Introduction
Stroke is one of the main causes of mortality and
permanent disability. The stroke rate doubles every decade and
frequently affects individuals aged ≥55 years (1). Of note, ~90% of stroke survivors
continue to suffer from chronic sequelae, and ~30% of these
patients are unable to perform daily activities independently
(2). Among these sequelae,
cognitive impairment, a common consequence of stroke, affects
>50% of individuals who survive for 6 months following a stroke
(3). Another study found that 83%
of patients had impairment in at least one cognitive domain, while
50% had cognitive impairment across multiple domains (4). The American Heart
Association/American Stroke Association (AHA/ASA) Guidelines for
Stroke Rehabilitation and Treatment in Adults (2016) state that the
majority of patients who suffer a clinical stroke undergo cognitive
assessment prior to hospital discharge (5). Cognitive impairment and cognitive
rehabilitation have been identified as top research priorities in
stroke survivors (top 10). The majority of available treatments aim
to either restore lost skills or to teach compensatory techniques;
however, the evidence has not been persuasive yet (6,7).
A previous systematic review article found that
targeted cognitive training in specific cognitive domains provides
several positive effects (8).
Starovasnik Žagavec et al (9) conducted a study with 11 stroke
survivors who underwent intensive training in selective attention.
The results of their study revealed a significant improvement from
a moderate to strong level in divided attention, and a mild effect
on alertness (9). Another study
highlighted the functional connectivity of the hippocampus with the
left frontal and parietal lobes through cognitive training,
indicating a key mechanism in cognitive recovery following a stroke
(10). Physical exercise is
another effective approach for improving the cognitive function of
patients. A previous systematic review examining the effects of
physical activity on cognitive function following a stroke
demonstrated that physical exercise exerted significant cognitive
benefits (11). Aerobic exercise
not only exerts beneficial effects in improving physical function
and reducing the risk of developing secondary complications, but
also reduces the risk of developing Alzheimer's disease and related
cognitive disorders (12). Aerobic
exercise has a positive effect on overall cognitive function, with
potential benefits to memory, attention and the visuospatial domain
in stroke survivors (13). The
improvement in cognitive function following exercise is probably
due to the regulation of angiogenic and neurotrophic growth
factors, which may facilitate neurogenesis, circuitry and synaptic
plasticity in the hippocampus and other cognitively relevant
cortical regions (14).
There is mounting evidence to indicate that
combining aerobic interventions with cognitive training may yield
additional benefits to cognitive performance, surpassing the
effects of a single type of training alone (15). Combining physical activity with
cognitive training leads to significant cognitive improvements and
reduces depression symptoms in older adults (16). A previous systematic review found
that this combination enhanced overall cognitive function, memory,
executive function and attention, in both cognitively impaired and
non-impaired populations (17). In
another study, in a population with cognitive impairment following
a stroke, the combination of exercise and cognitive training
exerted greater beneficial effects on cognitive function compared
to exercise alone (18). In
addition, a previous study posited that for exercise to effectively
produce combined neurological and cognitive benefits, it needs to
occur within a cognitively challenging environment (19). Performing aerobic sessions prior to
cognitive training prepares the brain for compensatory recruitment
during the subsequent cognitive training session (17). Aerobic exercise prior to cognitive
exercise can increase arousal levels, facilitate neurogenesis and
enhance memory consolidation, which may benefit memory retrieval
and cognitive task performance afterwards (20).
Several studies have evaluated the effects of
combining physical activity with cognitive training in older adults
or those with cognitive impairment (16-18).
However, evidence in older adults with mild cognitive impairment
(MCI) after stroke remains limited. The present study aimed to
evaluate a group of older adults for several reasons. First, the
incidence of stroke is higher among older adults. Older adults with
MCI following a stroke, if not addressed early, are at a risk of
further cognitive decline due to the degenerative process, which
may lead to the early onset of dementia and progress more severely.
Additionally, older adults generally engage in less physical
activity compared to younger individuals, rendering them more
likely to benefit from participation in a structured exercise
program, which can improve adherence to the training regimen.
In the present study, patient recruitment was
conducted at the National Geriatric Hospital in Hanoi, Vietnam,
with the target group primarily consisting of older adults. It was
observed that older adults with cognitive impairment following a
stroke often require long-term care, which places a notable burden
on both families and society. It should be noted that the
classification of the ‘older adult’ age group is not standardized
across various organizations. The present study followed the
definition provided by the National Institute on Aging (NIA) and
the classifications used in numerous Western countries and studies,
which define older adults as individuals aged ≥65 years.
Furthermore, the methods of combining these interventions need to
be carefully considered for optimal effectiveness. Patients
residing at a marked distance from the hospital or those with
limited access to prolonged in-hospital programs may derive fewer
benefits from the intervention compared to others. Therefore, the
present study not only evaluated the effectiveness of sequential
aerobic exercise and cognitive training, but also proposed an
intervention program with follow-up after hospital discharge. It
was hypothesized that the combination of aerobic exercise and
cognitive training would improve the cognitive function, physical
health and quality of life of patients than cognitive intervention
alone.
Patients and methods
Study type and population
The present study was a controlled prospective study
and adopted a convenience sampling method to evaluate the
effectiveness of a combined aerobic exercise in treating mild
cognitive impairment following a stroke.
The sample size formula for a two-independent-sample
study, with a type I error probability of 5%, a two-tailed test,
and 80% power, as proposed by Yeh et al (21), indicated a variance of σ²=4.92 for
the intervention group. A mean difference of ∆=4.7 between the two
groups was expected. The minimum sample size per group was 17, with
a 10% increase to ensure adequacy, resulting in 19 participants per
group.
Stroke survivors with MCI who met the inclusion
criteria were randomly assigned at a 1:1 ratio to either the
intervention or control group. Participants were first paired based
on similar characteristics, and subsequently, within each pair,
randomization was used to assign one participant to the
intervention group and the other to the control group. One
researcher was responsible for conducting the screening and
assessments both before and after the intervention, while another
researcher was assigned to randomize the participants and prescribe
the corresponding exercise protocols.
There were no modifications to the intervention
method following the recruitment of the participants. Each group
practiced for 60 min per day, 3 days per week, for 12 weeks. A
total of 36 sessions were divided into two phases as follows: The
first 4 weeks in the hospital and the following 8 weeks at home. No
interim analysis was performed during the study.
Participation and recruitment
The study was conducted at the Rehabilitation
Department of the National Geriatric Hospital in Hanoi, Vietnam.
Participants were provided with all information about the study and
signed a consent form after understanding the details of the
study.
The inclusion criteria were as follows: i) Patients
with a confirmed diagnosis of MCI following an ischemic stroke
[according to DSM-5 diagnostic criteria, Montreal cognitive
assessment (MoCA) score <26, mini-mental state examination score
≥19]; ii) patients with the first ischemic stroke occurring within
the past 6 months; iii) those with an age ≥65 years; iv) those with
the ability to participate in the program, including sufficient
mobility, balance, cognitive capacity to engage in and adhere to
the program, and the presence of a family member or caregiver to
monitor and supervise self-practice process; v) those who agreed to
provide informed consent and participate in the study.
The exclusion criteria were the following: i)
Patients with other neurological disorders, such as Parkinson's
disease, multiple sclerosis, etc.; ii) patients with injuries or
musculoskeletal diseases that markedly impaired mobility,
preventing participation in the program; ii) patients with acute or
chronic respiratory or cardiovascular diseases that were not stably
controlled or were at a risk of acute exacerbations during physical
activity.
The present study was approved by Hanoi Medical
University under Decision No. 3963/QĐ-ĐHYHN, dated September 26,
2022 and Hanoi Medical University Institutional Ethical Review
Board under Decision No. 1259/GCN-HDDDNCYSH-DHYHN, dated May 7,
2024. All patients provided a written consent to participate in
this study.
Clinical intervention, part 1:
Training program in the hospital (first 4 weeks). Aerobic
exercise
Patients in the intervention group performed a
30-min treadmill exercise, including 3 min of warm-up, and 25 min
of main exercise (walking on the treadmill) and ending with 2 min
of cool-down. The target intensity of the exercise was moderate,
assessed by aiming for a target heart rate during the aerobic
period that was 40% of the reserve heart rate of the patient,
calculated using the Karvonen formula. Additionally, the Borg
Perceived Exertion Scale was recorded during each session.
Depending on the ability and the physical activity level of each
individual, the 25-min main exercise was divided into 2-3
intervals, interspersed with short 2-min rest periods, to achieve
the target after 4 weeks. Achieving the target meant reaching the
target heart rate and completing 25 min of sustained exercise. All
sessions were conducted by physical therapists. The exercise was
fully monitored by rehabilitation doctors and physiotherapists to
observe the symptoms of the patient and adjust the intensity as
needed (Fig. 1).
Cognitive training. The cognitive training
program was conducted on paper, focusing on cognitive domains
commonly impaired in patients who have suffered a stroke, including
executive function, processing speed, concentration and attention,
memory, etc. Participants performed various tasks and the level was
gradually increased until an improvement was observed. Occupational
therapists conducted and monitored the sessions. The duration of
cognitive training was 30 min per day for the intervention group
and 60 min per day for the control group. The following exercises
were performed: i) Memory exercises: Interventions for memory
retrieval (mnemonic methods for language memory recovery;
organizing information into categories; using visual imagery to
enhance semantic memory; linking new information with previously
known information, etc.) and compensatory memory interventions
(using devices, schedules, etc.). ii) Attention, concentration and
processing speed exercises: Applying attention process training to
improve sustained, selective, alternating and divided attention.
iii) Executive function exercises: Applying metacognitive strategy
training to process tasks.
Clinical intervention, part 2: Home
training program (8 weeks)
The program was continued at home following the
instructions provided at the hospital. The duration of training was
8 weeks, comprising 24 sessions in total.
Aerobic exercise consisted of brisk walking on a
flat surface to achieve the target intensity. Cognitive exercises
involved instructing patients to prepare comic strips, images and
various objects related to different topics, similar to the tasks
practiced during their hospital sessions. In addition, patients
were provided with a document that recorded the exercises, along
with illustrative images of previously taught tasks. This allowed
patients to continue practicing at home with the support of family
members or caregivers.
Compliance monitoring. Groups of patients
were created on the phone app with 3-5 patients per group. All
participants were required to exercise within a specified time
frame each day and on specific days of the week. Researchers were
aware of the training schedules for each group and regularly
monitored and supervised each the training sessions of group via
video using phone software. A tracking sheet was used for each
patient. The evaluators verified the exercise sessions of the
patients according to the exercise schedule of each group and
marked the tracking sheet accordingly.
Outcome assessment
The outcomes were evaluated at two-time points as
follows: Once before the intervention began and again at 12 weeks
following the commencement of the intervention.
Primary outcome measures. Primary outcome
measures were cognitive functions, including the MoCA and the
frontal assessment battery (FAB). The MoCA is a brief screening
tool with a high sensitivity and specificity for detecting mild
cognitive impairment, assessing six domains: Memory, executive
function, attention, language, visuospatial ability and
orientation. The FAB includes six subdomains related to frontal
lobe function and is a simple tool for assessing frontal lobe
function.
Secondary outcome measures. Secondary
outcomes included measures of physical function and quality of
life. Physical functions were assessed using the Fugl-Meyer
Assessment Lower Extremity (FMA-LE) and the 6-min walk test (6MWT).
The FMA-LE is designed to evaluate motor function, balance,
sensation and joint function in patients of all ages with
post-stroke hemiplegia. The 6MWT measures the distance a patient
can walk in 6 min and is used as an indicator of walking endurance.
It is an important predictor of mobility and community integration
in stroke survivors.
Quality of life was assessed using the European
Quality of Life-5 Dimension-5 Level (EQ-5D-5L) instrument. This
tool evaluates five health aspects: Mobility, self-care, usual
daily activities, pain or discomfort and anxiety or depression.
Each aspect is rated on a five-level severity scale: No problems,
slight problems, moderate problems, severe problems and extreme
problems.
Statistical analysis
The data were analyzed using SPSS 20.0 software (IBM
Corp.). The Kolmogorov-Smirnov test was used to assess the
normality of the data. The basic characteristics of the
participants were compared using the Chi-squared (χ²) test and
Fisher's exact test. The Wilcoxon signed rank test was used to
compare pre- and post-intervention values within each group. To
compare the differences between the two groups, the Mann-Whitney U
test was applied. To evaluate the strength of the difference, the
effect size was calculated using the r coefficient for each
outcome: r≤0.1, small effect size; 0.1<r≤0.3, moderate effect
size; and r>0.3, large effect size. A value of P<0.05 was
considered to indicate a statistically significant difference. The
Bonferroni correction was applied to adjust the P-values, thereby
controlling the overall type I error rate at 0.05 across all
statistical tests conducted in the study.
Results
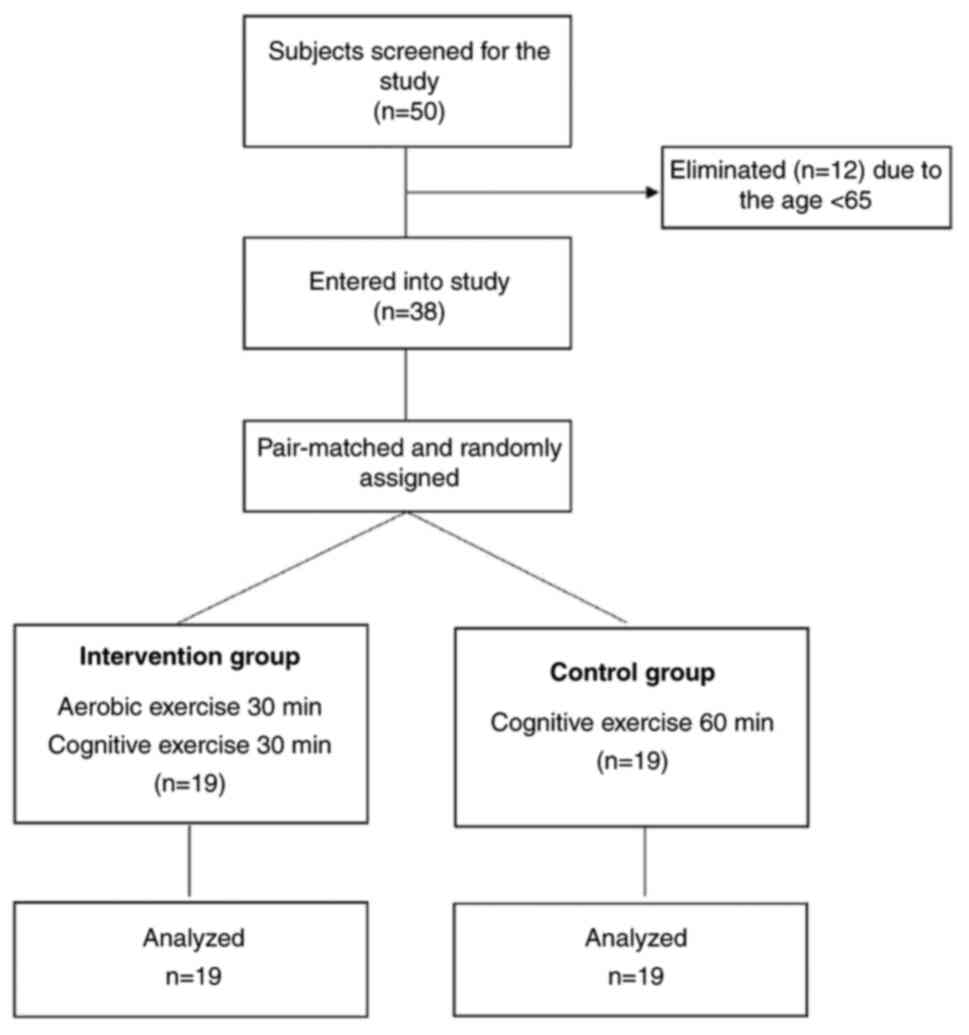
Initially, 50 ischemic stroke survivors were
selected, of whom 12 did not meet the age eligibility criteria.
Therefore, 38 stroke survivors with MCI were randomly assigned to
two groups as follows: An intervention groups (n=19) and a control
group (n=19) (Fig. 2). The
selection and follow-up period took place concurrently from
November 1, 2022, to May 31, 2023. During this period, participants
were enrolled, trained, and followed-up. The follow-up continued
until July 31, 2023. No changes were made to the study outcomes
after the study commenced. No adverse events were reported during
the study, and the study was conducted according to the planned
protocol until its completion. The results revealed that no
significant difference was observed in the demographic
characteristics between the two groups (P>0.05), apart from sex
(P<0.05; Table I).
 | Table IGeneral characteristics of the study
participants. |
Table I
General characteristics of the study
participants.
| Variables | Intervention group
(n=19) | Control group
(n=19) | P-value |
|---|
| Age (years) | 71.42±5.10 | 73.95±7.04 | 0.278 |
| Sex | | | 0.036 |
|
Male | 16 (84.2) | 10 (52.6) | |
|
Female | 3 (15.8) | 9 (47.4) | |
| BMI | 20.75±1.55 | 20.32±1.56 | 0.365 |
| NIHSS score | 6.47±1.98 | 5.68±1.89 | 0.218 |
| Side of brain
lesion | | | 0.103 |
|
Right | 6 (31.6) | 11 (57.9) | |
|
Left | 13 (68.4) | 8 (42.1) | |
| Education | | | 0.201 |
|
I (primary
school) | 8 (42.1) | 3 (15.8) | |
|
II (middle
school) | 5 (26.3) | 7 (36.8) | |
|
III
(secondary school) | 6 (31.6) | 9 (47.4) | |
Primary outcome measures
After applying the Bonferroni correction, the MoCA
score results indicated that both groups exhibited a significant
improvement following treatment, for the intervention group
(P=0.003) and the control group (P=0.014). However, the difference
in MoCA score changes between the intervention and control groups
was not statistically significant (P=0.041). As regards the FAB
scores, the intervention group exhibited a significantly greater
improvement compared to the control group (P=0.008). The FAB score
in the intervention group improved significantly (P=0.001); by
contrast, the improvement in the control group was not
statistically significant (P=0.068) (Table II).
 | Table IIPrimary outcome measures. |
Table II
Primary outcome measures.
| | Pre-training | Post-training | Wilcoxon signed rank
test P-valuea | Mann-Whitney U test
(∆) |
|---|
| Outcome | Intervention group
(n=19) | Control group
(n=19) | Intervention group
(n=19) | Control group
(n=19) | Intervention group
(n=19) | Control group
(n=19) | P-valueb | Effect size (r) |
|---|
| MoCA | 18.21±3.87 | 16.79±4.19 | 19.02±4.23 | 17.11±4.54 | 0.003 | 0.014 | 0.041 | 0.331 |
| FAB | 10.84±4.25 | 8.74±4.57 | 11.95±4.03 | 9.21±4.98 | 0.001 | 0.068 | 0.008 | 0.429 |
Secondary outcome measures
The data collected using the sub-measures in FMA-LE,
6MWT and EQ-5D-5L (Table III)
revealed that both groups experienced significant physical
improvement following treatment, with the intervention group
demonstrating a significant change compared to the control group.
After applying the Bonferroni correction, the intervention group
exhibited significant improvements in both FMA-LE and 6MWT, whereas
in the control group, only the 6MWT exhibited significant
improvements. As regards quality of life, only the intervention
group exhibited a significant improvement (P=0.002) (Table III).
 | Table IIISecondary outcome measures. |
Table III
Secondary outcome measures.
| | Pre-training | Post-training | Wilcoxon signed rank
test P-valuea | Mann-Whitney U test
(∆) |
|---|
| Outcome | Intervention group
(n=19) | Control group
(n=19) | Intervention group
(n=19) | Control group
(n=19) | Intervention group
(n=19) | Control group
(n=19) |
P-valueb | Effect size
(r) |
|---|
| FMA-LE | 17.58±3.50 | 17.42±3.61 | 20.21±3.77 | 17.74±3.86 | <0.001 | 0.034 | <0.001 | 0.759 |
| 6MWT (meters) | 41.47±12.28 | 38.84±11.62 | 71.42±14.97 | 52.16±16.16 | <0.001 | <0.001 | <0.001 | 0.797 |
| EQ–5D-5L | 0.78±0.04 | 0.77±0.04 | 0.81±0.41 | 0.78±0.05 | 0.002 | 0.128 | 0.011 | 0.41 |
Discussion
The results of the present study demonstrated that
the group participating in aerobic exercises experienced positive
improvements in general cognition compared to the group that
received cognitive training only. This supports the initial
hypothesis that incorporating aerobic exercise would lead to
greater overall cognitive improvement. The intervention group also
demonstrated notable improvements in physical function and quality
of life outcomes.
Aerobic exercise positively enhances cognitive
performance in patients who have suffered a stroke, both in general
cognitive function as measured using the MoCA scale and in specific
cognitive domains, including concentration, attention,
visual-spatial and executive function (18,21,22,23).
The findings of the present study are also partly in accordance
with the results of previous studies (13,21,22,23).
The findings indicate that combined aerobic exercise can help
patients enhance general cognitive function and improves executive
function, as assessed using FAB. In addition, aerobic exercise can
regulate angiogenic and neurotropic growth factors, which likely
facilitate neurogenesis, angiogenesis and synaptic plasticity.
Aerobic exercise combined with cognitive exercise likely has
synergistic or complementary effects on cognition at both the
neurobiological and behavioral levels (17,20).
The present study illustrated that the intervention of aerobic
exercise exerted a synergistic effect on improving cognitive
function in patients who had suffered a stroke.
The minimal clinically important difference (MCID)
for the MoCA score in patients who have suffered a stroke is
estimated to range from 1.22 to 2.15, based on both anchor-based
and distribution-based methods (24). In the present study, the changes in
MoCA scores before and after the intervention for each group were
as follows: Intervention group, from 18.21 to 19.02;
difference=0.81); control group, from 16.79 to 17.11;
difference=0.32). Neither group exceeded the MCID according to both
methods. It was deemed that the small sample size was a limitation.
Furthermore, the participants had MCI, which may explain the
limited change in the MoCA scores.
Aerobic intervention led to multiple positive
outcomes simultaneously. The difference from previous studies
(18,21,22),
is that the present study evaluated various outcomes related to
physical health and quality of life. The results indicated
significant improvements in both motor function and endurance in
patients who have suffered a stroke following aerobic intervention,
as assessed using the FMA-LE and 6MWT. Yeh et al (21) conducted a similar study with 56
patients assigned to three groups: Aerobic exercise, cognitive
exercise and a combination of aerobic and cognitive exercise. The
of their study results revealed improvements in endurance and
mobility (6MWT); however, their study did not evaluate quality of
life, daily functioning, or social engagement (21). Yeh et al (21) suggested that sequential training
may improve both general and specific cognitive domains, although
the effects were insufficient to transfer to activities of daily
living, quality of life or social participation. A previous
systematic review suggested that cognitive improvements appeared to
be limited to trained cognitive functions and do not generalize to
activities of daily living (25).
It was hypothesized that, once the aerobic effects are achieved at
the neurobiological level, they not only have a synergistic effect
on cognitive function, but also on other domains, specifically
physical function and daily living functions. The results of the
present study support this hypothesis. Incorporating aerobic
exercise was shown to improve cognitive function, lower limb
function, mobility and endurance, which, in turn enhances quality
of life. Quality of life was assessed using the EQ-5D-5L
instrument, which measures five dimensions. Improvements were
observed in two of these dimensions: Mobility and usual daily
activities.
The present study has several limitations which
should be mentioned. Firstly, the sample size was relatively small,
which limits generalizability and may have been influenced by
various confounding factors. The present study involved an unequal
sex distribution between the two groups. Considering that this is
an intervention study incorporating physical activity, the sex
imbalance may have influenced both the intervention process and the
outcomes. Future research with larger sample sizes is thus
required, along with the further exploration of how patient
characteristics may affect the results of the intervention.
Secondly, the cognitive training conducted on paper may have more
limitations compared to similar studies conducted on computers.
Lastly, the present study only evaluated the outcomes at the end of
the intervention. The present study did not monitor or assess
whether the effects of the sequential training could be maintained
over time. This remains an unknown factor that warrants further
investigation.
When comparing the findings of the present study
with those of previous studies (18,21,22),
it was observed that while these studies support the combined
approach of cognitive and physical training, the present study
differs in several ways: It focused on older adults, utilized a
paper-based cognitive intervention instead of a computer-based
program, and implemented a 4-week hospital program followed by 8
weeks of home-based intervention, rather than a 12-week
hospital-based program. These modifications were made to better
align with practical intervention programs commonly available at
most healthcare facilities, as numerous patients may find it
difficult to adhere to a 3-month hospital program. The home-based
approach, with monitoring, helps patients establish a routine that
can be maintained after the intervention ends. Nevertheless,
similar findings in cognitive and physical outcomes highlight the
consistency of the combined training approach across various
settings.
In conclusion, MCI following a stroke is prevalent
and significantly affects the recovery of patients. The present
study found that a sequential combination of aerobic and cognitive
exercise improved general cognitive function and frontal lobe
executive function, physical function and quality of life. However,
further studies with larger sample sizes are required to
investigate the factors influencing the effectiveness of
intervention in order for patients with MCI to fully benefit from
the intervention.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TNAN and VMP were involved in the conception and
design of the study, and performed the statistical analysis of the
data. TNAN and VMP were involved in the investigative aspects of
the study. TNAN and VMP were involved in the interpretation of the
data. TNAN and VMP were involved in the writing of original draft
of the manuscript, and the writing, reviewing and editing of the
manuscript. Both authors read and agreed to the published version
of the manuscript. TNAN and VMP confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The present study was approved by Hanoi Medical
University under Decision No. 3963/QĐ-ĐHYHN, dated September 26,
2022 and Hanoi Medical University Institutional Ethical Review
Board under Decision No. 1259/GCN-HDDDNCYSH-DHYHN, dated May 7,
2024. All patients provided a written consent to participate in
this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seshadri S and Wolf PA: Lifetime risk of
stroke and dementia: Current concepts, and estimates from the
Framingham Study. Lancet Neurol. 6:1106–1114. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Skolarus LE, Burke JF, Brown DL and
Freedman VA: Understanding stroke survivorship: Expanding the
concept of poststroke disability. Stroke. 45:224–230.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mellon L, Brewer L, Hall P, Horgan F,
Williams D and Hickey A: ASPIRE-S study group. Cognitive impairment
six months after ischaemic stroke: A profile from the ASPIRE-S
study. BMC Neurol. 15(31)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jokinen H, Melkas S, Ylikoski R,
Pohjasvaara T, Kaste M, Erkinjuntti T and Hietanen M: Post-stroke
cognitive impairment was common even after successful clinical
recovery. Eur J Neurol. 22:1288–1294. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Winstein CJ, Stein J, Warena R, Bates B,
Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, et
al: Guidelines for adult stroke rehabilitation and recovery: A
guideline for healthcare professionals from the American heart
Association/American stroke association. Stroke. 47:e98–e169.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cappa SF, Benke T, Clarke S, Rossi B,
Stemmer B and Van Heugten CM: Cognitive rehabilitation. Gilhus NE,
Barnes MP and Brainin M (eds). In: European Handbook of
Neurological Management. 2nd edition. Wiley-Blackwell, Oxford,
pp545-568, 2011.
|
|
7
|
Scottish Intercollegiate Guidelines
Network (SIGN). Management of Patients with Stroke: Rehabilitation,
Prevention and Management of Complications, and Discharge Planning,
SIGN Publication 118, 2010.
|
|
8
|
Gillespie DC, Bowen A, Chung CS, Cockburn
J, Knapp P and Pollock A: Rehabilitation for post-stroke cognitive
impairment: An overview of recommendations arising from systematic
reviews of current evidence. Clin Rehabil. 29:120–128.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Starovasnik Žagavec B, Mlinaric Lešnik V
and Goljar N: Training of selective attention in work-active stroke
patients. Int J Rehabil Res. 38(370)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin ZC, Tao J, Gao YL, Yin DZ, Chen AZ and
Chen LD: Analysis of central mechanism of cognitive training on
cognitive impairment after stroke: Resting-state functional
magnetic resonance imaging study. J Int Med Res. 42:659–668.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cumming TB, Tyedin K, Churilov L, Morris
ME and Bernhardt J: The effect of physical activity on cognitive
function after stroke: A systematic review. Int Psychogeriatr.
24:557–567. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Laurin D, Verreault R, Lindsay J,
MacPherson K and Rockwood K: Physical activity and risk of
cognitive impairment and dementia in elderly persons. Arch Neurol.
58:498–504. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zheng G, Zhou W, Xia R, Tao J and Chen L:
Aerobic exercises for cognition rehabilitation following stroke: A
systematic review. J Stroke Cerebrovasc Dis. 25:2780–2789.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Barber SE, Clegg AP and Young JB: Was
there a role for physical activity in preventing cognitive decline
in people with mild cognitive impairment? Age Ageing. 41:5–8.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Langdon KD and Corbett D: Improved working
memory following novel combinations of physical and cognitive
activity. Neurorehabil Neural Repair. 26:523–532. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Oswald WD, Gunzelmann T, Rupprecht R and
Hagen B: Differential effects of single versus combined cognitive
and physical training with older adults: The SimA study in a 5-year
perspective. Eur J Ageing. 3:179–1792. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Law LLF, Barnett F, Yau MK and Gray MA:
Effects of combined cognitive and exercise interventions on
cognition in older adults with and without cognitive impairment: A
systematic review. Ageing Res Rev. 15:61–75. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bo W, Lei M, Tao S, Jie LT, Qian L, Lin FQ
and Ping WX: Effects of combined intervention of physical exercise
and cognitive training on cognitive function in stroke survivors
with vascular cognitive impairment: A randomized controlled trial.
Clin Rehabil. 33:54–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Additive effects of physical exercise and
environmental enrichment on adult hippocampal neurogenesis in
mice-PubMed. Accessed September 23, 2022.
|
|
20
|
Labban JD and Etnier JL: Effects of acute
exercise on long-term memory. Res Q Exerc Sport. 82:712–721.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yeh TT, Chang KC, Wu CY, Chen CJ and
Chuang IC: Clinical efficacy of aerobic exercise combined with
computer-based cognitive training in stroke: A multicenter
randomized controlled trial. Topics in Stroke Rehabilitation.
29:255–264. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yeh TT, Chang KC and Wu CY: The active
ingredient of cognitive restoration: A multicenter randomized
controlled trial of sequential combination of aerobic exercise and
Computer-based cognitive training in stroke survivors with
cognitive decline. Arch Phys Med Rehabil. 100:821–827.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Marzolini S, Oh P, McIlroy W and Brooks D:
The effects of an aerobic and resistance exercise training program
on cognition following stroke. Neurorehabil Neural Repair.
27:392–402. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu CY, Hung SJ, Lin K, Chen KH, Chen P and
Tsay PK: Responsiveness, minimal clinically important difference,
and validity of the MoCA in stroke rehabilitation. Occup Ther Int.
2019(2517658)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lauenroth A, Ioannidis AE and Teichmann B:
Influence of combined physical and cognitive training on cognition:
A systematic review. BMC Geriatr. 16(141)2016.PubMed/NCBI View Article : Google Scholar
|