Introduction
Nephrotic syndrome (NS) is a common renal disease
affecting the pediatric population. This idiopathic condition is
responsive to steroid therapy; however, 40-50% of patients
experience frequent relapses or steroid dependence (1). Based on the response of the disease
to corticosteroids, NS can be categorized as steroid-sensitive NS
(SSNS) if full remission is achieved within 28 days of steroid
treatment, steroid-dependent NS (SDNS) following two consecutive
relapses or 14 days of treatment discontinuation, or
steroid-resistant NS (SRNS) if full remission is not achieved after
8 weeks of steroid treatment (2).
Although the KDIGO guidelines (3)
reduced the glucocorticoids dose (60 mg/m2/day) for the
initial 4 weeks, and then to 40 mg/m2 on alternate days
for 8 to 20 weeks to mitigate the adverse effects of steroids
(1), repeated and extended
treatments with corticosteroids may be considered during relapses
(4).
Among the severe adverse effects that are associated
with corticosteroid treatment are ocular manifestations. The
association between corticosteroid use and increased intraocular
pressure has been documented in the early 1950s (5,6). The
association of posterior subcapsular cataracts (PSCCs) with steroid
treatment has also been documented with a strong association with
the dose and duration of treatment (7). Patients with NS may also experience
other eye-related issues, such as skin atrophy in the eyelids,
ptosis, mydriasis, thinning of the cornea and sclera, keratitis,
recurrent episodes of hordeolum (8). Although severe complications have
been reported in patients with NS, a notable number of these
children are unable to detect any deterioration in their vision and
do not exhibit any symptoms that could potentially result in
blindness. There are currently no established guidelines for the
routine monitoring of patients with NS to facilitate the early
identification and treatment of these severe complications and
prevent the development of additional health complications
(9,10).
The prevalence of steroid-dependent and independent
ocular manifestations in children with NS has not yet been fully
determined, particularly in the context of steroid response
classification. Moreover, the association between the duration and
dosage of steroid treatment has produced conflicting outcomes in
these globally performed trials (11-13).
There is a scarcity of data that directly compares the ocular
manifestations in children with SRNS and SSNS in Iraq. The present
study aimed to investigate ocular manifestations in patients with
NS who had undergone prolonged treatments with steroids and to
compare the prevalence of these manifestations between those with
SSNS and those with SRNS.
Patients and methods
Study participants and
inclusion/exclusion criteria
The present cross-sectional study was conducted at
the Ibn AL-Haytham Teaching Eye Hospital (Baghdad, Iraq) during the
period between 2022 and 2023. The research received approval from
the Scientific and Ethics Committee of Alkadimayn Teaching Hospital
(no.=5542) and Ibn AL-Haytham Teaching Eye Hospital (no.=964) (both
in Baghdad, Iraq) in accordance with the Declaration of
Helsinki.
A total of 100 children were referred from the
pediatric nephrology clinic at the Alkadimayn Teaching Hospital
with a confirmed diagnosis of NS. The inclusion criteria were
pediatric patients aged 2-18 years receiving treatment with
steroids for NS who exhibited proteinuria >40 mg/h/m2
or >50 mg/kg/day, a protein/creatinine ratio >0.2 g/mmol
(>2 g/g), and hypoalbuminemia <25 g/l with or without edema
(14). The included patients had
negative serology test results for anti-dsDNA, ANA, ANCA, C3, C4,
CH50, anti-HIV, anti-HCV and HBS-Ag, and all had a
histopathological confirmed a diagnosis of focal segmental
glomerulosclerosis or minimal change disease. Written informed
consents from the parents were acquired after providing them with
all relevant information.
The participants who had received corticosteroids,
cyclophosphamide, or cyclosporine treatment within the previous 6
months, as well as those with secondary NS and NS with an onset at
a young age (<1 year) were precluded from the study.
Additionally, children with congenital cataracts, syndromic NS, or
any other eye condition that could cause complications such as
inflammation, infection, or damage were excluded from the study.
Furthermore, individuals who failed to comply with the requirements
of a thorough ophthalmological examination were not included.
The standard treatment for the first episode was
oral prednisolone at a dosage of 40-60 mg/m²/d, taken in split
doses for a period of 4-6 weeks. SRNS is defined as the inability
to attain remission after a 4-week period of treatment with
prednisone at a dosage of 60 mg/m2, with or without
three additional methylprednisolone pulses at a dosage of 10-20
mg/kg on 3 consecutive days (15,16).
The prednisolone dosage was decreased to 40 mg/m² every other day
for an extra duration of 4 weeks in all patients. Hypertension was
defined as BP ≥95th percentile for age, height and sex (17). Frequent relapsing NS is defined as
the occurrence of two or more episodes within a 6-month period or
four or more episodes within a single year (18).
Pediatric cases were recruited from nephrology
clinic at Alkadimayn Teaching Hospital by a specialist
pediatrician. These cases were then referred to Ibn AL-Haytham
Teaching Eye Hospital (specialist eye hospital) to be examined by a
specialist ophthalmologist to conduct proper ophthalmological
examinations.
A thorough ophthalmological examination included the
evaluation of visual acuity (VA) using the Snellen visual acuity or
another age-appropriate VA test. Visual impairment was graded
according to the revised visual impairment definitions of the
International Statistical Classification of Diseases (19). Intraocular pressure was measured
and slit lamp biomicroscopy was used to examine the anterior
segment of the eye in addition to dilated fundoscopy, and
cycloplegic refraction.
Statistical analysis
The Statistical Package for Social Sciences software
for Windows version 25 (IBM Corp.) was used for all statistical
analyses. Observational data are presented in the form of
frequencies and percentages. Continuous variables are expressed as
the mean and standard deviation (SD). Comparisons of nominal
variables of different groups were performed using the Chi-squared
test or Fisher's exact tests, as appropriate. Comparisons of
continuous variables were performed using the non-parametric
Mann-Whitney test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Of the 100 included children with NS, 75 children
had SSNS and 25 children were had SRNS. The median age of the
participants was 6.5 years, ranging between 2.2 and 15.2 years; no
significant differences were found between the SSNS and SRN groups
as regards age. The median duration of the disease was 33.6 months,
ranging between 2.4 and 144 months, with no significant differences
found between the study groups. Males constituted 70% of all
patients, accounting for the majority of both SSNS [50 (66.7%)] and
SRNS [20 (80%)] groups. Systolic and diastolic pressure levels were
significantly higher in the patients with SRNS, with a mean of
100.2±8.72 and 68.0±6.12, respectively. Frequent relapse was
reported in 11 (14.7%) patients in the SSNS group. The demographic
data of the patients are presented in Table I.
 | Table IDemographic data of the patients in
the SSNS and SRNS groups. |
Table I
Demographic data of the patients in
the SSNS and SRNS groups.
| Parameter | Patients with SSNS,
n=75 | Patients with SRNS,
n=25 | P-value |
|---|
| Age (years), mean ±
SD | 7.95±3.66 | 8.12±3.45 | 0.837 |
| Age at onset (years),
mean ± SD | 4.27±2.08 | 4.34±2.47 | 0.896 |
| Sex, n (%) | | | |
|
Male | 50 (66.7) | 20 (80.0) | 0.313a |
|
Female | 25 (33.3) | 5 (20.0) | |
| BMI
(kg/m2). Mean ± SD | 17.58±2.74 | 68.60±9.86 | <0.001 |
| Systolic BP (mmHg),
mean ± SD | 95.54±8.09 | 100.2±8.72 | 0.024 |
| Diastolic BP (mmHg),
mean ± SD | 60.27±8.3 | 68.0±6.12 | <0.001 |
| Duration of the
disease (months), mean ± SD | 44.2±32.3 | 45.3±35.9 | 0.936 |
| Frequent relapse, n
(%) | | | |
|
No | 64 (85.3) | 19(76) | 0.357a |
|
Yes | 11 (14.7) | 6(24) | |
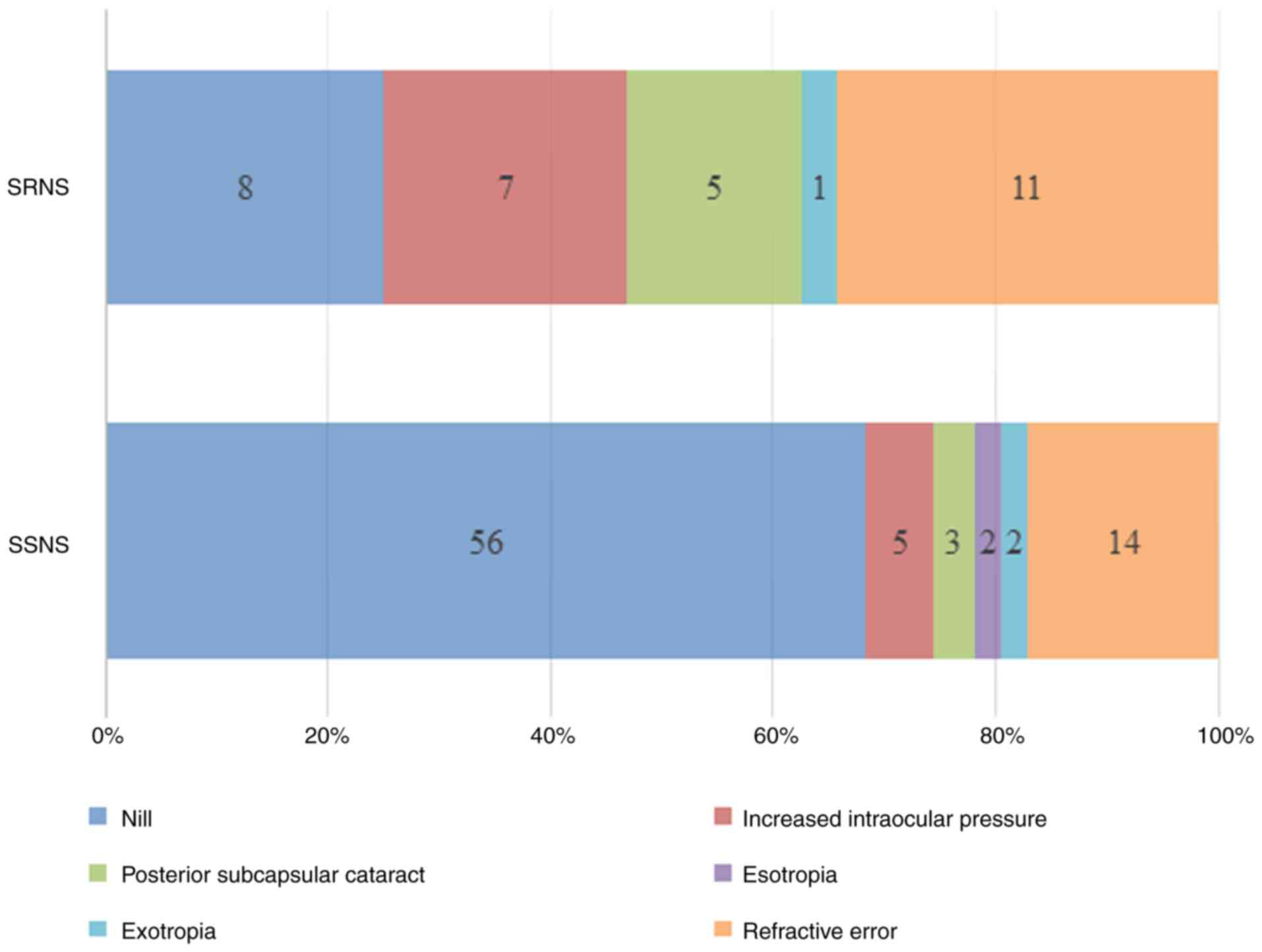
Ocular manifestations were more frequently observed
in the SRNS group; 17 (68%) of the patients with SRNS had such
manifestations compared to only 19 (25.3%) patients with SSNS. The
types and frequency of ocular findings are demonstrated in Fig. 1.
Compared with the SSNS group, the patients with SRNS
had a significantly higher rate of bilateral increased intraocular
pressure [7 (28%) vs. 5 (6.67)] and PSCC [5 (20%) vs. 3 (4%)], with
obtained P-values of 0.009 and 0.022, respectively. Similarly,
refractive errors were significantly more common in the patients
with SRNS, observed in 11 (44%) patients compared to 14 (18.7%)
patients in the SSNS group (P=0.011). Nonsteroid-dependent
manifestations, such as esotropia and exotropia were less
frequently observed, with no significant differences between the
groups. The frequency of ocular findings the patients are presented
in Table II.
 | Table IIFrequency of ocular findings in the
SSNS and SRNS groups of patients. |
Table II
Frequency of ocular findings in the
SSNS and SRNS groups of patients.
| Ocular findings | Total, n (%) | SSNS (n=75), n
(%) | SRNS (n=25), n
(%) | P-valuea |
|---|
| Increased intraocular
pressure | | | | |
|
No | 88(88) | 70 (93.3) | 18(72) | 0.009 |
|
Yes | 12 (12.0) | 5 (6.67) | 7 (28.0) | |
| Posterior subcapsular
cataract | | | | |
|
No | 92(92) | 72(96) | 20(80) | 0.022 |
|
Yes | 8 (8.0) | 3 (4.0) | 5 (20.0) | |
| Esotropia | | | | |
|
No | 98(98) | 73 (97.3) | 25(100) | NS |
|
Yes | 2 (2.0) | 2 (2.67) | 0 (0.0) | |
| Exotropia | | | | |
|
No | 97(97) | 73 (97.3) | 24(96) | NS |
|
Yes | 3 (3.0) | 2 (2.67) | 1 (4.0) | |
| Refractive error | | | | |
|
No | 75(75) | 61 (81.3) | 14(56) | 0.011 |
|
Yes | 25(25) | 14 (18.7) | 11(44) | |
| Mild VA
impairment | 22 (88.0) | 12 (85.7) | 10 (90.9) | NS |
| Moderate VA
impairment | 3(12) | 2 (14.3) | 1 (9.1) | |
PSCC and refractive errors were significantly
associated with older patients, with a mean age of 12.2±2.8
(P=0.002) and 10.3±3.8 (P=0.001) years, respectively. Refractive
errors exhibited a significant association with a prolonged
duration of the disease (P=0.004), while increased IOP (P<0.001)
and PSCC (P=0.003) were significantly associated with frequent
relapses. The three conditions exhibited a significant association
with increased higher systolic pressure, as shown in Table III.
 | Table IIIAssociation between ocular
manifestations and patient characteristics. |
Table III
Association between ocular
manifestations and patient characteristics.
| | Bilateral IOP
(mmHG) | PSCC | Refractive
error |
|---|
| Variables | Normal | High | P-value | Absent | Present | P-value | Absent | Present | P-value |
|---|
| Sex, n (%) | | | | | | | | | |
|
Male | 62 (88.6) | 8 (11.4) | 0.749a | 65 (92.9) | 5 (7.1) | 0.694a | 54 (77.1) | 16 (22.9) | 0.615a |
|
Female | 26 (86.7) | 4 (13.3) | | 27(90) | 3(10) | | 21(70) | 9(30) | |
| Age (years), mean
(SD) | 8 (3.6) | 8.2 (3.6) | 0.722 | 7.6 | 12.2 (2.8) | 0.002 | 7.2 (3.2) | 10.3 (3.8) | 0.001 |
| Duration of
disease, mean (SD) | 43.1 (31.1) | 54.5 (45.4) | 0.562 | 42.1 | 72 (49.8) | 0.088 | 39.2 (30.2) | 60 (36.6) | 0.004 |
| Systolic BP (mmHg),
mean (SD) | 100(16) | 103(7) | 0.05 | 99(12) | 122(28) | 0.009 | 97(10) | 113(20) |
<0.001 |
| Diastolic BP
(mmHg), mean (SD) | 53(16) | 40(26) | 0.206 | 52(18) | 45(17) | 0.269 | 52(17) | 49(20) | 0.732 |
| Frequent relapse, n
(%) | | | | | | | | | |
|
No | 79 (89.8) | 4 (33.3) |
<0.001a | 80(87) | 3 (37.5) |
0.003a | 81(84) | 2(80) | 0.759a |
|
Yes | 9 (10.2) | 8 (66.7 | | 12(13) | 5 (62.5) | | 17(16) | 5(20) | |
The patients with NS also suffered from
steroid-independent ocular pathologies, which included myopic
astigmatism, exotropia, esotropia and anisometropic amblyopia.
There was no statistically significant difference between the SSNS
and SRNS, as shown in Table
IV.
 | Table IVFrequency of steroid-independent
ocular findings in the SSNS and SRNS groups of patients. |
Table IV
Frequency of steroid-independent
ocular findings in the SSNS and SRNS groups of patients.
| Steroid-independent
ocular findings | SSNS (n=75), n
(%) | SRNS (n=25), n
(%) | Total, n (%) |
P-valuea |
|---|
| Myopic
astigmatism | | | | |
|
No | 65 (86.7) | 19(76) | 84(84) | 0.220 |
|
Yes | 10 (13.3) | 6 (24.0) | 16 (16.0) | |
| Exotropia | | | | |
|
No | 73 (97.3) | 24(96) | 97(97) | NS |
|
Yes | 2 (2.7) | 1 (4.0) | 3 (3.0) | |
| Esotropia | | | | |
|
No | 73 (97.3) | 25(100) | 98(98) | NS |
|
Yes | 2 (2.67) | 0 (0.0) | 2 (2.0) | |
| Anisometropic
amblyopia | | | | |
|
No | 73 (97.3) | 25(100) | 98(98) | NS |
|
Yes | 2 (2.67) | 0 (0.0) | 2 (2.0) | |
Discussion
The present study documented whether the ocular
manifestations in children with NS were steroid-sensitive or
steroid-resistant. It was found that ocular findings were more
frequent in SRNS (68%) compared to (24%) in SSNS, although a
significant difference in the duration of the disease or presence
of frequent relapses was not observed between the two groups. The
increased prevalence of ocular complications in children with SRNS
may be attributed to the fact that these patients are more likely
to receive intravenous pulse methylprednisolone before
transitioning to alternative treatments, which may expose them to
the potential adverse effects of corticosteroids. A previous study
reported that ocular abnormalities were more prevalent among
patients who received steroids irrationally (47%) than those who
received steroids according to the standard regimen (18%) (9). The rate reported by that study for
the standard regimen was low compared to the rate in the SSNS group
in the present study (18 vs. 24%); however, the median duration of
the disease from the time of onset to the date of ocular assessment
in the present study was 33.6 months, whereas this was not clear in
the study by Agrawal et al (9). Olonan et al (20) found that cataract formation was
more prevalent in pediatric patients who had been receiving steroid
therapy for an extended period, accounting for 13.6%.
In the present study, refractive error was
significantly higher in the SRNS group, accounting for almost half
of these patients compared to only 18% of the SSNS group. In their
study, Zulfiqar et al (6)
demonstrated that refractive error was the most prevalent ocular
manifestation, affecting 25% of children with NS and that it was
more commonly observed in those with SRNS; this is in agreement
with the findings of the present study. An Egyptian study reported
a higher rate of refractive error in children with NS, reaching 79%
(11).
Steroid-dependent findings were significantly more
common in those with SRNS, where 28% had increased IOP and 20%
developed PSCC compared to 6.67 and 4% in those with SSNS,
respectively. Zulfiqar et al (6) observed 2 cases of glaucoma, 1 case of
increased IOP in both eyes and 2 cases of PSCC. By contrast, an
Indian study reported a higher percentage of patients with PSCC and
elevated IOP (26.8 and 10.9%, respectively) (12). Elsharkawy et al (11) found no cases of increased IOP or
PSCC, despite a high rate of refractive errors in 79.2% of the
participants. An earlier Turkish study reported that ocular
complications were observed in 27% of those with SRNS, but in no
patients with SSNS, most of which were refractive errors while
steroid-dependent complications were only 9% (21). In a study addressing
steroid-induced glaucoma, Phulke et al (22) concluded that IOP elevation
typically occurs within the initial few weeks of steroid
administration in steroid-responsive patients. Nevertheless, it may
be elevated within 1 h or for a number of years following chronic
steroid use (22). Research
suggests that variable IOPs from steroid use can cause myopic
astigmatism by stretching the globe and lengthening the axial axis
(23). However, Kyrieleis et
al (24) discovered no causal
association in this occurrence.
Patients with glaucoma often do not notice the
gradual loss of vision until the disease has advanced, in contrast
to cataracts, which usually cause noticeable visual symptoms from
the beginning (25). Hence, ocular
examinations should be a part of the routine follow-up of patients
with NS to avoid irreversible damage, particularly since an
elevated IOP can be reversed (26). The necessity of commencing
ophthalmological surveillance in a timely manner was demonstrated
by a previous study, which found eye-related complications occurred
within 6 months of commencing steroid therapy (23).
In the present study, frequent relapses were
associated with increased IOP, PSCC and refractive error in
patients with NS. In addition, an increased age, duration of the
disease and systolic pressure were associated with refractive error
and PSCC. A recent study conducted on 45 Japanese children
diagnosed with NS found an association between the dose and
duration of the medication and the occurrence of PSCC; however,
there was no identified link between treatment and increased IOP
(23). Although in the present
study, the precise duration of steroid treatment could not be
included due to lack of data, an association was found between the
duration from the onset of the disease to the time of the eye
examination, as well as the frequency of the relapse; this could
indicate a prolonged duration of steroid treatment. In agreement
with the findings of the present study, Hayasaka et al
(23) found a strong association
between an older age and the development of PSCC.
The present study has some limitations which should
be mentioned. Due to the cross-sectional nature of the study, it
was not possible to determine the precise time of the start of
ocular disorders in the affected children. As a result, the impact
of the dosage and duration of corticosteroid therapy and the
development of ocular disorders may not be readily evident.
Differences in sample size, the racial makeup of the population
investigated, duration and the cumulative amount of corticosteroid
therapy may account for the discrepancies in the prevalence
found.
In conclusion, the findings of the present study
indicate that a considerable proportion of children with NS are
susceptible to corticosteroid-related ocular complications.
Nevertheless, it is important to take into account the possibility
of ocular involvement in children who have steroid-resistant
disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZNHAK was involved in the conception and design of
the study, in the literature search, in the analysis of clinical
data, data analysis and statistical analysis, as well as in the
preparation and reviewing of the manuscript. SHA was involved in
the conception and design of the study, in data analysis, and in
the preparation and reviewing of the manuscript. ZNHAK and SHA
confirm the authenticity of all the raw data. Both authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The research received approval from the Scientific
and Ethics Committee of Alkadimayn Teaching Hospital (no.=5542) and
Ibn AL-Haytham Teaching Eye Hospital (no.=964) (both in Baghdad,
Iraq) in accordance with the Declaration of Helsinki. Written
informed consents from the parents were acquired after providing
them with all relevant information.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Croitoru A and Balgradean M:
Treatment-associated side effects in patients with
steroid-dependent nephrotic syndrome. Maedica (Bucur). 17:285–290.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schijvens AM, Teeninga N, Dorresteijn EM,
Teerenstra S, Webb NJ and Schreuder MF: Steroid treatment for the
first episode of childhood nephrotic syndrome: Comparison of the 8
and 12 weeks regimen using an individual patient data
meta-analysis. Eur J Pediatr. 180:2849–2859. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Torres VE, Ahn C, Barten TRM, Brosnahan G,
Cadnapaphornchai MA, Chapman AB, Cornec-Le Gall E, Drenth JPH,
Gansevoort RT, Harris PC, et al: KDIGO 2025 clinical practice
guideline for the evaluation, management, and treatment of
autosomal dominant polycystic kidney disease (ADPKD): Executive
summary. Kidney Int. 107:234–254. 2025.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alssum LR: Repeated implants failure in
young patient with idiopathic nephrotic syndrome: A case report
with brief review of the literature. BMC Oral Health.
24(25)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thorn GW, Forsham PH, Frawley TF, Hill SR
Jr, Roche M, Staehelin D and Wilson DL: The clinical usefulness of
ACTH and cortisone. N Engl J Med. 242:824–834. 1950.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zulfiqar Z, Lanewala F, Khatri S, Bajeer
I, Aziz M and Hashmi S: Steroid induced ocular complications in
idiopathic nephrotic syndrome: A cross sectional single center
study. J Pak Med Assoc. 74:315–319. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
James ER: The etiology of steroid
cataract. J Ocul Pharmacol Ther. 23:403–420. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu D, Ahmet A, Ward L, Krishnamoorthy P,
Mandelcorn ED, Leigh R, Brown JP, Cohen A and Kim H: A practical
guide to the monitoring and management of the complications of
systemic corticosteroid therapy. Allergy Asthma Clin Immunol.
9(30)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Agrawal V, Devpura K, Mishra L and Agarwal
S: Study on steroid induced ocular findings in children with
nephrotic syndrome. J Clin Diagn Res. 11:SC05–SC06. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sato M, Ishikura K, Ando T, Kikunaga K,
Terano C, Hamada R, Ishimori S, Hamasaki Y, Araki Y, Gotoh Y, et
al: Prognosis and acute complications at the first onset of
idiopathic nephrotic syndrome in children: A nationwide survey in
Japan (JP-SHINE study). Nephrol Dial Transplant. 36:475–481.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Elsharkawy MM, Abd Elrahman HM, Omar KAA
and Deiaeldin YA: Assessment of ocular manifestations in children
with nephrotic syndrome during steroid treatment. Egypt J Hosp Med.
91:4672–4678. 2023.
|
|
12
|
Gaur S, Joseph M, Nityanandam S,
Subramanian S, Koshy AS, Vasudevan A, Phadke KD and Iyengar A:
Ocular complications in children with nephrotic syndrome on long
term oral steroids. Indian J Pediatr. 81:680–683. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Toruan YML, Trihono PP, Sitorus RS and
Dwipoerwantoro PG: Ocular complications in pediatric nephrotic
syndrome treated with corticosteroids. Paediatr Indones. 64:1–9.
2024.
|
|
14
|
Veltkamp F, Rensma LR and Bouts AHM:
LEARNS consortium. Incidence and relapse of idiopathic nephrotic
syndrome: Meta-analysis. Pediatrics.
148(e2020029249)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Niaudet P and Boyer O: Idiopathic
nephrotic syndrome in children: Clinical aspects. In: Avner E,
Harmon W, Niaudet P and Yoshikawa N (eds) Pediatric Nephrology.
Springer, Berlin, Heidelberg, pp667-702, 2009.
|
|
16
|
Zotta F, Vivarelli M and Emma F: Update on
the treatment of steroid-sensitive nephrotic syndrome. Pediatr
Nephrol. 37:303–314. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Falkner B, Gidding SS, Baker-Smith CM,
Brady TM, Flynn JT, Malle LM, South AM, Tran AH and Urbina EM:
American Heart Association Council on Hypertension et al.
Pediatric primary hypertension: An underrecognized condition: A
scientific statement from the American heart association.
Hypertension. 80:e101–e111. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lombel RM, Gipson DS and Hodson EM: Kidney
Disease. Improving Global Outcomes: Treatment of steroid-sensitive
nephrotic syndrome: New guidelines from KDIGO. Pediatr Nephrol.
28:415–426. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Al-Khafaji ZNH and Al Salam MSN:
VisualAcuity threshold for CataractsSurgeryat a tertiary eye center
in Iraq. Pak J Ophthalmol. 39:319–322. 2023.
|
|
20
|
Olonan LRN, Pangilinan CAG and Yatco MM:
Steroid-induced cataract and glaucoma in pediatric patients with
nephrotic syndrome. Philipp J Ophthalmol. 34:59–62. 2009.
|
|
21
|
Ozaltin F, Heeringa S, Poyraz CE, Bilginer
Y, Kadayifcilar S, Besbas N, Topaloglu R, Ozen S, Hildebrandt F and
Bakkaloglu A: Eye involvement in children with primary focal
segmental glomerulosclerosis. Pediatr Nephrol. 23:421–427.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Phulke S, Kaushik S, Kaur S and Pandav SS:
Steroid-induced glaucoma: An avoidable irreversible blindness. J
Curr Glaucoma Pract. 11:67–72. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hayasaka Y, Hayasaka S and Matsukura H:
Ocular findings in Japanese children with nephrotic syndrome
receiving prolonged corticosteroid therapy. Ophthalmologica.
220:181–185. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kyrieleis HA, Löwik MM, Pronk I, Cruysberg
HR, Kremer JA, Oyen WJ, van den Heuvel BL, Wetzels JF and
Levtchenko EN: Long-term outcome of biopsy-proven, frequently
relapsing minimal-change nephrotic syndrome in children. Clin J Am
Soc Nephrol. 4:1593–1600. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Green MB and Duker JS: Adverse ocular
effects of systemic medications. Life (Basel).
13(660)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mohan R and Muralidharan AR: Steroid
induced glaucoma and cataract. Indian J Ophthalmol. 37:13–16.
1989.PubMed/NCBI
|