Introduction
Acinetobacter baumannii (A.
baumannii), is one of the most potentially hazardous bacteria
in the domain of infections linked to healthcare. Once considered
to be a generally benign environmental microbe, this Gram-negative
coccobacillus has rapidly emerged as a major contributor to
nosocomial infections worldwide (1). A. baumannii poses significant
clinical challenges by driving persistent infections and organ
damage, such as endocarditis and pneumonia, particualrly in
immunocompromised individuals (2).
Infections caused by A. baumannii, are of significant
concern due to their ability to invade and damage vital organs,
such as the lungs, liver, kidneys and spleen (3). A baumannii is a pathogen of
key concern due to its ability to cause severe infections,
particularly in immunocompromised individuals (4).
Biofilm formation by A. baumannii poses
significant challenges in clinical settings, as it facilitates
bacterial adhesion to surfaces, such as heart valves and lung
tissue, resulting in persistent and difficult-to-treat infections
(5,6). The biofilm matrix serves as a
protective barrier, shielding bacteria from the host immune
response and antibiotic treatment. In the heart, this can lead to
endocarditis, marked by inflammation, tissue necrosis and the
development of lesions or abscesses on the heart valves (7). The role of biofilm is pivotal in
these processes, as it fosters the chronicity of the infection and
complicates pathogen eradication. Similarly, in the lungs, A.
baumannii biofilms can aggravate pulmonary infections, leading
to conditions such as bronchitis, pneumonia and pleuritis. The
dense bacterial communities within biofilms are linked to severe
pathological changes, including lung tissue consolidation and, in
extreme cases, abscess formation (8). These biofilm-related infections often
present with respiratory distress, coughing and fever, similar to
other bacterial infections, although with heightened resistance to
treatment due to the protective nature of the biofilm (9). The ability of A. baumannii to
form biofilms is closely linked to its virulence factors, which
enable it to evade the immune system and persist within the host.
The severity of infection, including the extent of tissue damage
and clinical outcomes, is influenced by factors, such as the
virulence of the strain, the bacterial load and the immune status
of the host. Understanding the role of biofilm formation in A.
baumannii infections is essential for devising more effective
treatment strategies, particularly for overcoming the challenges
posed by its antibiotic resistance (1). The interplay between biofilm
formation and antibiotic resistance is crucial for understanding
the pathogenesis of A. baumannii infections. Biofilms
confer increased resistance to conventional treatments, making it
difficult for antibiotics to penetrate and effectively kill the
bacterial cells (9). Consequently,
there is an urgent need for alternative therapeutic strategies that
target both the bacterial cells and their biofilm structures.
Research reveals a strong positive association
between biofilm formation and increased antibiotic resistance in
A. baumannii. Strains of A. baumannii exhibit high
resistance rates to ciprofloxacin, piperacillin and ceftazidime,
with the blaOXA-23-like gene detected in 93% of multidrug-resistant
(MDR) isolates (10).
Additionally, 50 isolates have been identified as carbapenemase
producers. Polymerase chain reaction (PCR) analysis has further
demonstrated the presence of key virulence genes: traT (80%)
associated with serum resistance, cvaC (34%) linked to colicin V
production, and iutA (16%) involved in aerobactin synthesis
(11). These findings underscore
the critical association between biofilm formation and MDR in A.
baumannii, highlighting the need for innovative approaches to
combat infections caused by this resilient pathogen.
Natural medicine offers a promising and effective
treatment option, potentially overcoming these challenges, while
providing safer solutions for managing infections. Plants are
extensively utilized in traditional medicine across the globe, as
plant-derived treatments are considered relatively safe and provide
more dependable and effective results. Compared to modern and
conventional medicines, plant-based remedies have been reported to
have fewer side-effects due to their natural origin (12). Plants produce secondary metabolites
and phyto-constituents, including alkaloids, flavonoids, phenols,
saponins, sterols, tannins and terpenoids, which can be used
therapeutically in plants, humans and animals (13). Rhamnus frangula (R.
frangula), frequently referred to as glossy buckthorn or alder
buckthorn, is a small tree or deciduous shrub that is a member of
the Rhamnaceae family. It is currently found in North
America, where it is frequently regarded as an invasive species,
having previously been native to Europe, North Africa, and Western
Asia. The rich anthraquinone, flavonoid and tannin content of R.
frangula is primarily responsible for its antimicrobial
activity (14). Emodin and
frangulin are two examples of anthraquinones that are well-known
for having antimicrobial qualities. These substances have the
ability to damage microbial cell walls, prevent the synthesis of
proteins and obstruct the metabolism of nucleic acids, all of which
can result in cell death. Furthermore, as flavonoids can disrupt
cell membranes, chelate metal ions and inhibit bacterial enzymes,
they broaden the antimicrobial activity spectrum of the plant
(15).
Biofilm formation not only supports bacterial
survival, but also amplifies pathogenicity by enhancing resistance
to antimicrobial treatments (16).
In vital organs such as the lungs, heart, liver and kidneys,
biofilms are often linked to chronic infections and play a crucial
role in disease progression. For instance, in the lungs, biofilms
formed by pathogens, such as A. baumannii play a critical
role in conditions such as cystic fibrosis and chronic obstructive
pulmonary disease (COPD), driving persistent inflammation and
causing tissue damage. Similarly, biofilm-associated bacteria in
the liver and kidneys can induce chronic infections, resulting in
marked organ dysfunction and disease progression (17). The role of biofilms in organ damage
is largely due to the ability of the bacteria to evade the immune
response and persist over long periods of time. This chronic
inflammation and infection compromise the integrity of the tissues,
leading to scarring, necrosis and functional impairment of the
organs. The interplay between biofilm formation and the immune
system is crucial in understanding the pathogenesis of these
infections and developing more effective treatment strategies to
prevent organ damage (18).
The present study aimed to evaluate the
antimicrobial and antibiofilm properties of R. frangula
against A. baumannii. Additionally, it sought to identify,
characterize, and analyze the subcellular pathological changes in
goat organs—an aspect that, to the best of our knowledge, has not
been previously explored. As a pioneering investigation, this
research examines both the antibiofilm effects of R.
frangula on A. baumannii and the pathological impact of
A. baumannii infusion in goat organs, utilizing an
innovative approach to assess its potential as a therapeutic
agent.
Materials and methods
Study design and sample
collection
The research study was conducted between March and
September, 2024, following approval from the Scientific Review
Board of Saveetha Dental College and Hospitals, Chennai, Tamil
Nadu, India (SRB/SDC/UG-2276/24/GPATH/076). The approval was
granted for the use of goat samples in the present study. R.
frangula herbal powder was sourced from a botanical garden in
Chennai, Tamil Nadu, India. To validate the authenticity, purity
and quality of the herbal sample, a certified botanist performed a
comprehensive examination and verified its identity. The
Authentication and Identification Certificate
(SVMC/BOT/272/2023-24) issued by Sri Vidya Mandir Arts &
Science College (Autonomous), Katteri, Krishnagiri District, Tamil
Nadu, India. This certification confirms that the sample was
authenticated based on its morphological characteristics. Following
authentication, the powder was stored under controlled conditions
for subsequent analysis and utilization in the study.
Sample extraction
To prepare the extract, 10 g powdered herbal R.
frangula were combined with 100 ml methanol (Rankem
Laboratories, LLC). Throughout the 48-h extraction period, a shaker
was used to periodically shake the mixture. Upon completing the
extraction process, the suspension was filtered, the temperature of
the water bath was regulated to 50˚C, and the methanol solvent was
removed from the filtrate. The material was weighed and stored at
4˚C for later use after drying.
Bacterial culture and conditions
The Malabar Cancer Center in Kerala, India, provided
the A. baumannii culture samples used in the present study.
Luria Bertani (LB) broth (HiMedia Laboratories, LLC) was used to
subculture the samples. Subsequently, for 24 h, A. baumannii
cultures were incubated at 37˚C in a shaking incubator with a speed
setting of 100 revolutions per minute (rpm). Both LB agar and
Nutrient agar exhiibted distinctive growth patterns. The laboratory
staff at Saveetha Dental College and Hospital in Chennai, Tamil
Nadu, India, used the VITEK 2 automated system for preliminary
identification to confirm the identity of A. baumannii,
following the methodology described by Bobenchik et al
(19).
Antimicrobial efficacy of R.
frangula
The antibacterial activity of R. frangula
extract was evaluated using the agar well-diffusion method,
according to previously established protocols (20). A bacterial culture of A.
baumannii was uniformly spread onto Mueller Hinton Agar (MHA)
plates (HiMedia, Mumbai, India) using a sterile swab moistened with
the bacterial suspension. A well with an 8-mm diameter was
carefully punched into the MHA medium using a sterile cork borer.
Subsequently, 40 µl R. frangula extract (prepared in DMSO at
a concentration of 20 mg/ml) were added to the well. DMSO alone was
used as a negative control. The plates were incubated at 37˚C for
24 h. Following the incubation period, the zones of inhibition
around the wells were measured in millimeters using a vernier
caliper to evaluate the antibacterial activity of the extract.
Drug susceptibility testing
The Kirby-Bauer disk diffusion method was employed
to evaluate the antimicrobial susceptibility of A.
baumannii, as previously described (21). A standardized suspension of A.
baumannii was evenly spread onto MHA plates using a sterile
swab. Disks impregnated with a range of antibiotics commonly used
against A. baumannii, including tetracycline,
piperacillin/tazobactam (PIT), cefixime, imipenem, ceftriaxone,
cefotaxime and meropenem (all from HiMedia Laboratories, LLC), were
placed on the agar surface. The plates were then incubated at 37˚C
for 24 h. Following incubation, the zones of inhibition around the
disks were measured in mm to determine bacterial
susceptibility.
Minimum inhibitory concentration assay
(MIC)
A. baumannii is a key nosocomial pathogen
known for its ability to develop MDR, rendering treatment options
limited (22). The present study
investigated the antimicrobial activity of R. frangula
extract against A. baumannii using the broth dilution method
(20,23). The MIC of the R. frangula
extract was determined using a 2-fold broth dilution method, with
concentrations ranging from 10 to 0.019 mg/ml. Briefly, a
standardized A. baumannii inoculum (1.5x108
CFU/ml) was added to LB broth containing the various extract
concentrations, and the samples were incubated at 37˚C for 24 h. To
monitor color changes and validate the findings, 2,3,5-triphenyl
tetrazolium chloride (TTC) was transferred to each tube following
incubation. A level of concentration at which no color change
occurred was considered to be the MIC. Further analysis was
conducted at the MIC endpoint for histopathological
examination.
Collection of organ samples
The goat heart, lung, liver, kidney and spleen were
carefully sourced from a slaughterhouse in Saidapet, Chennai, Tamil
Nadu, India, under the guidance of Professor M. Raman, a veterinary
expert from Saveetha Dental College and Hospitals, Chennai, Tamil
Nadu, India. Upon procurement, the organs were promptly covered
with sterile polyethylene to ensure hygiene and prevent any
potential contamination. These samples were then stored at a
temperature of 4˚C to preserve their integrity and prevent
bacterial growth. The samples were carefully transported to the
laboratory, ensuring that the organs remained undamaged during
transit. Upon arrival at the laboratory, the organs were placed
under refrigeration conditions at -15±4˚C to maintain their quality
and suitability for further analysis. This careful handling and
storage process ensured that the samples were in optimal condition
for subsequent experimental procedures and analysis.
Infusion of R. frangula extract and
bacteria into goat organs
At the end point of MIC of 5 mg/ml, R.
frangula extract was introduced into a sterile container with
brain heart infusion (BHI) (HiMedia Laboratories, LLC) medium. A
20-µl aliquot of A. baumannii was added to freshly prepared
BHI broth, followed by incubation at 37˚C for 18 h. Following this,
a wedge-shaped incision (3 mm) was made in the grossed piece of the
goat heart, lung, liver, kidney and spleen, which was subsequently
immersed in BHI broth. The previously incubated 20 µl of the
bacterial culture was inoculated into the broth. The container was
incubated at 37˚C for 24 h, alongside a control group consisting of
untreated organs. After 24 h, the organs were processed to assess
histopathological changes.
Histopathology of bacterial infused
organs
Hematoxylin and eosin (H&E) staining is a widely
utilized method in histopathology to visualize cellular components
of tissues. Herein, to preserve tissue structure and to prevent
degradation, tissue samples were fixed in 10% formalin (HiMedia
Laboratories, LLC) for at least 24 h. The fixed tissues were then
dehydrated through a series of increasing ethanol concentrations
(70, 80 and 100%) to remove water. Subsequently, the tissues were
treated with xylene to eliminate alcohol and prepare them for
paraffin infiltration. The tissues were then immersed in molten
paraffin wax, embedded in paraffin blocks and allowed to
solidify.
Thin tissue sections were obtained using a
microtome, cutting them to a thickness of 4-5 µm. These sections
were then floated on a water bath to flatten them before being
placed on glass slides. To remove the paraffin wax, the slides were
immersed in xylene, followed by rehydration through decreasing
concentrations of ethanol (100, 80 and 70%) and a rinse in
distilled water. The slides were then stained with hematoxylin
(Sigma-Aldrich, USA) at room temperature (25±2˚C) for 5-10 min,
rinsed in tap water, and differentiated in acid alcohol (1% HCl in
70% ethanol). Following another rinse in tap water, the slides were
‘blued’ by immersion in a weak alkaline solution (e.g., 0.1%
ammonia water) or tap water until the nuclei appear blue. The
slides were then stained with eosin (MilliporeSigma) at room
temperature (25±2˚C) for 1-3 min and briefly rinsed in tap water.
Dehydration is performed again by passing the slides through
increasing concentrations of ethanol (70, 80 and 100%) to remove
water, followed by immersion in xylene to eliminate ethanol and
prepare for mounting. A drop of mounting medium was applied to the
slide, and a coverslip was placed over the tissue sections. After
allowing the sections to dry, the stained sections were examined
under a light microscope (CX23, Olympus Corporation) to observe the
cellular and tissue structures (24).
Further experimental analysis was conducted to
evaluate the antibiofilm activity and growth curve of R.
frangula extract at sub-MIC concentrations against A.
baumannii.
Biofilm assay
To evaluate the effects of R. frangula
extract on A. baumannii biofilm formation, a crystal violet
staining assay was employed as previously described by Venkatraman
et al (25). A microtiter
plate containing 180 µl fresh LB medium was first filled with an
overnight culture of A. baumannii (20 µl). Subsequenlty,
R. frangula extract was added at concentrations ranging from
2.5 to 0.004 mg/ml, which is after the MIC point. For 48 h, the
mixture was incubated at 37˚C. Following incubation, the biofilm
that stuck to the surface was stained for 2 min at room temperature
using a 0.1% crystal violet solution (HiMedia Laboratories, LLC),
and the planktonic cells were eliminated by rinsing with sterile
distilled water at room temperature. The crystal violet-bound
biofilm was then eluted with 200 µl 70% ethanol after a 10-min
incubation. The absorbance of the eluted crystal violet was
measured at 520 nm using a UV-Vis spectrophotometer (JASCO UV/Vis,
India). Biofilm inhibition (%) was calculated using the following
formula:: (Control OD 520 nm - treated OD 520 nm)/control OD 520 nm
x100.
A. baumannii growth analysis
A. baumannii bacterial development was
examined using R. frangula extract at 1.25 mg/ml doses or
the extract. The culture was incubated at 37˚C for up to 24 h, and
the cell density was measured at OD 600 nm every hour.
Statistical analysis
All experiments were performed in triplicate, and
the biofilm assay (crystal violet assay), and growth curve
demonstrated statistical significance. The data were analyzed using
one-way ANOVA followed by Tukey's Honest Significant Difference
(HSD) test, performed with GraphPad Prism 10.1.0 software
(Dotmatics). A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Antimicrobial activity of R.
frangula
The antimicrobial potential of R. frangula
extract was evaluated using the agar well diffusion method against
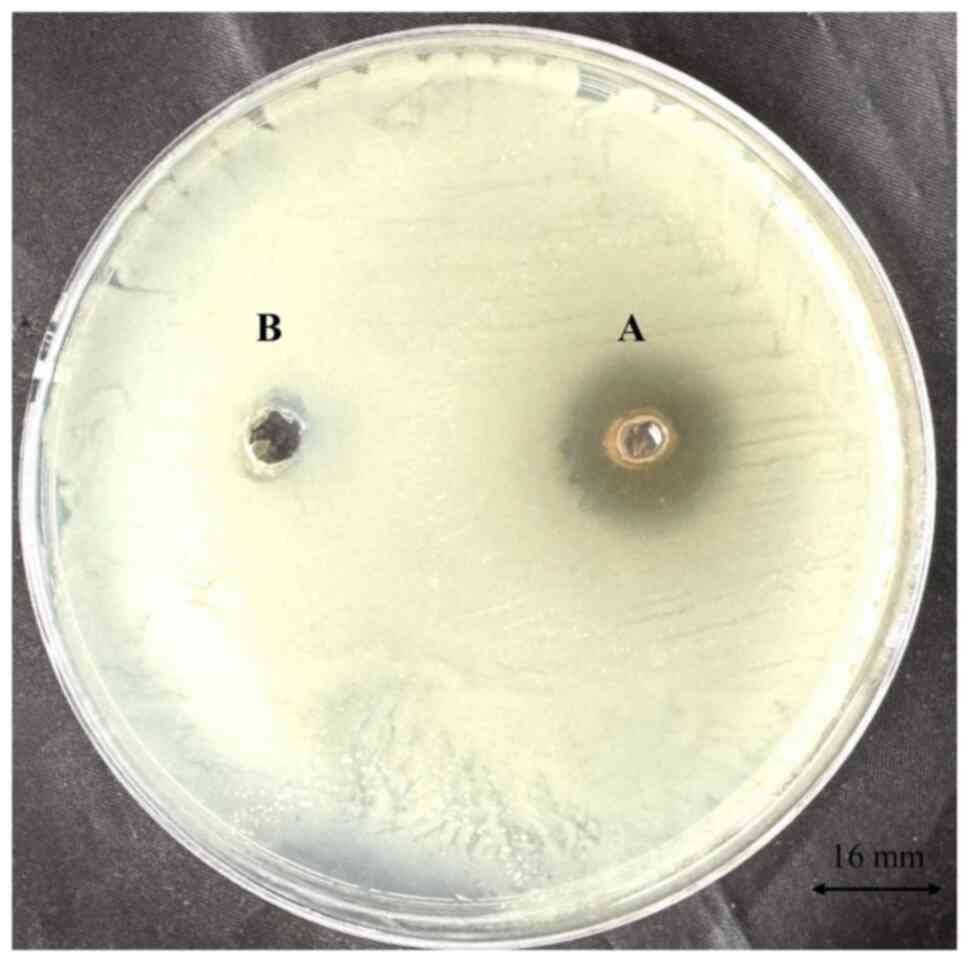
A. baumannii. The results revealed a distinct zone of
inhibition measuring 16 mm (Fig.
1), indicating a significant level of antimicrobial efficacy
against this pathogen. This suggests that R. frangula
extract possesses the capability to inhibit the growth of A.
baumannii, which is particularly relevant given the MDR nature
of the pathogen.
Antibiotic sensitivity patterns
The antibiotic resistance patterns of A.
baumannii were assessed using a panel of different antibiotic
discs. The findings indicated that the majority of the antibiotics
tested were ineffective against A. baumannii, highlighting a
notable level of MDR (Table I).
This extensive resistance complicates treatment options and
underscores the urgent need for new therapeutic strategies.
 | Table IAntibiogram of Acinetobacter
baumannii against several antibiotics. |
Table I
Antibiogram of Acinetobacter
baumannii against several antibiotics.
| Sample no. | Antibiotics | Acinetobacter
baumannii |
|---|
| 1 | Tetracycline | 10±0.8 |
| 2 | PIT | 11±0.8 |
| 3 | Cefixime | R |
| 4 | Imipenem | R |
| 5 | Ceftriaxone | R |
| 6 | Cefotaxime | R |
| 7 | Meropenem | R |
Bactericidal activity at the MIC
level
The inhibitory effect of R. frangula extract
was assessed using a 2-fold serial dilution method, with
concentrations ranging from 10 to 0.019 mg/ml. The results revealed
that the growth of A. baumannii was effectively inhibited at
a concentration of 5 mg/ml (Table
II). Subsequently, the potential antibiofilm properties of
R. frangula at sub-MIC concentrations were further
investigated. This additional analyses aimed to explore whether
lower, non-lethal concentrations of the extract could disrupt or
prevent biofilm formation, which is a key factor in the persistence
and resistance of A. baumannii in clinical settings.
 | Table IIMinimum inhibitory concentration of
R. frangula extract for the inhibition of A.
baumannii growth (final concentration of 5 mg/ml). |
Table II
Minimum inhibitory concentration of
R. frangula extract for the inhibition of A.
baumannii growth (final concentration of 5 mg/ml).
| Sample no. | Two-fold dilution
concentration (mg/ml) | Growth
measureda |
|---|
| 1 | 10 | - |
| 2 | 5 | - |
| 3 | 2.5 | + |
| 4 | 1.25 | + |
| 5 | 0.62 | + |
| 6 | 0.312 | + |
| 7 | 0.156 | + |
| 8 | 0.078 | + |
| 9 | 0.039 | + |
| 10 | 0.019 | + |
Findings of the histopathological
analysis
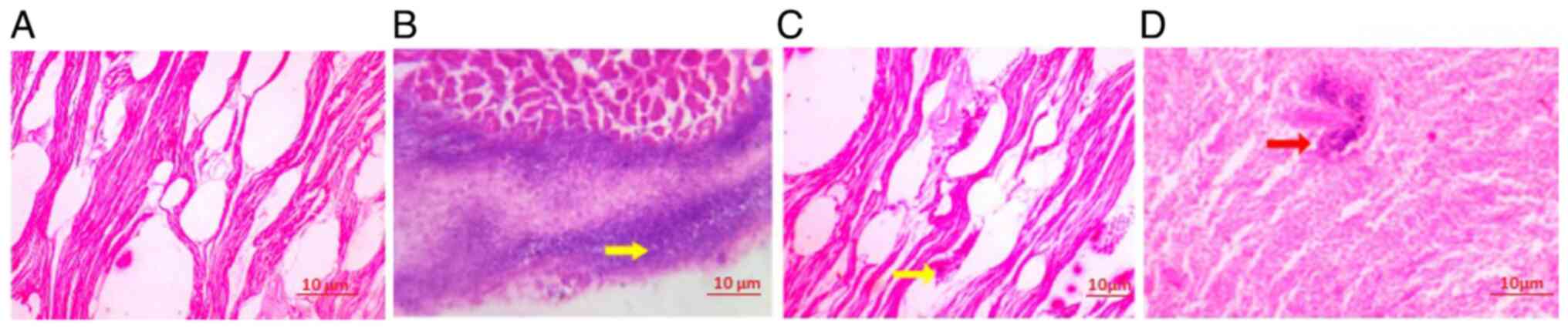
As evidenced herein, the infiltration of the
bacterium A. baumannii leads to the marked disintegration of
myofibrils. This bacterial presence triggers the degeneration of
myofibers, which is accompanied by a dense inflammatory infiltrate.
The extensive inflammatory response exacerbates the damage to
muscle tissue, resulting in the breakdown of myofibril structure
and contributing to overall muscle degeneration. This complex
pathological process underscores the aggressive nature of A.
baumannii infection and its detrimental effects on muscle
integrity (Fig. 2). As illustrated
in Fig. 2D, the cardiac tissue
treated with 5 mg/ml R. frangula extract exhibited a gradual
reduction in bacterial load and a corresponding decrease in
inflammatory cell infiltration.
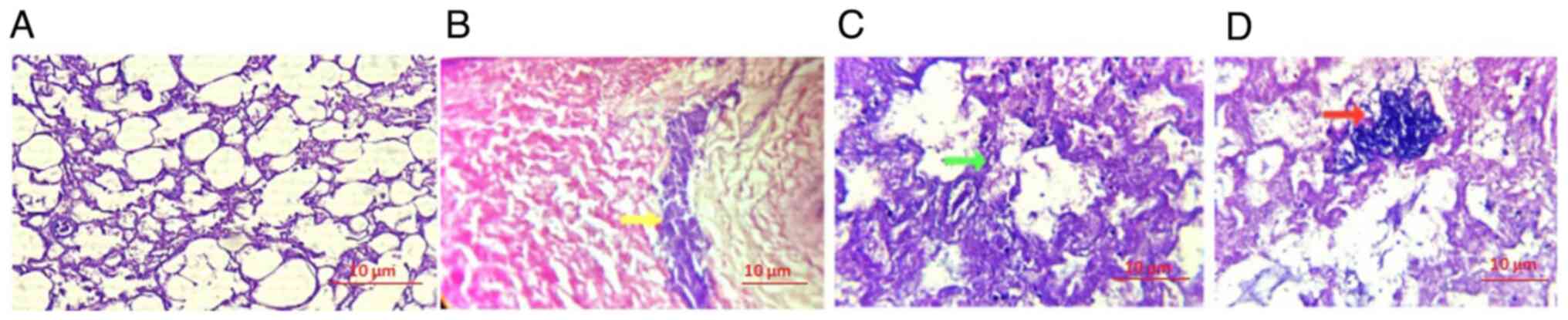
An H&E-stained section of the lung tissue
revealed diffuse alveolar damage accompanied by marked inflammatory
infiltrate. Following the infusion of the bacterium A.
baumannii, there was a marked increase in alveolar septal
thickening. This pathological alteration indicated severe lung
tissue injury, characterized by widespread damage to the alveolar
structures and intensified inflammatory response, leading to the
thickening of the alveolar walls. The histopathological findings
highlight the extensive impact of A. baumannii infection on
lung tissue architecture and function (Fig. 3). As illustrated in Fig. 3D, the lung tissue treated with the
endpoint MIC of R. frangula extract at 5 mg/ml exhibited a
reduction in bacterial load, along with a noticeable decrease in
inflammatory cell infiltration.
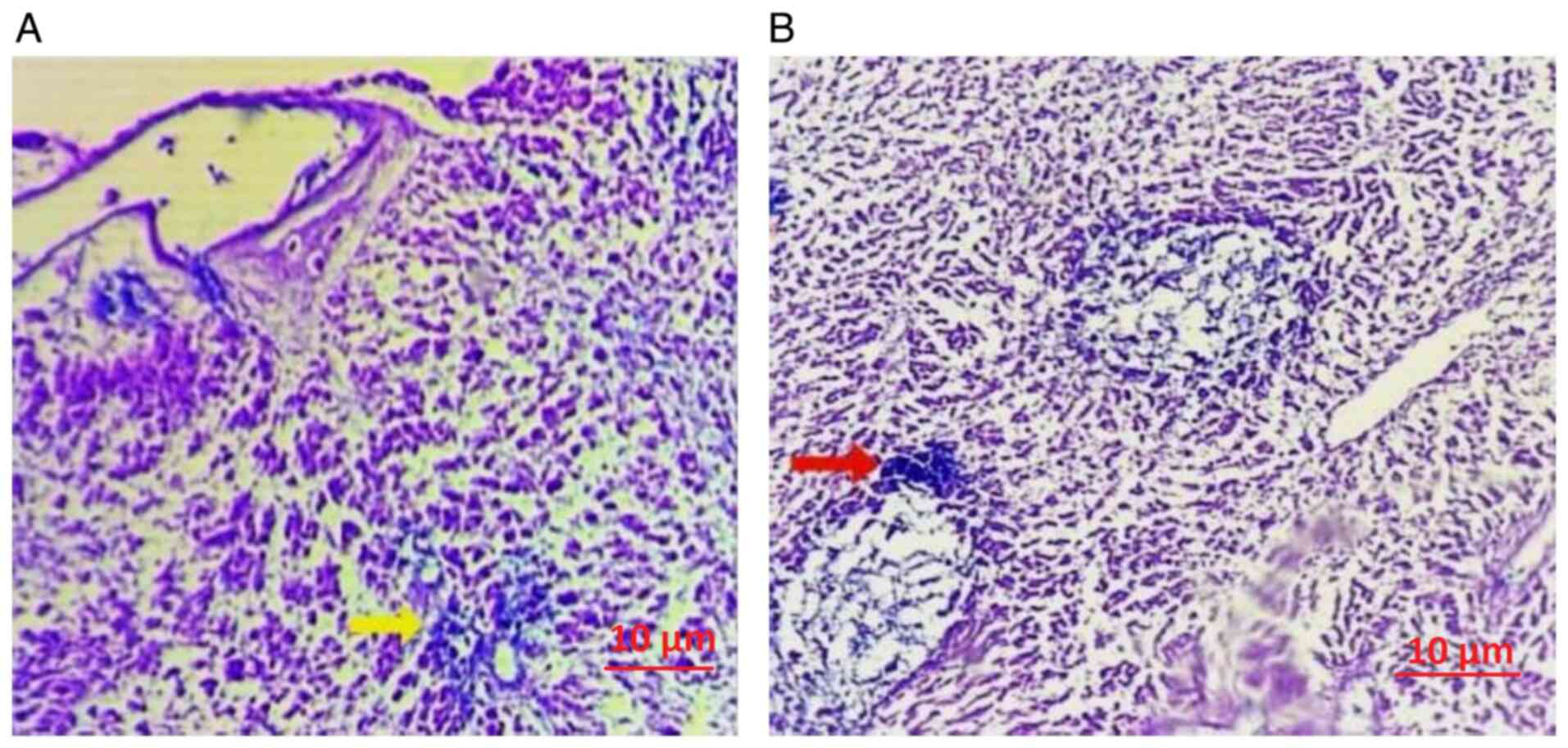
The hepatocytes demonstrated vacuolar degeneration
accompanied by the infiltration of A. baumannii (Fig. 4A). Following treatment with 5 mg/ml
R. frangula extract, there was a marked decrease in the
bacterial load (Fig. 4B). The
spleen exhibited widespread degeneration of the germinal centers,
accompanied by infiltration of A. baumannii (Fig. 5A). This was associated with a
significant bacterial load and a dense inflammatory infiltrate.
However, following treatment with 5 mg/ml R. frangula
extract, there was a notable reduction in the bacterial load
(Fig. 5B).
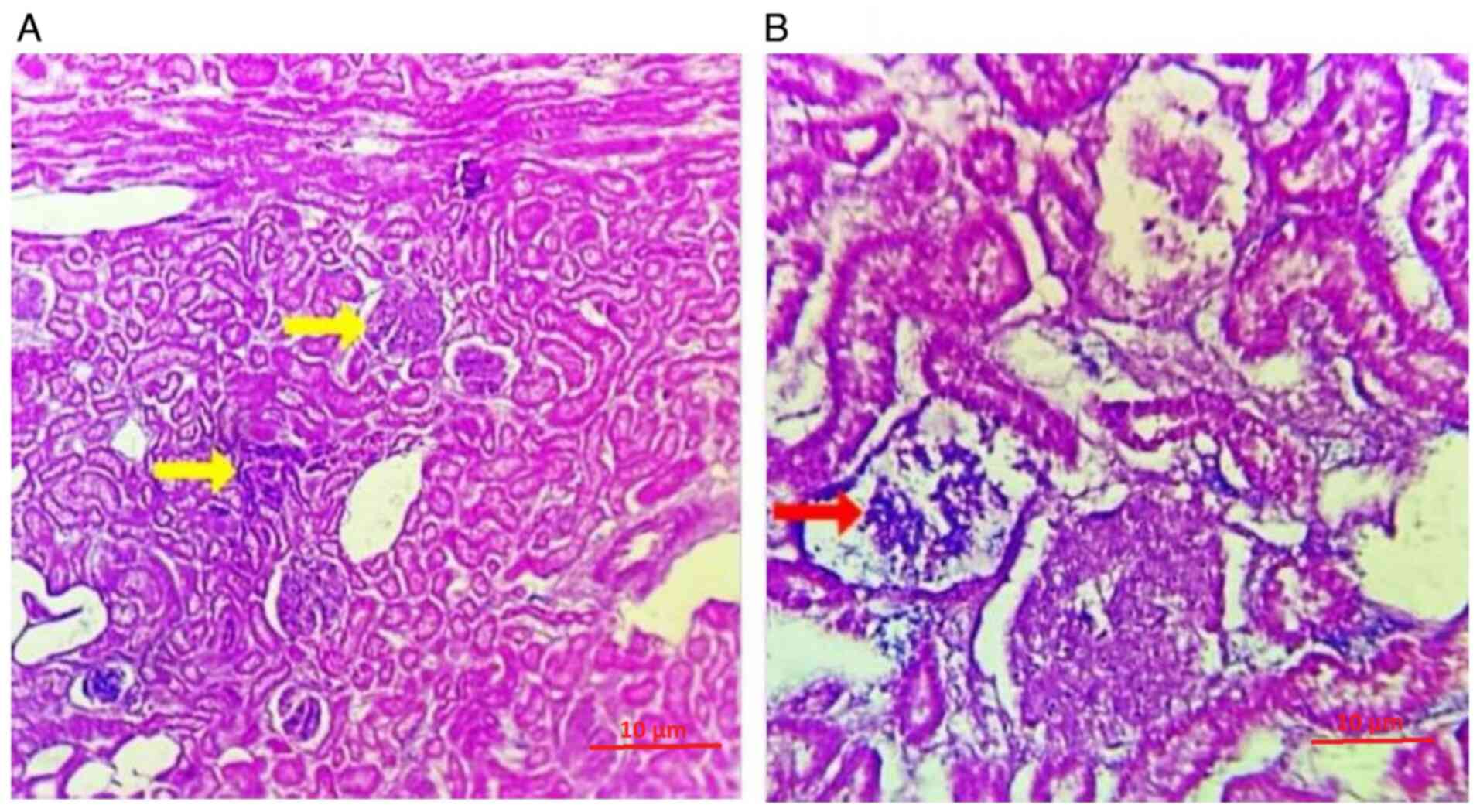
The spleen revealed generalized degeneration in the
kidney tubules, with certain regions of Bowman's capsule showing
infiltration by A. baumannii and a chronic inflammatory
response (Fig. 6A). Additionally,
areas of the kidney and Bowman's capsule exhibited vacuolar
degeneration. Following treatment with 5 mg/ml R. frangula
extract, there was a notable decrease in bacterial load, indicating
a therapeutic effect on the infected tissues (Fig. 6B).
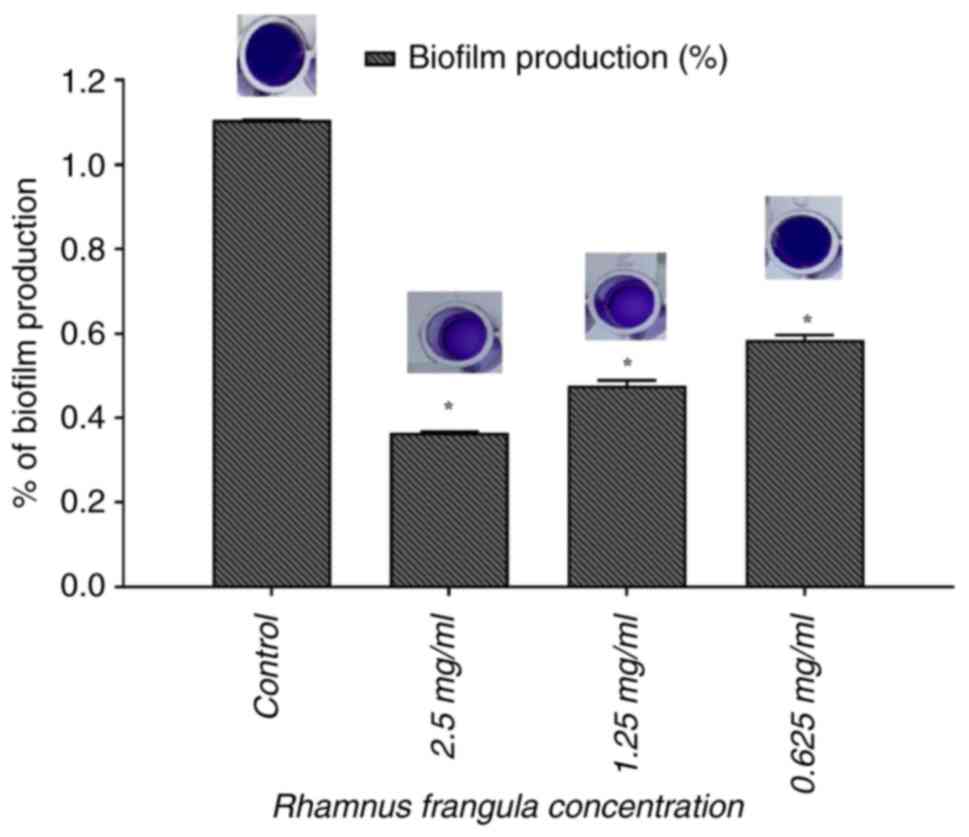
Inhibition of biofilm formation in A.
baumannii
The inhibitory effect of R. frangula extract
on the ability of A. baumannii to form biofilms was
investigated using the static microtiter plate method, with 0.1%
crystal violet staining to quantify biofilm biomass. The results
revealed that R. frangula extract significantly inhibited
biofilm formation (P<0.05, significant difference compared to
the untreated control) with spectrophotometric analysis revealing
67.26% inhibition at 2.5 mg/ml and 58.22% inhibition at 1.25 mg/ml
(Fig. 7).
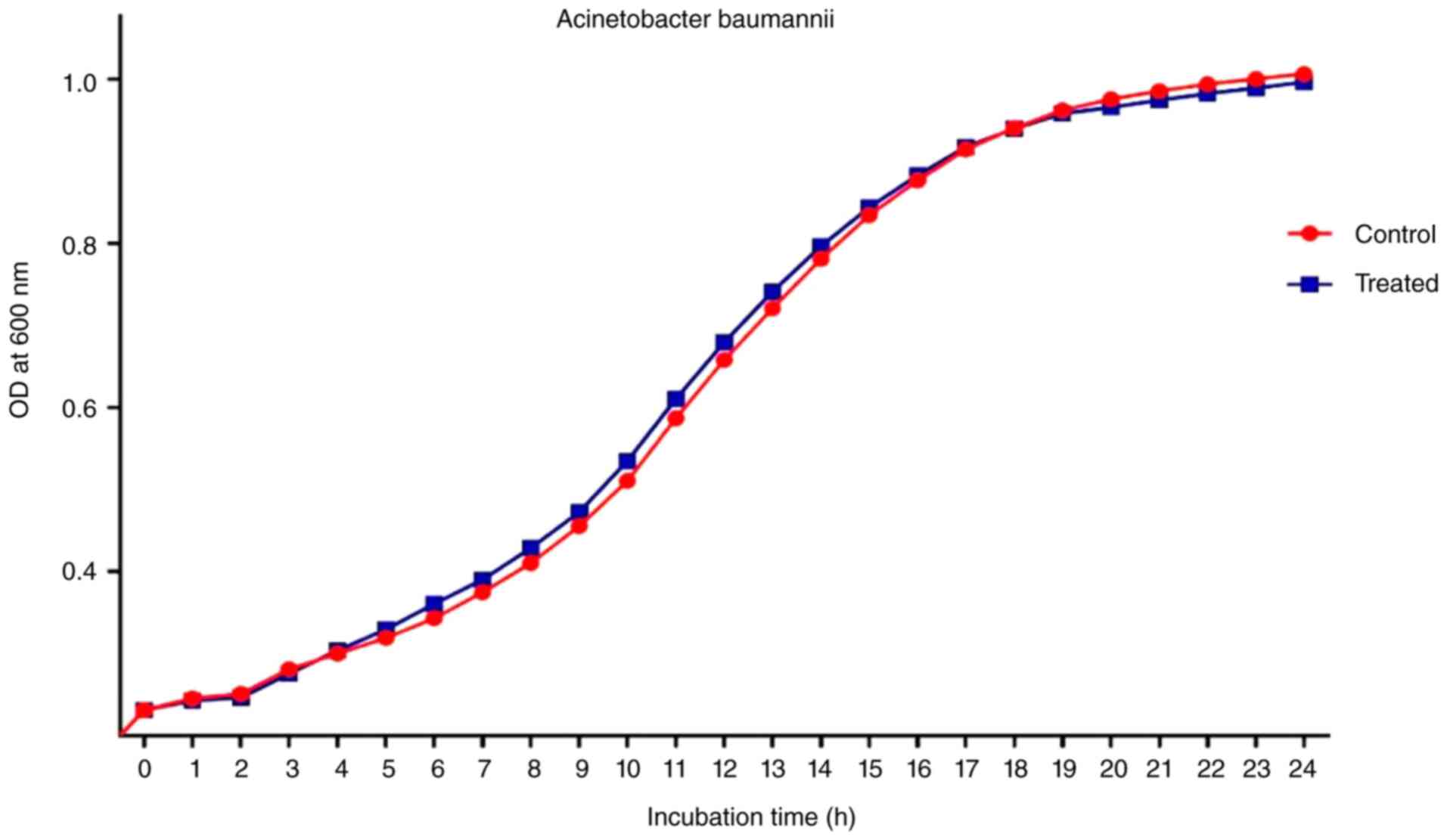
Analysis the growth of A.
baumannii
The growth curve analysis was applied both with and
without the R. frangula extract. Fig. 8 demonstrates that at a dose of 2.5
mg/ml, R. frangula extract did not inhibit the growth of
A. baumannii. Spectrophotometric measurements showed no
appreciable difference between the treated and control bacterial
cells at 600 nm. These findings suggest that R. frangula
extract does not inhibit the planktonic growth of A.
baumannii at 2.5 mg/ml also effectively reduces its ability to
form biofilms.
Discussion
A. baumannii is considered one of the most
hazardous bacteria associated with healthcare-related infections.
Its marked ability to acquire antibiotic resistance and persist in
diverse environments poses a significant challenge in clinical
settings. In the present study, a series of in vitro
experiments demonstrated that R. frangula extract
effectively reduced A. baumannii biofilm formation,
highlighting its potential as a therapeutic agent. Similarly, Zhou
et al (26) demonstrated
that P. aeruginosa three quorum sensing (QS) systems were
inhibited by hordenine, an herbal extract made from sprouting
barley.
In the present study, at a MIC of 5 mg/ml, the
initial results demonstrated that the bactericidal activity of
A. baumannii was inhibited by R. frangula extract.
This observation is consistent with the findings of a previous
study demonstrating that methanolic extracts of Cuminum
cyminum can inhibit Gram-negative bacteria at MIC levels
(27). Additionally, it has been
noted that aloe vera gel extract is more effective against
Gram-positive than Gram-negative bacteria, with ethanol and
methanol extracts exhibiting the highest activity, while acetone
extract exhibited the least inhibition (28). Furthermore, in the present study,
R. frangula extract inhibited QS-regulated biofilm formation
in A. baumannii in a concentration-dependent manner at
sub-MIC concentrations. Specifically, the crystal violet assay
revealed that a concentration of 2.5 mg/ml R. frangula
extract markedly decreased biofilm formation without influencing
planktonic cell growth (Figs. 7
and 8). Miyasaki et al
(29) also reported that specific
compounds in herbal extracts, such as flavones, tannins, and
phenolic compounds, are generally known for their
anti-Acinetobacter activity.
The discussion on biofilm formation and its
pathological implications in vital organs highlights the crucial
role of biofilms in chronic infections. Biofilm formation is a key
survival mechanism for bacteria, enhancing their resistance to
antimicrobial treatments and the immune system (30). The ability of bacteria to form
biofilms, particularly in organs such as the lungs, liver and
kidneys, contributes to persistent infections that are challenging
to eradicate, often leading to prolonged inflammation and organ
damage (31). Infections caused by
A. baumannii, a notorious biofilm producer, exemplify this
phenomenon, particularly in the lungs where biofilms are involved
in severe respiratory diseases such as cystic fibrosis and COPD
(32).
In the present study, A. baumannii
infiltrations induced notable pathological changes, including
disintegration of myofibrils in cardiac muscle and extensive damage
to alveolar structures in the lungs, which were effectively
mitigated with 5 mg/ml R. frangula extract. These findings
are consistent with those of previous research, demonstrating that
biofilm-producing bacteria exacerbate tissue damage by triggering
chronic inflammatory responses, which further deteriorate tissue
architecture (33). The persistent
presence of bacterial biofilms hinders the effectiveness of immune
clearance, allowing the bacteria to persist and cause scarring,
necrosis and the functional impairment of vital organs over time
(34).
An innovative feature of the present study lies in
its use of ex vivo goat models to evaluate the effects of
R. frangula extract on A. baumannii biofilms, an
approach that has not been extensively explored in previous
research to date, at least to the best of our knowledge. This
biologically relevant platform bridges the gap between in
vitro findings and potential in vivo applications,
providing a more comprehensive assessment of the extract's
efficacy. Furthermore, the holistic evaluation of antimicrobial and
antibiofilm activity, along with its impact on histopathological
changes across multiple organ systems (heart, lungs, liver, kidney
and spleen), underscores the therapeutic potential of the extract
and its ability to preserve tissue integrity.
The present study opens several promising avenues
for future research. The ex vivo goat organ model provides
valuable insight into the potential of R. frangula extract
for the management of A. baumannii-associated infections.
Expanding this research to include human clinical settings will
enable a more comprehensive evaluation of the extract's efficacy
and safety. Rigorous clinical trials could further establish its
therapeutic potential and bridge the gap to clinical applications.
Additionally, the present study investigated only a single isolate
of A. baumannii, which may limit the broader applicability
of the findings. Future studies are thus required to involve a
diverse range of clinical isolates to ensure the consistency and
generalizability of the results. Moreover, the findings of the
present study on biofilm inhibition create opportunities to delve
deeper into the molecular mechanisms behind this activity. A more
detailed exploration could illuminate the mode of action of the
extract, paving the way for innovative strategies, such as
combining R. frangula with conventional antibiotics to
enhance therapeutic outcomes.
Taken together, the findings of the present study
provide compelling evidence of the therapeutic potential of R.
frangula extract against A. baumannii biofilms, while
also recognizing its inherent strengths and limitations. These
findings contribute to the ongoing efforts to combat
antibiotic-resistant infections and underscore the importance of
further studies to fully explore and realize the clinical
applicability of this natural remedy.
In conclusion, the results of the present study
demonstrate the potential of R. frangula extract as a
beneficial treatment choice for A. baumannii infections. The
present study demonstrates the complex role of biofilms in
promoting bacterial persistence and tissue damage, while also
shedding light on the potential of natural compounds to mitigate
these effects. Further investigations are required however, to on
the molecular mechanisms underlying the biofilm-inhibitory
properties of R. frangula, contributing to the development
of novel therapeutic strategies for biofilm-associated
infections.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RMRV collected, managed the data and participated in
the writing of the manuscript. PR and NNP participated in writing
the proposal, performing data collection and in the writing of the
manuscript. PR and PSG were involved in data curation, data
analysis and in revising the manuscript. PR, NNP and PSG confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Scientific
Review Board of Saveetha Dental College and Hospitals, Chennai,
Tamil Nadu, India (SRB/SDC/UG-2276/24/GPATH/076). The approval was
granted for the use of goat samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ibrahim S, Al-Saryi N, Al-Kadmy IMS and
Aziz SN: Multidrug-resistant Acinetobacter baumannii as an emerging
concern in hospitals. Mol Biol Rep. 48:6987–6998. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pathoor NN, Ganesh PS and Gopal RK:
Microbiome interactions: Acinetobacter baumannii biofilms as a
co-factor in oral cancer progression. World J Microbiol Biotechnol.
40(398)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gedefie A, Demsis W, Ashagrie M, Kassa Y,
Tesfaye M, Tilahun M, Bisetegn H and Sahle Z: Biofilm formation and
its role in disease pathogenesis: A review. Infect Drug Resist.
14:3711–3719. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Müller C, Reuter S, Wille J, Xanthopoulou
K, Stefanik D, Grundmann H, Higgins PG and Seifert H: A global view
on carbapenem-resistant Acinetobacter baumannii. mBio.
14(e0226023)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Girija ASS: Acinetobacter baumannii as an
oro-dental pathogen: A red alert!! J Appl Oral Sci.
32(e20230382)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Loehfelm TW, Luke NR and Campagnari AA:
Identification and characterization of an Acinetobacter baumannii
biofilm-associated protein. J Bacteriol. 190:1036–1044.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pai L, Patil S, Liu S and Wen F: A growing
battlefield in the war against biofilm-induced antimicrobial
resistance: Insights from reviews on antibiotic resistance. Front
Cell Infect Microbiol. 13(1327069)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Smiline Girija SA: Hijacking the
epigenetic mechanisms of A. baumannii. Mol Biol Res Commun.
13:51–53. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pathoor NN, Viswanathan A, Wadhwa G and
Ganesh PS: Understanding the biofilm development of Acinetobacter
baumannii and novel strategies to combat infection. APMIS.
132:317–335. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mohajeri P, Farahani A, Feizabadi MM and
Norozi B: Clonal evolution multi-drug resistant Acinetobacter
baumannii by pulsed-field gel electrophoresis. Indian J Med
Microbiol. 33:87–91. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mohajeri P, Sharbati S, Farahani A and
Rezaei Z: Evaluate the frequency distribution of nonadhesive
virulence factors in carbapenemase-producing Acinetobacter
baumannii isolated from clinical samples in Kermanshah. J Nat Sci
Biol Med. 7:58–61. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nasim N, Sandeep IS and Mohanty S:
Plant-derived natural products for drug discovery: Current
approaches and prospects. Nucleus (Calcutta). 65:399–411.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kalinowska M, Gołębiewska E, Świderski G,
Męczyńska-Wielgosz S, Lewandowska H, Pietryczuk A, Cudowski A,
Astel A, Świsłocka R, Samsonowicz M, et al: Plant-derived and
dietary hydroxybenzoic acids-a comprehensive study of structural,
anti-/pro-oxidant, lipophilic, antimicrobial, and cytotoxic
activity in MDA-MB-231 and MCF-7 cell lines. Nutrients.
13(3107)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Greenleaf J, Karimzadeh R and Park YL:
Spatial patterns of Frangula alnus (Rosales: Rhamnaceae):
Implications for invasive plant management. Biology (Basel).
12(1393)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Górniak I, Bartoszewski R and Króliczewski
J: Comprehensive review of antimicrobial activities of plant
flavonoids. Phytochem Rev. 18:241–272. 2019.
|
|
16
|
Lahiri D, Nag M, Ray RR and Ghosh S (eds):
Biofilm-Associated Antimicrobial Resistance and Its Recovery. CRC
Press, Boca Raton, p391, 2023.
|
|
17
|
Kumar S, Chandra N, Singh L, Hashmi MZ and
Varma A (eds): Biofilms in Human Diseases: Treatment and Control.
Springer Nature, p318, 2019.
|
|
18
|
Labis V, Gaiduk I, Bazikyan E, Khmelenin
D, Zhigalina O, Dyachkova I, Zolotov D, Asadchikov V, Kravtsov I,
Polyakov N, et al: The role of metal nanoparticles in the
pathogenesis of stone formation. Int J Mol Sci.
25(9609)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bobenchik AM, Deak E, Hindler JA, Charlton
CL and Humphries RM: Performance of Vitek 2 for antimicrobial
susceptibility testing of Acinetobacter baumannii, Pseudomonas
aeruginosa, and Stenotrophomonas maltophilia with Vitek 2 (2009
FDA) and CLSI M100S 26th edition breakpoints. J Clin Microbiol.
55:450–456. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Soni M, Naseef Pathoor N, Viswanathan A,
Veeraragavan GR and Sankar Ganesh P: Exploring the antimicrobial
and antibiofilm activities of Artocarpus heterophyllus Lam. against
Pseudomonas aeruginosa PAO1. World Acad Sci J. 6(50)2024.
|
|
21
|
Hudzicki J: Kirby-Bauer Disk Diffusion
Susceptibility Test Protocol. American Society for Microbiology,
Washington, DC, pp55-63, 2009.
|
|
22
|
Howard A, O'Donoghue M, Feeney A and
Sleator RD: Acinetobacter baumannii: An emerging opportunistic
pathogen. Virulence. 3:243–250. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Peer Mohammed S, Pathoor N, Veeraragavan G
and Ganesh P: Unlocking the antibiofilm and anti-virulence
potential of Pithecellobium dulce against Chromobacterium violaceum
CV12472. World Acad Sci J. 7(14)2024.
|
|
24
|
Bancroft JD and Gamble M (eds): Theory and
practice of histological techniques. 6th edition. Elsevier Health
Sciences, p744, 2008.
|
|
25
|
Venkatramanan M, Sankar Ganesh P, Senthil
R, Akshay J, Veera Ravi A, Langeswaran K, Vadivelu J, Nagarajan S,
Rajendran K and Shankar EM: Inhibition of quorum sensing and
biofilm formation in Chromobacterium violaceum by fruit extracts of
Passiflora edulis. ACS Omega. 5:25605–25616. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou JW, Luo HZ, Jiang H, Jian TK, Chen ZQ
and Jia AQ: Hordenine: A novel quorum sensing inhibitor and
antibiofilm agent against Pseudomonas aeruginosa. J Agric Food
Chem. 66:1620–1628. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sybiya Vasantha Packiavathy IA,
Agilandeswari P, Musthafa KS, Karutha Pandian S and Veera Ravi A:
Antibiofilm and quorum sensing inhibitory potential of Cuminum
cyminum and its secondary metabolite methyl eugenol against
gram-negative bacterial pathogens. Food Res Int. 45:85–92.
2012.
|
|
28
|
Lawrence R, Tripathi P and Jeyakumar E:
Isolation, purification and evaluation of antibacterial agents from
Aloe vera. Braz J Microbiol. 40:906–915. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miyasaki Y, Rabenstein JD, Rhea J, Crouch
ML, Mocek UM, Kittell PE, Morgan MA, Nichols WS, Van Benschoten MM,
Hardy WD and Liu GY: Isolation and characterization of
antimicrobial compounds in plant extracts against
multidrug-resistant Acinetobacter baumannii. PLoS One.
8(e61594)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hu J, Shuai W, Sumner JT, Moghadam AA and
Hartmann EM: Clinically relevant pathogens on surfaces display
differences in survival and transcriptomic response in relation to
probiotic and traditional cleaning strategies. NPJ Biofilms
Microbiomes. 8(72)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li XZ, Elkins CA and Zgurskaya HI:
Efflux-mediated antimicrobial resistance in bacteria: mechanisms,
regulation and clinical implications. Springer, New York, NY, p848,
2016.
|
|
32
|
Grygiel I, Bajrak O, Wójcicki M, Krusiec
K, Jończyk-Matysiak E, Górski A, Majewska J and Letkiewicz S:
Comprehensive approaches to combatting Acinetobacter baumannii
biofilms: From biofilm structure to phage-based therapies.
Antibiotics (Basel). 13(1064)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mendhe S, Badge A, Ugemuge S and Chandi D:
Impact of biofilms on chronic infections and medical challenges.
Cureus. 15(e48204)2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kostakioti M, Hadjifrangiskou M and
Hultgren SJ: Bacterial biofilms: Development, dispersal, and
therapeutic strategies in the dawn of the postantibiotic era. Cold
Spring Harb Perspect Med. 3(a010306)2013.PubMed/NCBI View Article : Google Scholar
|