1. Introduction
Allergic disorders, such as allergic rhinitis,
atopic dermatitis (AD) and atopic asthma, exhibit an inherited
predisposition to sensitization by commonly encountered
environmental allergens and to the development of high levels of
immunoglobulin (Ig) E antibodies (1). It has been long recognized that Th2
lymphocytes and classical Th2 cell-derived cytokines, namely
interleukin (IL)-4, IL-5 and IL-13, play an important role in
orchestrating and amplifying allergic inflammation. Recently,
several lines of evidence suggested that the tissue levels of IL-21
and the IL-21 receptor were elevated in patients with AD (2,3). In
addition, tissue damage may be efficiently inhibited in murine
models of AD and allergic rhinitis by dampening IL-21 levels
(4), suggesting the involvement of
IL-21 in the pathogenesis of these diseases. Moreover, an
association of IL-21 polymorphisms with the susceptibility to
atopic asthma was reported (5). In
this review, we aimed to discuss the biological characteristics of
IL-21 and summarize the current progress on the role of IL-21 in
the regulation of allergic inflammation.
2. IL-21 and IL-21 receptor expression and
signaling
IL-21 is a recently discovered member of the type I
cytokine family, which is produced by activated CD4+ T
cells, NKT cells, Th17 cells and follicular helper T cells
(6,7). The biological functions of IL-21 are
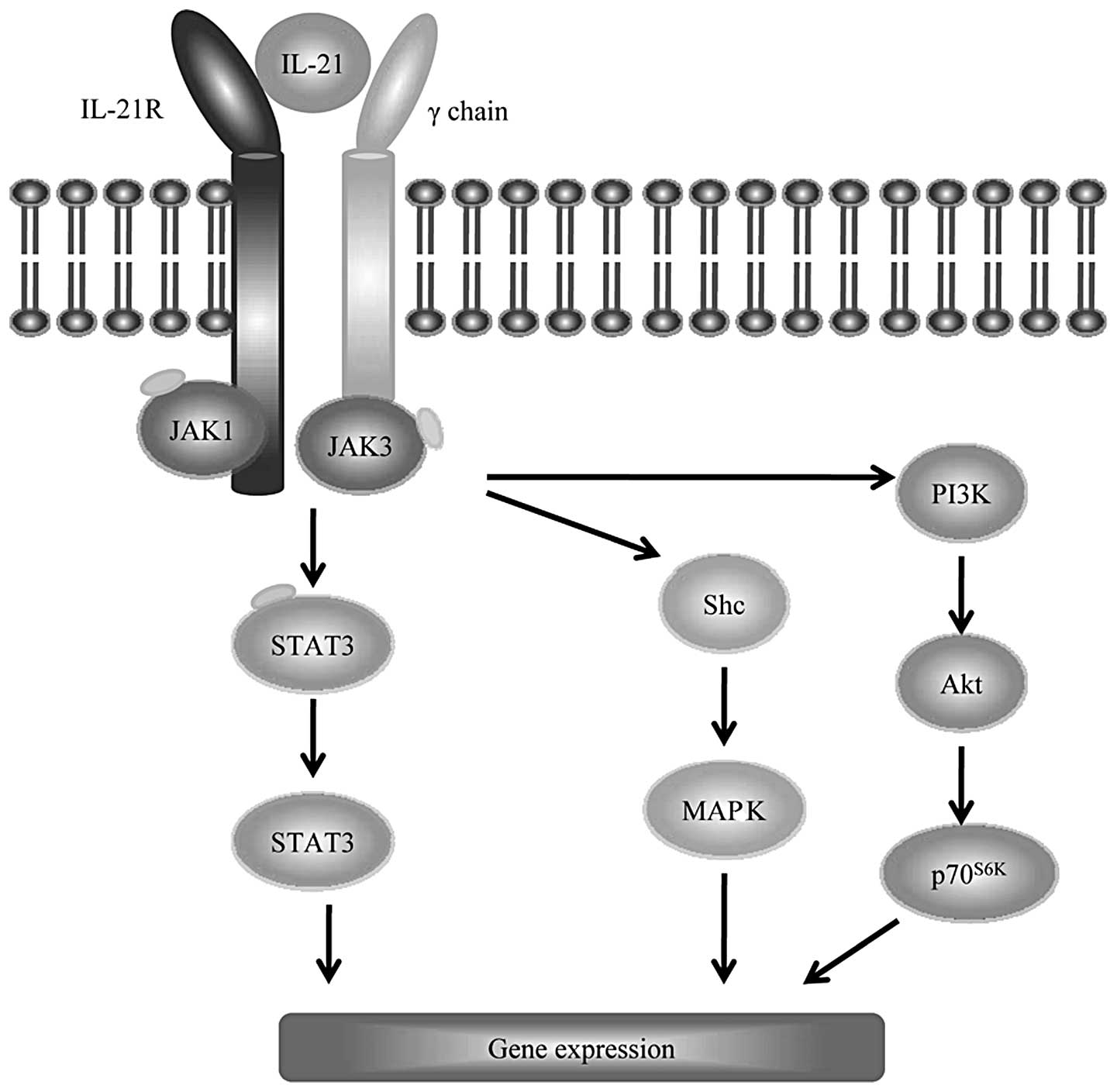
mediated by a heterodimeric receptor, formed by a common γ-chain
subunit, which is shared with the IL-2, IL-4, IL-7, IL-9 and IL-15
receptors, and its own unique receptor (designated IL-21R), a
member of the class I cytokine receptor family (Fig. 1) (7,8).
Although IL-21 production is restricted to lymphoid populations,
IL-21R is highly expressed on a range of immune (i.e., naïve and
activated T cells, B cells, NK cells, dendritic cells and
macrophages), as well as non-immune cells (i.e., keratinocytes and
endothelial cells) (7,8), indicating a broad spectrum of
functions. Of note, the expression of IL-21R on B cells was the
highest, even on resting cells (1).
Similar to other cytokines that signal through the common γ-chain,
IL-21 functions by activating the Janus kinases JAK1 and JAK3, with
JAK1 binding to IL-21R and JAK3 binding to the common γ-chain
(6). IL-21R/γc-driven
signaling leads to JAK1 and JAK3 autophosphorylation and subsequent
phosphorylation of signal transducer and activator of transcription
(STAT) 3, STAT1, STAT5a and STAT5b (Fig. 1) (6,9). Among
these STAT proteins, STAT3 appears to be the most important for
IL-21 signaling, since the lack of expression of STAT3 leads to
defective IL-21 signaling in T cells (9). Of note, the proliferation remained
markedly decreased in STAT knockout (KO) mice, suggesting the
involvement of other pathways in IL-21-mediated proliferation.
Indeed, the phosphatidylinositol 3-kinase (PI3K)/Akt and
mitogen-activated protein kinase (MAPK) pathways, together with the
JAK/STAT pathway, cooperatively contribute to the full
IL-21-mediated proliferative response (9).
3. IL-21 regulation of B-cell function
B cells contribute to the immunoreactivity of
allergic diseases by giving rise to high titers of immunoglobulin
(Ig) E antibodies. It was reported that IL-21 may not be essential
for B-cell development, as IL-21R KO mice exhibited no defects in
B-cell subsets within the bone marrow or in the periphery (10). However, IL-21 was shown to induce
apoptosis, proliferation and class-switching in mature B-cell
populations, depending on the co-stimulatory signals (11,12).
In the absence of a specific antigen or in the presence of a
non-specific polyclonal signal, IL-21 induces apoptosis of naïve B
cells, whereas IL-21 induces proliferation, isotype class-switching
and differentiation to memory B cells or terminally differentiated
plasma cells in the presence of a B-cell receptor signal and/or
co-stimulatory interactions with T cells. In the absence of IL-21,
the antibody isotype distribution is also disrupted. Naïve IL-21R
KO mice exhibited diminished levels of IgG1, IgG2b and IgG3 and
significantly higher levels of IgE upon immunization with
T-cell-dependent antigens (10).
This finding is consistent with results from in vivo and
in vitro studies on wild-type mice: IL-21 administered to
wild-type mice at the time of immunization may lead to reduced IgE
responses and previous in vitro experiments demonstrated
that IL-21 may reduce the levels of germline Cɛ transcripts,
leading to reduced IgE-specific switching (13). The IL-21-mediated downregulation of
IgE may result from the IL-21-induced expression of the
pro-apoptotic Bcl-2-modifying factor in IgE-expressing B cells
(14), the induction of inhibitor
of differentiation-2 in B cells or the reduction of germline Cɛ
transcripts, leading to reduced IgE-specific switching (15).
4. IL-21 in allergic inflammatory
diseases
IL-21 in allergic rhinitis
Allergic rhinitis represents the prototypical
chronic rhinitis diseases. The typical clinical manifestations are
nasal itching, sneezing, nasal running and nasal obstruction.
Currently, allergic rhinitis is considered to be a disease mediated
by IgE-mediated inflammation of the nasal mucosa, resulting in
eosinophilic and Th2-cell infiltration of the nasal lining
(16). Several cytokines produced
by Th2 cells (e.g., IL-3, IL-4, IL-5 and IL-13) contribute to the
induction and maintenance of the IgE production by plasma cells.
Due to its ability to regulate IgE production, IL-21 is involved in
the regulation of IgE-mediated allergic rhinitis responses. Indeed,
in an ovalbumin-induced mouse model of allergic rhinitis, the
intranasal administration of recombinant mouse IL-21 during the
initial antigen challenge significantly reduced the allergic
symptoms, with diminished antigen-specific serum IgE and reduced
Th2 cytokine (IL-4, IL-5 and IL-13) levels in nasal tissues
(4). Moreover, IL-21 acted on nasal
fibroblasts to inhibit the production of eotaxin, leading to
suppressed eosinophil migration into nasal tissues (4). To the best of our knowledge, there is
only one available prospective study addressing this subject in
humans. Huang et al(17)
indicated there was no significant difference in the serum IL-21
levels between allergic rhinitis patients and healthy controls and
that IL-21 was not associated with serum-specific IgE. However, due
to the limited sample of only 24 patients and the fact that IL-21
was detected only at the protein level, further studies, involving
a larger sample size, are required to investigate the precise role
of IL-21 in allergic rhinitis at the mRNA level.
IL-21 in AD
AD is a pruritic allergic inflammatory skin disease,
frequently associated with high plasma levels of IgE (18). AD affects 15–30% of children and
2–10% of the adult population worldwide (19). Although AD has been the subject of
numerous investigations, the pathophysiology of this disease has
not been fully elucidated (19).
Recently, a study by Jin et al(3) identified IL-21 as a critical regulator
of the processes that lead to sensitization and allergic
inflammation of the skin. By using a mouse model of allergic skin
inflammation, Jin et al(3)
demonstrated that the gene expression levels of IL-21 and IL-21R
were significantly upregulated in mouse skin subjected to tape
stripping, a surrogate for scratching. Moreover, IL-21R deficient
and wild-type mice treated with soluble IL-21R-IgG2aFc fusion
protein developed an impaired systemic response to epicutaneous
sensitization and disrupted trafficking of skin dendritic cells
(3). Further insight into this
defect was provided by the observation of impaired migration of
skin dendritic cells towards draining lymph nodes following antigen
capture in IL-21R-deficient mice. In addition, the expression of
CCR7/TARC by skin dendritic cells and MMP2 activation in the
epidermis following mechanical injury were hindered (3). These data offer insight into a
potential mechanism underlying the association of IL-21 signaling
with the pathogenesis of allergic dermatitis and contributing to
allergic skin inflammation.
As regards humans, elevated protein expression
levels of IL-21 and IL-21R were detected in acute skin lesions of
patients suffering from AD (3).
However, a previous study by Lin et al(20) revealed that the serum levels of
IL-21 in AD patients were significantly lower compared to those in
healthy controls. In addition, it was reported that serum IL-21
levels were inversely correlated with the severity of AD (20). The severity of the skin
manifestations was inversely correlated with the serum IL-21
titers. The differences in IL-21 expression between the skin
lesions and the serum may be attributed to the uneven distribution,
disease duration, or treatment regimen applied.
IL-21 in atopic asthma
Atopic asthma (also referred to as allergic asthma),
is a chronic airway inflammatory disease primarily characterized by
an abnormality in the IgE pathway, which is associated with the
inception of asthma and its acute deterioration (21). Evidence supporting a genetic basis
for asthma was obtained from recent research including case-control
and family studies (5). It was
reported that the exon-3 polymorphism C5250T of the IL-21 gene is
significantly associated with atopic asthma and serum total IgE.
Furthermore, the C5250T polymorphism was shown to affect the
concentration of serum IL-21 in atopic asthmatics (5). Of note, IL-21R-deficient mice
exhibited unexpectedly reduced airway hyperresponsiveness, although
the serum IgE levels were increased (22), although recent results obtained from
animal models suggested that IL-21 may diminish the severity of
allergy (4,5). A possible explanation for this
discrepancy is that IL-21 may play different roles, depending on
the immune setting and the combination with different cytokines.
Although further functional studies are required, it may be
hypothesized that IL-21 is involved in the pathogenesis of atopic
asthma.
5. Conclusion
The pleiotropic inflammatory functions of IL-21 and
the documented pathogenic effects of this cytokine in various
tissues suggest that the interruption of the IL-21 signaling
pathway merits extensive investigation as a therapeutic option for
the treatment of inflammatory and allergic diseases. However, mouse
models do not reflect the full spectrum of the complexity of human
disease. Therefore, further research is required to achieve
therapeutic efficacy in humans.
References
|
1
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caruso R, Botti E, Sarra M, et al:
Involvement of interleukin-21 in the epidermal hyperplasia of
psoriasis. Nat Med. 15:1013–1015. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin H, Oyoshi MK, Le Y, et al: IL-21R is
essential for epicutaneous sensitization and allergic skin
inflammation in humans and mice. J Clin Invest. 119:47–60.
2009.PubMed/NCBI
|
|
4
|
Hiromura Y, Kishida T, Nakano H, Hama T,
Imanishi J, Hisa Y and Mazda O: IL-21 administration into the
nostril alleviates murine allergic rhinitis. J Immunol.
179:7157–7165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chatterjee R, Batra J and Ghosh B: A
common exonic variant of interleukin 21 confers susceptibility to
atopic asthma. Int Arch Allergy Immunol. 148:137–146. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crotty S: Follicular helper CD4 T cells
(TFH). Annu Rev Immunol. 29:621–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spolski R and Leonard WJ: Interleukin-21:
basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarra M, Cupi ML, Pallone F and Monteleone
G: Interleukin-21 in immune and allergic diseases. Inflamm Allergy
Drug Targets. 11:313–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng R, Spolski R, Casas E, Zhu W, Levy DE
and Leonard WJ: The molecular basis of IL-21-mediated
proliferation. Blood. 109:4135–4142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozaki K, Spolski R, Feng CG, et al: A
critical role for IL-21 in regulating immunoglobulin production.
Science. 298:1630–1634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mehta DS, Wurster AL, Whitters MJ, Young
DA, Collins M and Grusby MJ: IL-21 induces the apoptosis of resting
and activated primary B cells. J Immunol. 170:4111–4118. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin H, Carrio R, Yu A and Malek TR:
Distinct activation signals determine whether IL-21 induces B cell
costimulation, growth arrest, or Bim-dependent apoptosis. J
Immunol. 173:657–665. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suto A, Nakajima H, Hirose K, et al:
Interleukin 21 prevents antigen-induced IgE production by
inhibiting germ line Cɛ transcription of IL-4-stimulated B cells.
Blood. 100:4565–4573. 2002.PubMed/NCBI
|
|
14
|
Harada M, Magara-Koyanagi K, Watarai H, et
al: IL-21-induced Bɛ cell apoptosis mediated by natural killer T
cells suppresses IgE responses. J Exp Med. 203:2929–2937. 2006.
|
|
15
|
Kishida T, Hiromura Y, Shin-Ya M, et al:
IL-21 induces inhibitor of differentiation 2 and leads to complete
abrogation of anaphylaxis in mice. J Immunol. 179:8554–8561. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Small P and Kim H: Allergic rhinitis.
Allergy Asthma Clin Immunol. 7(Suppl 1): S32011. View Article : Google Scholar
|
|
17
|
Huang X, Yang Q, Chen Y, Li P, Zhang G and
Li Y: Expressions of IL-17, IL-21 and IL-23 in the serum of
allergic rhinitis patients. J Med Biochem. 30:323–327. 2011.
View Article : Google Scholar
|
|
18
|
Hayashida S, Uchi H, Moroi Y and Furue M:
Decrease in circulating Th17 cells correlates with increased levels
of CCL17, IgE and eosinophils in atopic dermatitis. J Dermatol Sci.
61:180–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin SC, Chuang YH, Yang YH and Chiang BL:
Decrease in interleukin-21 in children suffering with severe atopic
dermatitis. Pediatr Allergy Immunol. 22:869–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wenzel SE: Asthma phenotypes: the
evolution from clinical to molecular approaches. Nat Med.
18:716–725. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fröhlich A, Marsland BJ, Sonderegger I,
Kurrer M, Hodge MR, Harris NL and Kopf M: IL-21 receptor signaling
is integral to the development of Th2 effector responses in vivo.
Blood. 109:2023–2031. 2007.PubMed/NCBI
|