Introduction
Human parechoviruses (HPeVs) are a discovered group
of non-enveloped, positive-sense single-stranded RNA viruses
belonging to the Picornaviridae family (1). Initially classified with
enteroviruses, they were later separated due to distinct
pathogenicity and cytopathic effects (1). Currently, 16 different serotypes of
HPeV have been identified, with two of these, HPeV1 and HPeV3,
being the most frequently found in association with human diseases
(2).
HPeVs mainly cause gastrointestinal and respiratory
syndromes; however, they are also associated with more severe
disorders, such as myocarditis, encephalitis and sepsis-like
syndrome, particularly in children <1 year of age (1,3-5).
In fact, HPeVs exhibit a high infection rate during the first year
of age, reaching 100% of HPeV-seropositive children in the age
group of 5-9 years (1). Moreover,
these viruses are more frequently detected during the peak
enterovirus season, from June to October in the Northern Hemisphere
(3,4).
The diagnosis of HPeV infection has traditionally
been carried out with viral cultures of different specimens;
however, due to the length of turnaround, viral culture may not be
the optimal diagnostic method, particularly in severe syndromes
(6). Therefore, molecular biology
techniques, such as reverse transcriptase polymerase chain reaction
(RT-PCR) and integrated systems with multiplex PCR able to test a
greater number of pathogens at once, have replaced viral cultures
and are currently routinely used with an even higher sensitivity
than the classic techniques (2,6,7).
Treatment commonly includes symptomatic therapy,
with complete recovery occurring within 1-2 weeks from the onset of
symptoms (3). HPeV, and
particularly HPeV3, has been recently identified as the cause of
epidemic myalgia in adult patients and has also been recognized,
although seldomly, as the etiology in severe syndromes, such as
myocarditis and pericarditis in the same population (8-13).
Occasionally, it was also found as the cause of encephalitis in
immunocompromised adult individuals (12).
The present study describes the case of an adult
immunocompetent patient with HPeV encephalitis and also provides a
review of the cases involving adults reported in the literature
(8-10,12-25).
The case report was written following the case report (CARE)
guidelines (26).
The aim of the present study was to raise awareness
against HPeV as a rare cause of severe disease in immunocompetent
adults.
Case report
A 49-year-old male presented to the emergency room
of the ‘Umberto I’ Hospital of Enna (Enna, Italy) in the
mid-October, 2023, complaining of fever with chills for 10 days,
with lower back pain, orchiodynia, strangury and hematuria. His
clinical history highlighted a colon diverticulosis diagnosed
several months prior to his admission and a recent (~1 month)
fracture of the head of the right radial bone; otherwise his
clinical history was negative. He denied having being in any
contact with children who were ill. At 3 days prior to this
episode, he underwent a urology consultation with an ultrasound
examination for the orchiodynia, which highlighted a prostatic
hypertrophy with the presence of calcifications and a diagnosis of
prostatitis was made, for which he was under treatment with
levofloxacin 500 mg qd, with no improvement.
He was therefore admitted to the Infectious Diseases
Ward of the aforementioned hospital. A physical examination
performed upon admission highlighted a suffering man, with no
specific signs, apart from a mild costovertebral angle tenderness.
The patient also complained of myalgia affecting the proximal and
distal muscles of the lower extremities. Antibiotic therapy with
piperacillin/tazobactam 4.5 g three times a day (tid) and
vancomycin 500 mg tid (20 mg/kg/day) was commenced. The results of
blood tests were also normal, with a white blood cell count of
12,310 cells/µl (70.5% neutrophils, 17.7% lymphocytes and 9.3%
monocytes). C-reactive protein and procalcitonin levels were also
normal. Mild hyponatremia (131 mEq/l), with normal kaliemia (3.9
mEq/l) was highlighted. Serological tests performed during the
first day of admission for cytomegalovirus (IgM and IgG),
Toxoplasma gondii (IgM and IgG), Epstein-Barr virus
(anti-VCA IgM and IgG), herpes simplex virus (HSV) 1 and 2 (IgM),
HSV-2 (IgG), rubeola (IgM), Mycoplasma pneumoniae (IgM and
IgG), Chlamydia pneumoniae (IgM and IgG) and Legionella
pneumophila (IgM and IgG) yielded negative results. HSV-1 (IgG)
and rubeola (IgG) tests yielded positive results. He also had
normal total and free prostate-specific antigen, and normal thyroid
hormone levels [free thyroxin (fT) 3, fT4 and thyroid stimulating
hormone]. Urine and blood cultures yielded negative results. An
interferon-gamma release assay for tuberculosis performed on day 6
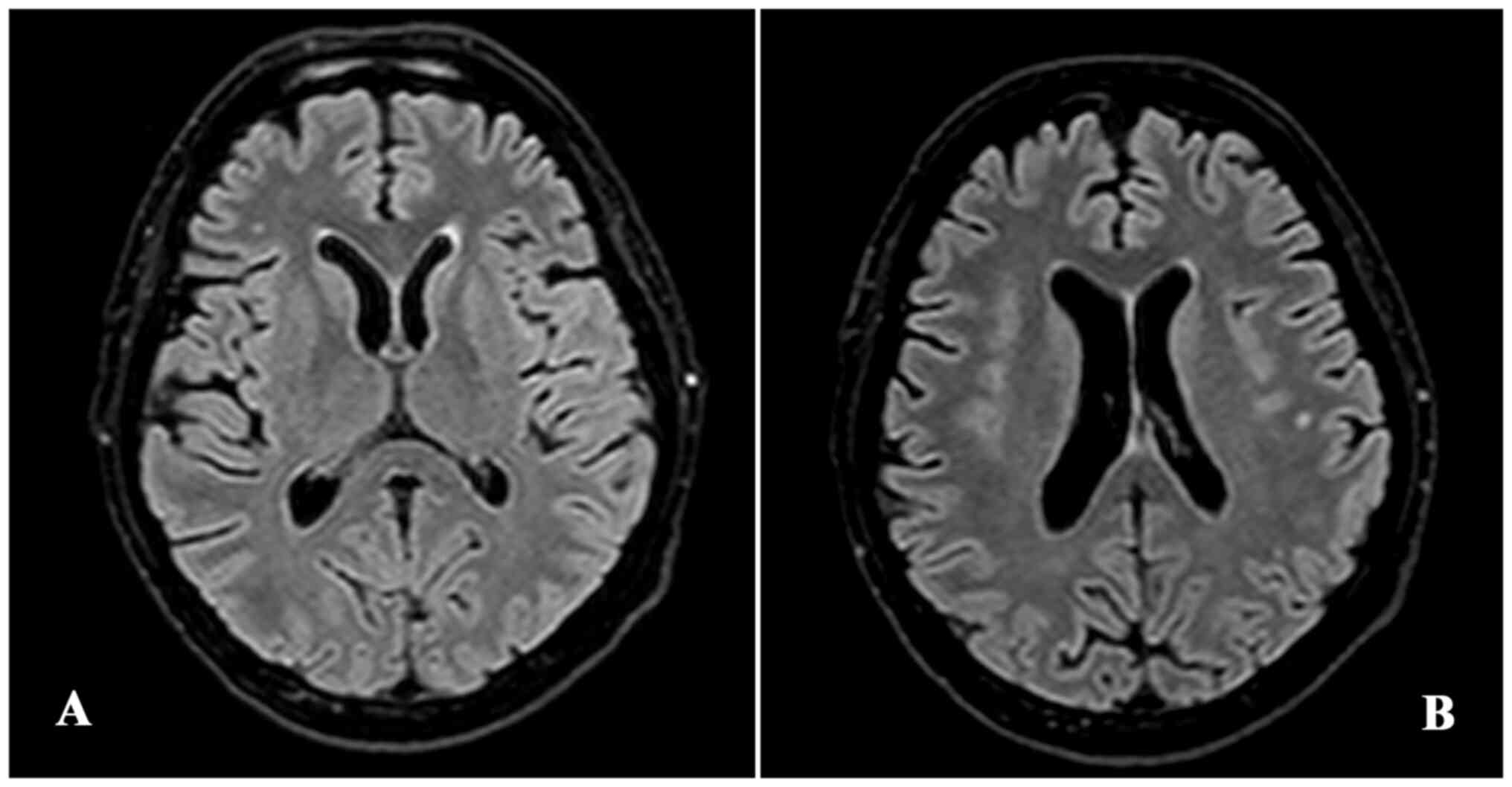
of admission yielded negative results. During the admission, for
the appearance of headache, a brain magnetic resonance imaging was
performed, highlighting the presence in the frontal lobe of
subcortical dotted areas of altered hyperintense signal in long
repetition time sequences, which had a non-specific gliotic
appearance (Fig. 1). On day 8 from
the time of admission, the patient underwent a positron emission
tomography-computed tomography with 18F-fludeoxyglucose (18F-FDG),
which yielded negative results. On day 9 from the time of
admission, for the persistence of the headache and fever, and the
appearance of a slight neck stiffness during the neurological
consultation, the patient underwent a lumbar puncture. The cerebral
spinal fluid (CSF) was clear, with 187 cells/µl, high protein
levels (126.8 mg/dl), a CSF albumin/serum albumin ratio of 0.02,
normal glucose levels, and a high concentration of IgG. The results
of Gram staining and culture were negative. A FilmArray for common
pathogens causing meningoencephalitis (Biofire®
FilmArray® Meningitis/Encephalitis Panel, Biomérieux)
was performed, which yielded positive results for HPeV. Antibiotic
treatment with piperacillin/tazobactam and vancomycin was then
terminated, and an anti-inflammatory treatment with dexamethasone 8
mg tid was commenced, with the immediate resolution of the headache
and fever. For the persistence of the myalgia at the lower
extremities, on day 12 from the time of admission, the patient
underwent and electromyography, which highlighted the normal
transmission of motor and sensitive signals. The patient was
discharged on day 22 from the time of admission, after 14 days of
steroid treatment.
At 1 week after being discharged, he complained of
photophobia and blurred vision. Thus, he underwent an
ophthalmologist consultation, which highlighted the presence of
papilledema. The papilledema was resolved following another cycle
of treatment with dexamethasone for 10 days. After 1 year since the
episode, the patient is in good general conditions and does not
complain of any neurological symptoms.
Discussion
As of the day of the submission of the present
study, 380 articles on viral encephalitis in adults were published
between 2020 and 2024, and indexed in Medline®,
excluding SARS-CoV-2 related ones. A total of 65 of these articles
are indexed as case reports, and ~30% of these cases were caused by
herpesviruses, followed by arboviruses (20%). The high number of
published articles indicates that the attention on encephalitis is
high, also due to the non-existence of a consensus over the
management of the disease, apart from herpesviruses. To the best of
our knowledge, this is the first report of an immunocompetent adult
affected by HPeV encephalitis.
HPeV are a rare cause of disease in immunocompetent
adults. A summary of all the cases reported in the literature in
adult patients and their outcomes is provided in Table I. Similar to the cases reported in
the studies by Mizuta et al (8-10),
the patient in the present study was a 49-year-old male, who
complained of myalgia and lower back pain, with orchiodynia, fever
and weakness. Although the majority of the reported cases
demonstrated that the onset of the symptoms followed interactions
with ill children, the patient in the present study denied any such
contacts.
 | Table ISummary of the adult HPeV infection
cases reported in the literature. |
Table I
Summary of the adult HPeV infection
cases reported in the literature.
| Sex | Age, years | Syndrome | Outcome | (Refs.) |
|---|
| M | 54 | Keratitis + anterior
uveitis | Recovery | (14) |
| M | 53 | Keratitis + anterior
uveitis | Recovery | (14) |
| F | 73 | Keratitis + anterior
uveitis + secondary glaucoma | Trabeculectomy | (14) |
| Ma | 37 | Keratitis +
panuveitis + retinitis + vasculitis + papillitis | Recovery | (14) |
| M | 38 | Myalgia + weakness +
orchiodynia + fever | Recovery | (8) |
| M | 41 | Myalgia + weakness +
orchiodynia + fever | Recovery | (8) |
| M | 41 | Myalgia + weakness +
fever + sore throat | Recovery | (8) |
| M | 31 | Myalgia + Weakness +
fever | Recovery | (8) |
| M | 35 | Myalgia + weakness +
sore throat | Recovery | (8) |
| M | 36 | Myalgia + weakness +
fever | Recovery | (9) |
| F | 66 | Myalgia +
weakness | Recovery | (9) |
| M | 25 | Myalgia + weakness
+ fever + orchiodynia | Recovery | (9) |
| M | 31 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| M | 32 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| M | 43 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| F | 30 | Myalgia + weakness
+ fever | Recovery | (9) |
| M | 28 | Myalgia + weakness
+ fever + sore throat + orchiodynia | Recovery | (9) |
| F | 39 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| M | 36 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| M | 35 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| M | 38 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| F | 38 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| F | 37 | Myalgia + weakness
+ seizures | Recovery | (9) |
| M | 48 | Myalgia + weakness
+ sore throat | Recovery | (9) |
| M | 41 | Myalgia + fever +
sore throat | Recovery | (9) |
| F | 26 | Myalgia + weakness
+ fever | Recovery | (9) |
| M | 36 | Myalgia + weakness
+ fever + sore throat + orchiodynia | Recovery | (9) |
| M | 23 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| M | 34 | Myalgia + weakness
+ fever | Recovery | (9) |
| F | 38 | Myalgia + weakness
+ fever + sore throat | Recovery | (9) |
| M | 55 | Myalgia + weakness
+ fever + sore throat + orchiodynia | Recovery | (9) |
| Fb | 28 | Myalgia +
arthralgia + fatigue | Recovery | (17) |
| M | 30 | Fever + myalgia +
weakness + orchiodynia | Recovery | (25) |
| M | 38 | Myalgia + Sore
throat + tongue pain | Recovery | (25) |
| M | 39 | Fever + sore throat
+ myalgia + weakness | Recovery | (25) |
| M | 26 | Myocarditis | Recovery | (13) |
| M | 19 | Pericarditis | Recovery | (16) |
| M | 32 | Fever + sore throat
+ mild diarrhea + orchiodynia | Recovery | (18) |
| Mc | 63 | Encephalitis | Recovery | (19) |
| M | 42 | Myalgia + sore
throat + orchiodynia | Recovery | (20) |
| M | 32 | Myalgia +
Orchiodynia + leg dysesthesia | Recovery | (21) |
| M | 47 | Myalgia | Recovery | (21) |
| F | 38 | Myalgia +
stomatitis + sore throat | Recovery | (21) |
| M | 34 | Myalgia + sore
throat | Recovery | (21) |
| M | 46 | Myalgia | Recovery | (21) |
| M | 42 | Myalgia +
orchiodynia + sore throat | Recovery | (21) |
| M | 38 | Myalgia +
orchiodynia + joint pain | Recovery | (21) |
| M | 22 | Myalgia + sepsis +
hepatitis + conjunctivitis + headache + fever + pharyngitis | Recovery | (15) |
| M | 44 | Myalgia + weakness
+ fever + stomatitis | Recovery | (10) |
| F | 30 | Myalgia + weakness
+ fever | Recovery | |
| M | 37 | Myalgia + sore
throat + stomatitis + orchiodynia | Recovery | (22) |
| Fd | 16 | Myocarditis +
encephalitis + myalgia + ophtalmoplegia | Slow recovery | (12) |
| F | 78 | Pneumonia | Death | (23) |
| F | 35 | Flu-like
syndrome | Recovery | (24) |
| M | 49 | Encephalitis +
myalgia + weakness + fever + orchiodynia + papilledema | Slow recovery | The present
case |
The case described herein suggests the importance of
performing a lumbar puncture even with mild neurological signs in
cases of fever of unknown origin and close to no radiological
signs. This is particularly true for HPeV, since Mizuta et
al (9) demonstrated that
serology has a low sensitivity in adults (~30%). Diagnosis was made
by FilmArray® (Biofire®, Biomérieux), a
multiplex PCR technique which allows the use of small amounts of
samples to collect information about the presence or absence of
bacterial and viral pathogens (7).
Although viral culture remains the gold-standard for diagnosis, its
long turnaround time renders it unpractical, particularly in severe
cases which involve the CNS. In fact, all the reported cases were
diagnosed using PCR techniques, both homemade and commercially
available, using various types of samples, particularly feces,
pharyngeal swabs and serum, with a higher sensitivity of feces
compared with serum and pharyngeal swab (8,9,17).
However, despite being extremely useful in having a rapid
etiological diagnosis, the FilmArray does not provide a serotype
differentiation. Therefore, in the present study, the serotype
affecting the patient could not be identified.
The study published by Piralla et al
(27) demonstrated that in 2012,
the most frequently isolated strand of HPeV was type 1 (57.9%),
followed by type 3 (36.8%) and type 6 (5.3%). On the other hand,
the study by Westerhuis et al (11) demonstrated that the seroprevalence
of HPeV3 was very low (<20%) in Finland and The Netherlands,
whereas HPeV1 and HPeV2 had a higher (80-100%) seroprevalence.
Almost all cases of epidemic myalgia reported from Japan have been
shown to be caused by HPeV3 (8-10,13,17,20).
Both HPeV1 and HPeV3 have been associated with encephalitis cases
in children, although this association is stronger with HPeV3
(5,11,28,29).
Given both clinical and epidemiological criteria, it can be
inferred that the patient in the present study was affected by
HPeV3.
Although notable, knowing the serotype however, does
not alter the management. In fact, there is no specific (or
aspecific) antiviral drug available against HPeV, and the review by
Kadambari et al (30)
highlights how randomized clinical trials (RCTs) for the most
commonly reported therapeutic approaches are lacking. An
intravenous immunoglobulin administration was attempted in two
reported cases of myocarditis, although its efficacy has not been
proven (13). In one case,
corticosteroids were added due to severe myalgia (21). However, in other cases the
treatment was reported as supportive (20). The patient described herein was
managed with symptomatic treatment, with the addition of
corticosteroids when encephalitis was diagnosed. The use of
corticosteroids in the treatment of viral encephalitis remains
controversial and is based on the decision of the single physician.
The only RCT on this matter has been terminated prematurely due to
the difficulty in enrolling patients, although dexamethasone is
anecdotally useful for the prevention of vascular edema, whenever
the patient is suspected for or diagnosed with intracranial
hypertension (31-33).
HPeV rarely causes permanent sequelae, and usually,
medical providers aim for a rapid (7-14 days) and full recovery.
The cases reported in literature all had a full recovery, apart
from one case affected by several comorbidities, who succumbed due
to of respiratory complications (23). In the case in the present study,
despite the patient being an immunocompetent adult, he had a slow
recovery, with the appearance of new symptoms even following the
termination of the corticosteroid therapy.
In conclusion, the present study reported the first
case of HPeV encephalitis in an immunocompetent adult. HPeVs
usually have a benign prognosis, although the elderly and
individuals with comorbidities which alter the normal function of
the immune system, such as diabetes and alcohol abuse, should be
aware they are at a higher risk of a negative prognosis. The use of
corticosteroids remains controversial, and a short course should be
preferred over a longer one in HPeV, when their use is deemed
necessary.
Further studies are warranted to determine whether
HPeV is a novel cause of encephalitis in immunocompetent adults.
The hypothesis is that climate changes may have contributed to this
shift in pathogenicity.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
MC, AG and FS conceptualized the study. MC, FS, DV,
SB, AN, LG and SDM were responsible for the treatments of the
patient and the patient's data. FS, DV, AG, SB, AN, LG and SDM were
involved in literature review. FS, DV, SDM, AG, LG and MC were
involved in the writing of the original draft of the manuscript. LG
and MC were involved in the writing, reviewing and editing of the
manuscript. MC, AG and AN were involved in table editing. All
authors have read and approved the final manuscript and confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Ethical committee approval was waived given the
observational and anecdotical nature of a case report. A written
informed consent for the use of anonymized data for research and
publishing purposes was obtained from the patient in the present
study.
Patient consent for publication
A written informed consent for the use of anonymized
data for research and publishing purposes was obtained from the
patient in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joki-Korpela P and Hyypiä T:
Parechoviruses, a novel group of human picornaviruses. Ann Med.
33:466–471. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mirand A, Archimbaud C, Chambon M,
Regagnon C, Brebion A, Bailly JL, Peigue-Lafeuille H and Henquell
C: Diagnosis of human parechovirus infections of the central
nervous system with a commercial real-time reverse
transcription-polymerase chain reaction kit and direct genotyping
of cerebrospinal fluid specimens. Diagn Microbiol Infect Dis.
74:78–80. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Centers for Disease Control and Prevention
(CDC). Nonpolio enterovirus and human parechovirus
surveillance-united states, 2006-2008. MMWR Morb Mortal Wkly Rep.
59:1577–1580. 2010.PubMed/NCBI
|
|
4
|
Nielsen ACY, Böttiger B, Midgley SE and
Nielsen LP: A novel enterovirus and parechovirus multiplex one-step
real-Time PCR-validation and clinical experience. J Virol Methods.
193:359–363. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Harvala H, McLeish N, K ondracka J,
McIntyre CL, Leitch ECM, Templeton K and Simmonds P: Comparison of
human parechovirus and enterovirus detection frequencies in
cerebrospinal fluid samples collected over a 5-year period in
edinburgh: HPeV type 3 identified as the most common picornavirus
type. J Méd Virol. 83:889–896. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Crom SC, Obihara CC, van Loon AM,
Argilagos-Alvarez AA, Peeters MF, van Furth AM and Rossen JW:
Detection of enterovirus RNA in cerebrospinal fluid: Comparison of
two molecular assays. J Virol Methods. 179:104–107. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Leber AL, Everhart K, Balada-Llasat JM,
Cullison J, Daly J, Holt S, Lephart P, Salimnia H, Schreckenberger
PC, DesJarlais S, et al: Multicenter evaluation of BioFire
FilmArray Meningitis/Encephalitis panel for detection of bacteria,
viruses, and yeast in cerebrospinal fluid specimens. J Clin
Microbiol. 54:2251–2261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mizuta K, Yamakawa T, Nagasawa H, Itagaki
T, Katsushima F, Katsushima Y, Shimizu Y, Ito S, Aoki Y, Ikeda T,
et al: Epidemic myalgia associated with human parechovirus type 3
infection among adults occurs during an outbreak among children:
Findings from Yamagata, Japan, in 2011. J Clin Virol. 58:188–193.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mizuta K, Kuroda M, Kurimura M, Yahata Y,
Sekizuka T, Aoki Y, Ikeda T, Abiko C, Noda M, Kimura H, et al:
Epidemic myalgia in adults associated with human parechovirus type
3 Infection, Yamagata, Japan, 2008. Emerg Infect Dis. 18:1787–1793.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mizuta K, Yamakawa T, Kurokawa K, Chikaoka
S, Shimizy Y, Itagaki T, Katsushima F, Katsushima Y, Ito S, Aoki Y,
et al: Epidemic myalgia and myositis associated with human
parechovirus type 3 Infections occur not only in adults but also in
children: Findings in Yamagata, Japan, 2014. Epidemiol Infect.
144:1286–1290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Westerhuis B, Kolehmainen P, Benschop K,
Nurminen N, Koen G, Koskiniemi M, Simell O, Knip M, Hyöty H,
Wolthers K and Tauriainen S: Human parechovirus seroprevalence in
finland and the netherlands. J Clin Virol. 58:211–215.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mardekian SK, Fortuna D, Nix A, Bhatti T,
Wiley CA, Flanders A, Urtecho J, Sloane J, Ahmad J and Curtis MT:
Severe human parechovirus type 3 myocarditis and encephalitis in an
adolescent with hypogammaglobulinemia. Int J Infect Dis. 36:6–8.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kong KL, Lau JSY, Goh SM, Wilson HL,
Catton M and Korman TM: Myocarditis caused by human parechovirus in
adult. Emerg Infect Dis. 23:1571–1573. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Groot-Mijnes JD, de Visser L, Zuurveen
S, Martinus RA, Völker R, ten Dam-van Loon NH, de Boer JH, Postma
G, de Groot RJ, van Loon AM and Rothova A: Identification of new
pathogens in the intraocular fluid of patients with uveitis. Am J
Ophthalmol. 150:628–636. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yamamoto SP, Kaida A, Naito T, Hosaka T,
Miyazato Y, Sumimoto S, Kohdera U, Ono A, Kubo H and Iritani N:
Human parechovirus infections and child myositis cases associated
with genotype 3 in Osaka City, Japan, 2014. J Méd Microbiol.
64:1415–1424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McGregor T, Bu'Lock FA, Wiselka M and Tang
JW: Human parechovirus infection as an undiagnosed cause of adult
pericarditis. J Infect. 75:596–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shinomoto M, Kawasaki T, Sugahara T,
Nakata K, Kotani T, Yoshitake H, Yuasa K, Saeki M and Fujiwara Y:
First report of human parechovirus type 3 infection in a pregnant
woman. Int J Infect Dis. 59:22–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nakamura K, Saito K, Hara Y, Aoyagi T,
Kitakawa K, Abe Y, Takemura H, Ikeda F, Kaku M and Kanemitsu K:
Severe epidemic myalgia with an elevated level of serum
Interleukin-6 caused by human parechovirus type 3: A case report
and brief review of the literature. BMC Infect Dis.
18(381)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chimunda T, Subramanian R, Smith J and
Mahony A: First reported case of human parechovirus encephalitis in
an adult patient complicated by refractory status epilepticus.
IDCases. 15(e00475)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Miyazaki M, Hara K, Takayoshi T, Kawase T,
Nakagawa Y, Arai T, Sugimoto T, Nishiyama K, Gonzalez G, Hanaoka N,
et al: Epidemic myalgia associated with human parechovirus type 3
infection. Intern Med. 59:739–744. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Orimo K, Hatano K, Sato N, Okabe S, Suzuki
A, Mori K, Chiba T and Hashida H: Clinical characteristics of
epidemic myalgia associated with human parechovirus type 3 during
the summer of 2019. Intern Med. 59:1721–1726. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Masuda S, Koizumi K, Sato M, Uojima H,
Kimura K, Nishino T, Ichita C, Sasaki A, Makazu M, Kobayashi M, et
al: Severe generalized epidemic myalgia in an adult due to human
parechovirus type 3: A case report. Cureus.
14(e30587)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abed Y and Boivin G: Human parechovirus
infections in Canada. Emerg Infect Dis. 12:969–975. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Watanabe K, Oie M, Higuchi M, Nishikawa M
and Fujii M: Isolation and characterization of novel human
parechovirus from clinical samples. Emerg Infect Dis. 13:889–895.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tanaka S, Kunishi Y, Ota M, Ono A, Kanno
N, Kurakami Y, Yanagibashi T, Matsubayashi M, Hao Y, Iwabuchi K, et
al: Severe human parechovirus type 3 infection in adults associated
with gastroenteritis in their children. Infect Dis (Lond).
49:772–774. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gagnier JJ, Kienle G, Altman DG, Moher D,
Sox H and Riley D: CARE Group. The CARE guidelines: Consensus-based
clinical case reporting guideline development. BMJ Case Rep.
2013(bcr2013201554)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Piralla A, Furione M, Rovida F, Marchi A,
Stronati M, Gerna G and Baldanti F: Human parechovirus infections
in patients admitted to hospital in Northern Italy, 2008-2010. J
Méd Virol. 84:686–690. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Benschop KSM, Schinkel J, Minnaar RP,
Pajkrt D, Spanjerberg L, Kraakman HC, Berkhout B, Zaaijer HL, Beld
MG and Wolthers KC: Human parechovirus infections in dutch children
and the association between serotype and disease severity. Clin
Infect Dis. 42:204–210. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Sharp J, Harrison CJ, Puckett K, Selvaraju
SB, Penaranda S, Nix WA, Oberste MS and Selvarangan R:
Characteristics of young infants in whom human parechovirus,
enterovirus or neither were detected in cerebrospinal fluid during
sepsis evaluations. Pediatr Infect Dis J. 32:213–216.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kadambari S, Harvala H, Simmonds P,
Pollard AJ and Sadarangani M: Strategies to improve detection and
management of human parechovirus infection in young infants. Lancet
Infect Dis. 19:e51–e58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Meyding-Lamadé U, Jacobi C,
Martinez-Torres F, Lenhard T, Kress B, Kieser M, Klose C, Einhäupl
K, Bösel J, Mackert MB, et al: The German trial on aciclovir and
corticosteroids in Herpes-simplex-virus-Encephalitis (GACHE): A
multicenter, randomized, double-blind, placebo-controlled trial.
Neurol Res Pract. 1(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moscatt V, Marino A, Ceccarelli M,
Cosentino F, Zagami A, Celesia BM, Nunnari G and Cacopardo B:
Possible role of low dose dexamethasone administration in listeria
monocytogenes meningoencephalitis: A case series. Biomed Rep.
17(73)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Beaman MH and Wesselingh SL: 4: Acute
Community-acquired meningitis and encephalitis. Méd J Aust.
176:389–396. 2002.PubMed/NCBI View Article : Google Scholar
|