Meningitis is particularly devastating due to its
potential to cause severe neurological complications (1). Despite worldwide efforts made for the
prevention and treatment of meningitis, the mortality rate
associated with the disease remains unacceptably high (2,3). The
management and outcomes of patients with meningitis are complicated
by various factors, including the post-neurosurgical condition
(4,5).
Post-operative bacterial meningitis (POBM) is
defined as meningitis that occurs following a neurosurgical
procedure. Despite the reported rate of only 10% (6,7),
POBM is associated with considerable mortality rates ranging from
20 to 50% (8). Overall, there are
no substantial differences in the clinical characteristics of
patients with POBM and those with community-acquired meningitis
(9). While there is extensive
knowledge about the factors affecting the outcomes of patients with
bacterial meningitis, POBM remains relatively underexplored.
Several publications have noted that the outcomes of
patients with bacterial meningitis are affected by factors, such as
comorbidities, initial Glasgow coma scale (GCS) score and an
advanced age (10-13).
Conversely, the majority of studies addressing the mortality rates
of patients with POBM have concentrated on surgical issues, such as
the volume of intraoperative bleeding, the duration of surgery and
post-operative care, including the use of mechanical ventilation
(8,14). However, numerous other factors
contribute to the complexity of the disease. Gram-negative
bacteria, which are more common in POBM compared to
community-acquired cases, are considered to be more resistant to
treatment (11,12,15-17).
Patients with underlying neurosurgical conditions may have more
severe baseline health or additional comorbidities, rendering their
condition more difficult to treat compared to community-acquired
cases (18). Certain cerebrospinal
fluid (CSF) parameters, specifically glucose and lactate levels,
indicate the severity of inflammation and may thus be associated
with increased mortality rates (19).
Existing knowledge about the effects of age,
presenting GCS scores, comorbidities, bacterial types and CSF
parameters on infectious cases prompted the authors to investigate
the effects of these factors on POBM. The present meta-analysis
aimed to determine whether these factors significantly contribute
to the mortality rate of patients with POBM.
A comprehensive literature search was performed on
the PubMed, ScienceDirect, The Cochrane Library and the Directory
of Open Access Journals databases to identify relevant studies.
Stringent inclusion and exclusion criteria were applied, and the
processes of research selection, data extraction and quality
assessment were conducted meticulously. Potential sources of
heterogeneity were extensively examined using subgroup and
sensitivity analyses.
The present meta-analysis encompasses clinical
trials, prospective studies, retrospective studies and case-control
studies. Case series with more than two cases were also included.
The included studies would have to present data on patients who had
undergone neurosurgery or neurosurgical interventions. Studies
involving patients who developed meningitis due to an underlying
neurological or neurosurgical condition, but were treated
conservatively were excluded. To be included in the analysis,
studies would have to provide data on at least one of the specified
factors.
The sampling method utilized in this research
involved an online literature search following the guidelines of
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA). Searches were conducted on the PubMed,
Cochrane, the Directory of Open Access Journals and ScienceDirect
databases. The detailed search strategy is outlined in Table I.
The search results were initially filtered based on
the relevance of their titles, followed by the relevance of their
abstracts. Non-English publications were automatically excluded.
Full-text articles were then evaluated by all authors to identify
potentially eligible studies. The reasons for exclusion were
documented and reported.
The present meta-analysis aimed to examine the
clinical, laboratory and microbiological factors affecting the
mortality rates of patients with POBM. The clinical factors
considered include age, comorbidities and the presenting GCS
scores. Laboratory factors encompassed CSF lactate and glucose
levels, while microbiological factors involved the Gram staining
status of the CSF. Data were extracted and presented in a table. In
the case that studies reported the median and range or median and
interquartile range for age, GCS, CSF lactate and CSF glucose,
these data were converted to the mean and standard deviation using
the mathematical methods described in the study by Wan et al
(20).
All authors evaluated the risk of bias. The biases
assessed were those outlined in the Cochrane Collaboration Tool for
Assessing Risk of Bias in Randomized Trials, published in
2011(21). Studies that were not
clinical trials were evaluated using Cochrane's ROBINS-I tool
(22).
Statistical analysis was performed using Review
Manager (RevMan) 5.4 and STATA, with the results presented as
forest plots. Confidence intervals (CI) and odds ratios (ORs) were
calculated automatically using the same software. The assessment of
heterogeneity was performed using the I2 value during
the construction of forest plots with RevMan 5.4 and STATA. In the
case that the I2 value was ≥50%, indicating significant
heterogeneity, the statistical model was adjusted to a random
effects model (21).
The bias of the remaining studies was evaluated
using the ROBINS-I tool. Several studies were identified as having
a high risk of confounding bias, primarily as they presented data
in median form rather than as the mean (14,23,26-29,33-35,38,45,47,53,55,59,61,62,64,65,70,72,74,77,80,81,88-90).
As aforementioned, these data were mathematically transformed into
mean values, which introduced a potential source of bias (20). Case series were inherently
considered to carry a high risk. Of note, three studies were judged
to have a high risk in the outcome domain due to incomplete data on
the non-survivors (57), unusually
large standard deviation (31),
and incomplete reporting of GCS scores (58). Studies that presented a high risk
in the selection domain typically focused solely on a specific
bacterial species or procedure. This approach may not accurately
represent the actual number of cases at the respective institutions
of the authors (Table IV).
A total of 70 studies reported the number of
non-survivors, allowing for a pooled analysis of the mortality
rates of patients with POBM. These studies cumulatively included
3,235 POBM cases with 853 fatalities. The pooled analysis indicated
a median mortality rate of 28% (95% CI, 23-32%). This analysis was
considered homogenous (I²=36.19%) (Fig. 2).
A total of 19 studies presented data on the presence
of comorbidities in both survivors and non-survivors. These studies
included 97 fatalities out of 235 cases with comorbidities and 129
fatalities out of 648 cases without comorbidities. The
meta-analysis indicated a risk ratio of 1.97 (95% CI, 1.58-2.46),
suggesting that patients with comorbidities have a higher risk of
mortality (Fig. 3).
A total of 32 studies reported the mean age of the
patients with POBM who survived and those who did not, encompassing
a total of 1,705 cases with 497 fatalities. The meta-analysis
revealed a small mean age difference of 4.65 years (95% CI,
1.78-7.52) between survivors and non-survivors (Fig. 4). This analysis was deemed
heterogeneous with an I² of 71% (Fig.
4).
In total, 15 studies provided data on CSF glucose
levels for both survivors and non-survivors, comprising a total of
164 non-survivors and 325 survivors. The meta-analysis indicated
that the mean CSF glucose level in survivors is 13.55 mg/dl lower
than that of non-survivors (95% CI, -20.95 to -6.15). This analysis
revealed heterogeneity (I²=67%). The pooled analysis revealed that
the mean CSF glucose level in survivors was 34.78 mg/dl (95% CI,
24.03-45.52 mg/dl) (I²=0%), while in non-survivors it was 27.29
mg/dl (95% CI, 12.96-41.63) (Fig.
5).
A total of 15 studies provided information on CSF
lactate levels, although none differentiated between survivors and
non-survivors. Consequently, a mean difference analysis could not
be conducted between these groups. Instead, a pooled analysis was
performed to determine the average CSF lactate level in POBM cases,
resulting in an average of 52.88 mg/dl (95% CI, 38.14-67.62)
(Fig. 6).
Only seven studies provided data on the Gram
staining status of both survivors and non-survivors. These studies
included 245 Gram-negative cases and 257 Gram-positive cases. The
meta-analysis produced a risk ratio of 1.42 (95% CI, 0.96-2.10;
I²=18%), indicating no significant difference in mortality risk
between the two groups (Fig.
7).
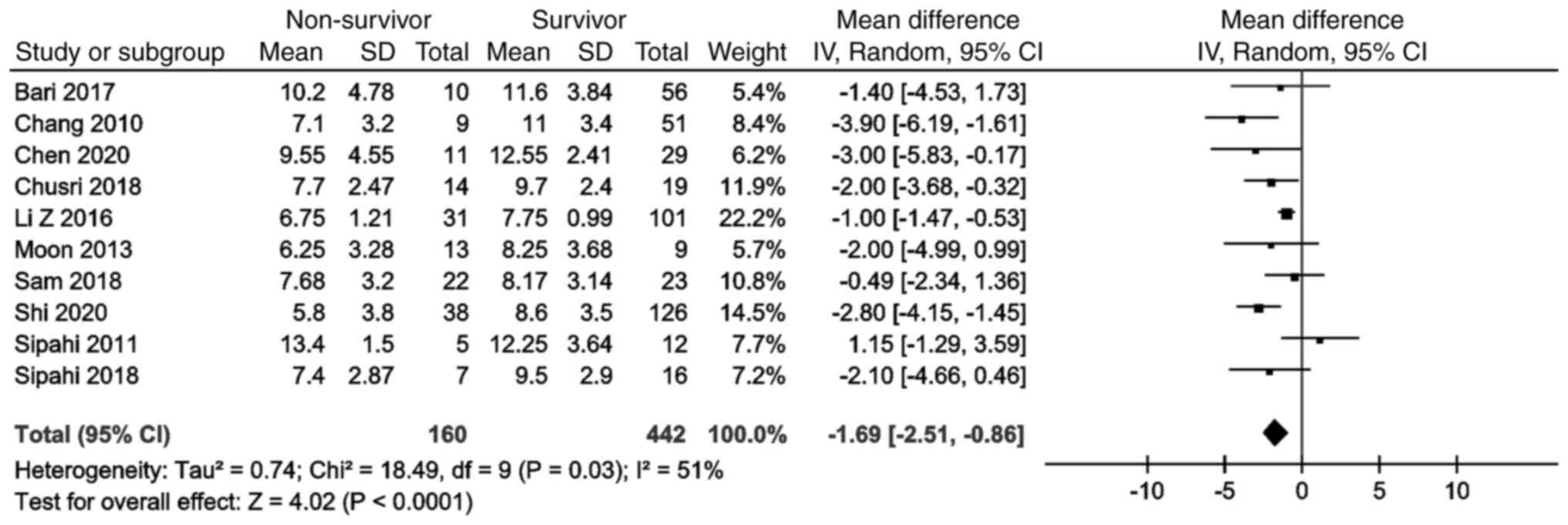
Of note, 10 studies provided data on the presenting
GCS scores of both survivors and non-survivors. These studies
included a total of 442 survivors and 160 non-survivors. The
meta-analysis revealed no significant difference between the two
groups (mean difference, -1.69 years; 95% CI, -2.51 to -0.86)
(Fig. 8).
There is a lack of sufficient studies on the
mortality rate of POBM. The present meta-analysis determined that
more than one quarter of POBM cases result in mortality (28%; 95%
CI, 23-32%). Chouhdari et al (8) reported mortality rate of 50% in their
study, while others have reported a considerably lower number
(0.3-10%) (4,102,103). This discrepancy may be attributed
to the sometimes non-specific clinical presentation and CSF
parameters of patients with POBM (104,105), which may cause
underreporting.
The present meta-analysis determined that, on
average, POBM non-survivors tend to be older. A similar pattern is
evident in community-acquired meningitis, where the mortality rate
is generally higher among elderly patients (106). In a previous retrospective study
on bacterial meningitis, Sunwoo et al (107) discovered that the mortality risk
for elderly patients did not differ significantly from that of
younger individuals (odds ratio, 1.03; 95% CI, 1.0-1.06). This
outcome contradicts the fundamental concept of aging and
immunology. It is well-established that aging is associated with
immunosenescence, particularly in individuals >60 years of age.
Immunosenescence weakens both innate and adaptive immune responses,
leading to more severe infections (108).
CSF lactate levels in meningitis cases have been
previously studied, with outcomes highlighting their diagnostic
value (112,113). Lotfi et al (45) identified a threshold value of 4
mmol/l (equivalent to 36.04 mg/dl) for the diagnosis of POBM. In a
previous study on tuberculous meningitis, Nuwagira et al
(114) determined that CSF
lactate was a crucial diagnostic test. However, it did not provide
any predictive value for mortality within the first 2 weeks
(114). There is a lack of
studies comparing CSF lactate levels between POBM survivors and
non-survivors, preventing any comparisons in the present
meta-analysis. However, the pooled analysis performed herein
indicated a CSF lactate value of 52.88 mg/dl (95% CI,
38.14-67.62).
CSF glucose levels in POBM cases have not been
extensively studied; yet, they are frequently used as a diagnostic
tool for bacterial meningitis. The present meta-analysis revealed
that non-survivors tend to have lower average CSF glucose levels.
Baud et al (19) found that
higher levels of CSF inflammation were linked to low CSF glucose in
bacterial meningitis. The notion of using CSF glucose as a
prognostic marker is based on the understanding that microbes
consume glucose for metabolism. Consequently, severe inflammation,
which demands higher levels of glucose, would lead to more severe
clinical symptoms and a poorer prognosis (19). The present meta-analysis revealed
that non-survivors had reduced levels of CSF glucose.
Despite the established knowledge that Gram-negative
bacteria are more virulent and resistant to antibiotics, the
present study did not identify a higher risk of mortality for
infections caused by Gram-negative bacteria compared to those
caused by Gram-positive bacteria. This outcome contradicts previous
concepts, which suggest that meningitis from Gram-negative bacteria
typically results in higher mortality rates (12). Additionally, patients with POBM
with Gram-negative infections are generally considered to be at a
greater risk of treatment failure and adverse outcomes (32). In theory, Gram-negative bacteria
are more difficult to treat due to their distinct characteristics,
such as the presence of an outer membrane and several unique
enzymes that aid in antibiotic resistance (115). The discrepancy between the
outcomes of the present meta-analysis and previous knowledge of
Gram-negative bacteria may stem from some studies reporting only
specific types of bacteria (28,30-33,35-37,39,41-43,47,51,57,69,71,72,75-77,79,81,83-85,87,88,93,96-100).
This selective reporting could lead to an unfair comparison between
meningitis caused by Gram-negative and Gram-positive bacteria.
In summary, the present study has several
limitations which should be mentioned: i) It did not include
subgroup analyses for different types of comorbidities; ii) it
lacks subgroup analyses for antibiotic usage; and iii) it did not
categorize patients based on the type of surgical intervention.
Thus, further research addressing these aspects is required in
order to provide a clearer understanding of the prognostic factors
for POBM.
In conclusion, the present meta-analysis provided
critical insight into the factors affecting mortality in patients
with POBM. The results presented herein can assist in clinical
decision-making and direct future research in the field of surgical
infectious complications. Based on these outcomes, the following
conclusions can be drawn: i) There is no marked difference between
POBM non-survivors and survivors in terms of age; ii) there is no
marked difference between POBM non-survivors and survivors in terms
of presenting GCS scores; iii) POBM non-survivors have an average
CSF glucose of <30 mg/dl; iv) patients with POBM with
comorbidities are at a higher risk of mortality; and v) more than
one quarter of patients with POBM do not survive.
Not applicable.
Funding: No funding was received.
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
WS constructed the basic concept of the study, and
was also involved in the editing and reviewing of the manuscript,
as well as in data extraction and study supervision. RM constructed
the basic concept of the study, and was also involved in the
editing of the manuscript, in the literature search, data
extraction and statistical analysis. MAP was involved in the
reviewing and editing of the manuscript, in the conception of the
study, in the literature search and in study supervision. BU was
involved in data extraction, in the literature search and in the
statistical analysis. AAF was involved in the reviewing of the
manuscript, in the literature search, in the statistical analysis,
and in study supervision. AHB was involved in the conception of the
study, in the reviewing and editing of the manuscript and in study
supervision. All authors have read and approved the final
manuscript. WS and RM confirm the authenticity of all the raw
data.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Jit M: The risk of sequelae due to
pneumococcal meningitis in high-income countries: a systematic
review and meta-analysis. J Infect. 61:114–124. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Edmond K, Clark A, Korczak VS, Sanderson
C, Griffiths UK and Rudan I: Global and regional risk of disabling
sequelae from bacterial meningitis: A systematic review and
meta-analysis. Lancet Infect Dis. 10:317–328. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stein-Zamir C, Shoob H, Sokolov I, Kunbar
A, Abramson N and Zimmerman D: The clinical features and long-term
sequelae of invasive meningococcal disease in children. Pediatr
Infect Dis J. 33:777–779. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van de Beek D, Drake JM and Tunkel AR:
Nosocomial bacterial meningitis. N Engl J Med. 362:146–154.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tunkel AR, Hasbun R, Bhimraj A, Byers K,
Kaplan SL, Scheld WM, van de Beek D, Bleck TP, Garton HJL and Zunt
JR: 2017 Infectious Diseases Society of America's Clinical Practice
Guidelines for Healthcare-Associated Ventriculitis and Meningitis.
Clin Infect Dis. 64:e34–e65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kono Y, Prevedello DM, Snyderman CH,
Gardner PA, Kassam AB, Carrau RL and Byers KE: One thousand
endoscopic skull base surgical procedures demystifying the
infection potential: Incidence and description of postoperative
meningitis and brain abscesses. Infect Control Hosp Epidemiol.
32:77–83. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
McClelland S III and Hall WA:
Postoperative central nervous system infection: Incidence and
associated factors in 2111 neurosurgical procedures. Clin Infect
Dis. 45:55–59. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Chouhdari A, Ebrahimzadeh K, Rezaei O,
Samadian M, Sharifi G and Hajiesmaeili M: Investigating related
factors with mortality rate in patients with postoperative
meningitis: One longitudinal follow up study in Iran. Iran J
Neurol. 17:82–85. 2018.PubMed/NCBI
|
|
9
|
Lin TY, Chen WJ, Hsieh MK, Lu ML, Tsai TT,
Lai PL, Fu TS, Niu CC and Chen LH: Postoperative meningitis after
spinal surgery: A review of 21 cases from 20,178 patients. BMC
Infect Dis. 14(220)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Koopmans MM, Brouwer MC, Bijlsma MW,
Bovenkerk S, Keijzers W, van der Ende A and van de Beek D: Listeria
monocytogenes sequence type 6 and increased rate of unfavorable
outcome in meningitis: Epidemiologic cohort study. Clin Infec Dis.
57:247–253. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Veen KEB, Brouwer MC, van der Ende A
and van de Beek D: Bacterial meningitis in diabetes patients: A
population-based prospective study. Sci Rep.
6(36996)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pomar V, de Benito N, Mauri A, Coll P,
Gurguí M and Domingo P: Characteristics and outcome of spontaneous
bacterial meningitis in patients with diabetes mellitus. BMC Infect
Dis. 20(292)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lucas MJ, Brouwer MC, van der Ende A and
van de Beek D: Outcome in patients with bacterial meningitis
presenting with a minimal Glasgow Coma Scale score. Neurol
Neuroimmunol Neuroinflamm. 1(e9)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kourbeti IS, Vakis AF, Ziakas P,
Karabetsos D, Potolidis E, Christou S and Samonis G: Infections in
patients undergoing craniotomy: Risk factors associated with
post-craniotomy meningitis. J Neurosurg. 122:1113–1119.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dzupova O, Rozsypal H, Prochazka B and
Benes J: Acute bacterial meningitis in adults: Predictors of
outcome. Scand J Infect Dis. 41:348–354. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brouwer MC, Tunkel AR and van de Beek D:
Epidemiology, diagnosis, and antimicrobial treatment of acute
bacterial meningitis. Clin Microbiol Rev. 23:467–492.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pomar V, Benito N, López-Contreras J, Coll
P, Gurguí M and Domingo P: Spontaneous gram-negative bacillary
meningitis in adult patients: Characteristics and outcome. BMC
Infect Dis. 13(451)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hussein K, Bitterman R, Shofty B, Paul M
and Neuberger A: Management of post-neurosurgical meningitis:
Narrative review. Clin Microbiol Infect. 23:621–628.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baud MO, Vitt JR, Robbins NM, Wabl R,
Wilson MR, Chow FC, Gelfand JM, Josephson SA and Miller S:
Pleocytosis is not fully responsible for low CSF glucose in
meningitis. Neurol Neuroimmunol Neuroinflamm.
5(e425)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wan X, Wang W, Liu J and Tong T:
Estimating the sample mean and standard deviation from the sample
size, median, range and/or interquartile range. BMC Med Res
Methodol. 14(135)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sterne JA, Hernán MA, Reeves BC, Savović
J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT,
Boutron I, et al: ROBINS-I: A tool for assessing risk of bias in
non-randomised studies of interventions. BMJ.
355(i4919)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ye L, Ji X, Song Z, Guan L, Zhao L, Wang W
and Du W: Clinical value of glycan changes in cerebrospinal fluid
for evaluation of post-neurosurgical bacterial meningitis with
hemorrhagic stroke patients. Diagnostics (Basel).
13(187)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mian SY, Mancuso-Marcello M, Kandasamy J,
Jamjoom AAB and Woodfield J: Comparison of external ventricular
drains with ventricular access devices for the emergency management
of adult hydrocephalus. World Neurosurg: Jun 26, 2023 (Epub ahead
of print).
|
|
25
|
Stevens AR, Gilbody H, Greig J, Usuah J,
Alagbe B, Preece A, Soon WC, Chowdhury YA, Toman E, Chelvarajah R,
et al: Cerebrospinal fluid diversion for refractory intracranial
hypertension in traumatic brain Injury: A single center experience.
World Neurosurg. 176:e265–e272. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Theofanopoulos A, Fermeli D, Vekios D,
Bizos A, Marangos M, Constantoyannis C, Panagiotopoulos V and
Assimakopoulos SF: Successful treatment of pan-drug resistant
Acinetobacter baumannii nosocomial meningitis/ventriculitis by
combined intravenous and intrathecal colistin-tigecycline
administration: A case series. Infez Med. 31:103–107.
2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kar M, Jamwal A, Dubey A, Sahu C and Patel
SS: Bacterial Meningitis among Intracranial Surgery Patients at a
University Hospital in Northern India. Indian J Crit Care Med.
26:1244–1252. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pilmis B, Péan de Ponfilly G, Farfour E,
Ranc AG, Fihman V, Bille E, Dortet L, Degand N, Morand P, Potron A,
et al: Epidemiology and clinical characteristics of Klebsiella spp.
meningitis in France. Infect Dis Now. 52:82–86. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pautova AK, Meglei AY, Chernevskaya EA,
Alexandrova IA and Beloborodova NV: 4-Hydroxyphenyllactic Acid in
Cerebrospinal Fluid as a Possible Marker of Post-Neurosurgical
Meningitis: Retrospective Study. J Pers Med. 12(399)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Solo-Peleteiro A, Diéguez P,
Pérez-Rodríguez MT, Galárraga RA, Pérez-Landeiro A and
Álvarez-Fernández M: Cerebrospinal fluid drainage-related
ventriculitis due to multidrug-resistant microorganisms. Enferm
Infecc Microbiol Clin (Engl Ed). 40:322–325. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zeinalizadeh M, Yazdani R, Feizabadi MM,
Shadkam M, Seifi A, Dehghan Manshadi SA, Abdollahi A and Salehi M:
Post-neurosurgical meningitis; gram negative bacilli vs. gram
positive cocci. Caspian J Intern Med. 13:469–474. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng G, Wang S, Lv H and Zhang G:
Nomogram analysis of clinical characteristics and mortality risk
factor of non-fermentative gram-negative bacteria-induced
post-neurosurgical meningitis. Infect Drug Resist. 15:6379–6389.
2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zheng G, Shi Y, Cao Y, Qian L, Lv H, Zhang
L and Zhang G: Clinical feature, therapy, antimicrobial resistance
gene distribution, and outcome of nosocomial meningitis induced by
multidrug-resistant Enterobacteriaceae-A longitudinal cohort study
from Two Neurosurgical Centers in Northern China. Front Cell Infect
Microbiol. 12(839257)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Goktas SY, Oral AY, Yılmaz E, Akalın EH,
Guvenc F, Ozkaya G, Kocaeli H, Dogan S, Yılmazlar S and Oral HB:
Diagnostic value of cerebrospinal fluid levels of D-lactate, tumour
necrosis factor-alpha and interleukin-6, -8, and -17 in suspected
nosocomial meningitis. Singapore Med J. 65:430–437. 2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Khan FY: Adult coagulase-negative
staphylococcal meningitis in qatar: Clinical characteristics and
therapeutic outcomes. Asian J Neurosurg. 16:714–718.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Y, Hu D, Ma X, Li D, Tian D, Gong Y and
Jiang X: Convergence of carbapenem resistance and hypervirulence
leads to high mortality in patients with postoperative Klebsiella
pneumoniae meningitis. J Glob Antimicrob Resist. 27:95–100.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen Y, Li F, Zhu M, Liu L and Luo Y:
Outcome and factors of patients with nosocomial meningitis by
multi-drug-resistant Gram-negative bacteria in a tertiary hospital
in China: A retrospective study. Br J Neurosurg. 34:324–328.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yousef Khan F: Enterococcal
meningitis/ventriculitis in Qatar-Experience with eight patients.
Qatar Med J. 2020(46)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Khanum I, Ilyas A and Ali F:
Stenotrophomonas maltophilia Meningitis-A case series and review of
the literature. Cureus. 12(e11221)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kul G, Sencan I, Kul H, Korkmaz N and
Altunay E: The role of cerebrospinal fluid biomarkers in the
diagnosis of post-neurosurgical meningitis. Turk Neurosurg.
30:513–519. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rodríguez-Lucas C, Fernández J,
Martínez-Sela M, Álvarez-Vega M, Moran N, Garcia A, Menendez C,
García-Prieto E and Rodríguez-Guardado A: Pseudomonas aeruginosa
nosocomial meningitis in neurosurgical patients with
intraventricular catheters: Therapeutic approach and review of the
literature. Enferm Infecc Microbiol Clin (Engl Ed). 38:54–58.
2020.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
42
|
Shi YJ, Zheng GH, Qian LY, Qsman RA, Li GG
and Zhang GJ: Longitudinal analysis of risk factors for clinical
outcomes of enterobacteriaceae meningitis/encephalitis in
post-neurosurgical patients: A comparative cohort study during
2014-2019. Infect Drug Resist. 13:2161–2170. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ye J, Tan LH, Shen ZP, Yu YS, Lai DM, Fan
J and Shu Q: Polymyxin for the treatment of intracranial infections
of extensively drug-resistant bacteria in children after
neurosurgical operation. World J Pediatr. 16:528–532.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zheng G, Li S, Zhao M, Yang X, Zhang Y,
Deng J, Luo Y, Lv H and Zhang G: Time to positive culture can
differentiate post-neurosurgical coagulase-negative Staphylococci
other than S epidermidis meningitis from contamination: A
case-control observational study. J Clin Lab Anal.
24(e23447)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lotfi R, Ines B, Aziz DM and Mohamed B:
Cerebrospinal fluid lactate as an indicator for post-neurosurgical
bacterial meningitis. Indian J Crit Care Med. 23:127–130.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Nisson PL, James WS, Gaub MB, Borgstrom M,
Weinand M and Anton R: Peripheral white blood cell count as a
screening tool for ventriculostomy-related infections. J Clin
Neurosci. 67:52–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chusri S, Sakarunchai I, Kositpantawong N,
Panthuwong S, Santimaleeworagun W, Pattharachayakul S, Singkhamanan
K and Doi Y: Outcomes of adjunctive therapy with intrathecal or
intraventricular administration of colistin for post-neurosurgical
meningitis and ventriculitis due to carbapenem-resistant
acinetobacter baumannii. Int J Antimicrob Agents. 51:646–650.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jin Y, Liu X, Gao L, Guo X, Wang Q, Bao X,
Deng K, Yao Y, Feng M, Lian W, et al: Risk factors and microbiology
of meningitis and/or bacteremia after transsphenoidal surgery for
pituitary adenoma. World Neurosurg. 110:e851–e863. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kammoun B, Kolsi F, Borni M, Abdelhedi A,
Abdelmouleh S, Jarraya F, Bouhamed O, Kammoun O, Elleuch E and
Boudawara MZ: Applicability, safety, and cost-effectiveness of
improvised external ventricular drainage: An observational study of
tunisian neurosurgery inpatients. World Neurosurg. 119:428–436.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sam JE, Lim CL, Sharda P and Wahab NA: The
organisms and factors affecting outcomes of external ventricular
drainage catheter-related ventriculitis: A penang experience. Asian
J Neurosurg. 13:250–257. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sipahi OR, Mermer S, Demirdal T, Ulu AC,
Fillatre P, Ozcem SB, Kaya Ş, Şener A, Bulut C, Tekin R, et al:
Tigecycline in the treatment of multidrug-resistant Acinetobacter
baumannii meningitis: Results of the Ege study. Clin Neurol
Neurosurg. 172:31–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bari ME, Haider G, Malik K, Waqas M,
Mahmood SF and Siddiqui M: Outcomes of post-neurosurgical
ventriculostomy-associated infections. Surg Neurol Int.
8(124)2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hoogmoed J, van de Beek D, Coert BA, Horn
J, Vandertop WP and Verbaan D: Clinical and laboratory
characteristics for the diagnosis of bacterial ventriculitis after
aneurysmal subarachnoid hemorrhage. Neurocrit Care. 26:362–370.
2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kurtaran B, Kuscu F, Ulu A, Inal AS, Komur
S, Kibar F, Cetinalp NE, Ozsoy KM, Arslan YK, Yilmaz DM, et al: The
causes of post-operative meningitis: The comparison of
gram-negative and gram-positive pathogens. Turk Neurosurg.
28:589–596. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hernández Ortiz OH, García García HI,
Muñoz Ramírez F, Cardona Flórez JS, Gil Valencia BA, Medina
Mantilla SE, Moreno Ochoa MJ, Sará Ochoa JE and Jaimes F:
Development of a prediction rule for diagnosing postoperative
meningitis: A cross-sectional study. J Neurosurg. 128:262–271.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Pagliano P, Caggiano C, Ascione T, Solari
D, Di Flumeri G, Cavallo LM, Tortora F and Cappabianca P:
Characteristics of meningitis following transsphenoidal endoscopic
surgery: A case series and a systematic literature review.
Infection. 45:841–848. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sun L, Wang X and Li Z: Successful
treatment of multidrug-resistant Acinetobacter baumannii meningitis
with ampicillin sulbactam in primary hospital. Br J Neurosurg.
32:642–645. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Fotakopoulos G, Makris D, Chatzi M,
Tsimitrea E, Zakynthinos E and Fountas K: Outcomes in
meningitis/ventriculitis treated with intravenous or
intraventricular plus intravenous colistin. Acta Neurochir (Wien).
158:603–610. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Li Z, Wu X, Yu J, Wu X, Du Z, Sun Y, Yuan
Q and Hu J: Empirical combination antibiotic therapy improves the
outcome of nosocomial meningitis or ventriculitis in neuro-critical
care unit patients. Surg Infect (Larchmt). 17:465–472.
2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Neuberger A, Shofty B, Bishop B, Naffaa
ME, Binawi T, Babich T, Rappaport ZH, Zaaroor M, Sviri G, Yahav D
and Paul M: Risk factors associated with death or neurological
deterioration among patients with Gram-negative postneurosurgical
meningitis. Clin Microbiol Infect. 22:573.e1–e4. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Shofty B, Neuberger A, Naffaa ME, Binawi
T, Babitch T, Rappaport ZH, Zaaroor M, Sviri G and Paul M:
Intrathecal or intraventricular therapy for post-neurosurgical
Gram-negative meningitis: Matched cohort study. Clin Microbiol
Infect. 22:66–70. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Soavi L, Rosina M, Stefini R, Fratianni A,
Cadeo B, Magri S, Latronico N, Fontanella M and Signorini L:
Post-neurosurgical meningitis: Management of cerebrospinal fluid
drainage catheters influences the evolution of infection. Surg
Neurol Int. 7 (Suppl 39):S927–S934. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang Y, Zhang J, Chen Y, Yu J, Cao G, Wu
X, Chen M, Wu J and Zhao X: Evaluation of meropenem penetration
into cerebrospinal fluid in patients with meningitis after
neurosurgery. World Neurosurg. 98:525–531. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chidambaram S, Nair MN, Krishnan SS, Cai
L, Gu W and Vasudevan MC: Postoperative central nervous system
infection after neurosurgery in a modernized, resource-limited
tertiary Neurosurgical Center in South Asia. World Neurosurg.
84:1668–1673. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mounier R, Lobo D, Cook F, Fratani A,
Attias A, Martin M, Chedevergne K, Bardon J, Tazi S, Nebbad B, et
al: Clinical, biological, and microbiological pattern associated
with ventriculostomy-related infection: a retrospective
longitudinal study. Acta Neurochir (Wien). 157:2209–2217;
discussion 2217. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Muñoz-Gómez S, Wirkowski E and Cunha BA:
Post craniotomy extra-ventricular drain (EVD) associated nosocomial
meningitis: CSF diagnostic criteria. Heart Lung. 44:158–160.
2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Tian R, Hao S, Hou Z, Gao Z and Liu B: The
characteristics of post-neurosurgical bacterial meningitis in
elective neurosurgery in 2012: A single institute study. Clin
Neurol Neurosurg. 139:41–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Li Y, Zhang G, Ma R, Du Y, Zhang L, Li F,
Fang F, Lv H, Wang Q, Zhang Y and Kang X: The diagnostic value of
cerebrospinal fluids procalcitonin and lactate for the differential
diagnosis of post-neurosurgical bacterial meningitis and aseptic
meningitis. Clin Biochem. 48:50–54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wang JH, Lin PC, Chou CH, Ho CM, Lin KH,
Tsai CT, Wang JH, Chi CY and Ho MW: Intraventricular antimicrobial
therapy in postneurosurgical Gram-negative bacillary meningitis or
ventriculitis: A hospital-based retrospective study. J Microbiol
Immunol Infect. 47:204–210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Williamson RA, Phillips-Bute BG, McDonagh
DL, Gray MC, Zomorodi AR, Olson DM, Britz GW, Laskowitz DT and
James ML: Predictors of extraventricular drain-associated bacterial
ventriculitis. J Crit Care. 29:77–82. 2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Huang CR, Chen SF, Tsai NW, Chang CC, Lu
CH, Chuang YC, Chien CC and Chang WN: Clinical characteristics of
Stenotrophomonas maltophilia meningitis in adults: A high incidence
in patients with a postneurosurgical state, long hospital staying
and antibiotic use. Clin Neurol Neurosurg. 115:1709–1715.
2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Khan FY, Abukhattab M and Anand D:
Nosocomial Escherichia coli meningitis in adults: Report of four
cases and literature review. J Neurosci Rural Pract. 4:349–351.
2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lai WA, Lu CH and Chang WN: Mixed
infection in adult post-neurosurgical bacterial meningitis: a
hospital-based study. Biomed J. 36:295–303. 2013.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Maskin LP, Capparelli F, Mora A, Hlavnicka
A, Orellana N, Díaz MF, Wainsztein N and Del Castillo M:
Cerebrospinal fluid lactate in post-neurosurgical bacterial
meningitis diagnosis. Clin Neurol Neurosurg. 115:1820–1825.
2013.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Moon C, Kwak YG, Kim BN, Kim ES and Lee
CS: Implications of postneurosurgical meningitis caused by
carbapenem-resistant Acinetobacter baumannii. J Infect Chemother.
19:916–919. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Khan FY, Abukhattab M and Baager K:
Nosocomial postneurosurgical Acinetobacter baumannii meningitis: A
retrospective study of six cases admitted to Hamad General
Hospital, Qatar. J Hosp Infect. 80:176–179. 2012.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Pintado V, Pazos R, Jiménez-Mejías ME,
Rodríguez-Guardado A, Gil A, García-Lechuz JM, Cabellos C, Chaves
F, Domingo P, Ramos A, et al: Methicillin-resistant Staphylococcus
aureus meningitis in adults: A multicenter study of 86 cases.
Medicine (Baltimore). 91:10–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Huang CR, Chen SF, Lu CH, Chuang YC, Tsai
NW, Chang CC, Wang HC, Chien CC and Chang WN: Clinical
characteristics and therapeutic outcomes of nosocomial
super-infection in adult bacterial meningitis. BMC Infect Dis.
11(133)2011.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Sipahi OR, Bardak S, Turhan T, Arda B,
Pullukcu H, Ruksen M, Aydemir S, Dalbasti T, Yurtseven T, Zileli M
and Ulusoy S: Linezolid in the treatment of methicillin-resistant
staphylococcal post-neurosurgical meningitis: A series of 17 cases.
Scand J Infect Dis. 43:757–764. 2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
van Mourik MS, Groenwold RH, Berkelbach
van der Sprenkel JW, van Solinge WW, Troelstra A and Bonten MJ:
Automated detection of external ventricular and lumbar
drain-related meningitis using laboratory and microbiology results
and medication data. PLoS One. 6(e22846)2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Chang WN, Lu CH, Huang CR, Chuang YC, Tsai
NW, Chang CC, Chen SF, Wang HC, Yang TM, Hsieh MJ and Chien CC:
Clinical characteristics of post-neurosurgical Klebsiella
pneumoniae meningitis in adults and a clinical comparison to the
spontaneous form in a Taiwanese population. J Clin Neurosci.
17:334–338. 2010.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Chang CJ, Ye JJ, Yang CC, Huang PY, Chiang
PC and Lee MH: Influence of third-generation cephalosporin
resistance on adult in-hospital mortality from post-neurosurgical
bacterial meningitis. J Microbiol Immunol Infect. 43:301–309.
2010.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Tuon FF, Penteado-Filho SR, Amarante D,
Andrade MA and Borba LA: Mortality rate in patients with nosocomial
Acinetobacter meningitis from a Brazilian hospital. Braz J Infect
Dis. 14:437–440. 2010.PubMed/NCBI
|
|
84
|
Rodríguez Guardado A, Blanco A, Asensi V,
Pérez F, Rial JC, Pintado V, Bustillo E, Lantero M, Tenza E,
Alvarez M, et al: Multidrug-resistant Acinetobacter meningitis in
neurosurgical patients with intraventricular catheters: Assessment
of different treatments. J Antimicrob Chemother. 61:908–913.
2008.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Metan G, Alp E, Aygen B and Sumerkan B:
Acinetobacter baumannii meningitis in post-neurosurgical patients:
Clinical outcome and impact of carbapenem resistance. J Antimicrob
Chemother. 60:197–199. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Guardado R, Asensi V, Torres JM, Pérez F,
Blanco A, Maradona JA and Cartón JA: Post-surgical enterococcal
meningitis: Clinical and epidemiological study of 20 cases. Scand J
Infect Dis. 38:584–588. 2006.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Arda B, Yamazhan T, Sipahi OR, Islekel S,
Buke C and Ulusoy S: Meningitis due to methicillin-resistant
Staphylococcus aureus (MRSA): Review of 10 cases. Int J Antimicrob
Agents. 25:414–418. 2005.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Briggs S, Ellis-Pegler R, Raymond N,
Thomas M and Wilkinson L: Gram-negative bacillary meningitis after
cranial surgery or trauma in adults. Scand J Infect Dis.
36:165–173. 2004.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Chang WN, Tsai YC, Chien CC, Huang CR and
Lu CH: Frequent association with neurosurgical conditions in adult
Proteus mirabilis meningitis: Report of five cases. Clin Neurol
Neurosurg. 104:121–124. 2002.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Huang CR, Lu CH and Chang WN: Adult
Enterobacter meningitis: A high incidence of coinfection with other
pathogens and frequent association with neurosurgical procedures.
Infection. 29:75–79. 2001.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chang WN, Lu CH, Huang CR and Chuang YC:
Mixed infection in adult bacterial meningitis. Infection. 28:8–12.
2000.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Lu CH, Chang WN and Chang HW: Adult
bacterial meningitis in Southern Taiwan: Epidemiologic trend and
prognostic factors. J Neurol Sci. 182:36–44. 2000.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Lu CH, Chang WN, Chuang YC and Chang HW:
Gram-negative bacillary meningitis in adult post-neurosurgical
patients. Surg Neurol. 52:438–444. 1999.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Jiménez-Mejías ME, Pachón J, Becerril B,
Palomino-Nicás J, Rodríguez-Cobacho A and Revuelta M: Treatment of
multidrug-resistant Acinetobacter baumannii meningitis with
ampicillin/sulbactam. Clin Infect Dis. 24:932–935. 1997.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Druel B, Vandenesch F, Greenland T,
Verneau V, Grando J, Salord F, Christen R and Etienne J: Aseptic
meningitis after neurosurgery: A demonstration of bacterial
involvement. Clin Microbiol Infect. 1:230–234. 1996.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Tang LM and Chen ST: Klebsiella oxytoca
meningitis: Frequent association with neurosurgical procedures.
Infection. 23:163–167. 1995.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Siegman-Igra Y, Bar-Yosef S, Gorea A and
Avram J: Nosocomial acinetobacter meningitis secondary to invasive
procedures: Report of 25 cases and review. Clin Infect Dis.
17:843–849. 1993.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Mancebo J, Domingo P, Blanch L, Coll P,
Net A and Nolla J: Post-neurosurgical and spontaneous gram-negative
bacillary meningitis in adults. Scand J Infect Dis. 18:533–538.
1986.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Mombelli G, Klastersky J, Coppens L,
Daneau D and Nubourgh Y: Gram-negative bacillary meningitis in
neurosurgical patients. J Neurosurg. 59:634–641. 1983.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Tacconelli E, Cataldo MA, Albanese A,
Tumbarello M, Arduini E, Spanu T, Fadda G, Anile C, Maira G,
Federico G and Cauda R: Vancomycin versus cefazolin prophylaxis for
cerebrospinal shunt placement in a hospital with a high prevalence
of meticillin-resistant Staphylococcus aureus. J Hosp Infect.
69:337–344. 2008.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Khan FY, Abukhattab M, AbuKamar M and
Anand D: Adult Klebsiella pneumoniae meningitis in Qatar: Clinical
pattern of ten cases. Asian Pac J Trop Biomed. 4:669–672.
2014.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Alghamri MS, McClellan BL, Hartlage CS,
Haase S, Faisal SM, Thalla R, Dabaja A, Banerjee K, Carney SV,
Mujeeb AA, et al: Targeting neuroinflammation in brain cancer:
Uncovering mechanisms, pharmacological targets, and
neuropharmaceutical developments. Front Pharmacol.
12(680021)2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Korinek AM, Baugnon T, Golmard JL, van
Effenterre R, Coriat P and Puybasset L: Risk factors for adult
nosocomial meningitis after craniotomy: role of antibiotic
prophylaxis. Neurosurgery 62 Suppl. 2:532–539. 2008.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Zarrouk V, Vassor I, Bert F, Bouccara D,
Kalamarides M, Bendersky N, Redondo A, Sterkers O and Fantin B:
Evaluation of the management of postoperative aseptic meningitis.
Clin Infect Dis. 44:1555–1559. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
105
|
Tängdén T, Enblad P, Ullberg M and Sjölin
J: Neurosurgical gram-negative bacillary ventriculitis and
meningitis: A retrospective study evaluating the efficacy of
intraventricular gentamicin therapy in 31 consecutive cases. Clin
Infect Dis. 52:1310–1316. 2011.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Cabellos C, Verdaguer R, Olmo M,
Fernández-Sabé N, Cisnal M, Ariza J, Gudiol F and Viladrich PF:
Community-Acquired bacterial meningitis in elderly patients:
Experience Over 30 Years. Medicine (Baltimore). 88:115–119.
2009.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Sunwoo JS, Shin HR, Lee HS, Moon J, Lee
ST, Jung KH, Park KI, Jung KY, Kim M, Lee SK and Chu K: A
hospital-based study on etiology and prognosis of bacterial
meningitis in adults. Sci Rep. 11(6028)2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Ponnappan S and Ponnappan U: Aging and
immune function: Molecular mechanisms to interventions. Antioxid
Redox Signal. 14:1551–1585. 2011.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Bekele F, Ahmed A, Kedir A and Sheleme T:
Treatment outcome and associated factors of bacterial meningitis at
pediatric wards of southwestern Ethiopian hospital: A prospective
observational study. J Pharm Health Care Sci. 7(41)2021.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Berbudi A, Rahmadika N, Tjahjadi AI and
Ruslami R: Type 2 diabetes and its impact on the immune system.
Curr Diabetes Rev. 16:442–449. 2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Betjes MGH: Immune cell dysfunction and
inflammation in end-stage renal disease. Nat Rev Nephrol.
9:255–265. 2013.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Cunha BA: Distinguishing bacterial from
viral meningitis: The critical importance of the CSF lactic acid
levels. Intensive Care Med. 32:1272–1273; author reply 1274.
2006.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Huy NT, Thao NT, Diep DT, Kikuchi M,
Zamora J and Hirayama K: Cerebrospinal fluid lactate concentration
to distinguish bacterial from aseptic meningitis: A systemic review
and meta-analysis. Crit Care. 14(R240)2010.PubMed/NCBI View
Article : Google Scholar
|
|
114
|
Nuwagira E, Huppler Hullsiek K, Jjunju S,
Rutakingirwa M, Kasibante J, Tadeo KK, Kagimu E, Tugume L,
Ssebambulidde K, Musubire AK, et al: Diagnostic and prognostic
value of cerebrospinal fluid lactate and glucose in HIV-Associated
tuberculosis meningitis. Microbiol Spectr.
10(e0161822)2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Lee CR, Lee JH, Park M, Park KS, Bae IK,
Kim YB, Cha CJ, Jeong BC and Lee SH: Biology of Acinetobacter
baumannii: Pathogenesis, antibiotic resistance mechanisms, and
prospective treatment options. Front Cell Infect Microbiol.
7(55)2017.PubMed/NCBI View Article : Google Scholar
|