance in BEL-7402/ADM
cells. It was observed that chrysin markedly decreased Nrf2
expression at both the mRNA and protein level via the
downregulation of the PI3K-Akt and ERK pathways (119). In another study, the combination
of chrysin and curcumin led to the apoptosis of HepG2 cells via the
p53 pathway (120) (Table II).
Gastric cancer is placed fifth for frequency and
fourth for mortality globally, and an estimated 769,000 deaths have
been recorded according to the GLOBOCAN report in 2020) (106). Conventional therapies are unable
to entirely cure cancer or considerably enhance the quality of life
of patients with cancer metastasis due to cancer cell migration. As
a result, innovative medicines that target the abnormal pathways
that contribute to cancer invasion or metastasis are being
developed (121). Chrysin has
been shown to exert significant effects in gastric cancer (Table V). Chrysin has been shown to
suppress both endogenous and inducible recepteur d'origine nantais
(RON), expression dose-dependently. The transcription factors,
Egr-1 and NF-κB, have a significant function in RON activity.
Furthermore, by inhibiting Egr-1 and NF-κB in AGS cells, chrysin
reduces RON expression at both levels (121) (Fig.
3). Chrysin instigated the movement of gastric cancer cells via
the downregulation of MMP9 and the downregulation of JNK and ERK
pathways (122). In gastric
cancer (MKN45) cells, the effect of chrysin on the expression
profile of TET proteins (TET1-3) was recently investigated. TET
enzymes play a role in DNA demethylation and, as a result, alter
gene expression via epigenetic mechanisms. It was found that TET1
expression in gastric cancer cells was enhanced by chrysin exposure
at both the transcript and protein levels. Chrysin, a HDAC
inhibitor, caused cytotoxicity, and also inhibited migration and
invasion. These effects were discovered to be mediated by TET1
overexpression induced by chrysin. TET1 overexpression in gastric
cancer cells replicated Chrysin-induced actions, leading to this
result. Notably, chrysin treatment induced apoptosis via the
modulation of Bax/Bcl-2, caused cell cycle detention at the sub G1
phase and repressed the metastasis of MKN-45 cells. In animal
experiments, chrysin decreased cancer development and increased
TET1 expression. Chrysin administration (20 mg/kg) for 14 days
significantly attenuated tumor development in a nude mouse
xenograft model of gastric cancer (123). These results demonstrate that
chrysin promotes programmed cell death in gastric cancer cells
initiated by the epigenetic player, TET1(42). Furthermore, dysregulation in miRNA
expression leads to the appearance of pathological circumstances,
particularly cancer (124,125).
Mohammadian et al (126)
reported that chrysin upregulated miR-9 and miR 22, and decreased
miR-18, miR-21 and miR-221 expression in the AGS cell line
(Table V). The use of chrysin and
PLGA-PEG nanoparticles has been shown to lead to the greater
promotion of miR-34a, miR-126 and miR-22 gene expression, in
comparison with free chrysin (127). In another study, the greater
decrease of miR-18a, miR-21 and miR-221 was attained by
chrysin-loaded PLGA-PEG nanoparticles (128). A previous study demonstrated that
chrysin upregulated miR-9 and Let-7a as onco-suppressor aspects in
cancer to hinder the propagation of gastric cancer cells.
Nanoparticles suggestively encourage the aptitude of chrysin in
upregulating miR-9 expression (129). It has been found that chrysin
with other chemotherapeutic agents enhances the anticancer effect.
The synergistic effect of chrysin and 5-fluorouracil (5-FU)
(Table II) was observed in
AGS/5-FU-resistant AGS cells with an enhanced p53-p21 activity,
with the arrest of the cell cycle at the G2/M phase (43). Chrysin and cisplatin used
concomitantly have been shown to lead to the self-programmed death
of HepG2 cells via both extracellular signals and mitochondrial
pathways by the activation of respective caspases) (120) (Table II). Chrysin has also been shown to
enhance the sensitivity of BEL-7402/ADM cells to doxorubicin
(121).
Colorectal cancer (CRC) is ranked third in terms of
frequency, but second in terms of cancer-related mortality. The
incidence rates are ~4-fold greater in developed countries than in
developing ones. The colon cancer incidence rates vary by ~9-fold
throughout the globe, with the highest rates observed in Europe,
New Zealand and North America, with a higher incidence in the male
population. However, Norway and Hungary also exhibit the highest
rates, although the incidence here among males and females is equal
(106). Previously, in in
vitro and in vivo experiments, the cytotoxic effects of
chrysin (Table V) and its line of
action were verified in colon cancer cells (CT26) (38). Chrysin exerted cytotoxic effects
and induced the apoptosis of CT26 cells in a
concentration-dependent manner (IC50, 80 µg/ml). Furthermore,
Chrysin-treated mice exhibited a considerable reduction in tumor
volume (38). A marked reduction
in the colon tumor volume in treated mice (8 and 10 mg/kg) was
observed as compared to the untreated mice. RT-PCR elucidated that
chrysin attenuated the tumor volume through the downregulation of
the Sall4 and the upregulation of Bax. Thus, the downregulation of
Sall4 and the increased expression of Bax were linked to a
decreased tumor volume (38). The
anticancer activity of chrysin has also been investigated in
studies involving the colon cancer cell lines, HT-55, HCA-7 and
LoVo. The IC50 values of chrysin against these cell lines ranged
from 0.4 to 0.8 mM with the modulation of various molecules, such
as ERK and AKT (42,130). Another study found that
irradiated chrysin induced the intrinsic apoptosis of H29 colon
cancer cells (131).
Chrysin in combination with other chemotherapeutic
agents has exhibited notable results. Drug resistance and adverse
effects have limited the use of 5-FU in the treatment of patients
with CRC (132,133). In the treatment of CRC, chrysin
has recently been proposed as a substitute for 5-FU. The use of
chrysin (5-50 M) has been linked to a considerable reduction in the
viability of CRC cells (134).
Autophagy is influenced by chrysin in the treatment if CRC,
according to a study of the molecular processes. Autophagy is a
‘self-digestion’ process that is triggered by stressful situations,
such endoplasmic reticulum stress, mitochondrial injury,
malnutrition, etc. (135).
Curcumin- and chrysin-loaded PLGA-PEG nanoparticles have been shown
to exert a collaborative effect and improve the cell death effects
of these bioactive agents against colorectal cancer cells (136). A previous study demonstrated that
apigenin used in combination with chrysin suppressed the
development and migration of CRC cells by reducing p38-MAPK/AKT
activity; however, at higher doses of chrysin (50 and 100 µM), the
effect was antagonistic (137)
(Table II).
Nanoparticle-based combinatorial chemotherapy has
been proposed as a potential technique for increasing intracellular
drug concentrations and achieving synergistic effects in anticancer
therapy. It has been shown that chrysin and curcumin alone exert a
dose-dependent cytotoxic effect on Caco-2 cells (138). However, curcumin-chrysin-loaded
nanoparticles, on the other hand, exert a significant inhibitory
effect on proliferation in comparison to the drugs used alone,
leading to a marked decrease in hTERT expression (138). Nano-encapsulated chrysin and
curcumin exert have been shown to exert a high synergistic effect
on SW480 cells cancer cells, as compared to their free versions.
The SW480 CRC cells subjected to a combination of chrysin and
curcumin in a nano-encapsulated form were found to exhibit a
significant inhibition of hTERT expression (136) (Fig.
5).
Ovarian cancer is not often detected, but is a very
fatal cancer and is the principal cause of mortality due to
gynecological cancers (139).
Ovarian cancer is divided into epithelial, germ cell, or stromal
tumors, based upon their location within the ovary (140), with the most common being the
epithelial type; this can be categorized into the benign, low
malignant and malignant type (141). The treatment regimen for ovarian
cancer is surgery followed by chemotherapy using carboplatin or
cisplatin or a combination of the two (140). However, due to the side-effects
associated with conventional therapies, it appears that
plant-derived agents are probable candidates for the treatment and
prevention ovarian cancer (40,142). Treatment with chrysin has been
shown to lead to the induction of cell death and the intrinsic
apoptosis of A2780cp cisplatin-resistant human ovarian cancer cells
(143). Another study
demonstrated that the treatment of ovarian cancer cells with
chrysin led to an upsurge in the concentration of ROS and
cytoplasmic Ca2+ and the activation of the MAPK/PI3K
pathways, that resulted in the induction of self-programed cell
death (40). However, by contrast,
a number of studies have demonstrated that the activation of the
PI3K/AKT pathway contributes to cell proliferation and metastasis,
and the inhibition of this pathway is a potential pathway for
targeted cancer therapy (4,56,144).
Thus, aforementioned study has depicted that chrysin suppresses
ovarian cancer via the activation of PI3K/AKT and MAPK (40). Hence, further research is warranted
in order to fully understand the exact modes of action of
chrysin.
Additionally, it has been reported that there is an
association between the incidence of cancer and diabetes (145); chrysin targets various disorders,
including diabetes and cancer. However, o date, to the best of our
knowledge, there are no data available demonstrating that the
anti-diabetic action of chrysin has prevented the occurrence of
cancer.
In conclusion, researchers are currently focusing
on finding the impact of various plant-derived agents on cancers
with the aim of developing cancer therapies. The results of this
research are aiding in the more in-depth understanding of the
causes and development of cancer. Although the effects of chrysin
have been shown in various types of cancer, the exact molecular
mechanisms through which chrysin controls the progression of
different types of cancer have not yet been fully eludicated.
Nevertheless, the majority of the data presented to date validate
the effects of chrysin on various types of cancer in vitro
and in animal models. However, the use of chrysin as a therapeutic
agent in clinical setups is still far from being realized, until
trials on humans are not performed and validated. In addition,
experiments to confirm its effects in combination with conventional
treatment agents and to enhance the treatment efficacy are
required.
Not applicable.
Funding: No funding was received.
Not applicable.
RR and AH were involved in the design of the study
and in the literature search for relevant references. RB edited the
manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hsieh YS, Yang SF, Sethi G and Hu DN:
Natural bioactives in cancer treatment and prevention. Biomed Res
Int. 2015(182835)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Montané X, Kowalczyk O, Reig-Vano B, Bajek
A, Roszkowski K, Tomczyk R, Pawliszak W, Giamberini M,
Mocek-Płóciniak A and Tylkowski B: Current perspectives of the
applications of polyphenols and flavonoids in cancer therapy.
Molecules. 25(3342)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raina R, Hussain A and Sharma R: Molecular
insight into apoptosis mediated by flavones in cancer (review).
World Acad Sci J. 2(6)2020.
|
|
4
|
Raina R, Afroze N, Sundaram MK, Haque S,
Bajbouj K, Hamad M and Hussain A: Chrysin inhibits propagation of
HeLa cells by attenuating cell survival and inducing apoptotic
pathways. Eur Rev Med Pharmacol Sci. 25:2206–2220. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Muthusami S, Prabakaran DS, An Z, Yu JR
and Park WY: EGCG suppresses fused toes homolog protein through p53
in cervical cancer cells. Mol Biol Rep. 40:5587–5596.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pratheeshkumar P, Sreekala C, Zhang Z,
Budhraja A, Ding S, Son YO, Wang X, Hitron A, Hyun-Jung K, Wang L,
et al: Cancer prevention with promising natural products:
Mechanisms of action and molecular targets. Anticancer Agents Med
Chem. 12:1159–1584. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kasala ER, Bodduluru LN, Barua CC, Madhana
RM, Dahiya V, Budhani MK, Mallugari RR, Maramreddy SR and Gogoi R:
Chemopreventive effect of chrysin, a dietary flavone against
benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice.
Pharmacol Rep. 68:310–318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh P, Tomar RS and Rath SK: Anticancer
potential of the histone deacetylase inhibitor-like effects of
flavones, a subclass of polyphenolic compounds: A review. Mol Biol
Rep. 42:1515–1531. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abdal Dayem A, Choi HY, Yang GM, Kim K,
Saha SK and Cho SG: The anti-cancer effect of polyphenols against
breast cancer and cancer stem cells: Molecular mechanisms.
Nutrients. 8(581)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kasala ER, Bodduluru LN, Madana RM, V AK,
Gogoi R and Barua CC: Chemopreventive and therapeutic potential of
chrysin in cancer: Mechanistic perspectives. Toxicol Lett.
233:214–225. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sak K: Characteristic features of
cytotoxic activity of flavonoids on human cervical cancer cells.
Asian Pacific J Cancer Prev. 15:8007–8019. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen
YM and Li H: Natural polyphenols for prevention and treatment of
cancer. Nutrients. 8(515)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu X, Li M, Xiao Z, Daglia M, Dragan S,
Delmas D, Vong T, Wang Y, Zhao Y, Shen J, et al: Dietary
polyphenols for managing cancers: What have we ignored? Trends Food
Sci Technol. 101:150–164. 2020.
|
|
14
|
Kopustinskiene DM, Jakstas V, Savickas A
and Bernatoniene J: Flavonoids as anticancer agents. Nutrients.
12(457)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Selvakumar P, Badgeley A, Murphy P, Anwar
H, Sharma U, Lawrence K and Lakshmikuttyamma A: Flavonoids and
other polyphenols act as epigenetic modifiers in breast cancer.
Nutrients. 12(761)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bodduluru LN, Kasala ER, Thota N, Barua
CC, Sistla R, Bodduluru LN, et al: Chemopreventive effect of
chrysin, a dietary flavone against benzo(a)pyrene induced lung
carcinogenesis in Swiss albino mice. Pharmacol Rep. 68:310–318.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ramírez-Espinosa JJ, Saldaña-Ríos J,
García-Jiménez S, Villalobos-Molina R, Ávila-Villarreal G,
Rodríguez-Ocampo AN, Bernal-Fernández G and Estrada-Soto S: Chrysin
induces antidiabetic, antidyslipidemic and anti-inflammatory
effects in athymic nude diabetic mice. Molecules.
23(67)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Naz S, Imran M, Rauf A, Orhan IE, Shariati
MA, Iahtisham-Ul-Haq IqraYasmin, Shahbaz M, Qaisrani TB, Shah ZA,
et al: Chrysin: Pharmacological and therapeutic properties. Life
Sci. 235(116797)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Balta C, Herman H, Boldura OM, Gasca I,
Rosu M, Ardelean A and Hermenean A: Chrysin attenuates liver
fibrosis and hepatic stellate cell activation through TGF-β/Smad
signaling pathway. Chem Biol Interact. 240:94–101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsuji PA and Walle T: Cytotoxic effects of
the dietary flavones chrysin and apigenin in a normal trout liver
cell line. Chem Biol Interact. 171:37–44. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Talebi M, Talebi M, Farkhondeh T,
Simal-Gandara J, Kopustinskiene DM, Bernatoniene J and
Samarghandian S: Emerging cellular and molecular mechanisms
underlying anticancer indications of chrysin. Cancer Cell Int.
21(214)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Galijatovic A, Otake Y, Walle UK and Walle
T: Extensive metabolism of the flavonoid chrysin by human Caco-2
and Hep G2 cells. Xenobiotica. 29:1241–1256. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ge S, Gao S, Yin T and Hu M: Determination
of pharmacokinetics of chrysin and its conjugates in wild-type FVB
and Bcrp1 knockout mice using a validated LC-MS/MS method. J Agric
Food Chem. 63:2902–2910. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee JA, Jung BG, Kim TH, Kim YM, Park MH,
Hyun PM, Jeon JW, Park JK, Cho CW, Suh GH and Lee BJ: Poly
D,L-lactide-co-glycolide (PLGA) nanoparticle-encapsulated honeybee
(Apis melifera) venom promotes clearance of Salmonella enterica
serovar Typhimurium infection in experimentally challenged pigs
through the up-regulation of T helper type 1 specific immune
responses. Vet Immunol Immunopathol. 161:193–204. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Arafa MG, Ghalwash D, El-Kersh DM and
Elmazar MM: Propolis-based niosomes as oromuco-adhesive films: A
randomized clinical trial of a therapeutic drug delivery platform
for the treatment of oral recurrent aphthous ulcers. Sci Rep.
8(18056)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mohammadinejad S, Akbarzadeh A,
Rahmati-Yamchi M, Hatam S, Kachalaki S, Zohreh S and Zarghami N:
Preparation and evaluation of chrysin encapsulated in PLGA-PEG
nanoparticles in the T47-D breast cancer cell line. Asian Pacific J
Cancer Prev. 16:3753–3758. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brown GA, Martini ER, Kohut ML, Franke WD,
Jackson DA and King DS and King DS: Endocrine and lipid responses
to chronic androstenediol-herbal supplementation in 30 to 58 year
old men. J Am Coll Nutr. 20:520–528. 2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Moghadam ER, Ang HL, Asnaf SE, Zabolian A,
Saleki H, Yavari M, Esmaeili H, Zarrabi A, Ashrafizadeh M and Kumar
AP: Broad-spectrum preclinical antitumor activity of chrysin:
Current trends and future perspectives. Biomolecules.
10(1374)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Slominski RM, Raman C, Chen JY and
Slominski AT: How cancer hijacks the body's homeostasis through the
neuroendocrine system. Trends Neurosci. 46:263–275. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mishra A, Mishra PS, Bandopadhyay R,
Khurana N, Angelopoulou E, Paudel YN and Piperi C: Neuroprotective
potential of chrysin: Mechanistic insights and therapeutic
potential for neurological disorders. Molecules.
26(6456)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
El-Sisi AE, El-Sayad ME and Abdelsalam NM:
Protective effects of mirtazapine and chrysin on experimentally
induced testicular damage in rats. Biomed Pharmacother.
95:1059–1066. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
El-Bassossy HM, Abo-Warda SM and Fahmy A:
Chrysin and luteolin attenuate diabetes-induced impairment in
endothelial-dependent relaxation: Effect on lipid profile, AGEs and
NO generation. Phyther Res. 27:1678–1684. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Satyanarayana K, Sravanthi K, Shaker I,
Ponnulakshmi R and Selvaraj J: Role of chrysin on expression of
insulin signaling molecules. J Ayurveda Integr Med. 6:248–258.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Samarghandian S, Farkhondeh T and
Azimi-Nezhad M: Protective effects of chrysin against drugs and
toxic agents. Dose Response. 15(1559325817711782)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mantawy EM, El-Bakly WM, Esmat A, Badr AM
and El-Demerdash E: Chrysin alleviates acute doxorubicin
cardiotoxicity in rats via suppression of oxidative stress,
inflammation and apoptosis. Eur J Pharmacol. 728:107–118.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tahir M and Sultana S: Chrysin modulates
ethanol metabolism in Wistar rats: A promising role against organ
toxicities. Alcohol Alcohol. 46:383–392. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Raina R, Almutary AG, Bagabir SA, Afroze
N, Fagoonee S, Haque S and Hussain A: Chrysin modulates aberrant
epigenetic variations and hampers migratory behavior of human
cervical (HeLa) cells. Front Genet. 12(768130)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bahadori M, Baharara J and Amini E:
Anticancer properties of chrysin on colon cancer cells, in vitro
and in vivo with modulation of caspase-3,-9, Bax and Sall4. Iran J
Biotechnol. 14:177–184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang Q, Ma S, Liu B, Liu J, Zhu R and Li
M: Chrysin induces cell apoptosis via activation of the
p53/Bcl-2/caspase-9 pathway in hepatocellular carcinoma cells. Exp
Ther Med. 12:469–474. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lim W, Ryu S, Bazer FW, Kim SM and Song G:
Chrysin attenuates progression of ovarian cancer cells by
regulating signaling cascades and mitochondrial dysfunction. J Cell
Physiol. 233:3129–3140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zaric M, Mitrovic M, Nikolic I, Baskic D,
Popovic S, Djurdjevic P, Milosavljevic Z and Zelen I: Chrysin
induces apoptosis in peripheral blood lymphocytes isolated from
human chronic lymphocytic leukemia. Anticancer Agents Med Chem.
15:189–195. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ganai SA, Sheikh FA and Baba ZA: Plant
flavone chrysin as an emerging histone deacetylase inhibitor for
prosperous epigenetic-based anticancer therapy. Phyther Res.
35:823–834. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee S, Lee SK and Jung J: Potentiating
activities of chrysin in the therapeutic efficacy of 5-fluorouracil
in gastric cancer cells. Oncol Lett. 21(24)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Y, Xie S, Wang Y, Luo K, Wang Y and
Cai Y: Liquiritigenin inhibits tumor growth and vascularization in
a mouse model of HeLa cells. Molecules. 17:7206–7216.
2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jin YM, Xu TM, Zhao YH, Wang YC and Cui
MH: In vitro and in vivo anti-cancer activity of formononetin on
human cervical cancer cell line HeLa. Tumor Biol. 35:2279–2284.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hussain A, Harish G, Prabhu SA, Mohsin J,
Khan MA, Rizvi TA and Sharma C: Inhibitory effect of genistein on
the invasive potential of human cervical cancer cells via
modulation of matrix metalloproteinase-9 and tissue inhibitiors of
matrix metalloproteinase-1 expression. Cancer Epidemiol.
36:e387–e393. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chou RH, Hsieh SC, Yu YL, Huang MH, Huang
YC and Hsieh YH: Fisetin inhibits migration and invasion of human
cervical cancer cells by down-regulating urokinase plasminogen
activator expression through suppressing the p38 MAPK-dependent
NF-κB signaling pathway. PLoS One. 8(e71983)2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen YJ, Kay N, Yang JM, Lin CT, Chang HL,
Wu YC, Fu CF, Chang Y, Lo S, Hou MF, et al: Total synthetic
protoapigenone WYC02 inhibits cervical cancer cell proliferation
and tumour growth through PIK3 signalling pathway. Basic Clin
Pharmacol Toxicol. 113:8–18. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Stelzle D, Tanaka LF, Lee KK, Ibrahim
Khalil A, Baussano I, Shah ASV, McAllister DA, Gottlieb SL, Klug
SJ, Winkler AS, et al: Estimates of the global burden of cervical
cancer associated with HIV. Lancet Glob Health. 9:e161–e169.
2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ham S, Kim KH, Kwon TH, Bak Y, Lee DH,
Song YS, Park SH, Park YS, Kim MS, Kang JW, et al: Luteolin induces
intrinsic apoptosis via inhibition of E6/E7 oncogenes and
activation of extrinsic and intrinsic signaling pathways in
HPV-18-associated cells. Oncol Rep. 31:2683–2691. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kim MS, Bak Y, Park YS, Lee DH, Kim JH,
Kang JW, Song HH, Oh SR and Yoon DY: Wogonin induces apoptosis by
suppressing E6 and E7 expressions and activating intrinsic
signaling pathways in HPV-16 cervical cancer cells. Cell Biol
Toxicol. 29:259–272. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Garcia FAR, Cornelison T, Nuño T,
Greenspan DL, Byron JW, Hsu CH, Alberts DS and Chow HH: Results of
a phase II randomized, double-blind, placebo-controlled trial of
Polyphenon E in women with persistent high-risk HPV infection and
low-grade cervical intraepithelial neoplasia. Gynecol Oncol.
132:377–382. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cherry JJ, Rietz A, Malinkevich A, Liu Y,
Xie M, Bartolowits M, Davisson VJ, Baleja JD and Androphy EJ:
Structure based identification and characterization of flavonoids
that disrupt human papillomavirus-16 E6 function. PLoS One.
8(e84506)2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Berman TA and Schiller JT: Human
papillomavirus in cervical cancer and oropharyngeal cancer: One
cause, two diseases. Cancer. 123:2219–2229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yin KB: Chrysin in PI3K/AKT and other
apoptosis signalling pathways, and its effect on HeLa cells,
2014.
|
|
56
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
von Brandenstein MG, Abety AN, Depping R,
Roth T, Koehler M, Dienes HP and Fries JWU: A p38-p65 transcription
complex induced by endothelin-1 mediates signal transduction in
cancer cells. Biochim Biophys Acta. 1783:1613–1622. 2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lirdprapamongkol K, Sakurai H, Abdelhamed
S, Yokoyama S, Athikomkulchai S, Viriyaroj A, Awale S, Ruchirawat
S, Svasti J and Saiki I: Chrysin overcomes TRAIL resistance of
cancer cells through Mcl-1 downregulation by inhibiting STAT3
phosphorylation. Int J Oncol. 43:329–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kanwal R, Datt M, Liu X and Gupta S:
Dietaryflavones as dual inhibitors of DNA methyltransferases and
histone methyltransferases. PLoS One. 11(e0162956)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Dong W, Chen A, Cao X, Li X, Cui YH, Xu C,
Cao J and Ning Y: Chrysin inhibits proinflammatory factor-induced
EMT phenotype and cancer stem cell-like features in HeLa cells by
blocking the NF-κB/Twist axis. Cell Physiol Biochem. 52:1236–1250.
2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pawar JS, Mustafa S and Ghosh I: Chrysin
and Capsaicin induces premature senescence and apoptosis via
mitochondrial dysfunction and p53 elevation in Cervical cancer
cells. Saudi J Biol Sci. 29:3838–3847. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Raina R, Hussain A, Almutary AG, Haque S,
Raza T, D'Souza AC, Subramani S and Sajeevan A: Co-administration
of chrysin and luteolin with cisplatin and topotecan exhibits a
variable therapeutic value in human cancer cells, HeLa. ACS Omega.
8:41204–41213. 2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Pandey K, An HJ, Kim SK, Lee SA, Kim S,
Lim SM, Kim GM, Sohn J and Moon YW: Molecular mechanisms of
resistance to CDK4/6 inhibitors in breast cancer: A review. Int J
Cancer. 145:1179–1188. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Pandey PR, Young KH, Kumar D and Jain N:
RNA-mediated immunotherapy regulating tumor immune
microenvironment: Next wave of cancer therapeutics. Mol Cancer.
21(58)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Shanmugam MK, Ahn KS, Hsu A, Woo CC, Yuan
Y, Tan KHB, Chinnathambi A, Alahmadi TA, Alharbi SA, Koh APF, et
al: Thymoquinone inhibits bone metastasis of breast cancer cells
through abrogation of the CXCR4 signaling axis. Front Pharmacol.
9(1294)2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Liu L, Ahn KS, Shanmugam MK, Wang H, Shen
H, Arfuso F, Chinnathambi A, Alharbi SA, Chang Y, Sethi G and Tang
FR: Oleuropein induces apoptosis via abrogating NF-κB activation
cascade in estrogen receptor-negative breast cancer cells. J Cell
Biochem. 120:4504–4513. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yang B, Huang J, Xiang T, Yin X, Luo X,
Huang J, Luo F, Li H, Li H and Ren G: Chrysin inhibits metastatic
potential of human triple-negative breast cancer cells by
modulating matrix metalloproteinase-10, epithelial to mesenchymal
transition, and PI3K/Akt signaling pathway. J Appl Toxicol.
34:105–112. 2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Rasouli S and Zarghami N: Synergistic
growth inhibitory effects of chrysin and metformin combination on
breast cancer cells through hTERT and cyclin D1 suppression. Asian
Pacific J Cancer Prev. 19:977–982. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Samarghandian S, Azimi-Nezhad M, Borji A,
Hasanzadeh M, Jabbari F, Farkhondeh T and Samini M: Inhibitory and
cytotoxic activities of Chrysin on human breast adenocarcinoma
cells by induction of apoptosis. Pharmacogn Mag. 12 (Suppl
4):S436–S440. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Sun LP, Chen AL, Hung HC, Chien YH, Huang
JS, Huang CY, Chen YW and Chen CN: Chrysin: A histone deacetylase 8
inhibitor with anticancer activity and a suitable candidate for the
standardization of Chinese propolis. J Agric Food Chem.
60:11748–11758. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lirdprapamongkol K, Sakurai H, Abdelhamed
S, Yokoyama S, Maruyama T, Athikomkulchai S, Viriyaroj A, Awale S,
Yagita H, Ruchirawat S, et al: A flavonoid chrysin suppresses
hypoxic survival and metastatic growth of mouse breast cancer
cells. Oncol Rep. 30:2357–2364. 2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Mohammadi Z, Sharif Zak M, Seidi K, Barati
M, Akbarzadeh A and Zarghami N: The effect of chrysin loaded
PLGA-PEG on metalloproteinase gene expression in mouse 4T1 tumor
model. Drug Res (Stuttg). 67:211–216. 2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Palakurthi S, Yellepeddi VK and Vangara
KK: Recent trends in cancer drug resistance reversal strategies
using nanoparticles. Expert Opin Drug Deliv. 9:287–301.
2012.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Javan Maasomi Z, Pilehvar Soltanahmadi Y,
Dadashpour M, Alipour Sh, Abolhasani S and Zarghami N: Synergistic
anticancer effects of silibinin and chrysin in T47D breast cancer
cells. Asian Pacific J Cancer Prev. 18:1283–1287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Roy S, Sil A and Chakraborty T:
Potentiating apoptosis and modulation of p53, Bcl2, and Bax by a
novel chrysin ruthenium complex for effective chemotherapeutic
efficacy against breast cancer. J Cell Physiol. 234:4888–4909.
2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Sulaiman GM, Jabir MS and Hameed AH:
Nanoscale modification of chrysin for improved of therapeutic
efficiency and cytotoxicity. Artif Cells Nanomed Biotechnol. 46
(Suppl 1):S708–S720. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Davaran S, Fazeli H, Ghamkhari A, Rahimi
F, Molavi O, Anzabi M and Salehi R: Synthesis and characterization
of novel P(HEMA-LA-MADQUAT) micelles for co-delivery of
methotrexate and chrysin in combination cancer chemotherapy. J
Biomater Sci Polym Ed. 29:1265–1286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Anari E, Akbarzadeh A and Zarghami N:
Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect
on the breast cancer cell line. Artif Cells Nanomed Biotechnol.
44:1410–1416. 2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Javan N, Khadem Ansari MH, Dadashpour M,
Khojastehfard M, Bastami M, Rahmati-Yamchi M and Zarghami N:
Synergistic antiproliferative effects of co-nanoencapsulated
curcumin and chrysin on MDA-MB-231 breast cancer cells through
upregulating miR-132 and miR-502c. Nutr Cancer. 71:1201–1213.
2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Shao JJ, Zhang AP, Qin W, Zheng L, Zhu YF
and Chen X: AMP-activated protein kinase (AMPK) activation is
involved in chrysin-induced growth inhibition and apoptosis in
cultured A549 lung cancer cells. Biochem Biophys Res Commun.
423:448–453. 2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhang Y, Xu X, Li W, Miao H, Huang S, Zhou
Y, Sun Y, Li Z, Guo Q and Zhao L: Activation of endoplasmic
reticulum stress and the extrinsic apoptotic pathway in human lung
cancer cells by the new synthetic flavonoid, LZ-205. Oncotarget.
7:87257–87270. 2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Pinsolle J, Terzi N, Ferrer L, Giaj Levra
M, Toffart AC and Moro-Sibilot D: Les avancées dans la prise en
charge des cancers bronchopulmonaires: Ce qui change pour le
réanimateur. Méd Intensive Réa. 28:290–299. 2019.
|
|
84
|
Mehdi SH, Zafaryab M, Nafees S, Khan A,
Ahmad I, Hafeez ZB and Rizvi MA: Chrysin sensitizes human lung
cancer cells to tumour necrosis factor related apoptosis-inducing
ligand (TRAIL) mediated apoptosis. Asian Pac J Cancer Biol.
4:27–33. 2019.
|
|
85
|
Maruhashi R, Eguchi H, Akizuki R, Hamada
S, Furuta T, Matsunaga T, Endo S, Ichihara K and Ikari A: Chrysin
enhances anticancer drug-induced toxicity mediated by the reduction
of claudin-1 and 11 expression in a spheroid culture model of lung
squamous cell carcinoma cells. Sci Rep. 9(13753)2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Lakshmi S, Suresh S, Rahul BS, Saikant R,
Maya V, Gopi M, Padmaja G and Remani P: In vitro and in vivo
studies of 5,7-dihydroxy flavones isolated from Alpinia galanga
(L.) against human lung cancer and ascetic lymphoma. Med Chem Res.
28:39–51. 2019.
|
|
87
|
Samarghandian S, Azimi Nezhad M and
Mohammadi G: Role of caspases, Bax and Bcl-2 in chrysin-induced
apoptosis in the A549 human lung adenocarcinoma epithelial cells.
Anticancer Agents Med Chem. 14:901–909. 2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wu TC, Chan ST, Chang CN, Yu PS, Chuang CH
and Yeh SL: Quercetin and chrysin inhibit nickel-induced invasion
and migration by downregulation of TLR4/NF-κB signaling in A549
cells. Chem Biol Interact. 292:101–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Lim HK, Kim KM, Jeong SY, Choi EK and Jung
J: Chrysin increases the therapeutic efficacy of docetaxel and
mitigates docetaxel-induced edema. Integr Cancer Ther. 16:496–504.
2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Mohammad P, Nosratollah Z, Mohammad R,
Abbas A and Javad R: The inhibitory effect of Curcuma longa extract
on telomerase activity in A549 lung cancer cell line. Afr J
Biotechnol. 9:912–919. 2010.
|
|
91
|
Brechbuhl HM, Kachadourian R, Min E, Chan
D and Day BJ: Chrysin enhances doxorubicin-induced cytotoxicity in
human lung epithelial cancer cell lines: The role of glutathione.
Toxicol Appl Pharmacol. 258:1–9. 2012.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Shahbaz M, Naeem H, Imran M, Ul Hassan H,
Alsagaby SA, Al Abdulmonem W, Waqar AB, Ghorab AH, Abdelgawad MA,
Ghoneim MM, et al: Chrysin a promising anticancer agent: Recent
perspectives. Int J Food Prop. 26:2294–2337. 2023.
|
|
93
|
Khazaei Z, Ghorat F, Jarrahi AM, Adineh
HA, Sohrabivafa M and Goodarzi E: Global incidence and mortality of
skin cancer by histological subtype and its relationship with the
human development index (HDI); an ecology study in 2018 2018. World
Cancer Res J. 6(e1265)2019.
|
|
94
|
Carr S, Smith C and Wernberg J:
Epidemiology and risk factors of melanoma. Surg Clin North Am.
100:1–12. 2020.
|
|
95
|
Islam MM, Nagaraja S, Hafsa NE, Meravanige
G, Asdaq SMB and Anwer MK: Polyphenol chrysin for management of
skin disorders: Current status and future opportunities. J King
Saud Univ Sci. 34(102026)2022.
|
|
96
|
Sassi A, Maatouk M, El gueder D, Bzéouich
IM, Abdelkefi-Ben Hatira S, Jemni-Yacoub S, Ghedira K and
Chekir-Ghedira L: Chrysin, a natural and biologically active
flavonoid suppresses tumor growth of mouse B16F10 melanoma cells:
In vitro and in vivo study. Chem Biol Interact. 283:10–19.
2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Bittner M, Meltzer P, Chen Y, Jiang Y,
Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, et
al: Molecular classification of cutaneous malignant melanoma by
gene expression profiling. Nature. 406:536–540. 2000.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Slominski RM, Sarna T, Płonka PM, Raman C,
Brożyna AA and Slominski AT: Melanoma, melanin, and melanogenesis:
The Yin and Yang relationship. Front Oncol.
12(842496)2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Slominski RM, Zmijewski MA and Slominski
AT: The role of melanin pigment in melanoma. Exp Dermatol.
24:258–259. 2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Yang HZ, Zhang J, Zeng J, Liu S, Zhou F,
Zhang F, Giampieri F, Cianciosi D, Forbes-Hernandez TY, Ansary J,
et al: Resveratrol inhibits the proliferation of melanoma cells by
modulating cell cycle. Int J Food Sci Nutr. 71:84–93.
2020.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Xue C, Chen Y, Hu D, Iacob C, Lu C and
Huang Z: Chrysin induces cell apoptosis in human uveal melanoma
cells via intrinsic apoptosis. Oncol Lett. 12:4813–4820.
2016.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Folgueras AR, Pendás AM, Sánchez LM and
López-Otín C: Matrix metalloproteinases in cancer: From new
functions to improved inhibition strategies. Int J Dev Biol.
48:411–424. 2004.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Chen HY, Jiang YW, Kuo CL, Way T Der, Chou
YC, Chang YS and Chung JG: Chrysin inhibit human melanoma A375.S2
cell migration and invasion via affecting MAPK signaling and NF-κB
signaling pathway in vitro. Environ Toxicol. 34:434–442.
2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Yufei Z, Yuqi W, Binyue H, Lingchen T, Xi
C, Hoffelt D and Fuliang H: Chrysin Inhibits melanoma tumor
metastasis via interfering with the FOXM1/β-catenin signaling. J
Agric Food Chem. 68:9358–9367. 2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Tavakoli F, Jahanban-Esfahlan R, Seidi K,
Jabbari M, Behzadi R, Pilehvar-Soltanahmadi Y and Zarghami N:
Effects of nano-encapsulated curcumin-chrysin on telomerase, MMPs
and TIMPs gene expression in mouse B16F10 melanoma tumour model.
Artif Cells Nanomed Biotechnol. 46 (Suppl 2):S75–S86.
2018.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Cai Z, Zhang F, Chen W, Zhang J and Li H:
Mirnas: A promising target in the chemoresistance of bladder
cancer. Onco Targets Ther. 12:11805–11816. 2019.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Korac-Prlic J, Degoricija M, Vilović K,
Haupt B, Ivanišević T, Franković L, Grivennikov S and Terzić J:
Targeting Stat3 signaling impairs the progression of bladder cancer
in a mouse model. Cancer Lett. 490:89–99. 2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Sun N, Liang Y, Chen Y, Wang L, Li D,
Liang Z, Sun L, Wang Y and Niu H: Glutamine affects T24 bladder
cancer cell proliferation by activating STAT3 through ROS and
glutaminolysis. Int J Mol Med. 44:2189–2200. 2019.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Anand V, Khandelwal M, Appunni S, Gupta N,
Seth A, Singh P, Mathur S and Sharma A: CD44 splice variant
(CD44v3) promotes progression of urothelial carcinoma of bladder
through Akt/ERK/STAT3 pathways: Novel therapeutic approach. J
Cancer Res Clin Oncol. 145:2649–2661. 2019.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Nagavally RR: Inhibition of epithelial
mesenchymal transition (EMT) and renal fibrosis by chrysin involves
modulation of Akt signaling. PhD dissertation. St. John's
University (New York) ProQuest Dissertations & Theses.
Publication no. 10170226, 2016. https://www.proquest.com/openview/9d262b69f271cffaa41ea9e6901fe3d0/1?pq-origsite=gscholar&cbl=18750.
|
|
112
|
Xu Y, Tong Y, Ying J, Lei Z, Wan L, Zhu X,
Ye F, Mao P, Wu X, Pan R, et al: Chrysin induces cell growth
arrest, apoptosis, and ER stress and inhibits the activation of
STAT3 through the generation of ROS in bladder cancer cells. Oncol
Lett. 15:9117–9125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Lima APB, Almeida TC, Barros TMB, Rocha
LCM, Garcia CCMH and Da Silva GN: Toxicogenetic and
antiproliferative effects of chrysin in urinary bladder cancer
cells. Mutagenesis. 35:361–371. 2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Talebi M, Talebi M, Kakouri E, Farkhondeh
T, Pourbagher-Shahri AM, Tarantilis PA and Samarghandian S:
Tantalizing role of p53 molecular pathways and its coherent
medications in neurodegenerative diseases. Int J Biol Macromol.
172:93–103. 2021.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Szliszka E, Gebka J, Bronikowska J and
Krol W: Dietary flavones enhance the effect of tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) on bladder cancer
cells. Cent Eur J Urol. 63:138–143. 2010.
|
|
116
|
Guo-Qing P, Yuan Y, Cai-Gao Z, Hongling Y,
Gonghua H and Yan T: A study of association between expression of
hOGG1, VDAC1, HK-2 and cervical carcinoma. J Exp Clin Cancer Res.
29(129)2010.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Khan MS, Devaraj H and Devaraj N: Chrysin
abrogates early hepatocarcinogenesis and induces apoptosis in
N-nitrosodiethylamine-induced preneoplastic nodules in rats.
Toxicol Appl Pharmacol. 251:85–94. 2011.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Xu D, Jin J, Yu H, Zhao Z, Ma D, Zhang C
and Jiang H: Chrysin inhibited tumor glycolysis and induced
apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J
Exp Clin Cancer Res. 36(44)2017.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Gao AM, Ke ZP, Shi F, Sun GC and Chen H:
Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin
by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem Biol
Interact. 206:100–108. 2013.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Li X, Huang JM, Wang JN, Xiong XK, Yang XF
and Zou F: Combination of chrysin and cisplatin promotes the
apoptosis of Hep G2 cells by up-regulating p53. Chem Biol Interact.
232:12–20. 2015.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Xia Y, Lian S, Khoi PN, Yoon HJ, Han JY,
Chay KO, Kim KK and Jung YD: Chrysin inhibits cell invasion by
inhibition of recepteur d'origine Nantais via suppressing early
growth response-1 and NF-κB transcription factor activities in
gastric cancer cells. Int J Oncol. 46:1835–1843. 2015.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Xia Y, Lian S, Khoi PN, Yoon HJ, Joo YE,
Chay KO, Kim KK and Do Jung Y: Chrysin inhibits tumor
promoter-induced MMP-9 expression by blocking AP-1 via suppression
of ERK and JNK pathways in gastric cancer cells. PLoS One.
10(e0124007)2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Zhong X, Liu D, Jiang Z, Li C, Chen L, Xia
Y, Liu D, Yao Q and Wang D: Chrysin induced cell apoptosis and
inhibited invasion through regulation of TET1 expression in gastric
cancer cells. Onco Targets Ther. 13:3277–3287. 2020.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Wang J, Zhang L, Jiang W, Zhang R, Zhang
B, Silayiding A and Duan X: MicroRNA-135a promotes proliferation,
migration, invasion and induces chemoresistance of endometrial
cancer cells. Eur J Obstet Gynecol Reprod Biol X.
5(100103)2019.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Xu C, Li B, Zhao S, Jin B, Jia R, Ge J and
Xu H: MicroRNA-186-5p inhibits proliferation and metastasis of
esophageal cancer by mediating HOXA9. Onco Targets Ther.
12:8905–8914. 2019.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Mohammadian F, Pilehvar-Soltanahmadi Y,
Alipour S, Dadashpour M and Zarghami N: Chrysin alters microRNAs
expression levels in gastric cancer cells: Possible molecular
mechanism. Drug Res (Stuttg). 67:509–514. 2017.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Mohammadian F, Abhari A, Dariushnejad H,
Nikanfar A, Pilehvar-Soltanahmadi Y and Zarghami N: Effects of
chrysin-PLGA-PEG nanoparticles on proliferation and gene expression
of miRNAs in gastric cancer cell line. Iran J Cancer Prev.
9(e4190)2016.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Mohammadian F, Pilehvar-Soltanahmadi Y,
Mofarrah M, Dastani-Habashi M and Zarghami N: Down regulation of
miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by
chrysin-loaded PLGA-PEG nanoparticles. Artif Cells Nanomed
Biotechnol. 44:1972–1978. 2016.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Mohammadian F, Pilehvar-Soltanahmadi Y,
Zarghami F, Akbarzadeh A and Zarghami N: Upregulation of miR-9 and
Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artif
Cells Nanomed Biotechnol. 45:1–6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Zhang MM, Huang SS, Long D and Lin X:
Anti-proliferative action of chrysin in colon cancer cells and its
effects on signaling pathways. Int J Clin Exp Med. 9:22784–22792.
2016.
|
|
131
|
Song HY, Kim HM, Mushtaq S, Kim WS, Kim
YJ, Lim ST and Byun EB: Gamma-irradiated chrysin improves
anticancer activity in HT-29 colon cancer cells through
mitochondria-related pathway. J Med Food. 22:713–721.
2019.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Lee CS, Ryan EJ and Doherty GA:
Gastro-intestinal toxicity of chemotherapeutics in colorectal
cancer: The role of inflammation. World J Gastroenterol.
20:3751–3761. 2014.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Crea F, Nobili S, Paolicchi E, Perrone G,
Napoli C, Landini I, Danesi R and Mini E: Epigenetics and
chemoresistance in colorectal cancer: An opportunity for treatment
tailoring and novel therapeutic strategies. Drug Resist Updat.
14:280–296. 2011.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Lin YM, Chen CI, Hsiang YP, Hsu YC, Cheng
KC, Chien PH, Pan HL, Lu CC and Chen YJ: Chrysin attenuates cell
viability of human colorectal cancer cells through autophagy
induction unlike 5-fluorouracil/oxaliplatin. Int J Mol Sci.
19(1763)2018.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Patra S, Mishra SR, Behera BP, Mahapatra
KK, Panigrahi DP, Bhol CS, Praharaj PP, Sethi G, Patra SK and
Bhutia SK: Autophagy-modulating phytochemicals in cancer
therapeutics: Current evidences and future perspectives. Semin
Cancer Biol. 80:205–217. 2022.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Bagheri R, Sanaat Z and Zarghami N:
Synergistic effect of free and nano-encapsulated chrysin-curcumin
on inhibition of hTERT gene expression in SW480 colorectal cancer
cell line. Drug Res (Stuttg). 68:335–343. 2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Zhang X, Zhang W, Chen F and Lu Z:
Combined effect of chrysin and apigenin on inhibiting the
development and progression of colorectal cancer by suppressing the
activity of P38-MAPK/AKT pathway. IUBMB Life. 73:774–783.
2021.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Lotfi-Attari J, Pilehvar-Soltanahmadi Y,
Dadashpour M, Alipour S, Farajzadeh R, Javidfar S and Zarghami N:
Co-delivery of curcumin and chrysin by polymeric nanoparticles
inhibit synergistically growth and hTERT gene expression in human
colorectal cancer cells. Nutr Cancer. 69:1290–1299. 2017.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Casper AC, Parsons MW, Chipman J, Burt LM
Jr, Suneja G, Maurer KA and Gaffney DK: Risk of secondary
malignancies in ovarian cancer survivors: 52,680 Patients analyzed
with over 40 years of follow-up. Gynecol Oncol. 162:454–460.
2021.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Jessmon P, Boulanger T, Zhou W and
Patwardhan P: Epidemiology and treatment patterns of epithelial
ovarian cancer. Expert Rev Anticancer Ther. 17:427–437.
2017.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Ulbright TM and Roth LM: Common epithelial
tumors of the ovary: Proliferating and of low malignant potential.
Semin Diagn Pathol. 2:2–15. 1985.PubMed/NCBI
|
|
142
|
Guo X, Mei J, Jing Y and Wang S:
Curcumin-loaded nanoparticles with low-intensity focused
ultrasound-induced phase transformation as tumor-targeted and
pH-sensitive theranostic nanoplatform of ovarian cancer. Nanoscale
Res Lett. 15(73)2020.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Amini E, Baharara J, Nikdel N and
Abdollahi FS: Cytotoxic and pro-apoptotic effects of honey bee
venom and chrysin on human ovarian cancer cells. Asia Pacific J Med
Toxicol. 4:68–73. 2015.
|
|
144
|
Tewari D, Patni P and Bishayee A, Sah AN
and Bishayee A: Natural products targeting the PI3K-Akt-mTOR
signaling pathway in cancer: A novel therapeutic strategy. Semin
Cancer Biol. 80:1–17. 2022.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Chang WC, Hsieh TC, Hsu WL, Chang FL, Tsai
HR and He MS: Diabetes and further risk of cancer: A nationwide
population-based study. BMC Med. 22(214)2024.PubMed/NCBI View Article : Google Scholar
|