1. Introduction
Severe acute respiratory syndrome (SARS) coronavirus
2 (SARS-CoV-2), which belongs to the coronaviridae family is the
source of the current coronavirus disease 2019 (COVID-19) pandemic,
which continues to affect humanity. The real-time observation of
ongoing evolutionary processes has provided a marked understanding
of SARS-CoV-2 diversification. Numerous variations have emerged as
a result of this diversification, each set apart by unique traits,
such as immunological evasion, severity and transmissibility.
Changes in immune profiles, human migration and infected
individuals are all part of the complex evolutionary path that is
intimately connected to ecological dynamics and the events of
transmission (1). Among the 180
identified species of ribonucleic acid (RNA) viruses capable of
infecting humans, an average of two new species emerge each year.
RNA viruses have extensively spread among humans, other mammals and
occasionally birds, across both epidemiological and evolutionary
timelines. Notably, 89% of human-infective species are zoonotic,
and a considerable proportion of the remaining species trace their
origins back to zoonotic sources. The pace at which mutations are
created and propagated across populations is the most critical
factor in viral evolution (2).
Natural selection also helps to fix favorable mutations and improve
transmissibility (3). However,
viral evolution becomes complex when viruses reproduce and develop
inside humans, while adapting to effective human-to-human
transmission. As viral lineages evolve, antigenically distinct
strains may emerge at higher organizational levels (4). The present review aimed to provide
insight into the evolutionary dynamics of SARS-CoV-2 across various
scales. This encompasses examining the stages of the COVID-19
pandemic, identifying crucial factors influencing the evolution of
the virus, exploring hypotheses surrounding the emergence of
statistically significant variants, and contemplating potential
evolutionary pathways that could affect public health in the
future. Considering the substantial role of SARS-CoV-2 in
triggering the COVID-19 pandemic, a comprehensive investigation
into the infection and its repercussions for public health is
essential. The present review also delves into the transmission of
SARS-CoV-2 from patient to host, the utilization of mathematical
models for predicting the risk of viral aerosol/droplet
transmission, potential pathways for viral entry into the human
host and the cellular mechanisms underlying these processes.
In addition, the present review highlights the
COVID-19 clinical symptoms and available diagnostic approaches for
detecting the virus. The requirement for effective treatment
techniques, such as vaccine development and medication repurposing,
is emphasized herein. Given the considerable amount of studies on
COVID-19 and the available literature, it appears difficult to
address each element. The present review aimed to provide a
comprehensive discussion of diverse facets concerning the COVID-19
outbreak. It covers a wide range of topics, such as preventative
measures against the virus, clinical characteristics of symptomatic
and asymptomatic individuals, estimations of the infection and
incubation periods, the immune responses that the virus elicits in
humans, and the association between pre-existing comorbidities and
COVID-19-associated mortality. Furthermore, the present review
provides a historical framework for understanding pandemics,
tracing their evolution from confined outbreaks to global
epidemics, starting in the 16th and 19th centuries. It delves into
zoonotic origins, elucidating the transmission of zoonoses from
animals to humans, with illustrative examples, such as human
immunodeficiency virus (HIV)/acquired immunodeficiency syndrome
(AIDS), Ebola and historical influenza strains. SARS-CoV-2 exhibits
significant genetic similarities to pangolin coronaviruses and bat
betacoronaviruses, indicating that the ongoing COVID-19 pandemic
has its origins in an animal reservoir (5). As the battle against COVID-19
continues, the acquisition of knowledge and understanding remains
indispensable in formulating efficacious strategies to safeguard
global public health.
2. Emergence and spread of COVID-19
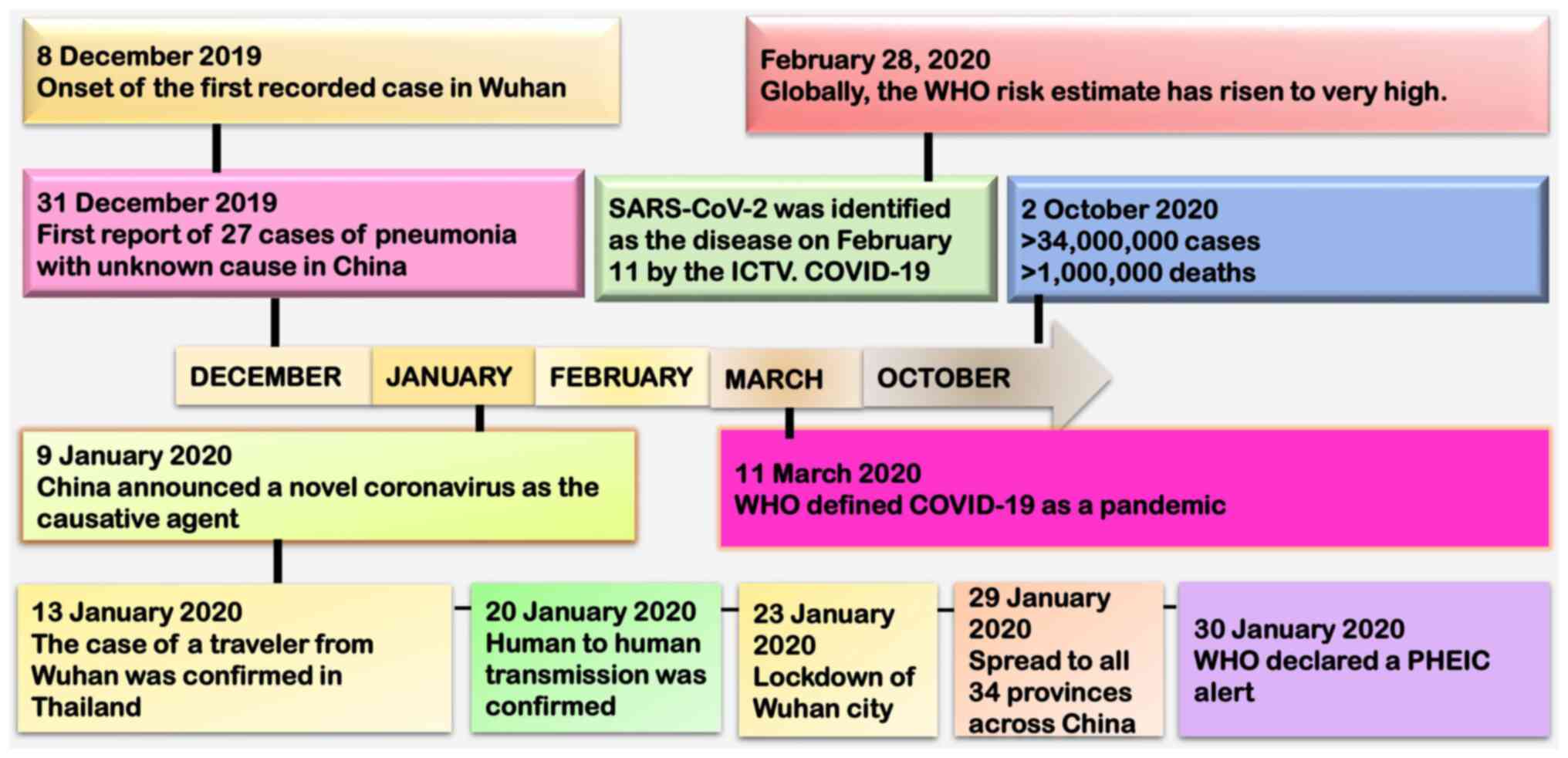
Several pneumonia cases with an unclear cause
emerged in late December, 2019, in Wuhan, Hubei Province, China
(6). The afflicted individuals
exhibited clinical signs of fever, cough, dyspnea, chest pain and
bilateral lung infiltration, symptoms of viral pneumonia, which
were comparable to those in SARS and Middle East respiratory
syndrome (MERS) (7). The Huanan
Seafood Wholesale Market, a wet market in downtown Wuhan known for
selling seafood and live animals, including poultry and wildlife,
was linked to the majority of the initial cases (8). On December 8, 2019, the earliest case
was recorded (9).
The World Health Organization (WHO) was formally
notified that the Wuhan Municipal Health Commission reported an
unknown pneumonia outbreak on December 31. Independent Chinese
scientific teams revealed a novel betacoronavirus as the cause of
this newly discovered disease (10). The first genome sequence of the
novel coronavirus was made available on January 10. The outbreak
coincided with the Lunar New Year celebrations, which led to more
individuals traveling and spreading the virus to additional Hubei
Province cities, and ultimately to other regions of China (11). The escalation in severity led the
WHO to declare the COVID-19 outbreak as a public health emergency
of international concern on January 30, 2020(12). The WHO officially designated the
illness as COVID-19 on February, 11 2020(13). A schematic diagram of the timeline
of these events is presented in Fig.
1. China imposed strict public health measures, such as a
city-wide lockdown of Wuhan on January 23, 2020 with travel and
transportation restrictions, to contain the outbreak (14). The high transmissibility of the
virus and global travel contributed to large clusters of infections
being reported in numerous countries. Consequently, on March 11,
2020, the WHO formally declared the COVID-19 outbreak to be a
pandemic (15,16). China was able to contain the virus
relatively well; however, the number of cases in the USA and Europe
increased rapidly (17).
As of August 20, 2024, the USA is experiencing a
high prevalence of the SARS-CoV-2 Omicron variants KP.2, KP.2.3,
KP.3, KP.3.1.1 and LB.1. The Center for Disease Control and
Prevention indicate that KP.3.1.1 is expected to comprise ~37% of
new COVID-19 cases in the USA. The estimated percentage of
illnesses caused by KP.2.3 remains at 14.4%, similar to the
previous 2-week period, and the estimated rate of diseases caused
by LB.1 also remains at 14.1%. JN.1 has been reported by 115
countries, rendering it the most reported VOI, representing 90.3%
of sequences in week 9, up from 89.4% in week 6. The parent lineage
of JN.1, BA.2.86, decreased and was responsible for 2.2% of
sequences in week 9, down from 3.0% in week 6 (https://covid.cdc.gov/covid-data-tracker/#variant-summary;
https://www.who.int/publications/m/item/covid-19-epidemiological-update-15-march-2024).
3. SARS and COVID-19: Similarities and
differences
There are notable similarities between the clinical
manifestations and modes of transmission of the 2019 COVID-19 and
SARS virus. Both infections have the potential to manifest as
rapidly progressing pneumonia. It appears that the primary mode of
transmission for both is infectious respiratory droplets that are
released from mucosal membranes (Table
I). The viruses exhibit comparable stability and degradation in
aerosols and on various surfaces (18). According to researchers, both
viruses can live for up to 2 days on stainless steel and 3 days on
plastic, and their viral titers on both surfaces exhibit comparable
decay patterns (19-21).
Both SARS and COVID-19 appear to have a median incubation period of
4 to 7 days from first exposure to the start of symptoms.
Furthermore, according to research, the maximum incubation time for
both might be up to 14 days (22-24).
This longer incubation time adds to the difficulty of preventing
the spread of these illnesses. Despite these similarities, it is
important to emphasize that SARS and COVID-19 are caused by
different viruses and are members of separate coronavirus
subfamilies. In summary, whereas SARS and COVID-19 share clinical
signs and transmission characteristics, they are caused by separate
viruses and have distinct characteristics that distinguish them as
distinct causative agents. Understanding these similarities and
differences appears critical for successful epidemic management and
prevention measures. The incubation time and length of viral
shedding are critical for determining the risk of transmission,
adopting isolation and quarantine measures, and developing
effective antiviral therapies for patients. According to recent
epidemiological research, the typical period of COVID-19 virus
shedding is ~20 days, with some survivors shedding for as long as
37 days (25). By contrast, viral
RNA remains detectable in non-survivors until mortality. Patients
with severe COVID-19 infection may suffer viral shedding for a
median of 31 days after the disease begins (26).
 | Table ISARS and COVID-19 comparisons. |
Table I
SARS and COVID-19 comparisons.
| Parameter | SARS | COVID-19 |
|---|
|
Pre-transmissibility | No | Yes |
| Mild case
transmissibility | No | Yes |
| Reproduction
Number | 1.7-1.9
(WHO) | 2.0-2.5
(WHO) |
| Number of reported
cases |
>8,000 | 692.52 million
(July 31, 2023) |
| Number of reported
deaths | 774 | 6,903,467 (July
31, 2023) |
| Mortality rate | 9% | 3.1% |
| The primary mode of
transmission | Infectious
respiratory droplets dispersed from mucous |
| Ability to survive
on surfaces | Yes |
| Median incubation
period | 4-7
days |
| Maximum incubation
period | 14 days |
| Potential to cause
severe respiratory infection | Yes |
| Potential to infect
the central nervous system and brain | Yes |
Betacoronaviruses and alphacoronaviruses have
natural hosts in bats. RaTG13, a bat coronavirus isolated from
Rhinolophus affinis in Yunnan province, China, is the
closest known match to SARS-CoV-2 to date (27). RaTG13 and SARS-CoV-2 share 96.2% of
the full-length genome sequence, demonstrating a strong genetic
similarity (28,29). The fact that SARS-CoV-2 and RaTG13
share >90% of their genome's sequence, including the variable
spike glycoprotein (S glycoprotein) and open reading frame (ORF)8
regions, is particularly notable (28). Their close association is
highlighted by phylogenetic analysis, which lends credence to the
theory that bats are the original host of SARS-CoV-2. SARS-CoV-2
and ‘RmYN02’, a recently discovered coronavirus found in a Yunnan
Rhinolophus malayanus bat, share 93.3% of their genome
(29). Notably, it shares a longer
1ab gene with SARS-CoV-2 with 97.2% identity, higher than
RaTG13(30). Furthermore, ZC45 and
ZXC21, two additional bat coronaviruses that were previously
discovered in eastern Chinese Rhinolophus pusillus bats, are
members of the SARS-CoV-2 lineage within the Sarbecovirus subgenus
(31). These findings highlight
the wide range of bat coronaviruses that are strongly associated
with SARS-CoV-2, indicating that bats may be the virus's possible
hosts. Recent investigations have revealed that the genetic
diversity observed in SARS-CoV-2 and its related bat coronaviruses
stems from over 20 years of sequence evolution (31,32).
Consequently, it is incorrect to categorize these bat coronaviruses
as the immediate progenitors of SARS-CoV-2, despite being likely
evolutionary ancestors.
Pangolins are another possible animal host connected
to SARS-CoV-2. Between 2017 and 2019, several viruses related to
SARS-CoV-2 were discovered in the tissues of pangolins (33). These pangolin viruses are from two
distinct sub-lineages and were independently traced in the
provinces, of Guangxi and Guangdong (34-37).
Pangolins linked to various smuggling incidents have been found to
have SARS-CoV-2-related coronavirus infections, suggesting that
these animals may serve as hosts for the viruses (38). Pangolins infected with
coronaviruses display clinical symptoms and histological changes,
such as multiple organ infiltration of inflammatory cells and
interstitial pneumonia, in contrast to bats, which typically carry
the virus without obvious damage (39).
Emerging coronaviruses that are derived from bats
require an intermediate host to proliferate. For example, dromedary
camels and palm civets served as intermediary hosts for SARS-CoV
and MERS-coronavirus (MERS-CoV), respectively (40). The viruses harbored by these hosts
share a genome sequence identity of >99% with the corresponding
viruses in humans (41). The role
of an intermediary host in the transmission of the SARS-CoV-2
virus, which is accountable for the COVID-19 pandemic, is under
scrutiny and remains unclear. Pangolin coronaviruses exhibit only a
92% genomic identity with SARS-CoV-2, despite displaying a marked
similar receptor-binding domain (RBD) (42). Consequently, it is challenging to
definitively ascertain whether pangolins acted as the intermediate
host for SARS-CoV-2 or if they were directly implicated in the
emergence of the virus. The animal source of SARS-CoV-2 is
presently poorly understood, with limited knowledge available on
this aspect. The reservoir hosts of the virus have yet not been
identified, nor it has been determined if an intermediate host was
involved in the transmission of the virus to humans. Significantly,
the discovery of pangolin coronaviruses, RaTG13, and RmYN02 implies
that SARS-CoV-2-like coronaviruses are prevalent in animals
(43-45).
In addition to wildlife, research has explored the
susceptibility of domesticated and laboratory animals to SARS-CoV-2
infection. Experimental findings have demonstrated that SARS-CoV-2
can effectively replicate in cats and ferrets, particularly in the
upper respiratory tract (46).
Conversely, dogs, pigs, chickens and ducks have exhibited immunity
to the virus (47). Notably, minks
have been observed to contract SARS-CoV-2, as evidenced by an
outbreak on mink farms in The Netherlands, leading to severe cases
of respiratory distress and interstitial pneumonia (48). Although devoid of symptoms, two
dogs in Hong Kong tested positive for spontaneous SARS-CoV-2
infection through serological and virological tests (49). Similarly, the analysis of blood
samples from cats in Wuhan revealed the presence of neutralizing
antibodies against SARS-CoV-2, confirming the infection in cat
populations. However, the possibility of transmission from cats to
humans remains uncertain (50).
Ongoing comprehensive research and surveillance on animal
susceptibility aim to provide a deeper understanding of potential
hosts and transmission dynamics of the virus.
4. Comparative insight into SARS-CoV-2:
Infectiousness, transmission and evolution
The virus accountable for acute respiratory illness,
SARS-CoV-2, belongs to the coronavirus family and carries a
non-segmented genome composed of positive-sense, single-stranded
RNA enveloped by the viral capsid (51). Coronaviruses (CoVs) are categorized
into four genera: α, β, γ and δ-CoV (52). While α- and β-CoV predominantly
infect mammals, they can also affect birds. Human-infecting
coronaviruses include HCoV-229E, SARS-CoV, HCoV-OC43, HCoV-NL63,
MERS-CoV and HCoV-HKU1(53).
Infections caused by HCoV-229E, HCoV-NL63, HCoV-HKU1 and HCoV-OC43
typically result in mild respiratory symptoms. By contrast,
SARS-CoV and MERS-CoV can lead to severe respiratory disease,
occasionally resulting in death due to multiple organ failure
(54). SARS-CoV-2 shares notable
similarities (>85%) with bat-derived SARS-like coronaviruses
identified as bat-SL-CoVZC45 and bat-SL-CoVZXC21(55). Compared to SARS-CoV and MERS-CoV,
it demonstrates ~79 and 50% homology, respectively (56). This evidence and phylogenetic
research strongly indicate that SARS-CoV-2 originated in bats and
potentially transmitted to humans through an unidentified
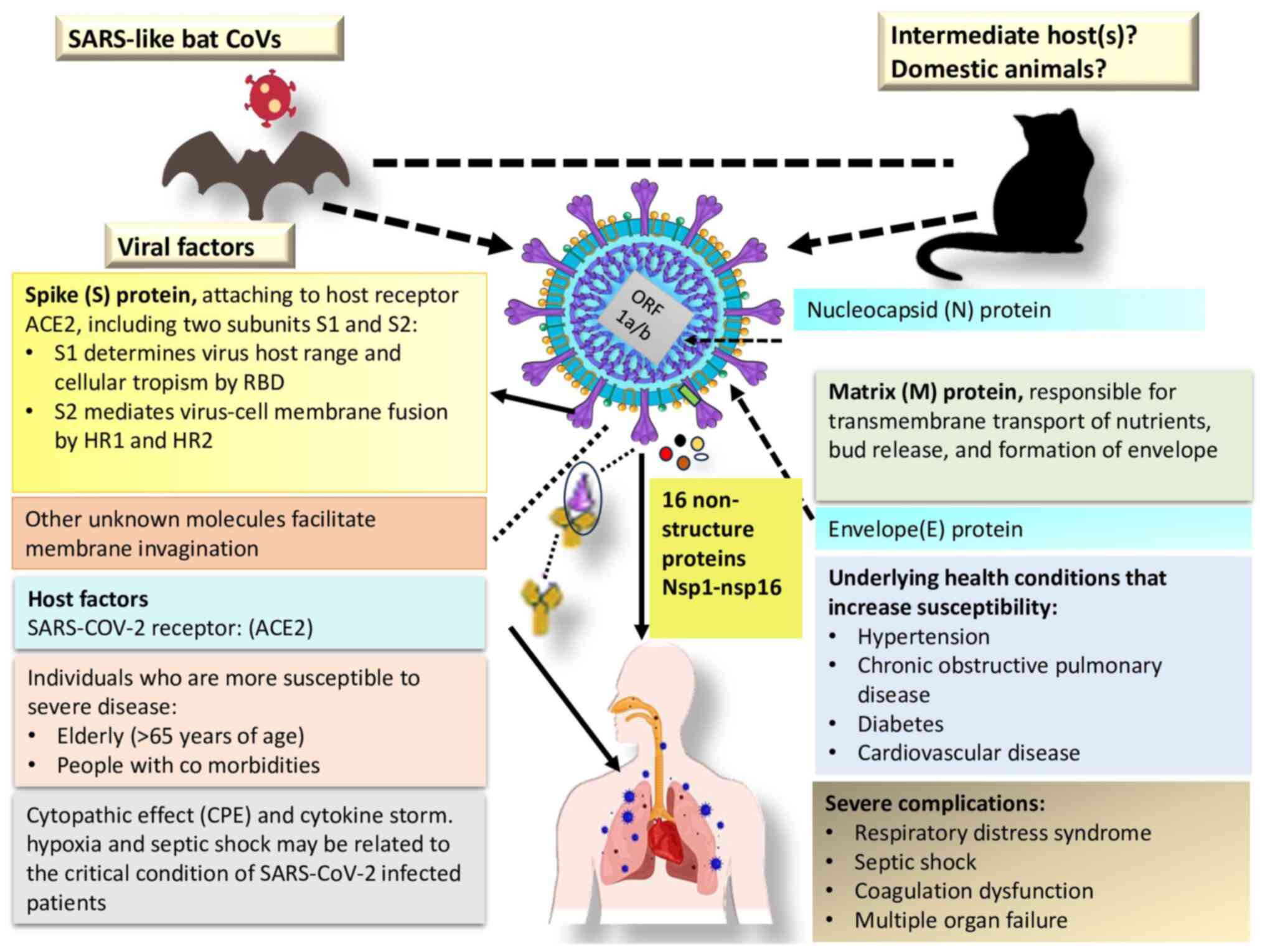
intermediate host species. The genomic structure, encoded
structural and non-structural proteins, and the primary host of
SARS-CoV-2 are illustrated in Fig.
2.
The pathogenesis of SARS-CoV-2 involves a complex
interplay of viral and host factors. As an enveloped positive-sense
single-stranded RNA virus, the genomic structure of SARS-CoV-2
comprises a significant portion (two thirds) dedicated to an ORF
(ORF 1a/b), encoding 16 non-structural proteins crucial for
replication. The remaining section of the genome encodes essential
structural proteins (S glycoprotein, small envelope protein, matrix
protein and nucleocapsid protein) and accessory proteins with
functions still under investigation. The S glycoprotein, essential
for host cell entry, binds to the angiotensin-converting enzyme 2
(ACE2) receptor. However, the precise mechanism of membrane
invagination for SARS-CoV-2 endocytosis remains unclear. Host
factors, particularly ACE2 expression, influence viral tropism. The
elderly and individuals with underlying health conditions are more
susceptible to severe infections, partly due to age-related immune
system changes and comorbidities. Host immune responses, both
innate and adaptive, play a crucial role, and dysregulated
responses can contribute to disease severity. Additionally, genetic
factors contribute to interindividual variability in susceptibility
and disease outcomes. A comprehensive understanding of these viral
and host elements is crucial for developing effective therapeutic
interventions and vaccines against SARS-CoV-2. Ongoing research
continues to unveil additional details about the intricate
virus-host interactions shaping the pathogenesis of COVID-19.
Without a doubt, expressive experimentation has shown that the
virus infects people by attaching itself to respiratory
system-expressed ACE2 receptors (57,58).
Overall, the findings from several investigations demonstrate that
SARS-CoV-2 is extremely infectious, with viral shedding commencing
before symptoms develop and the virus spreading through many
channels (15-17,59).
Controlling the spread of the disease is a main concern for public
health initiatives.
Although SARS-CoV-2 is less severe in terms of
morbidity and mortality than MERS and SARS, it is more contagious.
COVID-19 has a much lower mortality rate of 3.4% compared to 9.6
and 35% for SARS and MERS, respectively. COVID-19 primarily spreads
through person-to-person contact, particularly between close
friends and family members (59).
Numerous studies have demonstrated the critical role symptomatic
individuals play in COVID-19 transmission, mainly through
respiratory droplet expulsion from actions, such as coughing or
sneezing. On the other hand, nosocomial transmission was primarily
responsible for the spread of MERS-CoV and SARS-CoV among
healthcare personnel (60). In
MERS-CoV outbreaks, medical staff was responsible for 62-79% of
cases, whereas in the SARS case, they accounted for 33-42% of
cases. The most likely ways for a virus to spread are through
direct contact with the host or interactions with an unidentified
intermediate carrier (61).
The SARS-CoV-2 virus changes in a variety of ways as
it grows and spreads among the population. In December, 2020, a
noteworthy variant, VUI-202012/01, was examined due to 17 distinct
alterations or mutations in its DNA. Since the discovery of
SARS-CoV-2 in 2019, thousands of mutations have already manifested
in its genome (62). As the
pandemic continues, the continual mutation process in the
population may result in the production of immunologically relevant
mutations, thereby affecting vaccination effectiveness. These
mutations are resulting in novel viral combinations. The COVID-19
genomics UK consortium (COG-UK) has conducted extensive
epidemiological and virological investigations in response to the
significant surge in COVID-19 cases recently observed in the UK,
particularly in South East England (63). A novel variant was identified in
viral genome sequences, forming a distinct phylogenetic grouping.
This variant is distinguished by multiple spike protein mutations
(deletion 69-70, deletion 144, N501Y, A570D, D614G, P681H, T716I,
S982A and D1118H), accompanied by alterations in other genomic
regions (64). Although viral
mutations are normal, preliminary studies have indicated that this
variant in the UK may be critical for increased transmissibility
and is projected to possibly raise the reproductive number by 0.4
or more (65). Notably, this new
variety evolved during a period of increased family and social
gatherings. However, there is no indication that it causes more
severe infections than other variations.
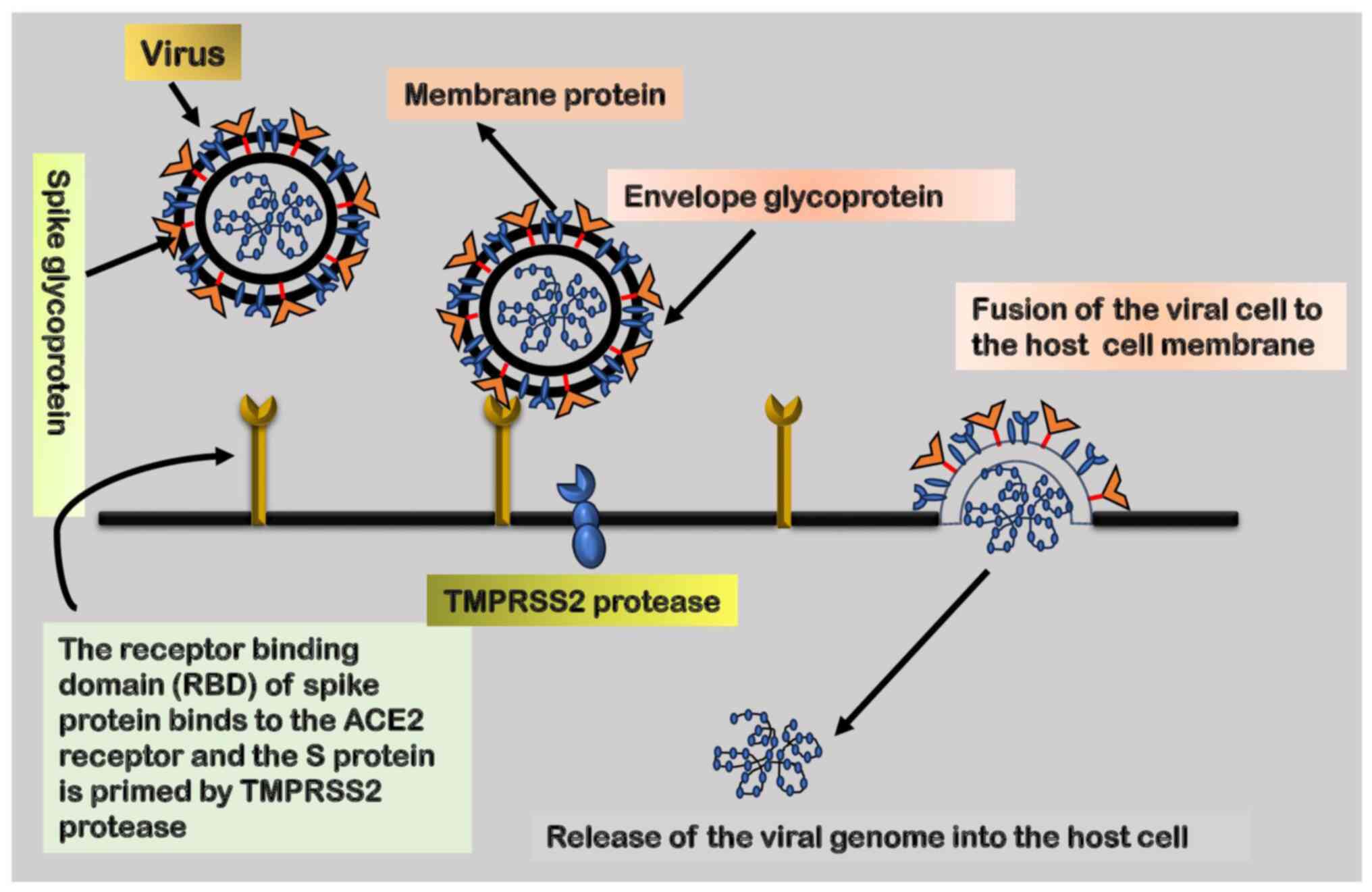
5. Role of ACE2 receptor, an
angiotensin-converting enzyme
SARS-CoV-2 gains entry into the human host through
receptor-mediated endocytosis, a mechanism through which viruses
bind to specific receptors on the cell surface of the host,
facilitating entry. The RBD of the virus establishes a connection
with the appropriate receptor on the host cell, enabling entry.
Both SARS-CoV and SARS-CoV-2 utilize the ACE2 receptor to infect
cells. Previous research has shown that the S protein of SARS-CoV
exhibits a strong affinity for the ACE2 receptor, serving as the
entry point for the virus into host cells (66). A schematic diagram depicting the
fusion of the virus with the host receptor is presented in Fig. 3.
The entry of SARS-CoV-2 into host cells is also
mediated by S protein priming by transmembrane protease serine-2
(TMPRSS2). This priming event is crucial for the fusion of the
viral envelope with the host cell membrane, enabling subsequent
viral entry. Therefore, the coordinated interplay between the ACE2
receptor and TMPRSS2 is essential for the efficient entry of
SARS-CoV-2 into the host environment. It is noteworthy that TMPRSS2
exhibits a higher expression and broader distribution compared to
ACE2 receptors, suggesting that ACE2 may function as a limiting
factor during the initial infection phase. While TMPRSS2 is a key
component for viral entry, alternate proteases, such as cathepsin
B/L, may act as substitutes for TMPRSS2. Hence, the simultaneous
inhibition of these proteases becomes crucial in preventing
cellular entry.
The structural characteristics of the S proteins of
SARS-CoV and SARS-CoV-2 facilitate the entry of the latter into
cells (67). Studies involving
human HeLa cells and animals with and without ACE2 expression
support the involvement of ACE2 receptors in the cellular entry of
the SARS-CoV-2 virus, particularly the Wuhan strain (68,69).
Research on the SARS-CoV-2 infection of BHK21 cells has indicated
higher infection rates when transfected with human and bat ACE2
receptors compared to BHK21 cells lacking ACE2 expression (70,71).
Biophysical and structural data suggest that the ACE2 binding
affinity of the SARS-CoV-2 S protein ectodomain is significantly
greater than that of the SARS-CoV S protein by a ratio of
10:20(72). This difference is
considered to contribute to the variance in contagiousness between
SARS-CoV-2 and SARS-CoV. Although the ACE2 and ACE-1 receptors
share similarities, the ACE2 receptor has a smaller active site and
a smaller binding pocket with different amino acids, making it
resistant to typical ACE inhibitors, such as lisinopril, enalapril,
and ramipril (73).
Furthermore, there is no evidence to suggest that
angiotensin receptor blockers (ARBs), such as losartan, disrupt the
activity of ACE2. TMPRSS2, identified as a type II transmembrane
protease, consists of distinct domains, including an intracellular
N-terminal domain, a transmembrane domain, an extracellularly
extending stem region, and a C-terminal domain facilitating its
serine protease (SP) function (74). The serine protease activity relies
on a catalytic triad, comprised of His296, Asp345 and Ser441,
responsible for cleaving basic amino acid residues, particularly
lysine or arginine residues, aligning with its role in cleaving the
S1/S2 site in SARS-CoV-2(75).
While TMPRSS2 has been recognized for its
involvement in prostate cancer and viral infections, such as
influenza, SARS and MERS (76), it
has recently gained attention from drug developers. Multiple
studies are underway to uncover strategies aimed at reducing
TMPRSS2 expression or blocking its activity in host cell membranes,
with the ultimate goal of inhibiting SARS-CoV-2 entry into host
cells (77,78).
6. Diagnostic and therapeutic approaches,
and strategies to inhibit viral entry
The molecular detection of SARS-CoV-2 nucleic acid
is the most accurate diagnostic approach (79). Various commercially available kits
for viral nucleic acid detection target different genes, including
ORF1ab (containing RdRp), N, E or S (80). The detection time may vary from a
few minutes to several hours depending on the technology utilized.
Although SARS-CoV-2 has been detected in throat swabs, posterior
oropharyngeal saliva, nasopharyngeal swabs, sputum and bronchial
fluid, the viral load is notably higher in samples from the lower
respiratory tract (81). Viral
nucleic acid has also been detected in intestinal and blood
samples, even in cases where respiratory tests yielded negative
results. The viral load may decrease from its peak at the onset of
the illness, potentially leading to false negatives when using oral
swabs (81). It is advisable to
employ multiple detection techniques to confirm a COVID-19
diagnosis.
To address the issue of false negatives, alternative
detection approaches have been utilized. Therefore, for individuals
with a robust clinical suspicion of COVID-19 despite an initial
negative nucleic acid screening, a combination of CT scans and
repeated swab testing has been recommended. Serological assays that
identify antibodies to the N or S protein of SARS-CoV-2 could
complement molecular diagnosis, particularly in the latter stages
of the illness or for retrospective research (76,82,83).
The magnitude and duration of immunological responses are still
unknown, and the sensitivity and specificity of existing
serological assays vary. When selecting and interpreting
serological testing, all these factors should be taken into
consideration, possibly even extending to future assays for T-cell
responses (84).
Currently, neither COVID-19 nor specific antivirals
that target SARS-CoV-2 have the potential to combat the disease.
However, several treatments have shown some promise. Manufacturers
and researchers are undertaking large clinical studies to examine
new COVID-19 therapy options.
The pharmaceutical interventions available for
COVID-19 therapy can be divided into several groups: Immunotherapy,
cellular therapy, antiviral and other drugs. Immunotherapy mainly
includes; immunoglobulins, interferons, convalescent plasma, and
monoclonal therapy. Human immunoglobulin is tested in patients with
pneumonia with COVID-19 and there are 478 clinical trials
concerning immunoglobulins; 18 of these trials have been completed
(https://www.clinicaltrials.gov/search?term=immunoglobulin&cond=Covid19&viewType=Table).
There are 87 clinical trials underway about
interferons against COVID-19; of these, six clinical trials have
been completed (https://www.clinicaltrials.gov/search?term=interferon&cond=Covid19&viewType=Table).
In addition, 140 clinical trials examining the efficacy and safety
of convalescent plasma for COVID-19 and, of these, nine trials have
been completed (https://www.clinicaltrials.gov/search?term=convalescent%20plasma&cond=Covid19&viewType=Table).
Currently, 47 clinical trials are being conducted
for monoclonal antibodies; none of have been completed (https://www.clinicaltrials.gov/search?term=monoclonal%20antibody&cond=Covid19&viewType=Table).
Cellular therapy primarily focuses on mesenchymal
stem cells (MSCs) and natural killer (NK) cells. MSCs are potent
anti-inflammatory and immunomodulatory tools of the immune system.
MSCs can reduce the burden of lung injury and respiratory distress
by preventing leakage of immune cells and proinflammatory cytokines
to the pulmonary tissue. Of note, there are 55 ongoing clinical
trials and two trials have been completed (https://www.clinicaltrials.gov/search?term=mesenchymal%20stem%20cell&cond=Covid19&viewType=Table).
As regards NK cells, they can identify the infected
cells, mediate antibody-dependent cytotoxicity and maintain
immunological homeostasis. A total of 28 clinical trials are
underway on NK cells (https://www.clinicaltrials.gov/search?term=natural%20killer%20cell&cond=Covid19&viewType=Table).
There are also ~84 clinical trials conducted with other drugs, and
of these, 9 trials have been completed (https://clinicaltrials.gov/search?term=chloroquine&cond=Covid19&viewType=Table).
Potential antiviral targets for the treatment of
COVID-19 are depicted in Fig. 3. A
crucial strategy in combatting SARS-CoV-2 infection is to hinder
viral entry. ACE2 exists in membrane-bound ACE2 (mACE2), located in
the gallbladder, heart, intestines, kidneys and testes (85). The virus uses human proteases as
entry activators to break through host cells through membrane
fusion, and it uses ACE2 as a receptor. Treatments aimed at this
entry mechanism have the potential for treating COVID-19.
Umifenovir, also known as Arbidol, is a drug approved for treating
respiratory viral infections and influenza in China and Russia. Its
mechanism of action involves preventing membrane fusion by
interfering with the interaction between the S protein and
ACE2(86). In vitro studies
have demonstrated its efficacy against SARS-CoV-2; clinical data
suggest that it may present a more effective treatment for COVID-19
when compared to lopinavir and ritonavir (87-91).
One notable drug that shows promise is camostat
mesylate, which is licensed in Japan for the treatment of
post-operative reflux esophagitis and pancreatitis (92). Previous studies have demonstrated
the ability of camostat mesylate to inhibit TMPRSS2 activity and
protect mice from fatal SARS-CoV infection (93). Recent studies have further
indicated that camostat mesylate can inhibit the entry of
SARS-CoV-2 into human lung cells (88). This suggests potential utility as
an antiviral drug against SARS-CoV-2 in the future, although
further clinical data is required to confirm its effectiveness
(92-95).
Other drugs used to treat autoimmune diseases and
prevent malaria, such as chloroquine and hydroxychloroquine, may
also influence SARS-CoV-2 entry. They function by preventing
membrane fusion by raising endosomal pH, interfering with the
interaction between virus and host receptor, and inhibiting the
glycosylation of cellular receptors (87). As regards their effectiveness in
treating COVID-19, there remains a lack of scientific consensus.
Despite concerns about an increased risk of cardiac arrest in
treated patients, two clinical investigations found no correlation
between these medications and patient mortality rates (96,97).
On June 15, 2020, due to documented adverse events, the US Food and
Drug Administration (FDA) revoked the emergency use authorization
for chloroquine and hydroxychloroquine in COVID-19 therapy
(98).
Another therapeutic approach involves the use of
soluble recombinant human ACE2 (hACE2), specific monoclonal
antibodies, or fusion inhibitors targeting the SARS-CoV-2 S protein
to prevent its binding to the ACE2 receptor (99). Examples of replication inhibitors
include remdesivir (GS-5734), favilavir (T-705), ribavirin,
lopinavir and ritonavir. The remaining three agents act on RdRp,
except for lopinavir and ritonavir, which inhibit 3CLpro. Potential
antiviral targets for COVID-19 treatment are illustrated in
Fig. 4. However, further clinical
research is required to evaluate the effectiveness and safety of
these approaches.
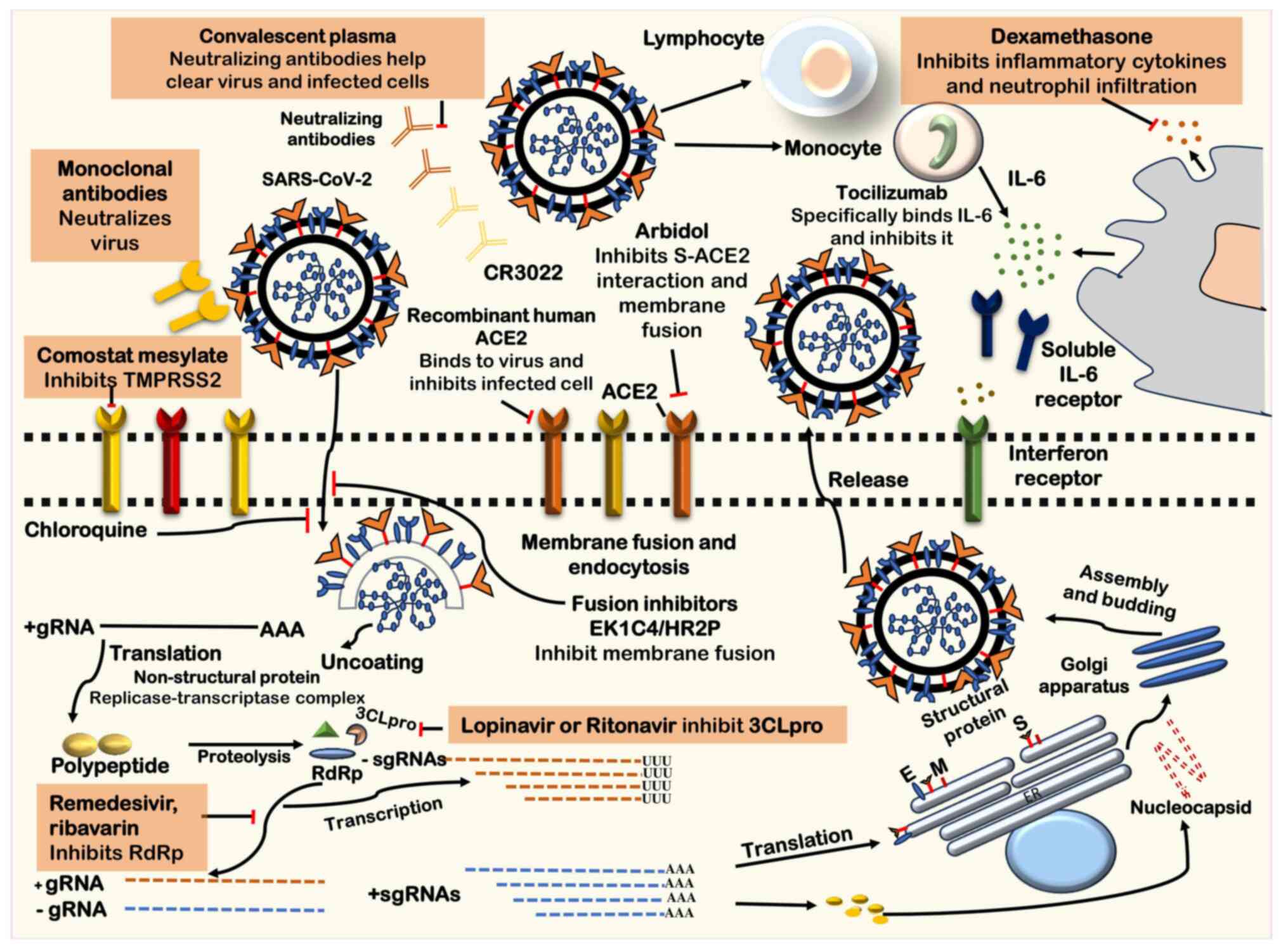 | Figure 4Potential antiviral interventions
against SARS-CoV-2. In addition to antiviral agents
immunomodulatory and immunoglobulin-based medications are potential
treatments. Key molecular targets implicated in the viral
replication cycle and potential treatments include ACE2, crucial
for the initial interaction of the virus during receptor binding;
3CLpro, a protease inhibited by lopinavir and ritonavir; CR3022, a
human monoclonal antibody targeting the SARS-CoV virus; envelope
protein (E), a potential target for disrupting viral replication;
endoplasmic reticulum (ER), involved in various stages of viral
replication and a potential therapeutic target; gRNA, a critical
component of the viral replication process; HR2P, peptides
considered for their potential in inhibiting viral fusion;
interferon-stimulated gene, targeted by immunomodulatory agents to
modulate the host immune response; M (membrane protein), a
potential target for disrupting viral replication; RdRp, the key
enzyme in the viral replication process targeted by antiviral
agents such as remdesivir, favilavir, and ribavirin; sgRNA,
involved in various stages of the replication cycle; S, a major
target for therapeutic intervention considering its role in
receptor binding and viral entry; and TMPRSS2, facilitating viral
entry into host cells and a potential target for antiviral
strategies. SARS-CoV-2, severe acute respiratory syndrome
coronavirus 2; 3CLpro, 3C-like protease; gRNA genomic RNA; HR2P,
SARS-CoV-2 spike protein derived peptides, heptad repeat 2; RdRp,
RNA-dependent RNA polymerase; sgRNA, subgenomic RNA; S, Spike
protein; TMPRSS2, transmembrane protease serine protease 2. |
Novel diagnostic approaches are currently aimed at
detecting COVID-19. Developing accessible point-of-care diagnostic
devices customized for low-resource settings remains an urgent
need. This can be accomplished through workflows that do not
require cell lysis, bioassays that are increasingly sensitive and
robust, improved methods for sample collection and processing that
minimize risks to healthcare workers, and kits that are
ready-to-use and do not require technical expertise, among other
potential innovations. RT-LAMP and CRISPR/Cas-based methods, such
as Specific High-Sensitivity Enzymatic Reporter UnLOCKing
(SHERLOCK) and DNA Endonuclease-Targeted CRISPR Trans Reporter
(DETECTR) are significant advancements that could be considered as
promising alternatives (100,101). As regards portable diagnostic
devices, few technologies hold as much promise as paper-based
microfluidics (102). Natural
remedies, such as Withania somnifera and Tinospora
cordifolia have been tested on experiment models of COVID-19;
research has shown their potency to limit the deterioration of the
health of patients by reducing inflammation (99). Furthermore, the researchers are
investigating the repurposing of drugs, such as procaine, which is
an antiviral agent. It has demonstrated the in vitro
inhibition of SARS-CoV-2, facilitating novel approaches for the
treatment of the disease (103).
The implementation of these strategies requires vast
capital investment. In light of concerns about a potential economic
downturn and financial breakdown, resilient and effective
leadership needs to emerge in healthcare, business, government, and
at the community level. There is a need for immediate relief
measures that should be adapted to assist the socioeconomically
weaker, who may be left behind.
7. Current management approaches for
COVID-19
Avoiding transmission should be the primary
objective of COVID-19 treatment, particularly in those with
moderate symptoms, given the uncertainty surrounding the
effectiveness of currently available antiviral medications.
Individuals receiving at-home care must be closely monitored, and
if their health worsens, therapy must be escalated right away.
Studies on the advantages of corticosteroids, weighing
anti-inflammatory effects with possible hazards of viral
replication, have shown conflicting findings (104). Corticosteroids may, however, be
taken into consideration in situations when there are other signs,
such as severe chronic obstructive pulmonary disease. Inhalers are
used over nebulized medicines, which produce aerosols, to reduce
the danger of airborne viral dissemination (105). Non-steroidal anti-inflammatory
drugs (NSAIDs) have generated controversy die to their ability to
affect epithelial cell ACE2 receptor levels and perhaps worsen
viral infection (106). The
specific effects of NSAID usage in COVID-19 remain uncertain. Some
suggest that NSAIDs may elevate the risk of developing acute
respiratory distress syndrome (ARDS) by triggering leukotriene
release and bronchoconstriction (107). However, the application of NSAIDs
for symptom management should be tailored to each individual.
Presently, the European Medicines Agency (EMA) and the WHO do not
advise against the use of NSAIDs (108). In hospital settings,
acetaminophen is often preferred over NSAIDs to minimize the risk
of bleeding and kidney damage (109).
Controversy has arisen regarding the use of ARBs and
ACE inhibitors in COVID-19. Nonetheless, the American Society of
Cardiology and the European Society of Cardiology presently do not
recommend initiating or discontinuing these drugs (110). The selection of antiviral and
anti-inflammatory therapies should be personalized according to
each the condition of each patient, guided by infectious disease
experts, and conducted within the context of a clinical trial or
registry. Oxygen therapy, encompassing methods, such as nasal
cannula and high-flow oxygen, is often beneficial for individuals
with mild to severe COVID-19(111). Non-invasive and invasive
mechanical ventilation are commonly required in situations of acute
respiratory failure. Positive airway pressure is an
aerosol-generating treatment; hence healthcare professionals have
and must use a greater degree of personal protective equipment
(112). Unless there are
particular contraindications, pharmaceutical prophylaxis is used
for these events and should be made available to hospitalized
patients with COVID-19 due to the elevated risk of venous
thromboembolism.
The PREDICT initiative by the US Agency for
International Development (USAID) has significantly improved the
local workforce and laboratories to identify emerging zoonotic
virus risks. Investment in spillover prevention in priority
countries remains a focus for USAID, with ongoing projects aimed at
building a ‘One Health Workforce’ in Asia and Africa and creating
interventions to halt spillover at critical high-risk human-animal
interfaces (113).
8. Preventing COVID-19: Progress in vaccine
advancements
Research has been conducted to evaluate a range of
innovative and repurposed medicines in the battle against
COVID-19(114). Among these
programs, vaccinations hold promise since they could stop the
spread of illness to a larger population. Before they may be used
widely, the safety and efficacy of these immunizations must first
be properly confirmed. It is impossible to overstate the importance
of this stage since subpar immunizations run the danger of doing
more damage than good via mechanisms including antibody-dependent
augmentation. Therefore, meticulous testing and verification are
crucial before the widespread adoption of any COVID-19
immunizations.
Technological approaches employed in
the development of COVID-19 vaccines
Numerous technologies are employed by scientists and
researchers globally in their endeavors to create a secure and
efficient vaccine for SARS-CoV-2. Among these technologies, gene
vaccines, inactivated vaccines, viral vector vaccines, and protein
subunit vaccines stand out as the most promising candidates
(115).
Vaccines based on protein
subunits
Protein subunit vaccines, frequently administered
through sophisticated systems, such as liposomes, virosomes, or
polymeric nanoparticles, harness components of the pathogen to
stimulate the immune system of the host (116). Liposomes and virosomes, serve as
effective adjuvants and carriers for antigens and are commonly
employed in the development of vaccines against SARS-CoV-2(117). For instance, research has
reflected upon a cationic liposome protein subunit vaccine that
incorporates the S1 component of the SARS-CoV-2 virus. This vaccine
also includes two adjuvants: Monophosphoryl lipid A (MPLA), acting
as a TLR4 and TLR9 agonist and CpG DNA (118). The inclusion of cationic elements
such as 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) enhances
the interaction of the liposome with antigen-presenting cells
(115). This liposome vaccine
demonstrated improved T-cell immunity, activating CD4+
and CD8+ cells and promoting IgA synthesis for potential
mucosal defense (119).
Virosomes, lipid vesicles containing viral proteins,
are preferred over liposomes as adjuvants due to their ability to
shield pharmaceutically active compounds from degradation in
endosomes until they reach the cytoplasm (120). Virosomes have previously been
utilized in the delivery of vaccines for SARS-CoV and MERS-CoV. The
Centre for Vaccine Development at Texas Children's Hospital, Baylor
College of Medicine, is working on a subunit vaccine against
SARS-CoV-2. This vaccine employs a recombinant S protein RBD,
likely combined with alum or glucopyranosyl lipid A (GLA), a
synthetic TLR4 agonist (121).
The Australia University of Queensland and Novavax
collaborated on the development of an immunogenic virus-like
nanoparticle vaccine, NVX-CoV2373, currently in phase 3 trials
(NCT04611802; https://fdaaa.trialstracker.net/trial/NCT04611802/).
This vaccine incorporates a recombinant S protein, demonstrating
minimal reactogenicity and eliciting a T-helper 1 response without
severe side-effects in the majority of individuals (122). Clover Biopharmaceuticals is also
working on a highly pure S-trimer vaccine using their Trimer-Tag
technology, previously employed in subunit vaccines for HIV,
respiratory syncytial virus and influenza. In collaboration with
GlaxoSmithKline (GSK) and Dynavax Technologies, Clover
Biopharmaceuticals has completed enrollment in a phase 1 study
(NCT04487210), employing the CpG 1018 adjuvant, a TLR9 agonist
known to activate CD4+ and CD8+ T-cells with
a favorable safety profile (123).
Vaccines with inactivated viruses
Weakened bacterial or viral pathogens used in
inactivated vaccinations stimulate the immune system without
actually infecting the recipient. Although these vaccinations do
not provide lifelong protection, booster injections are often
required to provide a long-term shielding effect. Large numbers of
viral particles are propagated, condensed, and then rendered
inactive using chemical and/or physical techniques to make
inactivated viral vaccines. Various techniques, such as the
application of ascorbic acid, binary ethylenimine, gamma
irradiation and high-temperature treatment, are commonly employed
to render viral particles inactive (124). The efficacy of these approaches
relies on ensuring complete deactivation of the specific virus. The
Wuhan Institute of Biological Products, affiliated with the China
National Pharmaceutical Group (Sinopharm), actively worked on one
of the initial inactivated COVID-19 vaccines (125). In the development of this
vaccine, the virus undergoes growth in the Vero cell line, followed
by inactivation using formalin or β-propiolactone, with alum
incorporated as an adjuvant (126). All participants in the phase 1/2
clinical trials developed antibodies in response to the
vaccination, with few negative side-effects (125).
The most typical adverse effects, such as discomfort
at the injection site and fever, were modest and self-limiting.
Phase 3 studies are currently being conducted to assess the
effectiveness and long-term safety of the vaccine. Sinovac Biotech
Ltd. in China is involved in developing CoronaVac (formerly
PiCoVacc), another inactivated vaccine. This vaccination places
genetic stability first, using the SARS-CoV-2 CN2 strain that was
isolated from bronchoalveolar lavage fluid samples of hospitalized
patients. The vaccine is presently in the midst of phase 3 clinical
trials (NCT04456595; https://covid19.trackvaccines.org/vaccines/7/),
involving a participant pool of 8,870 individuals (127). In a distinct development, the
University of Wisconsin, Madison, USA, has collaborated with
vaccine companies FluGen and Bharat Biotech to create an
inactivated vaccine named CoroFlu, designed for intranasal
delivery. Derived from FluGen's M2SR influenza vaccine, CoroFlu
leverages the immune response targeting influenza. The M2SR vaccine
has been adapted to incorporate the S protein gene sequences of
SARS-CoV-2, to elicit an immune response against the virus
(128,129). This non-invasive nasal
immunization approach exhibits potential in eliciting robust
mucosal and systemic immune responses to combat respiratory virus
infections, providing an alternative to traditional invasive
parenteral vaccination methods.
Adenovirus-based COVID-19
vaccines
Adenoviruses, with their icosahedral capsid and
double-stranded linear DNA, are essential for initiating both
innate and adaptive immunity in mammals. By increasing cytotoxic
T-lymphocytes and releasing pro-inflammatory cytokines, they aid in
the immune response. These lymphocytes are in charge of identifying
and getting rid of virus-infected cells (130). Building on this method,
adenoviral vectors have been extensively employed to combat a
variety of illnesses, including influenza, Ebola, SARS, HIV, and
recently COVID-19(131). Renowned
academic institutions and pharmaceutical companies including the
Jenner Institute at Oxford University, CanSino Biologics, and
Johnson & Johnson have led the development of COVID-19 vaccines
utilizing adenoviral vectors (132). Phase 2 clinical trials for
CanSino Biologics' Ad5-nCoV vaccine are presently underway
(NCT04526990; NCT04540419), and the results are promising (133). This vaccine carries the genetic
code for the S protein of the SARS-CoV-2 virus and employs the
non-replicating chimpanzee adenoviral vaccine vector, AZD1222.
Noteworthy is its suitability for vulnerable populations, such as
children, the elderly and individuals with pre-existing medical
conditions, as it necessitates only a single dose and triggers a
substantial immune response without causing illness (134). AstraZeneca and the University of
Oxford have conducted phase 1 and phase 2 studies on AZD1222,
demonstrating a promising safety profile and the successful
generation of neutralizing antibodies against SARS-CoV-2 (135,136).
Adenoviral vectors are still in the early stages of
development and have not yet been approved for use in the treatment
of infectious diseases in humans, even though they exhibit great
promise for COVID-19 vaccines. Concerns have been raised about
possible inflammatory responses, as reported in AstraZeneca
studies. Additionally, it is probable that individuals already have
some amount of resistance to adenoviral vectors owing to their
frequent exposure to them. While research and clinical trials
continue, the scientific community is dedicated to developing safe
and effective medicines to combat the COVID-19 pandemic and long
COVID.
Nucleic acid-based vaccines
DNA vaccines or mRNA vaccines promise to be more
effective than conventional immunizations. The direct
administration of DNA plasmids that encode particular target
antigens results in potent B- and T-cell responses with increased
safety (137). These vaccinations
are safe for those with impaired immune systems since they do not
include any infectious organisms. Synthetic DNA vaccines facilitate
the development process by enabling scalable manufacture, rapid
design and preclinical testing of several candidates, and simpler
regulatory approval for clinical use. Their stability at different
temperatures also guarantees a longer shelf life. Currently, a
gene-based vaccine is being developed that specifically targets the
S protein of SARS-CoV-2. The vaccine candidate from Inovio
Pharmaceuticals uses DNA-plasmid pGX9501, which was developed using
MERS-CoV vaccine constructions from the past. The vaccine is
currently in phase 2 clinical trials (NCT04447781; NCT04336410). It
is administered intradermally and then electroporated (138). Gene vaccines also use mRNA, which
operates in the cytoplasm without having to cross the nuclear
membrane, in addition to DNA. mRNA vaccines are less dose-intensive
than DNA vaccinations and produce strong immune system memory.
However, they are less stable due to their heat lability and
susceptibility to hydrolysis by circulating ribonucleases (139). This is addressed by the
formulation of mRNA vaccines as lipid nanoparticles, which improve
stability and host distribution. Examples include the
SARS-CoV-2-targeting drugs mRNA-1273 from Moderna and BNT162b1 from
Pfizer, both of which are in advanced clinical moderation (140). Despite the advancements, there
are still difficulties in the global production, distribution and
administration of COVID-19 vaccines.
Drugs approved for the treatment of
COVID-19
Ongoing extensive clinical trials are underway to
evaluate the potential effectiveness of several medications in the
treatment of patients with COVID-19. The selection of these
medications is based on the hypothesis that they may hinder the
virus from entering the host and replicating. Various compounds,
including some that have undergone human clinical trials, are
currently under assessment in clinical trials as potential COVID-19
treatments. Researchers are investigating the ability of
experimental drugs to impede the entry of the virus into the host
and subsequent replication. While certain medications have been
previously employed in treating SAR-CoV infections, others are
being utilized for the first time in the context of SARS-CoV-2
infections (Table II).
 | Table IICOVID-19 vaccinesa, their mechanisms of action,
advantages and disadvantages. |
Table II
COVID-19 vaccinesa, their mechanisms of action,
advantages and disadvantages.
| Form of
vaccine | Vaccine and
developer | Platform | Route of
administration |
Advantages/disadvantages and immune
action | Stage/trial
phase |
|---|
| Ribonucleic
acid-based vaccine | mRNA-1273,
Moderna/NIAIDb | mRNA encapsulated
in lipid nanoparticles | Intramuscular | Neutralizing
antibodies and responses CD4/8+ T-cell | Emergency use
authorization by the FDA |
| | BNT-162, BioNTech/
Fosun Pharma/Pfizer | Lipid nanoparticle
formulation encapsulated mRNA | | Neutralizing
antibody, and CD4/8+ T-cell activation | |
| | ARCoV, People's
Liberation Army, Academy of Military Sciences/Walvax
Biotechc | mRNA (expressing S
protein) | | Neutralizing
antibodies in primates | Phase 1
ChiCTR2000034112/ ChiCTR2000039212 |
| Deoxyribonucleic
acid-based vaccine | INO-4800, Inovio
Pharmaceuticals |
Electroporation-based Plasmid vaccine | Intradermal | High immune
response | Phase 1/2
NCT04447781; NCT04336410 |
| | ZyCoV-D/Zydus
Cadilac | DNA plasmid
(expressing S protein) | | Significant immune
responses in animal species | Phase 1/2
CTRI/2020/07/026352 |
| | COVID-19 Vaccine/
Takara Bio, Osaka University | DNA plasmid
(expressing S protein + adjuvant) | Intramuscular | Not applicable | Phase 1/2
NCT04463472; NCT04527081 |
| Non-replicating
viral vector | Ad5-nCoV, CanSino
Biological Incd. | Adenovirus serotype
5 expressing Spike protein | | Immunogenicity not
maintained | Phase 2/3
NCT04526990; NCT04540419 |
| | Ad26 Cov S1,
Janssen Pharmaceutical | Adenovirus serotype
26 expressing Spike protein | | Weakly
immunogenic. | Phase 3
NCT04505722; NCT04614948 |
| | Gam-COVID-Vac
Lyo/Gamaleya Research Institute | rAd26 + rAd5
expressing Spike protein | | Neutralizing and
antibody, and CD4/8+ T-cells | Phase 3
NCT04530396 |
| Replicating
virus | Institute
Pasteur/Themis/ Univ. of Pittsburgh Xiamen University | Measles vector
based | | Not applicable | Phase 1
NCT04497298 |
| Inactivated
virus |
PiCoVacc/Sinovac | whole virus
particles + adjuvant | | Neutralizing
antibodies | Phase 3
NCT04456595, 669/UN6. KEP/EC/2020, NCT04582344, NCT04617483 |
| | BBIBP-CorV
Sinopharm/ Wuhan Institute of Biological Products | Inactivated
SARS-CoV-2 | | High antibody
titers | Phase 3
ChiCTR2000034780, ChiCTR2000039000, NCT04612972 |
| | Institute of
Medical Biology, Chinese Academy of Science | Inactivated
SARS-CoV-2 | | Not applicable | Phase 1/2
NCT04470609 |
| Protein
subunit | Sanofi
Pasteur/GSK | S protein
(Baculovirus production) | | | Phase 1/2
NCT04473690 |
| | SCB-2019/Clover
Biopharmaceuticals In | Trimeric subunit
spike protein | | Neutralizing
antibodies in animal models | Phase 1/2
NCT04530357 |
| | Medigen Vaccine
Corporation/NIAID/ Dynavax | S-2P protein + CpG
1018 | | Not applicable | Phase 1
NCT04487210 |
Remdesivir, developed by Gilead Sciences, has
received FDA approval for the treatment of patients ≥12 of age with
COVID-19 requiring hospitalization. Remdesivir functions by
inhibiting the RNA-dependent RNA polymerase, disrupting its
interaction with the RNA of SARS-CoV-2 and thereby halting further
replication (141). In a previous
study, after receiving remdesivir intravenously, 36 out of 53
patients with COVID-19 exhibited an improvement, indicating
positive clinical outcomes (142). Although lopinavir and
ritonavir-based antiretroviral therapy have been investigated, it
has not been proven to be any more effective than standard care.
Umifenovir, which has been licensed for influenza prevention in
China and Russia, is used for the treatment of COVID-19 due to its
potential to inhibit the S protein/ACE2 interaction (143).
Research indicates that favipiravir, which inhibits
RNA polymerase and is approved for use against influenza in Japan,
leads to an improved clinical outcome in mild cases of COVID-19
than umifenovir (144). In
small-scale clinical trials conducted in China, chloroquine has
demonstrated potential in attenuating the progression of pneumonia
and viral replication in patients with COVID-19 (145,146). For patients with COVID-19, the
combined use of statins and ARBs holds promise for the prevention
of ARDS (147). Ongoing studies
are exploring the potential benefits of these combined treatments
in managing the severe consequences of the illness (Fig. 5). The strategy of employing
existing, approved drugs for COVID-19 treatment capitalizes on the
current pharmacopeia to swiftly address the urgent global health
crisis. This tactic comprises repurposing well-known
pharmaceuticals that were first authorized for a range of medical
conditions to target particular aspects of the SARS-CoV-2 virus or
the host immune system. To make the most of these medications
pharmacokinetically successful, it is important to understand the
mechanisms of action and to keep in mind the safety profiles of the
drug.
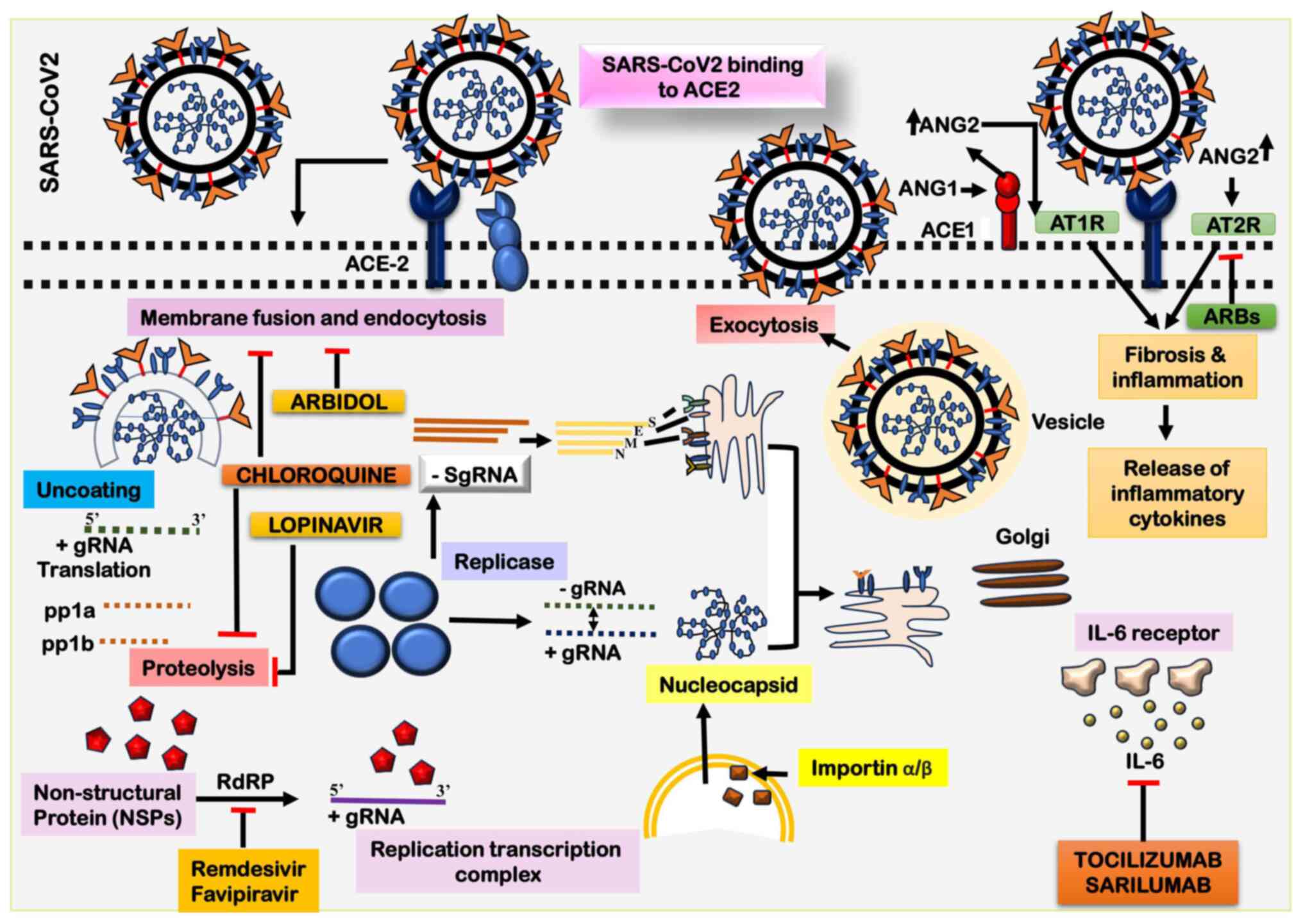 | Figure 5The illustration outlines the
potential steps involved in the entry and replication of
SARS-CoV-2. The sequence initiates with the conformational change
of the viral S protein, triggered by its binding to the cellular
ACE2 receptor. This interaction facilitates the fusion of the viral
envelope with the cell membrane through the endosome pathway. The
genomic RNA undergoes translation, leading to the synthesis of the
viral replicase polyproteins pp1a and 1ab. Viral proteases then
cleave these polyproteins, generating smaller functional products.
Following this, the viral polymerase transcribes irregularly,
resulting in the production of subgenomic mRNAs. These subgenomic
mRNAs, in turn, contribute to the translation of different viral
proteins. During the assembly phase, viral proteins and genomic RNA
combine to form virions within the endoplasmic reticulum (ER) and
Golgi apparatus. The ER-Golgi intermediate compartment (ERGIC)
plays a pivotal role in the maturation and transportation of
virions. Ultimately, assembled virions are encapsulated into
vesicles and released from the host cells. SARS-CoV-2, severe acute
respiratory syndrome coronavirus 2; ACE2, angiotensin-converting
enzyme 2; ARBs, angiotensin receptor blockers. |
Remdesivir, initially developed for Ebola, has been
repurposed as an antiviral for COVID-19. Its mechanism of action
involves inhibiting the viral RNA polymerase, thereby disrupting
viral replication (148).
Lopinavir/ritonavir, FDA-approved for the treatment of HIV, is
being explored for its ability to inhibit the 3CLpro enzyme in
SARS-CoV-2, disrupting viral replication (149). Agents with anti-inflammatory and
immunomodulatory properties, such as dexamethasone, a potent
corticosteroid with strong anti-inflammatory effects, are being
repurposed to alleviate the severe inflammatory responses observed
in patients critically ill with COVID-19, potentially reducing
mortality rates (150). The
anti-inflammatory characteristics of azithromycin, an antibiotic,
are currently under investigation for their ability to regulate the
immune system and mitigate inflammation in individuals with
COVID-19(151). Monoclonal
antibodies, designed to specifically target SARS-CoV-2, are
hypothesized to neutralize the virus, offering targeted therapeutic
intervention.
Convalescent plasma, derived from individuals who
have successfully recovered from COVID-19, contains antibodies that
may neutralize the virus in infected patients, thereby enhancing
the host's immune response (152). Antibiotics and antiparasitic
agents, including ivermectin, known for their well-established
safety profile, are undergoing examination for potential antiviral
effects against SARS-CoV-2. Additionally, azithromycin, an
antibiotic, is explored for its potential synergy with other
treatments in COVID-19 cases (153).
9. Challenges and future prospects
One major challenge is the ongoing emergence of new
SARS-CoV-2 variants. These variants may acquire increased
transmissibility, be resistant to immunity from previous infections
or vaccines, and may lead to more severe disease. Monitoring and
adapting to these variants will be an ongoing challenge. Ensuring
equitable and efficient distribution of COVID-19 vaccines in itself
remains one of the major challenges. Disparities in access to
vaccines can exacerbate the global health crisis and hinder efforts
to achieve herd immunity. Vaccine hesitancy and misinformation
continue to impede vaccination efforts. Promoting vaccine education
and addressing concerns is crucial to achieving widespread
vaccination and ending the pandemic. The long-term health effects
of COVID-19, also referred to as ‘long COVID’, are still difficult
to understand. Some individuals experience persistent symptoms and
complications long after recovering from the acute phase of the
disease (154,155). Healthcare systems in numerous
regions around the globe are still grappling with the strain of the
pandemic. Treating severe cases of COVID-19 can overwhelm hospitals
and lead to delays in providing care for other serious medical
conditions. The pandemic has caused severe economic and social
unrest, Global cooperation and coordination are necessary to combat
the pandemic effectively.
Research and the development of booster shots and
updated vaccines will likely continue to address emerging variants
and provide longer-lasting immunity. The development of effective
antiviral drugs to treat COVID-19 may improve outcomes for those
infected and reduce the severity of the disease. Achieving herd
immunity through vaccination remains a key goal for ending the
pandemic. Encouraging vaccination in underserved communities and
improving vaccine access are essential components of this effort
(156). The experience with
COVID-19 underscores the need for improved pandemic preparedness,
early warning systems, and global response mechanisms to mitigate
the impact of future infectious disease outbreaks. The pandemic has
accelerated the adoption of telemedicine and digital healthcare
solutions (157). These
innovations may continue to transform healthcare delivery and
improve access to medical care. Addressing the mental health
challenges arising from the pandemic will be a long-term prospect.
Investing in mental health services and support systems is crucial
for recovery. Promoting good hygiene habits and raising public
health awareness can be very effective in stopping the transmission
of contagious illnesses, such as COVID-19.
Numerous outbreaks of zoonotic origin have been
reported over the past century, most of which have presented with
severe illnesses and fatalities. One example is the aforementioned
SARS-CoV-2, the cause of COVID-19. Apart from the aforementioned
aspects, it is equally important to understand the preparedness
beyond COVID-19 and the implications of evolving zoonotic
spillovers. Vaccines are pivotal for reducing pandemic, and
post-pandemic consequences. The development of vaccines requires
in-depth research, large investments, and an integrated and
coordinated team of diverse scientists and clinicians. Viral
targets can be located and identified for their potential outbreaks
and candidates for vaccine design before any adverse event. Another
possibility is to lessen the emergence, by vaccinating wild
animals. This will reduce zoonotic spillovers, virus evolution and
amplified transmission. For example, the study by Keusch et
al (158) reported that
‘vaccinating poultry or swine for emerging animal influenza A
strains or camels for MERS-CoV in endemic countries’. In addition,
identifying and vaccinating potential hosts may reduce the threat
in endemic countries. Creating awareness among the masses,
clarifying misinformation, and providing recent research updates to
health workers at every level in the developed and developing world
will prepare them for COVID-19 and beyond (158).
Pandemic prevention can also be managed by reducing
the spillover risk by monitoring live markets, wildlife and
livestock trading. The expansion of these activities is associated
with the socio-economic status of the country. Some practices are a
part of traditional cultural events, and hence need to be tackled
mildly and judiciously. Still, these activities require strict
surveillance as they are the hotspots of infectious diseases. At
times trading activities link the geographical distributions,
further spreading the disease and connecting and increasing the
hotspots. The policy changes introduced should be evidence-based
and collaborative efforts and partnerships and should be
economically compatible and likely for the benefit of mankind
(159,160).
Socioeconomic status, public opinion, outreach
activities and communication strategies play a crucial role in
preventing and preparing for COVID-19, mainly due to political
polarization, variable public opinion and the associated risk
specifically in the low economic strata (161). The study by AlShurman et
al (162) reported that fake
news associated with exaggerated risk can increase susceptibility
to misinformation, and make vaccination less accepted among the
masses. Parents were reported to be hesitant to regarding the
vaccination of their children. Older individuals were also of the
belief that vaccination may enhance their morbid status. The same
study also mentioned that with proper counseling and knowledge,
individuals from different beliefs and low socioeconomic
distribution agreed to get vaccinated, although the number remained
lower than expected (162).
Along with the importance of identifying the
intersection between COVID-19 and socio-economic factors and taking
appropriate measures on them, it is equally important to spread
knowledge about the alternative methods or cutting-edge diagnostic
approaches that are being developed or currently in use. RT-PCR is
the primary method for detecting infection during the acute phase
of COVID-19. The WHO and the National Health Commission of China
have given the case definitions for COVID-19, which include a
positive serology test even when RT-PCR is negative. The RT-PCR
amplification is usually completed in a short timeframe; still the
protocol includes, extraction, running time, sample processing and
data management that in total requires up to 48 h. A large sample
testing area where samples are transported requires even more time.
RT-PCR is the gold standard for microbiological confirmation of
COVID-19. Some antigen-based rapid kits are arising, such as: i)
The novel Coronavirus (2019-nCov) Antigen Detection kit; ii)
CLMSRDL, Sichuan Mass Spectrometry Biotechnology Co., Ltd.; iii)
DIAQUICK COVID-19 Ag Cassette. These could provide rapid,
point-of-care antigen tests for diagnosis of SARS-CoV-2 infection
(163,164).
Infectious diseases are tackled differently,
employing different approaches depending on the geographical
distribution. To curb the infections some common legal measures are
adopted. These measures primarily focus on the view that the need
for protection is natural and necessary. Such interventions include
compulsory vaccination, screening, examination, isolation and
quarantines. Some studies and experts present a view that making
vaccination and other measures obligatory appears to violate the
patient's rights. Certain researchers are of the view, specifically
in the context of COVID-19, that enforcing vaccination is unethical
and does not take into consideration the ethical and religious
beliefs of patients. Individuals resist undergoing vaccination due
to diverse reasons, such as misinformation circulated by mass
media, a lack of communication between health providers and the
community, and individuals not being clear about the pros and cons
of vaccination protocols. The vaccine is the only hope for this
lethal infectious disease; however, it continues to face a number
of ethical challenges. The study by Jalilian et al (165) and others have reported that the
ethical considerations of COVID-19 fall under five categories as
follows: i) Autonomy and accountability; ii) the supply of vaccines
specifically to socio-economically weaker places; iii)
post-vaccination safety issues; iv) the use of standard vaccine; v)
fairness of reporting any adverse issues (164-166).
In principle, the equitable distribution of vaccines
may not be possible due to controlled approval and limited
production. Requirements, population density, policies and the
capacity of low/middle-income countries vary compared to the
high-income countries. To overcome these hurdles, COVAX prioritized
the vaccine-receiving population and included healthcare workers
and the elderly in vulnerable groups (167).
10. Conclusion and limitations
In conclusion, COVID-19 presents a range of
challenges; however, there is also hope for the future. Effective
vaccination, treatments, global cooperation and preparedness
efforts can contribute to bringing the pandemic under control and
better preparing the world to respond to future health crises. The
present comprehensive review provides an in-depth and enlightening
examination of the COVID-19. Millions of individuals have been
affected by the SARS-CoV-2 pandemic, which has created previously
unheard-of challenges for global health. The present review
elaborates on several aspects of the illness, starting with its
zoonotic origin and moving on to person-to-person transmission and
a map of its geographic distribution across continents. An key
subject included herein is the clinical manifestations COVID-19,
which may vary from modest respiratory symptoms to severe
instances, leading to pneumonia, ARDS and multi-organ failure. By
discussing the impact of the disease on different age groups and
vulnerable populations, the present review highlights the need for
specialist healthcare strategies to protect those who are most
susceptible. The present review also discusses several COVID-19
diagnostic methods, including molecular tests, such as PCR and
antigen assays, as well as serological testing for detecting
antibodies. These tests are necessary for controlling illnesses,
tracking down contacts and establishing containment procedures.
Effective therapies mainly include repurposed drugs, immunotherapy,
cellular therapy, antiviral and other drugs. The development and
administration of vaccines are observed as crucial strategies for
halting the pandemic and promoting herd immunity. The present
review acknowledges that significant advancements have beebn made
in the comprehension and management of COVID-19; however,
challenges and hurdles remain for the global healthcare systems.
The challenges include handling viral alterations understanding
novel varieties, combating false information and vaccination
resistance and preparing for impending outbreaks. The present
review provides a basis for further research and information for
public health activities, and scientists working at the molecular
level, helping to decrease the consequences of the epidemic and
prepare for any future health crises.
In the era of long-COVID-19 presented by the silent
persistence of the COVID-19 pandemic, there were a large number of
studies or research in progress or in the press that could not be
included or mentioned in the present review. However, the authors
tried to minimize this limitation by including studies close to the
discussed topic. Another limitation of the present study is that
there appears to be no standardized fixed formula to cover or
minimize the risk factors. Every government should accordingly make
judicious decisions for the benefit of mankind. The policies
adopted could not be discussed herein, as they may be
circumstantial. To prevent failures in policy making and
implementation, a collaborative effort among the stakeholders is
necessary. The COVID-19 crisis and mandatory vaccination have
become controversial.
Acknowledgements
The authors gratefully acknowledge the
infrastructure facilities provided by the Department of
Biochemistry, Jawaharlal Nehru Medical College, under the DST (FIST
& PURSE) program.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MM was involved in the conceptualization of the
study, in the curation and investigation of data from the
literature, as well as in the writing and preparation of the
original draft of the manuscript. KA and RA were involved in the
study design, and in the writing and editing of the manuscript. RA
was involved in the curation and manuscript preparation. WA was
involved in study supervision, and in the curation and
investigation of data from the literature. SI was involved in the
writing, review and editing of the manuscript. IQT was involved in
writing, reviewing and in providing literature resources. M was
involved in study supervision, in the conceptualization of the
study, and in the writing, review and editing of the manuscript.
MIH was involved in, study supervision and designing and image
preparation. MA, NU and SH were involved in the conceptualization
of the study, provision of literature and software resources, study
supervision, and in the writing, review and editing of the
manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheval S, Adamescu CM, Georgiadis T,
Herrnegger M, Piticar A and Legates DR: Observed and potential
impacts of the COVID-19 pandemic on the environment. Int J Environ
Res Public Health. 17(4140)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Woolhouse ME, Adair K and Brierley L: RNA
viruses: A case study of the biology of emerging infectious
diseases. Microbiol Spectr.
1(10.1128/microbiolspec.OH-0001-2012)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Markov PV, Ghafari M, Beer M, Lythgoe K,
Simmonds P, Stilianakis NI and Katzourakis A: The evolution of
SARS-CoV-2. Nat Rev Microbiol. 21:361–379. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chakraborty C, Chatterjee S, Bhattacharya
M, Chopra H, Bhattacharya P, Islam MA and Dhama K: The D614G
mutation helps to increase the transmissibility and reduce the
virulence of SARS-CoV-2 variants through natural selection. Int J
Surg. 109:171–174. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lythgoe KA, Hall M, Ferretti L, de Cesare
M, MacIntyre-Cockett G, Trebes A, Andersson M, Otecko N, Wise EL,
Moore N, et al: SARS-CoV-2 within-host diversity and transmission.
Science. 372(eabg0821)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wacharapluesadee S, Tan CW, Maneeorn P,
Duengkae P, Zhu F, Joyjinda Y, Kaewpom T, Chia WN, Ampoot W, Lim
BL, et al: Evidence for SARS-CoV-2 related coronaviruses
circulating in bats and pangolins in Southeast Asia. Nat Commun.
12(972)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
She J, Jiang J, Ye L, Hu L, Bai C and Song
Y: 2019 novel coronavirus of pneumonia in Wuhan, China: Emerging
attack and management strategies. Clin Transl Med.
9(19)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hu B, Guo H, Zhou P and Shi ZL:
Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol.
19:141–154. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Worobey M, Levy JI, Malpica Serrano L,
Crits-Christoph A, Pekar JE, Goldstein SA, Rasmussen AL, Kraemer
MUG, Newman C, Koopmans MPG, et al: The huanan seafood wholesale
market in Wuhan was the early epicenter of the COVID-19 pandemic.
Science. 377:951–959. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reis J, Le Faou A, Buguet A, Sandner G and
Spencer P: Covid-19: Early cases and disease spread. Ann Glob
Health. 88(83)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gralinski LE and Menachery VD: Return of
the coronavirus: 2019-nCoV. Viruses. 12(135)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eurosurveillance Editorial Team. Note from
the editors: World Health Organization declares novel coronavirus
(2019-nCoV) sixth public health emergency of international concern.
Euro Surveill. 25(200131e)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Coronaviridae Study Group of the
International Committee on Taxonomy of Viruses. The species severe
acute respiratory syndrome-related coronavirus: Classifying
2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 5:536–544.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zanin M, Xiao C, Liang T, Ling S, Zhao F,
Huang Z, Lin F, Lin X, Jiang Z and Wong SS: The public health
response to the COVID-19 outbreak in mainland China: A narrative
review. J Thorac Dis. 12:4434–4449. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Deng SQ and Peng HJ: Characteristics of
and public health responses to the coronavirus disease 2019
outbreak in China. J Clin Med. 9(575)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
World Health Organization: Coronavirus
disease 2019 (COVID-19). Situation report-51, 2020. Available at:
https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10.
|
|
18
|
Deslandes A, Berti V, Tandjaoui-Lambotte
Y, Alloui C, Carbonnelle E, Zahar JR, Brichler S and Cohen Y:
SARS-CoV-2 was already spreading in France in late December 2019.
Int J Antimicrob Agents. 55(106006)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pal M, Berhanu G, Desalegn C and Kandi V:
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): An
update. Cureus. 12(e7423)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Van Doremalen N, Bushmaker T, Morris DH,
Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL,
Thornburg NJ, Gerber SI, et al: Aerosol and surface stability of
SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med.
382:1564–1567. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aboubakr HA, Sharafeldin TA and Goyal SM:
Stability of SARS-CoV-2 and other coronaviruses in the environment
and on common touch surfaces and the influence of climatic
conditions: A review. Transbound Emerg Dis. 68:296–312.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kasloff SB, Leung A, Strong JE, Funk D and
Cutts T: Stability of SARS-CoV-2 on critical personal protective
equipment. Sci Rep. 11(984)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng
Q, Meredith HR, Azman AS, Reich NG and Lessler J: The incubation
period of coronavirus disease 2019 (COVID-19) from publicly
reported confirmed cases: Estimation and application. Ann Intern
Med. 172:577–582. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu Y, Kang L, Guo Z, Liu J, Liu M and
Liang W: Incubation period of COVID-19 caused by unique SARS-CoV-2
strains: A systematic review and meta-analysis. JAMA Netw Open.
5(e2228008)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang SW and Wang SF: SARS-CoV-2 entry
related viral and host genetic variations: Implications on COVID-19
severity, immune escape, and infectivity. Int J Mol Sci.
22(3060)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Men K, Li Y, Wang X, Zhang G, Hu J, Gao Y,
Han A, Liu W and Han H: Estimate the incubation period of
coronavirus 2019 (COVID-19). Comput Biol Med.
158(106794)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Batra A, Clark JR, Kang AK, Ali S, Patel
TR, Shlobin NA, Hoffman SC, Lim PH, Orban ZS, Visvabharathy L, et
al: Persistent viral RNA shedding of SARS-CoV-2 is associated with
delirium incidence and six-month mortality in hospitalized COVID-19
patients. Geroscience. 44:1241–1254. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Boni MF, Lemey P, Jiang X, Lam TT, Perry
BW, Castoe TA, Rambaut A and Robertson DL: Evolutionary origins of
the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19
pandemic. Nat Microbiol. 5:1408–1417. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song
ZG, Hu Y, Tao ZW, Tian JH, Pei YY, et al: A new coronavirus
associated with human respiratory disease in China. Nature.
579:265–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He B, Zhang Y, Xu L, Yang W, Yang F, Feng
Y, Xia L, Zhou J, Zhen W, Feng Y, et al: Identification of diverse
alphacoronaviruses and genomic characterization of a novel severe
acute respiratory syndrome-like coronavirus from bats in China. J
Virol. 88:7070–7082. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zheng J: SARS-CoV-2: An emerging
coronavirus that causes a global threat. Int J Biol Sci.
16:1678–1685. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Temmam S, Vongphayloth K, Baquero E,
Munier S, Bonomi M, Regnault B, Douangboubpha B, Karami Y, Chrétien
D, Sanamxay D, et al: Author Correction: Bat coronaviruses related
to SARS-CoV-2 and infectious for human cells. Nature.
607(E19)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou H, Chen X, Hu T, Li J, Song H, Liu Y,
Wang P, Liu D, Yang J, Holmes EC, et al: A novel bat coronavirus
closely related to SARS-CoV-2 contains natural insertions at the
S1/S2 cleavage site of the spike protein. Curr Biol.
30:2196–2203.e3. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou H, Ji J, Chen X, Bi Y, Li J, Wang Q,
Hu T, Song H, Zhao R, Chen Y, et al: Identification of novel bat
coronaviruses sheds light on the evolutionary origins of SARS-CoV-2
and related viruses. Cell. 184:4380–4391. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S,
Zhang Q, Shi X, Wang Q, Zhang L and Wang X: Structure of the
SARS-CoV-2 spike receptor-binding domain bound to the ACE2
receptor. Nature. 581:215–220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang H, Li X, Li T, Zhang S, Wang L, Wu X
and Liu J: The genetic sequence, origin, and diagnosis of
SARS-CoV-2. Eur J Clin Microbiol Infect Dis. 39:1629–1635.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nie J, Li Q, Zhang L, Cao Y, Zhang Y, Li
T, Wu J, Liu S, Zhang M, Zhao C, et al: Functional comparison of
SARS-CoV-2 with closely related pangolin and bat coronaviruses.
Cell Discov. 7(21)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yi C, Sun X, Ye J, Ding L, Liu M, Yang Z,
Lu X, Zhang Y, Ma L, Gu W, et al: Key residues of the receptor
binding motif in the spike protein of SARS-CoV-2 that interact with
ACE2 and neutralizing antibodies. Cell Mol Immunol. 17:621–630.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang S, Qiao S, Yu J, Zeng J, Shan S,
Tian L, Lan J, Zhang L and Wang X: Bat and pangolin coronavirus
spike glycoprotein structures provide insights into SARS-CoV-2
evolution. Nat Commun. 12(1607)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sharun K, Dhama K, Pawde AM, Gortázar C,
Tiwari R, Bonilla-Aldana DK, Rodriguez-Morales AJ, de la Fuente J,
Michalak I and Attia YA: SARS-CoV-2 in animals: Potential for
unknown reservoir hosts and public health implications. Vet Q.
41:181–201. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dhama K, Khan S, Tiwari R, Sircar S, Bhat
S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK and
Rodriguez-Morales AJ: Coronavirus disease 2019-COVID-19. Clin
Microbiol Rev. 33:e00028–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
De Wit E, Van Doremalen N, Falzarano D and
Munster VJ: SARS and MERS: Recent insights into emerging
coronaviruses. Nat Rev Microbiol. 14:523–534. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cui J, Li F and Shi ZL: Origin and
evolution of pathogenic coronaviruses. Nat Rev Microbiol.
17:181–192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wan Y, Shang J, Graham R, Baric RS and Li
F: Receptor recognition by the novel coronavirus from Wuhan: An
analysis based on decade-long structural studies of SARS
coronavirus. J Virol. 94:e00127–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Banerjee A, Doxey AC, Mossman K and Irving
AT: Unraveling the zoonotic origin and transmission of SARS-CoV-2.
Trends Ecol Evol. 36:180–184. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Focosi D and Maggi F: Recombination in
coronaviruses, with a focus on SARS-CoV-2. Viruses.
14(1239)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shi J, Wen Z, Zhong G, Yang H, Wang C,
Huang B, Liu R, He X, Shuai L, Sun Z, et al: Susceptibility of
ferrets, cats, dogs, and other domesticated animals to
SARS-coronavirus 2. Science. 368:1016–1020. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Oreshkova N, Molenaar RJ, Vreman S,
Harders F, Oude Munnink BB, Hakze-van der Honing RW, Gerhards N,
Tolsma P, Bouwstra R, Sikkema RS, et al: SARS-CoV-2 infection in
farmed minks, the Netherlands, April and May 2020. Euro Surveill.
25(2001005)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sit TH, Brackman CJ, Ip SM, Tam KWS, Law
PYT, To EMW, Yu VYT, Sims LD, Tsang DNC, Chu DKW, et al: Canine
SARS-CoV-2 infection. Nature. 586:776–778. 2020.
|
|
50
|
Zhang Q, Zhang H, Gao J, Huang K, Yang Y,
Hui X, He X, Li C, Gong W, Zhang Y, et al: A serological survey of
SARS-CoV-2 in cats in Wuhan. Emerg Microbes Infect. 9:2013–2019.
2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fehr AR and Perlman S: Coronaviruses: An
overview of their replication and pathogenesis. Methods Mol Biol.
1282:1–23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mohan SV, Hemalatha M, Kopperi H, Ranjith
I and Kumar AK: SARS-CoV-2 in environmental perspective:
Occurrence, persistence, surveillance, inactivation and challenges.
Chem Eng J. 405(126893)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Liu DX, Liang JQ and Fung TS: Human
coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae).
Encyclopedia Virol. (428)2021.
|
|
54
|
Wen C, Sun L, Zhao MC, Duan SX, Wang L and
Cui XW: Clinical study of human coronavirus NL63, OC43, 229E, HKU1
infections in hospitalized children from 2015 to 2020. Infect Drug
Resist. 15:1093–1101. 2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Rastogi M, Pandey N, Shukla A and Singh
SK: SARS coronavirus 2: From genome to infectome. Respir Res.
21:318. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhu Z, Lian X, Su X, Wu W, Marraro GA and
Zeng Y: From SARS and MERS to COVID-19: A brief summary and
comparison of severe acute respiratory infections caused by three
highly pathogenic human coronaviruses. Respir Res.
21(224)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bourgonje AR, Abdulle AE, Timens W,
Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors
AA, Osterhaus AD, et al: Angiotensin-converting enzyme 2 (ACE2),
SARS-CoV-2 and the pathophysiology of coronavirus disease 2019
(COVID-19). J Pathol. 251:228–248. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Gheblawi M, Wang K, Viveiros A, Nguyen Q,
Zhong JC, Turner AJ, Raizada MK, Grant MB and Oudit GY:
Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator
of the renin-angiotensin system: Celebrating the 20th anniversary
of the discovery of ACE2. Circ Res. 126:1456–1474. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Petrosillo N, Viceconte G, Ergonul O,
Ippolito G and Petersen E: COVID-19, SARS and MERS: Are they
closely related? Clin Microbiol Infect. 26:729–734. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Xiao J, Fang M, Chen Q and He B: SARS,
MERS and COVID-19 among healthcare workers: A narrative review. J
Infect Public Health. 13:843–848. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bharati S, Podder P, Mondal MR, Podder P
and Kose U: A review on epidemiology, genomic characteristics,
spread, and treatments of COVID-19. Data Sci COVID-19. 487–505.
2022.
|
|
62
|
Harvey WT, Carabelli AM, Jackson B, Gupta
RK, Thomson EC, Harrison EM, Ludden C, Reeve R and Rambaut A:
COVID-19 Genomics UK (COG-UK) Consortium et al. SARS-CoV-2
variants, spike mutations and immune escape. Nat Rev Microbiol.
19:409–424. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Marjanovic S, Romanelli RJ, Ali GC, Leach
B, Bonsu M, Rodriguez-Rincon D and Ling T: COVID-19 Genomics UK
(COG-UK) Consortium: Final Report. Rand Health Q.
9(24)2022.PubMed/NCBI
|
|
64
|
Khetran SR and Mustafa R: Mutations of
SARS-CoV-2 structural proteins in the alpha, beta, gamma, and delta
variants: Bioinformatics analysis. JMIR Bioinform Biotechnol.
4(e43906)2023.PubMed/NCBI View
Article : Google Scholar
|
|
65
|
European Centre for Disease Prevention and
Control: Threat Assessment Brief: Rapid increase of a SARS-CoV-2
variant with multiple spike protein mutations observed in the
United Kingdom. https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf.
|
|
66
|
Mahmoud IS, Jarrar YB, Alshaer W and
Ismail S: SARS-CoV-2 entry in host cells-multiple targets for
treatment and prevention. Biochimie. 175:93–98. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Xie Y, Karki CB, Du D, Li H, Wang J,
Sobitan A, Teng S, Tang Q and Li L: Spike proteins of SARS-CoV and
SARS-CoV-2 utilize different mechanisms to bind with human ACE2.
Front Mol Biosci. 7(591873)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Zamorano Cuervo N and Grandvaux N: ACE2:
Evidence of role as entry receptor for SARS-CoV-2 and implications
in comorbidities. Elife. 9(e61390)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wu J, Deng W, Li S and Yang X: Advances in
research on ACE2 as a receptor for 2019-CoV. Cell Mol Life Sci.
78:531–544. 2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Alipoor SD and Mirsaeidi M: SARS-CoV-2
cell entry beyond the ACE2 receptor. Mol Biol Rep. 49:10715–10727.
2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Guo H, Li A, Dong TY, Si HR, Hu B, Li B,
Zhu Y, Shi ZL and Letko M: Isolation of ACE2-dependent and
-independent sarbecoviruses from Chinese horseshoe bats. J Virol.
97(e0039523)2023.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shereen MA, Khan S, Kazmi A, Bashir N and
Siddique R: COVID-19 infection: Emergence, transmission, and
characteristics of human coronaviruses. J Adv Res. 24:91–98.
2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Najafi N, Davoudi A, Izadyar H, Alishahi
A, Mokhtariani A, Soleimanpourian B, Tabarrayi M, Moosazadeh M,
Daftarian Z and Ahangarkani F: The effect of ACE inhibitors and
ARBs on outcomes in hospitalized patients with COVID-19. Ir J Med
Sci. 192:1517–1523. 2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Liu K, Tan S, Niu S, Wang J, Wu L, Sun H,
Zhang Y, Pan X, Qu X, Du P, et al: Cross-species recognition of
SARS-CoV-2 to bat ACE2. Proc Natl Acad Sci USA.
118(e2020216118)2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Wettstein L, Kirchhoff F and Münch J: The
Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19
Treatment. Int J Mol Sci. 23(1351)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Guang C, Phillips RD, Jiang B and Milani
F: Three key proteases-angiotensin-I-converting enzyme (ACE), ACE2
and renin-within and beyond the renin-angiotensin system. Arch
Cardiovasc Dis. 105:373–385. 2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kucukoglu K, Faydalı N and Bul D: What are
the drugs having potential against COVID-19? Med Chem Res.
29:1935–1955. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Lee JS, Goldstein JM, Moon JL, Herzegh O,
Bagarozzi DA Jr, Oberste MS, Hughes H, Bedi K, Gerard D, Cameron B,
et al: Analysis of the initial lot of the CDC 2019-novel
coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. PLoS
One. 16(e0260487)2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sheikhzadeh E, Eissa S, Ismail A and
Zourob M: Diagnostic techniques for COVID-19 and new developments.
Talanta. 220(121392)2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Udugama B, Kadhiresan P, Kozlowski HN,
Malekjahani A, Osborne M, Li VYC, Chen H, Mubareka S, Gubbay JB and
Chan WCW: Diagnosing COVID-19: The disease and tools for detection.
ACS Nano. 14:3822–3535. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Xie X, Zhong Z, Zhao W, Zheng C, Wang F
and Liu J: Chest CT for typical coronavirus disease 2019 (COVID-19)
pneumonia: Relationship to negative RT-PCR testing. Radiology.
296:E41–E45. 2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Kanne JP: Chest CT findings in 2019 novel
coronavirus (2019-nCoV) infections from Wuhan, China: Key points
for the radiologist. Radiology. 295:16–17. 2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Machado BA, Hodel KV, Barbosa-Junior VG,
Soares MB and Badaro R: The main molecular and serological methods
for diagnosing COVID-19: An overview based on the literature.
Viruses. 13(40)2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Liu YX, Zhou YH, Jiang CH, Liu J and Chen
DQ: Prevention, treatment and potential mechanism of herbal
medicine for Corona viruses: A review. Bioengineered. 13:5480–5508.
2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Shuster A, Pechalrieu D, Jackson CB, Abegg
D, Choe H and Adibekian A: Clinical antiviral drug arbidol inhibits
infection by SARS-CoV-2 and variants through direct binding to the
spike protein. ACS Chem Biol. 16:2845–2851. 2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Kang CK, Seong MW, Choi SJ, Kim TS, Choe
PG, Song SH, Kim NJ, Park WB and Oh MD: In vitro activity of
lopinavir/ritonavir and hydroxychloroquine against severe acute
respiratory syndrome coronavirus 2 at concentrations achievable by
usual doses. Korean J Intern Med. 35(728)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
RECOVERY Collaborative Group.
Lopinavir-ritonavir in patients admitted to hospital with COVID-19
(RECOVERY): A randomized, controlled, open-label, platform trial.
Lancet. 396:1345–1352. 2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Nutho B, Mahalapbutr P, Hengphasatporn K,
Pattaranggoon NC, Simanon N, Shigeta Y, Hannongbua S and
Rungrotmongkol T: Why are lopinavir and ritonavir effective against
the newly emerged coronavirus 2019? Atomistic insights into the
inhibitory mechanisms. Biochemistry. 59:1769–1779. 2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Lian N, Xie H, Lin S, Huang J, Zhao J and
Lin Q: Umifenovir treatment is not associated with improved
outcomes in patients with coronavirus disease 2019: A retrospective
study. Clin Microbiol Infect. 26:917–921. 2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y,
Mo X, Wang J, Wang Y, Peng P, et al: Efficacy and safety of
lopinavir/ritonavir or arbidol in adult patients with mild/moderate
COVID-19: An exploratory randomized controlled trial. Med.
1:105–113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Djomkam ALZ, Olwal CO, Sala TB and Paemka
L: Commentary: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Front
Oncol. 10(1448)2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Li K, Meyerholz DK, Bartlett JA and McCray
PB Jr: The TMPRSS2 inhibitor nafamostat reduces SARS-CoV-2
pulmonary infection in mouse models of COVID-19. mBio.
12(e0097021)2021.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Chitsike L and Duerksen-Hughes P: Keep
out! SARS-CoV-2 entry inhibitors: Their role and utility as
COVID-19 therapeutics. Virol J. 18(154)2021.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Hoffmann M, Hofmann-Winkler H, Smith JC,
Krüger N, Arora P, Sørensen LK, Søgaard OS, Hasselstrøm JB, Winkler
M, Hempel T, et al: Camostat mesylate inhibits SARS-CoV-2
activation by TMPRSS2-related proteases and its metabolite GBPA
exerts antiviral activity. EBioMedicine. 65(103255)2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Tang W, Cao Z, Han M, Wang Z, Chen J, Sun
W, Wu Y, Xiao W, Liu S, Chen E, et al: Hydroxychloroquine in
patients with mainly mild to moderate coronavirus disease 2019:
Open label, randomised controlled trial. BMJ.
369(m1849)2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE,
et al: Hydroxychloroquine and azithromycin as a treatment of
COVID-19: Results of an open-label non-randomized clinical trial.
Int J Antimicrob Agents. 56(105949)2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Manivannan E, Karthikeyan C, Moorthy NSHN
and Chaturvedi SC: The rise and fall of
Chloroquine/Hydroxychloroquine as compassionate therapy of
COVID-19. Front Pharmacol. 12(584940)2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kumar S, Dutta D, Ravichandiran V and
Sukla S: Monoclonal antibodies: A remedial approach to prevent
SARS-CoV-2 infection. 3 Biotech. 12(227)2022.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Javalkote VS, Kancharla N, Bhadra B,
Shukla M, Soni B, Sapre A, Goodin M, Bandyopadhyay A and Dasgupta
S: CRISPR-based assays for rapid detection of SARS-CoV-2. Methods.
203:594–603. 2022.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Joung J, Ladha A, Saito M, Segel M,
Bruneau R, Huang MW, Kim NG, Yu X, Li J, Walker BD, et al:
Point-of-care testing for COVID-19 using SHERLOCK diagnostics.
medRxiv [Preprint]: May 8, 2020 doi:
10.1101/2020.05.04.20091231.
|
|
102
|
Yang SM, Lv S, Zhang W and Cui Y:
Microfluidic point-of-care (POC) devices in early diagnosis: A
review of opportunities and challenges. Sensors (Basel).
22(1620)2022.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Rizvi ZA, Babele P, Madan U, Sadhu S,
Tripathy MR, Goswami S, Mani S, Dikshit M and Awasthi A:
Pharmacological potential of Withania somnifera (L.) Dunal
and Tinospora cordifolia (Willd.) Miers on the experimental
models of COVID-19, T cell differentiation, and neutrophil
functions. Front Immunol. 14(1138215)2023.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Amponsah SK, Tagoe B, Adams I and Bugyei
KA: Efficacy and safety profile of corticosteroids and
non-steroidal anti-inflammatory drugs in COVID-19 management: A
narrative review. Front Pharmacol. 13(1063246)2022.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Falk JA, Minai OA and Mosenifar Z: Inhaled
and systemic corticosteroids in chronic obstructive pulmonary
disease. Proc Am Thorac Soc. 5:506–512. 2008.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Kushner P, McCarberg BH, Grange L, Kolosov
A, Haveric AL, Zucal V, Petruschke R and Bissonnette S: The use of
non-steroidal anti-inflammatory drugs (NSAIDs) in COVID-19. NPJ
Prim Care Respir Med. 32(35)2022.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Robb CT, Goepp M, Rossi AG and Yao C:
Non-steroidal anti-inflammatory drugs, prostaglandins, and
COVID-19. Br J Pharmacol. 177:4899–4920. 2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Sokolowska M, Rovati GE, Diamant Z,
Untersmayr E, Schwarze J, Lukasik Z, Sava F, Angelina A, Palomares
O, Akdis CA, et al: Effects of non-steroidal anti-inflammatory
drugs and other eicosanoid pathway modifiers on antiviral and
allergic responses: EAACI task force on eicosanoids consensus
report in times of COVID-19. Allergy. 77:2337–2354. 2022.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Kanchanasurakit S, Arsu A, Siriplabpla W,
Duangjai A and Saokaew S: Acetaminophen use and risk of renal
impairment: A systematic review and meta-analysis. Kidney Res Clin
Pract. 39(81)2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
De Spiegeleer A, Bronselaer A, Teo JT,
Byttebier G, De Tré G, Belmans L, Dobson R, Wynendaele E, Van De
Wiele C, Vandaele F, et al: The effects of ARBs, ACEis, and statins
on clinical outcomes of COVID-19 infection among nursing home
residents. J Am Med Dir Assoc. 21:909–914.e2. 2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Geng S, Mei Q, Zhu C, Yang T, Yang Y, Fang
X and Pan A: High flow nasal cannula is a good treatment option for
COVID-19. Heart Lung. 49:444–445. 2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Lockhart SL, Duggan LV, Wax RS, Saad S and
Grocott HP: Personal protective equipment (PPE) for both
anesthesiologists and other airway managers: Principles and
practice during the COVID-19 pandemic. Can J Anaesth. 67:1005–1015.
2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Gilardi KVK and Mazet JAK: The United
States Agency for international development emerging pandemic
threats PREDICT project-global detection of emerging wildlife viral
zoonoses. Fowler's Zoo Wild Animal Med Curr Ther. 9:110–116.
2019.
|
|
114
|
Chakraborty C, Sharma AR, Bhattacharya M,
Agoramoorthy G and Lee SS: The drug repurposing for COVID-19
clinical trials provide very effective therapeutic combinations:
Lessons learned from major clinical studies. Front Pharmacol.
12(704205)2021.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Okuyama R: mRNA and adenoviral vector
vaccine platforms utilized in COVID-19 vaccines: Technologies,
ecosystem, and future directions. Vaccines (Basel).
11(1737)2023.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Wang N, Chen M and Wang T: Liposomes used
as a vaccine Adjuvant-delivery system: From basics to clinical
immunization. J Control Release. 303:130–150. 2019.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Tretiakova DS and Vodovozova EL: Liposomes
as adjuvants and vaccine delivery systems. Biochem (Mosc) Suppl Ser
A Membr Cell Biol. 16:1–20. 2022.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Petkar KC, Patil SM, Chavhan SS, Kaneko K,
Sawant KK, Kunda NK and Saleem IY: An overview of Nanocarrier-based
adjuvants for vaccine delivery. Pharmaceutics.
13(455)2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Karunakaran B, Gupta R, Patel P, Salave S,
Sharma A, Desai D, Benival D and Kommineni N: Emerging trends in
lipid-based vaccine delivery: A special focus on developmental
strategies, fabrication methods, and applications. Vaccines
(Basel). 11(661)2023.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Ali H, Akbar M, Iqbal B, Ali F, Sharma NK,
Kumar N, Najmi A, Albratty M, Alhazmi HA, Madkhali OA, et al:
Virosome: An engineered virus for vaccine delivery. Saudi Pharm J.
31:752–764. 2023.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Nakanishi T, Hayashi A, Kunisawa J,
Tsutsumi Y, Tanaka K, Yashiro-Ohtani Y, Nakanishi M, Fujiwara H,
Hamaoka T and Mayumi T: Fusogenic liposomes efficiently deliver
exogenous antigen through the cytoplasm into the MHC class I
processing pathway. Eur J Immunol. 30:1740–1747. 2000.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Marchese AM, Zhou X, Kinol J, Underwood E,
Woo W, McGarry A, Beyhaghi H, Áñez G, Toback S and Dunkle LM:
NVX-CoV2373 vaccine efficacy against hospitalization: A post hoc
analysis of the PREVENT-19 phase 3, randomized, placebo-controlled
trial. Vaccine. 41:3461–3466. 2023.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Frederiksen LSF, Zhang Y, Foged C and
Thakur A: The long road toward COVID-19 herd immunity: Vaccine
platform technologies and mass immunization strategies. Front
Immunol. 11(1817)2020.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Sanders B, Koldijk M and Schuitemaker H:
Inactivated viral vaccines. Vaccine Analysis: Strategies Principles
Control: 45-80, 2014 doi: 10.1007/978-3-662-45024-6_2.
|
|
125
|
Wang C, Chen LY, Lu QB and Cui F:
Vaccination with the inactivated vaccine (Sinopharm BBIBP-CorV)
ensures protection against SARS-CoV-2 related disease. Vaccines
(Basel). 10(920)2022.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Hu L, Sun J, Wang Y, Tan D, Cao Z, Gao L,
Guan Y, Jia X and Mao J: A review of inactivated COVID-19 vaccine
development in China: Focusing on safety and efficacy in special
populations. Vaccines (Basel). 11(1045)2023.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Soysal A, Gönüllü E, Karabayır N, Alan S,
Atıcı S, Yıldız İ, Engin H, Çivilibal M and Karaböcüoğlu M:
Comparison of immunogenicity and reactogenicity of inactivated
SARS-CoV-2 vaccine (CoronaVac) in previously SARS-CoV-2 infected
and uninfected health care workers. Hum Vaccin Immunother.
17:3876–3880. 2021.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Pagliusi S, Jarrett S, Hayman B, Kreysa U,
Prasad SD, Reers M, Hong Thai P, Wu K, Zhang YT, Baek YO, et al:
Emerging manufacturers engagements in the COVID-19 vaccine
research, development and supply. Vaccine. 38:5418–5423.
2020.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Thanh Le T, Andreadakis Z, Kumar A, Gómez
Román R, Tollefsen S, Saville M and Mayhew S: The COVID-19 vaccine
development landscape. Nat Rev Drug Discov. 19:305–306.
2020.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Chaplin DD: Overview of the immune
response. J Allergy Clin Immunol. 125 (2 Suppl 2):S3–S23.
2010.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Chavda VP, Bezbaruah R, Valu D, Patel B,
Kumar A, Prasad S, Kakoti BB, Kaushik A and Jesawadawala M:
Adenoviral vector-based vaccine platform for COVID-19: Current
status. Vaccines (Basel). 11(432)2023.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Elkashif A, Alhashimi M, Sayedahmed EE,
Sambhara S and Mittal SK: Adenoviral vector-based platforms for
developing effective vaccines to combat respiratory viral
infections. Clin Transl Immunology. 10(e1345)2021.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Zhu FC, Guan XH, Li YH, Huang JY, Jiang T,
Hou LH, Li JX, Yang BF, Wang L, Wang WJ, et al: Immunogenicity and
safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine
in healthy adults aged 18 years or older: A randomised,
double-blind, placebo-controlled, phase 2 trial. Lancet.
396:479–488. 2020.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Joe CCD, Jiang J, Linke T, Li Y, Fedosyuk
S, Gupta G, Berg A, Segireddy RR, Mainwaring D, Joshi A, et al:
Manufacturing a chimpanzee adenovirus-vectored SARS-CoV-2 vaccine
to meet global needs. Biotechnol Bioeng. 119:48–58. 2022.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Asano M, Okada H, Itoh Y, Hirata H,
Ishikawa K, Yoshida E, Matsui A, Kelly EJ, Shoemaker K, Olsson U
and Vekemans J: Immunogenicity and safety of AZD1222 (ChAdOx1
nCoV-19) against SARS-CoV-2 in Japan: A double-blind, randomized
controlled phase 1/2 trial. Int J Infect Dis. 114:165–174.
2022.PubMed/NCBI View Article : Google Scholar
|
|
136
|
AstraZeneca: AZD1222 US Phase III primary
analysis confirms safety and efficacy. AstraZeneca, 2021. Available
at: https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html.
|
|
137
|
Khan KH: DNA vaccines: Roles against
diseases. Germs. 3:26–35. 2013.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Zhao G, Zhang Z, Ding Y, Hou J, Liu Y,
Zhang M, Sui C, Wang L, Xu X, Gao X and Kou Z: A DNA vaccine
encoding the full-length spike protein of beta variant (B.1.351)
elicited broader cross-reactive immune responses against other
SARS-CoV-2 variants. Vaccines (Basel). 11(513)2023.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Chavda VP, Jogi G, Dave S, Patel BM,
Vineela Nalla L and Koradia K: mRNA-Based vaccine for COVID-19:
They are new but not unknown! Vaccines (Basel).
11(507)2023.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Hussain A, Yang H, Zhang M, Liu Q,
Alotaibi G, Irfan M, He H, Chang J, Liang XJ, Weng Y and Huang Y:
mRNA vaccines for COVID-19 and diverse diseases. J Control Release.
345:314–333. 2022.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Gordon CJ, Tchesnokov EP, Woolner E, Perry
JK, Feng JY, Porter DP and Götte M: Remdesivir is a direct-acting
antiviral that inhibits RNA-dependent RNA polymerase from severe
acute respiratory syndrome coronavirus 2 with high potency. J Biol
Chem. 295:6785–6797. 2020.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Grein J, Ohmagari N, Shin D, Diaz G,
Asperges E, Castagna A, Feldt T, Green G, Green ML and Lescure FX:
Compassionate Use of Remdesivir for Patients with Severe Covid-19.
N Engl J Med. 382:2327–2336. 2020.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Chen C, Zhang Y, Huang J, Yin P, Cheng Z,
Wu J, Chen S, Zhang Y, Chen B, Lu M, et al: Favipiravir versus
arbidol for clinical recovery rate in moderate and severe adult
COVID-19 Patients: A prospective, multicenter, open-label,
randomized controlled clinical trial. Front Pharmacol.
12(683296)2021.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Joshi S, Parkar J, Ansari A, Vora A,
Talwar D, Tiwaskar M, Patil S and Barkate H: Role of favipiravir in
the treatment of COVID-19. Int J Infect Dis. 102:501–508.
2021.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Lei ZN, Wu ZX, Dong S, Yang DH, Zhang L,
Ke Z, Zou C and Chen ZS: Chloroquine and hydroxychloroquine in the
treatment of malaria and repurposing in treating COVID-19.
Pharmacol Ther. 216(107672)2020.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Mallat J, Hamed F, Balkis M, Mohamed MA,
Mooty M, Malik A, Nusair A and Bonilla MF: Hydroxychloroquine is
associated with slower viral clearance in clinical COVID-19
patients with mild to moderate disease. Medicine (Baltimore).
99(e23720)2020.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu
J, Cai J, Yang R, Han J, Huang Y and He S: Effects of Angiotensin
II receptor blockers and ACE (Angiotensin-Converting Enzyme)
inhibitors on virus infection, inflammatory status, and clinical
outcomes in patients with COVID-19 and hypertension: A
single-center retrospective study. Hypertension. 76:51–58.
2020.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Eastman RT, Roth JS, Brimacombe KR,
Simeonov A, Shen M, Patnaik S and Hall MD: Remdesivir: A review of
its discovery and development leading to emergency use
authorization for treatment of COVID-19. ACS Cent Sci. 6:672–683.
2020.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Uzunova K, Filipova E, Pavlova V and Vekov
T: Insights into antiviral mechanisms of remdesivir,
lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting
the new SARS-CoV-2. Biomed Pharmacother. 131(110668)2020.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Bahsoun A, Fakih Y, Zareef R, Bitar F and
Arabi M: Corticosteroids in COVID-19: Pros and cons. Front Med
(Lausanne). 10(1202504)2023.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Venditto VJ, Haydar D, Abdel-Latif A,
Gensel JC, Anstead MI, Pitts MG, Creameans J, Kopper TJ, Peng C and
Feola DJ: Immunomodulatory effects of azithromycin revisited:
Potential applications to COVID-19. Front Immunol.
12(574425)2021.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Peng HT, Rhind SG and Beckett A:
Convalescent plasma for the prevention and treatment of COVID-19: A
systematic review and quantitative analysis. JMIR Public Health
Surveill. 7(e25500)2021.PubMed/NCBI View
Article : Google Scholar
|
|
153
|
Pandey A, Nikam AN, Shreya AB, Mutalik SP,
Gopalan D, Kulkarni S, Padya BS, Fernandes G, Mutalik S and Prassl
R: Potential therapeutic targets for combating SARS-CoV-2: Drug
repurposing, clinical trials and recent advancements. Life Sci.
256(117883)2020.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Aiyegbusi OL, Hughes SE, Turner G, Rivera
SC, McMullan C, Chandan JS, Haroon S, Price G, Davies EH,
Nirantharakumar K, et al: Symptoms, complications and management of
long COVID: A review. J R Soc Med. 114:428–442. 2021.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Hosseinzadeh P, Zareipour M, Baljani E and
Moradali MR: Social consequences of the COVID-19 pandemic. A
systematic review. Invest Educ Enferm. 40(e10)2022.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Wouters OJ, Shadlen KC, Salcher-Konrad M,
Pollard AJ, Larson HJ, Teerawattananon Y and Jit M: Challenges in
ensuring global access to COVID-19 vaccines: Production,
affordability, allocation, and deployment. Lancet. 397:1023–1034.
2021.PubMed/NCBI View Article : Google Scholar
|
|
157
|
McNeely JA: Nature and COVID-19: The
pandemic, the environment, and the way ahead. Ambio. 50:767–781.
2021.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Keusch GT, Amuasi JH, Anderson DE, Daszak
P, Eckerle I, Field H, Koopmans M, Lam SK, Das Neves CG, Peiris M,
et al: Pandemic origins and a One Health approach to preparedness
and prevention: Solutions based on SARS-CoV-2 and other RNA
viruses. Proc Natl Acad Sci USA. 119(e2202871119)2022.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Morens DM, Taubenberger JK and Fauci AS:
Universal coronavirus vaccines-an urgent need. N Engl J Med.
386:297–299. 2022.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Loh EH, Zambrana-Torrelio C, Olival KJ,
Bogich TL, Johnson CK, Mazet JA, Karesh W and Daszak P: Targeting
transmission pathways for emerging zoonotic disease surveillance
and control. Vector Borne Zoonotic Dis. 15:432–437. 2015.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Pike J, Bogich T, Elwood S, Finnoff DC and
Daszak P: Economic optimization of a global strategy to address the
pandemic threat. Proc Natl Acad Sci USA. 111:18519–18523.
2014.PubMed/NCBI View Article : Google Scholar
|
|
162
|
AlShurman BA, Khan AF, Mac C, Majeed M and
Butt ZA: What demographic, social, and contextual factors influence
the intention to use COVID-19 vaccines: A scoping review. Int J
Environ Res Public Health. 18(9342)2021.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Dinnes J, Sharma P, Berhane S, van Wyk SS,
Nyaaba N, Domen J, Taylor M, Cunningham J, Davenport C, Dittrich S,
et al: Rapid, point-of-care antigen tests for diagnosis of
SARS-CoV-2 infection. Cochrane Database Syst Rev.
7(CD013705)2022.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Malekzadeh R, Abedi G, Ziapour A, Yıldırım
M and Amirkhanlou A: Analysis of ethical considerations of COVID-19
vaccination: Lessons for future. BMC Med Ethics.
24(91)2023.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Jalilian H, Amraei M, Javanshir E,
Jamebozorgi K and Faraji-Khiavi F: Ethical considerations of the
vaccine development process and vaccination: A scoping review. BMC
Health Serv Res. 23(255)2023.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Chung JY, Thone MN and Kwon YJ: COVID-19
vaccines: The status and perspectives in delivery points of view.
Adv Drug Deliv Rev. 170:1–25. 2021.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Forman R, Shah S, Jeurissen P, Jit M and
Mossialos E: COVID-19 vaccine challenges: What have we learned so
far and what remains to be done? Health Policy. 125:553–567.
2021.PubMed/NCBI View Article : Google Scholar
|