Introduction
Advanced glycation end products (AGEs) have recently
received increasing attention as substances that are directly
associated with the generation and development of diabetic
complications. The accumulation of AGEs has been reported to be
more apparent in the elderly and those with diabetic complications,
arteriosclerotic diseases and Alzheimer’s disease (1,2).
Numerous studies have revealed the association of AGEs with
neurodegenerative diseases (3–5), the
growth and metastasis of malignant tumors (6) and inflammatory reactions. In the
process of generating AGEs, Amadori rearrangement-induced compounds
generate 3-deoxyglucosone (3-DG) (7). 3-DG is considered to be a significant
reaction intermediate since its generation is associated with
oxidative stress, it is cytotoxic (8) to cancer and normal cells, and it
demonstrates mutagenic properties.
Natural products, such as crude drugs, reportedly
include organic compounds with significant antioxidant properties.
Among them, flavonoid compounds from Rutaceae, such as the
Citrus genus, exhibit biological activity in various fields
and their antioxidant properties are well known in the prevention
of chronic disease progression (9).
Daily consumption of flavonoids has also been reported to reduce
the risk of chronic diseases, including cardiovascular diseases and
cancer. In particular, polymethoxyflavone, which is a component of
citrus fruits (10), has been
reported to reduce inflammation (11) and have an antiproliferative effect
on human cancer cells (12).
Rosmarinic acid, which is a phenylpropanoid
derivative, is generally present in Boraginaceae and Lamiaceae and
exhibits a variety of biological activities, including significant
anti-inflammatory and antioxidant effects. With regards to the
mechanisms of the anti-inflammatory effect, inhibition of
5-lipoxygenase (13), inhibition of
histamine release from mast cells and the scavenging of superoxide
radicals (also known as the antioxidant effect) (14) have been reported previously.
The present study, based on the aforementioned, aim
to examine the inhibitory effects of flavonoids, stilbenes and
caffeic acid polymers, which are known as natural antioxidant
substances, on non-enzymatic amino acid glycation.
Materials and methods
Materials
Nobiletin (15),
piceatannol (16) were purified
from a peel of Citrus unshiu and Callistemon rigidus,
respectively. Rosmarinic acid and lithospermic acid (17) were purified from Lithospermum
erythrorhizon. Bovine serum albumin (BSA) and aminoguanidine
(Sigma-Aldrich, St. Louis, MO, USA); baicalin, quercetin and
naringin (Nacalai Tesque, Inc., Kyoto, Japan); and baicalein,
kaempferol, hesperidin and hesperetin (Wako Pure Chemical
Industries, Ltd., Osaka, Japan) were purchased. Lithospermic acid B
and epirabdosiin were provided by Dr Tabata (Hokkaido Mitsui
Chemicals, Inc., Hokkaido, Japan).
Measurement of
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging
activity
A modified version of a previous study (18) was used to measure the free
radical-scavenging activity of the pure compounds with DPPH. In a
96-well microplate, 100 μl sample at different concentrations (in
absolute MeOH) was added into wells containing 100 μl 0.06 mM DPPH
in MeOH and mixed well. The absorbance was measured at 510 nm
exactly by a microplate reader (ImmunoMini NJ-2300; Biotech Co.,
Ltd., Tokyo, Japan) after 30 min and the percentage inhibition was
calculated. IC50 values express the sample concentration
required to scavenge 50% of the DPPH-free radicals. All the samples
were assessed in triplicate.
AGE production inhibitory assay
The assay was carried out in accordance with a
previous study (19). D-Glucose
(10%) and BSA (1%) were dissolved in phosphate-buffered saline
(PBS) (pH 7.2). The samples were dissolved in PBS with 5% DMSO. The
glucose-BSA solution (900 μl) and sample solution (100 μl) were
mixed under asepsis and the mixture was incubated for 22 weeks at
37°C with 100% humidity. The assay samples were collected at 0, 1,
3, 5, 9 and 22 weeks. Fluorescence (F) was measured at 440 nm,
excited at 375 nm, following the dilution of the mixture (1:10).
The inhibitory activity was calculated as follows: Inhibitory
activity (%) = [1 − (F sample − F sampleBlank)/(F
control − F normal)] × 100. For the control (F control), the PBS
buffer was used instead of the sample solution in PBS. The F sample
constituted the sample solution and glucose-BSA solution; F
sampleBlank was the sample solution without incubation;
and F normal was the glucose-BSA solution without incubation. The
detection of 3-DG was performed in accordance with a previous study
(20). Briefly, following the
sample collection, 3-DG was labeled by 2,3-diaminonaphthalene for
14 h at 4°C, using 0.05% 2,3-butanedione as an internal standard.
The reaction mixture was analyzed by high-performance liquid
chromatography (HPLC) [column, COSMOSIL 5PE-MS, 4.6×250 mm (Nacalai
Tesque, Inc.); mobile phase, 50 mM phosphorus
acid-CH3CN-MeOH (60:20:20), 1.0 ml/min; detector, UV at
268 nm] at 20°C.
Statistics
IC50 values were expressed as the mean of
triplicate experiments in DPPH-radical scavenging activity. The
fluorescence values of advanced glycation-end products were
expressed as the mean of triplicate experiments. The values of 3-DG
formation were expressed as the mean ± standard error of the mean.
Significance was tested by assessing the effects of the compounds
compared to aminoguanidine, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of flavonoids and caffeic acid
polymers on DPPH radical-scavenging activity
The inhibitory effects of eight types of flavonoid,
two types of stilbene and five types of caffeic acid polymer were
examined on the DPPH radical-scavenging actvity. The results are
shown in Table I. The
IC50 of quercetin was the highest for the flavonoids, at
53.75 μM. Stilbenes showed stronger antioxidant effects than
flavonoids; the IC50 values of piceatannol and
resveratrol were 46.8 and 47.16 μM, respectively. Among the caffeic
acid polymers, lithospermic acid B exhibited the strongest
inhibitory effect, followed by epirabdosiin, lithospermic acid,
rosmarinic acid and caffeic acid. In particular, lithospermic acid
B showed the strongest radical-scavenging effect, with an
IC50 of 10.26 μM. The IC50 values of
epirabdosiin and lithospermic acid were 12.38 and 34.26 μM,
respectively, suggesting that the radical-scavenging effects are
enhanced depending on the degree of polymerization.
 | Table IDPPH-radical scavenging activity of
plant-polyphenolsa. |
Table I
DPPH-radical scavenging activity of
plant-polyphenolsa.
| Polyphenols | IC50,
μM |
|---|
| Baicalin | 60.06 |
| Baicalein | 72.96 |
| Nobiletin | >100 |
| Kaempferol | 173.18 |
| Quercetin | 53.75 |
| Naringin | >100 |
| Hesperidin | >100 |
| Hesperetin | >100 |
| Piceatannol | 46.80 |
| Resveratrol | 47.16 |
| Caffeic acid
(CA) | 93.78 |
| Rosmarinic acid
(RA) | 43.68 |
| Lithospermic acid
(LA) | 34.26 |
| Lithospermic acid B
(LAB) | 10.26 |
| Epirabdosiin
(RAB) | 12.38 |
Effects of flavonoids and caffeic acid
polymers on the generation of 3-DG
Sugar-dicarbonyls, such as 3-DG, are useful markers
for the effects of protein glycation inhibitors and defense
mechanisms against protein glycation in the body as they show
high-level reactivity. A sample was collected 20 weeks after
initiating the incubation and 3-DG was derivatized in the sample
with 2,3-diaminoaphthalene to examine the effect of the flavonoids,
stilbenes and caffeic acid polymers using HPLC. 2,3-Butanedione was
used as an internal standard. The amount of 3-DG generated by BSA
(50 mg/ml) and glucose (500 μM) was suppressed by the addition of
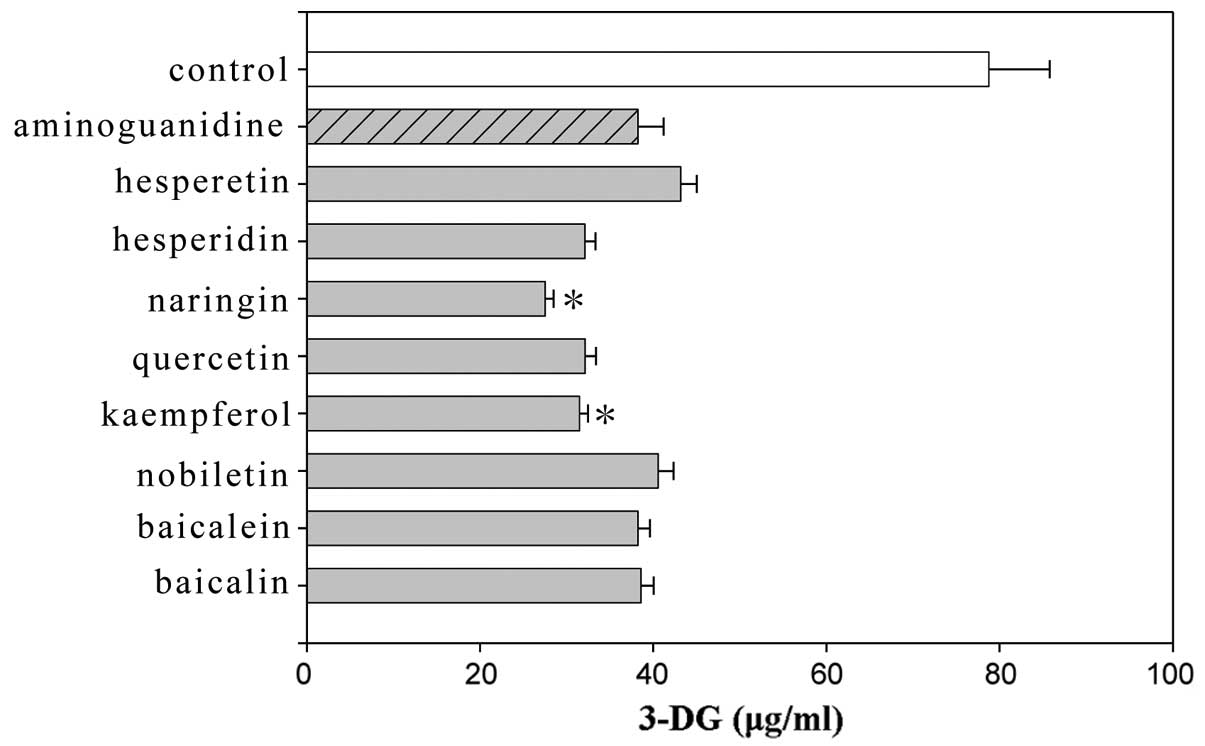
these compounds as compared to the control. Among the flavonoids,
kaempferol and naringin showed significant differences compared to
aminoguanidine (Fig. 1). Among the
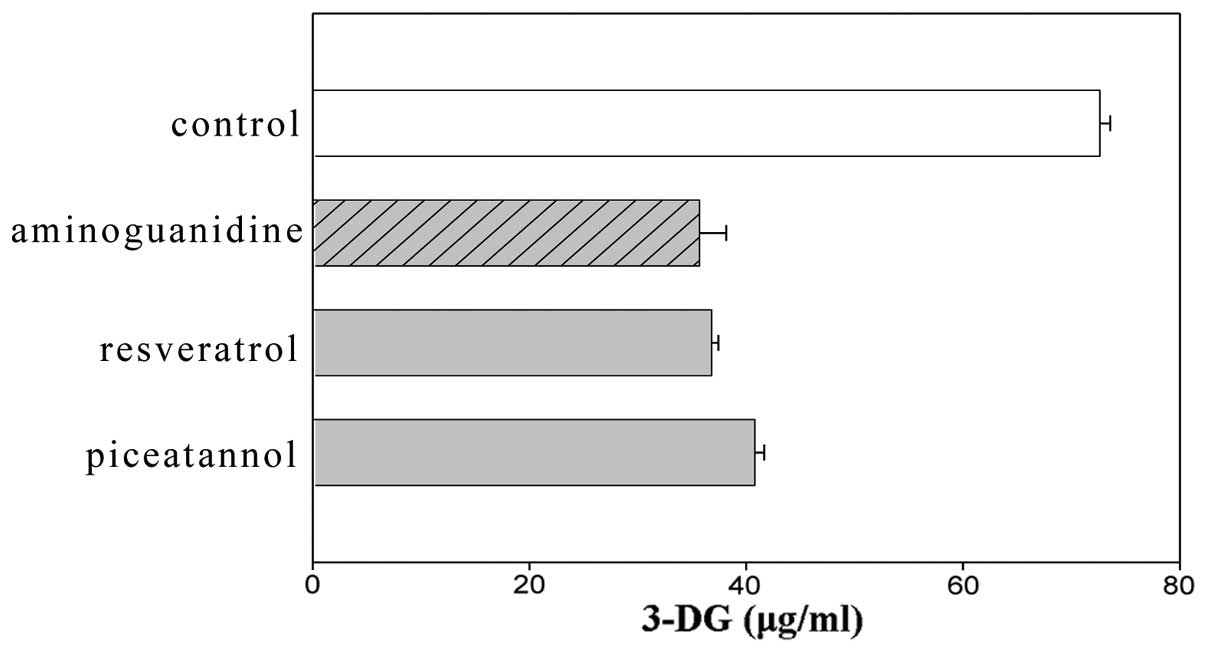
stilbenes, resveratrol and piceatannol exhibited suppressing
effects that were as strong as aminoguanidine (Fig. 2). Among the caffeic acid polymers,
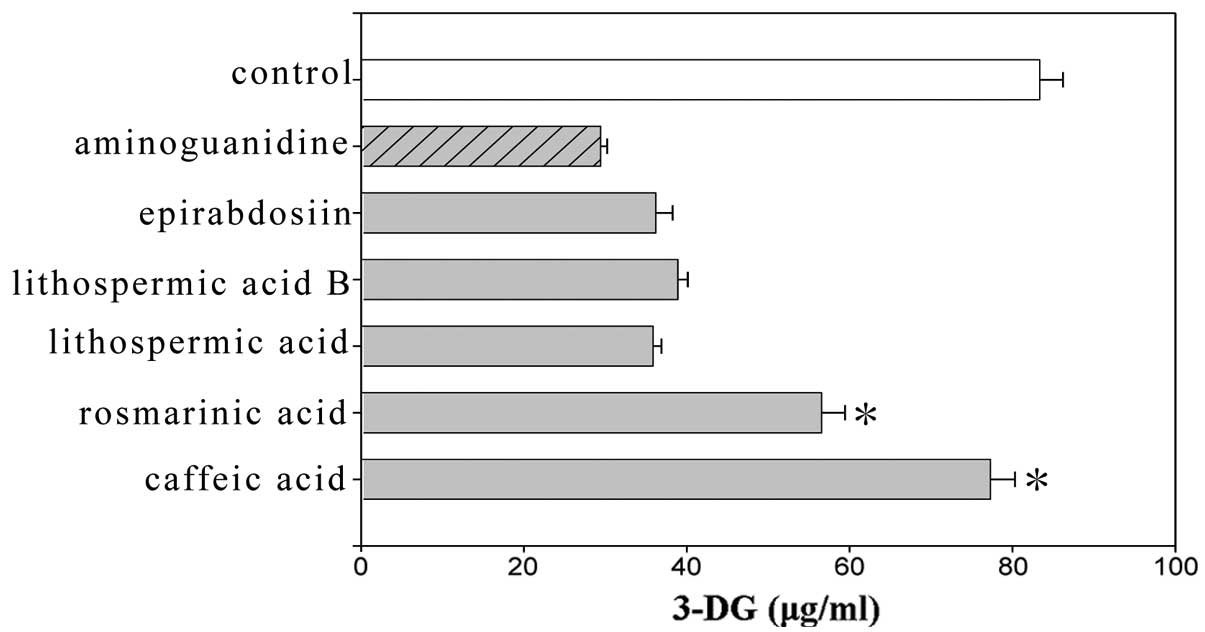
lithospermic acid, lithospermic acid B and epirabdosiin showed
significant differences, whereas caffeic acid and rosmarinic acid
did not. The amount of 3-DG generated with lithospermic acid,
lithospermic acid B and epirabdosiin was 35.84, 38.89 and 36.2
μg/ml, respectively, compared to 83.34 μg/ml with the control
(Fig. 3).
Effects of the flavonoids and caffeic
acid polymers on the generation of AGE-derived fluorescent
materials
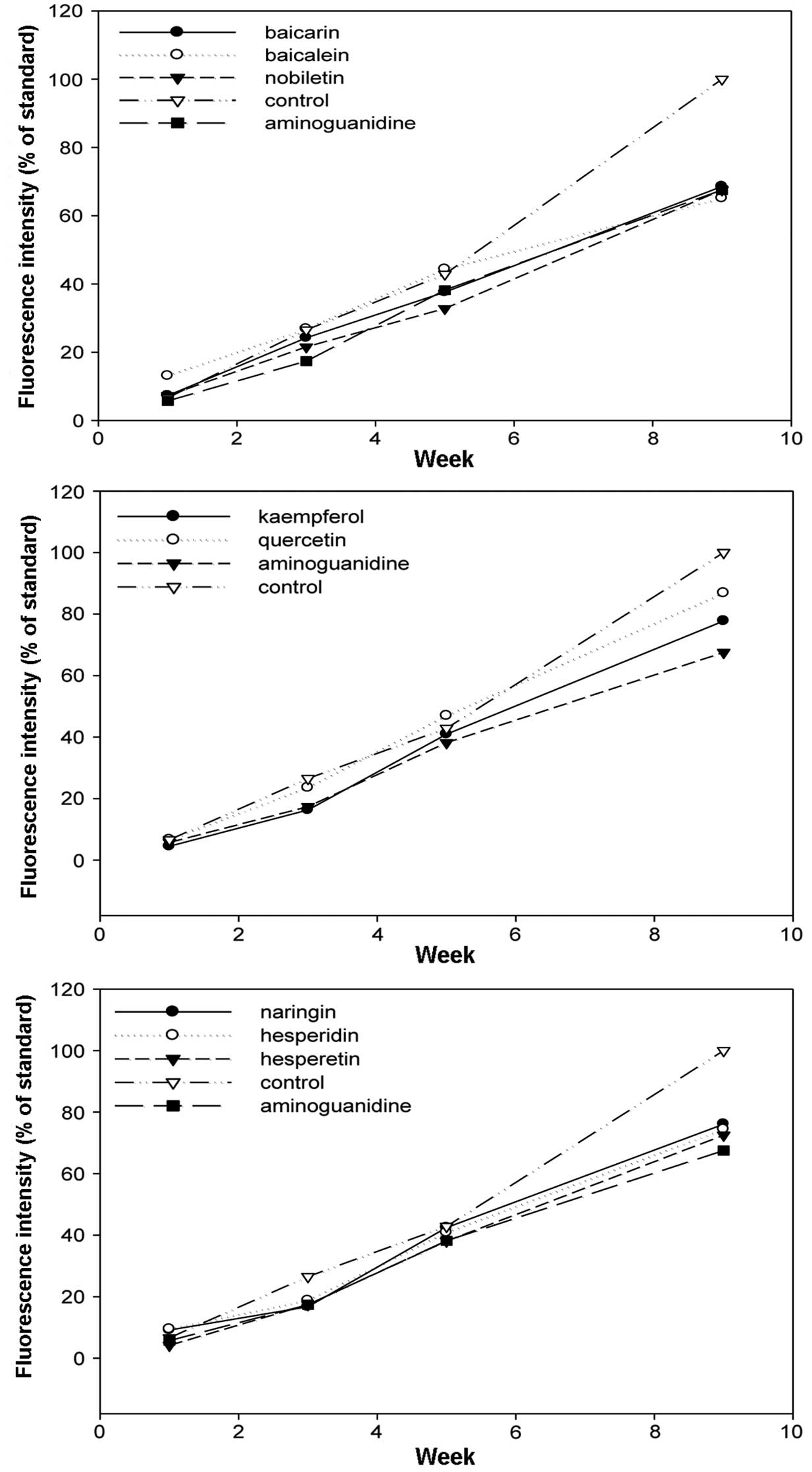
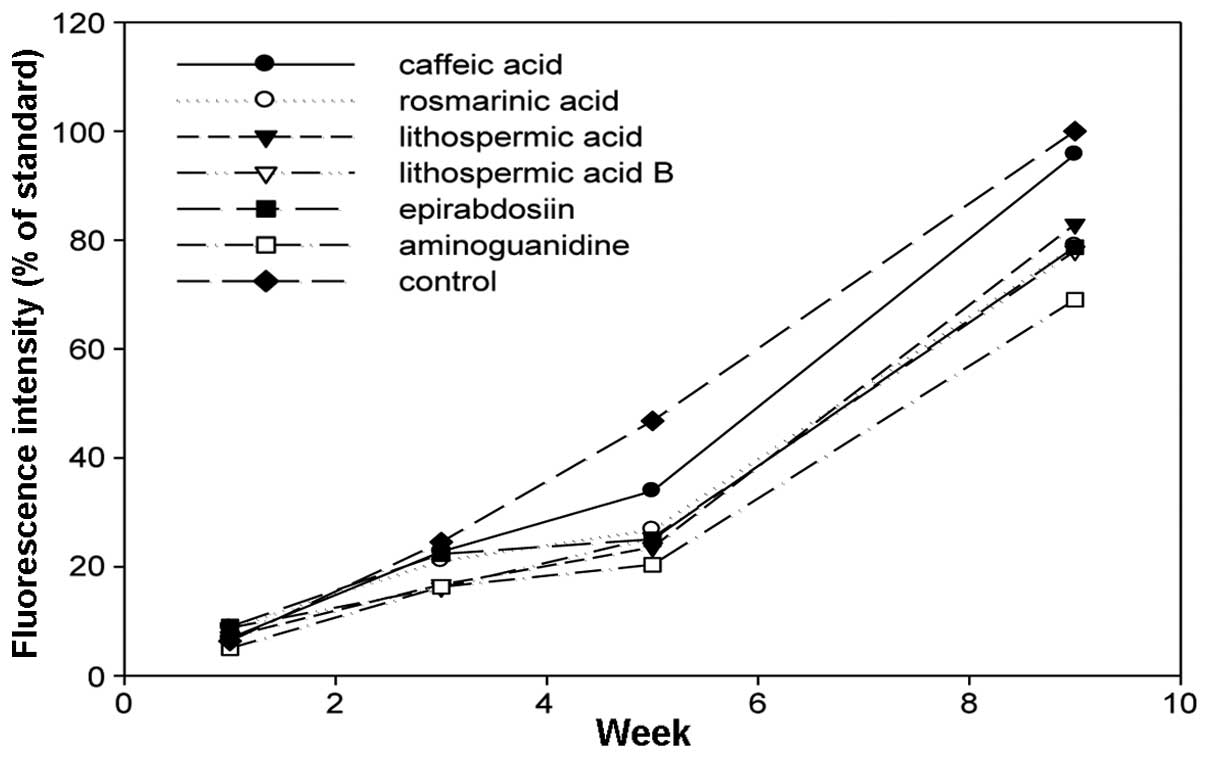
The increase of fluorescence attributed to
AGE-derived fluorescent materials was examined with fluorescence
spectrophotometry 1, 3, 4 and 9 weeks after the initiation of the
incubation. In all the flavonoids, stilbenes and caffeic acid
polymers compounds, the intensity of the fluorescence decreased
compared to the control (Figs.
4–6). Aminoguanidine, which was
a positive control, inhibited the intensity of AGE-derived
fluorescence by ~30% 9 weeks after the initiation of the
incubation. The amino groups of aminoguanidine bind to the keto
group of an Amadori product (ketoamine) in the early phase of
protein glycation and the keto group of 3-DG in the late phase, and
the subsequent reactions are considered to inhibit the progression
(21). Baicalin, baicalein and
nobiletin, which are flavonoids, inhibited the intensity of
AGE-derived fluorescence by 31.4, 34.8 and 32.4%, respectively. For
the stilbenes, piceatannol showed 27.8% inhibition, which was
similar to the inhibition exhibited by aminoguanidine, and
resveratrol showed 44.2% inhibition, which was stronger compared to
aminoguanidine. The inhibitory effects of the caffeic acid polymers
were low compared to those of the flavonoids and stilbenes, at
~20%.
Discussion
Flavonoids, stilbenes and caffeic acid polymers all
have antioxidant properties, and it is suggested that they inhibit
the generation of AGEs by inhibiting oxidative stress reactions
during the Maillard reaction and by suppressing the progression of
these reactions in the later phases. Oxidation is not involved in
the formation of Amadori rearrangement compounds, whereas oxidation
is associated with the generation of AGEs, including pentosidine
(22) and carboxymethyllysine
(23), and fluorescent as well as
bridged structures, which are certain AGE characteristics (24). As the Amadori compounds are
converted to AGEs by active oxygen, they become sources of active
oxygen. Highly-reactive intermediates, including 3-DG, rapidly
promote the generation of AGEs and AGEs that fluoresce by
generating 3-DG, such as pentosidine and pyrropyridine, increase as
the reaction progresses. The majority of the plant polyphenols used
in the present study strongly suppressed 3-DG generation and, as a
result, the generation of AGEs. However, AGEs are assumed to be
generated through the reaction pathway for the auto-oxidation of
glucose, which is not associated with 3-DG. In this pathway,
highly-reactive dicarbonyl compounds, including glyoxal and
methylglyoxal, react with proteins and generate AGEs (25). Therefore, a clear positive
correlation could not be shown between the antioxidant property and
inhibition of AGE generation. These examined compounds are assumed
to suppress AGE generation by inhibiting the increase of 3-DG
through a specific unknown mechanism in the early phase of the
Maillard reaction, by inhibiting the generation of active oxygen in
the later phase and by suppressing the progression of the reaction.
Further examinations are required to determine whether each
compound affects both the early and late phases, or only one of
them.
Flavonoids are oxidized by a direct reaction with
free radicals, the free radicals are stabilized and the active
oxygen species are directly eliminated. The possibility that these
plant polyphenols directly bind to proteins (26), which are substrates that would
inhibit the bonding of sugars and proteins and suppress the
promotion of the Maillard reaction, was also suggested. Caffeic
acid polymers have a tannic effect. The interactions between
tannins and proteins are associated with hydrogen bonding and
hydrophobic interactions based on van der Waals forces, and the
repelling of water and polymerization generally intensifies the
tannic effect (27). The
involvement of the carboxyl group and its steric structure, as well
as the degree of polymerization, have been suggested to have
important roles in inhibiting the Maillard reaction.
These compounds are generally consumed more as
multiple components rather than individually. Regular, daily
consumption of these compounds in the form of a food or extract is
expected to aid in the prevention or inhibition of non-enzymatic
amino acid glycation in the living body, which is possibly
associated with aging, diabetic complications, arteriosclerotic
diseases and Alzheimer’s disease, and they may also be effective
agents in cosmetics promoting anti-aging.
References
|
1
|
Barlovic DP, Soro-Paavonen A and
Jandeleit-Dahm KA: RAGE biology, atherosclerosis and diabetes. Clin
Sci (Lond). 121:43–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vitek MP, Bhattacharya K, Glendening JM,
Stopa E, Vlassara H, Bucala R, Manogue K and Cerami A: Advanced
glycation end products contribute to amyloidosis in Alzheimer
disease. Proc Natl Acad Sci USA. 91:4766–4770. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki N, Toki S, Chowei H, Saito T,
Nakano N, Hayashi Y, Takeuchi M and Makita Z: Immunohistochemical
distribution of the receptor for advanced glycation end products in
neurons and astrocytes in Alzheimer’s disease. Brain Res.
888:256–262. 2001.PubMed/NCBI
|
|
4
|
Sasaki N, Takeuchi M, Chowei H, Kikuchi S,
Hayashi Y, Nakano N, Ikeda H, Yamagishi S, Kitamoto T, Saito T and
Makita Z: Advanced glycation end products (AGE) and their receptor
(RAGE) in the brain of patients with Creutzfeldt-Jakob disease with
prion plaques. Neurosci Lett. 326:117–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kikuchi S, Shinpo K, Ogata A, Tsuji S,
Takeuchi M, Makita Z and Tashiro K: Detection of N
epsilon-(carboxymethyl)lysine (CML) and non-CML advanced glycation
end-products in the anterior horn of amyotrophic lateral sclerosis
spinal cord. Amytroph Lateral Scler Other Motor Neuron Disord.
3:63–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abe R, Shimizu T, Sugawara H, Watanabe H,
Nakamura H, Choei H, Sasaki N, Yamagishi S, Takeuchi M and Shimizu
H: Regulation of human melanoma growth and metastasis by AGE-AGE
receptor interactions. J Invest Dermatol. 122:461–467. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dyer DG, Blackledge JA, Thorpe SR and
Baynes JW: Formation of pentosidine during nonenzymatic browning of
proteins by glucose. Identification of glucose and other
carbohydrates as possible precursors of pentosidine in vivo. J Biol
Chem. 266:11654–11660. 1991.PubMed/NCBI
|
|
8
|
Kato H, van Chyuyen N, Shinoda T, Sekiya F
and Hayase F: Metabolism of 3-deoxyglucosone, an intermediate
compound in the Maillard reaction, administered orally or
intravenously to rats. Biochem Biophys Acta. 1035:71–76. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rice-Evans C, Spencer JP, Schroeter H and
Rechner AR: Bioavailability of flavonoids and potential bioactive
forms in vivo. Drug Metabol Drug Interact. 17:291–310. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang GJ, Han SC, Yi EJ, Kang HK and Yoo
ES: The inhibitory effect of premature Citrus unshiu extract
on atopic dermatitis in vitro and in vivo. Toxicol Res. 27:173–180.
2011.
|
|
11
|
Baek SH, Kim SM, Nam D, Lee JH and Ahn KS,
Choi SH, Kim SH, Shim BS, Chang IM and Ahn KS: Antimetastatic
effect of nobiletin through the down-regulation of CXC chemokine
receptor type 4 and matrix metallopeptidase-9. Pharm Biol.
50:1210–1218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manthey JA and Guthrie N:
Antiproliferative activities of citrus flavonoids against six human
cancer cell lines. J Agric Food Chem. 50:5837–5843. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto H, Sakakibara J, Nagatsu A and
Sekiya K: Inhibitors of arachidonate lipoxygenase from defatted
perilla seed. J Agric Food Chem. 46:862–865. 1998. View Article : Google Scholar
|
|
14
|
Nakamura Y, Ohto Y, Murakami A and
Ohigashi H: Inhibitory effects of curcumin and
tetrahydrocurcuminoids on the tumor promoter-induced reactive
oxygen species generation in leukocytes in vitro and in vivo. Jpn J
Cancer Res. 89:361–370. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasaki K and Yoshizaki F: Nobiletin as a
tyrosinase inhibitor from the peel of Citrus fruit. Biol Pharm
Bull. 25:806–808. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi K, Ishihara T, Khono E, Miyase T
and Yoshizaki F: Constituents of stem bark of Callistemon
rigidus showing inhibitory effects on mouse alpha-amylase
activity. Biol Pharm Bull. 29:1275–1277. 2006.PubMed/NCBI
|
|
17
|
Murata T, Sasaki K, Sato K, Yoshizaki F,
Yamada H, Mutoh H, Umehara K, Miyase T, Warashina T, Aoshima H, et
al: Matrix metalloproteinase-2 inhibitors from Clinopodium
chinense var. parviflorum. J Nat Prod. 72:1379–1384.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SK, Mbwambo ZH, Chung H, Luyengi L,
Gamez EJ, Mehta RG, Kinghorn AD and Pezzuto JM: Evaluation of the
antioxidant potential of natural products. Comb Chem High
Throughput Screen. 1:35–46. 1998.PubMed/NCBI
|
|
19
|
Valencia JV, Weldon SC, Quinn D, Kiers GH,
DeGroot J, TeKoppele JM and Hughes TE: Advanced glycation end
product ligands for the receptor for advanced glycation end
products: biochemical characterization and formation kinetics. Anal
Biochem. 324:68–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamada H, Miyata S, Igaki N, Yatabe H,
Miyauchi Y, Ohara T, Sakai M, Shoda H, Oimomi M and Kasuga M:
Increase in 3-deoxyglucosone levels in diabetic rat plasma.
Specific in vivo determination of intermediate in advanced Maillard
reaction. J Biol Chem. 269:20275–20280. 1994.PubMed/NCBI
|
|
21
|
Edelstein D and Brownlee M: Mechanistic
studies of advanced glycosylation end product inhibition by
aminoguanidine. Diabetes. 41:26–29. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sady C, Khosrof S and Nagaraj R: Advanced
Maillard reaction and crosslinking of corneal collagen in diabetes.
Biochem Biophys Res Commun. 214:793–797. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmed MU, Thorpe SR and Baynes JW:
Identification of N epsilon-carboxymethyllysine as a degradation
product of fructoselysine in glycated protein. J Biol Chem.
261:4889–4894. 1986.PubMed/NCBI
|
|
24
|
Reddy S, Bichler J, Wells-Knecht KJ,
Thorpe SR and Baynes JW: N epsilon-(carboxymethyl)lysine is a
dominant advanced glycation end product (AGE) antigen in tissue
proteins. Biochemistry. 34:10872–10878. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thornalley PJ, Langborg A and Minhas HS:
Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the
glycation of proteins by glucose. Biochem J. 344:109–116. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei J, Jin F, Wu Q, Jiang Y, Gao D and Liu
H: Molecular interaction study of flavonoid derivative 3d with
human serum albumin using multispectroscopic and molecular modeling
approach. Talanta. 126:116–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Richard T, Lefeuvre D, Descendit A,
Quideau S and Monti JP: Recognition characters in
peptide-polyphenol complex formation. Biochim Biophys Acta.
1760:951–958. 2006. View Article : Google Scholar : PubMed/NCBI
|